Introduction
There are a number of reasons why the development of novel anthelmintics is very necessary (Geary et al., 1999). In domestic animals, parasites cause serious loss of production and are a welfare concern. The control of these parasites requires changes in management practices to reduce the spread of infection and the use of therapeutic agents to treat affected animals. The development of vaccines against parasites is desirable but their development so far has been very limited. One notable exception is the vaccination of calves against infection by Dictyocaulus viviparous (lungworm) which has proved to be very effective (Bain, 1999). The global antiparasitic market for drugs and chemicals has been estimated as follows: 1) Plant pathogens, $12 billion; 2) Livestock and companion animals, $11 billion; 3) Human health, $0.5 Billion. In humans there are serious problems of morbidity and mortality associated with parasite infections. 1.6 billion People throughout the world are infected with ascariasis (Fig. 1A) and/or hookworm. Approximately one-third of the world’s population is suffering from the effects of intestinal nematode parasites, causing low growth-rates in infants, ill-thrift, diarrhea, and in 2% of cases, loss of life. Despite the huge number of affected individuals, the market for anti-parasitic drugs for humans is not big enough to foster the development of anthelmintics because most infestations that occur are in undeveloped countries that lack the ability to pay for the development of these drugs. The major economic motivator then, is for the development of animal anthelmintics.
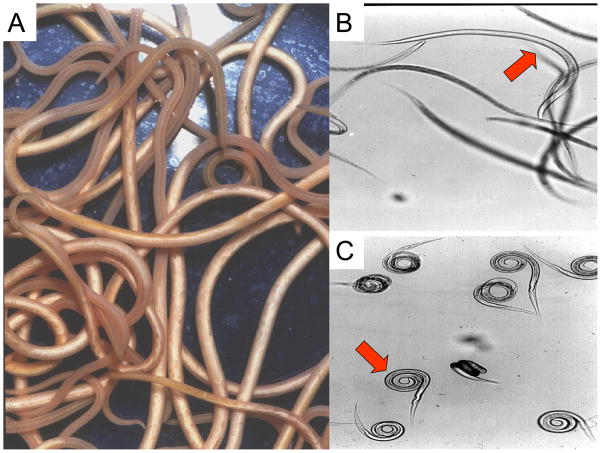
Fig. 1.
A: Adult Ascaris suum. These large intestinal nematode parasites of the pig are nearly identical to Ascaris lumbricoides found in the human intestine.
B: L3 exsheathed larvae of Ostertagia ostertagiae swimming freely in tap water
C: L3 exsheathed larvae of Ostertagia ostertagiae showing tight coiling or spastic paralysis following treatment with 10 μM levamisole.
In both domestic animals and now in humans, there is now a level of resistance to the available anthelmintic compounds (Bain, 1999). The resistance is either: constitutive, where a given species of parasite has never been sensitive to the compound; or acquired, where the resistance has developed through Darwinian selection fostered by the continued exposure to the antiparasitic drugs. The continued use of all anthelmintics has, and will, continue to increase the level of resistance. Cure rates are now often less than 100% and resistance of parasites to agents acting on the neuromuscular systems is present in a wide range of parasites of animals and humans hosts (Albonico et al., 2002) (Reynoldson et al., 1997).
In the face of this resistance the development of novel and effective agents is an urgent and imperative need. New drugs which act on the neuromuscular system have an advantage for medication for animals and humans because they have a rapid therapeutic effect within 3 hours of administration. Interestingly the world’s best selling antiparasitic drugs on the veterinary market are: ivermectin (effective against nematodes and some biting insects) and anti-insecticides (imidocloprid and fipronyl) act on neuromuscular ion-channels as do piperazine, pyrantel, and levamisole, showing that these are successful target sites. The effects on the neuromuscular system include: spastic paralysis with drugs like levamisole and pyrantel; flaccid paralysis as with piperazine; or disruption of other vital muscular activity as with ivermectin. Fig. 1B & C, illustrates an example of a spastic effect of levamisole on infectious L3 larvae of Ostertagia ostertagiae, a parasite of pigs. The effect was produced within minutes of the in vitro application of levamisole.
In this chapter we comment on the properties of existing agents that have been used to control nematode parasites and that have an action on neuromuscular systems. We then draw attention to resistance that has developed to these compounds, and comment on their toxicity and spectra of actions. We hope that some of the lessons that the use of these compounds has taught us may to be applied to any novel neuropeptide ligand that may be introduced. Our aim is then is to provide some warning signs for recognized but dangerous obstacles.
The existing antinematodal drugs with effects on neuromuscular systems
There are a limited number of classes of antinematodal drugs that are available (Martin, 1997) (Geary et al., 1999) (Kohler, 2001). It is usually assumed that compounds of the same class have the same mode of action, have similar spectra of actions, toxicities and can show cross-resistance. This assumption arises from the view that they have the same molecular sites of action. This is a commonly used assumption because it has the advantage of predicting spectra of actions and toxicities but is a limiting simplification because compounds that belong to the same class can have different receptor selectivities (Robertson et al., 2002) (Martin et al., 2003), can show differences in the details of their pharmacokinetic properties (Edwards & Breckenridge, 1988) (Baggot & McKellar, 1994) (Hennessy, 1997) toxicities and spectra of action. The different classes of anthelmintic drugs that have actions on nematode neuromuscular systems are now listed:
-
GABA agonists.
Piperazine is a GABA agonist that activates and gates GABA receptor channels on nematode muscle to produce an inhibitory effect and flaccid paralysis of the parasite (Martin, 1982; Martin, 1985). The drug is effective against larger nematodes found in the gastro-intestinal tract. The GABA agonists have not been developed beyond piperazine which has a limited spectrum of action. Although diethylcarbamazine is a piperazine derivative it is not a GABA agonist. Diethylcarbamazine appears to act as a lipooxygenase inhibitor and inhibits the production of leukotrienes so that it modifies the innate immune response (Piper & Temple, 1981). The modification of the innate immune response changes the host parasite balance and may be responsible for the elimination of microfilaria in the blood of hosts. Diethylcarbamazine is metabolized to a number of products in the mammalian host (Roy et al., 1981; Lee et al., 1997); one of these products is piperazine so that its mode of action against some nematodes, like hookworm, in the GI tract may be mediated by the generation of piperazine. Given the limited number of GABA agonists and the limited use of this class of compounds, it is possible this class of compounds could be developed in the future as novel anthelmintics. The use of piperazine has demonstrated that agents that cause muscle relaxation may be used as effective anthelmintics. Novel neuropeptide ligands that have similar effects (e.g. PF4 (Holden-Dye et al., 1997; Fellowes et al., 2000) but whose effects are mediated by other receptors (Purcell et al., 2002) might be developed.
-
Nicotinic agonists.
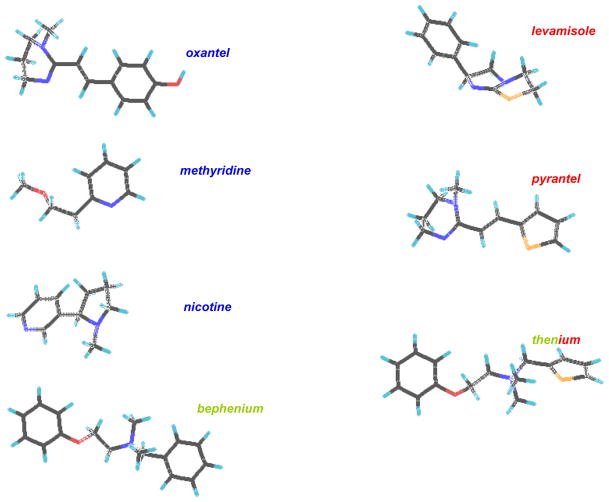
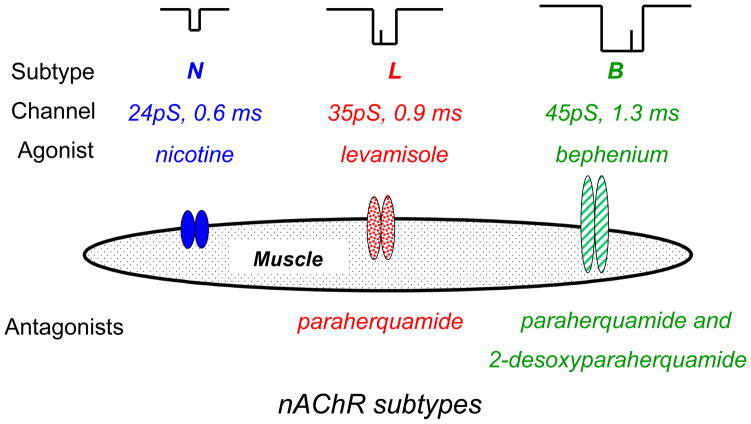
Levamisole and pyrantel are examples of nicotinic agonists (Fig. 2) that selectively gate acetylcholine ion-channels on nematode muscle and on nematode neurons to produce spastic paralysis and inhibition of egg-laying. There are a number of other nicotinic agonists including: butamisole, morantel, bephenium, oxantel and thenium that have been used or are still in current use. Their spectra of actions are broader than piperazine but depend on their pharmacokinetic distribution and receptor selectivity. Preparations of pyrantel (pamoate or embonate) are limited to the G.I tract oral administration and so are limited in action to effects on gastro-intestinal nematodes with no effect on lungworms. Levamisole, however, is distributed more widely, and can be administered by injection or by pour-on preparation. Levamisole reaches therapeutic concentrations in the lungs. It can be used to treat lungworm in some species like cattle. There are different subtypes of nicotinic receptor, in parasitic nematodes which have differences in pharmacological selectivities. We have observed 3 subtypes on Ascaris muscle (Fig. 3). There are the N-subtypes (nicotine and oxantel preferring), the L-subtype (levamisole and pyrantel preferring) and the B-subtypes (bephenium-preferring) in Ascaris suum (Qian et al., 2006). It is interesting that levamisole resistant Haemonchus contortus remain sensitive to bephenium (Sangster et al., 1991) and that Trichuris trichuris is not sensitive to pyrantel but is sensitive to oxantel (Lee et al., 1976). These observations illustrate the fact that even if the mode of action of a class of compounds appears to be similar, it is possible that the different members of the class of compounds have different selectivities for different subtypes of the receptor target site. The same phenomena could occur for novel neuropeptide ligands.
