Abstract
Bivalve species with exceptional longevity are newly introduced model systems in biogerontology to test evolutionarily conserved mechanisms of aging. Here, we tested predictions based on the oxidative stress hypothesis of aging using one of the tropical long-lived sessile giant clam species, the smooth giant clam (Tridacna derasa; predicted maximum life span: >100 years) and the short-lived Atlantic bay scallop (Argopecten irradians irradians; maximum life span: 2 years). The warm water–dwelling giant clams warrant attention because they challenge the commonly held view that the exceptional longevity of bivalves is a consequence of the cold water they reside in. No significant interspecific differences in production of H2O2 and 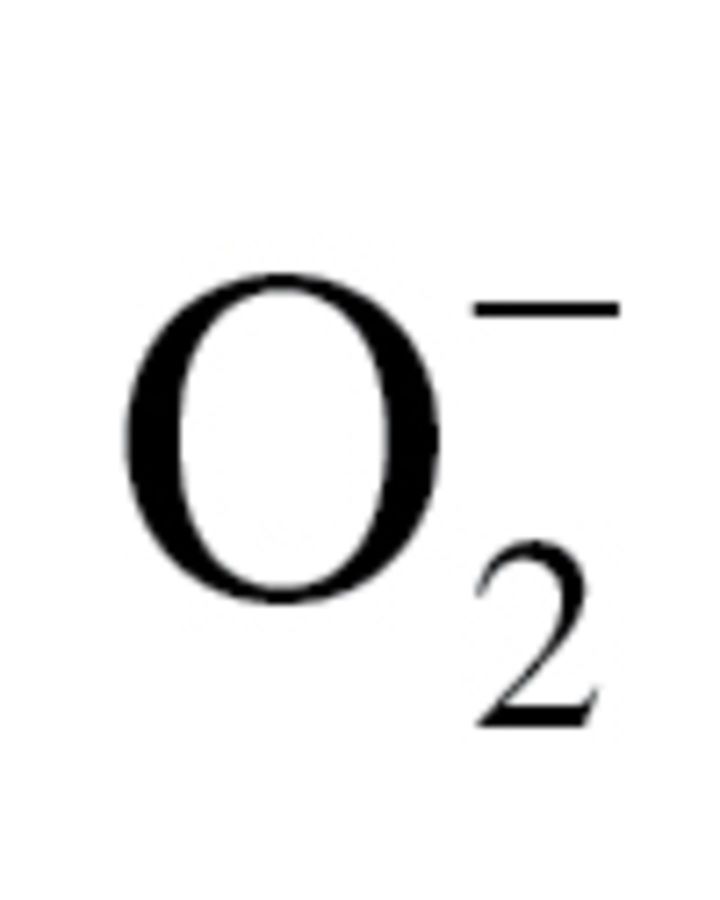 in the gills, heart, or adductor muscle were observed. Protein carbonyl content in gill and muscle tissues were similar in T derasa and A i irradians. In tissues of T derasa, neither basal antioxidant capacities nor superoxide dismutase and catalase activities were consistently greater than in A i irradians. We observed a positive association between longevity and resistance to mortality induced by exposure to tert-butyl hydroperoxide (TBHP). This finding is consistent with the prediction based on the oxidative stress hypothesis of aging. The findings that in tissues of T derasa, proteasome activities are significantly increased as compared with those in tissues of A i irradians warrant further studies to test the role of enhanced protein recycling activities in longevity of bivalves.
in the gills, heart, or adductor muscle were observed. Protein carbonyl content in gill and muscle tissues were similar in T derasa and A i irradians. In tissues of T derasa, neither basal antioxidant capacities nor superoxide dismutase and catalase activities were consistently greater than in A i irradians. We observed a positive association between longevity and resistance to mortality induced by exposure to tert-butyl hydroperoxide (TBHP). This finding is consistent with the prediction based on the oxidative stress hypothesis of aging. The findings that in tissues of T derasa, proteasome activities are significantly increased as compared with those in tissues of A i irradians warrant further studies to test the role of enhanced protein recycling activities in longevity of bivalves.
Key Words: Mollusc, Evolution, Oxidative stress resistance, Senescence, Oxidative stress theory.
The oxidative stress hypothesis of aging implies that organismal aging results from elevated levels of reactive oxygen species (ROS), generated as byproducts of mitochondrial respiration (1), which damage macromolecules and impair the function of cellular organelles (2). Although the oxidative stress hypothesis of aging continues to be among the most commonly adduced mechanistic hypotheses to explain variation in aging rate and age-related pathophysiological alterations (3–10), it is also a subject of ongoing debate (3–9). Significant controversy regarding the role of redox homeostasis in aging stems from the fact that most research on ROS-dependent mechanisms has been conducted on species that are relatively short lived and thus less successful at countering the aging process. Perhaps the strongest support for the oxidative stress hypothesis of aging comes from studies using a comparative approach as proposed by Austad (11–15) and others (16), which focus on phylogenetically diverse species with extreme longevity, identifying the causative mechanisms (eg, mitochondrial ROS production, antioxidant responses, resistance to oxidative stress) that distinguish them from shorter lived related species (17–19). In previous studies, the comparative approach proved to be especially useful to critically test the oxidative stress hypothesis of aging in mammalian and avian species (15,17,20–23).
Longevity evolved independently many times among the various phyla, and it is yet to be determined whether the role of cellular mechanisms involved in redox regulation and oxidative stress resistance determining the rate of aging and life span is conserved among these various groups. There is an growing interest in bivalve models of successful aging, as this invertebrate group includes species with the longest metazoan life spans with many species surviving for 100–400 years (including Arctica islandica, the longest living noncolony forming animal on earth; 13,14,24–33). Yet, the role of oxidative stress and antioxidant mechanisms in regulation of life span in bivalves is not as comprehensively understood as those in vertebrate models of aging (27–29,31–34). Recent studies have characterized several aspects of A islandica physiology (30–32,35) and comparison of A islandica with taxonomically related, short-lived clam species (eg, Mercenaria mercenaria) suggested that an association exists between longevity and resistance to oxidative stress–induced organismal and cellular death in burrowing clams (36). Moreover, extreme longevity in A islandica is also associated with an attenuated cellular H2O2 production and superoxide generation in the heart and gill (but not in the adductor muscle) (36) and a lower protein carbonyl concentration in gill tissue (36). These initial data on long-lived bivalves appeared to accord with predictions based on the oxidative stress hypothesis of aging. However, the aforementioned studies have entirely focused on a small number of long-lived species from the polar or temperate waters (30–32). Here, we have the rare opportunity to test predictions based on the oxidative stress hypothesis of aging using one of the tropical long-lived sessile giant clam species, the smooth giant clam (Tridacna derasa; predicted maximum life span: >100 years) and the short-lived Atlantic bay scallop (Argopecten irradians irradians; maximum life span: 2 years) as a longevity contrast pair (Table 1). The giant clams warrant attention because they are the outliers of the longevous bivalves, the only group of bivalves identified so far living in excess of a century, which reside outside the temperate waters; thereby challenging the commonly held view that the exceptional longevity of bivalves is a consequence of the cold water they reside in (27). The species of giant clam selected for this study (T derasa) is one of the largest giant clam species, which also maintains a symbiotic relationship with the photosynthesizing zooxanthellae, found associated with Pacific coral reefs, from a depth of 2 to 20 m in seawater temperatures of 20°C–30°C (37). For a comparative species, we used the shorter lived, epibenthic but active swimming A i irradians, which is found at depths of 0.3–10 m along the Eastern coast of the United States (38). Although found in slightly cooler water, there is a considerable overlap in the temperature ranges of these two species justifying their comparison. Previous studies reported that survival and development of eggs of A i irradians are optimal at water temperatures exceeding 20°C (39) and that cytoplasmic growth stage of gametogenesis is initiated once temperatures exceed 28°C (40).
