Abstract
Background
Animal studies have shown that memory deficits in the early post-anaesthetic period can be prevented by pre-treatment with an inverse agonist that preferentially inhibits α5 subunit-containing γ-aminobutyric acid type A (α5GABAA) receptors. The goal of this in vitro study was to determine whether inverse agonists that inhibit α5GABAA receptors reduce anaesthetic potentiation of GABAA receptor activity.
Methods
Cultures of hippocampal neurones were prepared from Swiss white mice, wild-type mice (genetic background C57BL/6J and Sv129Ev) and α5GABAA receptor null mutant (Gabra5−/−) mice. Whole-cell voltage clamp techniques were used to study the effects of the α5GABAA receptor-preferring inverse agonists L-655,708 and MRK-016 on anaesthetic potentiation of GABA-evoked currents.
Results
L-655,708 (50 nM) reduced sevoflurane potentiation of GABA-evoked current in wild-type neurones but not Gabra5−/− neurones, and produced a rightward shift in the sevoflurane concentration–response plot [sevoflurane EC50: 1.9 (0.1) mM; sevoflurane+L-655,708 EC50: 2.4 (0.2) mM, P<0.05]. Similarly, L-655,708 (50 nM) reduced isoflurane potentiation of GABA-evoked current [isoflurane: 4.0 (0.6) pA pF−1; isoflurane+L-655,708: 3.1 (0.5) pA pF−1, P<0.01]. MRK-016 also reduced sevoflurane and isoflurane enhancement of GABA-evoked current [sevoflurane: 1.5 (0.1) pA pF−1; sevoflurane+MRK-016 (10 nM): 1.2 (0.1) pA pF−1, P<0.05; isoflurane: 3.5 (0.3) pA pF−1; isoflurane+MRK-016 (1 nM): 2.9 (0.2) pA pF−1, P<0.05].
Conclusions
L-655,708 and MRK-016 reduced the potentiation by inhaled anaesthetics of GABAA receptor activated by a low concentration of GABA. Future studies are required to determine whether this effect contributes to the memory preserving properties of inverse agonists after anaesthesia.
Keywords: anaesthetics volatile, sevoflurane, isoflurane; brain, GABA; electrophysiology
Editor's key points.
Inhaled anaesthetics act by potentiating inhibitory type A GABA (GABAA) receptors to produce some of their desired, and perhaps undesired, effects.
Using cultured hippocampal neurones, inverse agonists selective for α5 subunit containing GABAA receptors reduced receptor potentiation by volatile anaesthetics.
Potentiation of α5 subunit-containing GABAA receptors might contribute to post-anaesthesia memory deficits, which might be reversible by selective inverse agonists.
One of the most potent effects of anaesthetics is their ability to cause profound memory blockade or amnesia.1 Most patients assume that their memory will rapidly return to baseline once the anaesthetic has been eliminated. However, some patients experience postoperative memory deficits that persist for days to months.2 Animal studies have shown that exposure to anaesthetics alone can induce long-term memory deficits3–5 through mechanisms that remain poorly understood.
Many of the desirable behavioural effects of anaesthetics are mediated by an increase in γ-aminobutyric acid type A (GABAA) receptor activity.6–9 Whether GABAA receptors also contribute to undesired side-effects of anaesthetics is a topic of on-going investigation.10 GABAA receptors are chloride-selective ion channels that assemble from a variety of subunits (α1–6, β1–3, γ1–3, δ, ɛ, π, θ, ρ1–3).11 The subunit composition regulates cellular distribution, agonist affinity and receptor kinetics of each GABAA receptor and determines its pharmacological properties.12 GABAA receptors mediate two distinct forms of inhibition: a transient synaptic inhibition and a persistent tonic inhibition.13 Recent attention regarding memory loss and anaesthesia has focused on high-affinity α5 subunit-containing GABAA (α5GABAA) receptors that generate a low amplitude tonic inhibitory conductance in the hippocampus.14
Inhaled anaesthetics, including isoflurane and sevoflurane, potently and effectively increase α5GABAA receptor function,15 an effect that might contribute to the acute memory blocking properties of these drugs.16,17 Intense enhancement of α5GABAA receptors might also trigger long-term post-anaesthetic memory loss that persists 24–48 h after the anaesthetic has been eliminated. We previously showed that genetic and pharmacologic interventions, that reduce α5GABAA receptor function during acute exposure to isoflurane prevent post-anaesthetic memory deficits that otherwise can last for days.18–20 Specifically, null-mutant mice lacking α5GABAA receptors (Gabra5−/−) were resistant to long-term memory deficits produced by sevoflurane and isoflurane, suggesting that α5GABAA receptors are necessary for post-anaesthetic memory deficits.18,19 Further, treatment of animals during exposure to isoflurane with an imidazobenzodiazepine inverse agonist that is selective for α5GABAA receptors, L-655,708 (FG-8094, C18H19N3O4), prevented memory deficits.20 These results suggest that inverse agonists can attenuate the potentiating effect of anaesthetics at α5GABAA receptors.
Inverse agonists act as negative allosteric modulators of α5GABAA receptors by binding to the benzodiazepine site and decreasing receptor function.21 Studies of recombinantly expressed GABAA receptors showed that the dissociation constant of L-655,708 for α5GABAA receptors is 50- to 100-fold lower than for GABAA receptors containing α1, α2, α3, or α6 subunits.20 The half-maximal inhibitory concentration of L-655,708 for α5GABAA receptors is 1.1 nM, a value that is considerably lower than for GABAA receptors containing the α1 (320 nM), α2 (135 nM), and α3 (960 nM) subunits.22 Another inverse agonist, MRK-016 (C17H20N8O2), which is structurally distinct from L-655,708, also preferentially inhibits α5GABAA receptors and improves memory performance.23,24
The aim of this in vitro study was to determine whether L-655,708 and MRK-016 inhibited sevoflurane- and isoflurane-mediated enhancement of GABAA receptor activity. We also sought to determine whether reversal of anaesthetic-mediated potentiation was specific for α5GABAA receptors.