-
Nicotinic antagonists.
Paraherquamide, desoxyparaherquamide and phenothiazine can act as nicotinic acetylcholine channel antagonists (Robertson et al., 2002) phenothiazine (Mitchell, 1994). Phenothiazine is the oldest of this group of compounds and its use is perhaps of only historical interest. It was used more extensively to treat gastro-intestinal nematodes of sheep, cattle and horses (Boddie, 1952) but resistance and toxicity were limiting factors. It has now fallen into disuse. Paraherquamide and desoxyparaherquamide have some useful anthelmintic effects but currently, have not been developed commercially for therapeutic purposes. All of these nicotinic antagonists inhibit muscle motility and lead to paralysis and elimination of the nematodes.
-
Organophosphorous compounds.
Metriphonate and dichlorvos are examples of organophosphorous cholinesterase antagonists that have selective effects against both insect pests and nematodes but fortunately have only a limited effect on host cholinesterases. The organophosphorous compounds selectively and irreversibly block the breakdown of acetylcholine by cholinesterases. There is then an accumulation of acetylcholine at nerve terminal sites and this over stimulates nicotinic and muscarinic receptors on post-synaptic nerves, muscle and glandular tissue. It fatally disrupts the normal operation of nerve and muscle systems of pests and parasites by gating nicotinic receptor channels open and stimulating G-protein activated acetylcholine receptors. Organophosphorous compounds produce uncoordinated muscle and enteric activity in the parasite or pest and destroy its normal physiological control. These compounds still have small but limited use for the treatment of internal insect parasites like stomach bots of equidae. Although their spectrum of action includes both insect and nematode parasites, the effect of the organophosphorous compounds on host cholinesterases at higher drug doses, means that host toxicity is a problem. More recently, organophosphorous drugs have also been found to be associated with demyelinating neurotoxicities in humans exposed to these compounds. These toxicities can appear seen several weeks after exposure. Any novel neuropeptide agent which acts on very similar target sites in the parasite and the host will obviously carry with it the potential for toxic effect. The selectivity for the parasite receptor must be much higher than for the host receptors.
-
Avermectins.
This class of compounds includes: ivermectin, milbemycin, moxidectin, doramectin, abamectin and selamectin. The mode of action of this group of compounds is considered to be due to the selective activation of glutamate-gated chloride channels that are only found in invertebrates including nematodes and insects (Arena et al., 1995; Cully et al., 1996; Geary et al., 1999) (Kohler, 2001; Martin et al., 2002). Unfortunately these glutamate-gated chloride channels are also found in fresh water invertebrates including crustaceans. There is a possibility of toxicity if the avermectins get into pond and river water. Avermectins are also found in animal feces and affects on the biology of the dung beetles have been of concern (Steel & Waring, 2002).
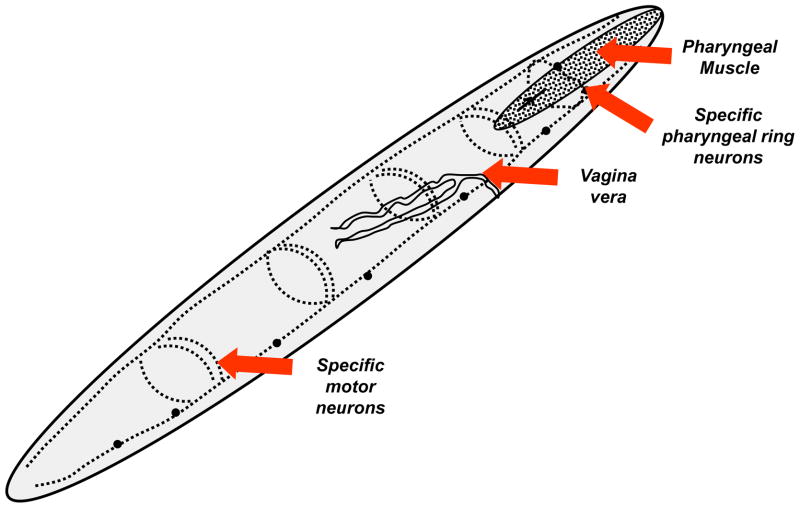
The avermectins can also activate some subtypes of GABAa ion-channel receptors at higher doses as well as inhibit N-subtype (ACR-16) α7-like nicotinic acetylcholine receptor channels (Raymond et al., 2000) of C. elegans. Depending on the species of nematode parasite, avermectins inhibit pharyngeal pumping and feeding (Geary et al., 1993; Martin, 1996; Sheriff et al., 2002), or will inhibit egg-laying, or will inhibit muscle motility (Gill et al., 1998). The effects on the different tissues of the nematode parasite might be explained by the presence of glutamate-gated chloride channels in these tissues (Fig. 4). The distribution of the glutamate-gated chloride channels appears to vary between nematode parasites and the sensitivity of the different tissues in the different nematodes also varies. The result is that the different species of nematode will be affected in different ways by a particular avermectin. For example pharyngeal pumping in Haemonchus contortus is very sensitive to ivermectin (Geary et al., 1993). The hookworm is less sensitive to avermectins (Richards et al., 1995) and Ancylostoma ceylanicum is 40–50 times more sensitive that Necator americanus. The avermectins have a very broad spectrum of action and that includes most nematode parasites, biting and sucking insect parasites but the avermectins do not have an action against trematodes like Fasciola hepatica or Schistosoma mansoni. The avermectins are much more potent than the other classes of anthelmintic. The very broad-spectrum of action and potency of the avermectin class of anthelmintics, has not so far not been bettered. The potency and very broad spectrum has given rise to their extensive use, resistance against these compounds, and the development of several therapeutic compounds from this class of drugs (Geary, 2005). Even though the avermectins are very potent, the development of resistance to them means that novel agents are still required, even if they are less potent and have a narrower spectrum of action than the avermectins.
-
Benzimidazoles.
Thiabendazole, albendazole and triclabendazole and others belonging to this class of anthelmintics, act by binding to β-tubulins and inhibiting the formation of microtubules (McKellar & Scott, 1990) (Lubega et al., 1994; Martin et al., 1997; Geary et al., 1998). The functions of microtubules are many and include intracellular transport, vesicular transport, cell division, cell shape and synapse formation. Benzimidazoles are slower acting than other anthelmintics and will upset the general biochemistry and homeostasis of nematodes and trematodes including their neuromuscular systems. In nematodes, they will inhibit egg production and muscle movement after a delay of 12 hours following administration of the drug to the patient or host animal. The spectrum of action of the more recent benzimidazoles like albendazole is broad (Gunawan et al., 1979). The benzimidazoles are effective against nematodes but hypobiotic nematodes and trematodes require higher doses. Trematodes are not sensitive to avermectins. Some benzimidazoles show evidence of teratogenic effects if they are administered in the first trimester of pregnancy. This toxic effect includes swollen chosto-chondral junctions (ribs) and an inability to extend limbs fully.
Parasites that are resistant to benzimidazoles are now fairly common and widespread in most countries and most host species. Resistance has been associated with changes in the structure of the β-tubulin genes with the β-tubulins becoming similar to host β-tubulin (Sangster et al., 1985) (Sangster & Gill, 1999).
-
Latrotoxin receptor agonist.
Emodepside (von Samson-Himmelstjerna et al., 2005), developed by Bayer under the influence of Achim Harder (Harder & von Samson-Himmelstjerna, 2002), inhibits neuromuscular transmission (Willson et al., 2003) and appears to act by activating a latrotoxin-like G-protein receptor that causes release of transmitter vesicles from synaptic terminals giving rise to paralysis. Emodepside has been introduced recently to the market. Because of its more limited spectrum of action it is combined with praziquantel to produce a broader spectrum product against both roundworms and tapeworms. This is an exciting novel agent and the further development of its use and perhaps other analogues might follow if this product is successful in the market.
-
Glutamate gated cation channels agonists and antagonists.
