Abstract
Hepatitis C viral protease inhibitors increase sustained virologic response rates compared to interferon and ribavirin but also add side effects. Telaprevir and boceprevir are structurally similar, and share cross-resistant mutations. This case report highlights successful management of telaprevir skin rash and anal discomfort by switching to boceprevir.
Keywords: hepatitis C virus, telaprevir, boceprevir, toxicity, duration
Hepatitis C virus (HCV) genotype 1 has historically been difficult to treat, with clinical trial success rates of pegylated interferon (peginterferon), and ribavirin much lower than for genotypes 2 or 3 (SVR in 46% vs 76%, respectively) [1]. Fortunately, new protease inhibitors are increasing efficacy, but side effects can result in treatment interruptions and failures. In treatment-naive noncirrhotic patients, sustained virologic response (SVR) rates of 75% and 66% have been shown for telaprevir-based and boceprevir-based regimens, respectively [2]. However, in one study, 56% of patients receiving telaprevir experienced a rash, 29% experienced anal discomfort (hemorrhoids, pain, or pruritus), and 17% discontinued therapy prematurely due to side effects, compared to only 4% in the placebo arm [3].
While minor side effects associated with telaprevir do not require telaprevir discontinuation, 5% of patients develop a severe rash that necessitates stopping telaprevir immediately. Drug-related eosinophilia and severe systemic illness (DRESS) and Stevens-Johnson syndrome (SJS) are life-threatening reactions that occur rarely with telaprevir [4]. The transition point from tolerable to life-threatening reactions is indistinct. Telaprevir and boceprevir are both linear α-ketoamides with striking molecular similarities. Cross-reaction is possible, especially when substituting one structurally similar drug for another, as in the case of protease inhibitors. Various β-lactams can exhibit cross-reactivity or molecular mimicry [5], but data for HCV protease inhibitors are unavailable.
The side-effect profile of protease inhibitors can cause alterations in the treatment plan. If telaprevir is discontinued before week 8, the effect on SVR and optimal treatment duration becomes unclear. While response-guided therapy can lead to SVR in 6 months, even 6 months may be unattainable for patients experiencing side effects. However, viral kinetic modeling suggests that only 12 weeks of telaprevir/peginterferon/ribavirin (T/P/R) therapy may be sufficient in compliant patients if the viral decline is rapid [6]. Challenging this modeling data is the result from the 12-week arm of the Protease Inhibition for Viral Evaluation (PROVE) 1 study of telaprevir, suggesting that <24 weeks of therapy is not optimal for all patients [7].
Presented here is a patient with a telaprevir rash and eosinophilia, who had resolution of dermatologic side effects after switching to boceprevir and achieved SVR with only 15 weeks of therapy.
CASE REPORT
The patient was a 58-year-old white man with chronic hepatitis C infection of >25 years’ duration. He was treatment naive and obese (body mass index = 38 kg/m2). A liver biopsy 5 years prior showed grade 2 scarring and stage 1 inflammation. Baseline labs showed an alanine aminotransferase level of 162 (normal, 0–65), platelet level of 225 (normal, 160–370), hemoglobin level of 14.6 (normal, 13.6–17.2), and a normal creatinine level, and interleukin 28B (IL-28B) testing at rs12979860 revealed he was heterozygous C/T. The patient's baseline viral load was 2.4 million IU/mL (Cobas TaqMan) and typed as genotype 1b (for protease gene sequence see Supplementary Figure 1). The patient was started on treatment for HCV with 750 mg of telaprevir every 8 hours, 180 ug peginterferon every week, and 1200 mg ribavirin every day (T/P/R).
After 8 days of T/P/R, the patient developed a rash over his legs and abdomen (Figure 1). His viral load decreased to 66 International Units/mL. He had no fever, no vesicular lesions, and no mucosal lesions to suggest a complicated drug reaction. Because his rash was mild, all 3 drugs were continued. The rash was treated conservatively with topical 1% cortisone cream and diphenhydramine orally as needed for pruritus.
Figure 1.
