Abstract
Background
Pre-session administration of d-cycloserine (DCS) has been found to augment exposure therapy outcomes in a variety of anxiety disorders. To be able to enhance learning only for successful exposure sessions, it would be beneficial to have the option of administering DCS after rather than before the session, a strategy encouraged by pre-clinical work. We believe the present study is the first published report on the efficacy of post-session administration of DCS in humans.
Method
Adults (N = 29) with a DSM-IV diagnosis of acrophobia were randomized to receive two sessions of virtual reality exposure therapy (VRE) in combination with placebo or 50 mg of DCS. Instead of administering the pill prior to each of the sessions, as has been done in extant work, we administered the pill immediately following each session. Measures of acrophobia severity were collected at baseline, at each treatment session, 1-week post-treatment, and at 1-month follow-up.
Results
Mixed-effects repeated-measures ANOVAs and GLMMs revealed significant improvement in all outcome measures over time, but no between-group differences were observed. At post-treatment, 63.5% of patients in the placebo condition vs. 60.0% of those in the DCS condition were in remission. At 1-month follow up, 63.4% of those in the placebo condition vs. 66.6% of those in the DCS condition were in remission.
Conclusions
These findings do not support the application of post-session DCS administration for augmenting the efficacy of exposure-based treatments. Possible reasons for these findings are discussed. Trial Registry: The Trial is registered at ClinicalTrials.gov (NCT01102803).
Keywords: Augmentation, CBT, Cognitive behavioral therapy, d-cycloserine, Exposure treatment, Randomized controlled trial, Acrophobia
1. Introduction
An exciting success of translational research is the use of d-cycloserine (DCS) as an augmentation strategy for exposure-based treatment (Anderson and Insel, 2006). Following pre-clinical research implicating the N-methyl d-aspartate (NMDA) receptor in fear conditioning and extinction learning (Baker and Azorlosa, 1996; Davis and Myers, 2002; Falls et al., 1992; Lee and Kim, 1998). Walker et al. (2002) demonstrated that rats administered DCS, a partial agonist at the NMDA receptor, prior to extinction training displayed enhanced dose-dependent extinction relative to rats administered saline. Replicated since (Myers and Davis, 2007), these findings encouraged the application of DCS for the augmentation of exposure treatment for anxiety disorders (Hofmann et al., 2011).
In the initial clinical trial, Ressler et al. (2004) enrolled 27 adults with DSM-III-R acrophobia into a 2-session virtual reality exposure (VRE) therapy protocol. The investigators selected this VRE protocol because VRE allows for carefully controlling exposure procedures and two 45-min sessions is considered a suboptimal dose, thus leaving sufficient room to detect augmentation effects. The efficacy of DCS augmentation was tested by randomly assigning patients to take either (a) pill placebo (PBO); (b) 50 mg of DCS; or (c) 500 mg of DCS 2–4 h prior to each of the 2 sessions. Relative to patients receiving PBO, those receiving DCS showed significantly greater reductions on in acrophobia severity during the 2-session intervention and the 3-month follow-up period with no differences between the two DCS doses.
The findings of the Ressler et al. (2004) study have since been replicated outside a VRE paradigm and extended across the anxiety disorders (Hofmann et al., 2006; Kushner et al., 2007; Otto et al., 2010; Wilhelm et al., 2008). In a meta-analysis of 10 trials involving clinical samples, Norberg et al. (2008) reported a significant advantage of DCS over PBO augmentation for exposure-based treatment efficacy (d = .60).