Table 1.
Chronological Age, Estimated Maximum Life Span, and Physiological Characteristics of the Marine Bivalve Species Used in This Study
| Species | Common Name | Average Chronological Age (yr) | Estimated Maximum Life span (yr) | Maximum Size (mm) | Growth Rate (k [vbgf]) | Mortality Rate (z) | Age at Maturity (yr) | Lifestyle | References |
|---|---|---|---|---|---|---|---|---|---|
| Tridacna derasa | Smooth giant clam | 4 | >100 | 500 | 0.1 | 0.04 | 10 | Epifaunal | 61 |
| Argopecten irradians irradians | Atlantic bay scallop | ~1 | 2 | 60 | N/A | 1.41 | 1 | Active swimmer | 62 |
Notes: N/A = not applicable; VBGF = von Bertalanffy growth function.
On the basis of the oxidative stress hypothesis of aging, it is predicted that cells of long-lived species exhibit lower generation of ROS than cells of shorter living ones. One would also expect that successfully aging species have increased tolerance for oxidative stress–induced cellular injury through superior cellular antioxidant defense mechanisms or increased elimination of damaged macromolecules. To test these hypotheses in the present study, we compared ROS production, resistance to oxidative stress, antioxidant defenses, and protein damage elimination processes (proteasome activities) in T derasa and A i irradians.
Methods
Clam Collection and Maintenance
The T derasa used in the present study were nursery grown in the Western Pacific and couriered to the Marine Aquatic Resources Center of the Marine Biological Laboratory (Woods Hole, MA), where they were kept at 27°C in 500-L tanks for more than 1 week prior to the studies. A i irradians used in the present study were collected in July 2010 in the coastal waters of New England and transported to the Marine Aquatic Resources Center of the Marine Biological Laboratory, where they were kept at 20°C in 500-L tanks for more than 1 week prior to the studies. On the day of the experiments, the bivalves were dissected, and the gill, heart, and adductor muscle were isolated using microsurgery instruments and a stereo operating microscope. Fresh tissue samples were obtained for measurements of ROS production ex vivo. Also, samples from the gill, heart, and mantle were frozen in liquid nitrogen for subsequent biochemical analysis.
Determination of Individual Age
Individual age of the clams used was determined from internal shell growth band increments as previously described (41).
Measurement of Tissue H2O2 and 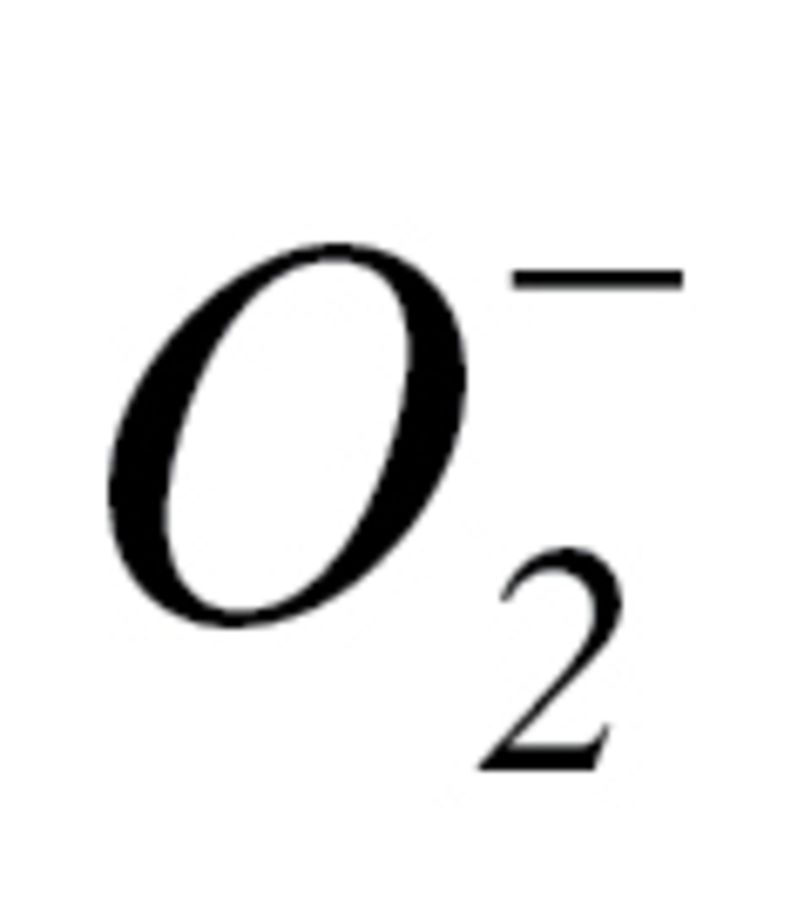 Production
Production
H2O2 production in the gill, heart, and adductor muscle tissue samples was measured fluorometrically using the Amplex Red–horseradish peroxidase assay as described (36). The rate of H2O2 generation was assessed by measuring resorufin fluorescence for 60 minutes by a Tecan Infinite M200 plate reader. Each experiment was run in triplicates. A calibration curve was constructed using H2O2, and the production of H2O2 in the samples was expressed as pmol H2O2 released per minute, normalized to tissue wet weight.
Production of 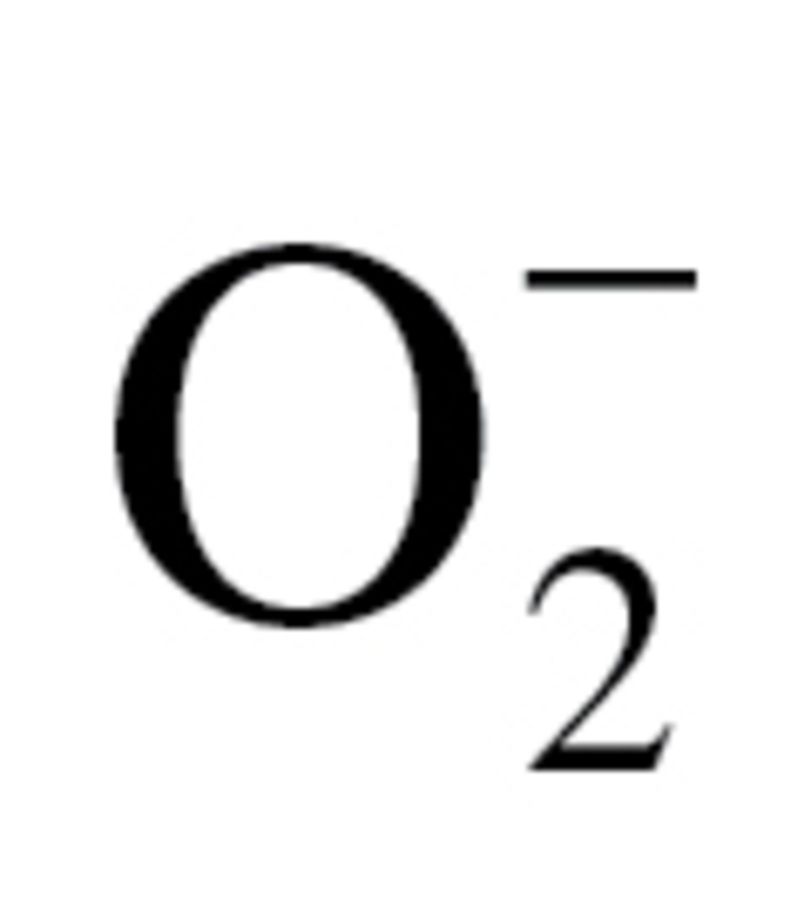 in the gill and the heart was determined using dihydroethidium, an oxidative fluorescent dye, as we previously reported (36,42). In brief, small tissue pieces were incubated with dihydroethidium (3 × 10−6 mol L−1; at room temperature, for 30 minutes). The tissues were then washed three times, embedded in optimal cutting temperature medium and cryosectioned. Optical sections were obtained and the red fluorescent images, captured at 20× magnification, were analyzed using the AutoMeasure function of the Axiovision (Carl Zeiss, Gottingen, Germany) imaging software. Four entire fields per tissue were analyzed. The mean fluorescence intensities of dihydroethidium-stained nuclei were calculated for each tissue. Thereafter, the intensity values for each animal in the group were averaged.
