Abstract
Increasing evidence suggests that enhanced dopamine (DA) neurotransmission in the nucleus accumbens (NAc) may play a role in mediating the reward and reinforcement produced by addictive drugs and in the attentional processing of drug-associated environmental cues. The meso-accumbens DA system is selectively enriched with DA D3 receptors, a DA receptor subtype increasingly implicated in reward-related brain and behavioural processes. From a variety of evidence, it has been suggested that selective DA D3 receptor antagonism may be a useful pharmacotherapeutic approach for treating addiction. The present experiments tested the efficacy of SB-277011A, a selective DA D3 receptor antagonist, in rat models of nicotine-enhanced electrical brain-stimulation reward (BSR), nicotine-induced conditioned locomotor activity (LMA), and nicotine-induced conditioned place preference (CPP). Nicotine was given subcutaneously within the dose range of 0.25–0.6 mg/kg (nicotine-free base). SB-277011A, given intraperitoneally within the dose range of 1–12 mg/kg, dose-dependently reduced nicotine-enhanced BSR, nicotine-induced conditioned LMA, and nicotine-induced CPP. The results suggest that selective D3 receptor antagonism constitutes a new and promising pharmacotherapeutic approach to the treatment of nicotine dependence.
Keywords: Brain-stimulation reward, conditioned locomotor activity, conditioned place preference, dopamine D3 receptors, nicotine
Introduction
Nicotine, like other addictive drugs such as amphetamines, cannabinoids, cocaine, ethanol, and opiates, enhances brain reward functions in the central meso-accumbens dopamine (DA) brain reward system (for reviews, see Clarke, 1990; Di Chiara, 2000; Gardner, 2005; Wise and Gardner, 2002). This nicotine-enhanced meso-accumbens brain reward is mediated by increased DA in the reward-related nucleus accumbens (NAc) (e.g. Imperato et al., 1986; Pontieri et al., 1996; Yoshida et al., 1993). The enhanced NAc DA is important to nicotine’s addictive effect (Di Chiara, 2000; Gardner, 2005; Pontieri et al., 1996; Wise and Gardner, 2002), and appears to result from increased meso-accumbens DA neuronal firing (Calabresi et al., 1989; Mereu et al., 1987; Nomikos et al., 2000). This most likely occurs via a site of action within the ventral tegmental area (VTA) (Corrigall et al., 1994; Nomikos et al., 2000; Wise and Gardner, 2002; Yeomans and Baptista, 1997), and may involve both D1 (Harrison et al., 2002) and D2 (Huston-Lyons et al., 1993) receptor mechanisms. Congruently, lesioning the DA-containing neurons that project from the VTA to the NAc through the meso-accumbens system attenuates nicotine self-administration in animals (Corrigall et al., 1992, 1994; Di Chiara, 2000; Stolerman and Shoaib, 1991). In humans, pharmacologically induced DA blockade produces a compensatory increase in cigarette smoking and nicotine intake (Caskey et al., 1999; 2002) while administration of a DA agonist produces a compensatory decrease in cigarette smoking and nicotine intake (Caskey et al., 2002; Jarvik et al., 2000), suggesting that nicotine self-administration is mediated by DA mechanisms in the same manner as other addictive drugs (e.g. de Wit and Wise, 1977; Gardner, 2000, 2005; Ikemoto and Wise, 2004; Wise and Rompré, 1989; Wise et al., 1995a,b; Yokel and Wise, 1975, 1976).
Considerable evidence indicates that D1 and D2 receptors play significant roles in drug-induced reward and incentive motivation (e.g. Baker et al., 1998; de Wit and Wise, 1977; Ettenberg et al., 1982; Gardner, 2005; Ikemoto et al., 1997; Nakajima, 1989; Nakajima et al., 1993; Spyraki et al., 1987; Wise and Gardner, 2002; Wise and Rompré, 1989). Recently, however, the DA D3 receptor has gained attention with respect to the meso-accumbens VTA–NAc DA system, drug-induced reward, motivation and relapse, and the behavioural manifestations of addiction (for reviews, see Heidbreder et al., 2004, 2005; Joyce and Millan, 2005; Newman et al., 2005). This has led to the suggestion that the DA D3 receptor may be a useful therapeutic target for anti-addiction medications (Caine and Koob, 1993, 1995; Caine et al., 1997; Heidbreder and Hagan, 2005; Levant, 1997; for reviews, see Heidbreder et al., 2004, 2005; Joyce and Millan, 2005; Le Foll et al., 2005a; Newman et al., 2005).
To date, studies of the involvement of DA D3 receptors in the neurobiology of drug addiction have been limited, due to a lack of suitably selective D3 compounds. Recently, however trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclo-hexyl]-4-quinolinecarboxamide (SB-277011A), a highly selective and potent D3 receptor antagonist with good brain-penetrant properties in rodents was synthesized (Reavill et al., 2000; Stemp et al., 2000). Recent research with SB-277011A suggests that selective DA D3 antagonism appears to have a promising anti-addiction profile against cocaine, heroin, and ethanol in animal models (for reviews, see Heidbreder et al., 2004, 2005). With respect to nicotine, recent studies have shown that nicotine-induced behavioural sensitization (Le Foll et al., 2003a) and nicotine-induced conditioned locomotion (Le Foll et al., 2003b) are both associated with significant increases in DA D3 receptor binding and mRNA levels in the NAc, without significant alteration in D1 or D2 receptor mRNA. The same studies also showed that nicotine cue-conditioned locomotor activity (LMA) is attenuated both by the D3 receptor antagonist SB-277011A and by BP-897, a partial D3 receptor agonist with D3 antagonist properties (Wicke and Garcia-Ladona, 2001; Wood et al., 2000). In addition, D3 receptor ligands have recently been shown to block nicotine-induced conditioned place preference (CPP) (Le Foll et al., 2005b), and we have recently shown that SB-277011A blocks nicotine-induced relapse to nicotine-seeking behaviour (Andreoli et al., 2003b). We take such findings to suggest that highly selective DA D3 receptor antagonists may prove useful as anti-nicotine addiction pharmacotherapeutic agents. Therefore, in the present series of experiments, we further investigated the effects of several doses of SB-277011A on nicotine-enhanced brain stimulation reward (BSR), nicotine-associated cue-conditioned LMA, and the expression of nicotine-induced CPP.
Materials and methods
Animals
For the nicotine-enhanced BSR experiments, male Long–Evans rats (Charles River Laboratories, Raleigh, NC, USA) experimentally naive at the start of experiments, and weighing 300–325 g at time of surgery, were used. They were housed individually in a climate-controlled environment with food and water freely available with the exception of the time spent each day in the test chambers. All BSR experiments were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the U.S. National Institutes of Health, and were carried out in compliance with applicable United States federal and Maryland state laws and regulations. For the cue-conditioned LMA experiments, male Wistar rats (Charles River Deutschland, Sulzfeld, Germany) experimentally naive at the start of experiments, and weighing 250–360 g at the start of drug-cue pairings, were used. They were housed individually in a climate-controlled environment with food and water freely available with the exception of the time spent each day in the test chambers. All cue-conditioned LMA experiments were conducted under a Project License obtained according to Italian law regulating animal experimentation (Article 7, Legislative Decree 116, 27 January 1992), which acknowledges European Directive 86/609/EEC for the protection of animals used for experimental and other scientific purposes. For the nicotine-induced CPP experiments, male Sprague–Dawley rats (Taconic Farms, Germantown, NY, USA) experimentally naive at the start of experiments, and weighing 200–210 g at the start of drug-cue pairings, were used. They were housed two per cage in a climate-controlled environment with food and water freely available with the exception of the time spent each day in the test chambers. All cue-conditioned CPP experiments were approved by the Animal Care and Use Committee of St John’s University, and were carried out in compliance with applicable United States federal and New York state laws and regulations. All experiments – whether conducted in Italy, New York, or Maryland – were carried out in accordance with the principles of laboratory animal care stipulated in the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, 1996).
Drugs and chemicals
(−)Nicotine (+)bitartrate salt was used in all experiments (Sigma-Aldrich Corporation, St Louis, MO, USA) and was dissolved in sterile physiological saline and the pH adjusted to 7.4 with NaOH. The doses of nicotine are expressed as free base. SB-277011A was provided by GlaxoSmithKline Pharmaceuticals (Verona, Italy, and Harlow, Essex, UK) and was placed into solution using 25% (for the BSR experiments), 10% (for the LMA experiments), or 20% (for the main CPP experiment testing SB-277011A’s effect on nicotine-induced CPP; see below) 2-hydroxypropyl-β-cyclodextrin (for the BSR and CPP experiments, obtained from Fluka Division of Sigma-Aldrich Corporation, St Gallen, Switzerland; for the LMA experiments, obtained from Wacker-Chemie GmbH, München, Germany). In two additional CPP experiments carried out to determine whether SB-277011A by itself produces appetitive or aversive effects (see below), SB-277011A was placed into solution using 2% w/v methylcellulose.
Behavioural methods and testing
BSR (nicotine-enhanced brain-stimulation reward) experiments
Surgery
Under an anaesthetic mixture of sodium pentobarbital and chloral hydrate, each rat was surgically implanted, using standard aseptic stereo-taxic technique, with a unilateral monopolar stainless-steel stimulating electrode (Plastics One, Roanoke, VA, USA) aimed at the medial forebrain bundle at the level of the lateral hypothalamus. The target implant stereotaxic coordinates were, from bregma, AP −2.5 mm, ML +1.7 and DV −8.4 mm, using the rat brain atlas of Paxinos and Watson (1998). The top of the electrode and the electrode connector (to which the wires from the brain stimulator connected via a quick-connect electrical mini-plug) were cemented to the skull with acrylic resin cement. A wire wrapped around a jeweller’s screw implanted in the skull and connected to a mini-pin in the electrical connector at the top of the electrode was used to accommodate return electrical current. The skin was drawn up around the acrylic mound and sutured, prophylactic antibiotics and post-surgical analgesics given, and each animal was kept warm and under observation until all anesthesia effects were gone. Rats were given 5 d to recover fully from surgery, under daily veterinary supervision, before experiments were started.
Apparatus
All training and testing occurred in standard operant chambers, each containing a retractable wall-mounted lever and a cue light immediately above the lever (MED Associates, Georgia, VT, USA). The operant chambers were enclosed in ventilated, sound-attenuating cabinets. Depression of the lever activated a stimulator programmed to deliver trains of 0.1 ms cathodal pulses, each pulse-train having 500 ms duration.