In all trials of DCS augmentation to date, the administration of DCS has been before instead of after the exposure session. However, post-session administration of DCS would offer the clinician and patient the advantage of select use of this augmentation strategy (i.e., only after sessions deemed successful). Indeed, the judicious use of DCS – administration following only the most successful exposure sessions – is supported by observations that repeated dosing appears to rapidly cause tolerance to DCS (Lopes et al., 1997; Parnas et al., 2005; Quartermain et al., 1994). Importantly, DCS dosing only after successful exposure sessions addresses the clinical concern that DCS may be applied to exposure sessions that accidentally sensitized the patient to fear cues. Recent research in fear reconsolidation validates this clinical concern. Specifically, when memories are retrieved, they become temporarily destabilized and vulnerable to intervention that may attenuate, modify, or stabilize the original memory (Lee, 2008; Monfils et al., 2009; Schiller et al., 2010). As such, during this time-limited state, memories are amenable to updating with new information, and hence sensitizing events or inadequate learning during exposure procedures may lead to reconsolidation. Research further suggests that, when the period of the initial conditioning sessions is brief, reconsolidation processes are dominant, whereas extinction processes dominate in longer sessions (Eisenberg et al., 2003; Lee et al., 2006). Therefore, DCS has the potential to augment extinction learning when the conditioned stimulus (CS) is re-exposed sufficient times for extinction to occur. In contrast, if stimulus re-exposure during memory reactivation is relatively brief compared to the strength of conditioning, little extinction is induced and DCS may augment reconsolidation (i.e., if the memory is in the labile state) (Lee et al., 2006). Although the bounds around reconsolidation effects in humans have yet to be investigated, research suggests that this period of lability, in which reconsolidation can occur, persists for approximately 6 h in animals (Nader et al., 2000). Hence, the application of post-session DCS administration only when exposure is judged to be adequate has the potential for avoiding unwittingly strengthening fear associations (Litz et al., 2012).
Pre-clinical data offers evidence in support of the potential efficacy of post-session DCS augmentation. For example, Ledgerwood et al. (2003) found that post-extinction subcutaneous administration of DCS in rats facilitated extinction and these effects were comparable to that observed with pre-extinction administration. Timing, similar to dosage, followed a linear trend such that peak extinction facilitation was observed when DCS was administered either immediately afterward or 30 min post-training, and diminished results were found at 120 min post-training administration. No benefits of DCS were observed at 240 min post-training administration (Ledgerwood et al., 2003). Parnas et al. (2005) have since replicated these findings.
The present study investigates whether post-session oral administration of DCS facilitates exposure-based CBT outcomes for anxiety disorders. Replicating the procedures of the first study examining the efficacy of pre-session DCS in humans (Ressler et al., 2004), we randomized 29 adults with height phobia to receive two sessions of VRE in combination with either PBO or 50 mg of DCS. Instead of administering the pill two to 4 h before each of the two VRE sessions, as was done in the Ressler et al. (2004) study, we administered the pill immediately after each of the two VRE sessions. Measures of acrophobia severity were collected at baseline, at each treatment session, 1-week post-treatment, and at 1-month follow-up. We hypothesized that, relative to participants receiving placebo-augmented VRE, those receiving DCS-augmented VRE would show decreased acrophobia symptoms at post-treatment and follow-up.
2. Materials and method
2.1. Participants
Participants (N = 29; Mage = 33.38) with acrophobia were recruited from Southern Methodist University and the greater Dallas area. Inclusion criteria were: (a) age 18–65; and (b) meeting DSM-IV-TR criteria for acrophobia. Exclusion criteria were: (a) a subjective distress score (SUDS) of <50 on the BAT; (b) a lifetime history of bipolar disorder, schizophrenia, psychosis, delusional disorders, or obsessive-compulsive disorder; current or recent diagnosis of substance or alcohol abuse or dependence; (c) current or recent suicidality or suicidal behavior; (d) current or recent diagnosis of PTSD, panic disorder, or eating disorder; (e) any physical or psychiatric condition that could interfere with the capacity to engage in therapy; (f) history of head trauma causing loss of consciousness, seizure, or ongoing cognitive impairment; (g) pregnancy; (h) concurrent psychotropic medication or psycho-therapy or prior non-response to exposure therapy for acrophobia.
2.2. Experimental design
Eligible participants were assigned to one of two blinded arms – VRE therapy plus pill placebo (N = 14) or 50 mg of DCS (N = 15). Randomization was done by research staff not involved in the trial using minimization procedures (Scott et al., 2002) and stratifying on gender, therapist, and time of day of treatment sessions (before or after 4 pm). Assessments occurred at baseline, prior to each treatment session, at one-week post-treatment, and at 1-month follow-up. Consistent with the original study (Ressler et al., 2004), the primary outcome measures were the BAT, AAVQ, AAQ (Cohen, 1977), ATHQ (Abelson and Curtis, 1989), and the CGI (Guy, 1970).