Kainate and quisqualate are found in significant therapeutic concentrations in seaweeds found in Asia (Davis, 1998). The extracts of the seaweeds were used successfully as anthelmintics before the active ingredients, kainate and quisqualate, were discovered to be agonists on excitatory glutamate receptors in the vertebrate C.N.S. Little development of these compounds as anthelmintics has been carried out. The presence of excitatory glutamate gated channels in C. elegans and transporters I Ascaris suggests that these ion-channel receptors or transporters might be considered as suitable target sites for potential drugs acting on the neuromuscular system of parasitic nematodes. If these compounds were to be developed for anthelmintic use, then selective compounds that are only selective for nematode glutamate receptor channels without an effect on host receptors will be necessary. MK-801 is a glutamate antagonist (Schaeffer et al., 1989) (Schaeffer et al., 1994) and that has a potent antinematodal action seen in C. elegans. The glutamate antagonists have not yet been developed as anthelmintics.
Fig. 2.
Chemical structure of nicotinic anthelmintics. The agonists that have a similar subtype selectivity are color coded: blue, N-type, red, L-type, and green, B-type
Fig. 3.
Subtypes of nicotinic acetylcholine receptor found on Ascaris muscle. There is the N-subtype, preferentially sensitive to nicotine, the L-subtype, preferentially sensitive to levamisole and antagonized by paraherquamide, and the B-subtype, preferentially sensitive to bephenium and antagonized by paraherquamide and desoxyparaherquamide. Diagram modified from Qian et al, 2006.
Fig. 4.
Diagram showing the predicted locations of avermectin receptors in a generalized parasitic nematode. The main locations include the pharynx, motor neurons, and the vagina vera. The diagram shows the two nerve cords and connecting commissures. Regions of receptor localization are marked with an arrow. Diagram modified from Martin et al., 2003.
Of the list of the 8 classes of drugs used for the treatment of parasites, 3 classes, (nicotinic agonists, avermectins and benzimidazoles) are used extensively to eliminate nematode parasites because of their efficacy, potency and wider spectrum of action. The remainder, GABA agonists, nicotinic antagonists, latrotoxin receptor agonists and glutamate agonists, currently, are not so widely used. Their use in the future will depend on the development of suitable agents. The organophosphorous compounds are less likely to be developed because of concerns over toxicity in animal hosts and humans.
Resistance is predicted
General comments
Resistance to most chemotherapeutic agents including any novel neuropeptide ligands that may be discovered and developed for the control of antiparasitic agents should be anticipated. Right from the days of Paul Erich, it was known that resistance to therapeutic agents could develop in parasitic species. Use of Trypan red by (Ehrlich & Shiga, 1904), to cure mice infected with mal de Caderas (trypanosomiasis), marked the beginning of modern ‘chemotherapy’, the process by which diseases, including those produced by parasites, are cured by treatment with chemical agents. Very soon after the discovery of the action of Trypan red, acquired resistance was observed. In 1905, Franke and Roehl, while working work with Ehrlïch, discovered that mice with trypanosomiasis, initially treated with Trypan red at low doses, became unresponsive to the original curative dose (Browning, 1907).
‘Resistance occurs when a greater frequency of individuals in a population of parasites, usually affected by a dose or concentration of compound are no longer affected’ and resistance is inherited, (Prichard, 1990). We have just mentioned how Franke and Roehl (Browning, 1907), while working work with Ehrlïch, discovered that mice with trypanosomiasis, treated with Trypan red at low doses, became unresponsive to the original curative dose. This is probably the first account of the development of resistance to chemotherapeutic agents, not only for nematode parasites but more generally for all therapeutic agents including bacteria viruses and malignant cancers.
The development of resistance to one agent within a chemical class, say albendazole, is expected to result in resistance to another member of that same class, say thiabendazole: we may refer to this as Cross-Resistance other individuals refer to this a side-resistance. Similar cross-resistance may be expected within the different classes of compound, say for example between the avermectins, ivermectin and doramectin. The cross-resistance may arise if the different compounds within the same class act on the protein target and its affinity for the drug class changes; or if uptake, metabolism or excretion of the drug class changes in the parasite to limit toxicity. If parasites become resistant to several different classes of anthelmintic then we refer to this as Multiple-resistance. Examples include Haemonchus contortus isolates, some of which show resistance to different classes including, avermectins, benzimidazoles and nicotinic anthelmintics (Waruiru et al., 1997; Gill et al., 1998; Sangster et al., 1999; Zajac & Gipson, 2000).
Polygenic resistance
Because resistance is inherited and carried genetically, it should be detectable by appropriate molecular markers or assays. However, to date, the development of molecular markers has met with limited success, perhaps because the resistance is polygenic (Martin & McKenzie, 1990; Jackson, 1993; Coles et al., 2006). It appears that many genes are involved in the therapeutic response and these have still be characterized. The detection of resistance to benzimidazoles has been possible in some isolates by looking for single nucleotide polymorphisms (SNPs) of the β-tubulin genes (Lacey & Prichard, 1986) in some species of parasitic nematodes (Lubega & Prichard, 1991; Geary et al., 1992; Roos et al., 1993; Lubega et al., 1994). A phenylalanine to tyrosine change at position 200 (P200Y) in isotype 1 β-tubulin (equivalent to TTC to TAC in the nucleotide of isotype 1 – there being 4 isotypes present in some parasitic nematodes but 6 in C. elegans) has been associated with resistance in some isolates of Haemonchus contortus. We know however, that all benzimidazole resistance is not associated with this change. Resistance to avermectins has been suggested to be involved with a number polymorphisms of genes including GABA subunit genes Blackhall, 2003 323 /id}, P-glycoproteins (Blackhall et al., 1998a) glutamate-gated chloride channel subunits (Blackhall et al., 1998b) polymorphism on GluClα3β (L256F) in some instances and innexins and dye filling genes (Dent et al., 2000). It seems likely that there will be many alleles and many SNPs that are associated with resistance, in part because the target sites of the anthelmintics are not single receptors because there is considerable redundancy with different isotypes of the receptors being present in the parasite. There are 4 β-tubulin genes producing 4 target sites for benzimidazoles in parasitic nematodes, and more than 4 genes (equivalent to C. elegans glc-1, glc-2, avr-14, and avr-15) that form the ion-channel target sites of the avermectins (Wolstenholme et al., 2004). The distribution of some of the proteins involved in avermectin resistance in C. elegans is illustrated in Fig. 5. If we consider the number of genes that are responsible for the expression and formation of the target site of an anthelmintic, along with genes that affect responses to the target site of the anthelmintic, like the down-stream effectors, then it is not surprising that anthelmintic resistance has been found to be polygenic in parasitic nematodes. Since resistance has followed the introduction of all anthelmintics, we should also anticipate that resistance to any novel anthelmintic that is a neuropeptide ligand will also occur and will involve changes in several genes.
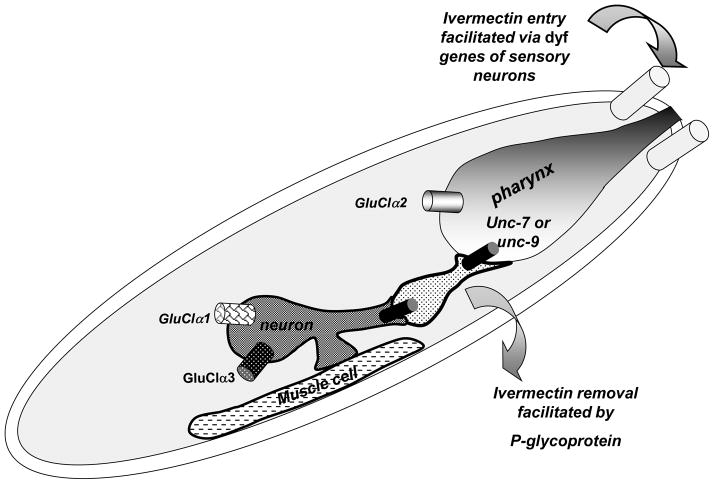
Fig. 5.
A diagram of the protein products of genes involved in ivermectin resistance in C. elegans. Derived from Dent et al., 2000
Fitness
It has usually been assumed that resistance is associated with a loss of fitness due to the loss of a drug target site. This loss of fitness is expected to allow reversion, a return to sensitivity when the anthelmintic is withdrawn. This logic ignores the possibility, and perhaps the necessity, of the parasites to retain its fitness in order to survive in vivo. So, in addition, the parasites are expected to accumulate other alleles (adaptation alleles) not directly needed for the resistance but that are required to recover the fitness of the parasite. Suppose that if a nematode parasite becomes resistant to levamisole by reducing the number of nicotinic acetylcholine receptors most sensitive to levamisole (L-subtype (Qian et al., 2006); to retain fitness, it might recover a level of fitness by increasing the number of muscarinic acetylcholine receptors on the muscle and/or reducing the number of GABA receptors on the muscle. So we predict that resistance is associated with two types of genetic modifications: the alleles associated directly with resistance and the alleles associated with adaptation or recovery of fitness. Both are required to allow a successful resistant nematode to survive. We would expect to see the same process to be associated with the development of resistance to any novel neuropeptide receptor anthelmintic.