Procedure
Animals (n=14) were placed once daily into the operant chambers, connected to the brain stimulator via their skull-mounted electrical connectors, and trained using an electrical BSR procedure slightly modified from studies that we have previously described (Lepore et al., 1996; Vorel et al., 2002). To find an animal’s optimal stimulation amperage, the animals were placed in the apparatus for 1 h per day and allowed to ‘auto-shape’ (i.e. learn by themselves) lever-pressing, on a FR-1 reinforcement schedule, for BSR at 300 μA and 124 Hz. The amperage was then increased gradually until the animal produced moderate rates of responding (45–60 responses/30 s). Our experience was that 300 μA was optimal for most animals; in no case did the amperage need to be increased to >350 μA to achieve the desired behavioural output of 45–60 responses/30 s. Once this training was complete, the rats were then transferred to a rate frequency procedure (Campbell et al., 1985) in which animals were allowed to lever press, on a FR-1 reinforcement schedule (which continued for the duration of experimentation), for a series of 16 different pulse frequencies, ranging from 141 to 25 Hz, presented to the animals in descending order. Each pulse frequency was preceded by a 500 ms priming stimulation to ‘inform’ the animal as to the frequency of the immediately following BSR, and then the rat was allowed to lever press for BSR during a 30-s period. Each pulse frequency was offered for two 30-s periods (time bins) before descending to the next frequency. We have, therefore, labelled such sweeps of pulse frequencies from 141 to 25 Hz as ‘twinned time-bin sweeps’. When one such ‘twinned time-bin sweep’ of pulse frequencies from 141 to 25 Hz was completed, the procedure was repeated – what we call a ‘double-sweep’. This double-sweep procedure then repeated itself twice more, for a total of three double-sweeps per day. The first double-sweep of each day was a ‘warm-up’ session and discarded from the data. The second double-sweep was taken as the baseline BSR session while the third was taken as the test BSR session. The four response rate measures at each stimulation frequency of the baseline BSR session were averaged, as were the four of the test BSR session, yielding an average baseline and an average test lever press rate at each frequency for the baseline BSR and test BSR sessions. Reward threshold, θ0(the frequency at which the animal failed to respond for rewarding stimulation), was then determined for the baseline BSR session and the test BSR session. This was done by mathematically fitting each rate-frequency curve (one for the baseline BSR session; one for the test BSR session) by iterative computer programs derived from the Gauss – Newton algorithm for nonlinear regression to three different sigmoidal curve-fitting mathematical growth models that appear to accurately fit rate-frequency electrical brain reward functions (Coulombe and Miliaressis, 1987): the Gompertz model (γ′=ae−e(b–cX)), the logistic model (γ′=a/[1+e(b–cX)]), and the Weibull function (γ′=a[1–e−(bX)c]). From each curve-fitting model, a θ0 solution was obtained. The three solutions for θ0 were averaged to produce a mean θ0 for each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies. When mean θ0 was stable (three successive days with each θ0 value within 10% of overall mean θ0), animals were injected, between the baseline and test BSR session with subcutaneous (s.c.) nicotine (0.25 mg/kg) and intra-peritoneal (i.p.) SB-277011A (0, 3, 6, or 12 mg/kg). SB-277011A or its vehicle was given 30 min, and nicotine 30 s before test sessions, 30 min being the time interval between the baseline and test BSR sessions (during which interval the animals remained in their operant test chambers). The sequence of the SB-277011A doses was counterbalanced.
A second group of animals (n=9) was also run in the BSR paradigm, and tested at a higher dose of nicotine (0.5 mg/kg s.c.). These animals were run using an inter-day, rather than an intra-day, testing protocol, with baseline and test BSR sessions on separate days. In this group, when mean θ0 was stable (5 successive days with each θ0 value within 10% of overall mean θ0), animals were given, on the same day, a ‘warm-up’ BSR session followed by a baseline BSR session. Then, when daily BSR sessions were again stable (3 more successive days with each θ0 value within 10% of overall mean θ0), animals were given, on the same day, a warm-up BSR session followed by a test BSR session. Successive warm-up and baseline BSR sessions then followed, with at least 3 days during which each θ0 value fell within 10% of overall mean θ0 being required before the next warm-up and test BSR session was run. Nicotine (0.5 mg/kg s.c.) and SB-277011A (0, 3, 6, or 12 mg/ kg i.p.) were administered 30 s and 30 min, respectively, before test sessions. Since warm-up BSR results were discarded (by definition, see above), drug effects on BSR were computed by comparing test BSR sessions against the immediately previous baseline BSR sessions. The sequence of the SB-277011A doses was counterbalanced.
Histology
Upon completion of experiments, animals were killed by anaesthetic overdose (sodium pentobarbital). The rats were then decapitated and their brains removed and stored in 10% buffered formalin solution. Brains were transferred to a 20% sucrose solution at least 24 h before sectioning. Coronal sections (50 µm thick) were made on a vibratome and stained with Cresyl Violet. Reconstructions of electrode tracks were made using a microscope and the rat brain atlas of Paxinos and Watson (1998).
Statistical analysis
Shifts in mean θ0 values produced by nicotine, nicotine+vehicle, or nicotine+ SB-277011A were analysed using Student’s t test where appropriate and also by one-way analysis of variance (ANOVA) for repeated measures followed by multiple comparison testing using the Newman– Keuls post-hoc test (Kirk, 1982; Winer, 1962). Minimally acceptable statistical significance was set at a probability level of p<0.05 for all tests.
LMA (nicotine cue-conditioned locomotor activity) experiments
Apparatus
Locomotor activity was recorded by 16 computerized AccuScan Versamax units (AccuScan Instruments Inc., Columbus, OH, USA). Each unit consisted of a clear Plexiglas cage (40 cm wide × 40 cm deep × 32 cm high) equipped with 32 infrared light beam sensors located 1.3 cm above the floor. Photo beam interruptions were monitored in 5-min periods (time bins) from each monitor and were analysed by AccuScan Analyzer computer software.
Procedure
The AccuScan Versamax activity monitoring units described immediately above were also used as cue-conditioning chambers following acute nicotine or saline administration. The conditioning phase consisted of 30-min conditioning (cue-pairing) sessions. There were four groups of animals (n=16 per group). Groups 1 and 2 were the saline- and nicotine-paired groups. Each rat in these two groups was injected with saline in the home cage and 3 h later with either saline (1 ml/kg s.c.) or nicotine (0.5 mg/kg s.c. in a volume of 1 ml/kg) and then immediately placed into a locomotor activity chamber (drug-paired environment) for 30 min. Groups 3 and 4 were control, unconditioned animals. Each rat in these two groups was injected with either saline (1 ml/kg s.c.) or nicotine (0.5 mg/kg s.c. in a volume of 1 ml/kg) in its home cage, and then 3 h later was injected with saline and immediately placed into a locomotor activity chamber (drug-unpaired environment) for 30 min. Groups 3 and 4 were, therefore, the saline- and nicotine-unpaired groups. This procedure was performed once a day for 4 d. The test phase occurred on the following day (day 5), and was conducted identically to the conditioning phase days, except that each animal was given an injection of saline and then immediately placed into a locomotor activity chamber. Thus, during the test phase, locomotor activity was induced only by the saline- or nicotine-associated environmental cues of the locomotor activity chambers. To assess the effects of D3 receptor antagonism on nicotine-cue-induced locomotor activity, on the test day animals were divided into two pharmacological treatment groups: 0 mg/kg i.p. SB-277011A (i.e. the β-cyclodextrin vehicle) or 10 mg/ kg i.p. SB-277011A (n=8 per group). In a further experiment, an additional 36 animals were trained as a nicotine-paired group only, as described above. On the test day these animals were divided into three pharmacological treatment groups: 0 (i.e. vehicle), 1, or 3 mg/kg i.p. SB-277011A. All pharmacological treatments were given 30 min prior to LMA testing.
Statistical analysis
Raw LMA data counts were converted to Area Under the Curve for the first 20 min of testing (AUC 0–20 min), using Simpson’s Rule for AUC calculation
[(MaxTime − MinTime)/3 * n] × [γ1+4 * (γ2+γ4+ … )+2 * (γ3+γ5+ … )+ γn],
where n represents the total number of time bins and γ represents the absolute value of each time bin in sequential order (see Epperson, 2001 for the theory and derivation of AUC calculations). Simpson’s Rule for AUC calculation was chosen over the Trapezoidal Rule for AUC calculation because it yields a more accurate numerical integration (Epperson, 2001). The data were then analysed by ANOVA followed by multiple comparison testing (Kirk, 1982; Winer, 1962). The effects of SB-277011A on LMA were analysed by three-way ANOVA with main factors of environment (paired vs. unpaired), conditioning drug (saline vs. nicotine), and treatment (vehicle or SB-277011A, 10 mg/kg), taking into account the following interactions: (1) drug × environment; (2) drug × treatment; (3) environment × treatment, and (4) drug × environment × treatment. Differences within each interaction were further analysed by performing planned comparisons using the ‘a priori t test’ (Kirk, 1982, pp. 95–97).
For the effects of SB-277011A (0, 1, 3 mg/kg i.p.) on the nicotine-paired group only, a one-way ANOVA was performed. Minimally acceptable statistical significance was set at a probability level of p<0.05 for all tests.
CPP (nicotine-induced conditioned place preference) experiments
Apparatus
An automated, three-chambered, Plexiglas CPP apparatus was used as previously described (Dewey et al., 1999; Horan et al., 2000), with modifications. The two pairing chambers of the apparatus were identical in dimensions (25 × 14 × 36 cm) and were separated by removable Plexiglas guillotine doors. The pairing chambers were composed of distinct visual and tactile cues. The walls of one of the pairing chambers were white with cage bedding on the floor and the walls of the second chamber consisted of alternating white and black boxes (1.2 × 1.8 cm) in a chessboard pattern and a Plexiglas floor. The two pairing chambers were separated by a third, connecting tunnel (7.5 × 7.5 × 36 cm). This third connecting tunnel was equipped with the same distinct visual and tactile cues as the two pairing chambers; the half of the connecting tunnel adjacent to the white-walled pairing chamber was similarly white-walled, while the half of the connecting tunnel adjacent to the chessboard-patterned wall was similarly chessboard patterned, and so forth. Thus, while strictly speaking a third chamber existed, a third set of visual and tactile cues did not.
