Abstract
The cDNA for the dopamine D3 receptor was isolated and characterized in 1990. Subsequent studies have indicated that D3 receptors, as well as D3 receptor mRNA, are primarily localized in limbic regions in mammals. This finding led to the postulate that D3 receptors may be involved in drug dependence and addiction. However, this hypothesis has been difficult to test due to the lack of compounds with high selectivity for central D3 receptors. The interpretation of results from studies using mixed D2/D3 agonists and/or antagonists is problematic because these agents have low selectivity for D3 over D2 receptors and it is likely that their actions are primarily related to D2 receptor antagonism and possibly interaction with other neurotransmitter receptors. Currently, with the synthesis and characterization of new highly selective D3 receptor antagonists such as SB-277011-A this difficulty has been surmounted. The purpose of the present article is to review, for the first time, the effects of various putative D3 receptor selective compounds in animal models of drug dependence and addiction. The results obtained with highly selective D3 receptor antagonists such as SB-277011-A, SB-414796, and NGB-2904 indicate that central D3 receptors may play an important role in drug-induced reward, drug-taking, and cue-, drug-, and stress-induced reinstatement of drug-seeking behavior. Provided these results can be extrapolated to human drug addicts, they suggest that selective DA D3 receptor antagonists may prove effective as potential pharmacotherapeutic agents to manage drug dependence and addiction.
Keywords: Addiction, Brain stimulation reward, Conditioned place preference, Dopamine D3 receptors, SB-277011-A, Self-administration
1. Introduction: drug addiction, the mesolimbic DA system, and the DA D3 receptor
Drug addiction is a dynamic phenomenon characterized by several key stages: (1) initiation or acquisition of drug-taking, (2) compulsive drug taking, and (3) drug taking coupled with a marked narrowing of the behavioral repertoire. The behavioral progression typically ends with excessive drug intake, loss of control over intake, and vulnerability to relapse [156]. One of the main challenges in drug dependence research is to understand the psychobiological dysregulation, and by extension, the molecular, cellular, and system processes that underlie these various phases.
In order to assess the neuroadaptations occurring within the brain reward systems in response to acute and repeated exposure to drugs of abuse, one must first understand the neurobiological bases of drug reward [183]. Consequently, molecular and cellular approaches have emphasized differences in the primary sites of action of drugs of abuse [52]. In contrast, the neural systems approach has explored the possible commonalities of the effects of such drugs [213]. The major focus of most of these investigations has been the mesocorticolimbic dopamine (DA) system originating from the ventral tegmental area (VTA) and projecting towards a wide range of limbic and telencephalic structures including the olfactory tubercle, the amygdala, frontal and limbic cortices, especially the medial prefrontal cortex (mPFC), and the nucleus accumbens (NAc). The NAc occupies a prominent position in the ventral striatum and is a main target of the mesotelencephalic DA system. As such, DA neurotransmission in the NAc provides the framework for theories exploring the chemoarchitectural substrates of reward and motivation, including aspects of drug addiction.
The role of mesolimbic DA in general reinforcement processes clearly pointed towards DA receptors as potential targets for the study of drug consumption and craving. After the cloning of the D1 and D2 receptors [39,204,315] several additional low-abundance DA receptors were identified. These new subtypes included the D3 and D4 receptors, which are homologous to the D2 receptor, and the D5 receptor, which is homologous to the D1 receptor [273,286,292]. A growing body of evidence suggests strongly that the DA D3 receptor is significantly involved in mechanisms of drug dependence and abuse [6,10,41,43,84,131,167,295]. These findings have underlined the need for selective tools to investigate the role of the DA D3 receptor in drug dependence. The present review will not cover molecular biological (gene organization, receptor synthesis, receptor isoforms, and protein structure) or cellular signaling mechanisms associated with the DA D3 or other DA receptor subtypes (see [175]), but will specifically focus on the rationale for the use of selective DA D3 receptor antagonists as potential pharmacotherapeutic agents to manage drug dependence and addiction. The review will start with a discussion of the selective distribution of the D3 receptor in the mesolimbic DA system. Functional pharmacological aspects of different DA D3 and mixed D3/D2 receptor agonists and antagonists will then be summarized in the context of drug addiction paradigms. Finally, potential sites of action of DA D3 receptor antagonists in the brain will be discussed.
2. Localization of DA D3 receptors
The DA D3 receptor was initially cloned from a rat cDNA library by using probes derived from the DA D2 receptor sequence [273]. The cloning of the human D3 receptor was reported thereafter [112], followed by the murine D3 receptor [100]. Molecular neurobiology techniques permitted the study of the D3 receptor in vitro by transfection in cells that do not normally express DA receptors. Molecular methods also allowed the study of receptor messenger ribonucleic acid (mRNA) in the brain.
2.1. Rodent DA D3 receptor localization under basal condition
The greatest densities of D3 mRNA in rat brain are found primarily in limbic brain areas such as the NAc (rostral pole and parts of shell), islands of Calleja, and olfactory tubercle [30,66,77,78,162,197,233,273]. Other brain areas reported to contain high levels of D3 mRNA include the medial and ventral lateral geniculate nuclei, mammillary nucleus, magnocellular preoptic nucleus, lateral substantia nigra pars compacta, dorsal cochlear nuclei, Purkinje cell layer of the vestibulocerebellum, paracentral thalamic nucleus, bed nucleus of the stria terminalis (BNST), and vertical limb of the diagonal band of Broca [30,77,197]. Bouthenet et al. [30] have reported moderate levels of D3 mRNA in the amygdala, ventral pallidum, various thalamic and hypothalamic nuclei, superior colliculus, inferior olivary nucleus, and nucleus of the horizontal limb of the diagonal band of Broca. Transcripts for the D3 receptor are located in the mesencephalic areas rich in DA cell bodies [77,78,282]. The restricted localization of the D3 receptor has led to the hypothesis that it may play an important role in emotion, cognition, and addiction [117,167,231,232,253,273,275].
In vitro homogenate and autoradiographic studies indicate that DA D3 receptors are expressed in highest densities in the islands of Calleja, olfactory bulb, NAc, and intermediate lobe of the pituitary [20,27,106,107,117,162, 173,177,179,182,252,258,282] (for reviews, see Refs. [175,258]). The lateral aspect of the substantia nigra pars compacta and the molecular layer of the vestibulocerebellum also contain moderate levels of D3 receptors.
2.2. Rodent DA D3 receptor localization following drug exposure
DA D3 mRNA and receptors are increased in cocaine cue conditioned locomotion [168]. A recent series of experiments has also shown that termination of a cocaine self-administration regimen increases DA D3 binding over time in the NAc core and ventral caudate–putamen, an adaptive change that may occur through regulatory responses to an increase in phasic DA levels associated with cocaine-taking and -seeking behavior [212]. In addition, nicotine-induced conditioned locomotion [169] and nicotine behavioral sensitization [170] are both associated with significant increases in D3 receptor binding and mRNA levels in the NAc shell without altering D1 or D2 receptor mRNA in the NAc shell or core subregions. Furthermore, twice daily morphine administration over 8 consecutive days with an escalating dosing regimen starting at 10 mg/kg was shown to produce a significant increase in D3 receptor mRNA in the caudate–putamen and ventral midbrain, including the substantia nigra and VTA. Morphine also produced a 25% decrease in D2 receptor mRNA in the caudate–putamen, but no alterations were seen in mRNA levels related to tyrosine hydroxylase or the DA transporter [278].
2.3. Human DA D3 receptor localization under basal condition
Expression of DA D3 receptor mRNA in the human brain follows a similar pattern as in the rodent brain [120,162, 194,287]. High levels are present in the islands of Calleja, ventral striatum/NAc, dentate gyrus, and striate cortex. Low to moderate densities are present in the caudate–putamen, anterior and medial thalamic nuclei, amygdala, hippocampal CA region, cortical regions (particularly the anterior cingulate and subcallosal gyrus), lateral geniculate body, substantia nigra pars compacta, locus ceruleus, and median raphé. Receptor binding data also indicate the presence of D3 receptors in the NAc, internal globus pallidus, ventral pallidum, septum, islands of Calleja, amygdala, and VTA [120,124,132,162,205]. Interestingly, significant inter-species differences have been reported in the distribution of D3 receptors [176]. For example, although rats, mice, guinea pigs, and humans show similar distributions of D3 sites in the basal ganglia and limbic forebrain, notable differences are typically observed in hypothalamic, thalamic, and mesencephalic brain areas. Furthermore, the detection of D3 sites in the vestibulocerebellum of the rat, but not other species suggests a possible role of DA D3 receptors in the control of posture, muscle tone, or eye movements in the rat.
2.4. Human DA D3 receptor localization following drug exposure
Postmortem studies using [3H]-(+)-7-OH-DPAT have shown an increase in the number of D3 receptors in the NAc [190,256] and specific areas of the striatum and substantia nigra [281] in cocaine overdose fatalities compared to drug-free and age-matched controls. A significant elevation in the levels of D3 receptor mRNA of human cocaine fatalities has also been reported [256] but was not corroborated by another study [195]. The discrepancy between these two studies might be related to the different methods used to determine levels of D3 receptor mRNA (reverse transcription-polymerase chain reaction (RT-PCR) [256] vs. in situ hybridization [195]). A human postmortem study comparing DA receptor density between smokers and nonsmokers revealed no significant differences in D3 receptors [63]. In that study, analysis of groups and areas for D3 receptor binding in the striatum indicated a significant difference between areas, but no difference between smokers, exsmokers and nonsmokers was observed and there was no interaction between groups and areas. However, [3H]-7-OH-DPAT binding was focused mainly on the putamen and caudate rather than the NAc. A recent study measured the expression of dopamine D3 receptor mRNA in peripheral blood lymphocytes by real-time polymerase chain reaction in smokers, former smokers, and nonsmoking control subjects [69]. The results of this study revealed a significant reduction in DA D3 receptor mRNA expression in smokers, but not former smokers compared with controls. Furthermore, the expression of DA D3 receptor mRNA in smokers was negatively correlated with daily number of cigarettes, suggesting a selective inhibiting effect of smoking on the expression of DA D3 receptor mRNA. Additional studies looking at the density of DA D3 receptors in smokers vs. nonsmokers are clearly warranted.
The above findings suggest that the distribution pattern of DA D3 receptors in both rodent and human brain is compatible with a major role in emotion, cognition, and processing of motor and sensory information. Postmortem and preclinical studies point to the possibility that chronic abuse of cocaine, nicotine, and opioids may be associated with an adaptive change in D3 receptors.
3. In vitro pharmacological characterization of DA D3 ligands
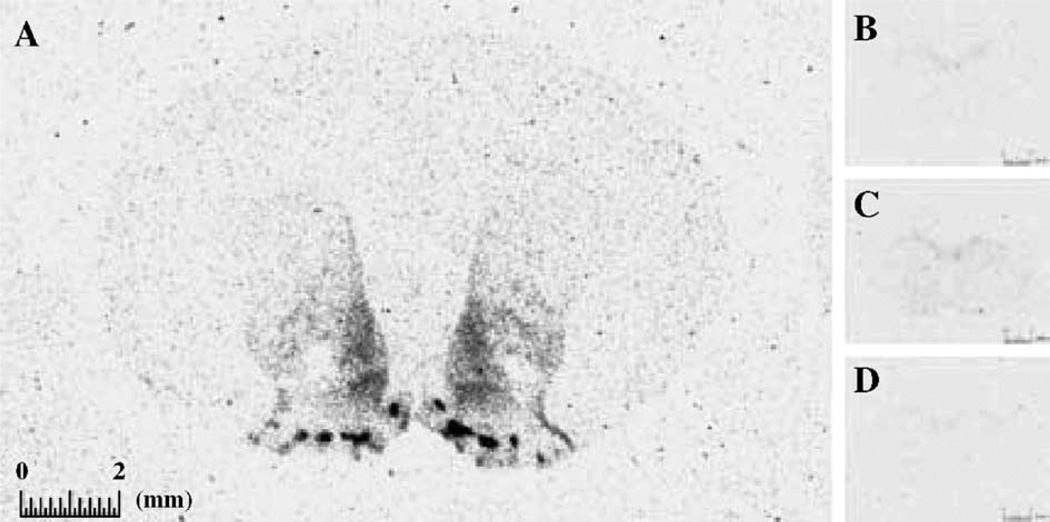
A number of agonists and antagonists have been used to characterize the pharmacology of DA D3 receptors in various expression systems and in the brain. However, results generated from these studies vary considerably and seem to be dependent upon the expression system or tissue, the radioligand, and the in vitro assay conditions used (for a detailed review, see Ref. [175]). For example, the observed D3 selectivity of several DA agonists may directly result from the use of in vitro conditions that disfavor the high-affinity conformation of the D2 receptor such as the inclusion of Na+ in some in vitro assay systems [40,180]. The greatest D2/D3 selectivity with [3H]-7-OH-DPAT and [3H]-PD128907 can also be obtained in the absence of Mg2+ and the presence of EDTA [40]. Studies have shown that although selective labeling of putative D3 sites may be reasonably obtained using [3H]-PD128907 or [3H]-7-OH-DPAT, labeling of the D2 site is also observed [20,114,250] as well as labeling of σ [250,296] and 5-HT1A receptors [72,198] in rat brain. In our hands, however, [3H]-7-OH-DPAT binding to rat brain slice preparations can be fully displaced by the highly selective DA D3 receptor antagonist SB-277011-A, at doses predicted by the affinity values of [3H]-7-OH-DPAT and SB-277011-A for rat D2 and D3 receptors [182,229], suggesting that appropriate conditions can be chosen to favor preferential [3H]-7-OH-DPAT binding to D3 receptors in receptor auto-radiography experiments (see Fig. 1 for further details).
Fig. 1.
Distribution of D3 receptors in rat brain, as revealed by [3H]-7-OH-DPAT autoradiography—displacement with the selective DA D3 receptor antagonist SB-277011. Coronal rat brain sections (at +1.70 mm from Bregma) were incubated in the presence of 0.5 nM [3H]7-OH-DPAT for 60 min at room temperature. (A) Total binding; (B) nonspecific binding, in the presence of 1 µM dopamine; (C) binding in the presence of 100 nM SB-277011-A; (D) binding in the presence of 1 µM SB-277011-A. Calibration bars correspond to 2 mm. Incubation with [3H]-7-OH-DPAT was performed in the presence of 1 mM EDTA, in order to abolish the radioligand binding to D2 receptors without affecting that to D3 receptors, as demonstrated in transfected cell lines [182]. The specificity of the radioligand binding to D3 receptors was confirmed by the distribution of [3H]-7-OH-DPAT binding sites, in line with that of D3 mRNA [30] and by displacement with the selective D3 antagonist SB-277011-A. As predicted by the affinity values of [3H]7-OH-DPAT and SB-277011-A for rat D2 and D3 receptors [182,229], 0.5 nM [3H]7-OH-DPAT binding was inhibited by 80% and 100% by 100 nM and 1 µM SB-277011-A, respectively.
In addition to radioligand binding studies, several functional assays including induction of Chinese hamster ovary (CHO) cell mitogenesis, melanocyte aggregation, or extracellular acidification rates in the microphysiometer assay have not only established the agonist or antagonist activity of a variety of DA compounds at the D3 receptor, but have also contributed to determining their D2/D3 selectivity. In contrast with the significant DA D3 selectivity reported in some binding studies, agonists such as DA, PD128907, 7-OH-DPAT, quinelorane, and (+)-UH232 exhibited only modest, if any, D3 selectivity in these functional tests [56,225,226] (for a direct comparison of selectivities obtained from radioligand binding vs. functional assays in CHO cells using the cytosensor microphysiometer, the reader is referred to Ref. [60]).
Overall, these findings clearly demonstrate that the rank order of potencies and selectivities in functional assays is not equivalent to the rank order of radioligand binding affinities and selectivities. These results further emphasize the need to consider both radioligand binding affinities and functional potencies as well as intrinsic activities in order to reliably interpret behavioral effects mediated by DA D2 and D3 receptors. Furthermore, they also further underline the need for caution in the use of in vitro binding data in the interpretation of in vivo or in vitro functional studies.
4. Role of DA D3 receptors in drug addiction: studies with mixed DA D2/D3 receptor agonists
The mixed D2/D3 agonists 7-OH-DPAT [41,42,102,219], quinpirole [41], quinelorane [43,219], pramipexole [43], and PD128907 [43] have all been shown to decrease cocaine self-administration in rats. However, the same mixed D2/D3 agonists have also led to discrepant findings in a wide range of paradigms. For example, 7-OH-DPAT (0.1 mg/kg) inhibits cocaine-seeking behavior as assessed by the conditioned place preference (CPP) paradigm [150] but reinstates intravenous (iv) cocaine self-administration behavior at doses of 3 and 10 mg/kg [257]. In contrast, Khroyan et al. [151] found that neither 7-OH-DPAT (0.01–0.1 mg/kg) nor PD128907 (0.01–0.1 mg/kg) alter cocaine-triggered reinstatement of drug-seeking behavior. Furthermore, 7-OH-DPAT (2.5–74 µg/kg) does not significantly alter brain stimulation reward (BSR) maintained by electrodes in the lateral hypothalamus in rats [128], and 7-OH-DPAT (0.05 mg/kg) given subcutaneously (sc) does not block the development of sensitization to cocaine [191]. However, 7-OH-DPAT can partially substitute for cocaine in the drug discrimination paradigm [1,105,146,161,208,269,280].
These mixed in vivo findings clearly demonstrate that the effect of the aforementioned mixed D2/D3 agonists (see Table 1) in drug addiction paradigms might be related to (1) their lack of selectivity for D3 over D2 receptors and/or (2) their ability to have incentive value per se. In support of the first argument (lack of selectivity) are the findings described in the previous section (i.e., radioligand binding studies vs. in vitro functional assays). In addition to lack of D3 receptor selectivity under in vitro assay conditions, both 7-OH-DPAT and PD128907 may activate D2 receptors in vivo in rats as a function of the doses used in different behavioral paradigms [2,19,36,71,73,95,146,159,180,181,203,228,293]. For example, behavioral characterizations of both 7-OH-DPAT and PD128907 up to 10 mg/kg revealed U-shaped dose– response curves for both compounds [2,71,228], suggesting activation of D3 receptors at low doses and increasing D2 receptor occupancy at higher doses. This hypothesis is further supported by studies based upon D2 receptor protection from N-ethoxycarbonyl-2-ethoxyl-1,2-dihydro-quinilone (EEDQ) alkylation, suggesting that 7-OH-DPAT doses below 0.3 mg/kg are devoid of significant D2 receptor occupancy [181]. Similarly, studies using DA D3 knockout mice showed that intraperitoneal (ip) doses of PD128907 in the range of 0.03–0.1 mg/kg affect DA release in wild type, but not knockout mice, suggesting D3-mediated effects of PD128907 when given at sufficiently low dose [314]. Furthermore, 7-OH-DPAT and PD128907 doses below 10 µg/kg have been reported to produce inhibition of novelty-stimulated locomotion in wild type, but not in D3 receptor knockout mice [227]. All other D3 knockout studies using 7-OH-DPAT or PD128907 doses at least 10-fold higher reported that neither 7-OH-DPAT nor PD128907 inhibit locomotion through selective D3 receptor stimulation [28,29], and hence concluded that locomotor inhibitory affects of 7-OH-DPAT are mediated through D2 autoreceptors or other receptors.
Table 1.