Methods
Cell culture
The experiments were approved by the Animal Care Committee of the University of Toronto (Protocol number: 20009455). Gabra5−/− mice were generated using a C57BL/6J and Sv129Ev background, as previously described.25 Swiss white mice (timed pregnant mice from Charles River, Toronto, ON, Canada), were also used for some experiments because the number of wild-type and Gabra5−/− mice available was limited. Hippocampal neuronal cultures were prepared as previously described.14 Briefly, fetal pups (embryonic day 18) were removed from mice that had been killed by cervical dislocation. The fetal hippocampus was collected, and neurones were dissociated by mechanical titration, plated at a density of 106 cells ml−1 on culture dishes that were coated with collagen or poly-d-lysine (Sigma-Aldrich Co., St Louis, MO, USA), and maintained in minimal essential medium (MEM) supplemented with 10% fetal bovine serum and 10% horse serum (both from Life Technologies, Grand Island, NY, USA) for the first 5 days. To reduce the number of dividing glial cells, a mixture of 5-fluorodeoxyuridine (4 mg) and uridine (10 mg) in 20 ml MEM was added. The media was further supplemented with 10% horse serum and was changed every 3 or 4 days. Cells were maintained in culture for 12–16 days before use. At this time, neurones develop the characteristic branching and synapses that resemble mature hippocampal pyramidal cells in vivo.26 Experiments were restricted to morphologically identified pyramidal neurones.25 For all reported results, data were acquired from cells from at least three different dissections. All electrophysiological recordings were performed at room temperature (20–23°C).
Electrophysiology
Whole-cell GABA currents were recorded under voltage-clamp (−60 mV) conditions using an Axopatch 1D amplifier (Molecular Devices, Sunnyvale, CA, USA) controlled with the pClamp 8.0 software (Molecular Devices, Sunnyvale, CA, USA) via a Digidata 1322 interface (Molecular Devices, Sunnyvale, CA, USA). Patch pipettes with open-tip resistances of 3–5 MΩ were pulled from thin-walled borosilicate glass capillary tubes. Patch electrodes were filled with an intracellular solution containing (in mM) 140 CsCl, 10 HEPES, 11 EGTA, 2 MgCl2, 1 CaCl2, 4 MgATP, and 2 TEA (adjusted to pH 7.3 with CsOH and to 285–295 mOsm with water). The extracellular solution contained (in mM) 140 NaCl, 1.3 CaCl2, 2 KCl, 1 MgCl2, 25 HEPES and 28 glucose (adjusted to pH 7.4 with NaOH and to 320–330 mOsm with water or glucose). Tetrodotoxin (TTX, 300 nM) was added to the extracellular solution to block voltage-gated sodium channels, and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 µM) and 2-amino-4-phosphonovaleric acid (D-AP5, 40 µM) were added to inhibit the ionotropic glutamate receptors. The extracellular solution was applied to neurones by a computer-controlled, multibarrelled perfusion system (SF-77B, Warner Instruments, Hamden, CT, USA).
Currents were analysed with the pClamp 10 software (Molecular Devices). Current amplitude was normalized by cell capacitance to determine current density (pA pF−1).27,28 To construct the concentration–response plot for sevoflurane, currents were normalized (I/Imax), where I is the current amplitude and Imax is the largest current recorded in the experiment. The concentration–response plot was fitted to the modified Michaelis–Menten equation: I=Imax/[1+(EC50/c)nH], where c is the concentration of sevoflurane, EC50 is the concentration of sevoflurane that produces currents with 50% of maximal amplitude, and nH is the estimated Hill coefficient.
Drugs and chemicals
Sevoflurane and isoflurane were obtained from Abbott Laboratories (North Chicago, IL, USA). CNQX and D-AP5 were obtained from Sigma-Aldrich (Oakville, ON, Canada). Tetrodotoxin was purchased from Alomone Labs (Jerusalem, Israel). L-655,708 and MRK-016 were obtained from Tocris Bioscience (Bristol, UK). Anaesthetic solutions were diluted from saturated aqueous phase of sevoflurane (11.8 mM)29 and isoflurane (2.5 mM)29 prepared at room temperature (20–23°C), as previously described.30 For the generation of the concentration–response curve, a range of sevoflurane concentrations were studied. For all other experiments concentrations equivalent to the minimal alveolar concentration (MAC) of sevoflurane31 and isoflurane15,29 were used. Stock solutions of all other reagents were prepared with distilled water.
Statistical analysis
Differences between groups were determined using a Student's t-test or a one-way analysis of variance (anova) with a Dunnett multiple-comparison post hoc test. P<0.05 was considered significant. GraphPad Prism 5 (GraphPad Software, Inc., CA, USA) was used to generate the bar charts. Results are presented as mean (sem).