Procedure
CPP expression was assessed as previously described (Horan et al., 2000; Vorel et al., 2002). Expression studies with acute SB-277011A or vehicle were divided into four phases: acclimation, handling, conditioning, and testing. The animals were not exposed to the chambers prior to the start of the pairings. During days 1–3, animals were acclimated to the animal facility. During handling (days 4–6), animals were transported to the laboratory and handled for 5 min each. During conditioning (days 7–22), animals were exposed to once-daily conditioning sessions. For each conditioning session, animals (n=40) were injected with nicotine (0.6 mg/kg s.c. in a volume of 1 ml/kg) or vehicle (1 ml/kg s.c.) and then immediately confined for 30 min in an appropriate cue-specific chamber. During conditioning, nicotine was always paired with one cue-specific environment, and vehicle was paired with the other; nicotine or vehicle exposure (and appropriate environmental pairing) alternated from day to day. This was done over a 16-d period, i.e. animals were given eight pairings with nicotine and there was a 24-h separation between exposure to vehicle and nicotine. The animals in each group were randomly assigned to a 2 × 2 factorial design with one factor being the pairing chamber and the other factor being the order of conditioning. In this counterbalanced procedure, the animals were randomly assigned to one of the two pairing chambers, so that half of the subjects received the drug in one compartment (white walls, with bedding on the floor) and the other half in the other compartment (alternating black and white squares on the walls, with a smooth chamber floor). This procedure resulted in the animals receiving equal exposure to the two compartments and due to random assignment, controlled for side preference. An additional group of 10 animals was paired with vehicle in both chambers of the apparatus during the conditioning phase.
On the test day (day 23), the 40 animals that had been paired in the CPP apparatus alternating with nicotine and vehicle during the conditioning phase were randomly divided into four groups of 10 and received either SB-277011A (1, 3, or 10 mg/kg i.p.) or vehicle in the home cage 30 min before they were placed in the CPP apparatus. Then, the guillotine doors were removed and the animals were allowed to move freely within the apparatus for 15 min. The amount of time spent in each chamber was determined using an automated timing system. Each of the pairing chambers contained infrared micro-beams (two per chamber) that were wired to an automated timer. When an animal entered a chamber, the beam was broken and a timer began recording. Once the animal left the chamber, the timer stopped. In addition, the circuitry had an internal timer that shut off after 15 min. The third small connecting chamber was so small that it was not possible for an animal to stay totally within it. Thus, the sum of the times spent in the two pairing chambers was always found to equal 15 min. On the test day, the 10 animals that had been paired in the CPP apparatus alternatingly with vehicle and vehicle during the conditioning phase were tested in identical manner to the 40 animals that had been paired in the CPP apparatus alternatingly with nicotine and vehicle during the conditioning phase.
To test for the possibility that SB-277011A alone might produce place preference or aversion, an additional CPP experiment was carried out with four separate groups of new animals (n=10 per group). In this additional experiment, injections of vehicle (1 ml/kg 2% w/v methylcellulose solution) or 1, 3, or 10 mg/kg SB-277011A were paired with one of the distinctive CPP cue chambers during CPP conditioning. These animals received four pairings of distinctive cue chamber and the appropriate dose of SB-277011A or vehicle, with each pairing separated by 24 h. On the test day, these animals received an i.p. injection of vehicle in the home cage 30 min before being placed into the CPP apparatus. Time spent in each cue-distinctive chamber was recorded. In all other methodological respects, this additional CPP experiment was carried out as detailed above for the main CPP experiment.
To test for the possibility that SB-277011A alone might produce behavioural effects on the CPP test day, a second additional CPP experiment was carried out with four separate groups of new animals (n=10 per group). In this second additional CPP experiment, the effects on CPP expression of vehicle or 1, 3, or 10 mg/kg of SB-277011A administered on the test day were assessed. For each CPP conditioning session, animals were injected with vehicle (1 ml/kg s.c. of deionized distilled water) and then immediately confined for 30 min in one of the cue-specific chambers. Twenty-four hours later, the animals were paired with vehicle in the other cue-specific chamber. This pairing cycle was repeated eight times over a 16-d period. Thus all animals received vehicle injection in both chambers. On the test day, animals received SB-277011A (1, 3, or 10 mg/kg i.p.) or vehicle in the home cage 30 min before being placed in the CPP apparatus. Time spent in each cue-distinctive chamber was recorded. In all other methodological respects, this additional CPP experiment was carried out as detailed above for the main CPP experiment.
Statistical analysis
Data were analysed using Student’s t test where appropriate (orthogonal observations, independent samples) (Ferguson, 1981) and also by one-way ANOVA (Kirk, 1982; Winer, 1962) followed by multiple comparison testing using the Newman– Keuls test (Kirk, 1982; Winer, 1962). Specifically, the times spent in each chamber by the different experimental groups of animals (i.e. vehicle/vehicle paired during training vs. vehicle/nicotine paired during training; vehicle/nicotine paired during training and SB-277011A 0 mg/kg on test day, vehicle/ nicotine paired during training and SB-277011A 1 mg/kg on test day, vehicle/nicotine paired during training and SB-277011A 3 mg/kg on test day, vehicle/nicotine paired during training and SB-277011A 10 mg/kg on test day) on test day (i.e. CPP expression) were analysed using the above-specified statistical tests. To analyse the data generated in the first additional CPP experiment (to study the possibility that SB-277011A alone might produce appetitive or aversive effects when paired with cue-distinctive chambers during CPP conditioning), ANOVA was used. To analyse the data generated in the second additional CPP experiment (to study the possibility that SB-277011A might by itself produce behavioural effects during CPP expression on the test day), ANOVA was used. In addition, to test even further for the possibility that SB-277011A might, on the test day, produce some form of ‘negative contrast’ between the positive CPP expected to be observed in the nicotine-paired compartment and the behaviour actually observed in the nicotine-paired compartment, the time spent in the nicotine-paired compartment by SB-277011A–treated rats was compared to the 7.5 min theoretical CPP ‘indifference level’ for each of the four tested groups of rats (vehicle, 1 mg/kg SB-277011A, 3 mg/kg SB-277011A, 10 mg/kg SB-277011A) using ‘one-sample t tests’ (Rosner, 2006). Minimally acceptable statistical significance was set at p<0.05 for all tests.
Results
BSR (nicotine-enhanced brain-stimulation reward) experiments
Histological verification of electrode implant locations revealed that all electrode tips were within 0.5 mm of the desired target locus in the brain, and (congruently) no animals had to be excluded from the experiment because of failure to reach satisfactory BSR levels and stability.
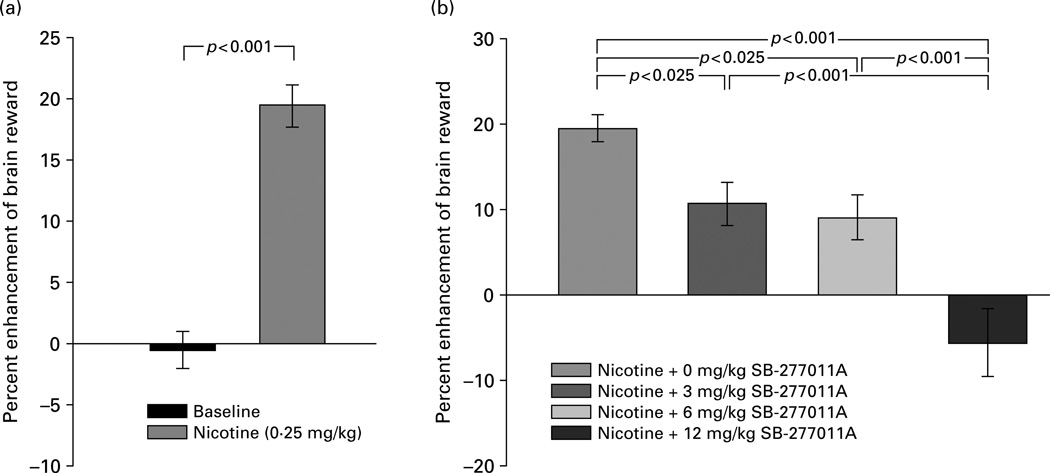
As shown in Figure 1, nicotine at a dose of 0.25 mg/kg significantly enhanced brain reward (i.e. lowered electrical BSR thresholds) compared to baseline (Figure 1a) (t=9.96, d.f.=13, p<0.001), and pretreatment with SB-277011A (3, 6, or 12 mg/kg) dose-dependently attenuated nicotine-enhanced BSR (Figure 1b). This effect of SB-277011A was confirmed by one-way ANOVA, which revealed a statistically significant SB-277011A main treatment effect (F3,39= 18.6, p<0.001). Follow-up individual pairwise comparisons using the Newman–Keuls post-hoc test confirmed that SB-277011A (3, 6, or 12 mg/kg i.p.) given prior to nicotine on the test day produced a statistically significant dose-dependent inhibition of nicotine-enhanced BSR relative to 0 mg/kg SB-277011A (Figure 1b).
Figure 1.
Effect of nicotine (0.25 mg/kg s.c.) and various doses of the DA D3 receptor antagonist SB-277011A (0, 3, 6, 12 mg/ kg i.p.) on electrical brain reward thresholds (θ0) in laboratory rats. Changes in brain reward threshold are plotted as percent enhancement or inhibition of brain reward ± s e m. Statistical significance levels for specific inter-treatment comparisons, wherein nicotine is compared to baseline by the paired Student’s t test (a) and other inter-treatment comparisons are made by post-ANOVA Newman–Keuls tests (b), are as shown directly on the figure.