Representative DA D2/D3 receptor agonists and antagonists, the partial agonist BP-897, and selective DA D3 receptor antagonistsa
| Ligand Name | Ki hD2 (nM) | Ki hD3 (nM) | D2:D3 Ki ratios | Chemical structure |
|---|---|---|---|---|
| Mixed DA D2/D3 agonists | ||||
| 7-OH-DPATb | [125I]-iodosulpiride = 92 | [3H]-PD128907 = 0.34 | [3]-PD128907 = 302 | 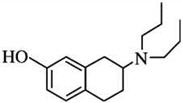 |
| [3H]-S14297= 1 | [3H]-S14297 = 103 | |||
| [125I]-iodosulpiride = 2.2 | [125I]-iodosulpiride = 47 | |||
| PD 128907c | [125I]-iodosulpiride = 339 | [3H]-PD128907= 1.33 | [3H]-PD128907 = 340 | 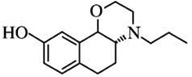 |
| [3H]-S14297 = 1.31 | [3H]-S14297 = 345 | |||
| [125]-iodosulpiride = 1.9 | [125I]-iodosulpiride = 239 | |||
| Partial DA D3 agonist | ||||
| BP-897d | [25I]-iodosulpiride = 61 | [125I]-iodosulpiride = 0.92 | [125I]-iodosulpiride = 66 | 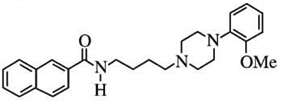 |
| Mixed DA D2/D3 antagonist | ||||
| U 99194-Ae | [125I]-iodosulpiride = 2281 | [3H]-PD128907 = 160 | [3H]-PD128907= 14 | 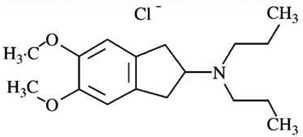 |
| [3H]-S14297 = 180 | [3H]-S14297= 13 | |||
| [125I]-iodosulpiride = 223 | [125I]-iodosulpiride = 10 | |||
| Nafadotridef | [125I]-iodosulpiride = 5 | [3H]-PD128907 = 0.52 | [3H]-PD128907 = 9 |  |
| [3H]-S14297 = 0.88 | [3H]-S14297 = 6 | |||
| [125I]-iodosulpiride = 0.81 | [125I]-iodosulpiride = 6 | |||
| DS 121g | [3H]-spiperone = 1140 | [3H]-spiperone = 249 | D2:D3Ki ratios = 4 |  |
| (+)-AJ 76 h | [125I]-iodosulpiride= 155 | [3H]-PD128907 = 26 | [3H]-PD128907 = 6 |  |
| [3H]-S14297 = 34 | [3H]-S14297 = 5 | |||
| R=H | [125I]-iodosulpiride = 70 | [125I]-iodosulpiride = 2 | ||
| (+)-UH-232i | [125]-iodosulpiride = 28 | [3H]-PD128907 = 3.3 | [3H]-PD128907 = 8 | 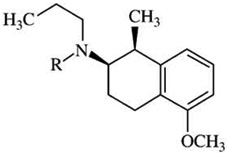 |
| [3H]-S14297 = 5.4 | [3H]-S14297 = 5 | |||
| R=propyl | [125I]-iodosulpiride = 7 | [125I]-iodosulpiride = 4 | ||
| S 14297j | [125I]-iodosulpiride = 297 | [3H]-PD128907 = 4.9 | [3H]-PD128907 = 6l | 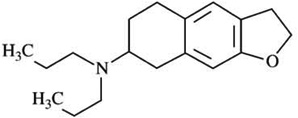 |
| [3H]-S14297 = 7.4 | [3H]-S14297 = 40 | |||
| [125I]-iodosulpiride = 13 | [125I]-iodosulpiride = 23 | |||
| GR 103691k | [125I]-iodosulpiride = 24 | [3H]-PD128907 = 0.4 | [3H]-PD128907 = 60 |  |
| [3H]-S14297 = 0.4 | [3H]-S14297 = 60 | |||
| [125I]-iodosulpiride = 0.4 | [125]-iodosulpiride = | |||
| Selective DA D3 antagonists | ||||
| SB-277011-Al | [125I]-iodosulpiride pKi=5.98 |
[125I]-iodosulpiride pKi=7.95 |
D2:D3Ki ratios = 120 |  |
| SB-414796m | [125I]-iodosulpiride pKi= 6.4 |
[125I]-iodosulpiride pKi = 8.4 |
D2:D3Ki ratios = 100 | 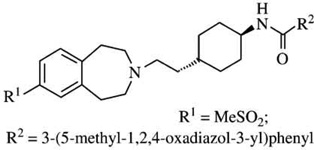 |
| NGB-2904n | [125I]-IABN = 911 | [125I]-IABN = 1.1 | D2:D3Ki ratios = 830 | 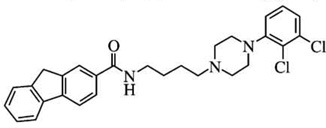 |
| S33084o | [125I]iodosulpride; pKi =7.54 | [125I]iodosulpride; pKi=9.59 | [125I]iodosulpride = 120 |  |
| [3H]spiperone; pKi = 7.28 | [3H]spiperone; pKi = 9.40 | [3H]spiperone = 125 | ||
| [35S]GTPγS; pKb = 7.75 | [35S]GTPγS; pKb = 9.61 | l35S]GTPγS = 66 | ||
| FAUC 365p | [3H]spiperone=3600 | [3H]spiperone = 0.5 | D2L:D3Ki ratios = |  |
| A-437203q | Ki D2L = 348 | Ki = 29 | D2L:D3 Ki ratios = 120 |  |
| KCH 1110r | [3H]-spiperone = 118.8 | [3H]-spiperone = 1.28 | D2:D3Ki ratios = 90 |  |
D2:D3Ki ratios are shown for different radioligands.
See Ref. [11].
See Ref. [11].
See Ref. [224].
See Ref. [11].
See Ref. [11].
See Ref. [276].
See Ref. [11].
See Ref. [11].
See Ref. [11].
See Ref. [11].
See Ref. [229].
See Ref. [186].
See Ref. [201].
See Ref. [23].
See Ref. [291].
See Ref. [216].
In support of the second argument (incentive value) is that 7-OH-DPAT alone (at 5 mg/kg [187]) or PD128907 alone (at 1 mg/kg [148]) have been reported to produce a significant CPP (however, see Refs. [147,149,150] for contrary findings). Furthermore, 7-OH-DPAT at 0.03 mg/kg [147] or at 0.05–0.1 mg/kg [121] has been reported to produce conditioned place aversion (CPA), as does PD128907 at 1 mg/kg sc [121]. It has also been shown that 7-OH-DPAT can attenuate the CPP response to morphine [245], d-amphetamine [149], and cocaine [150] in rats. However, evidence from Ukai et al. [290] indicates that administration of R(+)-7-OH-DPAT (0.001–0.1 mg/kg sc) produces an amnesic effect in the passive avoidance learning model in mice, and Smith et al. [270] have demonstrated that systemic administration of 7-OH-DPAT (6 or 25 µg/kg) induces cognitive impairment in the marmoset. Consequently, one cannot rule out the possibility that 7-OH-DPAT may interfere with the animals’ association of the appetitive value of these agents with the appropriate cues.
Together, these findings clearly indicate that the degree of functional selectivity of mixed D2/D3 agonists in recombinant systems is too low to allow meaningful discrimination in vivo between D2 and D3 receptors. The significant controversy around the outcome of in vivo studies using mixed DA D2/D3 agonists relates essentially to their lack of selectivity, but also to the possibility that these agents have intrinsic rewarding properties or produce nonspecific aversive effects.
5. Role of DA D3 receptors in drug addiction: studies with the partial DA D3 receptor agonist BP-897
A series of in vivo studies assessed the efficacy of the partial DA D3 receptor agonist BP-897 in animal models of drug addiction. In 1999, Pilla and colleagues [224] demonstrated that administration of BP-897 (0.05, 0.5, and 1 mg/kg) produces a significant dose-dependent decrease in the number of responses for cocaine in the first, but not the second interval of a second-order schedule of reinforcement without having intrinsic rewarding effects. In support of those findings [224], recent evidence suggests that BP-897 (1 mg/kg) can reduce cocaine-seeking behavior induced by the presentation of stimuli associated with and predictive of cocaine availability after a period of extinction and in the absence of cocaine itself [50]. However, it must be noted that, in contrast with the findings of Pilla et al. [224], a recent report found that, similar to ip administration of the D1 receptor antagonist SCH-23390 (0.1 and 0.2 mg/kg) or oral (po) administration of the D2 receptor antagonist haloperidol (0.2 and 0.5 mg/kg), BP-897 (1 mg/kg ip) increased cocaine self-administration under a continuous reinforcement schedule [102].
BP-897 (0.3, 1, or 3 mg/kg ip) reduces cocaine cue-induced hyperlocomotion in Swiss–Webster mice [168] as well as nicotine-induced conditioned locomotion [169] and nicotine-induced behavioral sensitization in rats [170]. Aujla et al. [12] also showed that BP-897 (1 mg/kg) blocked the expression of conditioned locomotor activity to amphetamine (2 mg/kg) in male Wistar rats without altering the acquisition of conditioning or the locomotor activating effect of amphetamine.
BP-897 significantly decreases the discriminative stimulus effects of d-amphetamine and cocaine [21] and attenuates the expression and acquisition of the CPP response to cocaine without altering the acquisition or expression of the CPP response to food or morphine [88]. However, BP-897 alone produced CPA, a finding congruent with that of Gyertyán and Gál [121], and may also have anxiolytic properties [246].
A recent paper by Campiani et al. [44] reported the pharmacological evaluation of a series of novel arylalkylpiperazine structures related to BP-897 as well as BP-897 itself. BP-897 (1 mg/kg) significantly reduced the number of active lever presses by male Sprague–Dawley rats following re-exposure to cocaine-associated stimuli. In contrast, compound 5q (N-[4-[4-(2,4-dichlorophenyl)piperazin-1-yl]bu-tyl]-5-chloroindole-2-carboxamide), a selective DA D3 receptor partial agonist (Ki D2 > 10000 nM; Ki D3 = 0.38±0.005nM;Ki5-HT1A>10000nM)didnotsignificantly alter cocaine-seeking behavior produced by environmental cues previously associated with cocaine self-administration. The explanation for the difference in the behavioral effects between these compounds remains to be elucidated.
It has been hypothesized that BP-897 exerts its anti-addictive actions via a selective partial agonism at D3 receptors. However, antagonism at D3 receptors and/or activity at other receptors might also explain some of the in vivo data. In support of the first argument (partial agonism vs. antagonism at D3 receptors) are pharmacological data (forskolin-induced increase in cAMP) indicating that in NG 108-15 cells expressing human D3 receptors, BP-897 behaves as a partial agonist compared to DA (59%) [224]. Similarly, in the mitogenesis assay, the maximum response elicited by BP-897 was 55% of that elicited by the full agonist quinpirole [23,224]. In cells expressing D2 receptors, BP-897 alone does not inhibit cAMP accumulation or elicit mitogenesis [224]. However, it reversibly antagonizes quinpirole-induced mitogenesis, but only at concentrations significantly greater than those required for D3 receptor stimulation [224]. Thus, based on the results obtained by Pilla et al. [224], BP-897 can be classified as a partial D3 receptor agonist. However, recent evidence from studies using microphysiometry shows that in CHO-K1 cells transfected with human D2 and D3 receptors, BP-897 behaves as a full antagonist at both DA D2 (pKb = 8.05) and D3 (pKb = 9.43) receptors [305]. In addition, in CHO cells transfected with human D3 receptors, BP-897 does not stimulate D3 receptors and displays antagonistic effects in a [35S]-GTPγS binding assay in cells expressing human D3 receptors [302]. Clearly, different results in the intrinsic activity of partial agonists can be obtained using different assay systems. The observed efficacy of partial agonists could be related to differences in the level of receptor reserve and efficiency of functional coupling. Wood et al. [305] identified BHT920 as a full agonist, but the same compound was identified as only a partial agonist by Pilla et al. [224]. There are also distinct differences between the microphysiometry and mitogenesis assays that are worth noting. The mitogenesis assay requires sustained activation that can be complicated by desensitization and stability of the agonists. The microphysiometry assay involves measurements carried out in real time. The response observed in the microphysiometry assay is likely a direct consequence of activation of D3 receptors whereas the response seen in the mitogenesis assay represents an adaptation of the cell to continuous receptor activation and may be complicated by the presence of endogenous receptors and regulators. Finally, in anesthetized rats, in vivo extracellular recording demonstrated that iv administration of BP-897 (maximum dose of 8.2 mg/kg) did not significantly alter the firing rate of spontaneously active substantia nigra pars compacta (A9) DA neurons, but did antagonize quinpirole-induced inhibition of firing of these neurons with an ED50 of 1.1 mg/kg [302]. Taken together, these results suggest that BP-897 may also exert antagonist actions at the DA D3 receptor and that one cannot exclude the possibility that BP-897’s antagonism of quinpirole’s action is partly related to antagonism at DA D2 receptors.
In support of the second argument (cross-selectivity profile) are data showing that BP-897 has affinity for other neurotransmitter receptors (for a detailed review on BP-897, the reader is referred to Ref. [103]). BP-897 shows moderate affinity for α1- and α2-adrenergic receptors (Ki = 60 and 83 nM, respectively), 5-HT1A (Ki = 84 nM), and DA D2 receptors (Ki = 61 nM) [224] (see also Ref. [44]). BP-897 also displays potent antagonistic action at 5-HT2A and α1-adrenergic receptors (M.J. Millan, personal communication). The interaction with 5-HT2A receptors may be important as a recent study in rats indicates that the 5-HT2A receptor antagonist M100,907 attenuates the ability of a priming injection of cocaine to reinstate lever pressing [101]. Thus, the effect of BP-897 in some animal models of addiction could be partially related to its antagonist action at 5-HT2A receptors. Finally, a recent study reported that the Ki of BP-897 for human D4 receptors is 39 nM and the human D4/D3 ratio is 28 [23].
Altogether, both in vitro and in vivo data suggest that BP-897 may act as a D3 receptor agonist or antagonist. In addition, one cannot rule out the possibility that part of BP-897’s action is mediated by interaction with other receptors.
6. Role of DA D3 receptors in drug addiction: studies with mixed DA D2/D3 receptor antagonists
A number of compounds were originally reported to be selective D3 receptor antagonists, including (+)-AJ-76 [273,274], (+)-UH-232 [273,274], U99194A [122], nafadotride [248], GR103691 [206], and DS-121 [153]. However, evidence from a variety of studies indicates that these compounds either lack sufficient in vitro and/or in vivo selectivity or interact with other receptors and therefore cannot be characterized as selective D3 receptor antagonists (see Table 1). For example, the in vitro selectivity of (+)-UH-232, (+)-AJ-76, nafadotride, and U99194A for D3/D2 is only 4–8, 2–6, 6–9, and 10–20-fold, respectively [118,175,272,298,307]. Functional and/or behavioral studies suggest that the aforementioned pharmacological agents produce effects associated with D2 receptor antagonism. For example, systemic administration of (+)-UH-232, (+)-AJ-76, and nafadotride can induce catalepsy and increase prolactin levels in rats [11]. The acute administration of U-99194A, nafadotride, and (+)-UH-232 significantly increases DA turnover in the striatum, NAc, frontal cortex and olfactory tubercle [11]. Levant and Vansell [178] also examined the in vivo occupancy of D2 receptors by nafadotride (0.1–10 mg/kg ip) in the EEDQ assay. Their results indicate that D2 receptor antagonism contributes to the pharmacological actions of nafadotride at sc doses above 1 mg/kg and ip doses above 3 mg/kg. A detailed summary of the main behavioral pharmacology of DS-121, nafadotride, U99194, S14297, and PD152255 can be found in Table 2.
Table 2.
Effect of mixed D2/D3 receptor antagonists in animal models of drug addictiona
| Compound name | D2:D3 selectivity | Paradigm | Dose (mg/kg) and route of administration |
Main finding | Reference |
|---|---|---|---|---|---|
| DS-121 | 3–5 | Cocaine-induced locomotor activity in cocaine tolerant rats |
0–7 (ip) | Potentiation | [91] |
| Spontaneous locomotor activity | 3.3–13.3 (sc) | Increase | [153] | ||
| Intra-NAc (0.05–53 µg/side) |
No effect | ||||
| Intra-VTA (0.05–53 µg/side) |
No effect | ||||
| ICV bilateral (66.3 µg/side) |
Increase | ||||
| CPP | 3.3–13.3 (sc) | Place preference | [153] | ||
| BSR | 3.3–13.3 (sc) | No effect | [153] | ||
| Discriminative stimulus properties of amphetamine (0.5 mg/kg) and cocaine (5 mg/kg) |
3.5–14 (sc) | Increase in amphetamine-like and cocaine-like responding |
[58] | ||
| Cocaine self-administration | 3–10 (sc) | Decrease | [238] | ||
| Progressive ratio breakpoint for cocaine self-administration |
15 (ip) | Decrease | [154] | ||
| Extracellular levels of DA in dorsal striatum |
15 (ip) | Increase | [154] | ||
| Potentiation of cocaine-induced increase in striatal DA levels |
|||||
| Nafadotride | 6–9 | Development of amphetamine sensitization |
25 µg/kg (sc) | Decrease | [230] |
| Expression of cocaine sensitization |
0.4 (ip) | Potentiation | [99] | ||
| Cocaine self-administration under FR-5 |
1–3 (sc) | Increase | [43] | ||
| Cue-induced cocaine-seeking behavior |
1 (ip) | Decrease | [299] | ||
| Apomorphine- and 7-OH-DPAT- induced reinstatement of food-seeking behavior |
1 (ip) | Decrease | [89] | ||
| No effect on food-primed food seeking | |||||
| Sensitization-induced facilitation of appetitive conditioning |
Intra-amygdala (20 µmol/ml; 0.5 µl/side) |
Retardation of conditioned response |
[221] | ||
| Isolation rearing-induced facilitation of Pavlovian learning |
Intra-amygdala (20 µmol/ml; 0.5 µl/side) |
Abolition of acquisition of Pavlovian-conditioned approach |
[222] | ||
| Spontaneous locomotor activity | 0.1–1 (sc) | Increase | [248] | ||
| 0.75–3 (ip) | Increase | [70] | |||
| Morphine (10 mg/kg)-induced hyperactivity | 0.3–1 (sc) | Blockade at 1 mg/kg | [61] | ||
| MK-801-induced hyperactivity | 0.3 and 1 (ip) | Blockade | [174] | ||
| Climbing behavior in mice | 0.1–1 (sc) | Increase | [248] | ||
| Conditioned reaction time | 0.1, 0.3, and 1 (sc) | Increased number of delayed responses at 1 mg/kg |
[271] | ||
| Catalepsy | AD50 = 16.4 (sc)b | [11] | |||
| DA turnover in dorsal striatum | AD50 = 0.5 (sc)c | [11] | |||
| Plasma prolactin levels | MED = 0.16 (sc)d | [11] | |||
| U99194 | 10–15 | Social interaction | 20 and 40 (sc) | Increase | [244] |
| Spontaneous locomotor activity and rearing | 5 and 10 (sc) | Increase | [108] | ||
| 20 and 40 (sc) | Decrease | [49,244,249,298] | |||
| Spontaneous and morphine-induced locomotor activity |
20 (ip) | Increase and blockade of morphine (20 mg/kg)-induced hyperactivity |
[188] | ||
| Development of amphetamine sensitization |
ICV (20 µg × 7 days) |
Decrease | [54] | ||
| Discriminative stimulus properties of 7-OH-DPAT (0.01–1 mg/kg) or PD128907 (0.03 mg/kg) |
2.5–20 (sc) | No effect/partial blockade |
[57,105] | ||
| Potentiation | [74] | ||||
| Amphetamine-induced enhancement of BSR |
5–20 (sc) | Increase | [49] | ||
| Discriminative stimulus properties of amphetamine (1 mg/kg) and cocaine (5 mg/kg) |
10–40 (sc) | No effect | [18,19] | ||
| Cocaine CPP | 12 and 24 (sc) | No effect Place preference per se |
[121] | ||
| Ethanol CPP | 10 and 20 (ip) | No effect | [86] | ||
| Enhancement | [31,32] | ||||
| Oral ethanol self-administration | 10 and 20 (ip) | No effect | [31,32] | ||
| Amphetamine (2.5 mg/kg)-induced contralateral rotation in |
5, 10, and 20 (sc) | >98% DA depletion (ipsilateral rotations): |
[241] | ||
| 6-OHDA-lesioned rats | no effect 80–97% DA depletion (contralateral rotations): blockade |
||||
| Drug discrimination | 5–35 (sc) | Generalization to UH-232, scopolamine, trihexyphenidyl, and clozapine |
[115] | ||
| Catalepsy | AD50 > 40 (sc)e | [11] | |||
| DA turnover in dorsal striatum | AD50 = 6.9 (sc)f | [11] | |||
| Plasma prolactin levels | MED = 40 (sc)g | [11] | |||
| S14297 | 23–61 | Catalepsy | AD50 > 20 (sc)h | [11,113,199] | |
| Plasma prolactin levels | MED > 40 (sc)i | [11] | |||
| Discriminative stimulus properties of U99194 |
3–8 (sc) | Partial substitution (66%) | [17] | ||
| Spontaneous locomotor activity | ED50 = 15.4 (ip) | Decrease | [62] | ||
| ED50 > 30 (sc) | No effect | ||||
| PD152255 | 40–50 | Amphetamine-induced locomotor activity |
1, 3, and 10 (ip) | Decrease | |
| 1, 3, and 10 (sc) | No effect | [62] | |||
| Discriminative stimulus properties of U99194 |
1–3 (ip) Suppression of responding at higher doses |
No generalization | [17] |
For a detailed ethologically based approach comparing the ethograms of U99194A, GR103691, and nafadotride, the reader is referred to Ref. [59].
AD50 was defined as the dose required for the induction of a half-maximal response (equivalent to 15 s).
AD50 was defined as an increase in DOPAC:DA ratios to 150% relative to vehicle values.
MED was defined as the lowest dose significantly different (P < 0.05) from vehicle control values.
AD50 was defined as the dose required for the induction of a half-maximal response (equivalent to 15 s).
AD50 was defined as an increase in DOPAC:DA ratios to 150% relative to vehicle values.
