Abstract
Peptidylgycine α-amidating monooxygenase (PAM), a highly conserved copper-dependent enzyme, is essential for the synthesis of all amidated neuropeptides. Biophysical studies revealed that the binding of copper to PAM affects its structure, and cell biological studies demonstrated that the endocytic trafficking of PAM was sensitive to copper. We review data indicating that genetic reduction of PAM expression and mild copper deficiency in mice cause similar alterations in several physiological functions known to be regulated by neuropeptides - thermal regulation, seizure sensitivity and anxiety-like behavior.
Keywords: amidation, peptide, nutrition
Key features of peptidergic systems
Highly conserved throughout evolution, peptides and their receptors assumed major regulatory roles in multicellular organisms like Planaria, Hydra, Schistosoma, and Drosophila (Asada et al., 2005; Grimmelikhuijzen et al., 1996; Kolhekar et al., 1997; Mair et al., 2004; Vos et al., 1995). The neuropeptides share a biosynthetic pathway with distinct differences from that of the small molecule transmitters (Fig.1). The mRNAs encoding prepropeptides are translated in the cell soma. Like other mRNAs encoding secreted proteins, they include an N-terminal signal sequence that is removed co-translationally. The propeptide is then modified by the same machinery used for the synthesis of integral membrane proteins and other secreted proteins to ensure proper folding, disulfide bond formation, phosphorylation and glycosylation.
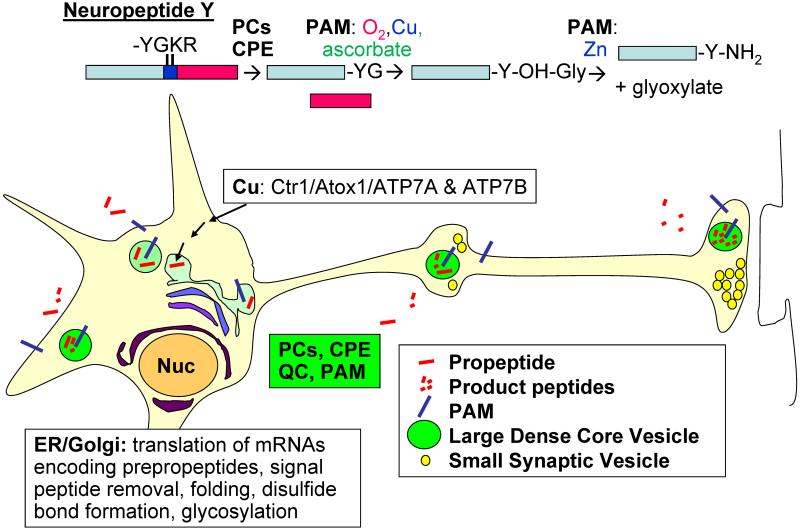
Fig. 1. Pathways for production, storage and secretion of peptides in neurons.
The biosynthetic pathway for a model neuropeptide, neuropeptide Y, is outlined with the necessary enzymatic steps. The key transporters/chaperones involved in providing copper to PAM are indicated. The subcellular locations at which different events occur are indicated.
The number of enzymes specialized for the production of neuropeptides is small (Fig.1). Prohormone convertases 1 and 2 (PC1, PC2) are subtilisin-like endoproteases with a prominent role in proneuropeptide cleavage (Fig.1). Carboxypeptidase E (CPE), glutaminyl cyclase (QC) and PAM are the other enzymes uniquely associated with neuropeptide synthesis. CPE removes basic amino acids from the C-terminus of the fragments generated by PC1 and PC2. If a Gly remains at the C-terminus, PAM uses molecular oxygen to catalyze its α-hydroxylation and subsequently cleaves the peptidyl-α-hydroxyglycine intermediate, amidating the penultimate amino acid and generating glyoxylate (Fig.1). QC catalyzes the cyclization of N-terminal Gln (Booth et al., 2003; Schilling et al., 2003). The pH of the luminal compartment plays an essential role in controlling propeptide processing and both PC1 and PC2 are activated in a low pH-dependent manner; until the PCs do their job, CPE, QC and PAM cannot do theirs.
Release of peptides from large dense core vesicles (LDCVs; also known as secretory granules) occurs in dendrites, the cell soma, along the axon and at the nerve terminal (Fig.1). Exocytosis of LDCVs requires a stronger stimulus (higher intracellular [Ca+2]) than release of small synaptic vesicles and many neuropeptides must diffuse some distance before encountering the receptors that identify their targets. Since the synthesis, storage and secretion of all neuropeptides require a common set of enzymes, manipulation of the genes encoding these enzymes has provided insight into the most sensitive elements of the peptidergic system.
PAM is essential for life
More than half of all neuropeptides must be α-amidated to be bioactive (Eipper et al., 1992; Eipper and Mains, 1988; In et al., 2001; Merkler, 1994). PAM (EC 1.14.17.3) is the only enzyme known to catalyze this reaction. In Drosophila, deletion of the PAM gene results in the absence of amidated peptides and in lethality at an early larval stage (Jiang et al., 2000; Kolhekar et al., 1997). Similarly, elimination of the murine PAM gene results in the absence of enzymatic activity and in lethality at mid-gestation (Czyzyk et al., 2005). The complete absence of amidation activity in PAM null mice and flies supports the argument that PAM is the only enzyme capable of post-translationally amidating peptides. PAM null mice bear a striking resemblance to adrenomedullin null mice, with severe edema, cardiac hypertrophy and thin arterial walls; adrenomedullin, a powerful hypotensive factor, must be amidated to be active (Caron and Smithies, 2001; Hay and Smith, 2001).
While mice lacking PAM do not survive gestation, mice lacking functional PC1, PC2 or CPE are viable (Dhanvantari and Brubaker, 1998; Hardiman et al., 2005; Jackson et al., 2003; Lloyd et al., 2006; Muller and Lindberg, 1999; Naggert et al., 1995; Woronowicz et al., 2009). For enzymes that function sequentially in a biosynthetic pathway, elimination of an upstream enzyme often has a more pronounced effect than elimination of a downstream enzyme. The fact that this is not true for these peptide biosynthetic enzymes may reflect the ability of other enzymes to substitute for PC1, PC2 or CPE and the inability of any other enzyme to substitute for PAM.
The binding of copper to PAM allows it to function as a copper sensor
Amidation occurs in two steps: peptidylglycine α-hydroxylating monooxygenase (PHM), which binds two copper ions, catalyzes the stereospecific hydroxylation of the α-carbon of the peptidylglycine substrate (Merkler et al., 1992; Noguchi et al., 1992; Prigge et al., 2000); peptidyl-α-hydroxyglycine α-amidating lyase (PAL) then catalyzes cleavage of the N-Cα bond, producing glyoxylate and the bioactive amidated peptide (Fig. 1). The individual PHM and PAL catalytic cores secreted by stably transfected CHO cells were purified and crystallized, providing high resolution structures for both enzymes (Chufan et al., 2009; Prigge et al., 2000; Prigge et al., 2004; Siebert et al., 2005). PHM has two domains connected by a single strand; each domain contains one copper binding site and the sites are separated by a large, solvent filled cleft. Copper is the only metal that supports peptide amidation; no other metal can substitute. The N-terminal CuH site is formed from three His residues, two of which are adjacent, and most resembles copper binding sites used for electron transfer (Prigge et al., 1997; Prigge et al., 1999; Siebert et al., 2005). The C-terminal CuM site is formed from two His residues and a Met (Met314) and binds molecular oxygen. Copper and iron are the two metals in biology that routinely participate in reactions which include splitting molecular oxygen.
The peptidylglycine substrate binds near CuM, with its α-carbon positioned to interact with molecular oxygen bound end-on to CuM. In each reaction cycle, both copper residues must be reduced from Cu(II) to Cu(I), a process normally catalyzed by ascorbic acid; the details of how CuH contributes to the reaction remain controversial (Chen et al., 2004; Francisco et al., 2003; Prigge et al., 1999; Prigge et al., 2004; Siebert et al., 2005). Mutation of Met314 to Ile inactivated the enzyme (Siebert et al., 2005). Analysis of this mutant revealed widespread structural effects, with alterations in the Gly299-Glu313 loop that precedes Met314 and in the CuH site. Despite its essential role, copper is not tightly bound to PHM (~0.06 μM); copper bound to PHM is lost during purification, necessitating the addition of exogenous copper to optimize catalytic activity (Bell et al., 2003). Coupled with the exposed nature of the CuH and CuM sites, this means that copper can easily be removed from and added back to PHM. PHM is unusual amongst cuproenzymes in using copper to fulfill both major structural and catalytic roles.