The development of resistance
The studies on the genome of parasitic nematodes including Haemonchus contortus have revealed the presence of: ‘Extreme polymorphism’ at the nucleotide level. The single nucleotide variation in nematodes is large (Van, V & de, 2004; Hoglund et al., 2004; Eng & Prichard, 2005; Hoglund et al., 2006) and varies from 0.05 to 2% in shotgun sequences. This implies that many alleles that could contribute to resistance are already present in the population of parasitic nematodes. These resistance alleles are present at a low level before the selection pressure of anthelmintics is applied but increase with the maintained use of the anthelmintic.
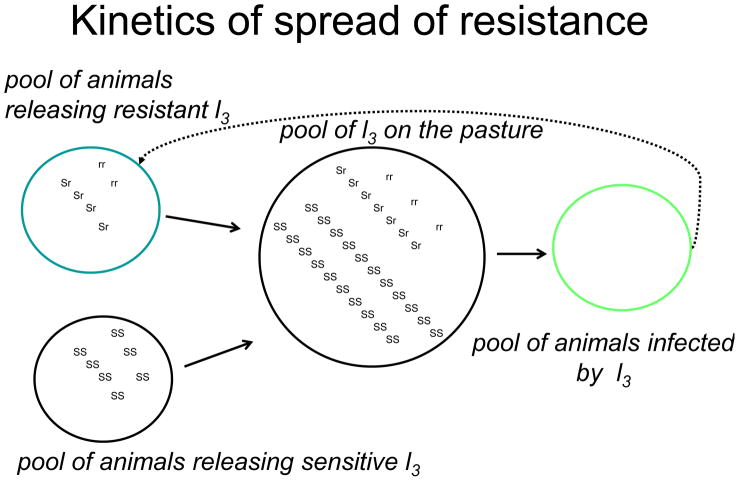
It is assumed that resistance in the parasite population occurs when a selective agent (the anthelmintic) kills susceptible worms but allows the resistant worms that carry resistance alleles to survive and to reproduce. The use of anthelmintics will provide a powerful selection pressure for the increase in the frequency of resistance alleles and encourage the dispersal of those alleles throughout the population. Fig. 6 illustrates how this may occur with the anthelmintic selecting for the resistant genes. If each resistance allele only contributes a small component to the resistance – one example might be an allele that increases the expression of a P-glycoprotein transporter which could reduce the concentration of the avermectins in the parasite by 50% – then a low dose of the anthelmintic will encourage the accumulation of this allele and other low level resistance alleles. If the dose of anthelmintic is then gradually increased with each passage of the life-cycle of the nematode, then the low level resistance genes will accumulate and concentrate in the surviving population. The simultaneous presence of lower-level resistance genes, particularly if they are recessive, will give rise to a much higher level of resistance (Dent et al., 2000). So we can start to see that there will be different stages of the development of resistance. We can start with emergence, the first stage, followed by development, the second stage with accumulation of resistance alleles, followed by full resistance, where clinical resistance is seen with little response to therapeutic doses of the anthelmintic. The resistance will develop more quickly in a population if low or under-dosing is practiced. Sub-therapeutic doses occur if the drug preparation has a very long half-life (t1/2) so that the drug concentrations tails off slowly over a long time so that there is a long period of sub-therapeutic concentrations present. The same sorts of problems and the development of resistance should be anticipated for a new product developed as a neuropeptide ligand.
Fig. 6.
Diagram showing the pool of resistance genes in L3 larvae on pasture and how they may be affected by contamination from animal grazing the pasture.
The rate of accumulation of resistance alleles for a novel neuropeptide anthelmintic ligand will depend on a number of factors (Wolstenholme et al., 2004). These include:
The use of low or sub-therapeutic anthelmintic doses.
The speed of the life cycle of the parasite – resistance will appear more quickly if there is rapid life cycle of the nematode parasite. It will also be faster if there is a direct life cycle. An indirect-life cycle will slow the accumulation of resistance alleles.
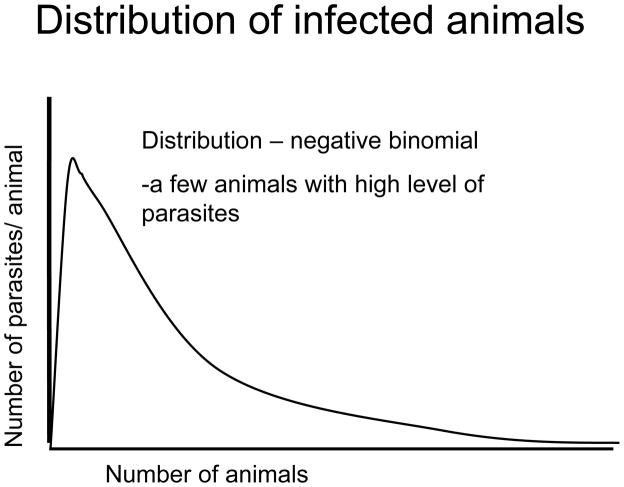
The presence of isolated pockets of sensitive parasites (refugia) that are not subject to the selection pressure. If these parasites have a fitness advantage they will continue to dilute the resistance alleles and limit the rate of appearance of resistance. Refugia will also dilute out the resistance alleles. Refugia will slow the accumulations of resistance alleles. One approach to limit the speed of development of resistance is to treat selected, clinically affected animals and leave those that are not clinically affected to provide refugia for sensitive alleles. Only a small proportion of the population, Fig. 7, shows a high level of infection, the remainder have a low level of infection. The distribution of infected animals is described by a negative binomial (few animals with high levels of infections others with decreasing levels of infection). It turns out that a number of factors, like resistance of individual host animals to parasite infection, the dose level of the infection, and the status of the host immune system means that few animals harbor high infection levels but most do not. To limit the accumulation of resistance it is considered better to treat those animals with high infection rates and to leave those with low infection rates to provide the refugia of sensitive parasites. However it will be necessary to treat animals or patients that are showing clinical signs. A particular scheme, FAMACHA (Kaplan et al., 2004) where this is practiced is in the control of Haemonchus contortus were many of the anthelmintics may become ineffective because of rapid development of resistance. In order to maintain the refugia and to control more pathogenic nematode parasites, narrow spectrum anthelmintics effective against the pathogenic species may be considered useful to introduce if the development costs can be reduced.
To reduce the probability of obtaining full clinical resistance, it is more desirable if the anthelmintic has several sites of action that require several genes changes to be present simultaneously before the resistance appears clinically. It would be useful if the novel neuropeptide ligand had an effect on multiple isotypes of receptor rather that a single receptor coded for by one gene. Targeting multiple protein receptors from genes that are genetically separate will require that any resistance is polygenic. The greater the number of separate genes then the slower will be the development of resistance. We have referred to an anthelmintic that requires the development of many genes to allow resistance as the MISA (multiple independent site of action) anthelmintics (Martin et al., 1998). A MISA neuropeptide ligand as an anthelmintic is more desirable and will limit the rate of appearance of resistance.
Combinations of two or more anthelmintic that have actions at target sites that are genetically separate will reduce the rate of accumulation of resistance. If possible a combination of novel neuropeptide receptor ligands would be desirable to reduce the rate of accumulation of resistance alleles. Pharmaceutical companies may be reluctant to do this because of licensing costs but they might be able to combine two active molecules into a single molecule to overcome the need to license two separate drugs.
Fig. 7.
Diagram of the negative binomial distribution that describes the distribution of the numbers of parasites in the animals of a flock or herd. Only a few animals have a high number of parasites; the remainder have lower levels of infection. It is suggested that these very highly parasitized animals should be treated selectively as per the FAMACHA system.
Cross resistance of novel neuropeptide anthelmintic with existing anthelmintics
We have mentioned already that cross-resistance can occur with the same class of anthelmintic agents. We may also expect to see cross-resistance if there is an overlap in the mechanism of action of the novel neuropeptide receptor ligand with existing agents. For example we know that levamisole and pyrantel activate subtypes on nicotinic acetylcholine channels on nematode muscle cells to produce contraction. Suppose a novel anthelmintic that is an agonist of the neuropeptide AF2 receptor were developed successfully as an anthelmintic. AF2 increases contractions in some model nematodes, presumably by activating a G-protein coupled receptor (Greenwood et al., 2005). Muscle contraction involves an increase in the cytosolic calcium which is coupled to the proteins that produce contraction. Suppose also that resistance to levamisole and pyrantel is produced by a null mutant or allele of a homologue of the unc-69 gene (Jones & Sattelle, 2004) that produces calcium induced calcium release to amplify the increase in cytosolic calcium and facilitates contraction. This resistant allele would be less sensitive to levamisole and pyrantel as well as our novel anthelmintics like AF2 receptor ligand and would show cross-resistance. So the lesson here would be to minimize the selection target sites for drug development that have the potential for overlapping mechanisms with existing anthelmintics.
Broad Spectrum or narrow spectrum
A small molecule neuropeptide receptor ligand may be easier to be developed that has a narrow spectrum perhaps with an activity against a few species of parasite. This is based on the knowledge that the distribution of the neuropeptides within the same neurons in different species of nematode appears to be unique (Yew et al., 2005). Many identical neurons in the parasitic nematode A. suum and in the model nematode C. elegans do not have the same neuropeptide transmitter present. This implies that the receptors of the neuropeptide may be different between nematode species and that the effect of a small molecule ligand agonist will vary between nematode species, depending on the distribution of the receptors. It will be necessary to overcome this potential limitation when developing an anthelmintic compound that is active against a wide range of species (broad spectrum).