Results
L-655,708 selectively inhibits α5GABAA receptors
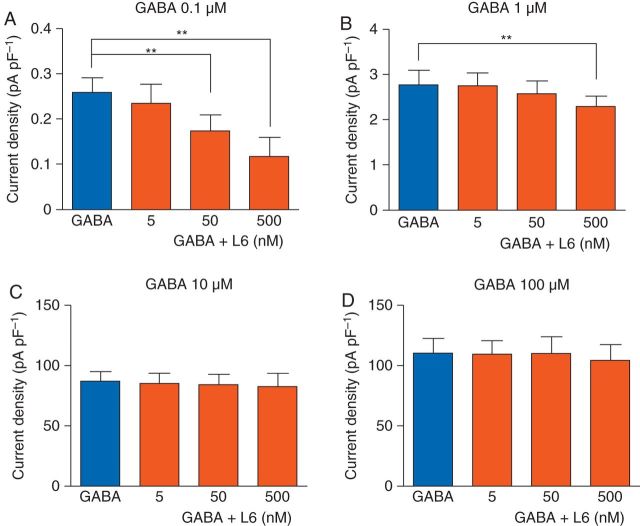
As α5GABAA receptors are preferentially activated by low GABA concentrations,32 we first studied whether currents evoked by low compared with high concentrations of GABA exhibit a greater sensitivity to L-655,708. The peak amplitude of current evoked by GABA (0.1 µM) was inhibited by L-655,708 (5, 50 and 500 nM) in a concentration-dependent manner [control: 0.26 (0.03) pA pF−1, L-655,708: 0.24 (0.04) pA pF−1 (5 nM); 0.17 (0.03) pA pF−1 (50 nM); 0.12 (0.04) pA pF−1 (500 nM), P<0.01, n=5 or 6, Fig. 1a]. When the concentration of GABA was increased to 1 µM, only the highest concentration of L-655,708 (500 nM) slightly inhibited the current to 85% of control [control: 2.7 (0.3) pA pF−1; L-655,708 (500 nM): 2.3 (0.2) pA pF−1, P<0.01, n=5 or 6, Fig. 1b]. When the concentration of GABA was further increased, L-655,708 had no effect (n=5 or 6, Fig. 1c and d). Collectively, the results show that L-655,708 effectively inhibits currents evoked by low but not high concentrations of GABA.
Fig 1.
L-655,708 inhibits currents evoked by low, but not high, concentrations of GABA in neurones from Swiss white mice. Summarized data show that L-655,708 (L6) has a concentration-dependent inhibitory effect on currents evoked by GABA (0.1 and 1 µM) but no effect on currents evoked by GABA (10 and 100 µM) (n=5–6). **P<0.01 vs GABA. Data are mean (sem).
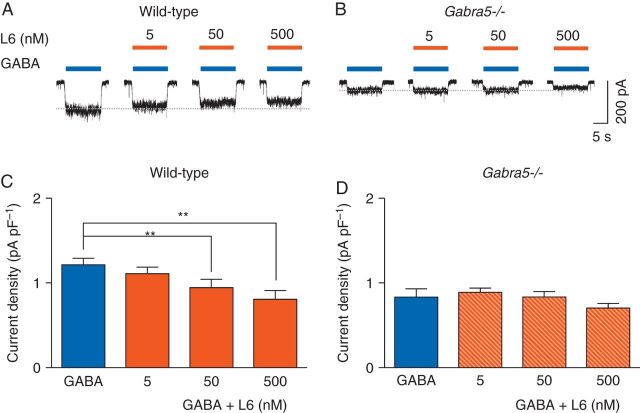
We next sought to determine whether the selectivity of L-655,708 is specific for α5GABAA receptors. GABA (0.5 µM)-evoked currents were smaller in Gabra5−/− neurones compared with wild-type neurones, as shown previously14 [0.8 (0.1) pA pF−1 and 1.2 (0.1) pA pF−1, respectively, P=0.024, n=6, Fig. 2a and b]. Cell capacitance was similar in the two genotypes [wild-type: 114.3 (11.2) pF, Gabra5−/−: 103.3 (12.9) pF, P=0.27, n=6]. In wild-type neurones, L-655,708 (50 and 500 nM) reduced GABA (0.5 µM)-evoked current, from 1.2 (0.1) pA pF−1 to 0.9 (0.1) pA pF−1 and 0.8 (0.1) pA pF−1, respectively (P<0.01, n=6, Fig. 2a and c), while a lower concentration of L-655,708 (5 nM) had no significant effect (n=6, Fig. 2a and c). In Gabra5−/− neurones, L-655,708 had no effect on GABA (0.5 µM)-evoked current, even at concentrations as high as 500 nM [control: 0.8 (0.1) pA pF−1; L-655,708: 0.7 (0.1) pA pF−1, n=5, Fig. 2b and d]. Thus, L-655,708 inhibits current evoked by low concentrations of GABA but only in wild-type neurones.
Fig 2.
L-655,708 inhibits GABA-evoked currents in neurones from wild-type but not Gabra5−/− mice. (a) and (b) The effects of L-655,708 (L6) on currents evoked by GABA (0.5 µM). (c) and (d) Summarized data showing the concentration-dependent effects of L-655,708 on GABA-evoked current (n=6). **P<0.01 vs GABA. Data are mean (sem).
L-655,708 attenuates sevoflurane-induced potentiation of GABA-evoked currents in wild-type but not Gabra5−/− neurones
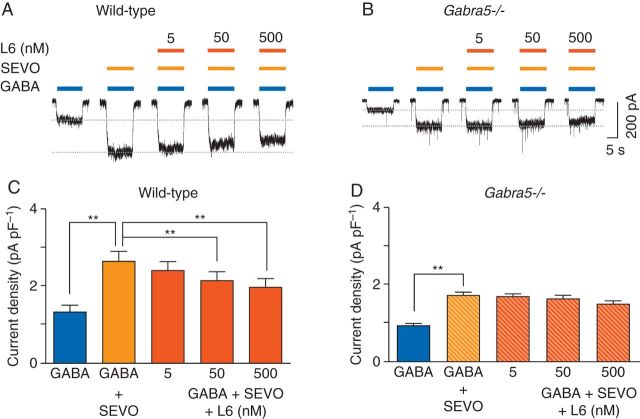
To determine whether L-655,708 inhibits the potentiating effects of inhaled anaesthetics, currents were evoked by a low concentration of GABA (0.5 µM) in the absence and presence of sevoflurane (266 µM). This concentration of sevoflurane was chosen as it is equivalent to that which causes immobility in 50% of subjects (MAC). Also, a comparable concentration of sevoflurane induces long-term memory deficits that could be prevented in mice by treatment with L-655,708.18,19 Sevoflurane caused a rapid and reversible increase in the GABA-evoked current [1.3 (0.2) pA pF−1 to 2.6 (0.3) pA pF−1, P<0.01, n=6, Fig. 3a and c]. Co-application of L-655,708 (50 and 500 nM) markedly reduced the sevoflurane potentiation of GABA-evoked current (2.1 (0.2) pA pF−1 and 1.9 (0.2) pA pF−1, respectively, P<0.01, n=6, Fig. 3a and c) whereas L-655,708 (5 nM) had no significant effect (n=6, Fig. 3a and c).