in the gill and the heart was determined using dihydroethidium, an oxidative fluorescent dye, as we previously reported (36,42). In brief, small tissue pieces were incubated with dihydroethidium (3 × 10−6 mol L−1; at room temperature, for 30 minutes). The tissues were then washed three times, embedded in optimal cutting temperature medium and cryosectioned. Optical sections were obtained and the red fluorescent images, captured at 20× magnification, were analyzed using the AutoMeasure function of the Axiovision (Carl Zeiss, Gottingen, Germany) imaging software. Four entire fields per tissue were analyzed. The mean fluorescence intensities of dihydroethidium-stained nuclei were calculated for each tissue. Thereafter, the intensity values for each animal in the group were averaged.
Determination of Protein Carbonylation
Protein carbonyl content was assessed in the gill and adductor tissues of T derasa and A i irradians using the Oxiselect Protein Carbonyl ELISA Kit (Cell Biolabs), as previously described (36).
Studies on Oxidative Stress Resistance
To assess resistance to oxidative stress, the bivalves were exposed to tert-butyl hydroperoxide (TBHP) in sea water as reported (36). TBHP is an organic peroxide that is highly stable in aqueous solutions. TBHP, known to cause cellular injury and induce apoptosis in a wide variety of eukaryotic cells by damaging DNA, lipids, and proteins, is a useful tool to assess cellular oxidative stress resistance. To study organismal resistance to oxidative stress, the survival of T derasa and A i irradians exposed to 10−4 mol/L TBHP was recorded for 10 days. Death was indicated by detachment of mantle from the shell, gaping mouth, and/or lack of light- and touch-responsive closing. To contrast oxidative stress–induced biochemical alterations in the gill and adductor of T derasa and A i irradians, the bivalves were exposed to 10−4 mol/L TBHP for 24 hours and their tissues sampled.
Apoptotic Cell Death
To compare cellular resistance to oxidative stress in T derasa and A i irradians, increases in the rate of apoptosis in response to TBHP (10−4 mol L−1, for 24 hours) were assessed. Gill segments and adductor muscle tissues were homogenized in lyses buffer, and caspase 3 activity, a useful measure of apoptosis, was measured as we reported (36), using the Caspase-Glo 3/7 assay system (Promega). Luminescent intensity was measured using an Infinite M200 plate reader and were normalized to the sample protein concentration.
Cellular Antioxidant Capacity
To compare the capacity of cellular antioxidant systems to counterbalance the deleterious effects of oxidative stress in tissues of T derasa and A i irradians, we assessed the Hydroxyl Radical Antioxidant Capacity (HORAC) and Oxygen Radical Absorbance Capacity (ORAC) using the Oxiselect HORAC Activity Assay (Cell Biolabs) and the Oxiselect ORAC Activity Assay (Cell Biolabs, San Diego, CA) as previously described (25,36). The HORAC activity assay is based on the oxidation-mediated quenching of a fluorescent probe by hydroxyl radicals produced by hydroxyl radical initiator and fenton reagent. The ORAC activity assay is based on the oxidation of a fluorescent probe by peroxyl radicals produced by a free radical initiator. Antioxidants present in the tissues delays the quenching of the fluorescent probe until the antioxidant activity in the sample is depleted. The antioxidant capacity of the quahog tissues was calculated on the basis of the area under the fluorescence decay curve, compared with an antioxidant standard curve obtained with gallic acid (for HORAC) or the water-soluble vitamin E analog Trolox (for ORAC), respectively. Sample protein concentration was used for normalization purposes.
Antioxidant Enzyme Activities
Activity of antioxidant enzymes in gill homogenates was measured using the Oxiselect Superoxide Dismutase Activity Assay Kit and the Oxiselect Catalase Activity Assay Kit (Cell Biolabs) and the Glutathione Peroxidase Assay Kit (Cayman Chemical, Ann Arbor, MI) as previously described (36).
Determination of Proteasome Activity
To compare protein recycling activities in tissues of T derasa and A i irradians, we assessed three types of protease activities associated with the proteasome complex in gill and adductor muscle samples using the Proteasome-Glo Chymotrypsin-Like, Trypsin-Like and Caspase-Like Assays (Promega, Madison, WI) as previously described (36).
Data Analysis
Where relevant criteria were met, data were analyzed using analysis of variance or t tests, and subsequently with Tukey’s or Fisher’s pair-wise tests. The Anderson–Darling Normality test was used to test if the data were normally distributed. Nonparametric data were analyzed using the Kruskal–Wallis one-way ANOVA test, with further pair-wise comparisons made using the Mann–Whitney test. Significance was considered to be at p < .05 level. Data are expressed as mean ± SEM, unless otherwise indicated.
Results
Age Determination
Following analysis of the internal growth increments, it was determined that the T derasa specimens had a mean age of 4 years, whereas the A i irradians specimens had a mean age of 12 months.
Cellular Production of Reactive Oxygen Species
Production of H2O2 in gill of T derasa tended to be less than in gill tissues from A i irradians, although the difference did not reach statistical significance (Figure 1A) (W = 68.0, p = .0923). Production of H2O2 in the adductor muscle (W = 52.0; p = .610) and the heart (W = 48.0; p = 0.272) did not differ significantly between the two species (Figure 1A). Analysis of nuclear dihydroethidium fluorescence intensities (Figure 1B) showed that cellular superoxide production also tended to decrease in the gill and the heart (W = 21.0; p = .081) of T derasa, although the difference did not reach statistical significance. In contrast, cellular 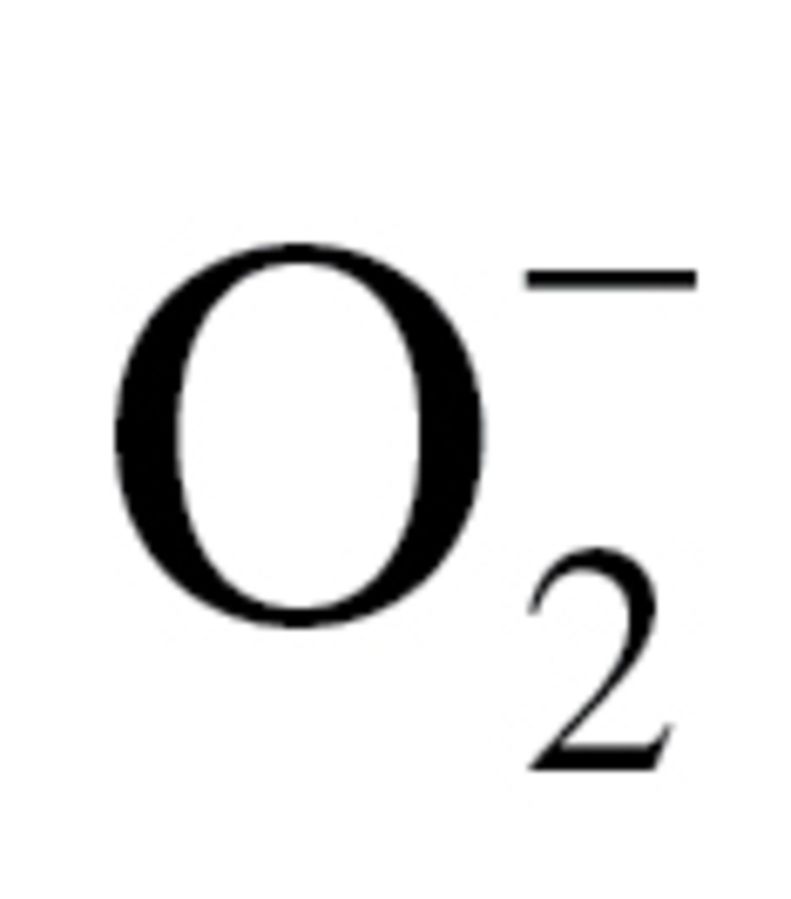 production tended to increase in the adductor muscle of T derasa (W = 50.0; p = .0137).
production tended to increase in the adductor muscle of T derasa (W = 50.0; p = .0137).