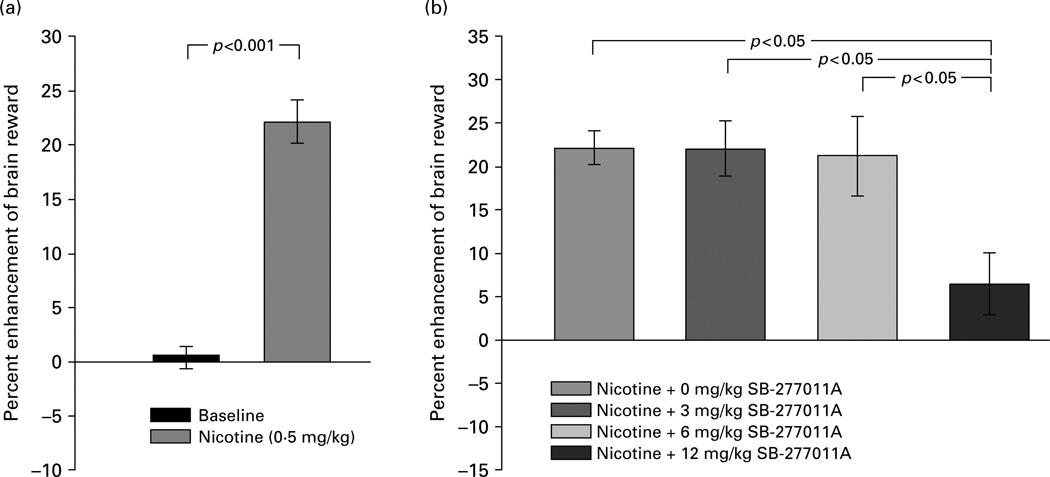
Figure 2 shows that nicotine at a dose of 0.5 mg/kg significantly enhanced brain reward (i.e. lowered electrical BSR thresholds) compared to baseline (Figure 2a) (t=9.35, d.f.=8, p<0.001), and that pretreatment with SB-277011A (3, 6, 12 mg/kg) attenuated nicotine-enhanced BSR (Figure 2b). This effect of SB-277011A was confirmed by one-way ANOVA, which revealed a statistically significant SB-277011A main treatment effect (F3,24=4.5, p<0.025). Follow-up individual pairwise comparisons using the Newman–Keuls post-hoc test showed that SB-277011A given prior to nicotine on the test day produced a statistically significant inhibition of nicotine-enhanced BSR, but only at a dose of 12 mg/kg SB-277011A (Figure 2b).
Figure 2.
Effect of nicotine (0.5 mg/kg s.c.) and various doses of the DA D3 receptor antagonist SB-277011A (0, 3, 6, 12 mg/kg i.p.) on electrical brain reward thresholds (θ0) in laboratory rats. Changes in brain reward threshold are plotted as percent enhancement or inhibition brain reward ± s e m. Statistical significance levels for specific inter-treatment comparisons, wherein nicotine is compared to baseline by the paired Student’s t test (a) and other inter-treatment comparisons are made by post-ANOVA Newman–Keuls tests (b), are as shown directly on the figure.
LMA (nicotine cue-conditioned locomotor activity) experiments
Effect of SB-277011A on LMA
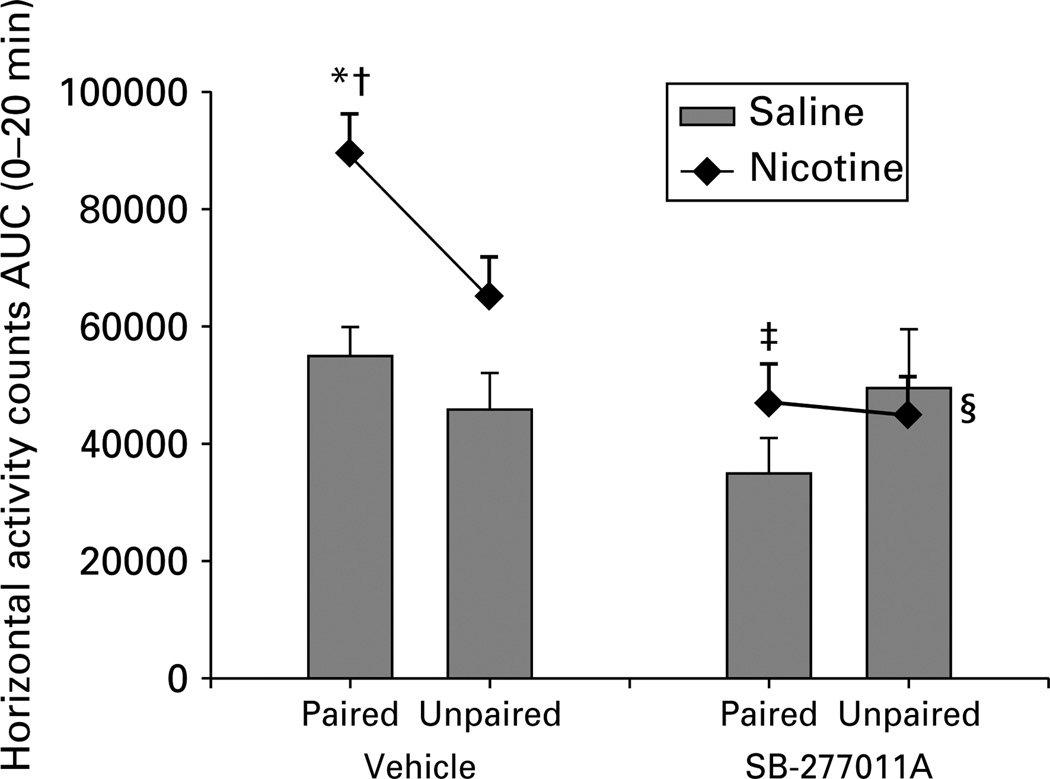
Rats that had received injections of nicotine in the locomotor activity chambers during conditioning sessions showed enhanced locomotor response, measured by horizontal activity counts, with respect to all other groups (Figure 3). To determine the role of each of the conditioning groups in the overall effects reported above, AUC 0–20 min data were analysed as detailed above (see ‘Behavioural methods and testing’).
Figure 3.
Nicotine (0.5 mg/kg s.c.) cue-conditioned locomotion in laboratory rats, shown as the area under the curve (AUC - see text) of horizontal activity counts for the first 20 min of locomotion in the four groups of animals treated with vehicle or with SB-277011A (10 mg/kg i.p.). Rats that received nicotine in the cue-specific test environment for 4 consecutive days (nicotine-paired) showed enhanced locomotor response compared to rats that received saline in the test environment (saline-paired), while there was no difference between the nicotine and the saline unpaired groups. SB-277011A produced an attenuation of nicotine-associated cue-induced locomotor activity and slightly reduced the nicotine unpaired response. * p < 0.05 vs. nicotine unpaired; †p <0.001 vs. vehicle saline paired; ‡p < 0.0001 vs. vehicle nicotine paired; §p = 0.04 vs. vehicle nicotine unpaired. Data are expressed as means ±s.e.m.
SB-277011A decreased nicotine cue-induced conditioned LMA (Figure 3). An ANOVA with main factors of environment, conditioning drug, and treatment revealed significant main effects of conditioning drug (F1,54 = 16.22, p<0.001) and treatment (F1,54 = 21.19, p<0.001), but no significant effect of environment: (F1,54 = 1.85, p=n.s.). Although the ANOVA showed no significant environment × conditioning drug interaction (F1,54 = 2.38, p = n.s.), a planned comparison showed that, in vehicle-treated animals, there was a statistically significant difference between the nicotine-paired and the nicotine-unpaired groups (p<0.05) as well as between the nicotine-paired and the saline-paired groups (p<0.001). There was no significant difference between the nicotine-unpaired and the saline-unpaired groups (p = 0.08) or between the saline-paired and saline-unpaired groups (p = 0.37). The ANOVA also showed no significant environment × conditioning drug × treatment interaction (F154 = 0.45, p=n.s.), but again, a planned comparison showed that pretreatment with SB-277011A (10 mg/kg) in the nicotine-paired group significantly reduced locomotor activity vs. the nicotine-paired group pretreated with vehicle (p<0.0001). Thus, treatment with SB-277011A abolished the difference between the nicotine-paired and the saline-paired groups, reducing an impressive degree of statistically significant difference (p< 0.001) to statistical insignificance (p = 0.26). Treatment with SB-277011A also produced a slight but significant effect in the unpaired groups (nicotine-unpaired vehicle-pretreated vs. nicotine-unpaired SB-277011A–pretreated, p = 0.04).
When lower doses of SB-277011A (1, 3 mg/kg i.p.) were tested for their effects on nicotine cue-conditioned LMA in a separate experiment, SB-277011A had no effect on cue-conditioned LMA (data not shown). Statistically, this was confirmed by an ANOVA of the AUC of horizontal activity counts (F2,33=1.2, p=n.s.).
CPP (nicotine-induced conditioned place preference) experiments
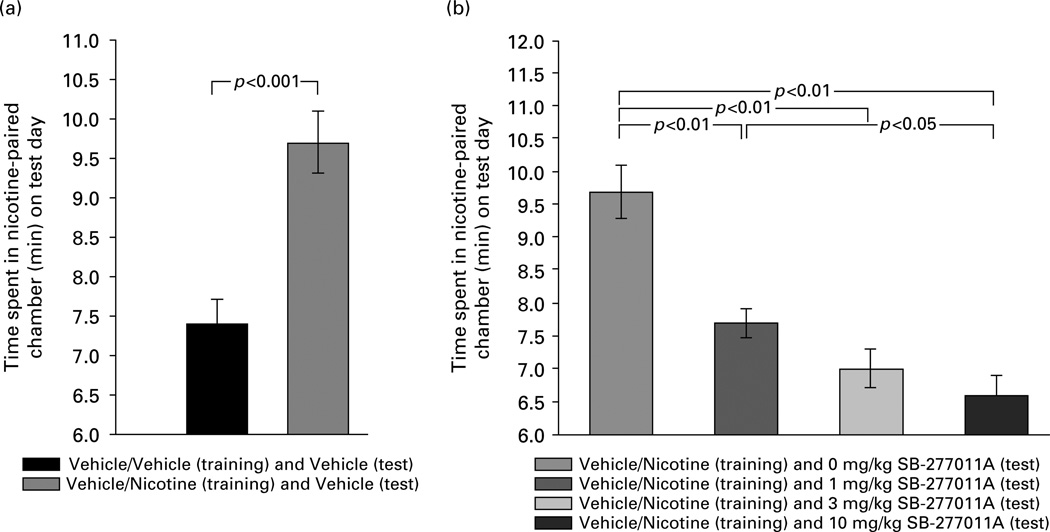
In the animals given vehicle/vehicle exposure (i.e. vehicle in both CPP compartments) on the training days, testing for CPP expression after exposure to vehicle on the test day produced no place preference (7.4 ± 0.3 min vs. 7.6 ± 0.3 min spent in the two CPP compartments respectively). This confirms that the CPP procedure used here was non-biased (i.e. no a-priori preferences among the animals for either chamber of the CPP apparatus prior to CPP training, and no a posteriori preferences for either chamber after vehicle exposure in both CPP compartments on the CPP training days). The unbiased CPP procedure is generally considered to be the preferred methodology, as the biased CPP procedure can yield false-positive results due to ceiling effects (Cunningham et al., 2003) or unconditioned motivational responses to the initially non-preferred environment (e.g. anxiety) (Tzschentke, 1998). As shown in Figure 4a, nicotine (0.6 mg/kg s.c.) produced a significant CPP (9.7 ± 0.4 min in the nicotine-paired cue-distinctive compartment) compared to vehicle (7.4 ± 0.3 min in the vehicle-paired cue-distinctive compartment), measured as CPP expression on the test day. This was confirmed by Student’s t test for the difference between the means (of time spent in the nicotine-paired CPP chamber on the test day) of the vehicle/ vehicle-paired group given vehicle on the test day vs. the vehicle/nicotine-paired group given vehicle on the test day (t=4.44, d.f.=18, p<0.001) (Figure 4a). Thus, the dose of nicotine used in the present experiments produced a statistically significant CPP.