Crystallization of PALcc, which contains molar equivalents of Zn(II) and Ca(II), revealed a six-bladed β-propeller with a central cavity (Chufan et al., 2009). Ca(II), which sits in the middle of the central cavity, plays a structural role. The active site sits in a cup formed by the loops connecting various β-strands. The active site includes a Zn(II) ion coordinated to three His residues. A catalytically essential Tyr654 (De et al., 2006) and Arg706 are situated in close proximity to the Zn(II). When substrate binds, the α-hydroxyl group is coordinated to the Zn(II). Divalent cation chelators inactivate PAL, increasing its protease and thermal sensitivity (Kolhekar et al., 2002). The β-propeller is constructed in such a way that the N- and C-terminal ends of PALcc are latched together. In full-length PAM, this arrangement forces the monooxygenase domain close to the secretory granule membrane. Since both copper and reducing equivalents must be delivered across the secretory granule membrane, this may be a particularly efficient way in which to facilitate the reaction.
PAM has both catalytic and non-catalytic functions
In vertebrates, the PAM gene encodes both PHM and PAL (Glauder et al., 1990; Ouafik et al., 1990; Vos et al., 1995). In Drosophila, Planaria, Schistoma and Hydra, PHM is encoded by a gene that does not encode PAL (Asada et al., 2005; Kolhekar et al., 1997; Mair et al., 2004). Alternative splicing of the mammalian PAM gene generates isoforms that differ in two important ways. In PAM-1, an endoprotease-sensitive linker domain (Exon 16) separates PHM from PAL; both PAM-1 and PAM-2 include a transmembrane domain and a cytosolic domain (CD) (Fig. 2) (Ciccotosto et al., 2000). Lacking the region encoded by Exon 16, the PHM and PAL domains of PAM-2 are rarely separated by proteolytic cleavage. PAM-3, which lacks Exon 16, also lacks the transmembrane domain (Exon 25) and is a soluble, secreted protein (Ciccotosto et al., 2000) (Fig. 2). The presence of a unique exon in the human PAM gene results in the production of two additional splice variants, but proteins equivalent to PAM-1, PAM-2 and PAM-3 predominate (Glauder et al., 1990; Vos et al., 1995).
Fig. 2. Major splice variants and products of PAM processing.
The proteins encoded by the 3 major PAM splice variants (PAM-1, -2, and -3) are shown above the dotted line: paired basic cleavage sites are indicated by vertical black ovals; the pro-region is removed early in the biosynthetic pathway; the transmembrane domain (TMD) is marked. The major protein products resulting from intragranular, cell surface and endosomal endoproteolytic processing of PAM-1 are shown below the dotted line; cleavage sites are marked by red arrows. The major fate of each isoform or cleavage product is indicated to the right of the diagram.
Tissue-specific endoproteolytic cleavage of PAM-1 generates soluble PHM and PAL, which are stored in large dense core vesicles and secreted upon stimulation (Fig. 2). Although their source is not clear, both PHM and PAL can be assayed in rodent and human serum (Mains et al., 1985; Wand et al., 1985) and are found in human cerebrospinal fluid (Gonzalez et al., 2009). In rats, serum levels of PAM activity were unaltered following hypophysectomy, adrenalectomy, sialectomy, or castration. Serum levels of PAM activity rose after thyroidectomy and declined after treatment with the ganglionic blocker chlorisondamine (Mains et al., 1985).
The sequence of the cytosolic domain of PAM is almost as well conserved as the sequences of PHM and PAL (Eipper et al., 1992). Initial investigations demonstrated an important role for the cytosolic domain in the entry of membrane PAM into LDCVs and in the recycling of membrane PAM deposited on the cell surface during exocytosis (Milgram et al., 1997) (Fig. 3). Using a rat hippocampal cDNA library and the yeast 2-hybrid system, three PAM-CD interactor proteins were identified (Alam et al., 1996): P-CIP1, a Ras association domain family member (Chen et al., 1998); Uhmk1 (P-CIP2), a Ser/Thr protein kinase which phosphorylates Ser949 in the PAM cytosolic domain and alters the endocytic trafficking of PAM (Caldwell et al., 1999; Steveson et al., 2001); and Kalirin, a Rho GDP/GTP exchange factor with multiple spectrin-like repeats. Trio, a Kalirin paralogue, was later identified as a PAM-CD interactor (Xin et al., 2005). In corticotrope tumor cells, Kalirin affects immature secretory granule formation (Ferraro et al., 2007), cytoskeletal organization (Alam et al., 1997; Mains et al., 1999) and the exocytosis of mature granules (Mains et al., 1999). Over-expression of PAM alone increased constitutive secretion of POMC products from pituitary cells, and eliminated regulated secretion; co-expression of both PAM and Kalirin restored and enhanced regulated secretion in corticotrope tumor cells (Mains et al., 1999). Using a corticotrope tumor cell line engineered so that expression of PAM-1 could be induced with doxycycline, PAM-1 was shown to cause alterations in cytoskeletal organization, hormone secretion and gene expression (Ciccotosto et al., 1999; Francone et al., 2010).
Fig. 3. The interactions of copper with PAM storage, processing and secretion.
The catalytic role of PAM (producing amidated product peptides) and the non-catalytic roles of PAM (affecting cytoskeletal organization, altering regulated secretion, responding to ambient copper levels) are diagramed. Amidated peptides generally act on target tissues by binding to G-Protein Coupled Receptors (GPCRs).
A soluble fragment of the PAM cytosolic domain (sf-CD) may play a key role in the ability of PAM to alter cell function (Figs. 2) (Rajagopal et al., 2009). A proteolytic cleavage that occurs within the transmembrane domain of PAM is thought to release sf-CD into the cytosol (Fig. 3). This proteasome-sensitive fragment was identified in the cytosol of PAM-1 AtT-20 cells and in pituitary and atrial nuclei. Consistent with this, fluorescently-tagged recombinant PAM-CD injected into the cytosol of AtT-20 cells is rapidly concentrated in the nucleus (Francone et al., 2010). Like the cleaved cytosolic domains of SREBP (Matthews et al., 2009), ATF6 (Tsukumo et al., 2007) and ICA512 (Trajkovski et al., 2004), sf-CD released from membrane PAM may serve as a means of conveying information about the status of the secretory pathway to the nucleus.
PAM is one of a small number of eukaryotic cuproenzymes
Copper dependent enzymes evolved with the appearance of molecular oxygen in the atmosphere (Crichton and Pierre, 2001; Ridge et al., 2008). In the absence of oxygen, copper was largely insoluble and not available for use in biological systems (Crichton and Pierre, 2001). In bacteria, cytochrome c oxidase (COX) is the most common, and often the only, cuproenzyme. On average, 0.3% of the proteins in a eukaryotic proteome bind copper but only about 25 enzymes require copper for activity (Andreini et al., 2008; Ridge et al., 2008). Eukaryotes retained ancient cuproenzymes and added new cuproenzymes like ceruloplasmin, hephaestin, several lysyl oxidases and a family of enzymes related to PAM (Andreini et al., 2008; Prigge et al., 2000); all function in the lumen of the secretory pathway or outside the cell. Included in the PHM family are dopamine β-monooxygenase (DBM or DBH, dopamine β-hydroxylase) (Blackburn et al., 1991; Klinman, 2008), monooxygenase X (MOX) and DBHL (Xin et al., 2005). DBM plays an essential role in the synthesis of both norepinephrine and epinephrine (Klinman, 2008), but the functions of MOX and DBHL remain unknown.
PHM appeared with the development of multicellular organisms, which rely on intercellular communication and need to be able to supply molecular oxygen and copper to their cells. Copper deficiency disrupts normal microvascular control mechanisms (Lominadze et al., 2004) and is often accompanied by cardiac hypertrophy (Prohaska et al., 2003; Prohaska et al., 2005; Prohaska and Broderius, 2006). If we look at PHM as a member of the copper proteome whose function is to enable the use of molecular oxygen, we gain a markedly different perspective on factors that might regulate PHM and non-catalytic functions that the larger PAM protein might have acquired. A recent study on hypoxia lends credence to this possibility. In rats, intermittent hypoxia caused an increase in brainstem PHM activity and an increase in tissue levels of immunoreactive Substance P and NPY, amidated peptides thought to play a role in obstructive sleep apnea (Sharma et al., 2009; Veasey, 2009). The increase in PHM activity resulted from increased proteolytic cleavage of the PAM precursor, which was triggered by an increase in reactive oxygen species (Sharma et al., 2009). PAM, which produces signaling molecules released into the vasculature, is perhaps uniquely equipped to detect and respond to changes in copper and molecular oxygen availability.
Lacking copper, apoceruloplasmin (Holtzman and Gaumnitz, 1970) and apohephaestin (Nittis and Gitlin, 2004) are degraded more rapidly than the corresponding holoenzyme. In copper-depleted corticotrope tumor cells, endocytosed PAM-1 is less likely to be degraded and is instead returned to secretory granules, where it can undergo endoproteolytic cleavage, leading to an increase in PHM secretion (De et al., 2007). Conversely, copper-loaded cells are more likely to degrade PAM after internalization, leading to a decrease in the secretion of soluble PHM. Copper availability affects both the non-catalytic and catalytic functions of PAM (Fig. 3). The fact that the conformation of PHM is sensitive to copper (Bell et al., 2003; Siebert et al., 2005) may play a key role in the ability of copper to affect the endocytic trafficking of PAM. Altered endocytic trafficking would be expected to affect both the generation of sf-CD, which signals to the nucleus, and the secretion of PHM. PAM is thus well equipped to serve as a sensor of copper levels.