Given the inevitable costs associated with discovering and developing a small molecule ligand it will be important to find compounds that have a broad spectrum that kill most parasites and all the significant pathogenic parasites. Drugs that can be used as a preventative as well as curative agent have a marketing advantage especially if they can act as an endo-parasiticide and an ecto-parasiticide like the avermectins (moxidectin and ivermectin). It will be necessary to focus on the neuropeptides and their receptor ligands that act across a range of parasites and phyla (Mousley et al., 2005). Some peptides have effects in arthropods as well as in A. suum (Mousley et al., 2004) but we do not know if they are mediated by the same receptor. In addition it will be important for the active agents to have effects in a range of domestic species, including cattle, which on a worldwide basis is the most significant economic species (in terms of potential revenue to the pharmaceutical company).
Potential problems associated with neuropeptide receptors as target sites for anthelmintics
We have described the mode of action of existing anthelmintics and we can see that many have an action on ligand-gated ion-channels. Only emodepside may activate a G-protein coupled receptor to exert its effect. Most of the neuropeptides activate receptors that are expected to be GPCRs (Greenwood et al., 2005). There are a number of potential problems that will need to be navigated to allow a successful anthelmintic to be developed.
RNAi knock down has been used to reduce the expression of putative neuropeptide receptors in the model nematode, C. elegans (Kamath et al., 2003). This large ranging RNA interference survey did not find many observable changes (phenotypes) that were produced by the RNAi. Another study aimed at 60 putative neuropeptide receptor GPCRs and neurotransmitters (Keating et al., 2003) found only 13 phenotypes produced by RNAi that were characterized by uncoordinated movement and changes in egg lying. The main point that can be drawn form these two studies is that RNAi experiments have failed to identify behavioral changes associated with knock down of most of the neuropeptides or putative GPCR receptors. We can predict on a basis of these observations that antagonists of neuropeptides or their receptors is unlikely to produce an effect. Agonists however are very different. We know that the neuropeptides including the FaRPs peptides produce dramatic effects when applied to neuromuscular preparation of nematodes (Maule et al., 1996). Thus we expect that antagonists of the neuropeptide receptors will show very little response and be less likely to be successful anthelmintics. In contrast agonists of the neuropeptide receptors are expected to be more successful as anthelmintics.
GPCRs are noted for their desensitization. It will be necessary to check receptor tachyphylaxis does not limit the novel neuropeptide agonist action as an anthelmintic.
Neuropeptides may activate several receptors either in the same species or across phyla (Greenwood et al., 2005; Mousley et al., 2005). A synthetic drug selected for activating one receptor may activate other receptors in the same species or across species and phyla. This will be and advantage if there is a synergistic effect between the activated receptors in the same species. There is also a potential for antagonism between activated receptors in the same species.
High-throughput screens for neuropeptide ligands
The selection of the anthelmintic neuropeptide agonist will probably be achieved through high-throughput primary screens rather than using slower primary screens based on animal studies (Geary et al., 1999; Maule et al., 2002; Geary et al., 2004). The consequence is that there is no-longer a minimal screen for toxicity and pharmacokinetics. It is missed and has to be done later. This means that methods for screening for active compounds can lead to more false starts. The development of the present successful anthelmintics (nicotinic anthelmintics, benzimidazoles and avermectins) were based on whole animal studies. The development of the avermectins for example used Nippostrongylus in mice as a screen.
The new high throughput screens rely on modern molecular and genetic techniques with cloning and expression of G Protein-coupled receptors and even some ion-channels into yeast, bacteria, and insect or mammalian cell systems. When these cell systems are grown in multi-well plates and the receptors coupled to growth or a fluorescent signal (Stables et al., 1997) they can be screened by thousands of compounds very rapidly.
It is pointed out that many of the potential GPCRs of C. elegans have been identified by bioinformatics, their natural ligand and functions in the nematodes remain to characterize in most instances. Expression of the nematode G Protein-coupled receptors remains in cells systems has not been easy. There have been difficulties with culture temperatures, perhaps affecting protein folding; there have been difficulties with protein trafficking with different signal sequence being present in nematodes and mammalian preparations (Geary & Kubiak, 2005). Despite this, let us take it that these systems can be developed for the screening of G protein receptors and ion-channels of nematode and insect neuropeptide receptors. Let us also take it that the high-throughput screening systems will allow an active ligand to be recognized and a significant ‘hit’ to be identified. It turns out there is still an enormous amount of work to convert the hit into an effective pharmaceutical product. That work will include minimizing any toxicity and getting the optimizing pharmacokinetics of the compounds for the best delivery of the drug.
Toxicity and safety
The three cardinal features required for successful therapeutic drug are safety (lack of significant toxicity to host and environment); efficacy (the drug must work in a high proportion of treatments or better than other agents available); and appropriate cost (the drug must be affordable by the users and allow the pharmaceutical companies to recover development, production and future investment costs). We will now comment on safety.
The anthelmintic agent will need to have minimal (ideally be free from) toxic effects to the host. The toxicities to be considered are both the acute toxicities and chronic toxicities so that there is a low risk of acute overdose and low risk associated with longer term administration since the drug may be administered over a protracted period. The freedom of toxicity is necessary to establish for all the species to which the anthelmintic is to be administered to as well as establishing safety guidelines for the use and handlers of the drug.
Drugs administered to animals that are used for meat or milk will require additional review for safety because of their consumption by humans. Residues and no-detectable effect levels will need to be established to determine how long the therapeutic drug requires to be eliminated from the meat and milk (McKellar, 1997). The aim is to determine how long a safety period between administration of the drug and the killing of the production animal needs to be for safe use (establish the pre-slaughter period). The requirement for the measurement of residues in food animals makes the introduction and licensing more expensive for food animals, so the development of drugs for companion animals that are not consumed is less expensive and may be introduced initially (c.f. emodepside for use in cats). There are some interesting examples of toxicities associated with specific anthelmintics. Some of the toxicities are associated with the mode of action of the drug and might be predicted for any novel neuropeptide receptor ligand that is developed.
Levamisole is a nicotinic anthelmintic that is widely distributed in the host animal so that it has effects on gastro-intestinal and lungworms present. It has a selective effect on the nematode nicotinic receptors but the therapeutic index in not high and depends on the species of the host. Horses are sensitive to levamisole (DiPietro & Todd, Jr., 1987) and show pronounced gastro-intestinal effects, presumably due to stimulation of neuronal nicotinic receptors of the parasympathetic and sympathetic nervous system. The effect includes sweating and colic. Other species are less sensitive but levamisole will produce symptoms if the therapeutic dose is increased by more that threefold. The other nicotinic anthelmintics are less likely to produce these symptoms because their pharmaceutical preparations require oral administration and limit the distribution of the drug to the gastro-intestinal tract. This means that they are safer but their action is confined to gastro-intestinal parasites.
The avermectin class of anthelmintics (e.g. ivermectin) is excluded from the central nervous system by the blood brain barrier which possesses an exclusion pump. If the avermectins cross the blood brain barrier they can have effects on ion-channels including the GABAa receptors and product C.N.S. depression. There are breed specificities, in Collie dogs (Hopper et al., 2002;Nelson et al., 2003)) and Murray cattle (Seaman et al., 1987) where it appears that a decreased blood brain barrier of the choroid plexus because there is a reduced P-glycoprotein like transporter present. This means that these species may allow high brain concentrations of the avermectins during treatment and thus show significant toxicity. There are several reports of neurological effects in humans following treatment with ivermectin for onchocerciasis. If we transfer this lesson to any novel neuropeptide receptor agonists, then we will have to be careful to keep in mind that in addition to species specific toxicities, we will have to consider the possibility of breed specific toxicities.
The benzimidazoles, including thiabendazole and albendazole, may produce teratogenic effects if they are administered in early of gestation (Whittaker & Faustman, 1991) (Capece et al., 2003). This toxic effect presumably relates to the disruption of vital microtubules required for the proper formation of the ribs and limbs. Again the use of any novel anthelmintic will need to tested for any teratogenic effects and avoided in necessary during pregnancy.
The environment is becoming a more important constituent that we must protect. The appearance of any drug residues, ether the metabolite or parent compound should not persist in the environment like the chlorinated hydrocarbons (dieldrin, aldrin and DDT) and should be quickly degraded. Even if they are degraded within a few days or a week, it will be important that toxic effects on environmentally sensitive species are not produced (McKellar, 1997). Here the avermectins serve as an example. Ivermectin has pronounced effects on insects and crustacean as well as the parasitic nematode that is under treatment. Contamination of water in rivers will have a deleterious effect on some crustaceans. Residues in fecal remains or ‘dung pats’ have the potential to damage and upset the live-cycles of insects, like the dung beetle. Again we can learn from the experience with the avermectins and anticipate and avoid any negative impact that may occur with a novel neuropeptide receptor agonists.