Figure 1.

(A) Production of H2O2 in the gill, heart, and adductor muscle of Argopecten irradians irradians and Tridacna derasa, as assessed by the Amplex Red/horseradish peroxidase assay. Data are mean ± SEM. n = 4–8 animals for each group. (B) Representative images showing red nuclear dihydroethidium fluorescence, representing cellular 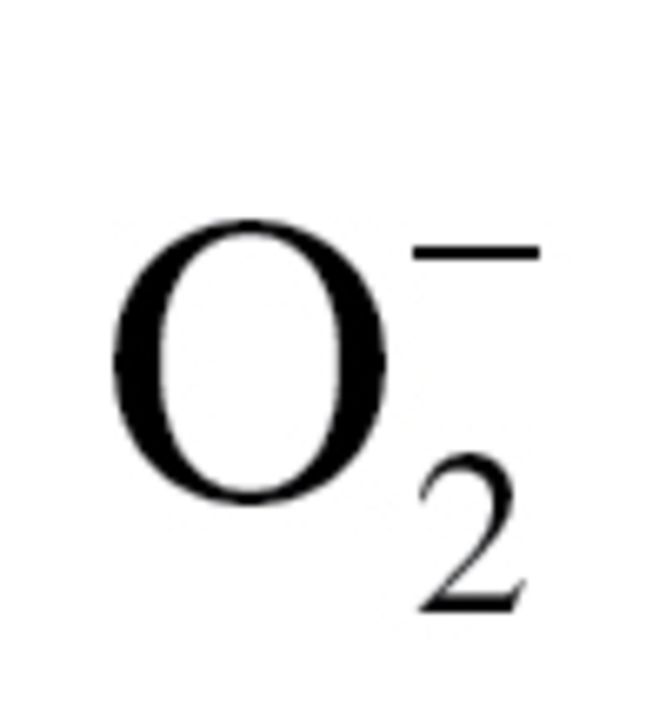 production, in sections of the gill, adductor muscle, and heart of A i irradians (left) and T derasa (right). Original magnification: 20×.
production, in sections of the gill, adductor muscle, and heart of A i irradians (left) and T derasa (right). Original magnification: 20×.
Protein Carbonyl Content
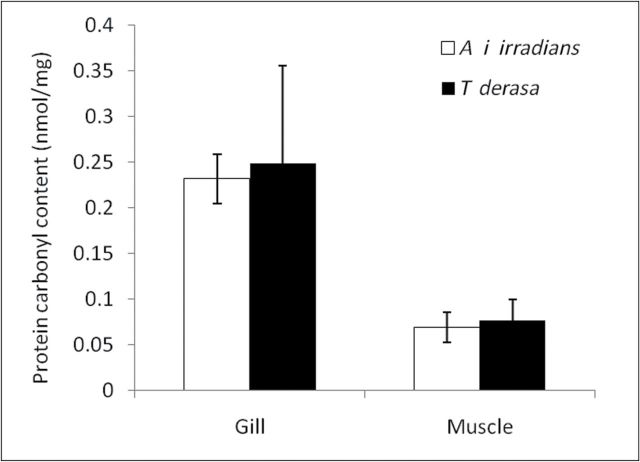
There was no difference in protein carbonyl content in the gill and adductor muscle between the two species (Figure 2).
Figure 2.
Carbonyl content in cellular proteins isolated from Argopecten irradians irradians and Tridacna derasa. Data are mean ± SEM (n = 5–8 in each group).
Survival
To assess resistance to oxidative stress, we obtained survival curves of the clams in the presence of TBHP. Analysis of the survival curves revealed that T derasa survived significantly longer than A i irradians in 0.1 mmol/L TBHP (p < .01; Figure 3). Comparison of the survival data to the recently published (36) survival curves of the extremely long-lived ocean quahog (A islandica, maximum life span: 405 years [41,43]) and the northern quahog (M mercenaria, maximum life span: ~106 years [36]) shows that both infaunal burrower hard clam species survive longer in TBHP than either T derasa or A i irradians.
Figure 3.

(A) Survival analysis of Argopecten irradians irradians and Tridacna derasa under exposure to 10−4 mol/L tert-butyl hydroperoxide (TBHP). (B) TBHP (10−4 mol/L, for 24h)-induced changes in caspase 3/7 activity in gills and adductor muscles of A i irradians and T derasa. Data ± SEM (n = 4–8 for each group). * indicates a significance (p < .05) difference between control and TBHP-exposed clams.
Apoptotic Cell Death
Short-term exposure to TBHP (10−4 mol/L, for 24 hours) elicited slight, although significant (p < .05) increases in caspase 3 activity in gill tissues but not in the adductor muscle of T derasa and A i irradians (Figure 3B). We attribute this difference to the limited diffusion of TBHP from the sea water into the muscle tissue, whereas the gill is directly exposed to the TBHP in sea water. We did not find any interspecies difference between the magnitude of TBHP-induced caspase activation in of T derasa and A i irradians gill tissues, likely due to the relatively short exposure time (the TBHP started to cause significant mortality after ~48 hours in both species).
Cellular Antioxidant Capacities
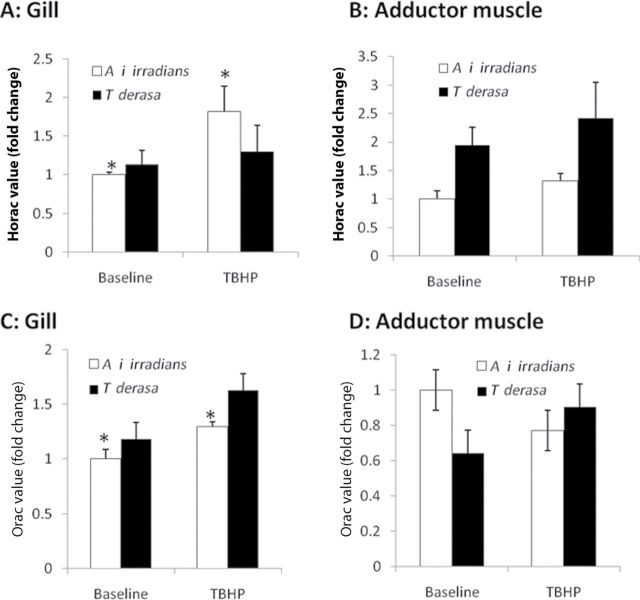
The baseline HORAC in the adductor muscle in T derasa is significantly higher than in A i irradians (W = 40.0; p = .0233) but no significant difference was observed between the gills of the two species (Figure 4). Following exposure of T derasa to TBHP, there was no significant response in the gill or adductor muscle. However, in A i irradians, there was a significant increase in HORAC in the gills following exposure to TBHP (W = 44.0; p = .0136).