MED was defined as the lowest dose significantly different (P < 0.05) from vehicle control values.
AD50 was defined as the dose required for the induction of a half-maximal response (equivalent to 15 s).
MED was defined as the lowest dose significantly different (P < 0.05) from vehicle control values.
7. Role of DA D3 receptors in drug addiction: studies with selective DA D3 receptor antagonists—SB-277011-A
Radioligand binding studies have shown that SB-277011-A is a selective DA D3 receptor antagonist with high affinity for the human (pKi 7.95) and rat (pKi 7.97) cloned DA D3 receptor. The ratio of the in vitro D3/D2 affinity of SB-277011-A for human and rat is 120 and 80, respectively [229]. SB-277011-A has a 100-fold selectivity over 66 other receptors, enzymes, and ion channels [229]. SB-277011-A is a potent and competitive antagonist, with pKb 8.4 (4 nM) in the microphysiometry in vitro functional assay using human cloned DA D3 receptors expressed in CHO cells, and it maintains selectivity with respect to DA D2 receptors (pKb 6.5). SB-277011-A has also been shown to readily penetrate the rat brain with a steady-state brain/ plasma ratio of 3.6:1 [229,283]. In the rat, SB-277011-A has an oral bioavailability of 43%, shows low clearance, and a half-life of 2.0 h. The drug appears to be metabolized by the liver enzyme hepatic aldehyde oxidase [16].
The effects of SB-277011-A per se in in vivo behavioral models are summarized in Table 3. SB-277011-A is devoid of typical DA D2/D3 receptor antagonist effects and does not show proconvulsant activity. Only very high doses of SB-277011-A (above 90 mg/kg po), which are significantly higher than those showing efficacy in models of addiction, produce weak sedative-like actions in the mouse Irwin test and impair performance in the rat rotarod test. In vivo brain microdialysis data indicate that administration of SB-277011-A (2.8 mg/kg po) reverses the decrease in extracellular DA levels produced by quinelorane (D2/D3 agonist) in the NAc, but not the dorsal striatum, a regional selectivity consistent with the distribution of DA D3 receptors in the rat brain [229]. A single ip administration of 10 mg/kg of SB-277011-A significantly increases extracellular levels of DA, norepinephrine (NE), and acetylcholine (ACh) in the anterior cingulate cortex in freely moving rats [160]. It should be pointed out that SB-277011-A does not produce a functional antagonism of D2 receptors in vivo as, unlike D2 receptor antagonists, its systemic administration in rats does not (1) elicit catalepsy at doses that exhibit anti-addiction action [229,295]; (2) produce a rightward shift in brain stimulation reward curve [229]; (3) increase plasma prolactin levels [295]; (4) inhibit spontaneous or stimulant-induced locomotion [295]; (5) antagonize the action of quinpirole in the dorsal striatum, a brain region with a high density of D2 receptors [295]; (6) increase DA levels in the striatum [295]; and (7) increase cocaine self-administration under a schedule of continuous reinforcement ([84,102,295]; see Section 7.4).
Table 3.
Effects of SB-277011-A alone in various pharmacology modelsa
| Experimental paradigm | Doses | Main finding |
|---|---|---|
| Spontaneous locomotor activity |
0–42.3 mg/kg po | No effect |
| Amphetamine-induced locomotor activity |
0–51.3 mg/kg po | No effect |
| Phencyclidine-induced locomotor activity |
0–51.3 mg/kg po | No effect |
| Apomorphine-induced climbing in mice |
0–42 mg/kg po | No effect |
| Quinelorane-induced locomotor hypoactivity |
0–41 mg/kg po | No effect |
| Quinelorane-induced reversal of amphetamine hyperactivity |
0–41 mg/kg po | No effect |
| Quinpirole-induced deficit in prepulse inhibition |
0–41 mg/kg po | No effect |
| Apomorphine-induced deficit in prepulse inhibition |
0–41 mg/kg po | No effect |
| Differential reinforcement of low response rates |
0–27.5 mg/kg po | No effect |
| Catalepsy | 0–78.8 mg/kg po | No effect |
| Haloperidol-induced catalepsy | 0–41 mg/kg po | No effect |
| Serum prolactin levels | 0–93 mg/kg po | No effect |
| Rotarod performance | 0–91.8 mg/kg po | No effect, except 91.8 mg/kg dose producing impairment in performance |
| Maximal electroshock seizure threshold |
0–92 mg/kg po | No effect |
| Mouse Irwin profile | 0–91.8 mg/kg po | No effect, except 91.8 mg/kg dose producing weak sedative-like effect |
| Delayed nonmatching test | 0–41 mg/kg po | No effect |
See Ref. [229].
The following subsections will summarize the effects of SB-277011-A in animal models of drug addiction (see also Table 4).
Table 4.
Effect of SB-277011-A in animal models of drug addiction
| Behavioral paradigm | Doses | Main finding | Reference |
|---|---|---|---|
| Nicotine self-administration (FR-1/FR-2) | 3–10 mg/kg ip | No effect | [6] |
| Nicotine-triggered relapse to nicotine seeking | 3–10 mg/kg ip | Blockade | [6] |
| Nicotine-induced enhancement of brain stimulation reward | 3, 6, and 12 mg/kg ip | Blockade | [111] |
| Nicotine-conditioned locomotor activity | 10 mg/kg ip | Blockade | [169] |
| Cocaine self-administration (FR-1) | 3, 6, and 12 mg/kg ip | No effect | [295] |
| 5 and 20 mg/kg po | No effect | [102] | |
| Cocaine-induced enhancement of brain stimulation reward | 3, 6, and 12 mg/kg ip | Blockade | [295] |
| Acquisition of cocaine-induced conditioned place preference | 0.3, 1, 3, and 10 mg/kg ip | Blockade | [295] |
| Expression of cocaine-induced conditioned place preference | 0.3, 1, 3, and 10 mg/kg ip | Blockade | [295] |
| Expression of food-induced conditioned place preference | 10 mg/kg ip | No effect | [295] |
| Cocaine-triggered relapse to cocaine seeking | 3, 6, and 12 mg/kg ip | Blockade | [295] |
| Cue-triggered relapse to cocaine seeking | 3, 10, and 30 mg/kg ip | Blockade | [51] |
| Cue-controlled cocaine seeking (2nd order reinforcement schedule) | 0.3, 1, 3, 10, 20, and 30 mg/kg ip | Blockade | [84] |
| Sucrose self-administration under 2nd order reinforcement schedule | 0.3, 1, 3, 10, 20, and 30 mg/kg ip | No effect | [84] |
| Cocaine self-administration under FR-10 and PR schedules of reinforcement | 3, 6, 12, and 24 mg/kg ip | Blockade | [111] |
| Stress-induced relapse to cocaine seeking | 3, 6, and 12 mg/kg ip | Blockade | [310] |
| 1.5 µg/0.5 µl/side NAc | Blockade | ||
| 1.5 µg/0.5 µl/side dorsal Striatum | No effect | ||
| Ethanol intake in ethanol-preferring vs. nonpreferring rats | 3, 10, and 30 mg/kg ip | Blockade | [235] |
| Oral ethanol self-administration | 10, 20, and 30 mg/kg ip | Blockade | [7] |
| Relapse to ethanol seeking | 10, 20, and 30 mg/kg ip | Blockade | [189] |
| Expression of heroin-induced conditioned place preference | 10 mg/kg ip | Blockade | [10] |
7.1. Effect of SB-277011-A on nicotine self-administration and nicotine-triggered relapse to nicotine-seeking behavior
Andreoli et al. [6] examined the effect of SB-277011-A on self-administration of nicotine (0.03 mg/kg) using an FR-1/FR-2 schedule in male Wistar rats. SB-277011-A (3 or 10 mg/kg ip) did not affect the number of infusions/h or the number of active lever presses/h compared with the vehicle group at any dose tested. SB-277011-A also failed to modify the number of inactive lever presses/h. The effect of SB-277011-A was also examined on noncontingent nicotine-triggered (0.15 mg/kg) reinstatement of extinguished responding on an operant lever, the depression of which previously resulted in iv nicotine infusions, 24 h after cessation of the self-administration of nicotine [6]. While acute administration of nicotine (0.15 mg/kg sc) produced a significant increase in nicotine-paired lever presses in the vehicle/nicotine group compared with the vehicle/saline control group, SB-277011-A (3 and 10 mg/ kg ip) produced a significant reduction in nicotine-paired lever presses compared to the vehicle/nicotine group. No significant changes in the number of inactive lever presses were seen during the noncontingent nicotine priming component of the experiment, suggesting a specific effect of SB-277011-A on drug-triggered drug-seeking behavior. These data suggest that SB-277011-A attenuates nicotine-triggered reinstatement of nicotine-seeking behavior without affecting stable maintenance of nicotine self-administration per se.
7.2. Effect of SB-277011-A on nicotine- and cocaine-induced enhancement of brain stimulation reward
The effects of SB-277011-A on nicotine-induced enhancement of BSR in male Long–Evans rats were also examined [45]. Rats were trained to lever press for BSR of the medial forebrain bundle at the level of the lateral hypothalamus and tested on a rate–frequency curve-shift brain reward paradigm. Administration of nicotine (0.5 mg/kg ip) robustly shifted BSR curves to the left, lowering brain reward thresholds by approximately 25%. Acute administration of SB-277011-A (3 and 6 mg/kg ip), given 1 h prior to BSR testing, failed to alter nicotine-enhanced BSR. However, 12 mg/kg of SB-277011-A significantly attenuated (70%) nicotine-induced enhancement of BSR. These data suggest that DA D3 receptors play an important role in mediating nicotine-enhanced brain reward.
Administration of SB-277011-A (3 mg/kg ip), given 30 min prior to BSR testing, also completely blocked the robust enhancement of BSR produced by cocaine (2 mg/kg ip) in rats [295]. This effect cannot be attributed to a D3 receptor antagonist-induced diminution of brain reward, as SB-277011-A alone did not significantly alter BSR thresholds at doses up to 12 mg/kg.
7.3. Effect of SB-277011-A on acquisition and expression of cocaine-induced conditioned place preference (CPP)
Acute systemic administration of SB-277011-A at all doses tested (0.3, 1, 3, and 10 mg/kg), 30 min prior to each administration of cocaine during the CPP acquisition phase, produced a significant blockade of the acquisition of cocaine-induced CPP [295]. This finding cannot be attributed to a D3-antagonist-induced place aversion, as SB-277011-A by itself produced neither a significant place preference nor a significant place aversion at doses up to 10 mg/kg. The finding that SB-277011-A by itself produces neither preference nor aversion was recently confirmed using oral doses of SB-277011-A (5 and 20 mg/kg po) [121]. A single injection of 1, 3, or 10 mg/kg ip of SB-277011-A, 30 min prior to behavioral testing, also produced a significant blockade of the expression of cocaine-induced CPP [295]. Finally, daily ip administration of SB-277011-A (3 mg/kg) for 14 days prior to testing for expression of cocaine-induced CPP produced a robust blockade of the expression of cocaine-induced CPP [295]. It should also be noted that acute administration of SB-277011-A (10 mg/kg ip) did not block the expression of food-induced CPP [295].
In contrast to our CPP findings with SB-277011-A [295], Gyertyán and Gál [121] have reported that administration of 5 or 20 mg/kg po of SB-277011-A (30 min prior to cocaine administration) did not significantly alter the acquisition of a CPP response to cocaine (10 mg/kg ip) in male Sprague– Dawley rats. The reason for the difference between our findings and those of Gyertán and Gál [121] is unknown, although there were significant differences in methodology including (1) preconditioning the animals to the CPP apparatus vs. no preconditioning; (2) time period of 4 h between the administration of saline and cocaine vs. 24 h; (3) SB-277011-A administered via the po vs. ip route; and (4) SB-277011-A suspended in 5% Tween 80 vs. 2% methylcellulose.
7.4. Effect of SB-277011-A on cocaine self-administration and cocaine-triggered relapse to cocaine-seeking behavior
Acute administration of SB-277011-A (3, 10, and 12 mg/kg ip) did not affect stable maintenance of cocaine self-administration under a continuous reinforcement schedule [84,295]. These findings were also recently confirmed by Gál and Gyertyán [102] who showed that acute administration of SB-277011-A (5 and 20 mg/kg po) does not affect cocaine self-administration under continuous reinforcement. In contrast, the same study showed that both the DA D1 receptor antagonist SCH-23390 (0.1 and 0.2 mg/kg ip) and the DA D2-preferring receptor antagonist haloperidol (0.2 and 0.5 mg/kg po) produced a compensatory increase in lever pressing, of the type classically known to be produced by DA antagonists [110,311]. Finally, the mixed D2/D3 agonists PD-128907 (1 mg/kg sc) and 7-OH-DPAT (0.1 and 0.5 mg/kg sc) produced a significant decrease in lever pressing for cocaine. Interestingly, the same study showed that BP-897 (1 mg/kg ip) significantly increased cocaine self-administration under a continuous reinforcement schedule, suggesting that this effect of BP-897 at a dose of 1 mg/kg is unlikely to be mediated through an action at DA D3 receptors. In addition to the findings of Gál and Gyertyán [102], it has been shown that cocaine self-administration is increased in a manner suggestive of a reduction in the reinforcing effects of the drug following administration of the nonselective D2/D3 or D1/D2 receptor antagonists YM-09–151-2 [37,279], spiper-one [62,137], sulpiride, metoclopramide, thioridiazine, chlorpromazine, haloperidol, pimozide, or alpha-flupentixol [238,239].
Although SB-277011-A did not alter the self-administration of cocaine or nicotine in animals under FR-1 reinforcement, we have seen that it does attenuate the CPP and enhanced BSR produced by cocaine and nicotine. There is evidence suggesting that the nigrostriatal dopaminergic system plays an important role in habit formation, allowing animals to acquire and maintain performance [142]. Since, as previously mentioned, the nigrostriatal system is virtually devoid of D3 receptors, it is possible that the inability of SB-277011-A to affect the self-administration of cocaine and nicotine is related to the relative lack of D3 receptors in the nigrostriatal system. However, this hypothesis remains to be tested. An alternative hypothesis, supported by our findings of inhibition of cocaine self-administration by SB-277011-A when the cocaine reinforcement schedule is changed from FR-1 to higher FR ratios (see below), is that drug reinforcement on an FR-1 ratio constitutes too powerful a reinforcer for D3 receptor antagonism to overcome.
A single noncontingent iv injection of 1 mg/kg cocaine produced robust reinstatement of extinguished operant behavior previously reinforced by iv cocaine injections. Acute pretreatment with SB-277011-A produced a dose-dependent attenuation of this cocaine-triggered reinstatement of extinguished cocaine-seeking behavior [295]. Importantly, SB-277011-A (3, 6, or 12 mg/kg) alone did not trigger reinstatement of cocaine seeking. Finally, over the dose range tested, SB-277011-A did not affect responses on the inactive lever.
7.5. Effect of SB-277011-A on cue-controlled cocaine-seeking behavior
The effect of SB-277011-A was also tested against cocaine-seeking behavior using second-order schedules of cocaine reinforcement, which provide an animal model of cue-controlled drug-seeking both prior to and after cocaine has been self-administered [84]. SB-277011-A (0.3, 3, 10, 20, and 30 mg/kg ip) produced a dose-dependent decrease in cocaine-seeking behavior maintained by a cocaine-associated conditioned reinforcer in both the first (drug-free) test interval and also following self-administration of cocaine (second interval) [84]. At higher doses, SB-277011-A also increased the latency to receive the first conditioned stimulus (CS) presentation and cocaine infusion, thereby decreasing the number of cocaine infusions self-administered under the second-order schedule of reinforcement. The decreased responding during the first and second intervals produced by pretreatment with SB-277011-A can be explained as an attenuation of the impact of the conditioned reinforcing properties of the drug-paired stimulus. Furthermore, the increase in latency to the first presentation of the contingent CS and the first cocaine infusion suggests that the decrease in cocaine intake under the second-order schedule is related to a decreased motivation to respond for cocaine. This suggestion is further strengthened by the observation that cocaine self-administration under an FR-1 schedule of reinforcement was not altered by SB-277011-A. Finally, the selectivity of D3 receptors in mediating cuecontrolled drug-seeking was further supported by the finding that SB-277011-A had no effect on responding for sucrose under similar second-order reinforcement.
Similar findings were reported in a model using male Sprague–Dawley rats trained to self-administer cocaine, while simultaneously establishing discriminative stimuli associated with, and predictive of, cocaine availability or nonavailability [51]. When given in doses ranging from 3 to 30 mg/kg ip, SB-277011-A decreased responding produced by re-introduction of cocaine-associated cues in a dose-dependent manner.
7.6. Effect of SB-277011-A on cocaine self-administration as determined by varying fixed-ratio and progressive-ratio reinforcement
The effect of SB-277011-A on cocaine self-administration under both fixed-ratio (FR) and progressive-ratio (PR) schedules of reinforcement in male Long–Evans rats was also examined [111]. The administration of SB-277011-A (3–24 mg/kg ip) did not significantly alter cocaine self-administration (0.75 mg/kg/injection) reinforced under an FR-1 schedule. However, SB-277011-A (24 mg/kg ip) produced a significant decrease in cocaine self-administration when (1) the unit dose of cocaine was decreased from 0.75 to 0.125–0.5 mg/kg, or (2) the work demand for cocaine was increased from an FR1 to FR10 schedule. Under a PR reinforcement schedule, SB-277011-A (6–24 mg/kg) produced a significant dose-dependent lowering of the PR breakpoint for cocaine self-administration. Furthermore, the 24 mg/kg dose significantly shifted the cocaine (0.25–1 mg/kg) dose–response breakpoint curve to the right. Finally, when SB-277011-A was substituted for cocaine in an FR schedule, it did not maintain cocaine self-administration behavior. Overall, these results indicate that antagonism of D3 receptors by SB-277011-A significantly inhibits acute cocaine-induced reinforcement in an FR schedule following a reduction in reinforcement potency or an increase in work requirement. Why SB-277011-A significantly attenuates cocaine self-administration when the unit dose of cocaine is decreased or the FR reinforcement schedule is increased from 1 to 10 remains unknown. Compared with the amount of cocaine administered in other paradigms such as BSR and CPP, animals readily self-administer a significantly larger amount of cocaine under a continuous FR1 schedule for a high unit dose of cocaine. Therefore, it is possible that the high cocaine intake might produce increases in extracellular DA which (by competitive inhibition produced by SB-277011-A binding to the D3 receptor) are too large for SB-277011-A to overcome. This hypothesis is partially supported by a study indicating a positive correlation between the amount of cocaine self-administered and extracellular NAc DA levels [220], and by the fact that the affinity of the D3 receptor for DA is greater than that of all other DA receptor subtypes. One may also suggest that, given the half-life of SB-277011-A (2 h) vs. the half-life of cocaine (20–40 min), SB-277011-A attenuates the “rush” effects induced by cocaine, and also possibly the negative compensatory effects that quickly follow the acute positive effects. Finally, it should also be noted that the FR1 schedule of reinforcement is useful for exploring patterns of rate of drug intake. However, the use of an FR1 schedule is less appropriate to assess changes in the reinforcing effects of drugs of abuse. In fact, rate of drug self-administration may be insensitive to changes in reinforcing efficacy and, even if changes are observed, there is little theoretical basis for interpreting these changes. The FR approach was mainly designed to explore how behavior changed when the contingencies between stimulus and response were altered; these procedures were not designed to estimate the magnitude of a reinforcer. Thus, FR responding is the equivalent to the rate of consumption and therefore corresponds to the rate of drug intake. This rate, however, is an ambiguous measure of drug efficacy. Since the PR breakpoint is an index of the relative strength of a reinforcer independent of response rate [8,135, 136,247], the shift in PR breakpoint produced by SB-277011-A indicates that SB-277011-A decreases the reinforcing value of cocaine in rats. Together, we conclude that SB-277011-A not only inhibits cocaine-induced incentive motivation and reinstatement, but also attenuates cocaine’s rewarding efficacy.
7.7. Effect of SB-277011-A on stress-triggered relapse to cocaine-seeking behavior
One of the factors that can lead to relapse to drug use is exposure to stress. It has been well established in both animals [3,38,92,165,259–263,266,284] and humans [157,267,268] that exposure to stressors can produce reinstatement of self-administration of addictive drugs and/ or drug-seeking behavior. Furthermore, studies in rats have indicated that morphine or cocaine CPP can be reactivated by footshock stress following drug-free periods [184,185,296]. Stressors such as food deprivation [265] and induction of a stress-like state by corticotropin-releasing factor (CRF) administration [261] reinstate heroin seeking in rats. As noted above, we have shown that acute administration of SB-277011-A significantly attenuates cocaine-induced reinstatement of cocaine-seeking behavior [295]. More recently, we have examined the effect of SB-277011-A on stress-induced reinstatement of cocaine seeking [310]. Administration of SB-277011-A (3–12 mg/kg ip) produced a dose-dependent decrease in the reinstatement of cocaine-seeking behavior produced by footshock stress. Furthermore, SB-277011-A microinjected intracranially into the NAc bilaterally (1.5 µg/0.5 µl/side) completely blocked stress-induced reinstatement of cocaine seeking, but micro-injections into the dorsal neostriatum failed to affect stress-triggered reinstatement. The SB-277011-A-induced attenuation of stress-induced relapse to cocaine-seeking would appear not to be the result of anxiolytic and/or analgesic action since (1) SB-277011-A is inactive in paradigms that are used as screens for anxiolytic agents, and (2) SB-277011-A, compared to vehicle-treated animals, did not significantly alter avoidance behaviors such as foot flicking, foot withdrawal, or jumping following the administration of intermittent footshock stimuli (Z.-X. Xi et al. unpublished observations; also see Ref. [260]).