Summary
We have considered the lessons that established anthelmintic drugs which act on neuromuscular systems can provide for us. These lessons predict that for any novel neuropeptide receptor ligand that is introduced will give rise to resistance but that we can delay the onset of that resistance by careful use. They also predict that species, breed and environmental toxicities will need to be considered along with the testing and review of residues in meat and milk to determine withdrawal periods and pre-slaughter times. The use and development of established anthelmintics also show us that the cost of drug development favors the marketing of drugs with a wide-spectrum of action. The possibility of differences in neuropeptide receptor functions in different species of nematode means that any neuropeptide receptor target or small molecule neuropeptide could limit the spectrum of action of the drug.
Acknowledgments
Supported by NIH R01 AI047194-06A1-06
References
- 1.ALBONICO M, BICKLE Q, HAJI HJ, RAMSAN M, KATRIB KJ, MONTRESOR A, SAVIOLI L, TAYLOR M. Evaluation of the efficacy of pyrantel-oxantel for the treatment of soil-transmitted nematode infections. 2002. pp. 685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ARENA JP, LIU KK, PARESS PS, FRAZIER EG, CULLY DF, MROZIK H, SCHAEFFER JM. The mechanism of action of avermectins in Caenorhabditis elegans: correlation between activation of glutamate-sensitive chloride current, membrane binding, and biological activity. J Parasitol. 1995;81:286–294. [PubMed] [Google Scholar]
- 3.BAGGOT JD, MCKELLAR QA. The absorption, distribution and elimination of anthelmintic drugs: the role of pharmacokinetics. J Vet Pharmacol Ther. 1994;17:409–419. doi: 10.1111/j.1365-2885.1994.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 4.BAIN RK. Irradiated vaccines for helminth control in livestock. Int J Parasitol. 1999;29:185–191. doi: 10.1016/s0020-7519(98)00187-8. [DOI] [PubMed] [Google Scholar]
- 5.BLACKHALL WJ, LIU HY, XU M, PRICHARD RK, BEECH RN. Selection at a P-glycoprotein gene in ivermectin- and moxidectin-selected strains of Haemonchus contortus. Mol Biochem Parasitol. 1998a;95:193–201. doi: 10.1016/s0166-6851(98)00087-5. [DOI] [PubMed] [Google Scholar]
- 6.BLACKHALL WJ, POULIOT JF, PRICHARD RK, BEECH RN. Haemonchus contortus: selection at a glutamate-gated chloride channel gene in ivermectin- and moxidectin-selected strains. Exp Parasitol. 1998b;90:42–48. doi: 10.1006/expr.1998.4316. [DOI] [PubMed] [Google Scholar]
- 7.BODDIE GF. Veterinary Therapeutics. Oliver and Boyd; London: 1952. [Google Scholar]
- 8.BROWNING C. Experimental chemotherapy in Trypanosome Infections. 1907. pp. 1405–1409. [Google Scholar]
- 9.CAPECE BP, NAVARRO M, ARCALIS T, CASTELLS G, TORIBIO L, PEREZ F, CARRETERO A, RUBERTE J, ARBOIX M, CRISTOFOL C. Albendazole sulphoxide enantiomers in pregnant rats’ embryo concentrations and developmental toxicity. Vet J. 2003;165:266–275. doi: 10.1016/s1090-0233(02)00158-2. [DOI] [PubMed] [Google Scholar]
- 10.COLES GC, JACKSON F, POMROY WE, PRICHARD RK, VON SAMSON-HIMMELSTJERNA G, SILVESTRE A, TAYLOR MA, VERCRUYSSE J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 2006;136:167–185. doi: 10.1016/j.vetpar.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 11.CULLY DF, PARESS PS, LIU KK, SCHAEFFER JM, ARENA JP. Identification of a Drosophila melanogaster glutamate-gated chloride channel sensitive to the antiparasitic agent avermectin. J Biol Chem. 1996;271:20187–20191. doi: 10.1074/jbc.271.33.20187. [DOI] [PubMed] [Google Scholar]
- 12.DAVIS RE. Action of excitatory amino acids on hypodermis and the motornervous system of Ascaris suum: pharmacological evidence for a glutamate transporter. Parasitology. 1998;116 (Pt 5):487–500. doi: 10.1017/s0031182098002479. [DOI] [PubMed] [Google Scholar]
- 13.DENT JA, SMITH MM, VASSILATIS DK, AVERY L. The genetics of ivermectin resistance in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2000;97:2674–2679. doi: 10.1073/pnas.97.6.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DIPIETRO JA, TODD KS., JR Anthelmintics used in treatment of parasitic infections of horses. Vet Clin North Am Equine Pract. 1987;3:1–14. doi: 10.1016/s0749-0739(17)30688-0. [DOI] [PubMed] [Google Scholar]
- 15.EDWARDS G, BRECKENRIDGE AM. Clinical pharmacokinetics of anthelmintic drugs. Clin Pharmacokinet. 1988;15:67–93. doi: 10.2165/00003088-198815020-00001. [DOI] [PubMed] [Google Scholar]
- 16.EHRLICH P, SHIGA K. Farbentherapeutische Versuche bei Trypanosomenerkrankung. 1904. pp. 329–392. [Google Scholar]
- 17.ENG JK, PRICHARD RK. A comparison of genetic polymorphism in populations of Onchocerca volvulus from untreated- and ivermectin-treated patients. Mol Biochem Parasitol. 2005;142:193–202. doi: 10.1016/j.molbiopara.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 18.FELLOWES RA, MAULE AG, MARKS NJ, GEARY TG, THOMPSON DP, HALTON DW. Nematode neuropeptide modulation of the vagina vera of Ascaris suum: in vitro effects of PF1, PF2, PF4, AF3 and AF4. Parasitology. 2000;120 (Pt 1):79–89. doi: 10.1017/s0031182099005260. [DOI] [PubMed] [Google Scholar]
- 19.GEARY TG. Ivermectin 20 years on: maturation of a wonder drug. Trends Parasitol. 2005;21:530–532. doi: 10.1016/j.pt.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 20.GEARY TG, CONDER GA, BISHOP B. The changing landscape of antiparasitic drug discovery for veterinary medicine. Trends Parasitol. 2004;20:449–455. doi: 10.1016/j.pt.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 21.GEARY TG, KUBIAK TM. Neuropeptide G-protein-coupled receptors, their cognate ligands and behavior in Caenorhabditis elegans. Trends Pharmacol Sci. 2005;26:56–58. doi: 10.1016/j.tips.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 22.GEARY TG, NULF SC, EXANDER-BOWMAN SJ, MAHMOUD BM, PRICHARD RK, KLEIN RD. Cloning and characterization of cDNAs encoding beta-tubulin from Dirofilaria immitis and Onchocerca volvulus. J Parasitol. 1998;84:356–360. [PubMed] [Google Scholar]
- 23.GEARY TG, NULF SC, FAVREAU MA, TANG L, PRICHARD RK, HATZENBUHLER NT, SHEA MH, ALEXANDER SJ, KLEIN RD. Three beta-tubulin cDNAs from the parasitic nematode Haemonchus contortus. Mol Biochem Parasitol. 1992;50:295–306. doi: 10.1016/0166-6851(92)90227-b. [DOI] [PubMed] [Google Scholar]
- 24.GEARY TG, SANGSTER NC, THOMPSON DP. Frontiers in anthelmintic pharmacology. Vet Parasitol. 1999;84:275–295. doi: 10.1016/s0304-4017(99)00042-4. [DOI] [PubMed] [Google Scholar]
- 25.GEARY TG, SIMS SM, THOMAS EM, VANOVER L, DAVIS JP, WINTERROWD CA, KLEIN RD, HO NF, THOMPSON DP. Haemonchus contortus: ivermectin-induced paralysis of the pharynx. Exp Parasitol. 1993;77:88–96. doi: 10.1006/expr.1993.1064. [DOI] [PubMed] [Google Scholar]
- 26.GILL JH, KERR CA, SHOOP WL, LACEY E. Evidence of multiple mechanisms of avermectin resistance in haemonchus contortus--comparison of selection protocols. Int J Parasitol. 1998;28:783–789. doi: 10.1016/s0020-7519(98)00015-0. [DOI] [PubMed] [Google Scholar]
- 27.GREENWOOD K, WILLIAMS T, GEARY T. Nematode neuropeptide receptors and their development as anthelmintic screens. Parasitology. 2005;131(Suppl):S169–S177. doi: 10.1017/S003118200500819X. [DOI] [PubMed] [Google Scholar]
- 28.GUNAWAN M, SANGSTER NC, KELLY JD, GRIFFIN D, WHITLOCK HV. The efficacy of fenbendazole and albendazole against immature and adult stages of benzimidazole-resistant sheep trichostrongylids. Res Vet Sci. 1979;27:111–115. [PubMed] [Google Scholar]