Figure 4.
Effect of nicotine (0.6 mg/kg s.c.) and various doses of the DA D3 receptor antagonist SB-277011A (0, 1, 3, 10 mg/kg i.p.) on the expression of conditioned place preference in laboratory rats. Times spent in the nicotine-paired chamber on test day are shown as means ± s e m. Statistical significance levels for specific inter-group comparisons, as determined by Student’s t test for independent samples (a) and by post-ANOVA Newman–Keuls tests (b), are as shown directly on the figure (also see text).
In addition, and referring directly to the effect of D3 receptor antagonism on nicotine-induced CPP, SB-277011A dose-dependently attenuated this statistically significant expression of nicotine-induced CPP (Figure 4b). Statistically this effect of SB-277011A was confirmed by one-way ANOVA over the four groups of animals shown in Figure 4b, which revealed a statistically significant main effect over all four groups (F3,36=18.3, p<0.0001). Follow-up individual pairwise group comparisons using the Newman– Keuls test showed that the vehicle/nicotine paired groups given SB-277011A (1, 3, or 10 mg/kg i.p.) on the test day each differed significantly from the vehicle/ nicotine paired group given vehicle (i.e. 0 mg/kg SB-277011A) on the test day (p<0.01 for all three pairwise comparisons). Newman–Keuls tests also revealed that the vehicle/nicotine paired group given SB-277011A at 1 mg/kg i.p. on the test day differed significantly from the vehicle/nicotine paired group given SB-277011A at 10 mg/kg i.p. on the test day (p<0.05) (Figure 4b). Thus, SB-277011A at all doses tested produced a statistically significant inhibition of nicotine-induced CPP, but SB-277011A at 10 mg/kg produced a significantly greater inhibition of nicotine-induced CPP than did SB-277011A at 1 mg/kg, indicating that SB-277011A’s attenuation of nicotine-induced CPP showed a significant dose-dependency.
Finally, SB-277011A by itself appeared not to produce aversion, ‘negative contrast’, or indeed any negative behavioural effects at all. In the first additional CPP experiment carried out to test this possibility (vehicle or SB-277011A given during CPP conditioning), the effects of vehicle or 1, 3, or 10 mg/kg SB-277011A on CPP acquisition in animals given vehicle on the test day were assessed. CPP expression on test day in these animals is shown in Table 1. Animals given SB-277011A in a cue-distinct environment developed neither a preference nor an aversion to that environment. Statistical analysis by ANOVA of the data shown in Table 1 indicate that repeated pairing of SB-277011A with a given cue-distinctive chamber of the CPP apparatus did not significantly alter the amount of time spent, on test day, in the SB-277011A–paired chambers compared to the vehicle-paired chamber (F3,34=1.88, p=0.15). In the second additional CPP experiment carried out to test this possibility (vehicle or SB-277011A given during CPP expression), the effects of vehicle or 1, 3, or 10 mg/kg SB-277011A on CPP expression in animals given vehicle on conditioning days were assessed. CPP expression on test day in these animals is shown in Table 2. Animals given SB-277011A 30 min prior to CPP expression-testing on the test day displayed no alterations in their behaviour. Statistical analysis by ANOVA of the data shown in Table 2 indicate that SB-277011A produced no statistically significant alterations in behaviour on the test day (F3,36=0.34, p=0.8). This was further confirmed by the results of the one-sample t tests (Rosner, 2006) carried out on times spent in the nicotine-paired compartment by SB-277011A–treated rats on the test day as compared to the 7.5 min theoretical CPP ‘indifference level’ for each of the four tested groups of rats (vehicle, 1 mg/kg SB-277011A, 3 mg/kg SB-277011A, 10 mg/kg SB-277011A). From those one-sample t tests, it was clear that SB-277011A did not produce any negative behavioural effect on the test day (Vehicle group: t=0.26, d.f.=9, p=n.s.; 1 mg/kg SB-277011A group: t=1.19, d.f.=9, p=n.s.; 3 mg/ kg SB-277011A group: t=0.85, d.f.=9, p=n.s.; 10 mg/ kg SB-277011A group: t=0.17, d.f.=9, p=n.s.).
Table 1.
Place conditioning response to SB-277011A (SB) in male Sprague–Dawley rats
| Treatment pairings | Treatment given on the test day |
Time spent in the chambers (min) |
No. of crossings |
|
|---|---|---|---|---|
| Paired | Unpaired | |||
| Vehicle/SB, 1 mg/kg | Vehiclea | 8.3±1.4b | 6.7±1.4 | 28.0±3.2 |
| Vehicle/SB, 3 mg/kg | Vehicle | 9.4±1.0 | 5.6±1.0 | 29.9±1.8 |
| Vehicle/SB, 10 mg/kg | Vehicle | 9.8±0.9 | 5.2±0.9 | 32.8±3.5 |
| Vehicle/vehicle | Vehicle | 7.5±0.4 | 7.5±0.4 | 27.0±2.6 |
Vehicle was 1 ml/kg of a 2% methylcellulose solution (w/v).
Each value represents the mean number of minutes spent in each chamber ± s.e.m. A total of 9–10 rats were examined for each treatment pairing. All animals received four pairings with the appropriate dose of SB/vehicle with each pairing being separated by a 24-h period. On the test day, animals received vehicle 30 min before being placed into the CPP apparatus.
Table 2.
Effect of vehicle or SB-277011A (SB) on expression of the conditioned place preference response to vehicle in male Sprague–Dawley rats
| Time spent in chambers (min) |
|||
|---|---|---|---|
| Treatment pairings | Treatment given on the test day |
Vehicle chamber 1 |
Vehicle chamber 2 |
| Vehicle/vehicle | Vehiclea | 7.6 ± 0.3b | 7.4 ± 0.3 |
| Vehicle/vehicle | SB, 1 mg/kg | 7.8 ± 0.3 | 7.2 ± 0.3 |
| Vehicle/vehicle | SB, 3 mg/kg | 7.8 ± 0.3 | 7.2 ± 0.3 |
| Vehicle/vehicle | SB, 10 mg/kg | 7.5 ± 0.3 | 7.5 ± 0.3 |
The vehicle used during treatment pairings was 1 ml/kg s.c. of distilled water. The vehicle used on the test day was 1 ml/kg i.p. of 2% methylcellulose.
Each value represents the mean number of minutes spent in each chamber ± s.e.m. A total of 10 rats were examined for each treatment pairing. On the test day, animals received either 1 ml/kg i.p. vehicle (2 % methylcellulose) or SB-277011A (1, 3 or 10 mg/kg i.p.) 30 min before being placed into the CPP apparatus. The ‘Time spent in chambers’ represents the times spent, on the test day, in the two different chambers that were each paired with vehicle on the CPP acquisition days (‘Treatment pairings’).
Discussion
The present experiments demonstrate that pre-treatment with the potent and selective D3 receptor antagonist SB-277011A significantly reduces nicotine’s effects on BSR and significantly attenuates nicotine-associated environmental-cue effects on LMA and CPP. The observed effects of SB-277011A support the hypothesis that D3 receptors play an important role in mediating nicotine-induced enhancement of brain reward and nicotine-associated cue-induced incentive motivational effects.
The present finding that nicotine significantly enhances the rewarding efficacy of BSR is consistent with previous reports (e.g. Bauco and Wise, 1994; Bozarth et al., 1998; Huston-Lyons and Kornetsky, 1992; Ivanová and Greenshaw, 1997; Panagis et al., 2000; Wise et al., 1992, 1998). Some of those previous reports have gone further than merely demonstrating that nicotine enhances BSR, and have implicated DA mechanisms in such enhancement. Thus, nicotine’s enhancement of BSR has been shown to be attenuated by DA receptor antagonists (Huston-Lyons et al., 1993; Ivanová and Greenshaw, 1997) and nicotinic receptors expressed on DA neurons have been shown to be essential to nicotine’s augmentation of BSR (Wise et al., 1998). However, no previous work has implicated D3 receptor mechanisms in nicotine’s effects on brain-reward substrates. Thus, the present findings with SB-277011A constitute the first demonstration that DA D3 receptor mechanisms are involved in nicotine’s effects on brain reward and that selective D3 receptor antagonism attenuates nicotine-enhanced BSR.
The present finding that nicotine-associated environmental cues come to elicit LMA is consistent with previous findings (Bevins et al., 2001; Bevins and Palmatier, 2003; Le Foll et al., 2003b; Palmatier and Bevins, 2002; Palmatier et al., 2003; Walter and Kuschinsky, 1989). In some of those previous studies, it was reported that the expression of nicotine-paired cue-induced LMA was blocked by DA receptor antagonists (Bevins et al., 2001), by the D3 receptor partial agonist BP-897 (Le Foll et al., 2003b), and by SB-277011A (Le Foll et al., 2003b). However, in those previous studies, the effects of the various DA-active compounds were not assessed in appropriate unpaired control groups (i.e. animals that received vehicle or nicotine in their home cages rather than in the cue-specific locomotor activity chambers). Thus, it is not established that the pharmacological effects reported in those studies were due to specific action on nicotine-paired cue-induced LMA, since pharmacological effects on nicotine-unpaired cue-induced LMA were not tested. The present data show that SB-277011A–induced D3 receptor antagonism appears to robustly attenuate nicotine-paired cue-induced LMA (p<0.0001), although SB-277011A treatment also slightly but significantly affected locomotor activity in the unpaired group (p=0.04).