7.8. Effect of SB-277011-A on ethanol self-administration and relapse to ethanol-seeking behavior
We have examined the effect of SB-277011-A on the intake of ethanol in ethanol-preferring (P) and non-ethanol-preferring (NP) rats [235]. A single administration of SB-277011-A (3 mg/kg po) did not significantly alter ethanol intake in P or NP rats compared to vehicle-treated rats. However, compared to vehicle-treated animals, a single po administration of 10 or 30 mg/kg of SB-277011-A significantly decreased ethanol intake in P rats, and a single po administration of 30 mg/kg of SB-277011-A significantly decreased ethanol intake in both P and NP rats.
We also examined the effect of a single ip injection of 10, 20, or 30 mg/kg of SB-277011-A, and its vehicle, on the number of oral ethanol reinforcements and ethanol intake in adult male C57BL/6N mice [7]. Acute administration of either 10 or 20 mg/kg ip of SB-277011-A did not significantly alter oral ethanol self-administration compared to vehicle-treated animals. However, a single administration of 30 mg/kg ip of SB-277011-A significantly decreased the number of reinforcements (by 71%) and the amount of ethanol consumed (by 72%) compared to vehicle-treated animals.
In contrast to our findings, the mixed D2/D3 receptor antagonist U99194 enhances ethanol CPP but does not affect oral alcohol self-administration in Swiss–Webster mice [31,32]. In contrast, U99194 fails to alter ethanol CPP in DBA/2J mice [86]. Moreover, Narita et al. [209] reported that in D3 receptor knockout mice, physical dependence to ethanol is increased, although another study indicates that deleting the D3 receptor in C57BL/6J mice does not significantly alter the rewarding effects of ethanol as assessed by operant ethanol self-administration [33]. These findings are in direct contrast to our data indicating that selective antagonism at D3 receptors by SB-277011-A significantly decreases the intake of ethanol by rats and mice compared to animals treated with vehicle. The discrepant findings with D3 receptor knockout studies might be explained by changes during the development of the genetically modified animal to compensate for the absence of the D3 receptor. In support of this suggestion are findings that haloperidol-treated animals acquire ethanol CPP normally [234] whereas DA D2 receptor knockout mice fail to acquire the CPP response [65]. These findings demonstrate that the behavioral effects produced by a receptor antagonist are not always compatible with those produced by genetically deleting the receptor at which the antagonist acts. For a detailed review on the role of DA D3 receptors in the addictive properties of ethanol, the reader is referred to Ref. [131].
Using a new model of relapse to ethanol-seeking behavior in mice that we have recently developed [189], we have found that noncontingent ethanol administration or ethanol-associated cues can robustly reinstate ethanol-seeking behavior in mice behaviorally extinguished from their previous ethanol self-administration behavior. Acute pretreatment with SB-277011-A (10, 20, and 30 mg/kg ip) produced a dose-dependent attenuation of reinstatement of extinguished ethanol-seeking behavior [189].
7.9. Effect of SB-277011-A on opiate-induced conditioned place preference (CPP)
We have recently shown that the administration of 10 mg/kg ip of SB-277011-A significantly attenuates the acquisition and expression of the CPP response to 1.5 mg/ kg ip of heroin in adult male Sprague–Dawley rats [10]. As previously discussed, SB-277011-A alone does not produce place preference or aversion or shift the BSR curve, suggesting that the effect of SB-277011-A on heroin CPP cannot be related to SB-277011-A itself producing reward or aversion. In contrast, others report that the incentive motivating effect of morphine is significantly enhanced in D3 receptor knockout mice ([210], but see discussion above).
7.10. Summary of effects of SB-277011-A on addictive drug action
The effects of SB-277011-A in several animal models of drug addiction are summarized in Table 4. Together, these findings indicate that SB-277011-A can reduce cocaine-, nicotine-, ethanol-, and heroin-seeking behaviors. These effects are most likely related to the selective antagonism of D3 receptors, as SB-277011-A is a selective high affinity D3 receptor antagonist. In addition, the effects of SB-277011-A are unlikely to result from (1) SB-277011-A producing aversive effects as it does not produce a dysphoric shift in the BSR curve and does not produce CPA, or (2) SB-277011-A producing a rewarding/reinforcing effect as it does not produce a significant left shift of the BSR curve, is not self-administered, and does not produce CPP. SB-277011-A completely blocked the acquisition and expression of the CPP to cocaine. Since the acquisition phase involves storage and encoding of contextual stimuli, and the expression phase involves retrieval of memories of contextual stimuli, it is possible that SB-277011-A may block CPP by interfering with various aspects of encoding and retrieving memories. However, SB-277011-A does not appear to alter memory (as measured using a delayed nonmatched position test, D. Jones and J.J. Hagan, personal communication). Furthermore, acute administration of SB-277011-A significantly increases ACh levels in the anterior cingulate cortex [160] and reverses scopolamine-induced memory deficits as assessed by the threechoice point water labyrinth test [163]. Both of these effects would be expected to improve rather than to interfere with memory. Finally, SB-277011-A does not produce catalepsy or significantly alter locomotor activity [229] at the doses used for the BSR, reinstatement, and CPP experiments, suggesting that the effects observed in these paradigms were not due to interference with normal locomotion/coordination.
The minimum effective dose of SB-277011-A to attenuate cocaine-induced CPP (0.3 mg/kg) was significantly lower than the minimum effective dose to attenuate cocaine-triggered reinstatement (6 mg/kg). This difference could be related to the fact that the reinstatement-triggering dose of cocaine (1 mg/kg iv) may have been significantly supra-threshold (see, e.g., Ref. [76]) and therefore more difficult to overcome. Another explanation could be that cocaine-induced CPP is fundamentally more sensitive to D3 antagonism than cocaine-induced reinstatement. In this regard, the absence of SB-277011-A dose-dependence in blocking cocaine-induced CPP within the dose range used in the reported experiments may be relevant; dose dependence may exist at lower doses. Similarly, it is possible that cocaine-associated cue-induced increases in forebrain DA are substantially lower than cocaine-induced increases [35,109], and perhaps easier for D3 antagonism to surmount.
The different experimental animal paradigms described in previous sections of the present review and summarized in Table 4 each have unique relevance for different aspects of human cocaine addiction. BSR presumably measures the direct rewarding properties of addictive drugs and may come closest to modeling the drug-induced subjective “high”. CPP presumably measures drug-seeking behavior specifically evoked by the incentive salience [24,25,85, 138,285] acquired by environmental cues after repeated association with an addictive drug. Reinstatement presumably measures drug-seeking behavior specifically evoked by re-exposure to drugs, cues, or stressors after behavioral extinction and pharmacological detoxification. The present data suggest that selective DA D3 antagonism may hold highest promise for attenuating cue-evoked relapse to addictive drug use. To date, few other potential pharmaco-therapies have been found which block cue-triggered reinstatement (see, however, [75]). Therefore, a relatively unique therapeutic utility may exist for selective DA D3 antagonists.
Our results are also the first to show that the acute systemic administration of a potent and highly selective brain penetrant D3 receptor antagonist significantly decreases stress-induced reinstatement to iv drug-seeking behavior. We therefore suggest that D3 receptors in the brain are involved in mediating/modulating stress-induced relapse. This suggestion is novel, as stress-triggered relapse has heretofore appeared to be predominantly mediated by noradrenergic and CRF neurotransmitter mechanisms (for reviews, see Refs. [223,262,264,266]). Importantly, however, a role for DA has not been ruled out. For example, acute stress rapidly activates DA neurons in the VTA [144] and increases DA release in the NAc [143,260,289] and mPFC [277,312]. Stress-induced elevation of NAc DA correlates temporally with reinstatement of heroin seeking [259]. Stress appears to stimulate NAc DA release by activating an excitatory projection from the mPFC to glutamate receptors on VTA DA neurons [203]. In addition, stress may also activate mesolimbic DA via CRF release in the midbrain and amygdala [262]. Intracerebroventricular infusion of CRF mimics stress-induced heroin seeking [261] and enhances DA release in the hypothalamus and mPFC [164], although CRF effects on DA release in the NAc have not been reported. Finally, it has been postulated that DA may play an indirect/modulatory role in footshock stress-induced reinstatement [262,266]. Overall, it is likely that multiple neurochemical and anatomical substrates are involved in stress-induced relapse. While our findings with NAc microinjections of SB-277011-A suggest that the NAc may be involved in stress-triggered relapse, there is an important caveat. The NAc is close to the BNST, a region implicated in stress-triggered relapse [262,266,284]. The latency between microinjections and testing in the experiments described in the present review appears sufficient for diffusion of SB-277011-A from NAc to BNST. As discussed earlier, the BNST is a brain area that has a moderate to high density of D3 receptor mRNA, although this area seems to have few or no D3 receptors as ascertained by radioligand binding studies. Thus, additional studies are needed to localize the intracerebral site of action for SB-277011-A’s protective effects against stress-triggered reinstatement of drug-seeking behavior.
8. Role of DA D3 receptors in drug addiction: further confirmation with similar and structurally diverse selective D3 receptor antagonists
Confirmation that it is D3 receptor blockade that is important in mediating the effects of SB-277011-A is provided by reports that structurally dissimilar D3 receptor antagonists possess similar in vivo properties. Recent studies have shown that trans-3-(2-(4-((3-(3-(5-methyl-1,2,4-oxidiazolyl))phenyl)carboxamido)cyclohexyl)ethyl)-7-methylsulfonyl-2,3,4,5-tetrahydro-1H-3-benzapine (SB-414796), another potent and selective DA D3 receptor antagonist, can also block the expression of cocaine-induced CPP [186]. Furthermore, the effect of the selective D3 receptor antagonist NGB-2904 [236,313] has recently been examined in animal models of addiction [309]. It has been reported that, using baculovirus expression of rat DA receptors in vitro, the selectivity of NGB-2904 for D3 vs. D2 is 830 as determined by D2 and D3 receptor binding and use of the radioligand [125I]-IABN [214]. NGB-2904 was also shown to be a functional antagonist in the mitogenesis assay using human D3-transfected CHO cells [236]. It was found that in male Long–Evans rats, acute ip administration of NGB-2904 (1) dose-dependently (0.1, 1, or 5 mg/kg) attenuated iv cocaine self-administration maintained by an FR-2 schedule of reinforcement; (2) significantly reduced motivation for cocaine reward (1 or 5 mg/kg), manifested as a significant reduction in progressive-ratio breakpoint for iv cocaine self-administration; and (3) inhibited cocaine-triggered reinstatement of cocaine-seeking behavior in a dose-dependent manner [309]. The inhibitory effect of NGB-2904 on cocaine-taking and cocaine-seeking behavior was prolonged (2–3 days) after a single ip injection. This phenomenon may result from lipid sequestration of NGB-2904, which is highly lipophilic (clogD = 6.94). Importantly, NGB-2904 (1 mg/kg/infusion) cannot by itself maintain iv self-administration when substituted for cocaine. Finally, when tested over a broad dose range (0.1–10 mg/ kg), NGB-2904 alone had no significant effects on rat locomotor behavior.
Together, the results obtained with the selective DA D3 receptor antagonists SB-414796 [186], NGB-2904 [309], and compound 5p (N-[4-[4-(2,4-dichlorophenyl)piperazin1-yl]butyl]indole-2-carboxamide) [44] all confirm our previous findings with SB-277011-A and further strengthen the hypothesis that central D3 receptors play an important role in the rewarding and incentive motivating effects of cocaine.
Other compounds have been reported to exhibit high in vitro selectivity for D3 receptors whose anti-addiction profiles remain to be characterized. As two previous excellent reviews by Crider and Scheideler [64] and by Hackling and Stark [123] have been published on the medicinal chemistry and selectivity of various compounds for the D3 receptor, we will only discuss the profiles of some recently synthesized compounds that may be worth examining in models of addiction.
Using [3H]-antagonist radioligands, it has been reported that 2-(3-[4-(2-tert-butyl-6-trifluoromethyl-pyrimidin-4-yl)-piperazin-1-yl]-propyl-sulfanyl)-3H-pyrimidin-4-one fuma-rate (A-437203) has D2S/D3 and D2L/D3 receptor selectivity ratios of 45 and 120, respectively, using HEK293 cells transfected with human D2 and D3 receptors [291] (see also Ref. [53]). Functional studies in cellular systems transfected with human D3 receptors indicate that A-437203 lacks intrinsic activity at the D3 receptor but antagonizes agonist-induced actions with pK values of 9 and 7.5 [291]. This compound does not produce catalepsy at doses as high as 464 mg/kg po. Systemic administration of A-437203 induces c-fos expression in the NAc and islands of Calleja, an effect reported for other selective D3 receptor antagonists, and blocks the quinpirole-induced decrease of extracellular DA in the mPFC and NAc [87]. Furthermore, chronic administration of A-437203 for 21 days produces a significant and selective decrease in the number of spontaneously active VTA DA neurons in anesthetized rats [85], a finding similar to that reported for SB-277011-A [9].
The compound 3aR, 9bS-N-[4-(8-cyano-1,3a,4,9b–tetrahydro-3H-benzopyrano[3,4]pyrrole-2-yl)-butyl]-(4-phenyl) benzamide (S33084) has been reported to be a selective D3 receptor antagonist [67,68,90,200,201] with more than 200-fold selectivity compared to 40 other binding sites [200]. Binding studies using cloned human D3 and D2 receptors indicate that the D2/D3 receptor selectivity ratios using [125I]-iodosulpiride and [3H]-spiperone are 120 and 125, respectively [200]. S33084 also exhibits high selectivity for cloned and native rat D3 receptors [68]. S33084 alone does not significantly modify [35S]GTPγS binding at human D3 receptors [200]. In addition, S33084 does not activate the ERK1 and ERK2 species of MAP kinase in CHO cells transfected with human D3 receptors, but antagonizes the induction of MAP by DA [200]. The pA2 value of S33084 to antagonize the stimulation of D3 receptors by DA is 9.69. S33084 has low affinity for a number of other neuro-transmitter receptors, including D1 and D4 receptors [200]. Behavioral studies in rats indicate that S33084 does not induce catalepsy, significantly alter locomotor responses to amphetamine or cocaine, or antagonize methylphenidate-induced gnawing [201]. Furthermore, S33084 does not increase prolactin secretion in rats [201]. Thus, these functional studies suggest that S33084 is not a D2 receptor antagonist. Similarly, the compound 2(R,S)-(di-n-propylamino)-6-(4-methoxyphenylsufonyl methyl)-1,2,3,4-tetrahy-dronaphthalene (GR218,231) [207] is a selective hD3 vs. hD2 receptor antagonist with hD2/hD3 receptor selectivity ratios using [125I]-sulpiride and [3H]-spiperone of 60 and 100, respectively, and a neurochemical profile similar to that of S33084 [200,201].
A series of studies published by Austin and colleagues reports the synthesis of various novel compounds with excellent in vitro selectivity for D3 vs. D2 receptors. A novel substituted 1,2,3,4-tetrahydroisoquinoline with a 7-CF3SO2O substituent and a 3-indolylpropenamido group has a D2/D3 receptor selectivity ratio of 150 as determined in CHO cells transfected with human D2 and D3 receptors [13]. A 2,3,4,5-tetrahydro-1H-3-benzazepine derivative (“compound 20”) has a 130-fold selectivity for D3 vs. D2 receptors [14]. In addition, a 5-substi-tutued-2,3-dihydro-1H–isoindole has at least 100-fold selectivity for D3 receptors over other aminergic receptors [15].
Recently, it has been reported that the benzothiophenes designated FAUC346 and FAUC365 have high affinity for human D3 receptors, with Ki values of 0.23 and 0.5 nM, respectively [23]. The hD2L/hD3 receptor selectivity ratios for FAUC346 and FAUC365 are 380 and 7200, respectively. In the mitogenesis assay in CHO cells transfected with hD3 receptors, FAUC346 displayed partial agonist action whereas FAUC365 lacked intrinsic action, suggesting that it is a D3 receptor antagonist. The compounds designated 1c, 3a, 3b, 3e, and 3f have hD2L/hD3 receptor selectivity ratios of 72, 100, 210, 93, and 170, respectively [23].
Wright et al. [306] reported the synthesis of benzimidazole derivatives, one of which, 3-[4[1-[4-[2-[4-(3-diethylamino-propoxy)phenyl]-benzoimidazol-1-yl-butyl]-1H–benzoimidazol-2-yl]-phenoxy]propyl]diethylamide (PD 58491), was found to be a selective D3 receptor antagonist. A subsequent study, using CHO cells transfected with human D3 and D2L receptors, indicated that PD 58491 has a D2/D3 receptor selectivity ratio of 120 [300]. PD 58491 antagonized the quinpirole-induced stimulation of [3H]-thymidine uptake in CHOpro-5 cells but lacked significant intrinsic activity by itself [300]. PD 58491 only partially blocked the decrease in DA synthesis in the striatum and mesolimbic system of rats produced by the putative selective D3 receptor agonist PD 128907. This finding may be related to stimulation of D2 autoreceptors by PD 128907, and PD 58491 would not antagonize this action, a hypothesis congruent with studies suggesting that PD 128907 can activate D2 receptors in the rat brain. It was also hypothesized that there may be a high presynaptic receptor reserve of D3 receptors, although this must still be determined.
Park et al. [216] have reported that the compound 1-(2-ethoxy-phenyl)-4-[3-(3-thiphen-2[yl-isoxzaolin-5-yl)-propyl]-piperazine (KCH-1110) has an in vitro hD3/hD2 ratio of 90. However, it is likely that this compound is an antagonist at D2 receptors in vivo as it significantly (1) decreases basal locomotor activity in mice at doses of 2.5 and 5 mg/kg; (2) antagonizes apomorphine-induced climbing in mice; (3) increases serum prolactin levels in rats at a dose of 10 mg/kg; (4) antagonizes 7-OH-DPAT-induced hypothermia in mice; and (5) induces catalepsy, albeit mild, at 30 mg/kg. These effects are generally indicative of D2 receptor antagonism. In addition, these effects of KCH-1110 are not seen after the administration of high doses of SB-277011-A [229]. The authors did not examine the effect of KCH-1110 against drugs of abuse in animal models of addiction (e.g., self-administration, CPP, reinstatement). Interestingly, the pre-treatment of mice with 1 mg/kg of KCH-1110 significantly attenuated cocaine-induced hyperlocomotion. However, there is no significant correlation between a compound’s efficacy to increase locomotor activity and its abuse potential. Finally, it should be noted that Park et al. [216] did not report whether KCH-1110 interacted with other CNS receptors to any significant degree.
9. Conclusions
The two compounds that have been systematically tested in animal models of drug addiction are the partialDAD3 receptor agonist BP-897 and the selective DA D3 receptor antagonist SB-277011-A (see Table 4). The sites of action of SB-277011-A and BP-897 in the CNS remain to be elucidated. There is considerable evidence that DA plays an important role in the addictive properties of drugs of abuse (for reviews, see Refs. [22,34,96–98,104,138,171,237,242,303,304]). Indeed, virtually all abusable substances activate the mesolimbic DA system [46,47,79,139,140,220,240,288] (for reviews, see Refs. [104,155]). However, the concept that the mesolimbic DA system simply encodes hedonic tone has been called into question. Analysis of response patterns of single DA neurons to reward presentation has led Schultz and colleagues to suggest that mesolimbic DA may be more involved in prediction of reward and the use of such information to strengthen behaviors and increase their future likelihood. Schultz and colleagues have suggested that the DA signal may constitute an alert message about reward prediction error that rapidly informs postsynaptic structures about unexpected rewards or reward omissions, without detailed information about the nature of the reward per se. The advantage of such a reward alert signal would be to allow rapid behavioral reactions towards rewarding stimuli, while the exact nature of the reward would be evaluated by slower systems during the approach behavior to the rewarding stimulus (for a recent review, see Ref. [251]). On the other hand, Wise and colleagues have argued compellingly for a crucial role for meso-accumbens DA in the actual mediation of reward—if not a final common path for all rewards, at least an intermediate common path for most rewards [303], and we [104] and others [34,46,79,139,140,220,288] have argued that elevation of brain DA constitutes a neurochemical substrate of the reward produced by addictive drugs (and by drug-associated environmental cues; see e.g., Refs. [82,109,141]), not merely a correlate.