- 29.HARDER A, VON SAMSON-HIMMELSTJERNA G. Cyclooctadepsipeptides--a new class of anthelmintically active compounds. Parasitol Res. 2002;88:481–488. doi: 10.1007/s00436-002-0619-2. [DOI] [PubMed] [Google Scholar]
- 30.HENNESSY DR. Physiology, pharmacology and parasitology. Int J Parasitol. 1997;27:145–152. doi: 10.1016/s0020-7519(96)00144-0. [DOI] [PubMed] [Google Scholar]
- 31.HOGLUND J, ENGSTROM A, MORRISON DA, MATTSSON JG. Genetic diversity assessed by amplified fragment length polymorphism analysis of the parasitic nematode Dictyocaulus viviparus the lungworm of cattle. Int J Parasitol. 2004;34:475–484. doi: 10.1016/j.ijpara.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 32.HOGLUND J, MORRISON DA, MATTSSON JG, ENGSTROM A. Population genetics of the bovine/cattle lungworm (Dictyocaulus viviparus) based on mtDNA and AFLP marker techniques. Parasitology. 2006;133:89–99. doi: 10.1017/S0031182006009991. [DOI] [PubMed] [Google Scholar]
- 33.HOLDEN-DYE L, BROWNLEE DJ, WALKER RJ. The effects of the peptide KPNFIRFamide (PF4) on the somatic muscle cells of the parasitic nematode Ascaris suum. Br J Pharmacol. 1997;120:379–386. doi: 10.1038/sj.bjp.0700906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.HOPPER K, ALDRICH J, HASKINS SC. Ivermectin toxicity in 17 collies. J Vet Intern Med. 2002;16:89–94. doi: 10.1892/0891-6640(2002)016<0089:itic>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 35.JACKSON F. Anthelmintic resistance--the state of play. Br Vet J. 1993;149:123–138. doi: 10.1016/S0007-1935(05)80083-1. [DOI] [PubMed] [Google Scholar]
- 36.JONES AK, SATTELLE DB. Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode, Caenorhabditis elegans. Bioessays. 2004;26:39–49. doi: 10.1002/bies.10377. [DOI] [PubMed] [Google Scholar]
- 37.KAMATH RS, FRASER AG, DONG Y, POULIN G, DURBIN R, GOTTA M, KANAPIN A, LE BN, MORENO S, SOHRMANN M, WELCHMAN DP, ZIPPERLEN P, AHRINGER J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 38.KAPLAN RM, BURKE JM, TERRILL TH, MILLER JE, GETZ WR, MOBINI S, VALENCIA E, WILLIAMS MJ, WILLIAMSON LH, LARSEN M, VATTA AF. Validation of the FAMACHA eye color chart for detecting clinical anemia in sheep and goats on farms in the southern United States. Vet Parasitol. 2004;123:105–120. doi: 10.1016/j.vetpar.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 39.KEATING CD, KRIEK N, DANIELS M, ASHCROFT NR, HOPPER NA, SINEY EJ, HOLDEN-DYE L, BURKE JF. Whole-genome analysis of 60 G protein-coupled receptors in Caenorhabditis elegans by gene knockout with RNAi. Curr Biol. 2003;13:1715–1720. doi: 10.1016/j.cub.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 40.KOHLER P. The biochemical basis of anthelmintic action and resistance. Int J Parasitol. 2001;31:336–345. doi: 10.1016/s0020-7519(01)00131-x. [DOI] [PubMed] [Google Scholar]
- 41.LACEY E, PRICHARD RK. Interactions of benzimidazoles (BZ) with tubulin from BZ-sensitive and BZ-resistant isolates of Haemonchus contortus. Mol Biochem Parasitol. 1986;19:171–181. doi: 10.1016/0166-6851(86)90122-2. [DOI] [PubMed] [Google Scholar]
- 42.LEE EL, IYNGKARAN N, GRIEVE AW, ROBINSON MJ, DISSANAIKE AS. Therapeutic evaluation of oxantel pamoate (1, 4, 5, 6-tetrahydro-1-methyl-2-[trans-3-hydroxystyryl] pyrimidine pamoate) in severe Trichuris trichiura infection. Am J Trop Med Hyg. 1976;25:563–567. doi: 10.4269/ajtmh.1976.25.563. [DOI] [PubMed] [Google Scholar]
- 43.LEE S, CASTEEL DA, FLECKENSTEIN L. Specific gas chromatographic analysis of diethylcarbamazine in human plasma using solid-phase extraction. J Chromatogr B Biomed Sci Appl. 1997;704:181–185. doi: 10.1016/s0378-4347(97)00424-6. [DOI] [PubMed] [Google Scholar]
- 44.LUBEGA GW, KLEIN RD, GEARY TG, PRICHARD RK. Haemonchus contortus: the role of two beta-tubulin gene subfamilies in the resistance to benzimidazole anthelmintics. Biochem Pharmacol. 1994;47:1705–1715. doi: 10.1016/0006-2952(94)90551-7. [DOI] [PubMed] [Google Scholar]
- 45.LUBEGA GW, PRICHARD RK. Beta-tubulin and benzimidazole resistance in the sheep nematode Haemonchus contortus. Mol Biochem Parasitol. 1991;47:129–137. doi: 10.1016/0166-6851(91)90155-y. [DOI] [PubMed] [Google Scholar]
- 46.MARTIN PJ, MCKENZIE JA. Levamisole resistance in Trichostrongylus colubriformis: a sex-linked recessive character. Int J Parasitol. 1990;20:867–872. doi: 10.1016/0020-7519(90)90024-h. [DOI] [PubMed] [Google Scholar]
- 47.MARTIN RJ. Electrophysiological effects of piperazine and diethylcarbamazine on Ascaris suum somatic muscle. Br J Pharmacol. 1982;77:255–265. doi: 10.1111/j.1476-5381.1982.tb09294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MARTIN RJ. gamma-Aminobutyric acid- and piperazine-activated single-channel currents from Ascaris suum body muscle. Br J Pharmacol. 1985;84:445–461. doi: 10.1111/j.1476-5381.1985.tb12929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MARTIN RJ. An electrophysiological preparation of Ascaris suum pharyngeal muscle reveals a glutamate-gated chloride channel sensitive to the avermectin analogue, milbemycin D. Parasitology. 1996;112(Pt 2):247–252. doi: 10.1017/s0031182000084833. [DOI] [PubMed] [Google Scholar]
- 50.MARTIN RJ. Modes of action of anthelmintic drugs. Vet J. 1997;154:11–34. doi: 10.1016/s1090-0233(05)80005-x. [DOI] [PubMed] [Google Scholar]
- 51.MARTIN RJ, BAI G, CLARK CL, ROBERTSON AP. Methyridine (2-[2-methoxyethyl]-pyridine]) and levamisole activate different ACh receptor subtypes in nematode parasites: a new lead for levamisole-resistance. Br J Pharmacol. 2003;140:1068–1076. doi: 10.1038/sj.bjp.0705528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MARTIN RJ, MURRAY I, ROBERTSON AP, BJORN H, SANGSTER N. Anthelmintics and ion-channels: after a puncture, use a patch. Int J Parasitol. 1998;28:849–862. doi: 10.1016/s0020-7519(98)00048-4. [DOI] [PubMed] [Google Scholar]
- 53.MARTIN RJ, ROBERTSON AP, BJORN H. Target sites of anthelmintics. Parasitology. 1997;114(Suppl):S111–S124. [PubMed] [Google Scholar]
- 54.MARTIN RJ, ROBERTSON AP, WOLSTENHOLME AJ. In: Mode of action of the macrocyclic lactones. Vercruysse J, Rew RS, editors. Wallingford: CABI; 2002. pp. 125–140. [Google Scholar]
- 55.MAULE AG, BOWMAN JW, THOMPSON DP, MARKS NJ, FRIEDMAN AR, GEARY TG. FMRFamide-related peptides (FaRPs) in nematodes: occurrence and neuromuscular physiology. Parasitology. 1996;113(Suppl):S119–S135. doi: 10.1017/s0031182000077933. [DOI] [PubMed] [Google Scholar]
- 56.MAULE AG, MOUSLEY A, MARKS NJ, DAY TA, THOMPSON DP, GEARY TG, HALTON DW. Neuropeptide signaling systems - potential drug targets for parasite and pest control. Curr Top Med Chem. 2002;2:733–758. doi: 10.2174/1568026023393697. [DOI] [PubMed] [Google Scholar]
- 57.MCKELLAR QA. Ecotoxicology and residues of anthelmintic compounds. Vet Parasitol. 1997;72:413–426. doi: 10.1016/s0304-4017(97)00108-8. [DOI] [PubMed] [Google Scholar]
- 58.MCKELLAR QA, SCOTT EW. The benzimidazole anthelmintic agents--a review. J Vet Pharmacol Ther. 1990;13:223–247. doi: 10.1111/j.1365-2885.1990.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 59.MITCHELL SC. The toxicity of phenothiazine. Drug Metabol Drug Interact. 1994;11:201–235. doi: 10.1515/dmdi.1994.11.3.201. [DOI] [PubMed] [Google Scholar]