Fig 3.
L-655,708 attenuates the potentiating effects of sevoflurane in neurones from wild-type but not Gabra5−/− mice. (a) and (b) The effects of L-655,708 (L6) on sevoflurane (SEVO, 266 μM)-mediated enhancement of currents evoked by GABA (0.5 μM). (c) and (d) The concentration-dependent effects of L-655,708 on sevoflurane-mediated enhancement of GABA-evoked current are shown (n=6). **P<0.01 vs GABA+SEVO. Data are mean (sem).
To determine whether L-655,708 attenuation of sevoflurane-induced potentiation of current required α5GABAA receptors, the experiments were repeated with neurones from Gabra5−/− mice. As shown previously,15 under baseline conditions, currents evoked by GABA (0.5 µM) were smaller in Gabra5−/− neurones than in wild-type neurones [0.9 (0.1) pA pF−1 and 1.3 (0.2) pA pF−1, respectively, P=0.024, n=6, Fig. 3a and b], whereas cell capacitance was similar [Gabra5−/−: 76.3 (8.6) pF, wild-type: 66.0 (11.5) pF, P=0.48, n=6]. Application of sevoflurane (266 µM) to Gabra5−/− neurones caused a 2-fold increase in current [control: 0.9 (0.1) pA pF−1; sevoflurane: 1.7 (0.1) pA pF−1, P<0.05, n=6, Fig. 3b and d]. Potentiation of GABA-evoked current in Gabra5−/− neurones was lower than in wild-type neurones [1.7 (0.1) pA pF−1 and 2.6 (0.3) pA pF−1, respectively, P=0.0017, n=6, Fig. 3b and d], while the relative increase in current was similar between the two genotypes (wild-type: 200% of control, Gabra5−/−: 180% of control). As predicted, co-application of L-655,708 had no effect on sevoflurane-mediated potentiation in Gabra5−/− neurones (n=6, Fig. 3b and d). Collectively, these results indicate that L-655,708 attenuates sevoflurane potentiation of GABA-evoked current in a concentration-dependent manner in wild-type but not Gabra5−/− neurones, and that the sevoflurane potentiation of current is smaller in Gabra5−/− neurones.
Next, the effects of L-655,708 on sevoflurane concentration–response plots were studied (Fig. 4). Co-application of L-655,708 (50 nM) with sevoflurane (2 and 3 mM) caused a significant inhibition of sevoflurane potentiation [34 (9) % and 20 (6) %, respectively, P<0.05, n=6]. On the other hand, co-application of L-655,708 with higher sevoflurane concentrations (6 and 8 mM) had no effect. L-655,708 shifted the sevoflurane concentration–response plot to the right without affecting the maximal response (Fig. 4b). The EC50 was increased from 1.9 (0.1) mM to 2.4 (0.2) mM (P<0.05, n=6) in the absence and presence of L-655,708, respectively. These results demonstrate that L-655,708 reduced the potency of sevoflurane, but had no effect on its efficacy at high concentrations.
Fig 4.
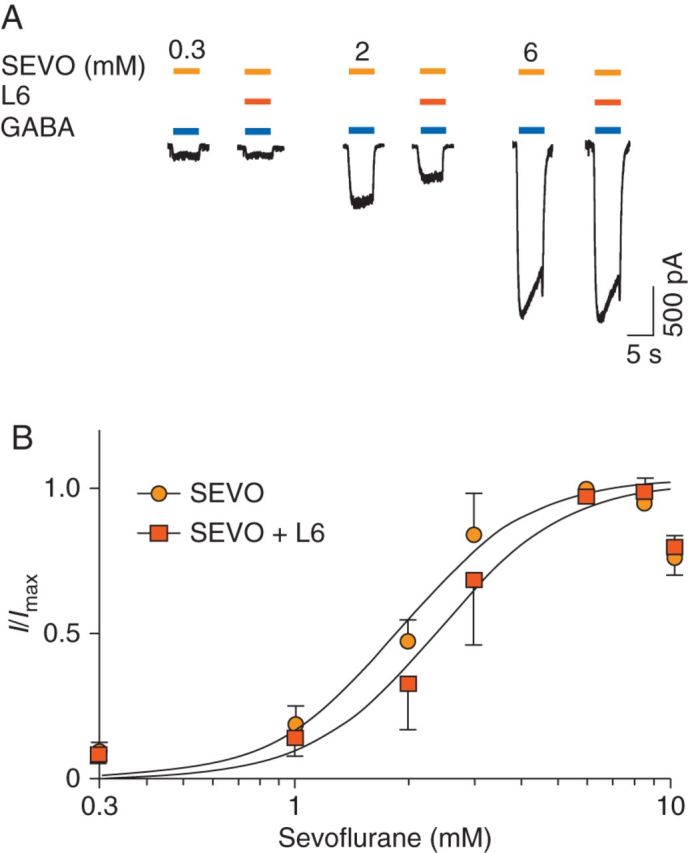
L-655,708 decreases the potency of sevoflurane for increasing GABAA receptor activity in neurones from Swiss white mice. (a) The effects of L-655,708 (L6, 50 nM) on sevoflurane (SEVO)-mediated enhancement of currents evoked by GABA (0.5 µM). As shown previously,39 a tail current was observed during drug washout at higher concentrations of sevoflurane (6, 8, and 10 mM). (b) The corresponding concentration–response plots for sevoflurane potentiation of GABA-evoked current in the absence or presence of L-655,708 (L6). L-655,708 increased the EC50 from 1.9 (0.1) to 2.4 (0.2) (P<0.05, n=6) without affecting the maximal response. Application of sevoflurane (10 mM) caused a decrease in current, which is consistent with previous studies.39 Data points for sevoflurane (10 mM) were excluded from the curve-fitting analyses. Data are mean (sem).