The circuitry that mediates reinstatement of drug-seeking behavior is complex [4,5,26,116,118,131,177,211,218,270, 301,303], but key elements involve DA mechanisms. For example, the presentation of a drug-associated CS to animals can induce large conditioned increases in NAc DA [82,141], suggesting that DA in the NAc is involved in cue-controlled drug seeking [308] through interactions with limbic afferents to the NAc [81,83]. Cue incentive properties [24,242] appear mediated by hippocampal and amygdaloid mechanisms [55,152,196]. The amygdala plays an important role [94], particularly in drug-enhanced stimulus– reward associations [127,243] which may, in conjunction with enhanced stimulus–response associations [79], underlie drug craving and compulsive drug taking at the human level [80,215]. Lesions of the central amygdala (CeA) or infusion of 7-OH-DPAT into this brain area can attenuate the acquisition of a Pavlovian approach response [133,134, 217] and can impair the enhancement of instrumental behavior by presentation of a Pavlovian CS [125,126]. Interestingly, lesions of the basolateral amygdala (BLA) can impair the acquisition of cocaine seeking under a second-order schedule [301]. In addition, reversible inactivation of the BLA by lidocaine can block both the acquisition and expression of the response-maintaining properties of a CS under both second-order and reinstatement conditions [119,145,158]. Recent studies have also shown that direct microinfusion of d-amphetamine into the BLA can potentiate cue-triggered relapse to cocaine seeking in a dose-dependent manner without affecting extinction responding [166]. Furthermore, electrical or chemical stimulation of the BLA can reinstate cocaine-seeking behavior following behavioral extinction of the cocaine-seeking habit [129], as can electrical stimulation of the hippocampus, another brain structure implicated in the storage and retrieval of memories underlying the cue-incentive properties of addictive drugs [294]. Within the amygdala, direct infusion of the nonselective muscarinic receptor antagonist scopolamine into the BLA during the acquisition of conditioned pairing of a light/tone stimulus with cocaine self-administration can also disrupt cocaine-seeking behavior maintained by the cocaine-associated cues [255]. In contrast, the same scopolamine treatment given just prior to the reinstatement test fails to affect conditioned cue-induced relapse to cocaine seeking [255]. Reversible inactivation of the BLA, anterior cingulate cortex, or prelimbic cortex just prior to the reinstatement test impairs the ability of a light/tone stimulus to reinstate extinguished lever pressing for cocaine-paired stimuli [193]. These results are congruent with the finding that neural activity in the VTA, anterior cingulate cortex, NAc core, and ventral pallidum is necessary for cocaine-induced reinstatement of drug-seeking behavior [192]. It has also been shown that stress-induced relapse may be mediated by the BNST and CeA [93,173,264].
Such findings as those noted above point toward an important role of the BLA in stimulus–reward associations that mediate cue-triggered reinstatement of cocaine-seeking behavior. Enhanced monoaminergic tone in the BLA appears to increase the motivational properties or salience of cocaine-associated cues during reinstatement of cocaine-seeking behavior, whereas inactivation of the BLA produces the reverse effect. The CeA may mediate conditioned increases in DA measured in the NAc following the noncontingent presentation of a CS [82,141] perhaps via projections to the VTA [218] and seems to play a key role in stress-triggered relapse to cocaine-seeking behavior. Finally, the anterior cingulate cortex seems to serve as a common link in the neural circuitry underlying reinstatement of drug-seeking behaviors, perhaps because the anterior cingulate cortex is critically involved in the discrimination of multiple stimuli on the basis of their association with reward [48], in shifting away from spatial locations previously associated with reward (response perseveration), attention, and the ability to adequately plan actions involved in fear responding (for a complete review, see Ref. [130]). Thus, these results point towards the anterior cingulate cortex as a key component involved in the temporal patterning of behavioral sequences.
Given that SB-277011-A is effective in attenuating the action of a number of addictive drugs in various paradigms, one might hypothesize that (1) the cue-, drug-, and stress-induced reinstatement circuits may, in fact, have a common final pathway and that the efficacy of SB-277011-A is due to blockade of D3 receptors in this pathway, and/or (2) D3 receptors are located at critical junctions in the pathways involved in cue-, drug-, and stress-induced reinstatement. Indeed, DA D3 receptors are present in moderate to high densities in the amygdala, NAc, VTA, BNST, and mPFC. In a recent series of pharmacological magnetic resonance imaging (MRI) experiments, we have shown that pretreatment with SB-277011-A, at a dose behaviorally effective against drug-seeking, potentiates the rapid relative cerebral blood volume (rCBV) response to amphetamine in regions including, but extending beyond, the D3-rich areas, while SB-277011-A itself produced only limited activation and gradual rCBV changes commensurate with its pharmacokinetics [254]. Importantly, the regions showing increased rCBV due to SB-277011-A alone did not correspond to the foci of dense D3 receptor distribution and thus did not coincide with those foci showing a potentiated response to amphetamine. SB-277011-A itself produced focal bilateral activation in the entorhinal cortex, lateral globus pallidus, and CeA. The pattern of potentiation was more widespread and included the NAc, islands of Calleja, anterior cingulate and retrosplenial cortices, thalamus, dorsal striatum, BNST, and ventral subiculum. These findings suggest that the efficacy of selective DA D3 receptor antagonists in attenuating reinstatement of drug-seeking behavior may in fact proceed via increased activity in both the extended amygdala and the mesolimbic DA system. Additional studies must be conducted in order to determine the specific brain areas involved in the effects of SB-277011-A in the CPP, BSR, and reinstatement paradigms. Studies examining the effect of SB-277011-A microinjections into areas such as the amygdala, VTA, NAc, and anterior cingulate cortex are warranted and have been initiated.
Acknowledgments
We thank Sharon Buie for assistance with manuscript preparation and Arlene C. Pak and Jeremy Gilbert for helpful comments, criticisms, and assistance with reference citations.
References
- 1.Acri JB, Carter SR, Alling K, Geter-Douglass B, Dijkstra D, Wikström H, Katz JL, Witkin JM. Assessment of cocaine-like discriminative stimulus effects of dopamine D3 receptor ligands. Eur. J. Pharmacol. 1995;281:R7–R9. doi: 10.1016/0014-2999(95)00411-d. [DOI] [PubMed] [Google Scholar]
- 2.Ahlenius S, Salmi P. Behavioral and biochemical effects of the dopamine D3 receptor-selective ligand, 7-OH-DPAT, in the normal and the reserpine-treated rat. Eur. J. Pharmacol. 260(1994):177–181. doi: 10.1016/0014-2999(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed SH, Koob GF. Cocaine-but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berlin) 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- 4.Alderson HL, Robbins TW, Everitt BJ. The effects of excitotoxic lesions of the basolateral amygdala on the acquisition of heroin-seeking behaviour in rats. Psychopharmacology (Berlin) 2000;153:111–119. doi: 10.1007/s002130000527. [DOI] [PubMed] [Google Scholar]
- 5.Alderson HL, Parkinson JA, Robbins TW, Everitt BJ. The effects of excitotoxic lesions of the nucleus accumbens core or shell regions on intravenous heroin self-administration in rats. Psychopharmacology (Berlin) 2001;153:455–463. doi: 10.1007/s002130000634. [DOI] [PubMed] [Google Scholar]
- 6.Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003;28:1272–1280. doi: 10.1038/sj.npp.1300183. [DOI] [PubMed] [Google Scholar]
- 7.Andreoli M, Marcon C, Hagan JJ, Heidbreder CA. Effect of selective antagonism at dopamine D3 receptor by SB-277011-A on oral alcohol self-administration in mice. Eur. Neuropsychopharmacol. 2003;13(Suppl. 1):S17. [Google Scholar]
- 8.Arnold JM, Roberts DCS. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol. Biochem. Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- 9.Ashby CR, Jr., Minabe Y, Stemp G, Hagan JJ, Middlemiss DN. Acute and chronic administration of the selective D3 receptor antagonist SB-277011-A alters activity of midbrain dopamine neurons in rats: an in vivo electrophysiological study. J. Pharmacol. Exp. Ther. 2000;294:1166–1174. [PubMed] [Google Scholar]
- 10.Ashby CR, Jr., Paul M, Gardner EL, Heidbreder CA, Hagan JJ. Acute administration of the selective D3 receptor antagonist SB-277011-A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 2003;48:154–156. doi: 10.1002/syn.10188. [DOI] [PubMed] [Google Scholar]
- 11.Audinot V, Newman-Tancredi A, Gobert A, Rivet J-M, Brocco M, Lejeune F, Gluck L, Desposte I, Bervoets K, Dekeyne A, Millan MJ. A. Dekeyne, M.J. Millan, A comparative in vitro and in vivo pharmacological characterization of the novel dopamine D3 receptor antagonists (+)-S 14297, nafadotride, GR 103,691 and U 99194. J. Pharmacol. Exp. Ther. 1998;287:187–197. [PubMed] [Google Scholar]
- 12.Aujla H, Sokoloff P, Beninger RJ. A dopamine D3 receptor partial agonist blocks the expression of conditioned activity. NeuroReport. 2002;13:173–176. doi: 10.1097/00001756-200201210-00039. [DOI] [PubMed] [Google Scholar]
- 13.Austin NE, Avenell KY, Boyfield I, Branch CL, Coldwell MC, Hadley MS, Jeffrey P, Johns A, Johnson CN, Nash DJ, Riley GJ, Smith SA, Stacey RC, Stemp G, Thewlis KM, Vong AKK. Novel 1,2,3,4-tetrahydroisoquinolines with high affinity and selectivity for the dopamine D3 receptor. Bioorg. Med. Chem. Lett. 1999;9:179–184. doi: 10.1016/s0960-894x(98)00699-4. [DOI] [PubMed] [Google Scholar]
- 14.Austin NE, Avenell KY, Boyfield I, Branch CL, Hadley MS, Jeffrey P, Johnson CN, Macdonald GJ, Nash DJ, Riley GJ, Smith AB, Stemp G, Thewlis KM, Vong AKK, Wood M. Novel 2,3,4,5-tetrahydro-1H-3-benzazepines with high affinity and selectivity for the dopamine D3 receptor. Bioorg. Med. Chem. Lett. 2000;10:2553–2555. doi: 10.1016/s0960-894x(00)00505-9. [DOI] [PubMed] [Google Scholar]
- 15.Austin NE, Avenell KY, Boyfield I, Branch CL, Hadley MS, Jeffrey P, Johnson CN, Macdonald GJ, Nash DJ, Riley GJ, Smith AB, Stemp G, Thewlis KM, Vong AKK, Wood MD. Design and synthesis of novel 2,3-dihydro-1H–isoindoles with high affinity and selectivity for the dopamine D3 receptor. Bioorg. Med. Chem. Lett. 2001;11:685–688. doi: 10.1016/s0960-894x(01)00037-3. [DOI] [PubMed] [Google Scholar]
- 16.Austin NE, Baldwin SJ, Cutler L, Deeks N, Kelly PJ, Nash M, Shardlow CE, Stemp G, Thewlis K, Ayrton A, Jeffrey P. Pharmacokinetics of the novel, high-affinity and selective dopamine D3 receptor antagonist SB-277011 in rat, dog and monkey: in vitro/in vivo correlation and the role of aldehyde oxidase. Xenobiotica. 2001;31:677–686. doi: 10.1080/00498250110056531. [DOI] [PubMed] [Google Scholar]
- 17.Baker LE, Prus AJ. Reevaluation of PNU-99194A discriminative stimulus effects: potentiation by both a D2 and a D3/D2 agonist. Pharmacol. Biochem. Behav. 2002;73:753–758. doi: 10.1016/s0091-3057(02)00882-1. [DOI] [PubMed] [Google Scholar]
- 18.Baker LE, Svensson KA, Garner KJ, Goodwin AK. The dopamine D3 receptor antagonist PNU-99194A fails to block (+)-7-OH-DPAT substitution for d-amphetamine or cocaine. Eur. J. Pharmacol. 1998;358:101–109. doi: 10.1016/s0014-2999(98)00582-2. [DOI] [PubMed] [Google Scholar]
- 19.Baker LE, Hood CA, Heideman AM. Assessment of D3 versus D2 receptor modulation of the discriminative stimulus effects of (+)-7-OH-DPAT in rats. Behav. Pharmacol. 1999;10:717–722. doi: 10.1097/00008877-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Bancroft GN, Morgan KA, Fliestra RJ, Levant B. [3H]PD 128907, a putatively selective ligand for the D3 dopamine receptor, in rat brain: a receptor binding and quantitative autoradiographic study. Neuropsychopharmacology. 1998;18:305–316. doi: 10.1016/S0893-133X(97)00162-0. [DOI] [PubMed] [Google Scholar]
- 21.Beardsley PM, Sokoloff P, Balster RL, Schwartz J-C. The D3R partial agonist, BP 897, attenuates the discriminative stimulus effects of cocaine and d-amphetamine and is not self-administered. Behav. Pharmacol. 2001;12:1–12. doi: 10.1097/00008877-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning or incentive salience. Brain Res., Brain Res. Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 23.Bettinetti L, Schlotter K, Hübner H, Gmeiner P. Interactive SAR studies: rational discovery of super-potent and highly selective dopamine D3 receptor antagonists and partial agonists. J. Med. Chem. 2002;45:4594–4597. doi: 10.1021/jm025558r. [DOI] [PubMed] [Google Scholar]
- 24.Bindra D. Neuropsychological interpretation of the effects of drive and incentive-motivation on general activity and instrumental behavior. Psychol. Rev. 1968;75:1–22. [Google Scholar]
- 25.Bolles RC. Reinforcement, expectancy, and learning. Psychol. Rev. 1972;79:394–409. [Google Scholar]
- 26.Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metclafe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 27.Booze RM, Wallace DR. Dopamine D2 and D3 receptors in the rat striatum and nucleus accumbens: use of 7-OH-DPAT and [125I]-iodosulpiride. Synapse. 1995;19:1–13. doi: 10.1002/syn.890190102. [DOI] [PubMed] [Google Scholar]
- 28.Boulay D, Depoortere R, Perrault G, Borrelli E, Sanger DJ. Dopamine D2 receptor knockout mice are insensitive to the hypolocomotor and hypothermic effects of dopamine D2/D3 receptor agonists. Neuropharmacology. 1999;38:1389–1396. doi: 10.1016/s0028-3908(99)00064-7. [DOI] [PubMed] [Google Scholar]
- 29.Boulay D, Depoortere R, Rostene W, Perrault G, Sanger DJ. Dopamine D3 receptor agonists produce similar decreases in body temperature and locomotor activity in D3 knock-out and wild-type mice. Neuropharmacology. 1999;38:555–565. doi: 10.1016/s0028-3908(98)00213-5. [DOI] [PubMed] [Google Scholar]
- 30.Bouthenet M-L, Souil E, Martres M-P, Sokoloff P, Giros B, Schwartz J-C. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- 31.Boyce JM, Risinger FO. Enhancement of ethanol reward by dopamine D3 receptor blockade. Brain Res. 2000;880:202–206. doi: 10.1016/s0006-8993(00)02801-8. [DOI] [PubMed] [Google Scholar]
- 32.Boyce JM, Risinger FO. Dopamine D3 receptor antagonist effects on the motivational effects of ethanol. Alcohol. 2002;28:47–55. doi: 10.1016/s0741-8329(02)00237-9. [DOI] [PubMed] [Google Scholar]
- 33.Boyce-Rustay JM, Risinger FO. Dopamine D3 receptor knockout mice and the motivational effect of ethanol. Pharmacol. Biochem. Behav. 2003;75:373–379. doi: 10.1016/s0091-3057(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 34.Bozarth MA. The mesolimbic dopamine system as a model reward system. In: Willner P, Scheel-Kruger J, editors. The Mesolimbic Dopamine System: From Motivation to Action. Chichester, UK: Wiley; 1991. pp. 301–330. [Google Scholar]
- 35.Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J. Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bristow LJ, Cook GP, Patel S, Curtis N, Mawer I, Kulagowsk JJ. Discriminative stimulus properties of the putative dopamine D3 receptor agonist, (+)-PD 128907: role of presynaptic dopamine D2 autoreceptors. Neuropharmacology. 1998;37:793–802. doi: 10.1016/s0028-3908(98)00066-5. [DOI] [PubMed] [Google Scholar]
- 37.Britton DR, Curzon P, MacKenzie RG, Kebabian JW, Williams JEG, Kerkman D. Evidence for involvement of both D1 and D2 receptors in maintaining cocaine self-administration. Pharmacol. Biochem. Behav. 1991;39:911–915. doi: 10.1016/0091-3057(91)90052-4. [DOI] [PubMed] [Google Scholar]
- 38.Buczek Y, Le AD, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berlin) 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- 39.Bunzow JR, Van Tol HHM, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988;336:783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- 40.Burris KD, Pacheco MA, Filtz TM, Kung M-P, Kung HF, Molinoff PB. Lack of discrimination by agonists for D2 and D3 dopamine receptors. Neuropsychopharmacology. 1995;12:335–345. doi: 10.1016/0893-133X(94)00099-L. [DOI] [PubMed] [Google Scholar]
- 41.Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- 42.Caine SB, Koob GF. Pretreatment with the dopamine agonist 7-OH DPAT shifts the cocaine self-administration dose-effect function to the left under different schedules in the rat. Behav. Pharmacol. 1995;6:333–347. [PubMed] [Google Scholar]
- 43.Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz J-C, Sokoloff P. D3 receptor test in vitro predicts decreased cocaine self-administration in rats. NeuroReport. 1997;8:2373–2377. doi: 10.1097/00001756-199707070-00054. [DOI] [PubMed] [Google Scholar]
- 44.Campiani G, Butini S, Trotta F, Fattorusso C, Catalanotti B, Aiello F, Gemma S, Nacci V, Novellino E, Stark JA, Cagnotto A, Fumagalli E, Carnovali F, Cervo L, Mennini T. Synthesis and pharmacological evaluation of potent and highly selective D3 receptor ligands: inhibition of cocaine-seeking behavior and the role of dopamine D3/D2 receptors. J. Med. Chem. 2003;46:3822–3839. doi: 10.1021/jm0211220. [DOI] [PubMed] [Google Scholar]
- 45.Campos AC, Xi Z-X, Gilbert J, Ashby CR, Jr., Heidbreder CA, L E. Gardner, The dopamine D3 receptor antagonist SB277011A antagonizes nicotine-enhanced brain-stimulation reward in rat. Abstr.-Soc. Neurosci. 29(2003) (abstract 322.8) [Google Scholar]
- 46.Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- 47.Carboni E, Silvagni A, Rolando MTP, Di Chiara G. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J. Neurosci. 20(2000):RC102. doi: 10.1523/JNEUROSCI.20-20-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardinal RN, Parkinson JA, Marbini HD, Toner AJ, Bussey TJ, Robbins TW, Everitt BJ. Role of the anterior cingulate cortex in the control over behavior by Pavlovian conditioned stimuli in rats. Behav. Neurosci. 2003;117:566–587. doi: 10.1037/0735-7044.117.3.566. [DOI] [PubMed] [Google Scholar]
- 49.Carr KD, Yamamoto N, Omura M, Cabeza de Vaca S, Krahne L. Effects of the D3 dopamine receptor antagonist, U99194A, on brain stimulation and d-amphetamine reward, motor activity, and c-fos expression in ad libitum fed and food-restricted rats. Psychopharmacology (Berlin) 2002;163:76–84. doi: 10.1007/s00213-002-1132-0. [DOI] [PubMed] [Google Scholar]
- 50.Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28:1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- 51.Cervo L, Cocco A, Petrella C, Heidbreder CA, Bendotti C, Mennini T. SB-277011-A, a selective dopamine D3 receptor antagonist, reduces cocaine-seeking behavior in response to drug-associated stimuli in rats. Abstr.-Soc. Neurosci. 2003;29 (abstract 420.3) [Google Scholar]
- 52.Chao J, Nestler EJ. Molecular neurobiology of drug addiction. Annu. Rev. Med. 55(2004):113–132. doi: 10.1146/annurev.med.55.091902.103730. [DOI] [PubMed] [Google Scholar]
- 53.Chaperon F, Tricklebank MD, Unger L, Neijt HC. Evidence for regulation of body temperature in rats by dopamine D2 receptor and possible influence of D1 but not D3 and D4 receptors. Neuropharmacology. 2003;44:1047–1053. doi: 10.1016/s0028-3908(03)00113-8. [DOI] [PubMed] [Google Scholar]