Figure 4.
(A,B) Hydroxyl radical antioxidant capacity (HORAC) and (C,D) oxygen radical absorbance capacity (ORAC) in homogenates of gill tissues (A,C) and adductor muscles (B,D) from Argopecten irradians irradians and Tridacna derasa maintained under control conditions (baseline) or exposed to tert-butyl hydroperoxide (TBHP; 10−4 mol/L, for 24h). Data are mean ± SEM fold change (n = 4–8 in each group). * p < .05 vs T derasa.
Baseline data demonstrated no interspecies differences in the ORAC of the gill (W = 45.0; p = .270) and adductor muscle (W = 22.0; p = .067). Following the pattern observed in the HORAC analysis, the ORAC of both T derasa tissues exhibited no significant response after exposure to TBHP (Figure 4C and 4D). Similarly, ORAC values in the gills of A i irradians significantly increased following exposure to TBHP, mirroring the response observed in the HORAC data (W = 48; p = .046).
Antioxidant Enzyme Activities
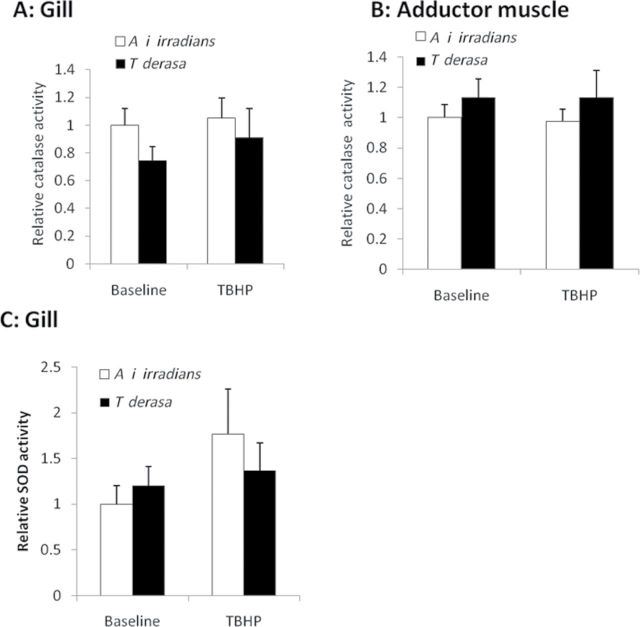
Analyses of the specific antioxidant enzyme activities revealed no significant interspecies differences. There was no significant difference in the baseline catalase activity when comparing gill and adductor muscle between the two species (Figure 5A and 5B) and also exposure to TBHP did not initiate any significant changes in catalase activity in either of tissue. There were no significant interspecies differences between superoxide dismutase activities in the gill tissues either under baseline conditions or following exposure to TBHP (Figure 5C).
Figure 5.
Relative antioxidant enzyme activities in Argopecten irradians irradians and Tridacna derasa. Catalase activity was assessed in homogenates of gill tissues (A) and adductor muscles (B) and superoxide dismutase activity was assessed in homogenates of gill tissues (C) A i irradians and T derasa maintained under control conditions (baseline) or exposed to tert-butyl hydroperoxide (TBHP; 10−4 mol/L, for 24h). Data are mean ± SEM (n = 4–8 in each group). * indicates a significance (p < .05) difference between control and TBHP-exposed clams.).
Proteasome Activity
Relative proteasome activity demonstrated the most contrasting set of results between the two species (Figure 6A–6E). Both chymotrypsin-like and trypsin-like activity in the gills of T derasa was significantly higher than in the gills of A i irradians (chymotrypsin-like: W = 36; p = .0043; trypsin-like: W = 41; p = .0338). In addition, the chymotrypsin-like activity in the adductor muscle of T derasa was significantly higher than in A i irradians (muscle: W = 42; p = .0481).
Figure 6.
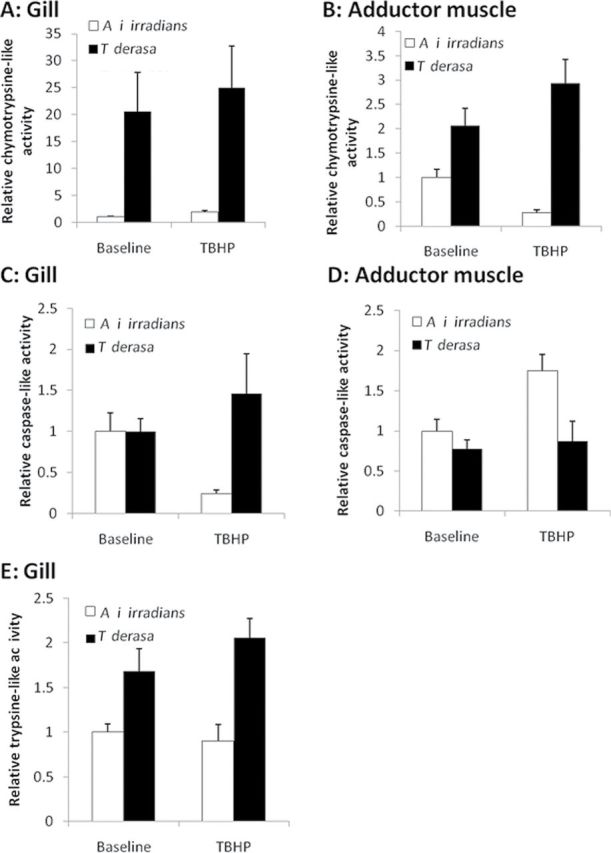
Relative proteasome activity in Argopecten irradians irradians and Tridacna derasa. Chymotrypsin-like activity (A,B), caspase-like activity (C,D), and trypsin-like activity (E) were assessed in homogenates of gill tissues (A,C,E) and adductor muscles (B,D) from Mercenaria. mercenaria and Arctica islandica maintained under control conditions (baseline) or exposed to tert-butyl hydroperoxide (TBHP; 10−4 mol/L, for 24h). Data are mean ± SEM (n = 8 in each group).
Following exposure to TBHP, T derasa exhibited no significant responses, whereas A i irradians exhibited a matching response in the case of chymotrypsin-like and caspase-like activity. In both instances, significant increases in activity in gill tissue and concomitant significant decreases in activity in adductor muscle tissue were observed (chymotrypsin-like activity—gill: W = 42; p = .0072; muscle: W = 42; p = .0072; caspase-like activity—gill: W = 46; p = .0239; muscle: W = 94; p = .0074). There were no significant differences in trypsin-like activity between baseline and response to TBHP exposure in either species or tissue.