Genetic and dietary copper deficiency have widespread effects on the nervous system
In the central nervous system, ATP7A, a P-type ATPase, is largely responsible for transporting Cu(I) into the lumen of the secretory pathway, where it can be used by cuproenzymes like PAM and DBM (Fig. 1). ATP7A receives Cu(I) from Atox1, a cytoplasmic copper chaperone; the roles of the many transporters and chaperones involved in the uptake and distribution of dietary copper are summarized in recent reviews (Goldstein et al., 2009; Kaler et al., 2008; Kim et al., 2008; Madsen and Gitlin, 2007; Walker et al., 2004). Mutations of ATP7A cause Menkes Disease, with low levels of copper in the brain and severe neurodegeneration (Bahi-Buisson et al., 2006; Goldstein et al., 2009; Kaler et al., 2008; Tumer and Horn, 1997). Seizures and hypothermia are major clinical features of Menkes disease (Al-Bitar et al., 2005; Bahi-Buisson et al., 2006; Maury et al., 2007; Ozawa et al., 2002). In the mottled/brindled mouse, a model for Menkes Disease, both peptide amidation (Niciu et al., 2007; Steveson et al., 2003; Strausak et al., 2001) and norepinephrine production (Goldstein et al., 2009; Kaler et al., 2008) are compromised. Levels of amidated joining peptide, cholecystokinin and PACAP are reduced in a peptide and brain-region specific manner in these mutant mice (Niciu et al., 2007; Steveson et al., 2003). Purkinje cell synaptogenesis and axon extension are compromised in this mouse model of Menkes disease (Niciu et al., 2007). Increased expression of ATP7A in the capillary endothelial cells of mottled/brindled mice is observed, along with increased association of astrocytes and microglia with the blood-brain barrier (Niciu et al., 2007).
Using a zebrafish model, Gitlin and coworkers uncovered a continuum of essential copper-dependent processes that vary in both their sensitivity to copper deprivation and in the developmental time periods and tissues in which they are essential (Madsen and Gitlin, 2007; Madsen and Gitlin, 2008; Mendelsohn et al., 2006). The most sensitive mutations were calamity, a mutation in the Menkes protein ATP7A, and catastrophe, a mutation in the Atp6v0d1 subunit that links the cytosolic complex of the vesicular proton pump to its partner, a membrane complex. Although a similar range of sensitivities to copper deprivation almost certainly exists in mammals, there is no consensus on biomarkers that can reliably identify mild copper deficiency. The fact that PAM requires copper to function suggests that functions dependent on amidated neuropeptides can be compromised through a combination of genetic and dietary deficits.
Most Americans consume a level of copper greater than the minimal recommended dietary intake. Major dietary sources of copper include shellfish, white potatoes, tomatoes, nuts, dark chocolate, and beer. The recommended dietary intake of copper for adult men and women is 900 μg copper per day (Scientific Committee on Food and European Commission on Health and Consumer Protection Directorate, 2003). The median intake of dietary copper reported in the US National Health and Nutrition Survey and Continuing Survey of Food Intakes ranged from 1200 and 1600 μg copper per day, with upper levels of 8000 and 10,000 μg copper per day (Scientific Committee on Food and European Commission on Health and Consumer Protection Directorate, 2003). Malabsorption following bariatric surgery and prolonged intravenous feeding or parenteral nutrition can cause copper deficiency (Tokuda et al., 2006; von Drygalski and Andris, 2009). Copper deficiency due to overuse of denture creams containing zinc may cause permanent neuropathies and may be more widespread than previously believed (AssociatedPress, 2009; Sanders, 2009).
Copper plays a critical role in the early post-natal development of rodents, and severe dietary copper deficiency during this time period results in low body weight, cardiac hypertrophy and decreased survival (Penland and Prohaska, 2004; Prohaska and Wells, 1974). ATP7A expression in the central nervous system peaks around postnatal day 4 and mottled/brindled mice die within 2 weeks of birth. Consistent with this, the ability of copper supplementation to ameliorate the deficits associated with Menkes Disease or the deficits apparent in mottled/brindled mice requires delivery early in development (Christodoulou et al., 1998; Kaler, 1998; Nagara et al., 1981; Sheela et al., 2005).
In adults, copper deficiency causes ataxia, peripheral neuropathy, hyperexcitability/seizures and may contribute to Alzheimer disease (Bahi-Buisson et al., 2006; Bayer et al., 2006; Hung et al., 2009; Kessler et al., 2006; Strausak et al., 2001; Tan et al., 2006). The cardiovascular system is affected; cardiac hypertrophy, hypertension, elevated cholesterol, myocardial infarction and coronary artery disease are associated with copper deficiency (Klevay, 2000a; Klevay, 2000b; Prohaska et al., 2005). Other symptoms include hypothermia, glucose intolerance, and anemia/ liver iron accumulation (Al-Bitar et al., 2005; Maury et al., 2007; Ozawa et al., 2002; Prohaska and Broderius, 2006; Pyatskowit and Prohaska, 2008). Neuropeptides are known to play key roles in many of these processes, including thermoregulation, seizure sensitivity and control of glucose metabolism.
PAM heterozygosity causes behavioral deficits
Given that PAM null mice are not viable, we examined PAM heterozygotes (PAM+/−) more closely. As expected, tissues from PAM+/− mice contained reduced levels of amidation activity. PAM+/− mice exhibit normal motor ability, body weight and reproductive behavior (Czyzyk et al., 2005). Mild glucose intolerance and increased adiposity are apparent in old PAM+/− mice. Based on immunoassays specific for TRH-Gly and TRH, inactive TRH-Gly accumulates in the paraventricular nucleus and preoptic area of PAM+/− mice, but significant amounts of TRH are still present (Bousquet-Moore et al., 2009a). We reasoned that further analysis of PAM+/− mice could provide a means of identifying peptidergic pathways with the least “safety margin”. Based on deficits associated with Menkes Disease and copper deficiency, we selected thermoregulation, anxiety-like behavior and seizure-sensitivity for our initial studies. Despite the rather modest changes observed in peptide amidation in PAM+/− mice, behavioral deficits were readily apparent.
Core body temperature is one of the best guarded biological constants (Silva, 2006). When placed into a cold environment (4°C for 2 h), PAM+/− mice are unable to thermoregulate, largely due to an impairment in cold-induced vasoconstriction of peripheral vessels (Bousquet-Moore et al., 2009a). Impaired ability to produce and secrete amidated peptides like NPY and TRH could contribute to the phenotype observed (Dark and Pelz, 2008; Pelz and Dark, 2007) (Hwa et al., 1999). Pretreatment with NPY enhances the cold-induced vasoconstriction associated with sympathetic excitation in ex vivo studies of the rabbit central ear artery (Padilla et al., 1997; Shintani et al., 2005). Elderly individuals often exhibit deficits in cold-induced peripheral vasoconstriction, a process which relies heavily on peptidergic transmission (Stephens et al., 2004).
PAM+/− mice exhibit increased anxiety-like behavior (Bousquet-Moore et al., 2009b). Anxiety-like behavior is thought to be altered by changes in neuronal excitation and inhibition in the basolateral amygdala, specifically through modulation of a distinct population of peptide-expressing interneurons (Truitt et al., 2009). Amongst the amidated peptides known to diminish anxiety-like behavior are NPY (Broqua et al., 1995) and TRH (Gutierrez-Mariscal et al., 2008). PAM+/− mice exhibit increased sensitivity to pentylenetetrazole (PTZ) induced seizures (Bousquet-Moore et al., 2009b). PAM is most prevalent in GABAergic interneurons (Ma et al., 2008), which exert their inhibitory effects through PTZ-sensitive GABAA receptors and through GABAB receptors (Sperk et al., 2004). A number of amidated peptides have been implicated in the modulation of neural excitation, inhibition and seizures. PreproTRH mRNA levels in the dentate gyrus of the hippocampus, frontal and occipital cortex and striatum of rats were profoundly increased following seizure induction with kainate (Jaworska Feil et al., 1999a; Jaworska Feil et al., 1999b). Intranasal delivery of a TRH analog (3-methyl-histidine TRH) shortly before amygdalar stimulation attenuated the resulting seizures in electrically-kindled rats (Veronesi et al., 2007). TRH has been found to reduce excitation by inhibiting potassium-stimulated glutamate and aspartate release from hippocampal slices in vitro (Nie et al., 2005). Levels of NPY mRNA increase in the hippocampus after acute and repeated electroconvulsive shock. Levels of NPY peptide also increase following repeated electroconvulsive shock, accumulating in mossy fibers and dentate granule cell dendrites (Ma et al., 2002). Both acute and chronic seizures are suppressed following virally mediated increases in NPY expression in the hippocampus (Richichi et al., 2004). NPY can diffuse to non-activated neighboring excitatory synapses and decrease glutamate release in a paracrine fashion (Sorensen et al., 2008; Sorensen et al., 2009). NPY activates G-protein coupled inwardly rectifying potassium currents, dampening excitability within the lateral amygdala (Sosulina et al., 2008). Understanding whether any of these specific mechanisms contribute to the seizure sensitivity observed in PAM+/− mice will require detailed electrophysiological studies of both hippocampus and amygdala.