L-655,708 attenuates isoflurane-induced potentiation of GABA-evoked currents
To determine whether the inhibitory effect of inverse agonists on anaesthetic-induced potentiation could be generalized to different volatile anaesthetics, the effect of L-655,708 on isoflurane potentiation of GABA-evoked currents was studied. For these studies, hippocampal neurones from Swiss white mice were used. L-655,708 caused a concentration-dependent decrease in the GABA (0.5 µM)-evoked current [control: 1.7 (0.2) pA pF−1; L-655,708: 1.6 (0.2) pA pF−1 (5 nM); 1.4 (0.2) pA pF−1 (50 nM), P<0.05; 1.1 (0.1) pA pF−1 (500 nM), P<0.001, n=6, Fig. 7a and c]. Isoflurane, studied at a concentration of ∼1 MAC (250 µM), enhanced GABA (0.5 µM)-evoked currents [control: 1.8 (0.3) pA pF−1, isoflurane: 4.0 (0.6) pA pF−1, P<0.001, n=6, Fig. 5]. Co-application of L-655,708 caused a concentration-dependent reduction in the effect of isoflurane [L-655,708: 3.6 (0.5) pA pF−1 (5 nM), n=6; L-655,708: 3.1 (0.5) pA pF−1 (50 nM), P<0.01, n=6; 2.4 (0.3) pA pF−1 (500 nM), P<0.001, n=6, Fig. 5). Thus, L-655,708 effectively attenuated potentiation of the GABA current by isoflurane.
Fig 7.
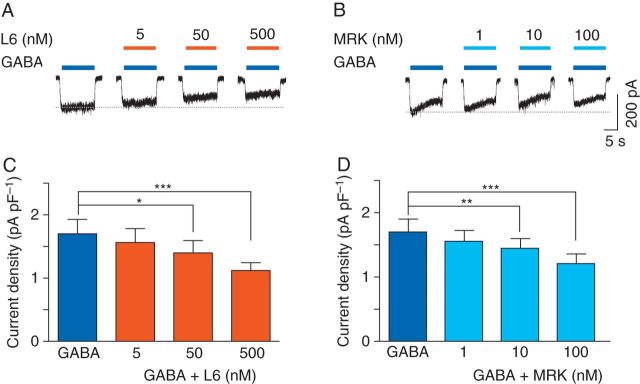
L-655,708 and MRK-016 inhibit GABA-evoked currents in neurones from Swiss white mice. (a) and (b) The effects of L-655,708 (L6) and MRK-016 (MRK) on currents evoked by GABA (0.5 µM). (c) and (d) Summarized data showing the concentration-dependent inhibitory effects of L-655,708 and MRK-016 on GABA-evoked current (n=6). *P<0.05, **P<0.01, and ***P<0.001 vs GABA. Data are mean (sem).
Fig 5.
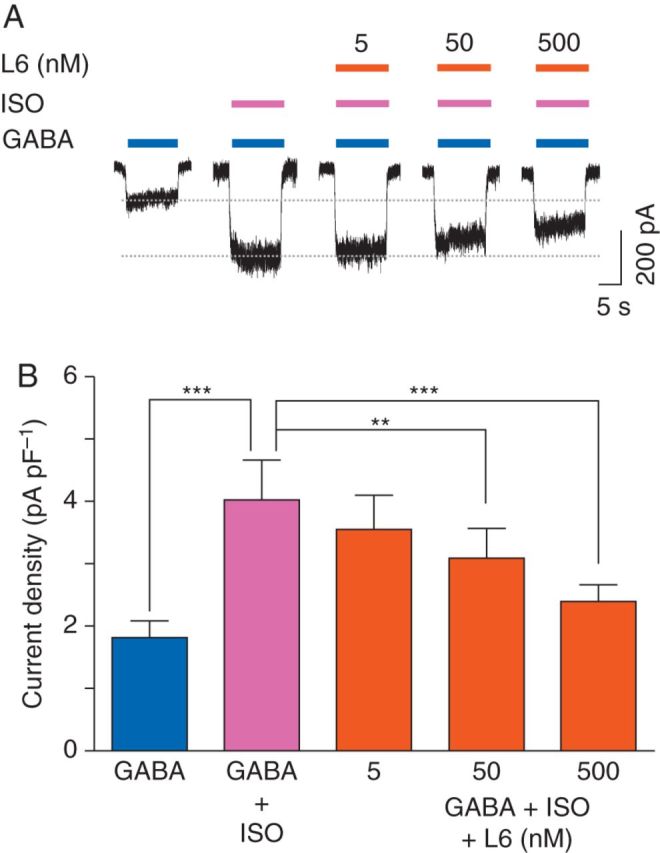
L-655,708 inhibits the potentiating effects of isoflurane on GABA currents in neurones from Swiss white mice. (a) The effects of L-655,708 (L6) on isoflurane (ISO, 250 µM)-mediated enhancement of currents evoked by GABA (0.5 µM). (b) Summarized data showing the concentration-dependent inhibitory effects of L-655,708 on isoflurane-mediated enhancement of GABA-evoked currents (n=6). **P<0.01, ***P<0.001 vs GABA+ISO. Data are mean (sem).