Discussion
One of the seminal questions in biogerontology is why different species age at different rates. One prediction based on the oxidative stress hypothesis of aging is that the vast life-span variation among animal species is, in part, due to interspecies variation in cellular ROS production. We hypothesized that if in bivalve mollusk species, cellular production of ROS is a determinant in the rate of aging, then cells of successfully aging clams should produce less ROS than the short-lived ones. The data from the present study demonstrate that mitochondria-rich gill tissues from long-lived T derasa tend to produce less H2O2 and 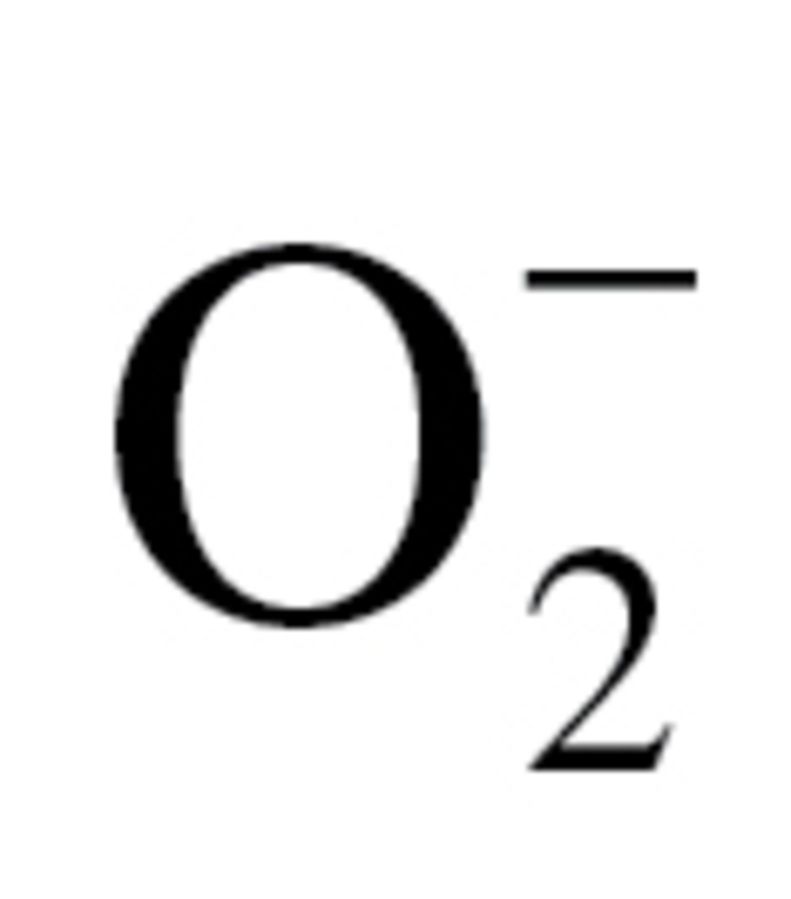 than gill tissues from short-lived A i irradians (Figure 1). These observations are consistent with the recent data obtained in gill tissues (36) and isolated mitochondria derived from shorter and longer living burrowing clams (29). Yet, contrary to our expectation, we did not find the same pattern in the heart and adductor muscle of T derasa and A i irradians (Figure 1). These data suggest that important tissue-specific differences exist in cellular ROS production in clams. Taken together, the observed cellular ROS production signatures in the T derasa and A i irradians longevity contrast pair are not consistent with the prediction of the oxidative stress hypothesis of aging.
than gill tissues from short-lived A i irradians (Figure 1). These observations are consistent with the recent data obtained in gill tissues (36) and isolated mitochondria derived from shorter and longer living burrowing clams (29). Yet, contrary to our expectation, we did not find the same pattern in the heart and adductor muscle of T derasa and A i irradians (Figure 1). These data suggest that important tissue-specific differences exist in cellular ROS production in clams. Taken together, the observed cellular ROS production signatures in the T derasa and A i irradians longevity contrast pair are not consistent with the prediction of the oxidative stress hypothesis of aging.
Another prediction based on the oxidative stress hypothesis of aging is that the long-lived species should show relatively low oxidative macromolecular damage even at young ages. The findings that protein carbonyl content in gill and muscle tissues were similar in T derasa and A i irradians (Figure 2) are not consistent with this prediction. Different conclusions were reached by previous studies on burrowing clams showing that reduced ROS production in long-living A islandica is associated with a lower level of accumulated macromolecular damage as compared with the shorter living M mercenaria (33,36).
Accumulating empirical data obtained in diverse vertebrate and invertebrate model systems suggest that resistance to the aging process is often reflected in resistance to oxidative stressors both at the organismal and cellular levels (11,44). Our previous studies demonstrated that the extreme longevity in A islandica is also associated with increased resistance to oxidative stress–induced mortality (36), raising the possibility that evolutionarily highly conserved pathways are involved in both cellular stress resistance and life-span regulation. In the present study, we exposed T derasa and A i irradians to organic peroxide treatment and observed an association between longevity and resistance to oxidative stress–induced mortality (Figure 3). These findings are consistent with the prediction based on the oxidative stress hypothesis of aging. Further studies are needed to assess indices of biomolecular damage in T derasa and A i irradians. Comparisons with infaunal bivalves (eg, A islandica and M mercenaria) are warranted to assess cellular responses to a range of diverse stressors to determine whether epibenthic species are more sensitive to oxidative injury, which will have ecotoxicological implications.
Contrary to our predictions based on the oxidative stress hypothesis of aging, we found that in T derasa neither basal antioxidant capacities (Figure 4) nor specific antioxidant enzyme activities (Figure 5) were consistently greater than in A i irradians. These results extend our previous findings (36) and those of Abele and colleagues (31) showing no difference in antioxidant capacities among cells of long-lived A islandica and other bivalve species. In that context, it is interesting to note that in mice with overexpression or genetic knockout of major antioxidant enzymes (including MnSOD, Cu, ZnSOD, catalase, and glutathione peroxidase), there is no correlation between alterations of cellular antioxidant capacity and life span (3,4). Because in response to oxidative stressors in eukaryotic cells, an evolutionarily conserved antioxidant response can be manifested, we also analyzed these antioxidant systems in tissues of clams exposed to TBHP. In contrast to our expectation, we found that T derasa did not exhibit consistently a more pronounced homeostatic antioxidant response than A i irradians (Figures 4 and 5). Recent studies also failed to find any consistent relationship between age-related changes in antioxidant enzyme activities and life span in various clams (36,45). In conclusion, the aforementioned data does not support a predominant role of superior free radical detoxification systems in the longevity of bivalve species.
Effective removal of oxidatively damaged proteins by the proteasome is thought to be a critical determinant of life span (23,46,47). Previous studies reported that in various species, proteasome activity declines with age (48–51) and this aging-induced proteasome dysfunction was proposed to be involved in the etiology and/or progression of various age-related diseases (52,53). The findings that in tissues of T derasa, proteasome activities are significantly increased as compared with those in tissues of A i irradians (Figure 5) raise the possibility that enhanced protein recycling activities may contribute to the longevity of giant clams. It should be noted that previously we could not demonstrate any significant differences between proteasome activities in tissues of long-lived A islandica and short-lived M mercenaria (36). There are several articles showing that lipofuscin in marine invertebrates increases in a faster rate at higher temperatures, thus at warmer temperatures repair mechanisms (eg, proteasomal activity) might be especially important to ensure cellular functioning over a long life span. Higher proteasome activities in T derasa as compared with that in A i irradians may also be due to higher protein turnover rates, which is characteristic to species living at higher temperatures (54). Further studies are evidently needed to compare additional mechanisms involved in maintenance of protein stability and integrity (23,55) and to investigate interspecies differences in protein repair and proteasomal degradation capacities in a larger cohort of bivalve species with disparate longevity.
Limitations of the Study
Although the giant clams (T derasa, Tridacna gigas, and Tridacna tevoroa) are believed to live in excess of 100 years, no precise information is available (56). Anecdotal information on the life span of single clams passed through generations in artisanal fishing communities suggests a maximum life span in excess of 100 years, and more recently Watanabe and his colleagues (57) published a 60-year isotopic record from the shell of a 93-cm long T gigas, below the maximum shell length of 137cm quoted for the species (37). Furthermore, analysis of the growth curve indicates that the asymptotic shell height had not been reached, suggesting the animal could be expected to live considerably longer. Demographic analysis of Tridacna populations by the Bombay Natural History Society also indicated a life span in excess of 100 years (Dr Deepak Apte, personal communication, 2010). Although the oft quoted maximal life span of 100 years remains unsubstantiated, there is considerable supporting evidence.