A number of SNPs have been identified in the coding and non-coding regions of the human PAM gene (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=5066), and genetic studies of experimental animals link alcohol and nicotine use to changes in PAM expression (Jahng et al., 1997; Sandberg et al., 2000). Whether these mutations in human PAM affect mRNA or protein expression will require further study, but the fact that a two-fold drop in PAM expression is of functional significance in our mouse model suggests that further study is warranted.
In vivo gene-nutrient interactions between PAM and copper
Given the ease with which behavioral deficits were identified in the PAM+/− mice, we reasoned that these same peptidergic systems might be sensitive to dietary copper intake. In order to explore the interaction of dietary copper with peptide amidation under physiologically relevant conditions, we exposed adult PAM+/− mice and their WT littermate controls to a mildly copper deficient diet (0.6 ppm vs 16 ppm Cu for two weeks) or to a normal diet supplemented or not with 70 ppm Cu in the drinking water (Bousquet-Moore et al., 2009a; Bousquet-Moore et al., 2009b). The results of a similar experiment using synthetic diets differing in copper content are shown in Fig. 4. Growth of male and female mice was unaltered and all mice were healthy (Fig. 4A). Ceruloplasmin levels dropped about 3-fold, consistent with creation of mild copper deficiency (Fig. 4B). Levels of TRH-Gly increased in copper deficient WT mice, but were not diminished when PAM+/− mice were supplied with supplemental copper (Bousquet-Moore et al., 2009b).
Fig. 4. PAM+/− and copper deficient WT mice exhibit similar deficits in thermoregulation and anxiety-like behavior.
Equal numbers of WT male and female mice were maintained for 8 weeks on a synthetic, copper-replete diet (16 ppm Cu; Teklad # TD.09301) or a synthetic copper-deficient diet (no copper included, but otherwise identical; Teklad #TD.97190) before experimentation. A. Body weights for male and female mice kept on the normal and copper-deficient diets were indistinguishable. B. Serum ceruloplasmin activity fell to below half of control values for both WT and PAM+/− mice on the copper deficient diet (p<0.0005 for both genotypes); pooled data for males and females. C. WT mice on the Cu-deficient diet spent significantly less time in the open arms of the elevated zero maze (EZM; 5 min) than did WT mice on the control diet; the behavior of the PAM+/− mice was not affected by the copper deficient diet; N=10-11 mice per diet (p<0.0005); pooled data for males and females. D. Core body temperatures taken after a 2 h exposure to 4°C are shown. WT mice kept on the normal diet maintained core body temperature during a 2 hour exposure to 4°C; when kept on the Cu-deficient diet, their core body temperature dropped significantly (*: one-way ANOVA, p=0.014). PAM+/− mice kept on the control diet were less able than WT mice to maintain body temperature in the cold (**: one-way ANOVA, p=0.001). Copper deficiency did not further impair temperature regulation in PAM+/− mice; pooled data for males and females.
Both PAM+/− mice and WT mice kept on the copper-deficient diet for 8 weeks demonstrate an increase in anxiety-like behavior, as seen by decreased time in the open arm of the elevated zero maze (Fig. 4C). The increased anxiety-like behavior observed in PAM+/− mice was not exacerbated by the copper-deficient diet, but was eliminated when drinking water that contained supplemental copper was provided (Bousquet-Moore et al., 2009b). Copper deficient WT mice and PAM+/− mice spent similar amounts of time in the open arm of the elevated zero maze.
When placed into a 4°C environment for 2 h, PAM+/− mice and WT mice kept on the copper-deficient diet for 8 weeks exhibited similar impairments in their ability to maintain body temperature (Fig. 4D). The decreased ability of PAM+/− mice to thermoregulate was not further exacerbated by the copper-deficient diet, but was eliminated when supplemental copper was provided in the drinking water (Bousquet-Moore et al., 2009b). The impaired peripheral vasoconstriction observed in PAM+/− mice was reversed when supplemental copper was provided (Bousquet-Moore et al., 2009a). Copper deficiency impaired cold-induced vasoconstriction in both WT and PAM+/− mice (Bousquet-Moore et al., 2009b).
Like PAM+/− mice, copper-deficient WT mice exhibit increased sensitivity to PTZ-induced seizures (Bousquet-Moore et al., 2009b). Unlike anxiety-like behavior and thermoregulation, providing supplemental copper to PAM+/− mice did not diminish their altered sensitivity to PTZ (Bousquet-Moore et al., 2009b).
PAM and copper homeostasis
As hypothesized, supplementary dietary copper ameliorated some of the deficits observed in PAM+/− mice (Bousquet-Moore et al., 2009a; Bousquet-Moore et al., 2009b). However, the extent to which copper-mediated changes in peptide amidation are responsible for these effects is not at all clear since dietary copper availability will affect all cuproenzymes. The modest effects of PAM heterozygosity on peptide amidation led us to explore two additional mechanisms through which alterations in PAM expression could affect function. Studies of purified PHM and cell lines expressing membrane PAM revealed an effect of copper on the conformation of PHM and on the endocytic trafficking of membrane PAM. These studies led us to consider the possibility that PAM could function as a copper sensor. Alterations in dietary copper that led to changes in PAM cleavage, trafficking and secretion could play an essential role in adjusting to copper availability.
Copper deficient WT mice had higher levels of serum PAM activity than WT mice on the control diet (Bousquet-Moore et al., 2009b). An increase in serum PAM activity in copper deficient mice was predicted by the effects of copper on PAM trafficking and endoproteolytic cleavage in AtT-20 corticotrope tumor cells (De et al., 2007). PAM+/− mice had lower levels of serum PAM activity than WT mice and were less sensitive to copper deficiency. In addition, dietary copper deficiency altered the metabolism of PAM in atrial myocytes, resulting in increased cleavage of PAM-1 and decreased levels of PAM activity.
Using a corticotrope tumor cell line in which expression of PAM-1 can be increased by the addition of doxycycline, we identified a set of PAM-responsive transcripts (Francone et al., 2010). Included in the set of corticotrope transcripts whose levels are increased by PAM expression are three cytosolic copper chaperones (Atox1, CCS and Cox17) along with Ctr1 and ATP7A; expression of cuproenzymes like SOD1 and SOD3 was not affected (Ciccotosto et al., 1999; Francone et al., 2010). qPCR analysis of RNA prepared from the pituitaries of WT and PAM+/− mice revealed higher levels of Atox1 and Cox17 in WT pituitaries, as predicted from the studies in PAM-1 AtT-20 cells. Tissue specific changes of this type may underlie the slight increase in liver copper levels observed in PAM+/− mice (Bousquet-Moore et al., 2009b). While the mechanisms through which PAM alters gene expression have not been elucidated, sf-CD, the cytosolic fragment of PAM identified in pituitary and atrial nuclei (Rajagopal et al., 2009) is likely to play a key role. Taken together, current data support the hypothesis that PAM is a physiologically relevant copper sensor.