- 54.Chiang Y-C, Chen P-C, Chen J-C. D3 dopamine receptors are down-regulated in amphetamine sensitized rats and their putative antagonists modulate the locomotor sensitization to amphetamine. Brain Res. 2003;972:159–167. doi: 10.1016/s0006-8993(03)02522-8. [DOI] [PubMed] [Google Scholar]
- 55.Childress AR, Mozley PD, McElgwin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am. J. Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chio CL, Lajiness ME, Huff RM. Activation of heterologously expressed D3 dopamine receptors: comparison with D2 dopamine receptors. Mol. Pharmacol. 1994;45:51–60. [PubMed] [Google Scholar]
- 57.Christian AJ, Goodwin AK, Baker LE. Antagonism of the discriminative stimulus effects of (+)-7-OH-DPAT by remoxipride but not PNU-99194A. Pharmacol. Biochem. Behav. 2001;68:371–377. doi: 10.1016/s0091-3057(00)00470-6. [DOI] [PubMed] [Google Scholar]
- 58.Clark D, Exner M, Furmidge LJ, Svensson K, Sonesson C. Effects of the dopamine autoreceptor antagonist (−)-DS121 on the discriminative stimulus properties of d-amphetamine and cocaine. Eur. J. Pharmacol. 1995;275:67–74. doi: 10.1016/0014-2999(94)00747-u. [DOI] [PubMed] [Google Scholar]
- 59.Clifford JJ, Waddington JL. Heterogeneity of behavioural profile between three new putative selective D3 dopamine receptor antagonists using an ethologically based approach. Psychopharmacology (Berlin) 1998;136:284–290. doi: 10.1007/s002130050567. [DOI] [PubMed] [Google Scholar]
- 60.Coldwell MC, Boyfield I, Brown AM, Stemp G, Middlemiss DN. Pharmacological characterization of extracellular acidification rate responses in human D2(long), D3 and D4.4 receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1999;127:1135–1144. doi: 10.1038/sj.bjp.0702657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cook CD, Beardsley PM. The modulatory actions of dopamine D2/3 agonists and antagonists on the locomotor-activating effects of morphine and caffeine in mice. Pharmacol. Biochem. Behav. 2003;75:363–371. doi: 10.1016/s0091-3057(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 62.Corbin AE, Pugsley TA, Akunne HC, Whetzel SZ, Zoski KT, Georgic LM, Nelson CB, Wright JL, Wise LD, Heffner TG. Pharmacologic characterization of PD 152225, a novel dimeric benzimidazole dopamine D3 antagonist. Pharmacol. Biochem. Behav. 1998;59:487–493. doi: 10.1016/s0091-3057(97)00442-5. [DOI] [PubMed] [Google Scholar]
- 63.Court JA, Lloyd S, Thomas N, Piggott MA, Marshall EF, Morris CM, Lamb H, Perry RH, Johnson M, Perry EK. Dopamine and nicotinic receptor binding and the levels of dopamine and homovanillic acid in human brain related to tobacco use. Neuroscience. 1998;87:63–78. doi: 10.1016/s0306-4522(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 64.Crider AM, Scheideler MA. Recent advances in the development of dopamine D3 receptor agonists and antagonists. Mini Rev. Med. Chem. 2001;1:89–99. doi: 10.2174/1389557013407287. [DOI] [PubMed] [Google Scholar]
- 65.Cunningham CL, Howard MA, Gill SJ, Rubinstein M, Low MJ, Grandy DK. Ethanol-conditioned place preference is reduced in dopamine D2 receptor-deficient mice. Pharmacol. Biochem. Behav. 2000;67:693–699. doi: 10.1016/s0091-3057(00)00414-7. [DOI] [PubMed] [Google Scholar]
- 66.Curran EJ, Watson SJ., Jr. Dopamine receptor mRNA expression patterns by opioid peptide cells in the nucleus accumbens of the rat: a double in situ hybridization study. J. Comp. Neurol. 1995;361:57–76. doi: 10.1002/cne.903610106. [DOI] [PubMed] [Google Scholar]
- 67.Cussac D, Newman-Tancredi A, Sezgin L, Millan MJ. [ H]S33084: a novel, selective and potent radioligand at cloned, human dopamine D3 receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000;361:569–572. doi: 10.1007/s002100000217. [DOI] [PubMed] [Google Scholar]
- 68.Cussac D, Newman-Tancredi A, Sezgin L, Millan MJ. The novel antagonist, S33084, and GR218,231 interact selectively with cloned and native, rat dopamine D3 receptors as compared with native, rat dopamine D2 receptors. Eur. J. Pharmacol. 2000;394:47–50. doi: 10.1016/s0014-2999(00)00149-7. [DOI] [PubMed] [Google Scholar]
- 69.Czermak C, Lehofer M, Wagner EM, Prietl B, Gorkiewicz G, Lemonis L, Rohrhofer A, Legl T, Schauenstein K, Liebmann PM. Reduced dopamine D3 receptor expression in blood lymphocytes of smokers is negatively correlated with daily number of smoked cigarettes: a peripheral correlate of dopaminergic alterations in smokers. Nicotine Tob. Res. 2004;6:49–54. doi: 10.1080/14622200310001656858. [DOI] [PubMed] [Google Scholar]
- 70.Dall’Olio R, Gaggi R, Voltattorni M, Tanda O, Gandolfi O. Nafadotride administration increases D1 and D1/D2 dopamine receptor mediated behaviors. Behav. Pharmacol. 2002;13:633–638. doi: 10.1097/00008877-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Daly SA, Waddington JL. Behavioural effects of the putative D-3 dopamine receptor agonist 7-OH-DPAT in relationship to other “D-2-like” agonists. Neuropharmacology. 1993;32:509–510. doi: 10.1016/0028-3908(93)90177-5. [DOI] [PubMed] [Google Scholar]
- 72.Dekeyne A, Rivet J-M, Gobert A, Millan MJ. Generalization of the serotonin (5-HT)1A agonists and the antipsychotics, clozapine, ziprasidone and S16924, but not haloperidol, to the discriminative stimuli elicited by PD128,907 and 7-OH-DPAT. Neuropharmacology. 2001;40:899–910. doi: 10.1016/s0028-3908(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 73.Depoortere R, Perrault G, Sanger DJ. Behavioural effects in the rat of the putative dopamine D3 receptor agonist 7-OH-DPAT: comparison with quinpirole and apomorphine. Psychopharmacology (Berlin) 1996;124:231–240. doi: 10.1007/BF02246662. [DOI] [PubMed] [Google Scholar]
- 74.Depoortere R, Perrault G, Sanger DJ. The D3 antagonist PNU-99194A potentiates the discriminative cue produced by the D3 agonist 7-OH-DPAT. Pharmacol. Biochem. Behav. 2000;65:31–34. doi: 10.1016/s0091-3057(99)00120-3. [DOI] [PubMed] [Google Scholar]
- 75.De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN. A cannabinoid mechanism in relapse to cocaine seeking. Nat. Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- 76.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berlin) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 77.Diaz J, Lévesque D, Lammers CH, Griffon N, Martres M-P, Schwartz J-C, Sokoloff P. Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience. 1995;65:731–745. doi: 10.1016/0306-4522(94)00527-c. [DOI] [PubMed] [Google Scholar]
- 78.Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz J-C, Sokoloff P. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J. Neurosci. 2000;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J. Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- 80.Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning: role of nucleus accumbens shell/extended amygdala dopamine. Ann. N. Y. Acad. Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- 81.Di Ciano P, Everitt BJ. Dissociable effect of antagonism of NMDA and AMPA receptors in the nucleus accumbens core and shell on cocaine-seeking behaviour. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 82.Di Ciano P, Blaha CD, Phillips AG. Conditioned changes in dopamine oxidation currents in the nucleus accumbens of rats by stimuli paired with self-administration or yoked administration of d-amphetamine. Eur. J. Neurosci. 1998;10:1121–1127. doi: 10.1046/j.1460-9568.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- 83.Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of Pavlovian approach behavior. J. Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- 85.Dickinson A, Balleine B. Motivational control of goal-directed action. Anim. Learn. Behav. 1994;22:1–18. [Google Scholar]
- 86.Dickinson SD, Lee EL, Rindal K, Cunningham CL. Lack of effect of dopamine receptor blockade on expression of ethanol-induced conditioned place preference in mice. Psychopharmacology (Berlin) 2003;165:238–244. doi: 10.1007/s00213-002-1270-4. [DOI] [PubMed] [Google Scholar]
- 87.Drescher KU, Garcia-Ladona FJ, Teschendorf HJ, Traut M, Unger L, Wicke KM, Weddige FK, Freeman AS, Gross G. In vivo effects of the selective dopamine D3 receptor antagonist A-437203. Abstr.-Soc. Neurosci. 2002;28 (abstract 894.6) [Google Scholar]
- 88.Duarte C, Lefebvre C, Chaperon F, Hamon M, Thiébot M-H. Effects of a dopamine D3 receptor ligand, BP 897, on acquisition and expression of food-, morphine-, and cocaine-induced conditioned place preference, and food-seeking behavior in rats. Neuropsychopharmacology. 2003;28:1903–1915. doi: 10.1038/sj.npp.1300276. [DOI] [PubMed] [Google Scholar]
- 89.Duarte C, Biala G, Le Bihan C, Hamon M, Thiébot M-H. Respective roles of dopamine D2 and D3 receptors in food-seeking behaviour in rats. Psychopharmacology (Berlin) 2003;166:19–32. doi: 10.1007/s00213-002-1310-0. [DOI] [PubMed] [Google Scholar]
- 90.Dubuffet T, Newman-Tancredi A, Cussac D, Audinot V, Loutz A, Millan MJ, Lavielle G. Novel benzopyrano[3,4-c]pyrrole derivatives as potent and selective dopamine D3 receptor antagonists. Bioorg. Med. Chem. Lett. 1999;19:2059–2064. doi: 10.1016/s0960-894x(99)00312-1. [DOI] [PubMed] [Google Scholar]
- 91.Ellinwood EH, King GR, Davidson C, Lee TH. The dopamine D2/D3 antagonist DS121 potentiates the effect of cocaine on locomotion and reduces tolerance in cocaine-tolerant rats. Behav. Brain Res. 2000;116:169–175. doi: 10.1016/s0166-4328(00)00270-9. [DOI] [PubMed] [Google Scholar]
- 92.Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psycho-pharmacology (Berlin) 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- 93.Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berlin) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- 94.Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward: the role of amygdala-ventral striatal subsystems. Ann. N. Y. Acad. Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 95.Ferrari F, Giuliani D. Behavioural effects of the dopamine D3 receptor agonist 7-OH-DPAT in rats. Pharmacol. Res. 1995;32:63–68. doi: 10.1016/s1043-6618(95)80010-7. [DOI] [PubMed] [Google Scholar]
- 96.Fibiger HC. Drugs and reinforcement mechanisms: a critical review of the catecholamine theory. Annu. Rev. Pharmacol. Toxicol. 1978;18:37–56. doi: 10.1146/annurev.pa.18.040178.000345. [DOI] [PubMed] [Google Scholar]
- 97.Fibiger HC, Phillips AG. Reward, motivation, cognition: psycho-biology of mesotelencephalic dopamine systems. In: Mount-castle VB, Bloom FE, Geiger SR, editors. Handbook of Physiology, The Nervous System. vol. 4. Bethesda, MD: American Physiological Society; 1986. pp. 647–675. [Google Scholar]
- 98.Fibiger HC, Phillips AG. Mesocorticolimbic dopamine systems and reward. Ann. N. Y. Acad. Sci. 1988;537:207–215. doi: 10.1111/j.1749-6632.1988.tb42107.x. [DOI] [PubMed] [Google Scholar]
- 99.Filip M, Papla I, Czepiel K. Role of dopamine D3 receptors in controlling the expression of cocaine sensitization in rats. Pol. J. Pharmacol. 2002;54:687–691. [PubMed] [Google Scholar]
- 100.Fishburn CS, Belleli D, David C, Carmon S, Fuchs S. A novel short isoform of the D3 dopamine receptor generated by alternative splicing in the third cytoplasmic loop. J. Biol. Chem. 1993;268:5872–5878. [PubMed] [Google Scholar]
- 101.Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5HT2A receptor antagonist M100,907 and the 5-HT2C receptor antagonist SB242,084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–584. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 102.Gál K, Gyertyán I. Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self-administration in rats. Brain Res. Bull. 2003;61:595–601. doi: 10.1016/s0361-9230(03)00217-x. [DOI] [PubMed] [Google Scholar]
- 103.Garcia-Ladona FJ, Cox BF. BP-897, a selective dopamine D3 receptor ligand with therapeutic potential for the treatment of cocaine addiction. CNS Drug Rev. 2003;9:141–158. doi: 10.1111/j.1527-3458.2003.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gardner EL. What we have learned about addiction from animal models of drug self-administration. Am. J. Addict. 2000;9:285–313. doi: 10.1080/105504900750047355. [DOI] [PubMed] [Google Scholar]
- 105.Garner KJ, Baker LE. Analysis of D2 and D3 receptor-selective ligands in rats trained to discriminate cocaine from saline. Pharmacol. Biochem. Behav. 1999;64:373–378. doi: 10.1016/s0091-3057(99)00064-7. [DOI] [PubMed] [Google Scholar]
- 106.Gehlert DR. Quantitative autoradiography of Gpp(NH)p sensitive and insensitive [3H]quinpirole binding sites in the rat brain. Synapse. 1992;14:113–120. doi: 10.1002/syn.890140203. [DOI] [PubMed] [Google Scholar]
- 107.Gehlert DR, Gackenheimer SL, Seeman P, Schaus J. Autoradiographic localization of [3H]-quinpirole binding to dopamine D2 and D3 receptors in rat brain. Eur. J. Pharmacol. 1992;211:189–194. doi: 10.1016/0014-2999(92)90528-c. [DOI] [PubMed] [Google Scholar]
- 108.Gendreau PL, Petitto JM, Schnauss R, Frantz KJ, Van Hartesveldt C, Gariepy J-L, Lewis MH. Effects of the putative dopamine D3 receptor antagonist PNU 991194A on motor behavior and emotional reactivity in C57BL/6J mice. Eur. J. Pharmacol. 1997;337:147–155. doi: 10.1016/s0014-2999(97)01324-1. [DOI] [PubMed] [Google Scholar]
- 109.Gerasimov MR, Schiffer WK, Gardner EL, Marsteller DA, Lennon IC, Taylor SJ, Brodie JD, Ashby CR, Jr., Dewey SL. GABAergic blockade of cocaine-associated cue-induced increases in nucleus accumbens dopamine. Eur. J. Pharmacol. 2001;414:205–209. doi: 10.1016/s0014-2999(01)00800-7. [DOI] [PubMed] [Google Scholar]
- 110.Gerber GJ, Wise RA. Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: a variable dose paradigm. Pharmacol. Biochem. Behav. 1989;32:527–531. doi: 10.1016/0091-3057(89)90192-5. [DOI] [PubMed] [Google Scholar]
- 111.Gilbert J, Xi Z-X, Campos AC, Ashby CR, Jr., Heidbreder CA, Gardner EL. The dopamine D3 receptor antagonist SB277011A inhibits cocaine reinforcement under fixed ratio and progressive ratio schedules. Abstr.-Soc. Neurosci. 2003;29 (abstract 422.10) [Google Scholar]
- 112.Giros B, Martres M-P, Sokoloff P, Schwartz J-C. Clonage du géne récepteur dopaminergique D3 humain et identification de son chromosome. C. R. Acad. Sci III Sci. Vie. 1990;311:501–508. [PubMed] [Google Scholar]
- 113.Gobert A, Rivet J-M, Audinot V, Cistarelli L, Spedding M, Vian J, Peglion J-L, Millan MJ. Functional correlates of dopamine D3 receptor activation in the rat in vivo and their modulation by the selective antagonist, (+)-S 14297: II. Both D2 and “silent” D3 autoreceptors control synthesis and release in mesolimbic, mesocortical and nigrostriatal pathways. J. Pharmacol. Exp. Ther. 1995;275:899–913. [PubMed] [Google Scholar]
- 114.Gonzales AM, Sibley DR. [3H]7-OH-DPAT is capable of labeling dopamine D2 as well as D3 receptors. Eur. J. Pharmacol. 1995;272:R1–R3. doi: 10.1016/0014-2999(94)00738-s. [DOI] [PubMed] [Google Scholar]
- 115.Goudie AJ, Baker LE, Smith JA, Prus AJ, Svensson KA, Cortes-Burgos LA, Wong EH, Haadsma-Svensson S. Common discriminative stimulus properties in rats of muscarinic antagonists, clozapine and the D3 preferring antagonist PNU-99194A: an analysis of possible mechanisms. Behav. Pharmacol. 2001;12:303–315. doi: 10.1097/00008877-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 116.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. U. S. A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Griffon N, Diaz J, Lévesque D, Sautel F, Schwartz J-C, Sokoloff P, Simon P, Costentin J, Garrido F, Mann A, Wermuth C. Localization, regulation and role of the dopamine D3 receptor are distinct from those of the D2 receptor. Clin. Neuropharmacol. 1995;18(Suppl. 1):S130–S142. [Google Scholar]
- 118.Griffon N, Pilon C, Schwartz J-C, Sokoloff P. The preferential D3 receptor ligand, (+)-UH 232, is a partial agonist. Eur. J. Pharmacol. 1995;282:R3–R4. doi: 10.1016/0014-2999(95)00460-3. [DOI] [PubMed] [Google Scholar]
- 119.Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology. 2000;22:473–479. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- 120.Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20:60–80. doi: 10.1016/S0893-133X(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 121.Gyertyán I, Gál K. Dopamine D3 receptor ligands show place conditioning effect but do not influence cocaine-induced place preference. NeuroReport. 2003;14:93–98. doi: 10.1097/00001756-200301200-00018. [DOI] [PubMed] [Google Scholar]
- 122.Haadsma-Svensson SR, Smith MW, Svensson K, Waters N, Carlsson A. The chemical structure of U99194A. J. Neural Transm.: Gen. Sect. 1995;99:I. doi: 10.1007/BF01271465. [DOI] [PubMed] [Google Scholar]
- 123.Hackling AE, Stark H. Dopamine D3 receptor ligands with antagonist properties. ChemBioChem. 2002;3:946–961. doi: 10.1002/1439-7633(20021004)3:10<946::AID-CBIC946>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 124.Hall H, Halldin C, Dijkstra D, Wikström H, Wise LD, Pugsley TA, Sokoloff P, Pauli S, Farde L, Sedvall G. Autoradio-graphic localisation of D3-dopamine receptors in the human brain using the selective D3-dopamine receptor agonist (+)-[3H]PD 128907. Psychopharmacology (Berlin) 1996;128:240–247. doi: 10.1007/s002130050131. [DOI] [PubMed] [Google Scholar]
- 125.Hall J, Parkinson JA, Hall TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur. J. Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- 126.Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J. Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Harmer CJ, Phillips GD. Enhanced dopamine efflux in the amygdala by a predictive, but not a non-predictive, stimulus: facilitation by prior repeated d-amphetamine. Neuroscience. 1999;90:119–130. doi: 10.1016/s0306-4522(98)00464-3. [DOI] [PubMed] [Google Scholar]
- 128.Hatcher JP, Hagan JJ. The effects of dopamine D3/D2 receptor agonists on intracranial self stimulation in the rat. Psychopharmacology (Berlin) 1998;140:405–410. doi: 10.1007/s002130050782. [DOI] [PubMed] [Google Scholar]
- 129.Hayes RJ, Vorel SR, Spector J, Liu X, Gardner EL. Electrical and chemical stimulation of the basolateral complex of the amygdala reinstates cocaine-seeking behavior in the rat. Psychopharmacology (Berlin) 2003;168:75–83. doi: 10.1007/s00213-002-1328-3. [DOI] [PubMed] [Google Scholar]
- 130.Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci. Biobehav. Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 131.Heidbreder CA, Andreoli M, Marcon C, Thanos PK, Ashby CR, Jr., Gardner EL. Role of dopamine D3 receptors in the addictive properties of ethanol. Drugs Today (Barc.) 2004;40:355–365. doi: 10.1358/dot.2004.40.4.820081. [DOI] [PubMed] [Google Scholar]
- 132.Herroelen L, De Backer J-P, Wilczak N, Flamez A, Vaunquelin G, De Keyser J. Autoradiographic distribution of D3-type dopamine receptors in human brain using [3H]7-hydroxy-N, N-di-n-propyl-2-aminotetralin. Brain Res. 1994;648:222–228. doi: 10.1016/0006-8993(94)91121-5. [DOI] [PubMed] [Google Scholar]
- 133.Hitchcott PK, Phillips GD. Effects of intra-amygdala R(+) 7-OH-DPAT on intra-accumbens d-amphetamine-associated learning. I. Pavlovian conditioning. Psychopharmacology (Berlin) 1998;140:300–309. doi: 10.1007/s002130050771. [DOI] [PubMed] [Google Scholar]
- 134.Hitchcott PK, Phillips GD. Effects of intra-amygdala R(+) 7-OH-DPAT on intra-accumbens d-amphetamine-associated learning. II. Instrumental conditioning. Psychopharmacology (Berlin) 1998;140:310–318. doi: 10.1007/s002130050772. [DOI] [PubMed] [Google Scholar]