2.3. Procedures
2.3.1. Screening
Respondents to study advertisements were briefly screened by a research assistant before being invited to the clinic for formal eligibility screening. After signing informed consent, participants were administered the Structured Clinical Interview for DSM-IV (SCID) (First et al., 2001) and clinician-rated instruments. The study physician reviewed the medical history information and prescribed all study medications. The senior author (JAJS) verified assessment of entry criteria and diagnoses.
2.3.2. Treatment
The two-session VRE protocol was identical to the protocol used by Ressler et al. (2004). Treatment started one week following baseline assessment. Assessment of clinical status and safety occurred prior to each session. In the first session (60 min), therapists reviewed the rationale for exposure and the cognitive-behavioral model of acrophobia. Therapists then led participants through their first graded VRE exposures. The virtual reality environment simulated a hotel with a glass elevator, and a series of catwalks, balconies, and rooftop in which participants could stand and look around while wearing a virtual reality helmet and goggles. Participants reported their subjective fear levels (i.e., subjective units of distress [SUDS]) (Wolpe, 1958) on a 0–100 scale (0 indicates no fear, 100 indicates extreme fear or panic) every 5 min throughout the exposures. Exposure sessions began at the floor where the participants first reported SUDS of 50 or greater on the BAT. Therapists then led participants through each exposure (i.e., teaching them to approach, not avoid, and challenging beliefs), until meaningful SUDS decrease occurred (>50% reduction), at which time the therapist would lead the participant up to the next floor and repeat these procedures for a total of 30 min of VRE exposures. At the second session, completed one week after the first session, participant completed another 30 min of VRE exposures. At the conclusion of each of the two sessions, participants were administered the pill (DCS or PBO). Blind to the condition, therapists were advanced doctoral-student level therapists trained and supervised by the senior author (JAJS).
2.3.3. Medication
The 50 mg DCS and PBO capsules (size #0) were compounded by the Abrams Royal Pharmacy in Dallas, TX. The DCS capsules contained d-cycloserine 50 mg (derived from Seromycin 250 mg capsules) and polyethylene glycol 3350 powder. The matching placebo capsules contained only the polyethylene glycol 3350 powder. We selected a 50 mg dose of DCS because this dosage was as effective as a large dose (500 mg) in the original clinical trial we replicated (Ressler et al., 2004) and because this dosage has been applied successfully in other trials by our group examining the effects of pre-session administration of DCS (Hofmann et al., 2006; Otto et al., 2009, 2010). All capsules were identical in appearance to maintain the blind.
3. Measures
3.1. SCID
The SCID was administered for inclusion and exclusion DSM-IV criteria at screening (First et al., 2001). The specific phobia module of the SCID was completed again at the post-treatment and follow-up assessments in order to inform clinical global impression ratings. All SCID interviews were conducted by certified doctoral students. The certification procedure required (a) observing 3–4 videotaped and live SCID administrations by trained and certified interviewers with the comparison of the trainees’ ratings to that of the trained and certified interviewer, and (b) administer 3–5 SCID interviews in the presence of the trained and certified interviewer with the requirement of the trainees’ diagnosis match that of the trained and certified interviewer on at least 3 consecutive administrations.
3.2. Behavioral avoidance test (BAT)
During the initial screen, at post-treatment, and at follow-up, participants underwent a behavioral avoidance test in the virtual reality height environment. Participants reported on a 0–100 scale (100 being the most intense fear) their SUDS for floors 1, 2, 3, 4, 9, 19 of the virtual glass elevator and balconies. This test has been used successfully as a measure of treatment gains in previous studies of acrophobia research (Ressler et al., 2004). For the outcome analyses, we included the level of fear reported at the highest floor of the virtual elevator environment (19th floor).
3.3. Acrophobia questionnaire with avoidance (AAVQ) and anxiety (AAQ) scales
This questionnaire (Cohen, 1977) describes 20 situations and assesses levels of avoidance (0–3) and anxiety (0–6). These scales widely used measure of acrophobia with adequate retest reliability (r = .82–.86) and validity (Baker et al., 1973).