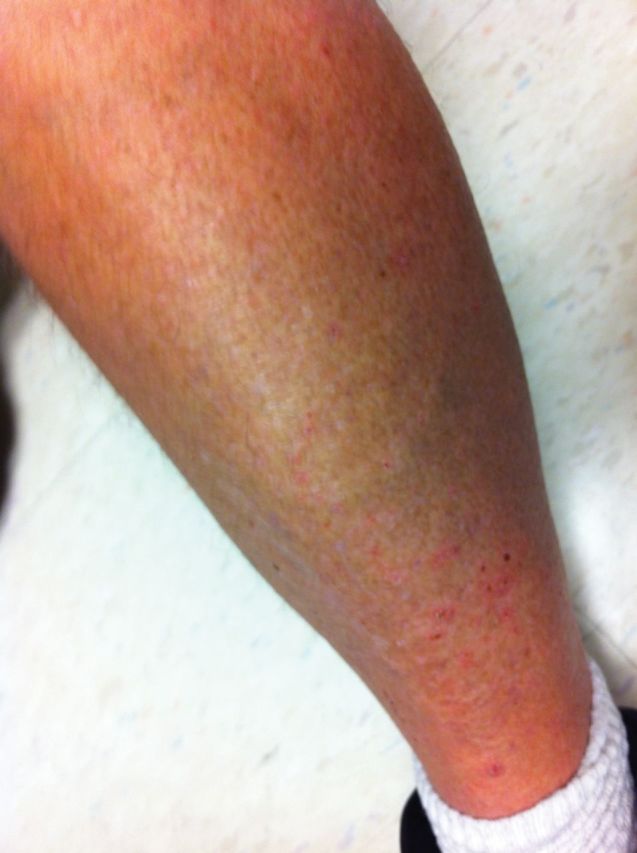
Case patient's rash 8 days into treatment course.
After 4 weeks of T/P/R, the patient developed severe rectal pain and bleeding. At that time his rash was unchanged and HCV RNA was undetectable (Supplementary Figure 2). Monitoring of his eosinophils revealed an increase to 9% (absolute eosinophil count of 480). Despite conservative measures (sitz baths, hemorrhoid creams), the patient's rectal pain became intolerable.
At this point the patient wanted to stop telaprevir; however, his odds of achieving SVR on dual therapy were thought to be suboptimal owing to his C/T IL28B polymorphism. Therefore, he was changed from telaprevir to boceprevir and continued on peginterferon and ribavirin (B/P/R). A prior authorization for boceprevir was placed before telaprevir was discontinued to avoid an interruption in protease inhibitor therapy. Despite the continued use of a chemically similar protease inhibitor, the itching, skin rash, and anal pain resolved in <1 week, along with normalization of eosinophil count over the next 2 weeks. Other than mild anemia (hemoglobin nadir 12), he did not develop problems clearly associated with boceprevir.
At week 10 of therapy the patient developed worsening attacks of chest discomfort, shortness of breath, and anxiety after interferon injections. Cardiopulmonary workup was unrevealing. Symptoms would dissipate about 2–3 days after an injection, but were slightly more severe with each injection. The patient did not want to start any new medications for symptomatic treatment. Ultimately all 3 drugs were stopped after 15 weeks of total therapy for HCV (5 weeks T/P/R and 10 weeks B/P/R). HCV RNA remained undetectable throughout the 10 weeks of B/P/R therapy and remained negative 20 and 28 weeks after all therapy was stopped.
DISCUSSION
Approximately 56% of patients will develop rash on telaprevir but only 5% of patients develop a severe rash [2]. In most cases the rash is mild to moderate and telaprevir can be continued safely [8]. For a severe rash, classified by vesicular lesions, involvement of >50% of body surface area, or any signs of SJS or DRESS, telaprevir should be stopped immediately (Supplementary Table 1) [9]. The case patient here did not yet have DRESS but he was clearly intolerant of telaprevir with a persistent rash, rising eosinophil count, and severe rectal pain necessitating a change in therapy.
In the PROVE 1 trial, patients who received triple therapy for only 12 weeks achieved SVR rates of approximately 35% [7]. Considering the case patient's race and IL-28B haplotype C/T, his chance of achieving SVR was 33% with dual therapy, compared to 69% had he been C/C [2]. On the other hand, the rate of SVR in C/T haplotypes for boceprevir-based therapy is 71% [2]. Because of the concern for potential relapse on dual therapy, we chose to lengthen time on triple therapy by switching telaprevir for boceprevir. This change was both safe and effective. The side effects he developed from telaprevir resolved with this change, he did not have any cross-reactive side effects with boceprevir, and he achieved SVR with only 15 weeks of total therapy despite his IL-28B C/T status. Although we cannot rule out the possibility that the patient would have achieved SVR without boceprevir, this would likely have required >15 weeks of interferon therapy based on his IL-28B C/T polymorphism.