Reference List
- Al-Bitar Y, Azam JS, Azam T. Menke’s kinky hair syndrome-a rare medical condition. J. Pak. Med. Assoc. 2005;55:40–42. [PubMed] [Google Scholar]
- Alam MR, Caldwell BD, Johnson RC, Darlington DN, Mains RE, Eipper BA. Novel proteins that interact with the COOH-terminal cytosolic routing determinants of an integral membrane peptide-processing enzyme. J. Biol. Chem. 1996;271:28636–28640. doi: 10.1074/jbc.271.45.28636. [DOI] [PubMed] [Google Scholar]
- Alam MR, Johnson RC, Darlington DN, Hand TA, Mains RE, Eipper BA. Kalirin, a cytosolic protein with spectrin-like and GDP/GTP exchange factor-like domains that interacts with peptidylglycine α-amidating monooxygenase, an integral membrane peptide-processing enzyme. J. Biol. Chem. 1997;272:12667–12675. doi: 10.1074/jbc.272.19.12667. [DOI] [PubMed] [Google Scholar]
- Andreini C, Banci L, Bertini I, Rosato A. Occurence of copper proteins through the three domains of life: a bioinformatic approach. J. Proteome Res. 2008;7:209–216. doi: 10.1021/pr070480u. [DOI] [PubMed] [Google Scholar]
- Asada A, Orii H, Watanabe K, Tsubaki M. Planarian peptidylglycine-hydroxylating monooxygenase, a neuropeptide processing enzyme, colocalizes with cytochrome b561 along the central nervous system. FEBS J. 2005;272:942–955. doi: 10.1111/j.1742-4658.2004.04528.x. [DOI] [PubMed] [Google Scholar]
- AssociatedPress AP Overuse of denture cream with zinc sparks lawsuits. N. Y. Times. 2009 Sept.18,2009. [Google Scholar]
- Bahi-Buisson N, Kaminska A, Nabbout R, Barnerias C, Desquere I, De Lonlay P, Mayer M, Plouin P, Dulac O, Chiron C. Epilepsy in Menkes disease: analysis of clinical stages. Epilepsia. 2006;47:380–386. doi: 10.1111/j.1528-1167.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Schafer S, Breyhan H, Wirths O, Treiber C, Multhaup G. A vicious circle: role of oxidative stress, intraneuronal Abeta and Cu in Alzheimer’s disease. Clin. Neuropathol. 2006;25:163–171. [PubMed] [Google Scholar]
- Bell J, El Meskini R, D’Amato D, Mains RE, Eipper BA. Mechanistic investigation of peptidylglycine alpha-hydroxylating monooxygenase via intrinsic tryptophan fluorescence and mutagenesis. Biochemistry. 2003;42:7133–7142. doi: 10.1021/bi034247v. [DOI] [PubMed] [Google Scholar]
- Blackburn NJ, Hasnain SS, Pettingill TM, Stranger RW. Copper K-extended X-ray absorption fine structure studies of oxidized and reduced dopamine beta-hydroxylase. J Biol. Chem. 1991;266:23127. [PubMed] [Google Scholar]
- Booth RE, Misquitta SA, Bateman RC. Human pituitary glutaminyl cyclase: expression in insect cells and dye affinity purification. Protein Expr. Purif. 2003;32:141–146. doi: 10.1016/S1046-5928(03)00226-2. [DOI] [PubMed] [Google Scholar]
- Bousquet-Moore D, Ma XM, Nillni EA, Czyzyk TA, Pintar JE, Eipper BA, Mains RE. Reversal of physiological deficits caused by diminished levels of peptidylglycine alpha-amidating monooxygenase by dietary copper. Endocrinology. 2009a;150:1739–1756. doi: 10.1210/en.2008-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet-Moore D, Prohaska JR, Nillni EA, Czyzyk TA, Wetsel WC, Mains RE, Eipper BA. Interactions of Peptide Amidation and Copper: Novel Biomarkers and Mechanisms of Neural Dysfunction. Neurobiol. Dis. 2009b doi: 10.1016/j.nbd.2009.09.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broqua P, Wettstein JG, Rocher MN, Gauthier-Martin B, Junien JL. Behavioral effects of Neuropeptide Y receptor antagonists in the elevated plus-maze and fear-potentiated startle procedures. Behav. Pharmacol. 1995;6:215–222. [PubMed] [Google Scholar]
- Caldwell BD, Darlington DN, Penzes P, Johnson RC, Eipper BA, Mains RE. The novel kinase P-CIP2 interacts with the cytosolic routing determinants of the peptide processing enzyme peptidylglycine α-amidating monooxygenase. J Biol. Chem. 1999;274:34646–34656. doi: 10.1074/jbc.274.49.34646. [DOI] [PubMed] [Google Scholar]
- Caron KM, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional adrenomedullin gene. Proc. Natl. Acad. Sci. U. S. A. 2001;98:615–619. doi: 10.1073/pnas.021548898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Johnson RC, Milgram SL. P-CIP1, a novel protein that interacts with the cytosolic domain of peptidyglycine α-amidating monooxygenase, is associated with endosomes. J Biol. Chem. 1998;273:33524–33532. doi: 10.1074/jbc.273.50.33524. [DOI] [PubMed] [Google Scholar]
- Chen P, Bell J, Eipper BA, Solomon EI. Oxygen activation by the noncoupled binuclear copper site in peptidylglycine alpha-hydroxylating monooxygenase: spectroscopic definition of the resting sates and the putative CuIIM-OOH intermediate. Biochemistry. 2004;43:5735–5747. doi: 10.1021/bi0362830. [DOI] [PubMed] [Google Scholar]
- Christodoulou J, Danks DM, Sarkar B, Baerloucher KE, Casey R, Horn N, Tumer Z, Clarke JT. Early treatment of Menkes disease with parenteral copper-histidine: long-term follow-up of four treated patients. Am. J Med. Genet. 1998;72:154–164. [PubMed] [Google Scholar]
- Chufan EE, De M, Eipper BA, Mains RE, Amzel LM. Amidation of bioactive peptides: the structure of the lyase domain of the amidating enzyme. Structure. 2009;17:1–9. doi: 10.1016/j.str.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccotosto GD, Hand TA, Mains RE, Eipper BA. Breeding stock-specific variation in PAM mRNA splicing in rat pituitary. Endocrinology. 2000;141:476–486. doi: 10.1210/endo.141.2.7337. [DOI] [PubMed] [Google Scholar]
- Ciccotosto GD, Schiller MR, Eipper BA, Mains RE. Induction of integral membrane PAM expression in AtT-20 cells alters the storage and trafficking of POMC and PC1. J. Cell Biol. 1999;144:459–471. doi: 10.1083/jcb.144.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton RR, Pierre JL. Old iron, young copper: from Mars to Venus. Biometals. 2001;14:99–112. doi: 10.1023/a:1016710810701. [DOI] [PubMed] [Google Scholar]
- Czyzyk TA, Ning Y, Hsu MS, Peng B, Mains RE, Eipper BA, Pintar JE. Deletion of peptide amidation enzymatic activity leads to edema and embryonic lethality in the mouse. Dev. Biol. 2005;287:301–313. doi: 10.1016/j.ydbio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Dark J, Pelz KM. NPY Y1 receptor antagonist prevents NPY-induced torporlike hypothermia in cold-acclimated Siberian hamsters. Am. J Physiol. Reg. Integr. Comp. Physiol. 2008;294:R236–R245. doi: 10.1152/ajpregu.00587.2007. [DOI] [PubMed] [Google Scholar]
- De M, Ciccotosto GD, Mains RE, Eipper BA. Trafficking of a secretory granule membrane protein is sensitive to copper. J. Biol. Chem. 2007;282:23362–23371. doi: 10.1074/jbc.M702891200. [DOI] [PubMed] [Google Scholar]
- De M, Bell J, Blackburn NJ, Mains RE, Eipper BA. Role for an essential tyrosine in peptide amidation. J Biol. Chem. 2006;281:20873–20882. doi: 10.1074/jbc.M513886200. [DOI] [PubMed] [Google Scholar]
- Dhanvantari S, Brubaker PL. Proglucagon processing in an islet cell line: effects of PC1 overexpression and PC2 depletion. Endocrinology. 1998;139:1630–1637. doi: 10.1210/endo.139.4.5936. [DOI] [PubMed] [Google Scholar]
- Eipper BA, Mains RE. Peptide alpha-amidation. Annu. Rev. Physiol. 1988;50:333–344. doi: 10.1146/annurev.ph.50.030188.002001. [DOI] [PubMed] [Google Scholar]
- Eipper BA, Stoffers DA, Mains RE. The biosynthesis of neuropeptides: peptide alpha-amidation. Annu. Rev. Neurosci. 1992;15:57–85. doi: 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- Ferraro F, Ma XM, Sobota JA, Eipper BA, Mains RE. Kalirin/Trio Rho guanine nucleotide exchange factors regulate a novel step in secretory granule maturation. Mol. Biol. Cell. 2007;18:4813–4825. doi: 10.1091/mbc.E07-05-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco WA, Blackburn NJ, Klinman JP. Oxygen and hydrogen isotope effects in an active site tyrosine to phenylalanine mutant of peptidylglycine alpha-hydroxylating monooxygenase: mechanistic implications. Biochemistry. 2003;42:1813–1819. doi: 10.1021/bi020592t. [DOI] [PubMed] [Google Scholar]