MRK-016 attenuates sevoflurane- and isoflurane-induced potentiation of GABA-evoked currents
We next studied the effects of MRK-016 to determine whether other inverse agonists also reduce anaesthetic enhancement of the GABAA receptor activity. MRK-016 caused a concentration-dependent decrease in GABA current [control: 1.7 (0.2) pA pF−1; MRK-016: 1.5 (0.2) pA pF−1 (1 nM); 1.4 (0.2) pA pF−1 (10 nM), P<0.01; 1.2 (0.1) pA pF−1 (100 nM), P<0.001, n=6, Fig. 7b and d].
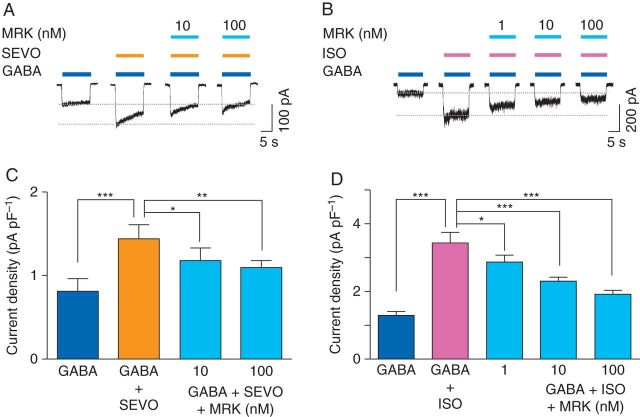
Next, the effect of MRK-016 on sevoflurane (266 µM)-induced potentiation of GABA current was examined. Sevoflurane alone increased the current evoked by GABA (0.5 µM) from 0.8 (0.1) pA pF−1 to 1.5 (0.1) pA pF−1 (P<0.001, n=6, Fig. 6a and c). Co-application of MRK-016 (10 or 100 nM) reduced the effect of sevoflurane in a concentration-dependent manner [1.2 (0.1) pA pF−1 (10 nM), P<0.05, n=6; 1.1 (0.1) pA pF−1 (100 nM), P<0.01, n=6, Fig. 6a and c]. Similarly, isoflurane (250 µM) increased currents evoked by GABA (0.5 µM) from 1.3 (0.1) pA pF−1 to 3.5 (0.3) pA pF−1 (P<0.001, n=6, Fig. 6b and d). Co-application of MRK-016 reduced isoflurane potentiation of GABA-evoked current [2.9 (0.2) pA pF−1 (1 nM), P<0.05, n=6; 2.3 (0.1) pA pF−1 (10 nM), P<0.001, n=6; 1.9 (0.1) pA pF−1 (100 nM), P<0.001, n=6, Fig. 6b and d].
Fig 6.
MRK-016 attenuates the potentiating effects of sevoflurane and isoflurane in neurones from Swiss white mice. (a) and (b) The effects of MRK-016 (MRK) on sevoflurane (SEVO, 266 μM) and isoflurane (ISO, 250 μM) enhancement of currents evoked by GABA (0.5 μM). (c) and (d) Summarized data showing the concentration-dependent inhibitory effects of MRK-016 on sevoflurane- and isoflurane-mediated enhancement of GABA current (n=6). *P<0.05, **P<0.01, ***P<0.001 vs GABA+SEVO or GABA+ISO. Data are mean (sem).
Discussion
This study provides the first evidence that inverse agonists attenuate the potentiation of GABAA receptor activity by inhaled general anaesthetics. L-655,708 and MRK-016 caused a concentration-dependent reduction in the effects of both sevoflurane and isoflurane on GABA-evoked current in mouse hippocampal neurones. The decrease in sevoflurane enhancement of GABA-evoked current by L-655,708 was dependent on the expression of α5GABAA receptors, as this effect was only observed in wild-type neurones but not Gabra5−/− neurones.
The concentration of L-655,708 is a key determinant of its selectivity for α5GABAA receptors. In addition, GABA-evoked whole-cell currents in hippocampal neurones are generated by heterogeneous populations of GABAA receptors with different subunit compositions and different affinities for GABA.13,32 Thus, the effects of several L-655,708 concentrations (5–500 nM) on currents evoked by a range of GABA concentrations (0.1–100 μM) were studied. L-655,708 reduced current evoked by low (0.1 and 1 μM) but not high (10 and 100 μM) concentrations of GABA, consistent with the relative selectivity of L-655,708 for high-affinity GABAA receptors.13 Notably, the lowest concentration of L-655,708 (5 nM) was insufficient to inhibit GABA-evoked currents. This result contrasts with previous studies done in recombinant human GABAA receptors that showed a half-maximal inhibitory concentration of 1.1 nM for L-655,708.22 Nevertheless, L-655,708 (50 and 500 nM) appears to inhibit current primarily generated by α5GABAA receptors because the inhibitory effects of L-655,708 were absent in neurones from Gabra5−/− mice. These results suggest that the potency of L-655,708 could be lower for native GABAA receptors or that the low concentrations of GABA activated a heterogeneous population of GABAA receptors in the cultured neurones.