Due to logistical and legislative limitations, it was impossible to investigate age-related changes in the biology of giant clams. These clam species are listed as vulnerable on the IUCN redlist (58), which prevents the collection of older individuals from the wild and dictates a reliance on young nursery grown specimens. The giant clams used in our study were actively growing (below their asymptotic shell length), reproductively mature adults. Thus, they were in a life stage that is comparable to that of A i irradians specimens used in the same study. Due to the limited availability of T derasa, we could not test organismal and cellular resistance to stress challenges that cause cellular damage primarily via protein misfolding (eg, by inducing endoplasmic reticulum stress). We would also like to acknowledge that the primary cause of death in the survival studies is unknown. The time course of T derasa mortality upon TBHP administration suggests that the primary cause of organismal death does not involve TBHP-induced injury to the zooxanthellae and subsequent starvation. A previous study (59) assessing the antioxidant and oxidative damage response of the bivalve Ruditapes decussatus to exposure to organophosphorus pesticides, which are known to increase cellular ROS levels (60), suggest that the gill tissue is very sensitive to oxidative stressors. Yet, because the vital functions of bivalves depend on an intact fluid transport system, future studies investigating organismal stress resistance in clams should consider interspecies differences in cellular resistance to the cardiotoxicity of the stress challenges applied as well.
Conclusions
To our knowledge, this is the first comparative study that investigates cellular mechanisms involved in the process of aging using a species from the long-lived giant clam group. Our findings demonstrate diverse, tissue-specific ROS production signatures in the bivalves studied and a positive association between longevity and resistance to oxidative stress–induced organismal death in T derasa. These findings therefore only partially accord with predictions based on the oxidative stress hypothesis of aging. Our results warrant further studies to test the association between proteasomal activity and resistance to stress challenges that cause protein misfolding using a larger cohort of bivalve mollusk species with disparate longevity.
Funding
This work was supported by grants from the American Heart Association (to Z.U. and A.C.), American Federation for Aging Research (to A.C.), the Biotechnology and Biological Sciences Research Council (BBSRC, to I.D.R.), the Oklahoma Center for the Advancement of Science and Technology (to A.C. and Z.U.), the National Institutes of Health (NIH; AG031085 to A.C.; AT006526 to Z.U.; AG038747, NS056218, and P01 AG11370 to W. E. S.; AG022873 and AG025063 to S.N.A.), the Ellison Medical Foundation and the Arkansas Claude Pepper Older Americans Independence Center at University of Arkansas Medical Center (to A.C.). The experiments took place during the 2010 Biology of Aging Course at the Marine Biological Laboratory (Woods Hole, MA) organized by S.N.A., for which we thank The Ellison Medical Foundation.
Acknowledgments
We would like to thank Dr. Katherine Schafer-Hales, Mr. Christopher Rieken (Carl Zeiss Microimaging), and Mr. Ed Enos (Marine Aquatic Resource Center, Marine Biological Laboratory, Woods Hole, MA), for their invaluable help with the imaging experiments and the acquisition and maintenance of the bivalves used in the present study, respectively.
References
- 1. Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147 [DOI] [PubMed] [Google Scholar]
- 2. Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300 [DOI] [PubMed] [Google Scholar]
- 3. Jang YC, Pérez VI, Song W, et al. Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol A Biol Sci Med Sci. 2009;64:1114–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pérez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sentman ML, Granström M, Jakobson H, Reaume A, Basu S, Marklund SL. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem. 2006;281:6904–6909 [DOI] [PubMed] [Google Scholar]
- 6. Mansouri A, Muller FL, Liu Y, et al. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev. 2006;127:298–306 [DOI] [PubMed] [Google Scholar]
- 7. Van Remmen H, Ikeno Y, Hamilton M, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37 [DOI] [PubMed] [Google Scholar]
- 8. Wu S, Li Q, Du M, Li SY, Ren J. Cardiac-specific overexpression of catalase prolongs lifespan and attenuates ageing-induced cardiomyocyte contractile dysfunction and protein damage. Clin Exp Pharmacol Physiol. 2007;34:81–87 [DOI] [PubMed] [Google Scholar]
- 9. Mele J, Van Remmen H, Vijg J, Richardson A. Characterization of transgenic mice that overexpress both copper zinc superoxide dismutase and catalase. Antioxid Redox Signal. 2006;8:628–638 [DOI] [PubMed] [Google Scholar]
- 10. Buttemer WA, Abele D, Constantini D. From bivalves to birds: oxidative stress and longevity. Funct Ecol. 2010;24:971–98310.1111/j.1365-2435.2010.01740.x. [Google Scholar]
- 11. Austad SN. An experimental paradigm for the study of slowly aging organisms. Exp Gerontol. 2001;36:599–605 [DOI] [PubMed] [Google Scholar]
- 12. Austad SN. Diverse aging rates in metazoans: targets for functional genomics. Mech Ageing Dev. 2005;126:43–49 [DOI] [PubMed] [Google Scholar]
- 13. Austad SN. Comparative biology of aging. J Gerontol A Biol Sci Med Sci. 2009;64:199–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Austad SN. Is there a role for new invertebrate models for aging research? J Gerontol A Biol Sci Med Sci. 2009;64:192–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Austad SN. Methusaleh’s Zoo: how nature provides us with clues for extending human health span. J Comp Pathol. 2010;142(Suppl 1): S10–S21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barja G. Mitochondrial free radical production and aging in mammals and birds. Ann N Y Acad Sci. 1998;854:224–238 [DOI] [PubMed] [Google Scholar]
- 17. Lambert AJ, Boysen HM, Buckingham JA, et al. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell. 2007;6:607–618 [DOI] [PubMed] [Google Scholar]
- 18. Labinskyy N, Csiszar A, Orosz Z, et al. Comparison of endothelial function, O2-* and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Heart Circ Physiol. 2006;291:H2698–H2704 [DOI] [PubMed] [Google Scholar]
- 19. Sohal RS, Ku HH, Agarwal S. Biochemical correlates of longevity in two closely related rodent species. Biochem Biophys Res Commun. 1993;196:7–11 [DOI] [PubMed] [Google Scholar]
- 20. Csiszar A, Labinskyy N, Zhao X, et al. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007;6:783–797 [DOI] [PubMed] [Google Scholar]
- 21. Labinskyy N, Mukhopadhyay P, Toth J, et al. Longevity is associated with increased vascular resistance to high glucose-induced oxidative stress and inflammatory gene expression in Peromyscus leucopus. Am J Physiol Heart Circ Physiol. 2009;296:H946–H956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ogburn CE, Carlberg K, Ottinger MA, Holmes DJ, Martin GM, Austad SN. Exceptional cellular resistance to oxidative damage in long-lived birds requires active gene expression. J Gerontol A Biol Sci Med Sci. 2001;56:B468–B474 [DOI] [PubMed] [Google Scholar]