- Francone VP, Ifrim MF, Rajagopal C, Wang Y, Carson JH, Mains RE, Eipper BA. Signaling from the secretory granule to the nucleus: PAM and Uhmk1. Mol. Endocrinol. 2010 doi: 10.1210/me.2009-0381. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauder J, Ragg H, Rauch J, Engels JW. Human peptidylglycine α–amidating monooxygenase: cDNA, cloning, and expression of a truncated form in COS cells. Biochem. Biophys. Res. Commun. 1990;169:551–558. doi: 10.1016/0006-291x(90)90366-u. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Holmes CS, Kaler SG. Relative efficiencies of plasma catechol levels and ratios for neonatal diagnosis of menkes disease. Neurochem. Res. 2009;34:1464–1468. doi: 10.1007/s11064-009-9933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H, Ottervald J, Nilsson KC, Sjogren N, Olsson T, Franzen B. Identification of novel candidate protein biomarkers for the post-polio syndrome - Implications for diagnosis, neurodegeneration and neuroinflammation. J. Proteomics. 2009;71:670–681. doi: 10.1016/j.jprot.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Grimmelikhuijzen CJP, Leviev I, Carstensen K. Peptides in the Nervous Systems of Cnidarians: Structure, Function and Biosynthesis. Int Rev Cytology. 1996;167:37–89. doi: 10.1016/s0074-7696(08)61345-5. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Mariscal M, de Gortari P, Lopez-Rubaicava C, Joseph-Bravo P. Analysis of the anxiolytic-like effect of TRH and the response of amygdalar TRHergic neurons in anxiety. Psychoneuroendocrinology. 2008;33:198–213. doi: 10.1016/j.psyneuen.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Hardiman A, Friedman TC, Grunwald WC, Furuta M, Zhu Z, Steiner DF, Cool DR. Endocrinomic profile of neurointermediate lobe pituitary prohormone processing in PC1/3 and PC2-null mice using SELDI-TOF mass spectrometry. J Mol. Endocrinol. 2005;34:751. doi: 10.1677/jme.1.01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DL, Smith DM. Knockouts and transgenics confirm the importance of adrenomedullin in the vasculature. Trends Pharmacol. Sci. 2001;22:57–59. doi: 10.1016/s0165-6147(00)01617-5. [DOI] [PubMed] [Google Scholar]
- Holtzman NA, Gaumnitz BM. Studies of the rate of release and turnover of ceruloplasmin and apoceruloplasmin in rat plasma. J Biol. Chem. 1970;245:2354–2358. [PubMed] [Google Scholar]
- Hung YH, Robb EL, Volitakis I, Ho M, Cherny RA, Bush AI. Paradoxical condensation of copper with elevated beta-amyloid in lipid rafts under cellular copper deficiency conditions: implications for Alzheimer disease. J. Biol. Chem. 2009;284:21899–21907. doi: 10.1074/jbc.M109.019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa J, Witten M, Williams P, Ghibaudi L, Gao J, Salisbury B, Mullins D, Hamud F, Strader CD, Parker EM. Activation of the NPY Y5 receptor regulates both feeding and energy expenditure. Am. J Physiol. Reg. Integr. Comp. Physiol. 1999;277:R1428–R1434. doi: 10.1152/ajpregu.1999.277.5.R1428. [DOI] [PubMed] [Google Scholar]
- In Y, Fujii M, Sasada Y, Ishida T. Structural studies on C-amidated amino acids and peptides: structures of hydrochloride salts of C-amidated Ile-Val, Thr, Ser, Met, Trp, Gln, and Arg, and comparison with their C-unamidated counterparts. Acta Crystallographica - Section B, Structural Science. 2001;203:631–639. doi: 10.1107/s0108768100013975. [DOI] [PubMed] [Google Scholar]
- Jackson RS, Creemers JWM, Farooqui S, Raffin-Sanson ML, Varro A, Dockary GJ, Holst JJ, Brubaker PL, Corvol P, Polonsky KS, Ostrega D, Becker KL, Bertagna X, Hutton JC, White A, Dattani MT, Hussain K, Middleton SJ, Nicole TM, Milla PJ, Lindley KJ, O’Rahilly S. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J Clin. Invest. 2003;112:1550–1560. doi: 10.1172/JCI18784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng JW, Houpt TA, Joh TH, Wessell TC. Expression of catecholamine-synthesizing enzymes, peptidylglycine alpha-amidating monooxygenase, and neuropeptide mRNA in the rat adrenal medulla after acute systemic nicotine. J. Mol. Neurosci. 1997;8:45–52. doi: 10.1007/BF02736862. [DOI] [PubMed] [Google Scholar]
- Jaworska Feil L, Turchan J, Przewlocka B, Budziszewska B, Leskiewicz M, Lason W. Effects of pentylenetetrazole-induced kindling on thyrotropin-releasing hormone biosynthesis and receptors in rat brain. Neuroscience. 1999a;90:695–704. doi: 10.1016/s0306-4522(98)00446-1. [DOI] [PubMed] [Google Scholar]
- Jaworska Feil L, Turchan J, Przewlocka B, Budziszewska B, Leskiewicz M, Lason W. Effects of pilocarpine- and kainate-induced seizures on thyrotropin-releasing hormone biosynthesis and receptors in the rat brain. J. Neural Transm. 1999b;106:395–407. doi: 10.1007/s007020050167. [DOI] [PubMed] [Google Scholar]
- Jiang N, Kolhekar AS, Jacobs PS, Mains RE, Eipper BA, Taghert PH. PHM is required for normal developmental transitions and for biosynthesis of secretory peptides in drosophila. Dev. Biol. 2000;226:118–136. doi: 10.1006/dbio.2000.9832. [DOI] [PubMed] [Google Scholar]
- Kaler SG. Diagnosis and therapy of Menkes syndrome, a genetic form of copper deficiency. Am. J Clin. Nutr. 1998;67:1029S–1034S. doi: 10.1093/ajcn/67.5.1029S. [DOI] [PubMed] [Google Scholar]
- Kaler SG, Holmes CS, Goldstein DS, Tang J, Godwin SC, Patronas N. Neonatal diagnosis and treatment of Menkes disease. N. Engl. J. Med. 2008;358:605–614. doi: 10.1056/NEJMoa070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler H, Pajonk FG, Meisser P, Schneider-Axmann T, Hoffman KH, Supprian T, Herrman W, Obeid R, Multhaup G, Falkai P, Bayer TA. Cerebrospinal fluid diagnostic markers correlate with lower plasma copper and ceruloplasmin in patients with Alzheimer’s disease. J Neural. Transm. 2006;113:1763–1769. doi: 10.1007/s00702-006-0485-7. [DOI] [PubMed] [Google Scholar]
- Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008;4:176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- Klevay LM. Cardiovascular disease from copper deficiency - a history. J Nutr. 2000a;130:489S–492S. doi: 10.1093/jn/130.2.489S. [DOI] [PubMed] [Google Scholar]
- Klevay LM. Dietary copper and risk of coronary heart disease. Am. J Clin. Nutr. 2000b;71:1213–1214. doi: 10.1093/ajcn/71.5.1213. [DOI] [PubMed] [Google Scholar]
- Klinman JP. The copper-family of dopamine β-monooxygenase and peptidylglycine α-amidating. J Biol. Chem. 2008;281:3016. doi: 10.1074/jbc.R500011200. [DOI] [PubMed] [Google Scholar]
- Kolhekar AS, Bell J, Shiozaki EN, Jin L, Keutmann HT, Hand TA, Mains RE, Eipper BA. Essential features of the catalytic core of peptidyl-α-hydroxyglycine α-amidating lyase. Biochemistry. 2002;41:12384–12394. doi: 10.1021/bi0260280. [DOI] [PubMed] [Google Scholar]
- Kolhekar AS, Roberts MS, Jiang N, Johnson RC, Mains RE, Eipper BA, Taghert PH. Neuropeptide amidation in drosophila: Separate genes encode the two enzymes catalyzing amidation. J. Neurosci. 1997;17:1363–1376. doi: 10.1523/JNEUROSCI.17-04-01363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DJ, Bohan S, Gekakis N. Obesity, hyperphagia, and increased metabolic efficiency in Pc1 mutant mice. Hum. Mol. Genet. 2006;15:1884–1893. doi: 10.1093/hmg/ddl111. [DOI] [PubMed] [Google Scholar]
- Lominadze D, Saari JT, Pericival SS, Schuschke DA. Proinflammatory effects of copper deficiency on neutrophils and lung endothelial cells. Immunology and Cell Biol. 2004;82:238. doi: 10.1046/j.1440-1711.2004.01231.x. [DOI] [PubMed] [Google Scholar]
- Ma XM, Mains RE, Eipper BA. Plasticity in hippocampal peptidergic systems induced by repeated electroconvulsive shock. Neuropsychopharmacology. 2002;27:55–71. doi: 10.1016/S0893-133X(02)00284-1. [DOI] [PubMed] [Google Scholar]
- Ma XM, Wang Y, Ferraro F, Mains RE, Eipper BA. Kalirin-7 Is an Essential Component of both Shaft and Spine Excitatory Synapses in Hippocampal Interneurons. J. Neurosci. 2008;28:711–724. doi: 10.1523/JNEUROSCI.5283-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu. Rev. Neurosci. 2007;30:317–337. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- Madsen E, Gitlin JD. Zebrafish mutants calamity and catastrophe define critical pathways of gene-nutrient interactions in developmental copper metabolism. PLOS Genet. 2008;4:1–10. doi: 10.1371/journal.pgen.1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains RE, Alam MR, Johnson RC, Darlington DN, Back N, Hand TA, Eipper BA. Kalirin, a multifunctional PAM COOH-terminal domain interactor protein, affects cytoskeletal organization and ACTH secretion from AtT-20 cells. J. Biol. Chem. 1999;274:2929–2937. doi: 10.1074/jbc.274.5.2929. [DOI] [PubMed] [Google Scholar]
- Mains RE, Myers AC, Eipper BA. Hormonal, drug, and dietary factors affecting peptidyl glycine alpha-amidating monooxygenase activity in various tissues of the adult male rat. Endocrinology. 1985;116:2505–2515. doi: 10.1210/endo-116-6-2505. [DOI] [PubMed] [Google Scholar]