The mechanisms underlying the interaction(s) between inverse agonists and volatile anaesthetics on the GABAA receptor complex are unknown but likely result from reciprocal allosteric coupling between the benzodiazepine binding site and the anaesthetic modulatory domain.33 L-655,708 exerts its inhibitory effect by acting on a specific benzodiazepine binding site of GABAA receptors.20 Electrophysiological experiments showed that positive agonists of the benzodiazepine site, such as diazepam, allosterically increase the opening frequency of the GABAA receptors and thereby increase chloride influx and shunting inhibition.34,35 As L-655,708 acts on the same binding site as benzodiazepines, L-655,708 might also reduce frequency of channel opening.16 In contrast, volatile anaesthetics act at binding cavities that are distinct from the benzodiazepine site.36,37 Positive benzodiazepine modulators such as midazolam can synergistically interact with anaesthetics to enhance GABAA receptor function.38,39 Our results demonstrate that inverse agonists exert the opposite effect to positive benzodiazepine allosteric modulators possibly via reciprocal allosteric coupling.
A growing body of evidence implicates α5GABAA receptors in triggering anaesthetic-induced memory deficits that persist after the drugs have been eliminated.18,19 These receptors are highly expressed in hippocampus8 where they primarily mediate persistent, tonic inhibition.9 Under physiological conditions, α5GABAA receptors play a pivotal role in learning and memory processes,14 as genetic deletion or pharmacological inhibition of α5GABAA receptors can improve memory performance.40 41 Increasing the function of these receptors reduces neuronal excitability, decreases the firing of action potentials,25 reduces synaptic plasticity in the hippocampus, and can cause memory deficits.42 The specific molecular mechanisms by which anaesthetic-induced enhancement of α5GABAA receptor function can induce long-term memory deficits are unknown and are a topic for future studies. However, our results support the intriguing notion that L-655,708 and related agents might be useful for preventing memory deficits after anaesthesia.
Declaration of interest
None declared.
Funding
This work was supported by operating grants from the Canadian Institutes of Health Research (MOP-38028, MOP-79428) and a grant from the Canadian Anesthesiologists’ Society to B.A.O. I.L. was supported by a Savoy Foundation studentship. B.A.O. holds a Canada Research Chair.
Acknowledgements
We are grateful to Ella Czerwinski for her technical assistance with cell culture. We also thank Agnieszka A. Zurek for her helpful suggestions.
References
- 1.Wang DS, Orser BA. Inhibition of learning and memory by general anaesthetics. Can J Anaesth. 2011;58:167–77. doi: 10.1007/s12630-010-9428-8. [DOI] [PubMed] [Google Scholar]
- 2.Caza N, Taha R, Qi Y, Blaise G. The effects of surgery and anesthesia on memory and cognition. Prog Brain Res. 2008;169:409–22. doi: 10.1016/S0079-6123(07)00026-X. [DOI] [PubMed] [Google Scholar]
- 3.Culley DJ, Baxter M, Yukhananov R, Crosby G. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth Analg. 2003;96:1004–9. doi: 10.1213/01.ANE.0000052712.67573.12. table of contents. [DOI] [PubMed] [Google Scholar]
- 4.Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100:309–14. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Callaway JK, Jones NC, Royse CF. Isoflurane induces cognitive deficits in the Morris water maze task in rats. Eur J Anaesthesiol. 2012;29:239–45. doi: 10.1097/EJA.0b013e32835103c1. [DOI] [PubMed] [Google Scholar]
- 6.Harrison NL, Kugler JL, Jones MV, Greenblatt EP, Pritchett DB. Positive modulation of human γ-aminobutyric acid type A and glycine receptors by the inhalation anesthetic isoflurane. Mol Pharmacol. 1993;44:628–32. [PubMed] [Google Scholar]
- 7.Nakahiro M, Yeh JZ, Brunner E, Narahashi T. General anaesthetics modulate GABA receptor channel complex in rat dorsal root ganglion neurons. FASEB J. 1989;3:1850–4. doi: 10.1096/fasebj.3.7.2541038. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins A, Franks NP, Lieb WR. Effects of temperature and volatile anaesthetics on GABAA receptors. Anesthesiology. 1999;90:484–91. doi: 10.1097/00000542-199902000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anaesthetics. N Engl J Med. 2003;348:2110–24. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- 10.Grasshoff C, Drexler B, Rudolph U, Antkowiak B. Anaesthetic drugs: linking molecular actions to clinical effects. Curr Pharm Des. 2006;12:3665–79. doi: 10.2174/138161206778522038. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 12.Barnard EA, Skolnick P, Olsen RW, et al. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acid A receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 13.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 14.Caraiscos VB, Elliott EM, You-Ten KE, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci. 2004;101:3662–7. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caraiscos VB, Newell JG, You-Ten KE, et al. Selective enhancement of tonic GABAergic inhibition in murine hippocampal neurons by low concentrations of the volatile anesthetic isoflurane. J Neurosci. 2004;24:8454–8. doi: 10.1523/JNEUROSCI.2063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munglani R, Andrade J, Sapsford DJ, Baddeley A, Jones JG. A measure of consciousness and memory during isoflurane administration: the coherent frequency. Br J Anaesth. 1993;71:633–41. doi: 10.1093/bja/71.5.633. [DOI] [PubMed] [Google Scholar]