- 23. Salmon AB, Leonard S, Masamsetti V, et al. The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis. FASEB J. 2009;23:2317–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ridgway ID, Richardson CA, Austad SN. Maximum shell size, growth rate, and maturation age correlate with longevity in bivalve molluscs. J Gerontol A Biol Sci Med Sci. 2011;66:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ungvari Z, Bailey-Downs L, Gautam T, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ungvari Z, Philipp EE. Comparative gerontology–from mussels to man. J Gerontol A Biol Sci Med Sci. 2011;66:295–297 [DOI] [PubMed] [Google Scholar]
- 27. Philipp E, Brey T, Pörtner HO, Abele D. Chronological and physiological ageing in a polar and a temperate mud clam. Mech Ageing Dev. 2005;126:598–609 [DOI] [PubMed] [Google Scholar]
- 28. Philipp E, Heilmayer O, Brey T, Abele D, Portner HO. Physiological ageing in a polar and a temperate swimming scallop. Mar Ecol Prog Ser. 2006;307:187–198 [Google Scholar]
- 29. Philipp E, Pörtner HO, Abele D. Mitochondrial ageing of a polar and a temperate mud clam. Mech Ageing Dev. 2005;126:610–619 [DOI] [PubMed] [Google Scholar]
- 30. Philipp EE, Abele D. Masters of longevity: lessons from long-lived bivalves–a mini-review. Gerontology. 2010;56:55–65 [DOI] [PubMed] [Google Scholar]
- 31. Abele D, Brey T, Philipp E. Bivalve models of aging and the determination of molluscan lifespans. Exp Gerontol. 2009;44:307–315 [DOI] [PubMed] [Google Scholar]
- 32. Abele D, Strahl J, Brey T, Philipp EE. Imperceptible senescence: ageing in the ocean quahog Arctica islandica. Free Radic Res. 2008;42:474–480 [DOI] [PubMed] [Google Scholar]
- 33. Strahl J, Philipp EE, Brey T, Broeg K, Abele D. Physiological aging in the Icelandic population of the ocean quahog Arctica islandica. Aquatic Biology. 2007;1:77–84 [Google Scholar]
- 34. Strahl J, Abele D. Cell turnover in tissues of the long-lived ocean quahog Arctica islandica and the short-lived scallop Aequipecten opercularis. Marine Biology. 2010;157:1283–1290 [Google Scholar]
- 35. Ridgway ID, Richardson CA. Arctica islandica: the longest lived non colonial animal known to science. Rev Fish Biol Fisher. 2010;21:297–31010.1007/s11160-11010-19171-11169. [Google Scholar]
- 36. Ungvari Z, Ridgway I, Philipp EE, et al. Extreme longevity is associated with increased resistance to oxidative stress in Arctica islandica, the longest-living non-colonial animal. J Gerontol A Biol Sci Med Sci. 2011;66:741–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hardy JT, Hardy SA. Ecology of Tridacna in Palau. Pac Sci. 1969;23:467–472 [Google Scholar]
- 38. Broom MJ. Synopsis of biological data on scallops. FAO Fish Synopsis No 114 (FI RS/S114). 1976:43 [Google Scholar]
- 39. Castagna M. Culture of the bay scallop, Argopecten i rradians, in Virginia. Mar Fish Rev. 1975;37:19–24 [Google Scholar]
- 40. Sastry AN. The relationships among food, temperature, and gonad development of the bay scallop. Physiol Zool. 1968;41:44–53 [Google Scholar]
- 41. Wanamaker AD, Heinemeier J, Scourse JD, et al. Very long-lived molluscs confirm 17th century AD tephra-based radiocarbon reservoir ages for north Icelandic shelf waters. Radiocarbon. 2008;50:1–14 [Google Scholar]
- 42. Ungvari Z, Gautam T, Koncz P, et al. Vasoprotective effects of life span-extending peripubertal GH replacement in Lewis dwarf rats. J Gerontol A Biol Sci Med Sci. 2010;65:1145–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thórarinsdóttir GG, Einarsson ST. Distribution, abundance, population structure and meat yield of the ocean quahog, Arctica islandica, in Icelandic waters. J Mar Biol Assoc UK. 1996;76:1107–1114 [Google Scholar]
- 44. Kapahi P, Boulton ME, Kirkwood TB. Positive correlation between mammalian life span and cellular resistance to stress. Free Radic Biol Med. 1999;26:495–500 [DOI] [PubMed] [Google Scholar]
- 45. Fernández C, San Miguel E, Fernández-Briera A. Superoxide dismutase and catalase: tissue activities and relation with age in the long-lived species Margaritifera margaritifera. Biol Res. 2009;42:57–68 [PubMed] [Google Scholar]
- 46. Pérez VI, Buffenstein R, Masamsetti V, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci USA. 2009;106:3059–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kruegel U, Robison B, Dange T, et al. Elevated proteasome capacity extends replicative lifespan in Saccharomyces cerevisiae. PLoS Genet. 2011;7:e1002253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferrington DA, Husom AD, Thompson LV. Altered proteasome structure, function, and oxidation in aged muscle. FASEB J. 2005;19:644–646 [DOI] [PubMed] [Google Scholar]
- 49. Rodriguez KA, Gaczynska M, Osmulski PA. Molecular mechanisms of proteasome plasticity in aging. Mech Ageing Dev. 2010;131: 144–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dasuri K, Zhang L, Ebenezer P, Liu Y, Fernandez-Kim SO, Keller JN. Aging and dietary restriction alter proteasome biogenesis and composition in the brain and liver. Mech Ageing Dev. 2009;130:777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bulteau AL, Szweda LI, Friguet B. Age-dependent declines in proteasome activity in the heart. Arch Biochem Biophys. 2002;397:298–304 [DOI] [PubMed] [Google Scholar]
- 52. Gavilán MP, Pintado C, Gavilán E, et al. Dysfunction of the unfolded protein response increases neurodegeneration in aged rat hippocampus following proteasome inhibition. Aging Cell. 2009;8:654–665 [DOI] [PubMed] [Google Scholar]
- 53. Marfella R, Di Filippo C, Laieta MT, et al. Effects of ubiquitin-proteasome system deregulation on the vascular senescence and atherosclerosis process in elderly patients. J Gerontol A Biol Sci Med Sci. 2008;63:200–203 [DOI] [PubMed] [Google Scholar]
- 54. Clarke A, Fraser KP. Why does metabolism scale with temperature? Func Ecol. 2004;18:243–251 [Google Scholar]
- 55. Salway KD, Page MM, Faure PA, Burness G, Stuart JA. Enhanced protein repair and recycling are not correlated with longevity in 15 vertebrate endotherm species. Age (Dordr). 2011;33:33–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ramadoss K. Giant clam (Tridacna) resources. CMFRI Bulletin. 1983;34:79–80 [Google Scholar]
- 57. Watanabe T, Suzuki A, Kawahata H, Kan H, Ogawa S. A 60-year isotopic record from a mid-Holocene fossil giant clam (Tridacna gigas) in the Ryukyu Islands: physiological and paleoclimatic implications. Palaeogeo Palaeoclimatol Palaeoecol. 2004;212:343–354 [Google Scholar]
- 58.Wells S. Tridacna derasa. In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.1 www.iucnredlist.org Downloaded on 25 July 2012.
- 59. Abdel-Nabi IM, El-Shenawy NS, Taha IA, Moawad TS. Oxidative stress biomarkers and bioconcentration of reldan and roundup in the edible clam Ruditapes decussates. Curr Zool. 2007;53:910–920 [Google Scholar]
- 60. El-Shenawy NS, Moawad TS, Mohallal ME, Abdel-Nabi IM, Taha IA. Histopathologic biomarker response of clam, Ruditapes decussates, to organophosphorous pesticides reldan and roundup: A laboratory study. Ocean Sci J. 2009;44:27–34 [Google Scholar]
- 61. Pearson RG, Munro JL. Growth, mortality and recruitment rates of giant clams, Tridacna gigas and T. derasa, at Michaelmas Reef, central Great Barrier Reef, Australia. Aust J Mar Freshwater Res. 1991;42:241–262 [Google Scholar]
- 62. Bricelj VM, Eppa J, Maloufa RE. Comparative physiology of young and old cohorts of bay scallop Argopecten irradians irradians (Lamarck): mortality, growth, and oxygen consumption. J Exp Mar Biol Ecol. 1987;112:73–91 [Google Scholar]