- Mair GR, Niciu MJ, Stewart MT, Brennan G, Omar H, Halton DW, Mains RE, Eipper BA, Maule AG, Day TA. A functionally atypical amidating enzyme from the human parasite Schistosoma mansoni. FASEB J. 2004;18:114–121. doi: 10.1096/fj.03-0429com. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Kunte AS, Tambe-Ebot E, Rawson RB. Alternative Activation of SREBP During Larval Development in Drosophila melanogaster. Genetics. 2009;181:119–128. doi: 10.1534/genetics.108.093450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury A, Payen V, Toutain A, Guiraud P, Saliba E, Labarthe F. Neonatal onset of Menkes disease: diagnosis interest of cupremia and microscopic examination of the hairs. Arch. Pediatr. 2007;14:1216–1218. doi: 10.1016/j.arcped.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Mendelsohn BA, Yin C, Johnson SL, Wilm TP, Solnica-Krezel L, Gitlin JD. ATP7a determines a hierarchy of copper metabolism essential for notochord development. Cell Metab. 2006;4:155–162. doi: 10.1016/j.cmet.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Merkler DJ. C-terminal amidated peptides: Production by the in vitro enzymatic amdiation of glycine-extended peptides and the importance of the amide to biological activity. Ezyme Microb. Technol. 1994;16:450–456. doi: 10.1016/0141-0229(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Merkler DJ, Kulathila R, Consavo AP, Young SD, Ash DE. 18O isotopic 13C NMR shift as proof th bifunctional peptidylglycine α-amidating enzyme is a monooxygenase. Biochemistry. 1992;31:7282–7288. doi: 10.1021/bi00147a011. [DOI] [PubMed] [Google Scholar]
- Milgram SL, Kho ST, Martin GV, Mains RE, Eipper BA. Localization of integral membrane peptidylglycine α-amidating monooxygenase in neuroendocrine cells. J. Cell Sci. 1997;110:695–706. doi: 10.1242/jcs.110.6.695. [DOI] [PubMed] [Google Scholar]
- Muller L, Lindberg I. The cell biology of the prohormone convertases PC1 and PC2. Prog. Nucleic Acid Res. Mol. Biol. 1999;63:69–108. doi: 10.1016/s0079-6603(08)60720-5. [DOI] [PubMed] [Google Scholar]
- Nagara H, Yajima K, Suzuki K. The effect of copper supplementation on the brindled mouse. J. Neuro. Exp. Neurol. 1981;40:428–446. doi: 10.1097/00005072-198107000-00006. [DOI] [PubMed] [Google Scholar]
- Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH. Hyperinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat. Genet. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Ma XM, El Meskini R, Pachter JS, Mains RE, Eipper BA. Altered ATP7a expression and other compensatory responses in a murine model of Menkes disease. Neurobiol. Dis. 2007;27:278–291. doi: 10.1016/j.nbd.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, Shoepp DD, Klaunig JG, Yard M, Lahiri DK, Kubek MJ. Thyrotropin-releasing hormone (protirelin) inhibits potassium-stimulated glutamate and aspartate release from hippocampal slices in vitro. Brain Res. 2005;1054:45–54. doi: 10.1016/j.brainres.2005.06.077. [DOI] [PubMed] [Google Scholar]
- Nittis T, Gitlin JD. Role of copper in the proteosome-mediated degradation of the multicopper oxidase hephaestin. J Biol. Chem. 2004;279:25696–25702. doi: 10.1074/jbc.M401151200. [DOI] [PubMed] [Google Scholar]
- Noguchi H, Seino H, Kochi H, Okamoto H, Tanaka T, Hirama M. The source of the oxygen aton in the α-hydroxyglycine intermediate of the peptidylglycine α-amidating reaction. Biochem J. 1992;283:883–888. doi: 10.1042/bj2830883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouafik LH, May V, Saffen DW, Eipper BA. Thyroid hormone regulation of peptidylglycine α-amidating monooxygenase expression in anterior pituitary gland. Mol. Endocrinol. 1990;4:1497–1505. doi: 10.1210/mend-4-10-1497. [DOI] [PubMed] [Google Scholar]
- Ozawa H, Nakamoto N, Kodama H. Clinical manifestations for early diagnosis of the patient with classical Menkes disease. No To Hattatsu. 2002;34:387–390. [PubMed] [Google Scholar]
- Padilla J, Garcia-Villalon AL, Monge L, Garcia JL, Fernandez N, Gomez B, Diequez G. Peptidergic modulation of the sympathetic contraction in the rabbit ear artery: effects of temperature. Br. J Pharmacol. 1997;121:21–28. doi: 10.1038/sj.bjp.0701094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelz KM, Dark J. ICV NPY Y1 agonist but not Y5 agonist induces torpor-like hypothermia in cold-acclimated Siberian hamsters. Am. J Physiol. Reg. Integr. Comp. Physiol. 2007;292:R2299–R2311. doi: 10.1152/ajpregu.00790.2006. [DOI] [PubMed] [Google Scholar]
- Penland JG, Prohaska JR. Abnormal motor function persists following recovery from perinatal copper deficiency in rats. J. Nutr. 2004;134:1984–1988. doi: 10.1093/jn/134.8.1984. [DOI] [PubMed] [Google Scholar]
- Prigge ST, Eipper BA, Mains RE, Amzel LM. Dioxygen binds end-on to mononuclear copper in a precatalytic enzyme complex. Science. 2004;304:864–867. doi: 10.1126/science.1094583. [DOI] [PubMed] [Google Scholar]
- Prigge ST, Kolhekar AS, Eipper BA, Mains RE, Amzel LM. Amidation of bioactive peptides: the structure of peptidylglycine α-hydroxylating monooxygenase. Science. 1997;278:1300–1305. doi: 10.1126/science.278.5341.1300. [DOI] [PubMed] [Google Scholar]
- Prigge ST, Kolhekar AS, Eipper BA, Mains RE, Amzel LM. Substrate-mediated electron transfer in peptidylglycine alpha-hydroxylating monooxygenase. Nat. Struct. Biol. 1999;6:976–983. doi: 10.1038/13351. [DOI] [PubMed] [Google Scholar]
- Prigge ST, Mains RE, Eipper BA, Amzel LM. New insights into copper monooxygenases and peptide amidation: structure, mechanism and function. Cell Mol. Life Sci. 2000;57:1236–1259. doi: 10.1007/PL00000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska JR, Broderius M. Plasma peptidylglycine alpha-amidating monooxygenase (PAM) and ceruloplasmin are affected by age and copper status in rats and mice. Comp. Biochem. Physiol B Biochem. Mol. Biol. 2006;143:360–366. doi: 10.1016/j.cbpb.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska JR, Broderius M, Brokate B. Metallochaperone for Cu,Zn-superoxide dismutase (CCS) protein, but not mRNA is higher in organs from copper deficient mice and rats. Arch. Biochem. Biophys. 2003;417:227–234. doi: 10.1016/s0003-9861(03)00364-3. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Gybina AA, Broderius M, Brokate B. Peptidylglycine-alpha-amidating monooxygenase activity and protein are lower in copper-deficient rats and suckling copper-deficient mice. Arch. Biochem. Biophys. 2005;434:212–220. doi: 10.1016/j.abb.2004.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska JR, Wells WW. Copper deficiency in the developing rat brain: a possible model for Menkes’ steely hair disease. J Neurochem. 1974;23:91–98. doi: 10.1111/j.1471-4159.1974.tb06920.x. [DOI] [PubMed] [Google Scholar]
- Pyatskowit JW, Prohaska JR. Iron injection restores brain iron and hemoglobin deficits in perinatal copper-deficient rats. J Nutr. 2008;138:1880–1886. doi: 10.1093/jn/138.10.1880. [DOI] [PubMed] [Google Scholar]
- Rajagopal C, Stone KL, Francone V, Mains RE, Eipper BA. Secretory Granule to the Nucleus: ROLE OF A MULTIPLY PHOSPHORYLATED INTRINSICALLY UNSTRUCTURED DOMAIN. J. Biol. Chem. 2009;284:25723–25734. doi: 10.1074/jbc.M109.035782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richichi C, Lin ED, Stefanin D, Colella D, Ravizza T, Grignaschi G, Veglianese P, Sperk G, During MJ, Vezzani A. Anticonvulsant and antiepileptic effects mediated by adeno-associated virus vector Neuropeptide Y expression in the rat hippocampus. J Neurosci. 2004;24:3051–3059. doi: 10.1523/JNEUROSCI.4056-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge PG, Zhang Y, Gladeyshev VN. Comparative genomic analyses of copper transporters and cuproproteomes reveal evolutionary dynamics of copper utilization and its link to oxygen. PLoS ONE. 2008;3:e1378. doi: 10.1371/journal.pone.0001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg R, Yasuda R, Pankratz D, Carter T, Lockhart D, Barlow C. Regional and strain-specific gene expression mapping in the adult mouse brain. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11043. doi: 10.1073/pnas.97.20.11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L. Fear of falling. N. Y. Times Magazine. 2009 Sept.2,2009. [Google Scholar]
- Schilling S, Niestroj AJ, Rahfeld JU, Hoffmann T, Wermann M, Zunkel K, Wasternack C, Demuth HU. Identification of human glutaminyl cyclase as a metalloenzyme. Potent inhibition by imidazole derivatives and heterocyclic chelators. J. Biol. Chem. 2003;278:49773–49779. doi: 10.1074/jbc.M309077200. [DOI] [PubMed] [Google Scholar]
- Scientific Committee on Food. European Commission on Health. Consumer Protection Directorate Opinion of the Scientific Committee on Food on the tolerable upper level intake of copper. 2003.