- 17.Galinkin JL, Janiszewski D, Young CJ, et al. Subjective, psychomotor, cognitive, and analgesic effects of subanesthetic concentrations of sevoflurane and nitrous oxide. Anesthesiology. 1997;87:1082–8. doi: 10.1097/00000542-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Saab BJ, Maclean AJ, Kanisek M, et al. Short-term memory impairment after isoflurane in mice is prevented by the α5 γ-aminobutyric acid type A receptor inverse agonist L-655,708. Anesthesiology. 2010;113:1061–71. doi: 10.1097/ALN.0b013e3181f56228. [DOI] [PubMed] [Google Scholar]
- 19.Zurek AA, Bridgwater EM, Orser BA. Inhibition of α5 γ-Aminobutyric acid type A receptors restores recognition memory after general anesthesia. Anesth Analg. 2012;114:845–55. doi: 10.1213/ANE.0b013e31824720da. [DOI] [PubMed] [Google Scholar]
- 20.Quirk K, Blurton P, Fletcher S, et al. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the α5 subunit. Neuropharmacology. 1996;35:1331–5. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- 21.Casula MA, Bromidge FA, Pillai GV, et al. Identification of amino acid residues responsible for the α5 subunit binding selectivity of L-655,708, a benzodiazepine binding site ligand at the GABAA receptor. J Neurochem. 2001;77:445–51. doi: 10.1046/j.1471-4159.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- 22.Atack JR, Bayley PJ, Seabrook GR, et al. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for α5-containing GABAA receptors. Neuropharmacology. 2006;51:1023–9. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Chambers MS, Atack JR, Carling RW, et al. An orally bioavailable, functionally selective inverse agonist at the benzodiazepine site of GABAA α5 receptors with cognition enhancing properties. J Med Chem. 2004;47:5829–32. doi: 10.1021/jm040863t. [DOI] [PubMed] [Google Scholar]
- 24.Atack JR, Maubach KA, Wafford KA, et al. In vitro and in vivo properties of 3-tert-butyl-7-(5-methylisoxazol-3-yl)-2-(1-methyl- 1H-1,2,4-triazol-5-ylmethoxy)-pyrazolo[1,5-d]-[1,2,4]triazine (MRK-016), a GABAA receptor α5 subtype-selective inverse agonist. J Pharmacol Exp Ther. 2009;331:470–84. doi: 10.1124/jpet.109.157636. [DOI] [PubMed] [Google Scholar]
- 25.Bonin RP, Martin LJ, MacDonald JF, Orser BA. α5GABAA receptors regulate the intrinsic excitability of mouse hippocampal pyramidal neurons. J Neurophysiol. 2007;98:2244–54. doi: 10.1152/jn.00482.2007. [DOI] [PubMed] [Google Scholar]
- 26.Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–42. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- 27.Cammack JN, Schwartz EA. Channel behavior in a γ-aminobutyrate transporter. Proc Natl Acad Sci. 1996;93:723–7. doi: 10.1073/pnas.93.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song I, Savtchenko L, Semyanov A. Tonic excitation or inhibition is set by GABAA conductance in hippocampal interneurons. Nat Commun. 2011;2:376. doi: 10.1038/ncomms1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franks NP, Lieb WR. Which molecular targets are most relevant to general anaesthesia? Toxicol Lett. 1998;100–101:1–8. doi: 10.1016/s0378-4274(98)00158-1. [DOI] [PubMed] [Google Scholar]
- 30.Joo DT, Gong D, Sonner JM, et al. Blockade of AMPA receptors and volatile anaesthetics: reduced anesthetic requirements in GluR2 null mutant mice for loss of the righting reflex and antinociception but not minimum alveolar concentration. Anesthesiology. 2001;94:478–88. doi: 10.1097/00000542-200103000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Watts MT, Escarzaga M, Williams CH. Gas chromatographic headspace analysis of sevoflurane in blood. J Chromatogr. 1992;577:289–98. doi: 10.1016/0378-4347(92)80250-t. [DOI] [PubMed] [Google Scholar]
- 32.Yeung JY, Canning KJ, Zhu G, et al. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]
- 33.Olsen RW, Sapp DM, Bureau MH, Turner DM, Kokka N. Allosteric actions of central nervous system depressants including anaesthetics on subtypes of the inhibitory γ-aminobutyric acid A receptor-chloride channel complex. Ann N Y Acad Sci. 1991;625:145–54. doi: 10.1111/j.1749-6632.1991.tb33838.x. [DOI] [PubMed] [Google Scholar]
- 34.Rogers CJ, Twyman RE, Macdonald RL. Benzodiazepine and β-carboline regulation of single GABAA receptor channels of mouse spinal neurones in culture. J Physiol. 1994;475:69–82. doi: 10.1113/jphysiol.1994.sp020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Study RE, Barker JL. Diazepam and pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of γ-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci. 1981;78:7180–4. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mihic SJ, Ye Q, Wick MJ, et al. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–9. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 37.Hemmings HC, Jr, Akabas MH, Goldstein PA, et al. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 38.McAdam LC, MacDonald JF, Orser BA. Isobolographic analysis of the interactions between midazolam and propofol at GABAA receptors in embryonic mouse neurons. Anesthesiology. 1998;89:1444–54. doi: 10.1097/00000542-199812000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Wu J, Harata N, Akaike N. Potentiation by sevoflurane of the γ-aminobutyric acid-induced chloride current in acutely dissociated CA1 pyramidal neurones from rat hippocampus. Br J Pharmacol. 1996;119:1013–21. doi: 10.1111/j.1476-5381.1996.tb15772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng VY, Martin LJ, Elliott EM, et al. α5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci. 2006;26:3713–20. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin LJ, Oh GH, Orser BA. Etomidate targets α5 γ-aminobutyric acid subtype A receptors to regulate synaptic plasticity and memory blockade. Anesthesiology. 2009;111:1025–35. doi: 10.1097/ALN.0b013e3181bbc961. [DOI] [PubMed] [Google Scholar]
- 42.Martin LJ, Zurek AA, MacDonald JF, et al. α5GABAA receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory. J Neurosci. 2010;30:5269–82. doi: 10.1523/JNEUROSCI.4209-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]