The present finding that nicotine produces a robust CPP in male rats is consistent with previous findings from both our laboratory (Ashby et al., 2002; Dewey et al., 1999; Horan et al., 1997, 2001) and other groups (for review, see Le Foll and Goldberg, 2005). Interestingly, most previous reports of nicotine-induced CPP have used the biased CPP methodology, rather than the unbiased procedure used in the present experiments (for review, see Le Foll and Goldberg, 2005). The present findings suggest that nicotine-induced CPP is not dependent upon the biased methodology. The present findings also show that SB-277011A dose-dependently attenuated the expression of nicotine-induced CPP. In previous studies, it has been reported that the D3 receptor partial agonist BP-897 and the putative D3 receptor antagonist ST-198 significantly attenuated nicotine-induced CPP (Le Foll et al., 2005b). However, it should be noted that BP-897 alone has been reported to produce conditioned place aversion (Duarte et al., 2003; Gyertyán and Ga´l, 2003) and, congruently, to inhibit electrical BSR, an aversive-like effect (Campos et al., 2004). Aversive-like effects produced by BP-897 could easily confound the interpretation of experiments (e.g. Le Foll et al., 2005b) in which BP-897 was used as a putative D3 receptor antagonist. In contrast, SB-277011A in the present experiments did not by itself produce a significant place preference or aversion, and SB-277011A administered on the test day following repeated vehicle/vehicle pairings on acquisition days did not produce any significant alteration in the rats’ behaviour. Also, BP-897 has been shown to have affinity for neurotransmitter receptors other than D3 (e.g. D2, a1 adrenergic, a2 adrenergic, 5-HT1A, 5-HT2A) (Cussac et al., 2000; Heidbreder et al., 2005; Pilla et al., 1999; Xi et al., 2005), and data obtained using cloned human receptors show that BP-897 has potent partial agonist effects at 5-HT1A receptors and potent antagonist effects at a1 and a2 receptors (Cussac et al., 2000). Thus, action at one or more of those sites may be responsible for BP-897’s effects in the CPP paradigm. ST-198 may also possess D2 antagonist action, as (like BP-897) it significantly attenuates L-dopa-induced dyskinesias in monkeys (Bézard et al., 2003). Therefore, the present findings with SB-277011A appear to constitute the first clear demonstration that selective DA D3 receptor antagonism attenuates nicotine-induced CPP.
Non-selective D1- or D2-like receptor antagonists inhibit addictive-drug-induced enhancement of brain reward (e.g. Nakajima, 1989; Ranaldi and Wise, 2001; Wise, 1994; Wise and Bozarth, 1985; Wise and Rompré, 1989) as well as addictive-drug-associated cue-induced behavioural effects (e.g. Cervo et al., 2003; Ciccocioppo et al., 2001; Weiss et al., 2000). Such findings raise the issue of whether the presently observed inhibitory effects of SB-277011A on nicotine-enhanced BSR and nicotine-associated cue-induced LMA and CPP might be attributable to D1 or D2 receptor-selective antagonism rather than D3 receptor-selective antagonism. However, accumulating evidence does not support this notion: (1) SB-277011A is a highly potent and highly selective D3 receptor antagonist with 80- to 100-fold selectivity for D3 over other DA receptors; high affinity for the human (pKi=7.95) and rat (pKi=7.97) cloned DA D3 receptor; and 100-fold selectivity over 66 other receptors, enzymes, ion channels, and transporters in the central nervous system (Reavill et al., 2000; Stemp et al., 2000); (2) the effects of SB-277011A in various animal models relating to addiction are significantly different from those produced by D1- or D2-preferring antagonists (Heidbreder et al., 2005); for example, D1 and D2 antagonists significantly elevate electrical BSR thresholds (Baldo et al., 1999; Harrison et al., 2002; Hunt et al., 1994; Nakajima and O’Regan, 1991; Panagis and Spyraki, 1996; Stein, 1962; Stein and Ray, 1960), while SB-277011A does not alter BSR thresholds (Vorel et al., 2002); similarly, D1- and D2-preferring antagonists produce aversion in the CPP paradigm (e.g. Shippenberg and Herz, 1988; for review, see Tzschentke, 1998), while SB-277011A produces neither reward nor aversion in the CPP paradigm (Gyertyán and Gál, 2003; Vorel et al., 2002; Xi et al., 2005); (3) SB-277011A does not significantly alter locomotor activity (Reavill et al., 2000; Xi et al., 2005), whereas locomotor inhibition is a classic property of D2 antagonists; (4) at doses of many-fold those used in the present experiments, SB-277011A does not block quinelorane-induced decreases in DA in the dorsal striatum where a high density of D2 receptors are located, and (5) SB-277011A does not significantly alter prolactin levels (Reavill et al., 2000), whereas alteration of prolactin levels is a classic property of D2 antagonists. SB-277011A, but not D2 antagonists, significantly increases acetylcholine levels in the rat frontal cortex (Lacroix et al., 2003).
If the presently observed inhibitory effects of SB-277011A on nicotine-enhanced BSR and on nicotine-associated cue-induced LMA and CPP cannot be attributed to D1 or D2 antagonism, might they be attributable to non-specific behavioural inhibition? Again, the evidence seems against such a possibility. SB-277011A, within the dose range used in the present experiments, has been demonstrated to have no effect on locomotor activity or on food- or sucrose-taking behaviour (Heidbreder et al., 2005; Reavill et al., 2000; Vorel et al., 2002) and to not induce sedation or catalepsy (Vorel et al., 2002; Xi et al., 2005). Since expression of nicotine-associated cue-induced LMA or CPP may be presumed to involve encoding and storage of cue-induced associations, plus memory retrieval of such cue-induced associations, the possibility arises that SB-277011A’s inhibition of cue-induced LMA or CPP might be mediated by interference with general aspects of memory storage and retrieval. However, this again seems unlikely, as SB-277011A reverses scopolamine-induced memory deficits as assessed by a three-choice-point water labyrinth test (Laszy et al., 2005) and significantly increases extracellular levels of acetylcholine in the anterior cingulate cortex (Lacroix et al., 2003). Both of these effects would be expected to improve rather than to interfere with memory. Indeed, a comprehensive review of the scientific literature shows that SB-277011A appears to be singularly devoid of nonspecific behavioural effects (Heidbreder et al., 2005), and it is, therefore, unlikely that SB-277011A’s effects in the present study were due to non-specific effects.
The present findings with nicotine should be understood within the context of accumulating evidence that D3 receptor antagonism may be a useful addition to the pharmacotherapeutic armamentarium against addiction. We and others have shown that SB-277011A attenuates: (1) cocaine-enhanced electrical BSR (Vorel et al., 2002); (2) acquisition and expression of cocaine-seeking under second-order reinforcement (Di Ciano et al., 2003); (3) cocaine- or heroin-induced CPP (Ashby et al., 2003; Vorel et al., 2002); (4) cocaine self-administration under progressive ratio and variable-cost/variable-payoff fixed-ratio reinforcement (Gilbert et al., 2003; Xi et al., 2005); (5) cocaine- or nicotine-triggered relapse to drug-seeking behaviour as assessed by the reinstatement model (Vorel et al., 2002; Andreoli et al., 2003b); (6) stress-triggered relapse to cocaine-seeking behaviour as assessed by the reinstatement model (Xi et al., 2004); (7) drug-associated environmental cue-triggered relapse to cocaine-seeking behaviour as assessed by the reinstatement model (Gilbert et al., 2005); (8) oral ethanol intake (Andreoli et al., 2003a; Thanos et al., 2005); and (9) relapse to ethanol-seeking behaviour in animals extinguished from ethanol-taking behaviour (Marcon et al., 2003). Consistent with such findings, the present study shows that SB-277011A, within the same dose range as in those prior experiments, inhibits nicotine-enhanced BSR and two forms of nicotine-induced Pavlovian cue-association learning (LMA, CPP) that may underlie nicotine-associated cue-induced drug-seeking behaviour. It is of note that SB-277011A by itself, within the same dose range, has no effect on BSR (Vorel et al., 2002), does not produce preference or aversion in the CPP paradigm (present data), and does not sustain intravenous self-administration behaviour in rats (Xi et al., 2005). Thus, it may be inferred to lack addictive potential.
In conclusion, the present study demonstrates that selective blockade of DA D3 receptors by SB-277011A inhibits nicotine-enhanced BSR and nicotine-paired cue-induced LMA and CPP. Overall, the present findings suggest that DA D3 receptors play an important role in the neural and behavioural substrates of nicotine addiction, and that selective DA D3 receptor antagonists merit further investigation as anti-nicotine pharmacotherapies, especially as current therapeutic interventions for smoking cessation are inadequate (Vaszar et al., 2002).
Acknowledgements
This research was supported in part by the Intramural Research Program of the U.S. National Institutes of Health, National Institute on Drug Abuse, and in part by GlaxoSmithKline Pharmaceuticals. We thank Dr Roy A. Wise for insightful comments on Pavlovian pairing and the LMA and CPP paradigms. We also thank Enzo Valerio and Paolo Repeto for expert technical and statistical support respectively.
Statement of Interest
Dr Heidbreder and Dr Pilla are employees of GlaxoSmithKline Pharmaceuticals, the developer of SB-277011A. Dr Ashby and Dr Gardner have been recipients of research contract and research grant support from GlaxoSmithKline Pharmaceuticals.
Footnotes
Some of the data presented in this article were previously presented, in abbreviated abstract form, at the 2003 and 2004 annual meetings of the Society for Neuroscience, at the 2004 annual meeting of the College on Problems of Drug Dependence, and at the 2004 annual meeting of the American College of Neuropsychopharmacology.