Once a divergence from current protease inhibitor therapy guidelines is made, optimal duration of therapy becomes unclear. What is clear is that an approximately 10% viral relapse between week 12 and 24 occurs when telaprevir is used according to current guidelines, attributable to telaprevir no longer being part of the regimen [7]. Because telaprevir is not approved for >12 weeks of therapy, secondary to increasing toxicity beyond 12 weeks, this rate of viral relapse cannot be lowered by extended telaprevir duration. On the other hand, in patients who are converted from telaprevir to boceprevir, virologic control may be preserved. While the optimal duration of protease inhibitor therapy in this circumstance is unknown, the combined duration of protease inhibitor therapy in this patient, who achieved SVR, was 15 weeks. This is shorter than a response-guided 28-week boceprevir regimen but longer than a 12-week telaprevir/P/R that performed poorly in the PROVE 1 trial [7]. Remarkably, the amount of interferon needed to achieve SVR in this patient was minimal (15 doses, compared to 24 doses or more in most treatment regimens).
Rapid virologic response (RVR) and having a genotype 1b virus (rather than 1a) likely allowed our patient to achieve SVR with only 15 weeks of therapy. In the ADVANCE trial, RVR (undetectable viral load at 4 weeks) was associated with SVR, even if patients only had 8 weeks of telaprevir (SVR in 78% of patients with RVR, compared to 51% in patients with detectable virus at 4 weeks) [10]. In both the 8-week arm and the 12-week arm, viral relapse was more common in genotype 1a vs 1b. The case patient's genotype 1b virus likely had a higher barrier to resistance than most genotype 1a viruses (genotype 1b requires 2 mutations for protease inhibitor resistance, compared to genotype 1a, which requires only 1 mutation) [11]. Although there is a case report of a genotype 1b responding to telaprevir monotherapy [12], the HCV isolate reported here did not have the Q195K substitution suggested as potentially responsible for the monotherapy success [11].
The treatment of HCV is lengthy with a side-effect profile reviewed here. Genetic characterization of HCV treatment failures and successes may aid in future decisions regarding optimal length of therapy. This case highlights the concept that in patients with RVR, a telaprevir to boceprevir substitution may be successful for drug intolerance and may decrease the length of therapy needed to achieve SVR.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the Office of Research and Development, Biomedical Laboratory R&D Service, Department of Veterans Affairs.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–71. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 2.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–44. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butt AA, Kanwal F. Boceprevir and telaprevir in the management of hepatitis C virus-infected patients. Clin Infect Dis. 2012;54:96–104. doi: 10.1093/cid/cir774. [DOI] [PubMed] [Google Scholar]
- 4.Montaudié H, Passeron T, Cardot-Leccia N, Sebbag N, Lacour JP. Drug rash with eosinophilia and systemic symptoms due to telaprevir. Dermatology (Basel) 2010;221:303–5. doi: 10.1159/000318904. [DOI] [PubMed] [Google Scholar]
- 5.Frumin J, Gallagher JC. Allergic cross-sensitivity between penicillin, carbapenem, and monobactam antibiotics: what are the chances? Ann Pharmacother. 2009;43:304–15. doi: 10.1345/aph.1L486. [DOI] [PubMed] [Google Scholar]
- 6.Guedj J, Perelson AS. Second-phase hepatitis C virus RNA decline during telaprevir-based therapy increases with drug effectiveness: implications for treatment duration. Hepatology. 2011;53:1801–8. doi: 10.1002/hep.24272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827–38. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- 8.Incivek (Telaprevir manufacturer) A guide to help you assess and manage rash. 2012. Available at: http://www.incivek.com/hcp/assess-and-manage-rash . Accessed 1 August 2012. [Google Scholar]
- 9.Picard O, Cacoub P. Dermatological adverse effects during genotype-1 hepatitis C treatment with the protease inhibitors telaprevir and boceprevir. Patient management. Clin Res Hepatol Gastroenterol. 2012;36:437–40. doi: 10.1016/j.clinre.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 11.Kieffer TL, Sarrazin C, Miller JS, et al. Telaprevir and pegylated interferon–alpha-2a inhibit wild-type and resistant genotype 1 hepatitis C virus replication in patients. Hepatology. 2007;46:631–9. doi: 10.1002/hep.21781. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki F, Suzuki Y, Akuta N, et al. Sustained virological response in a patient with chronic hepatitis C treated by monotherapy with the NS3-4A protease inhibitor telaprevir. J Clin Virol. 2010;47:76–8. doi: 10.1016/j.jcv.2009.09.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