- 60.MOUSLEY A, MARKS NJ, HALTON DW, GEARY TG, THOMPSON DP, MAULE AG. Arthropod FMRFamide-related peptides modulate muscle activity in helminths. Int J Parasitol. 2004;34:755–768. doi: 10.1016/j.ijpara.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 61.MOUSLEY A, MAULE AG, HALTON DW, MARKS NJ. Inter-phyla studies on neuropeptides: the potential for broad-spectrum anthelmintic and/or endectocide discovery. Parasitology. 2005;131(Suppl):S143–S167. doi: 10.1017/S0031182005008553. [DOI] [PubMed] [Google Scholar]
- 62.NELSON OL, CARSTEN E, BENTJEN SA, MEALEY KL. Ivermectin toxicity in an Australian Shepherd dog with the MDR1 mutation associated with ivermectin sensitivity in Collies. J Vet Intern Med. 2003;17:354–356. [PubMed] [Google Scholar]
- 63.PIPER PJ, TEMPLE DM. The effect of lipoxygenase inhibitors and diethylcarbamazine on the immunological release of slow reacting substance of anaphylaxis (SRS-A) from guinea-pig chopped lung. J Pharm Pharmacol. 1981;33:384–386. doi: 10.1111/j.2042-7158.1981.tb13810.x. [DOI] [PubMed] [Google Scholar]
- 64.PRICHARD RK. Anthelmintic resistance in nematodes: extent, recent understanding and future directions for control and research. Int J Parasitol. 1990;20:515–523. doi: 10.1016/0020-7519(90)90199-w. [DOI] [PubMed] [Google Scholar]
- 65.PURCELL J, ROBERTSON AP, THOMPSON DP, MARTIN RJ. PF4, a FMRFamide-related peptide, gates low-conductance Cl(−) channels in Ascaris suum. Eur J Pharmacol. 2002;456:11–17. doi: 10.1016/s0014-2999(02)02622-5. [DOI] [PubMed] [Google Scholar]
- 66.QIAN H, MARTIN RJ, ROBERTSON AP. Pharmacology of N-, L- and B- subtypes of nematode nAChR resolved and the single channel level. FASEB, Journal. 2006;20:E1–E9. doi: 10.1096/fj.06-6264fje. [DOI] [PubMed] [Google Scholar]
- 67.RAYMOND V, MONGAN NP, SATTELLE DB. Anthelmintic actions on homomer-forming nicotinic acetylcholine receptor subunits: chicken alpha7 and ACR-16 from the nematode Caenorhabditis elegans. Neuroscience. 2000;101:785–791. doi: 10.1016/s0306-4522(00)00279-7. [DOI] [PubMed] [Google Scholar]
- 68.REYNOLDSON JA, BEHNKE JM, PALLANT LJ, MACNISH MG, GILBERT F, GILES S, SPARGO RJ, THOMPSON RC. Failure of pyrantel in treatment of human hookworm infections (Ancylostoma duodenale) in the Kimberley region of north west Australia. Acta Trop. 1997;68:301–312. doi: 10.1016/s0001-706x(97)00106-x. [DOI] [PubMed] [Google Scholar]
- 69.RICHARDS JC, BEHNKE JM, DUCE IR. In vitro studies on the relative sensitivity to ivermectin of Necator americanus and Ancylostoma ceylanicum. Int J Parasitol. 1995;25:1185–1191. doi: 10.1016/0020-7519(95)00036-2. [DOI] [PubMed] [Google Scholar]
- 70.ROBERTSON AP, CLARK CL, BURNS TA, THOMPSON DP, GEARY TG, TRAILOVIC SM, MARTIN RJ. Paraherquamide and 2-deoxy-paraherquamide distinguish cholinergic receptor subtypes in Ascaris muscle. J Pharmacol Exp Ther. 2002;302:853–860. doi: 10.1124/jpet.102.034272. [DOI] [PubMed] [Google Scholar]
- 71.ROOS MH, KWA MS, VEENSTRA JG, KOOYMAN FN, BOERSEMA JH. Molecular aspects of drug resistance in parasitic helminths. Pharmacol Ther. 1993;60:331–336. doi: 10.1016/0163-7258(93)90014-5. [DOI] [PubMed] [Google Scholar]
- 72.ROY TK, SHARMA S, SRIVASTAVA VM. Comparative tissue distribution and urinary excretion of diethylcarbamazine and centperazine. Indian J Med Res. 1981;74:565–571. [PubMed] [Google Scholar]
- 73.SANGSTER NC, BANNAN SC, WEISS AS, NULF SC, KLEIN RD, GEARY TG. Haemonchus contortus: sequence heterogeneity of internucleotide binding domains from P-glycoproteins. Exp Parasitol. 1999;91:250–257. doi: 10.1006/expr.1998.4373. [DOI] [PubMed] [Google Scholar]
- 74.SANGSTER NC, DAVIS CW, COLLINS GH. Effects of cholinergic drugs on longitudinal contraction in levamisole-susceptible and -resistant Haemonchus contortus. Int J Parasitol. 1991;21:689–695. doi: 10.1016/0020-7519(91)90080-q. [DOI] [PubMed] [Google Scholar]
- 75.SANGSTER NC, GILL J. Pharmacology of anthelmintic resistance. Parasitol Today. 1999;15:141–146. doi: 10.1016/s0169-4758(99)01413-1. [DOI] [PubMed] [Google Scholar]
- 76.SANGSTER NC, PRICHARD RK, LACEY E. Tubulin and benzimidazole-resistance in Trichostrongylus colubriformis (Nematoda) J Parasitol. 1985;71:645–651. [PubMed] [Google Scholar]
- 77.SCHAEFFER JM, BERGSTROM AR, FRAZIER EG, UNDERWOOD D. Nematocidal activity of MK-801 analogs and related drugs. Structure-activity relationships. Biochem Pharmacol. 1994;48:411–418. doi: 10.1016/0006-2952(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 78.SCHAEFFER JM, BERGSTROM AR, TURNER MJ. MK-801 is a potent nematocidal agent. Characterization of MK-801 binding sites in. Caenorhabditis elegans Biochem J. 1989;260:923–926. doi: 10.1042/bj2600923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.SEAMAN JT, EAGLESON JS, CARRIGAN MJ, WEBB RF. Avermectin B1 toxicity in a herd of Murray Grey cattle. Aust Vet J. 1987;64:284–285. doi: 10.1111/j.1751-0813.1987.tb15963.x. [DOI] [PubMed] [Google Scholar]
- 80.SHERIFF JC, KOTZE AC, SANGSTER NC, MARTIN RJ. Effects of macrocyclic lactone anthelmintics on feeding and pharyngeal pumping in Trichostrongylus colubriformis in vitro. Parasitology. 2002;125:477–484. doi: 10.1017/s0031182002002251. [DOI] [PubMed] [Google Scholar]
- 81.STABLES J, GREEN A, MARSHALL F, FRASER N, KNIGHT E, SAUTEL M, MILLIGAN G, LEE M, REES S. A bioluminescent assay for agonist activity at potentially any G-protein-coupled receptor. Anal Biochem. 1997;252:115–126. doi: 10.1006/abio.1997.2308. [DOI] [PubMed] [Google Scholar]
- 82.STEEL JW, WARING RH. Ecological Impact of Macrocyclic lactones on Dung Fauna. In: Vercruysse J, Rew RS, editors. Macrocyclic lactones in antiparasitic therapy. Wallingford: CABI; 2002. pp. 141–162. [Google Scholar]
- 83.VAN DV, DE VE. A single nucleotide polymorphism map of the mitochondrial genome of the parasitic nematode Cooperia oncophora. Parasitology. 2004;128:421–431. doi: 10.1017/s0031182003004633. [DOI] [PubMed] [Google Scholar]
- 84.VON SAMSON-HIMMELSTJERNA G, HARDER A, SANGSTER NC, COLES GC. Efficacy of two cyclooctadepsipeptides, PF1022A and emodepside, against anthelmintic-resistant nematodes in sheep and cattle. Parasitology. 2005;130:343–347. doi: 10.1017/s0031182004006523. [DOI] [PubMed] [Google Scholar]
- 85.WARUIRU RM, NGOTHO JW, MUKIRI JG. Multiple anthelmintic resistance in Haemonchus contortus on a sheep farm in Kenya. Vet Res Commun. 1997;21:483–491. doi: 10.1023/a:1005990303552. [DOI] [PubMed] [Google Scholar]
- 86.WHITTAKER SG, FAUSTMAN EM. Effects of albendazole and albendazole sulfoxide on cultures of differentiating rodent embryonic cells. Toxicol Appl Pharmacol. 1991;109:73–84. doi: 10.1016/0041-008x(91)90192-h. [DOI] [PubMed] [Google Scholar]
- 87.WILLSON J, AMLIWALA K, HARDER A, HOLDEN-DYE L, WALKER RJ. The effect of the anthelmintic emodepside at the neuromuscular junction of the parasitic nematode Ascaris suum. Parasitology. 2003;126:79–86. doi: 10.1017/s0031182002002639. [DOI] [PubMed] [Google Scholar]
- 88.WOLSTENHOLME AJ, FAIRWEATHER I, PRICHARD R, VON SAMSON-HIMMELSTJERNA G, SANGSTER NC. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 89.YEW JY, KUTZ KK, DIKLER S, MESSINGER L, LI L, STRETTON AO. Mass spectrometric map of neuropeptide expression in Ascaris suum. J Comp Neurol. 2005;488:396–413. doi: 10.1002/cne.20587. [DOI] [PubMed] [Google Scholar]
- 90.ZAJAC AM, GIPSON TA. Multiple anthelmintic resistance in a goat herd. Vet Parasitol. 2000;87:163–172. doi: 10.1016/s0304-4017(99)00174-0. [DOI] [PubMed] [Google Scholar]