References
- Andreoli M, Marcon C, Hagan JJ, Heidbreder CA. Effect of selective antagonism of dopamine D3 receptor by SB-277011-A on oral alcohol self-administration in mice. European Neuropsychopharmacology. 2003a;13(Suppl. 1):S17. [Google Scholar]
- Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003b;28:1272–1280. doi: 10.1038/sj.npp.1300183. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Paul M, Gardner EL, Gerasimov MR, Dewey SL, Lennon IC, Taylor SJC. Systemic administration of 1R,4S-4-amino-cyclopent-2-ene-carboxylic acid, a reversible inhibitor of GABA transaminase, blocks expression of conditioned place preference to cocaine and nicotine in rats. Synapse. 2002;44:61–63. doi: 10.1002/syn.10052. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Paul M, Gardner EL, Heidbreder CA, Hagan JJ. Acute administration of the selective D3 receptor antagonist SB-277011A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 2003;48:154–156. doi: 10.1002/syn.10188. [DOI] [PubMed] [Google Scholar]
- Baker DA, Fuchs RA, Specio SE, Khroyan TV, Neisewander JL. Effects of intraaccumbens administration of SCH-23390 on cocaine-induced locomotion and conditioned place preference. Synapse. 1998;30:181–193. doi: 10.1002/(SICI)1098-2396(199810)30:2<181::AID-SYN8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Jain K, Veraldi L, Koob GF, Markou A. A dopamine D1 agonist elevates self-stimulation thresholds: comparison to other dopamine-selective drugs. Pharmacology, Biochemistry, and Behavior. 1999;62:659–672. doi: 10.1016/s0091-3057(98)00206-8. [DOI] [PubMed] [Google Scholar]
- Bauco P, Wise RA. Potentiation of lateral hypothalamic and midline mesencephalic brain stimulation reinforcement by nicotine: examination of repeated treatment. Journal of Pharmacology and Experimental Therapeutics. 1994;271:294–301. [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: dopaminergic and GABAergic influences on conditioned expression. Pharmacology, Biochemistry, and Behavior. 2001;68:135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Nicotine-conditioned locomotor sensitization in rats: assessment of the US-preexposure effect. Behavioural Brain Research. 2003;143:65–74. doi: 10.1016/s0166-4328(03)00009-3. [DOI] [PubMed] [Google Scholar]
- Bézard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, Gross C, Sokoloff P. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nature Medicine. 2003;9:762–767. doi: 10.1038/nm875. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Pudiak CM, KuoLee R. Effect of chronic nicotine on brain stimulation reward. I. Effect of daily injections. Behavioural Brain Research. 1998;96:185–188. doi: 10.1016/s0166-4328(98)00050-3. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Pretreatment with the dopamine agonist 7-OH-DPAT shifts the cocaine self-administration dose-effect function to the left under different schedules in the rat. Behavioural Pharmacology. 1995;6:333–347. [PubMed] [Google Scholar]
- Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz J-C, Sokoloff P. D3 receptor test in vitro predicts decreased cocaine self-administration in rats. Neuroreport. 1997;8:2373–2377. doi: 10.1097/00001756-199707070-00054. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Lacey MG, North RA. Nicotinic excitation of rat ventral tegmental area neurones in vitro studied by intracellular recording. British Journal of Pharmacology. 1989;98:135–140. doi: 10.1111/j.1476-5381.1989.tb16873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KA, Evans G, Gallistel CR. A microcomputer-based method for physiologically interpretable measurement of the rewarding efficacy of brain stimulation. Physiology & Behavior. 1985;35:395–403. doi: 10.1016/0031-9384(85)90315-4. [DOI] [PubMed] [Google Scholar]
- Campos A, Xi Z-X, Gilbert J, Ashby CR, Jr, Heidbreder CA, Newman AH, Gardner EL. Abstracts of the 34th Annual Meeting of the Society for Neuroscience, 2004 Abstract Viewer/Itinerary Planner (abstract 691.6) Washington DC: Society for Neuroscience; 2004. Blockade of dopamine D3 receptors by SB277011A, NGB2904 or BP897 attenuates nicotine-enhanced brain stimulation reward in rat. Published online: http://sfn.scholarone.com/itin2004/index.html. [Google Scholar]
- Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Experimental and Clinical Psychopharmacology. 1999;7:72–78. doi: 10.1037//1064-1297.7.1.72. [DOI] [PubMed] [Google Scholar]
- Caskey NH, Jarvik ME, Wirshing WC, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Eisenberger NI, Huerta L, Terrace SM. Modulating tobacco smoking rates by dopaminergic stimulation and blockade. Nicotine & Tobacco Research. 2002;4:259–266. doi: 10.1080/14622200210153830. [DOI] [PubMed] [Google Scholar]
- Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28:1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D1 antagonists. Proceedings of the National Academy of Sciences USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PBS. Mesolimbic dopamine activation – the key to nicotine reinforcement? In: Bock G, Marsh J, editors. The Biology of Nicotine Dependence (Ciba Foundation Symposium. Vol. 152. New York: Wiley; 1990. pp. 153–168. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Research. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KBJ, Coen KM, Clarke PBS. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Coulombe D, Miliaressis E. Fitting intracranial self-stimulation data with growth models. Behavioral Neuroscience. 1987;101:209–214. doi: 10.1037//0735-7044.101.2.209. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology. 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cussac D, Newman-Tancredi A, Audinot V, Nicolas J-P, Boutin J, Gobert A, Millan MJ. The novel dopamine D3 receptor partial agonist, BP897, is a potent ligand at diverse adrenergic and serotonergic receptors. Society for Neuroscience Abstracts. 2000;26:2154. [Google Scholar]
- de Wit H, Wise RA. Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Canadian Journal of Psychology. 1977;31:195–203. doi: 10.1037/h0081662. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Brodie JD, Gerasimov M, Horan B, Gardner EL, Ashby CR., Jr A pharmacologic strategy for the treatment of nicotine addiction. Synapse. 1999;31:76–86. doi: 10.1002/(SICI)1098-2396(199901)31:1<76::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. European Journal of Pharmacology. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Duarte C, Lefebvre C, Chaperon F, Hamon M, Thie´bot M-H. Effects of a dopamine D3 receptor ligand, BP 897, on acquisition and expression of food-, morphine-, and cocaine-induced conditioned place preference, and food-seeking behavior in rats. Neuropsychopharmacology. 2003;28:1903–1915. doi: 10.1038/sj.npp.1300276. [DOI] [PubMed] [Google Scholar]
- Epperson JF. An Introduction to Numerical Methods and Analysis. New York: Wiley; 2001. [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Ferguson GA. Statistical Analysis in Psychology and Education. 5th edn. New York: McGraw-Hill; 1981. [Google Scholar]
- Gardner EL. What we have learned about addiction from animal models of drug self-administration. American Journal on Addictions. 2000;9:285–313. doi: 10.1080/105504900750047355. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Brain reward mechanisms. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance Abuse: A Comprehensive Textbook. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 48–97. [Google Scholar]
- Gilbert J, Newman AH, Gardner EL, Ashby CR, Jr, Heidbreder CA, Pak AC, Peng X-Q, Xi Z-X. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J, Xi Z-X, Campos A, Ashby CR, Jr, Heidbreder CA, Gardner EL. Abstracts of the 33rd Annual Meeting of the Society for Neuroscience, 2003 Abstract Viewer/Itinerary Planner (abstract 422.10) Washington, DC: Society for Neuroscience; 2003. The dopamine D3 receptor antagonist SB277011A inhibits cocaine reinforcement under fixed-ratio and progressive-ratio schedules. Published online: http://sfn.scholarone.com/itin2003/index.html. [Google Scholar]
- Gyertya´n I, Ga´l K. Dopamine D3 receptor ligands show place conditioning effect but do not influence cocaine-induced place preference. Neuroreport. 2003;14:93–98. doi: 10.1097/00001756-200301200-00018. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DhbE and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology. 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Hagan JJ. Novel pharmacotherapeutic approaches for the treatment of drug addiction and craving. Current Opinion in Pharmacology. 2005;5:107–118. doi: 10.1016/j.coph.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Andreoli M, Marcon C, Thanos PK, Ashby CR, Jr, Gardner EL. Role of dopamine D3 receptors in the addictive properties of ethanol. Drugs of Today. 2004;40:355–365. doi: 10.1358/dot.2004.40.4.820081. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi Z-X, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Research Reviews. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan B, Gardner EL, Ashby CR., Jr Enhancement of conditioned place preference response to cocaine in rats following subchronic administration of 3,4-methylenedioxymethamphetamine (MDMA) Synapse. 2000;35:160–162. doi: 10.1002/(SICI)1098-2396(200002)35:2<160::AID-SYN9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Horan B, Gardner EL, Dewey SL, Brodie JD, Ashby CR., Jr The selective s1 receptor agonist, 1-(3,4-dimethoxyphenethyl)-4-(phenylpropyl) piperazine (SA4503), blocks the acquisition of the conditioned place preference response to (−)-nicotine in rats. European Journal of Pharmacology. 2001;426:R1–R2. doi: 10.1016/s0014-2999(01)01229-8. [DOI] [PubMed] [Google Scholar]
- Horan B, Smith M, Gardner EL, Lepore M, Ashby CR., Jr (–)-Nicotine produces conditioned place preference in Lewis, but not Fischer 344 rats. Synapse. 1997;26:93–94. doi: 10.1002/(SICI)1098-2396(199705)26:1<93::AID-SYN10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Hunt GE, Atrens DM, Jackson DM. Reward summation and the effects of D1 and D2 agonists and antagonists on fixed-interval responding for brain stimulation. Pharmacology, Biochemistry, and Behavior. 1994;48:853–862. doi: 10.1016/0091-3057(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Kornetsky C. Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacology, Biochemistry, and Behavior. 1992;41:755–759. doi: 10.1016/0091-3057(92)90223-3. [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Sarkar M, Kornetsky C. Nicotine and brain-stimulation reward: interactions with morphine, amphetamine and pimozide. Pharmacology, Biochemistry, and Behavior. 1993;46:453–457. doi: 10.1016/0091-3057(93)90378-7. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. Journal of Neuroscience. 1997;17:8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47(Suppl. 1):190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. European Journal of Pharmacology. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Ivanová S, Greenshaw AJ. Nicotine-induced decreases in VTA electrical self-stimulation thresholds: blockade by haloperidol and mecamylamine but not scopolamine or ondansetron. Psychopharmacology. 1997;134:187–192. doi: 10.1007/s002130050441. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Caskey NH, Wirshing WC, Madsen DC, Iwamoto-Schaap PN, Elins JL, Eisenberger NI, Olmstead RE. Bromocriptine reduces cigarette smoking. Addiction. 2000;95:1173–1183. doi: 10.1046/j.1360-0443.2000.95811734.x. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Millan MJ. Dopamine D3 receptor antagonists as therapeutic agents. Drug Discovery Today. 2005;10:917–925. doi: 10.1016/S1359-6446(05)03491-4. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. 2nd edn. Belmont, CA: Brooks/Cole Publishing Company; 1982. [Google Scholar]