- 135.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 136.Hodos W, Kalman G. Effects of increment size and reinforcer volume on progressive-ratio performance. J. Exp. Anal. Behav. 1963;6:387–392. doi: 10.1901/jeab.1963.6-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hubner CB, Moreton JE. Effects of selective D1 and D2 dopamine antagonists on cocaine self-administration in the rat. Psychopharmacology (Berlin) 1991;105:151–156. doi: 10.1007/BF02244301. [DOI] [PubMed] [Google Scholar]
- 138.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res., Brain Res. Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 139.Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J. Pharmacol. Exp. Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- 140.Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur. J. Pharmacol. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- 141.Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J. Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J. Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- 144.Kaneyuki H, Yokoo H, Tsuda A, Yoshida M, Mizuki Y, Yamada M, Tanaka M. Psychological stress increases dopamine turnover selectively in mesoprefrontal dopamine neurons of rats: reversal by diazepam. Brain Res. 1991;557:154–161. doi: 10.1016/0006-8993(91)90129-j. [DOI] [PubMed] [Google Scholar]
- 145.Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J. Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Katz JL, Alling KL. Discriminative stimulus effects of putative D3 dopamine receptor agonists in rats. Behav. Pharmacol. 2000;11:483–493. doi: 10.1097/00008877-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 147.Khroyan TV, Baker DA, Neisewander JL. Dose-dependent effects of the D3-preferring agonist 7-OH-DPAT on motor behaviors and place conditioning. Psychopharmacology (Berlin) 1995;122:351–357. doi: 10.1007/BF02246265. [DOI] [PubMed] [Google Scholar]
- 148.Khroyan TV, Fuchs RA, Baker DA, Neisewander JL. Effects of D3-preferring agonists 7-OH-PIPAT and PD-128,907 on motor behaviors and place conditioning. Behav. Pharmacol. 1997;8:65–74. [PubMed] [Google Scholar]
- 149.Khroyan TV, Baker DA, Fuchs RA, Manders N, Neisewander JL. Differential effects of 7-OH-DPAT on amphetamine-induced stereotypy and conditioned place preference. Psychopharmacology (Berlin) 1998;139:332–341. doi: 10.1007/s002130050724. [DOI] [PubMed] [Google Scholar]
- 150.Khroyan TV, Fuchs RA, Beck AM, Groff RS, Neisewander JL. Behavioral interactions produced by co-administration of 7-OH-DPAT with cocaine or apomorphine in the rat. Psychopharmacology (Berlin) 1999;142:383–392. doi: 10.1007/s002130050903. [DOI] [PubMed] [Google Scholar]
- 151.Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J. Pharmacol. Exp. Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- 152.Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, G Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch. Gen. Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 153.Kling-Petersen T, Ljung E, Wollter L, Svensson K. Effects of the dopamine D3- and autoreceptor preferring antagonist (−)-DS121 on locomotor activity, conditioned place preference and intracranial self-stimulation in the rat. Behav. Pharmacol. 1995;6:107–115. [PubMed] [Google Scholar]
- 154.Koch S, Piercey MF, Galloway MP, Svensson KA. Interactions between cocaine and (−)-DS 121: studies with 2-deoxyglucose autoradiography and microdialysis in the rat brain. Eur. J. Pharmacol. 1997;319:173–180. doi: 10.1016/s0014-2999(96)00852-7. [DOI] [PubMed] [Google Scholar]
- 155.Koob GF, Nestler EJ. The neurobiology of drug addiction. J. Neuropsychiatry Clin. Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- 156.Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci. Biobehav. Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 157.Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 158.Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J. Neurosci. 2001;21:RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kurashima M, Yamada K, Nagashima M, Shirakawa K, Furukawa T. Effects of putative dopamine D3 receptor agonists, 7-OH-DPAT and quinpirole, on yawning, stereotypy, and body temperature in rats. Pharmacol. Biochem. Behav. 1995;52:503–508. doi: 10.1016/0091-3057(95)00103-4. [DOI] [PubMed] [Google Scholar]
- 160.Lacroix LL, Hows MA, Shah AJ, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors enhances mono-aminergic and cholinergic neurotransmission in the rat anterior cingulate cortex. Neuropsychopharmacology. 2003;28:839–849. doi: 10.1038/sj.npp.1300114. [DOI] [PubMed] [Google Scholar]
- 161.Lamas X, Negus SS, Nader MA, Mello NK. Effects of the putative dopamine D3 receptor agonist 7-OH-DPAT in rhesus monkeys trained to discriminate cocaine from saline. Psychopharmacology (Berlin) 1996;124:306–314. doi: 10.1007/BF02247435. [DOI] [PubMed] [Google Scholar]
- 162.Landwehrmeyer B, Mengod G, Palacios JM. Dopamine D3 receptor mRNA and binding sites in human brain. Brain Res., Mol. Brain Res. 1993;18:187–192. doi: 10.1016/0169-328x(93)90188-u. [DOI] [PubMed] [Google Scholar]
- 163.Laszy J, Gyertyan I, Laszlovszky I, Szombathely Z. Dopamine D3 receptor antagonists show cognitive enhancer activity; Prague. Proceedings of the Sixth IBRO World Congress of Neuroscience 10–15 July, International Brain Research Organization; 2003. p. 45. (abstract 1126) [Google Scholar]
- 164.Lavicky J, Dunn AJ. Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis. J. Neurochem. 1993;60:602–612. doi: 10.1111/j.1471-4159.1993.tb03191.x. [DOI] [PubMed] [Google Scholar]
- 165.Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berlin) 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- 166.Ledford CC, Fuchs RA, See RE. Potentiated reinstatement of cocaine-seeking behavior following d-amphetamine infusion into the basolateral amygdala. Neuropsychopharmacology. 2003;28:1721–1729. doi: 10.1038/sj.npp.1300249. [DOI] [PubMed] [Google Scholar]
- 167.Le Foll B, Schwartz J-C, Sokoloff P. Dopamine D3 receptor agents as potential new medications for drug addiction. Eur. Psychiatr. 2000;15:140–146. doi: 10.1016/s0924-9338(00)00219-4. [DOI] [PubMed] [Google Scholar]
- 168.Le Foll B, Frances H, Diaz J, Schwartz J-C, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur. J. Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- 169.Le Foll B, Schwartz J-C, Sokoloff P. Disruption of nicotine conditioning by dopamine D3 receptor ligands. Mol. Psychiatry. 2003;8:225–230. doi: 10.1038/sj.mp.4001202. [DOI] [PubMed] [Google Scholar]
- 170.Le Foll B, Diaz J, Sokoloff P. Increased dopamine D3 receptor expression accompanying behavioral sensitization to nicotine in rats. Synapse. 2003;47:176–183. doi: 10.1002/syn.10170. [DOI] [PubMed] [Google Scholar]
- 171.Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol. Rev. 1991;7:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- 172.Le Moine C, Bloch B. Expression of the D3 dopamine receptor in peptidergic neurons of the nucleus accumbens: comparison with the D1 and D2 dopamine receptors. Neuroscience. 1996;73:131–143. doi: 10.1016/0306-4522(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 173.Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J. Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Leriche L, Schwartz J-C, Sokoloff P. The dopamine D3 receptor mediates locomotor hyperactivity induced by NMDA receptor blockade. Neuropharmacology. 2003;45:174–181. doi: 10.1016/s0028-3908(03)00145-x. [DOI] [PubMed] [Google Scholar]
- 175.Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol. Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- 176.Levant B. Differential distribution of D3 dopamine receptors in the brains of several mammalian species. Brain Res. 1998;800:269–274. doi: 10.1016/s0006-8993(98)00529-0. [DOI] [PubMed] [Google Scholar]
- 177.Levant B, De Souza EB. Differential pharmacological profile of striatal and cerebellar dopamine receptors labeled by [3H]quinpirole: identification of a discrete population of putative D3 receptors. Synapse. 1993;14:90–95. doi: 10.1002/syn.890140112. [DOI] [PubMed] [Google Scholar]
- 178.Levant B, Vansell NR. In vivo occupancy of D2 dopamine receptors by nafadotride. Neuropsychopharmacology. 1997;17:67–71. doi: 10.1016/S0893-133X(97)00024-9. [DOI] [PubMed] [Google Scholar]
- 179.Levant B, Grigoriadis DE, De Souza EB. [3H]Quinpirole binding to putative D2 and D3 dopamine receptors in rat brain and pituitary gland: a quantitative autoradiographic study. J. Pharmacol. Exp. Ther. 1993;264:991–1001. [PubMed] [Google Scholar]
- 180.Levant B, Grigoriadis DE, De Souza EB. Relative affinities of dopaminergic drugs at dopamine D2 and D3 receptors. Eur. J. Pharmacol. 1995;278:243–247. doi: 10.1016/0014-2999(95)00160-m. [DOI] [PubMed] [Google Scholar]
- 181.Levant B, Bancroft GN, Selkirk CM. In vivo occupancy of D2 dopamine receptors by 7-OH-DPAT. Synapse. 1996;24:60–64. doi: 10.1002/(SICI)1098-2396(199609)24:1<60::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 182.Lévesque D, Diaz J, Pilon C, Martres M-P, Giros B, Souil E, Schott D, Morgat J-L, Schwartz J-C, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N, N-di-n-propyl-2-aminotetralin. Proc. Natl. Acad. Sci. U. S. A. 1992;89:8155–8199. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lingford-Hughes A, Nutt D. Neurobiology of addiction and implications for treatment. Br. J. Psychiatry. 2003;182:97–100. doi: 10.1192/bjp.182.2.97. [DOI] [PubMed] [Google Scholar]
- 184.Lu L, Ceng X, Huang M. Corticotropin-releasing factor receptor type I mediates stress-induced relapse to opiate dependence. Neuro-Report. 2000;11:2373–2378. doi: 10.1097/00001756-200008030-00008. [DOI] [PubMed] [Google Scholar]
- 185.Lu L, Liu D, Ceng X. Corticotropin-releasing factor receptor type I mediates stress-induced relapse to cocaine-conditioned place preference in rat. Eur. J. Pharmacol. 2001;415:203–208. doi: 10.1016/s0014-2999(01)00840-8. [DOI] [PubMed] [Google Scholar]
- 186.Macdonald GJ, Branch CL, Hadley MS, Johnson CN, Nash DJ, Smith AB, Stemp G, Thewlis KM, Vong AKK, Austin NE, Jeffrey P, Winborn KY, Boyfield I, Hagan JJ, Middlemiss DN, Reavill C, Riley GJ, Watson JM, Wood M, Parker SG, Ashby CR., Jr Design and synthesis of trans-3-(2-(4-((3-(3-(5-methyl-1,2,4-oxadiazolyl))-phenyl)carboxamido)cyclohexy-l)ethyl)-7-methylsulfonyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SB-414796): a potent and selective dopamine D3 receptor antagonist. J. Med. Chem. 2003;46:4952–4964. doi: 10.1021/jm030817d. [DOI] [PubMed] [Google Scholar]
- 187.Mallet PE, Beninger RJ. 7-OH-DPAT produces place conditioning in rats. Eur. J. Pharmacol. 1994;261:R5–R6. doi: 10.1016/0014-2999(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 188.Manzanedo C, Aguilar MA, Min˜arro J. The effects of dopamine D2 and D3 antagonists on spontaneous motor activity and morphine-induced hyperactivity in male mice. Psychopharmacology (Berlin) 1999;143:82–88. doi: 10.1007/s002130050922. [DOI] [PubMed] [Google Scholar]
- 189.Marcon C, Andreoli M, Pilla M, Tessari M, Heidbreder CA. A new model to assess drug and cue-induced relapse to ethanol self-administration in mice. Behav. Pharmacol. 2003;14(Suppl. 1):S66. [Google Scholar]
- 190.Mash DC, Staley JK. D3 dopamine and kappa opioid receptor alterations in human brain of cocaine-overdose victims. Ann. N. Y. Acad. Sci. 1999;877:507–522. doi: 10.1111/j.1749-6632.1999.tb09286.x. [DOI] [PubMed] [Google Scholar]
- 191.Mattingly BA, Caudill A, Abel M. Differential effect of 7-OH-DPAT on the development of behavioral sensitization to apomorphine and cocaine. Pharmacol. Biochem. Behav. 2001;68:417–426. doi: 10.1016/s0091-3057(00)00471-8. [DOI] [PubMed] [Google Scholar]
- 192.McFarland K, Kalivas P. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berlin) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- 194.Meador-Woodruff JH, Grandy DK, Van Tol HHM, Damask SP, Little KY, Civelli O, Watson SJ., Jr. Dopamine receptor gene expression in the human medial temporal lobe. Neuropsychophar-macology. 1994;10:239–248. doi: 10.1038/npp.1994.27. [DOI] [PubMed] [Google Scholar]
- 195.Meador-Woodruff JH, Little KY, Damask SP, Watson SJ. Effects of cocaine on D3 and D4 receptor expression in the human striatum. Biol. Psychiatry. 1995;38:263–266. doi: 10.1016/0006-3223(95)00099-3. [DOI] [PubMed] [Google Scholar]
- 196.Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav. Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- 197.Mengod G, Villaro´ MT, Landwehrmeyer GB, Martinez-Mir MI, Niznik HB, Sunahara RK, Seeman P, O’Dowd BF, Probst A, M J. Palacios, Visualization of dopamine D1, D2 and D3 receptor mRNAs in human and rat brain. Neurochem. Int. 1992;20(Suppl. 1):33S–43S. doi: 10.1016/0197-0186(92)90208-9. [DOI] [PubMed] [Google Scholar]
- 198.Millan MJ, Peglion J-L, Vian J, Rivet J-M, Brocco M, Gobert A, Newman-Tancredi A, Dacquet C, Bervoets K, Girardon S. Functional correlates of dopamine D3 receptor activation in the rat in vivo and their modulation by the selective antagonist, (+)-S14297. 1. Activation of postsynaptic D3 receptors mediates hypothermia whereas blockade of D2 receptors elicits prolactin secretion and catalepsy. J. Pharmacol. Exp. Ther. 1995;275:885–898. [PubMed] [Google Scholar]
- 199.Millan MJ, Gressier H, Brocco M. The dopamine D3 receptor antagonist, (+)-S 14297, blocks the cataleptic properties of haloperidol in rats. Eur. J. Pharmacol. 1997;321:R7–R9. doi: 10.1016/s0014-2999(97)00049-6. [DOI] [PubMed] [Google Scholar]
- 200.Millan MJ, Gobert A, Newman-Tancredi A, Lejeune F, Cussac D, Rivet J-M, Audinot V, Dubuffett T, Lavielle G. S33084, a novel, potent, selective, and competitive antagonist at dopamine D3-receptors: I. Receptorial, electrophysiological and neurochemical profile compared with GR218,231 and L741,626, J. Pharmacol. Exp. Ther. 2000;293:1048–1062. [PubMed] [Google Scholar]
- 201.Millan MJ, Dekeyne A, Rivet J-M, Dubuffet T, Lavielle G, Brocco M. S33084, a novel, potent, selective, and competitive antagonist at dopamine D3-receptors: II. Functional and behavioral profile compared with GR218,231 and L741,626. J. Pharmacol. Exp. Ther. 2000;263:1063–1073. [PubMed] [Google Scholar]
- 202.Millan MJ, Girardon S, Monneyron S, Dekeyne A. Discriminative stimulus properties of the dopamine D3 receptor agonists, PD128907 and 7-OH-DPAT: a comparative characterization with novel ligands at D3 versus D2 receptors. Neuropharmacology. 2000;39:586–598. doi: 10.1016/s0028-3908(99)00180-x. [DOI] [PubMed] [Google Scholar]
- 203.Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol. Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- 204.Monsma FJ, Mahan LC, Jr, McVittie LD, Gerfen CR, Sibley DR. Molecular cloning and expression of a D1 receptor linked to adenylyl cyclase activation. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6723–6727. doi: 10.1073/pnas.87.17.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Murray AM, Ryoo HL, Gurevich E, Joyce JN. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc. Natl. Acad. Sci. U. S. A. 1994;91:11271–11275. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Murray PJ, Harrison LA, Johnson MR, Robertson GM, Scopes DIC, Bull DR, Graham EA, Hayes AG, Kilpatrick GJ, Den Daas I, Large C, Sheehan MJ, Stubbs CM, Turpin MP. A novel series of arylpiperazines with high affinity and selectivity for the dopamine D3 receptor. Bioorg. Med. Chem. Lett. 1995;5:219–222. [Google Scholar]
- 207.Murray PJ, Helden RM, Johnson MR, Robertson GM, Scopes DIC, Stokes M, Wadman S, Whitehead JWJ, Hayes AG, Kilpatrick GJ, Large C, Stubbs CM, Turpin MP. Novel 6-substituted 2-aminotetralins with potent and selective affinity for the D3 dopamine receptor. Bioorg. Med. Chem. Lett. 1996;6:403–408. [Google Scholar]
- 208.Nader MA, Mach RH. Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology (Berlin) 1996;125:13–22. doi: 10.1007/BF02247388. [DOI] [PubMed] [Google Scholar]
- 209.Narita M, Soma M, Tamaki H, Suzuki T. Intensification of the development of ethanol dependence in mice lacking dopamine D3 receptor. Neurosci. Lett. 2002;324:129–132. doi: 10.1016/s0304-3940(02)00235-5. [DOI] [PubMed] [Google Scholar]
- 210.Narita M, Mizuo K, Mizoguchi H, Sakata M, Narita M, Tseng LF, Suzuki T. Molecular evidence for the functional role of dopamine D3 receptor in the morphine-induced rewarding effect and hyperlocomotion. J. Neurosci. 2003;23:1006–1012. doi: 10.1523/JNEUROSCI.23-03-01006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Mars J, Hall F. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J. Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Neisewander JL, Fuchs RA, Tran-Nguyen LT, Weber SM, Coffey GP, Joyce JN. Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1479–1487. doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- 213.Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol. Learn. Mem. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- 214.Newman AH, Cao J, Bennett CJ, Robarge MJ, Freeman RA, Luedtke RR. N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl, butenyl and butynyl}arylcarboxamides as novel dopamine D3 receptor antagonists. Bioorg. Med. Chem. Lett. 2003;13:2179–2183. doi: 10.1016/s0960-894x(03)00389-5. [DOI] [PubMed] [Google Scholar]
- 215.O’Brien CP, Childress AR, Ehrman RN, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J. Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 216.Park W-K, Jeong D, Yun C-W, Lee S, Cho H, Kim G-D, Koh HY, Pae AN, Cho YS, Choi KI, Jung JY, Jung SH, Kong JY. Pharmacological actions of a novel and selective dopamine D3 receptor antagonist, KCH-1110. Pharmacol. Res. 2003;48:615–622. doi: 10.1016/s1043-6618(03)00242-1. [DOI] [PubMed] [Google Scholar]
- 217.Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive Pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by d-amphetamine. J. Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Parkinson JA, Cardinal RN, Everitt BJ. Limbic cortico-ventral striatal systems underlying appetitive conditioning. Prog. Brain Res. 2000;126:263–285. doi: 10.1016/S0079-6123(00)26019-6. [DOI] [PubMed] [Google Scholar]
- 219.Parsons LH, Caine SB, Sokoloff P, Schwartz J-C, Koob GF, Weiss F. Neurochemical evidence that postsynaptic nucleus accumbens D3 receptor stimulation enhances cocaine reinforcement. J. Neurochem. 1996;67:1078–1089. doi: 10.1046/j.1471-4159.1996.67031078.x. [DOI] [PubMed] [Google Scholar]
- 220.Pettit HO, Justice BJ., Jr Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo micro dialysis. Pharmacol. Biochem. Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- 221.Phillips GD, Harmer CJ, Hitchcott PK. Blockade of sensitisation-induced facilitation of appetitive conditioning by post-session intra-amygdala nafadotride. Behav. Brain Res. 2002;134:249–257. doi: 10.1016/s0166-4328(02)00034-7. [DOI] [PubMed] [Google Scholar]
- 222.Phillips GD, Harmer CJ, Hitchcott PK. Isolation rearing-induced facilitation of Pavlovian learning: abolition by postsession intra-amygdala nafadotride. Physiol. Behav. 2002;76:677–684. doi: 10.1016/s0031-9384(02)00802-8. [DOI] [PubMed] [Google Scholar]