3.4. Attitudes toward heights questionnaire (ATHQ)
This questionnaire (Abelson and Curtis, 1989) includes six heights situations and assesses attitudes toward these situations using a 0–10 scale. Reported internal consistency is sound (α = .81) and validity is acceptable (Coelho et al., 2008; Davis et al., 2006; Rothbaum, 1995).
3.5. Clinical global impressions severity and improvement scales (CGI-S and CGI-I)
The CGI-S and CGI-I are widely used measures of global psychopathology severity and improvement initially developed for the study of psychotropic drugs (Guy, 1970). In order to obtain CGI ratings, the therapists (blind to study condition) interviewed the participant and used the SCID (including the specific phobia module) as well as the additional measures of acrophobia symptoms (BAT, AAQ, AAVQ, and ATHQ). Response was defined as either “very much improved” or “much improved” on CGI-I (score ≤ 2). Remission was defined as either “normal” or “minimally ill” on CGI-S (score ≤ 2).
4. Data analysis
All data were evaluated for differences between the groups at baseline (DCS vs. PBO; see Table 1). Study hypothesis was tested using a series of mixed-effects repeated-measures analyses of variance (ANOVAs), which allows all participants to be included in the analyses regardless of missing data and allows specification of a more complex covariance matrix for the correlation of the errors of the repeated measures (Singer and Willett, 2003). A growth curve model (e.g., linear or quadratic) was not employed since recent research has shown that constraining changes over time to fit a specific growth curve can lead to misleading results (Liu et al., 2012). Time (baseline, week 1, week 2, post-treatment, and 1 month follow-up) was entered as the within-person factor, and Treatment Condition (0 = PBO,1 = DCS) as the between-subjects factor. Hence, a significant Time by Treatment Condition interaction on outcome measures would provide support for the study hypothesis. For outcomes that were dichotomous (response and remission), generalized linear mixed models (GLMMs) were performed, using a logistic linking function for the dependent variable. Accordingly, all analyses were based on an intent-to-treat design, including all randomized individuals (N = 29).
Table 1.
Baseline data.
| Variable | DCS M (SD) (n = 15) |
PBO M (SD) (n = 14) |
P value |
|---|---|---|---|
| Age | 29.33 (14.67) | 37.71 (16.81) | .16 |
| Behavioral avoidance test (BAT) | 68.07 (14.78) | 71.29 (19.94) | .62 |
| Acrophobia avoidance questionnaire (AAVQ) | 20.73 (7.74) | 23.06 (10.60) | .50 |
| Acrophobia anxiety questionnaire (AAQ) | 48.27 (20.98) | 58.36 (25.71) | .26 |
| Attitude toward heights questionnaire (ATHQ) | 45.67 (8.53) | 47.71 (12.98) | .62 |
| Clinical global impression of severity (CGI) | 4.13 (.52) | 4.14 (.36) | .96 |
An a-priori power analysis revealed that a sample size of 16 (for 5 time points; CGI-S)–22 (for 3 time points; BAT) yields adequate power (i.e., .80) to detect a medium effect size (f2 = .25) at p < .05 for within–between interaction in a two-group design using repeated-measures ANOVAs (G*Power 3) (Faul et al., 2007). We selected a sample size (N = 29) that exceeded the minimum needed to detect clinically meaningful differences in order to approximate the methodological approach employed in the work being replicated (Ressler et al., 2004).