- Lacroix LP, Hows MEP, Shah AJ, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors enhances monoaminergic and cholinergic neurotransmission in the rat anterior cingulate cortex. Neuropsychopharmacology. 2003;28:839–849. doi: 10.1038/sj.npp.1300114. [DOI] [PubMed] [Google Scholar]
- Laszy J, Laszlovszky I, Gyertyán I. Dopamine D3 receptor antagonists improve the learning performance of memory-impaired rats. Psychopharmacology. 2005;179:567–575. doi: 10.1007/s00213-004-2096-z. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. Increased dopamine D3 receptor expression accompanying behavioral sensitization to nicotine in rats. Synapse. 2003a;47:176–183. doi: 10.1002/syn.10170. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology. 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005a;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Schwartz J-C, Sokoloff P. Disruption of nicotine conditioning by dopamine D3 receptor ligands. Molecular Psychiatry. 2003b;8:225–230. doi: 10.1038/sj.mp.4001202. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 receptor ligands block nicotine-induced conditioned place preferences through a mechanism that does not involve discriminative-stimulus or antidepressant-like effects. Neuropsychopharmacology. 2005b;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- Lepore M, Liu X, Savage V, Matalon D, Gardner EL. Genetic differences in Δ9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life Sciences [Pharmacology Letters] 1996;58:PL365–PL372. doi: 10.1016/0024-3205(96)00237-8. [DOI] [PubMed] [Google Scholar]
- Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacological Reviews. 1997;49:231–252. [PubMed] [Google Scholar]
- Marcon C, Andreoli M, Pilla M, Tessari M, Heidbreder CA. A new model to assess drug and cue-induced relapse to ethanol self-administration in mice. Behavioural Pharmacology. 2003;14(Suppl. 1):S66. [Google Scholar]
- Mereu G, Yoon K-WP, Boi V, Gessa GL, Naes L, Westfall TC. Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. European Journal of Pharmacology. 1987;141:395–400. doi: 10.1016/0014-2999(87)90556-5. [DOI] [PubMed] [Google Scholar]
- Nakajima S. Subtypes of dopamine receptors involved in the mechanism of reinforcement. Neuroscience and Biobehavioral Reviews. 1989;13:123–128. doi: 10.1016/s0149-7634(89)80020-x. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Liu X, Lau CL. Synergistic interaction of D1 and D2 dopamine receptors in the modulation of the reinforcing effect of brain stimulation. Behavioral Neuroscience. 1993;107:161–165. doi: 10.1037//0735-7044.107.1.161. [DOI] [PubMed] [Google Scholar]
- Nakajima S, O’Regan NB. The effects of dopaminergic agonists and antagonists on the frequency-response function for hypothalamic self-stimulation in the rat. Pharmacology, Biochemistry, and Behavior. 1991;39:465–468. doi: 10.1016/0091-3057(91)90209-k. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences (National Research Council, Commission on Life Sciences, Institute of Laboratory Animal Resources) Guide for the Care and Use of Laboratory Animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. Journal of Medicinal Chemistry. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Schilström B, Hildebrand BE, Panagis G, Grenhoff J, Svensson TH. Role of a7 nicotinic receptors in nicotine dependence and implications for psychiatric illness. Behavioural Brain Research. 2000;113:97–103. doi: 10.1016/s0166-4328(00)00204-7. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA. Examination of GABAergic and dopaminergic compounds in the acquisition of nicotine-conditioned hyperactivity in rats. Neuropsychobiology. 2002;45:87–94. doi: 10.1159/000048682. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Fung EY, Bevins RA. Effects of chronic caffeine pre-exposure on conditioned and unconditioned psychomotor activity induced by nicotine and amphetamine in rats. Behavioural Pharmacology. 2003;14:191–198. doi: 10.1097/00008877-200305000-00002. [DOI] [PubMed] [Google Scholar]
- Panagis G, Kastellakis A, Spyraki C, Nomikos G. Effects of methyllycaconitine (MLA), an a 7 nicotinic receptor antagonist, on nicotine- and cocaine-induced potentiation of brain stimulation reward. Psychopharmacology. 2000;149:388–396. doi: 10.1007/s002130000384. [DOI] [PubMed] [Google Scholar]
- Panagis G, Spyraki C. Neuropharmacological evidence for the role of dopamine in ventral pallidum self-stimulation. Psychopharmacology. 1996;123:280–288. doi: 10.1007/BF02246582. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th edn. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz J-C, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Wise RA. Blockade of D1 dopamine receptors in the ventral tegmental area decreases cocaine reward: possible role for dendritically released dopamine. Journal of Neuroscience. 2001;21:5841–5846. doi: 10.1523/JNEUROSCI.21-15-05841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, Boyfield I, Branch CL, Cilia J, Coldwell MC, et al. Pharmacological actions of a novel, high-affinity, and selective human dopamine D3 receptor antagonist, SB-277011-A. Journal of Pharmacology and Experimental Therapeutics. 2000;294:1154–1165. [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. 6th edn. Belmont, CA: Thomson Brooks/Cole; 2006. [Google Scholar]
- Shippenberg TS, Herz A. Motivational effects of opioids: influence of D-1 versus D-2 receptor antagonists. European Journal of Pharmacology. 1988;151:233–242. doi: 10.1016/0014-2999(88)90803-5. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Nomikos GG, Varonos DD. Intravenous cocaine-induced place preference: attenuation by haloperidol. Behavioural Brain Research. 1987;26:57–62. doi: 10.1016/0166-4328(87)90016-7. [DOI] [PubMed] [Google Scholar]
- Stein L. Effects and interactions of imipramine, chlorpromazine, reserpine, and amphetamine on self-stimulation: possible neurophysiological basis of depression. Recent Advances in Biological Psychiatry. 1962;4:297–311. doi: 10.1007/978-1-4684-8306-2_27. [DOI] [PubMed] [Google Scholar]
- Stein L, Ray OS. Brain stimulation reward ‘thresholds’ self-determined in rat. Psychopharmacologia. 1960;1:251–256. doi: 10.1007/BF00402746. [DOI] [PubMed] [Google Scholar]
- Stemp G, Ashmeade T, Branch CL, Hadley MS, Hunter AJ, Johnson CN, Nash DJ, Thewlis KM, Vong AKK, Austin NE, et al. Design and synthesis of trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]–4-quinolinecarboxamide (SB-277011): a potent and selective dopamine D3 receptor antagonist with high oral bioavailability and CNS penetration in the rat. Journal of Medicinal Chemistry. 2000;43:1878–1885. doi: 10.1021/jm000090i. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Shoaib M. The neurobiology of tobacco addiction. Trends in Pharmacological Sciences. 1991;12:467–473. doi: 10.1016/0165-6147(91)90638-9. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Katana JM, Ashby CR, Jr, Michaelides M, Gardner EL, Heidbreder CA, Volkow ND. The selective dopamine D3 receptor antagonist SB-277011-A attenuates ethanol consumption in ethanol preferring (P) and non-preferring (NP) rats. Pharmacology, Biochemistry, and Behavior. 2005;81:190–197. doi: 10.1016/j.pbb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Progress in Neurobiology. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Vaszar LT, Sarinas PS, Lillington GA. Achieving tobacco cessation: current status, current problems, future possibilities. Respiration (Herrlisheim) 2002;69:381–384. doi: 10.1159/000064014. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. Journal of Neuroscience. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Kuschinsky K. Conditioning of nicotine effects on motility and behaviour in rats. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1989;339:208–213. doi: 10.1007/BF00165145. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proceedings of the National Academy of Sciences of the USA. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicke K, Garcia-Ladona J. The dopamine D3 receptor partial agonist, BP 897, is an antagonist at human dopamine D3 receptors and at rat somatodendritic dopamine D3 receptors. European Journal of Pharmacology. 2001;424:85–90. doi: 10.1016/s0014-2999(01)01054-8. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1962. [Google Scholar]
- Wise RA. Cocaine reward and cocaine craving: the role of dopamine in perspective. NIDA Research Monograph. 1994;145:191–206. [PubMed] [Google Scholar]
- Wise RA, Bauco P, Carlezon WA, Jr, Trojniar W. Self-stimulation and drug reward mechanisms. Annals of the New York Academy of Sciences. 1992;29:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiatric Medicine. 1985;3:445–460. [PubMed] [Google Scholar]
- Wise RA, Gardner EL. Functional anatomy of substance-related disorders. In: D’haenen H, den Boer JA, Willner P, editors. Biological Psychiatry. New York: Wiley; 2002. pp. 509–522. [Google Scholar]
- Wise RA, Leone P, Rivest R, Leeb K. Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse. 1995a;21:140–148. doi: 10.1002/syn.890210207. [DOI] [PubMed] [Google Scholar]
- Wise RA, Marcangione C, Bauco P. Blockade of the reward-potentiating effect of nicotine on lateral hypothalamic brain stimulation by chlorisondamine. Synapse. 1998;29:72–79. doi: 10.1002/(SICI)1098-2396(199805)29:1<72::AID-SYN6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology. 1995b;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre´ P-P. Brain dopamine and reward. Annual Review of Psychology. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wood MD, Boyfield I, Nash DJ, Jewitt FR, Avenell KY, Riley GJ. Evidence for antagonist activity of the dopamine D3 receptor partial agonist, BP 897, at human dopamine D3 receptor. European Journal of Pharmacology. 2000;407:47–51. doi: 10.1016/s0014-2999(00)00732-9. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost – variable-payoff fixed-ratio cocaine self-administration in rats. European Journal of Neuroscience. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans J, Baptista M. Both nicotinic and muscarinic receptors in ventral tegmental area contribute to brain-stimulation reward. Pharmacology, Biochemistry, and Behavior. 1997;57:915–921. doi: 10.1016/s0091-3057(96)00467-4. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Attenuation of intravenous amphetamine reinforcement by central dopamine blockade in rats. Psychopharmacology. 1976;48:311–318. doi: 10.1007/BF00496868. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yokoo H, Tanaka T, Mizoguchi K, Emoto H, Ishii H, Tanaka M. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1993;187:547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]