- 223.Piazza PV, Le Moal M. Pathophysiological basis of vulnerability to drug abuse: interaction between stress, glucocorticoids, and dopaminergic neurons. Annu. Rev. Pharmacol. Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- 224.Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz J-C, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- 225.Pilon C, Lévesque D, Dimitriadou V, Griffon N, Martres M-P, Schwartz J-C, Sokoloff P. Functional coupling of the human dopamine D3 receptor in a transfected NG 108-15 neuroblastoma-glioma hybrid cell line. Eur. J. Pharmacol. 1994;268:129–139. doi: 10.1016/0922-4106(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 226.Potenza MN, Graminski GF, Schmauss C, Lerner MR. Functional expression and characterization of human D2 and D3 dopamine receptors. J. Neurosci. 1994;14:1463–1476. doi: 10.1523/JNEUROSCI.14-03-01463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pritchard LM, Logue AD, Hayes S, Weige JA, Xu M, Zhang J, Berger SP, Richtand NM. 7-OH-DPAT and PD 128907 selectively activate the D3 dopamine receptor in a novel environment. Neuro-psychopharmacology. 2003;28:100–107. doi: 10.1038/sj.npp.1300018. [DOI] [PubMed] [Google Scholar]
- 228.Pugsley TA, Davis MD, Akunne HC, MacKenzie RG, Shih YH, Damsma G, Wikström H, Wetzel SZ, Georgic LM, Cooke LW, Demattos SB, Corbin AE, Glase SA, Wise LD, Dijkstra D, Heffner TG. Neurochemical and functional characterization of the preferentially selective dopamine D3 agonist PD 128907. J. Pharmacol. Exp. Ther. 1995;275:1355–1366. [PubMed] [Google Scholar]
- 229.Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, Boyfield I, Branch CL, Cilia J, Coldwell MC, Hadley MS, Hunter AJ, Jeffrey P, Jewitt F, Johnson CN, Jones DNC, Medhurst AD, Middlemiss DN, Nash DJ, Riley GJ, Routledge C, Stemp G, Thewlis KM, Trail B, Vong AKK, Hagan JJ. Pharmacological actions of a novel, high-affinity, and selective human dopamine D3 receptor antagonist, SB-277011-A. J. Pharmacol. Exp. Ther. 2000;294:1154–1165. [PubMed] [Google Scholar]
- 230.Richtand NM, Logue AD, Welge JA, Perdiue J, Tubbs LJ, Spitzer RH, Sethuraman G, Geracioti TD. The dopamine D3 receptor antagonist nafadotride inhibits development of locomotor sensitization to amphetamine. Brain Res. 2000;867:239–242. doi: 10.1016/s0006-8993(00)02247-2. [DOI] [PubMed] [Google Scholar]
- 231.Richtand NM, Goldsmith RJ, Nolan JE, Berger SP. The dopamine D3 receptor and substance dependence. J. Addict. Res. 2001;20:19–32. doi: 10.1300/J069v20n03_03. [DOI] [PubMed] [Google Scholar]
- 232.Richtand NM, Woods SC, Berger SP, Strakowski SM. D3 dopamine receptor, behavioral sensitization, and psychosis. Neuro-sci. Biobehav. Rev. 2001;25:427–443. doi: 10.1016/s0149-7634(01)00023-9. [DOI] [PubMed] [Google Scholar]
- 233.Ridray S, Griffon N, Mignon V, Souil E, Carboni S, Diaz J, Schwartz J-C, Sokoloff P. Coexpression of dopamine D1 and D3 receptors in islands of Calleja and shell of nucleus accumbens of the rat: opposite and synergistic functional interactions. Eur. J. Neurosci. 1998;10:1676–1786. doi: 10.1046/j.1460-9568.1998.00173.x. [DOI] [PubMed] [Google Scholar]
- 234.Risinger FO, Dickinson SD, Cunningham CL. Haloperidol reduces ethanol-induced motor activity stimulation but not conditioned place preference. Psychopharmacology (Berlin) 1992;107:453–456. doi: 10.1007/BF02245175. [DOI] [PubMed] [Google Scholar]
- 235.Rivera SN, Katana J, Ashby CR, Jr., Piyis YS, Gardner EL, Pena LA, Heidbreder C, Volkow ND, Thanos PK. A novel dopamine D3 receptor antagonist (SB-2770110-A) attenuates ethanol consumption in ethanol preferring (P) and non-preferring (NP) Rats. Abstr.-Soc. Neurosci. 2003;29 doi: 10.1016/j.pbb.2005.03.013. (abstract 665.7) [DOI] [PubMed] [Google Scholar]
- 236.Robarge MJ, Husbands SM, Kieltyka A, Brodbeck R, Thurkauf A, Newman AH. Design and synthesis of [(2,3-dichlor-ophenyl)piperazin-1-yl]alkylfluorenylcarboxamides as novel ligands selective for the dopamine D3 receptor. J. Med. Chem. 2001;44:3175–3186. doi: 10.1021/jm010146o. [DOI] [PubMed] [Google Scholar]
- 237.Robbins TW, Everitt BJ. Neurobehavioral mechanisms of reward and motivation. Curr. Opin. Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 238.Roberts DCS, Ranaldi R. Effect of dopaminergic drugs on cocaine reinforcement. Clin. Neuropharmacol. 1995;18(Suppl. 1):S84–S95. [Google Scholar]
- 239.Roberts DCS, Vickers G. Atypical neuroleptics increase self-administration of cocaine: an evaluation of a behavioural screen for antipsychotic activity. Psychopharmacology (Berlin) 1984;82:135–139. doi: 10.1007/BF00426397. [DOI] [PubMed] [Google Scholar]
- 240.Roberts DCS, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol. Biochem. Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- 241.Robinet PM, Bardo MT. Dopamine D3 receptors are involved in amphetamine-induced contralateral rotation in 6-OHDA lesioned rats. Pharmacol. Biochem. Behav. 2001;70:43–54. doi: 10.1016/s0091-3057(01)00581-0. [DOI] [PubMed] [Google Scholar]
- 242.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res., Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 243.Robledo P, Robbins TW, Everitt BJ. Effects of excitotoxic lesions of the central amygdaloid nucleus on the potentiation of reward-related stimuli by intra-accumbens amphetamine. Behav. Neurosci. 1996;110:981–990. doi: 10.1037//0735-7044.110.5.981. [DOI] [PubMed] [Google Scholar]
- 244.Rodríguez-Arias M, Felip CM, Broseta I, Miñarro J. The dopamine D3 antagonist U-99194A maleate increases social behaviors of isolation-induced aggressive male mice. Psychopharmacology (Berlin) 1999;144:90–94. doi: 10.1007/s002130050981. [DOI] [PubMed] [Google Scholar]
- 245.Rodríguez de Fonseca F, Rubio P, Martín-Calderon JL, Caine SB, Koob GF, Navarro M. The dopamine receptor agonist 7-OH-DPAT modulates the acquisition and expression of morphine-induced place preference. Eur. J. Pharmacol. 1995;274:47–55. doi: 10.1016/0014-2999(94)00708-f. [DOI] [PubMed] [Google Scholar]
- 246.Rogoz Z, Skuza G, Klodzinska A. Anxiolytic-like effects of preferential dopamine D3 receptor agonists in an animal model. Pol. J. Pharmacol. 2003;55:449–454. [PubMed] [Google Scholar]
- 247.Rowlett JK. A labor-supply analysis of cocaine self-administration under progressive ratio schedules: antecedents, methodologies, and perspectives. Psychopharmacology (Berlin) 2000;153:1–16. doi: 10.1007/s002130000610. [DOI] [PubMed] [Google Scholar]
- 248.Sautel F, Griffon N, Sokoloff P, Schwartz J-C, Launay C, Simon P, Costentin J, Schoenfelder A, Garrido F, Mann A. Mann, Nafadotride, a potent preferential dopamine D3 receptor antagonist, activates locomotion in rodents. J. Pharmacol. Exp. Ther. 1995;275:1239–1246. [PubMed] [Google Scholar]
- 249.Sautel F, Griffon N, Lévesque D, Pilon C, Schwartz J-C, Sokoloff P. A functional test identifies dopamine agonists selective for D3 versus D2 receptors. NeuroReport. 1995;6:329–332. doi: 10.1097/00001756-199501000-00026. [DOI] [PubMed] [Google Scholar]
- 250.Schoemaker H. [3H]7-OH-DPAT labels both dopamine D3 receptors and A sites in the bovine caudate nucleus. Eur. J. Pharmacol. 1993;242:R1–R2. doi: 10.1016/0014-2999(93)90259-k. [DOI] [PubMed] [Google Scholar]
- 251.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 252.Schwartz J-C, Lévesque D, Martres M-P, Sokoloff P. Dopamine D3 receptor: basic and clinical aspects. Clin. Neuropharmacol. 1993;16:295–314. doi: 10.1097/00002826-199308000-00002. [DOI] [PubMed] [Google Scholar]
- 253.Schwartz J-C, Diaz J, Pilon C, Sokoloff P. Possible implications of the dopamine D3 receptor in schizophrenia and in antipsychotic drug actions. Brain Res., Brain Res. Rev. 2000;31:277–287. doi: 10.1016/s0165-0173(99)00043-0. [DOI] [PubMed] [Google Scholar]
- 254.Schwarz AJ, Gozzi A, Reese T, Bertani S, Crestan V, Hagan JJ, Heidbreder C, Bifone A. Selective dopamine D3 receptor antagonist SB-277011-A potentiates phMRI response to acute amphetamine challenge in the rat brain. Synapse. 2004;54:1–10. doi: 10.1002/syn.20055. [DOI] [PubMed] [Google Scholar]
- 255.See RE, McLaughlin J, Fuchs RA. Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine-stimulus association in a model of relapse to cocaine-seeking behavior in rats. Neuroscience. 2003;117:477–483. doi: 10.1016/s0306-4522(02)00665-6. [DOI] [PubMed] [Google Scholar]
- 256.Segal DM, Moraes CT, Mash DC. Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Res., Mol. Brain Res. 1997;45:335–339. doi: 10.1016/s0169-328x(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 257.Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- 258.Shafer RA, Levant B. The D3 dopamine receptor in cellular and organismal function. Psychopharmacology (Berlin) 1998;135:1–16. doi: 10.1007/s002130050479. [DOI] [PubMed] [Google Scholar]
- 259.Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychophar-macology (Berlin) 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- 260.Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology (Berlin) 1996;125:385–391. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- 261.Shaham Y, Funk D, Erb S, Brown TJ, Walker C-D, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J. Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res. Brain Res. Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 263.Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus ceruleus noradrenergic neurons. Eur. J. Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- 264.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berlin) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 265.Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology (Berlin) 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- 266.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol. Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 267.Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berlin) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- 268.Sinha R, Catapano D, O’Mally S. Stress-induced craving and stress responses in cocaine dependent individuals. Psychopharmacology (Berlin) 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- 269.Sinnott RS, Mach RH, Nader MA. Dopamine D2/D3 receptors modulate cocaine’s reinforcing and discriminative stimulus effects in rhesus monkeys. Drug Alcohol Depend. 1999;54:97–110. doi: 10.1016/s0376-8716(98)00162-8. [DOI] [PubMed] [Google Scholar]
- 270.Smith AG, Neill JC, Costall B. The dopamine D3/D2 receptor agonist 7-OH-DPAT induces cognitive impairment in the marmoset. Pharmacol. Biochem. Behav. 1999;63:201–211. doi: 10.1016/s0091-3057(98)00230-5. [DOI] [PubMed] [Google Scholar]
- 271.Smith AD, Smith DL, Zigmond MJ, Amalric M, Koob GF. Differential effects of dopamine receptor subtype blockade on performance of rats in a reaction-time paradigm. Psychopharmaco-logy (Berlin) 2000;148:355–360. doi: 10.1007/s002130050063. [DOI] [PubMed] [Google Scholar]
- 272.Sokoloff P, Schwartz J-C. Novel dopamine receptors half a decade later. Trends Pharmacol. Sci. 1995;16:270–275. doi: 10.1016/s0165-6147(00)89044-6. [DOI] [PubMed] [Google Scholar]
- 273.Sokoloff P, Giros B, Martres M-P, Bouthenet M-L, Schwartz J-C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 274.Sokoloff P, Andrieux M, Besançon R, Pilon C, Martres M-P, Giros B, Schwartz J-C. Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: comparison with D2 receptor. Eur. J. Pharmacol. 1992;225:331–337. doi: 10.1016/0922-4106(92)90107-7. [DOI] [PubMed] [Google Scholar]
- 275.Sokoloff P, Giros B, Martres M-P, Andrieux M, Besançon R, Pilon C, Bouthenet M-L, Souil E, Schwartz J-C. Localization and function of the D3 dopamine receptor. Arzneimittel-Forschung. 1992;42:224–230. [PubMed] [Google Scholar]
- 276.Sonesson C, Lin C-H, Hansson L, Waters N, Svensson K, Calsson A, Smith MW, Wikström H. Substituted (S)-phenylpiperidines and rigid congeners as preferential dopamine autoreceptor antagonists: synthesis and structure-activity relationships. J. Med. Chem. 1994;37:2735–2753. doi: 10.1021/jm00043a013. [DOI] [PubMed] [Google Scholar]
- 277.Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the medial prefrontal cortex. Neuroscience. 1993;53:695–703. doi: 10.1016/0306-4522(93)90617-o. [DOI] [PubMed] [Google Scholar]
- 278.Spangler R, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Elevated D3 dopamine receptor mRNA in dopaminergic and dopaminoceptive regions of the rat brain in response to morphine. Brain Res., Mol. Brain Res. 2003;111:74–83. doi: 10.1016/s0169-328x(02)00671-x. [DOI] [PubMed] [Google Scholar]
- 279.Spealman RD. Antagonism of behavioral effects of cocaine by selective dopamine receptor blockers. Psychopharmacology (Berlin) 1990;101:142–145. doi: 10.1007/BF02253732. [DOI] [PubMed] [Google Scholar]
- 280.Spealman RD. Dopamine D3 receptor agonists partially reproduce the discriminative stimulus effects of cocaine in squirrel monkeys. J. Pharmacol. Exp. Ther. 1996;278:1128–1137. [PubMed] [Google Scholar]
- 281.Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J. Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Stanwood GD, Artymyshyn RP, Kung M-P, Kung HF, Lucki I, McGonigle P. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [125I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J. Pharmacol. Exp. Ther. 2000;295:1223–1231. [PubMed] [Google Scholar]
- 283.Stemp G, Ashmeade T, Branch CL, Hadley MS, Hunter AJ, Johnson CN, Nash DJ, Thewlis KM, Vong AKK, Austin NE, Jeffrey P, Avenell KY, Boyfield I, Hagan JJ, Middlemiss DN, Reavill C, Riley GJ, Routledge C, Wood M. Design and synthesis of trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolinecarboxamide (SB-277011A): a potent and selective dopamine D3 receptor antagonist with high oral bioavailability and CNS penetration in the rat. J. Med. Chem. 2000;43:1878–1885. doi: 10.1021/jm000090i. [DOI] [PubMed] [Google Scholar]
- 284.Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J. Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- 285.Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol. Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- 286.Sunahara R, Guan H-C, O’Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HHM, Niznik HB. Cloning of the gene for a human D5 receptor with higher affinity for dopamine than D1 . Nature. 1991;350:614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- 287.Suzuki M, Hurd YL, Sokoloff P, Schwartz J-C, Sedvall G. D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Res. 1998;779:58–74. doi: 10.1016/s0006-8993(97)01078-0. [DOI] [PubMed] [Google Scholar]
- 288.Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common µ1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 289.Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- 290.Ukai M, Tanaka T, Kameyama T. Effects of the dopamine D3 receptor agonist, R(+)-hydroxy-N,N-di-n-propyl-2-aminotetralin, on memory processes in mice. Eur. J. Pharmacol. 1997;324:147–151. doi: 10.1016/s0014-2999(97)00075-7. [DOI] [PubMed] [Google Scholar]
- 291.Unger L, Garcia-Ladona FJ, Wernet W, Sokoloff P, Wicke KM, Gross G. In vitro characterization of the selective dopamine D3 receptor antagonist A-437203. Abstr.-Soc. Neurosci. 2002;28 (abstract 894.5) [Google Scholar]
- 292.Van Tol HHM, Bunzow JR, Guan H-C, Sunahara RK, Seeman P, Niznik HB, Civelli O. Cloning the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- 293.Varty GB, Higgins GA. Investigations into the nature of a 7-OH-DPAT discriminative cue: comparison with d-amphetamine. Eur. J. Pharmacol. 1997;339:101–107. doi: 10.1016/s0014-2999(97)01388-5. [DOI] [PubMed] [Google Scholar]
- 294.Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- 295.Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J. Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wallace DR, Booze RM. Identification of D3 and σ receptors in the rat striatum and nucleus accumbens using (±)-7-hydroxy-N, N-di-n-[3H]propyl-2-aminotetralin and carbetapentane. J. Neurochem. 1995;64:700–710. doi: 10.1046/j.1471-4159.1995.64020700.x. [DOI] [PubMed] [Google Scholar]
- 297.Wang X, Cen X, Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur. J. Pharmacol. 2001;432:153–161. doi: 10.1016/s0014-2999(01)01487-x. [DOI] [PubMed] [Google Scholar]
- 298.Waters N, Svensson K, Haadsma-Svensson SR, Smith MW, Carlsson A. The dopamine D3-receptor: a postsynaptic receptor inhibitory on rat locomotor activity. J. Neural Transm.: Gen. Sect. 1993;94:11–19. doi: 10.1007/BF01244979. [DOI] [PubMed] [Google Scholar]
- 299.Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25:361–372. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- 300.Whetzel SZ, Shih YH, Georgic LM, Akunne HC, Pugsley TA. Effects of the dopamine D3 antagonist PD 58491 and its interaction with the dopamine D3 agonist PD 128907 on brain dopamine synthesis in rat. J. Neurochem. 1997;69:2363–2368. doi: 10.1046/j.1471-4159.1997.69062363.x. [DOI] [PubMed] [Google Scholar]
- 301.Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology (Berlin) 1996;127:213–224. [PubMed] [Google Scholar]
- 302.Wicke K, Garcia-Ladona J. The dopamine D3 receptor partial agonist, BP 897, is an antagonist at human dopamine D3 receptors and at rat somatodendritic dopamine D3 receptors. Eur. J. Pharmacol. 2001;424:85–90. doi: 10.1016/s0014-2999(01)01054-8. [DOI] [PubMed] [Google Scholar]
- 303.Wise RA. Dopamine, learning and motivation. Nat. Rev., Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 304.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 305.Wood MD, Boyfield I, Nash DJ, Jewitt FR, Avenell KY, Riley GJ. Evidence for antagonist activity of human dopamine D3 receptor partial agonist, BP 897, at human dopamine D3 receptor. Eur. J. Pharmacol. 2000;407:47–51. doi: 10.1016/s0014-2999(00)00732-9. [DOI] [PubMed] [Google Scholar]
- 306.Wright JL, Downing DM, Feng MR, Hayes RN, Heffner TG, MacKenzie RG, Meltzer LT, Pugsley TA, Wise LD. Identification, characterization and pharmacological profile of three metabolites of (R)-(+)-1,2,3,6-tetrahydro-4-phenyl-1-[(3-phenylcyclohexen-1-yl)methyl]pyridine (CI-1007), a dopamine autoreceptor agonist and potential antipsychotic agent. J. Med. Chem. 1995;38:5007–5014. doi: 10.1021/jm00026a007. [DOI] [PubMed] [Google Scholar]
- 307.Wustrow DJ, Wise LD. Progress in developing dopamine D3 ligands as potential antipsychotic agents. Curr. Pharm. Des. 1997;3:391–404. [Google Scholar]
- 308.Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J. Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Xi Z-X, Gilbert J, Campos AC, Ashby CR, Jr, Gardner EL, Newman AH. The dopamine D3 receptor antagonist NGB 2904 inhibits cocaine reward and cocaine-triggered reinstatement of cocaine-seeking behavior. Abstr.-Soc. Neurosci. 2003;29 (abstract 422.9) [Google Scholar]
- 310.Xi Z-X, Gilbert J, Campos AC, Kline N, Ashby CR, Jr., Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology (Berlin) 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]
- 312.Yoshioka M, Matsumoto M, Togashi H, Saito H. Effect of conditioned fear stress on dopamine release in the prefrontal cortex. Neurosci. Lett. 1996;209:201–203. doi: 10.1016/0304-3940(96)12631-8. [DOI] [PubMed] [Google Scholar]
- 313.Yuan J, Chen X, Brodbeck R, Primus R, Braun J, Wasley JWF, Thurkauf A. Highly selective dopamine D3 receptor antagonists. Bioorg. Med. Chem. Lett. 1998;8:2715–2718. doi: 10.1016/s0960-894x(98)00469-7. [DOI] [PubMed] [Google Scholar]
- 314.Zapata A, Witkin JM, Shippenberg TS. Selective D3 dopamine receptor agonist effects of (+)-PD 128907 on dialysate dopamine at low doses. Neuropharmacology. 2001;41:351–359. doi: 10.1016/s0028-3908(01)00069-7. [DOI] [PubMed] [Google Scholar]
- 315.Zhou Q-Y, Grandy DK, Thambi L, Kushner JA, Van Tol HHM, Cone R, Pribnow D, Salon J, Bunzow JR, Civelli O. Cloning and expression of human and rat D1 dopamine receptors. Nature. 1990;347:76–80. doi: 10.1038/347076a0. [DOI] [PubMed] [Google Scholar]