5. Results
5.1. Study flow and baseline characteristics
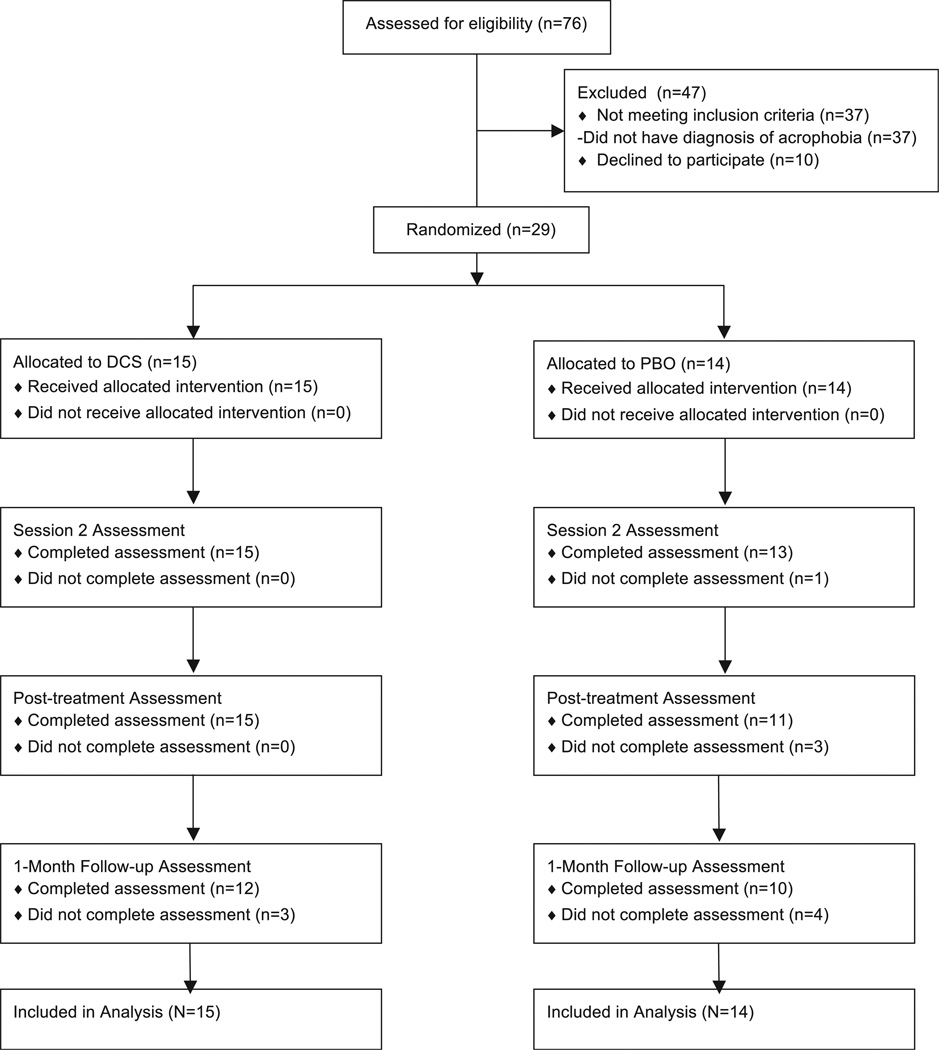
Five hundred and sixty-two people completed the Internet screen and 76 came in for a clinical interview (see Fig. 1). Of these, 29 participants were eligible and randomized (PBO = 14; DCS = 15) and thus included in the analyses. On average, the sample presented as “moderately ill” at baseline (see Table 1). There were no differences between DCS and PBO on any of the measures at baseline (see Table 1). By post-treatment, a total of 3 patients in the PBO condition (21.4%) and none in the DCS condition had dropped out. At follow-up, 4 patients in PBO (28.6%) and 3 in DCS (20.0%) had dropped out (see Fig. 1). None of these differences was significant (ps > .10).
Fig 1.
Patient flow diagram.
5.2. Treatment effects
Means and standard deviations for all outcome measures at the different time points are reported in Table 2.
Table 2.