- Sharma SD, Raghuraman G, Lee MS, Prabhakar NR, Kumar GK. Intermittent hypoxia activates peptidylglycine alpha-amidating monooxygenase in rat brain stem via reactive oxygen species-mediated proteolytic processing. J Appl. Physiol. 2009;106:12–19. doi: 10.1152/japplphysiol.90702.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheela SR, Latha M, Lui P, Lem K, Kaler SG. Copper-replacement treatment for symptomatic Menkes disease: ethical considerations. Clin. Genet. 2005;68:278–283. doi: 10.1111/j.1399-0004.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- Shintani M, Tamura Y, Monden M, Shiomi H. Thyrotropin-releasing hormone induced thermogenesis in Syrian hamsters: site of action and receptor subtype. Brain Res. 2005;1039:22–29. doi: 10.1016/j.brainres.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Siebert X, Eipper BA, Mains RE, Prigge ST, Blackburn NJ, Amzel LM. The catalytic copper of peptidylglycine alpha-hydroxylating monooxygenase also plays a critical structural role. Biophysical J. 2005;89:3312–3319. doi: 10.1529/biophysj.105.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- Sorensen AT, Kanter-Schlifke I, Lin ED, During MJ, Kokaia M. Activity-dependent volume transmission by transgene NPY attenuates glutamate release and LTP in the subiculum. Mol. Cell. Neurosci. 2008;39:229–237. doi: 10.1016/j.mcn.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Sorensen AT, Nikitidou L, Ledri M, Lin ED, During MJ, Kanter-Schlifke I, Kokaia M. Hippocampal NPY gene transfer attenuates seizures without affecting epilepsy-induced impairment of LTP. Exp. Neurol. 2009;215:328–333. doi: 10.1016/j.expneurol.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosulina L, Schwesig G, Seifert G, Pape HC. Neuropeptide Y activates a G-protein-coupled inwardly rectifying potassium current and dampens excitability in the lateral amygdala. Mol. Cell. Neurosci. 2008;39:498. doi: 10.1016/j.mcn.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Sperk G, Furtinger S, Schwarzer C, Pirker S. GABA and its receptors in epilepsy. Adv. Exp. Med. Biol. 2004;548:92–103. doi: 10.1007/978-1-4757-6376-8_7. [DOI] [PubMed] [Google Scholar]
- Stephens DP, Saad AR, Bennett LA, Kosiba WA, Johnson JM. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am. J Physiol. Heart Circ. Physiol. 2004;287:H1404–H1409. doi: 10.1152/ajpheart.00061.2004. [DOI] [PubMed] [Google Scholar]
- Steveson TC, Ciccotosto GD, Ma XM, Mueller GP, Mains RE, Eipper BA. Menkes protein contributes to the function of peptidylglycine alpha-hydroxylating monooxygenase. Endocrinology. 2003;144:188–200. doi: 10.1210/en.2002-220716. [DOI] [PubMed] [Google Scholar]
- Steveson TC, Zhao GC, Keutmann HT, Mains RE, Eipper BA. Access of a membrane protein to secretory granules is facilitated by phosphorylation. J. Biol. Chem. 2001;276:40326–40337. doi: 10.1074/jbc.M011460200. [DOI] [PubMed] [Google Scholar]
- Strausak D, Mercer JF, Dieter HH, Stremmel W, Multhaup G. Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res. Bull. 2001;55:175–185. doi: 10.1016/s0361-9230(01)00454-3. [DOI] [PubMed] [Google Scholar]
- Tan JC, Burns DL, Jones HR. Severe ataxia, myelopathy, and peripheral neuropathy due to acquired copper deficiency in a patient with history of gastrectomy. J Parenter. Enteral. Nutr. 2006;30:446–450. doi: 10.1177/0148607106030005446. [DOI] [PubMed] [Google Scholar]
- Tokuda Y, Kashima M, Kayo M, Nakazato N, Stein GH. Cocoa supplementation for copper deficiency associated with tube feeding nutrition. Intern Med. 2006;45:1079–1085. doi: 10.2169/internalmedicine.45.1525. [DOI] [PubMed] [Google Scholar]
- Trajkovski M, Mziaut H, Altkrüger A, Ouwendijk J, Knoch KP, Müller S, Solimena M. Nuclear translocation of an ICA512 cytosolic fragment couples granule exocytosis and insulin expression in {beta}-cells. J Cell Biol. 2004;16:1063–1074. doi: 10.1083/jcb.200408172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt WA, Johnson PL, Dietrich AD, Fitz SD, Shekar A. Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience. 2009;160:284–294. doi: 10.1016/j.neuroscience.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukumo Y, Tomida A, Kitahara O, Nakamura Y, Asada S, Mori K, Tsuruo T. Nucleobindin 1 controls the unfolded protein response by inhibiting ATF6 activation. J Biol Chem. 2007;282:29264–29272. doi: 10.1074/jbc.M705038200. [DOI] [PubMed] [Google Scholar]
- Tumer Z, Horn N. Menkes disease: recent advances and new aspects. J Med. Genet. 1997;34:265–274. doi: 10.1136/jmg.34.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey S. Peptide amidation: a push me, don’t pull you for the morbidities in sleep apnea. J Appl. Physiol. 2009;106:4. doi: 10.1152/japplphysiol.91422.2008. [DOI] [PubMed] [Google Scholar]
- Veronesi MC, Kubek DJ, Kubek MJ. Intranasal delivery of a thyrotropin-releasing hormone analog attenuates seizures in the amygdala-kindled rat. Epilepsia. 2007;48:2280–2286. doi: 10.1111/j.1528-1167.2007.01218.x. [DOI] [PubMed] [Google Scholar]
- von Drygalski A, Andris DA. Anemia after bariatric surgery: more than just iron deficiency. Nutr Clin Pract. 2009;24:217–226. doi: 10.1177/0884533609332174. [DOI] [PubMed] [Google Scholar]
- Vos MD, Jones JE, Treston AM. Human peptidylglycine alpha-amidating monooxygenase transcripts derived by alternative mRNA splicing of an unreported exon. Gene. 1995;163:307–311. doi: 10.1016/0378-1119(95)00364-c. [DOI] [PubMed] [Google Scholar]
- Walker JM, Huster D, Ralle M, Morgan CT, Blackburn NJ. The N-terminal metal binding 2 of the Wilson’s disease protein plays a key role in the transfer of copper from Atox-1. J Biol. Chem. 2004;279:15376–15384. doi: 10.1074/jbc.M400053200. [DOI] [PubMed] [Google Scholar]
- Wand GS, Ney RL, Baylin S, Eipper BA, Mains RE. Characterization of a peptide alpha-amidation activity in human plasma and tissues. Metabolism. 1985;34:1044–1052. doi: 10.1016/0026-0495(85)90077-0. [DOI] [PubMed] [Google Scholar]
- Woronowicz A, Cawley NX, Chang SY, Koshimizu H, Phillips AW, Ziong ZG, Loh YP. Carboxypeptidase E knockout mice exhibit abnormal dendritic arborization and spine morphology in central nervous system neurons. J. Neurosci. Res. 2009 doi: 10.1002/jnr.22174. PMID: 19598241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Mains RE, Eipper BA. Monooxygenase X, a member of the copper-dependent monooxygenase family localized to the endoplasmic reticulum. J. Biol. Chem. 2005;279:48159–48167. doi: 10.1074/jbc.M407486200. [DOI] [PubMed] [Google Scholar]