Means and standard deviations of dependent variables at each time point.
| Measure | Mean (SD) | ||||
|---|---|---|---|---|---|
| Baseline | Session 1 | Session 2 | Post-treatment | Follow-up | |
| BAT | |||||
| DCS | 68.07 (14.78) | – | – | 29.73 (25.67) | 25.59 (23.98) |
| PBO | 71.29 (19.94) | – | – | 35.55 (25.18) | 23.80 (19.86) |
| AAVQ | |||||
| DCS | 20.73 (7.74) | – | 16.87 (9.90) | 9.00 (7.41) | 6.83 (7.76) |
| PBO | 23.06 (10.60) | – | 14.85 (10.75) | 12.38 (9.72) | 7.73 (6.28) |
| AAQ | |||||
| DCS | 48.27 (20.98) | – | 37.67 (18.66) | 25.47 (14.83) | 17.75 (12.74) |
| PBO | 58.36 (25.71) | – | 41.15 (20.44) | 33.08 (22.54) | 26.27 (16.80) |
| ATHQ | |||||
| DCS | 45.67 (8.53) | – | 38.13 (13.28) | 45.67 (8.53) | 26.83 (13.11) |
| PBO | 47.71 (12.98) | – | 37.08 (11.06) | 47.71 (12.98) | 27.55 (10.30) |
| CGI-S | |||||
| DCS | 4.13 (.52) | 4.15 (.38) | 3.60 (.91) | 2.53 (1.13) | 2.17 (.94) |
| PBO | 4.14 (.36) | 4.09 (.54) | 3.62 (.65) | 2.36 (.81) | 2.50 (.97) |
| CGI-I | |||||
| DCS | – | 4.00 (.00) | 3.00 (.93) | 2.27 (.96) | 1.92 (1.00) |
| PBO | – | 3.91 (.30) | 2.92 (.86) | 2.00 (.89) | 1.90 (.99) |
Note. BAT = Behavioral avoidance test, AAVQ = Acrophobia avoidance questionnaire; AAQ = Acrophobia anxiety questionnaire; ATHQ = Attitudes toward heights questionnaire; CGI-S = Clinician’s global impression of severity; CGI-I = Clinician’s global impression of improvement.
5.2.1. Changes in behavioral measure of fear of heights
There was a significant main effect of Time on BAT SUDs [F(2,49) = 59.26, p < .001] such that participants’ self-reported fear on the top floor of the virtual reality environment decreased over time. There was no significant interaction between Time and Treatment Condition, suggesting no differences between treatment conditions in improvement on the BAT over time [p = .76].
5.2.2. Changes in self-reported avoidance of height situations
There was a significant main effect of Time [F(3,73) = 22.22, p < .001] such that self-reported avoidance of height situations (AAVQ) decreased over time. The Time by Treatment Condition interaction was not significant [p = .07], indicating that there were no differences between DCS and PBO in improvements on AAVQ scores.1
5.2.3. Changes in self-reported fear of heights situations
There was a significant main effect of Time [F(3,72) = 25.90, p < .001] such self-reported fear of height situations (AAQ) decreased over time. There was no significant Time by Treatment Condition interaction [p = .83], suggesting that there were no differences between treatment conditions in AAQ scores over time.
5.2.4. Changes in cognitions related to heights
There was a significant main effect of Time [F(3,72) = 28.53, p < .001] such that negative cognitions about height situations (ATHQ) decreased over time. There was no significant Time by Treatment Condition interaction [p = .83], suggesting that there were no differences between DCS and PBO in ATHQ scores over time.
5.2.5. Changes in clinician-rated improvement of acrophobia
There was a significant main effect of Time on CGI-I [F(3,44) = 33.92, p < .001] such that clinician-rated global improvement scores of acrophobia decreased over time, but there were no differences between treatment conditions on CGI-I scores changes [p = .90]. GLMM analyses showed an 81.8% response rate for PBO and 66.7% response rate for DCS at post-treatment. At 1-month follow up, response rates were 80.1% and 75.0% for PBO and DCS, respectively. Between-group differences were not significant [ps ≥ .40].
5.2.6. Changes in clinician-rated severity of acrophobia
There was a significant main effect of Time [F(4,86) = 32.47, p < .001] such that participants’ clinician-rated global severity scores (CGI-S) of acrophobia decreased over time. Again, there was no significant interaction between Time and Treatment condition [p = .49]. GLMM analyses indicated remission rates of 63.5% for PBO and 60.0% for DCS condition at possttreatment. At 1-month follow up, remission rates were 63.4% and 66.6% for PBO and DCS, respectively. These between-group differences were not significant [ps > .75].
6. Discussion
To our knowledge, this is the first published examination of whether post-exposure session administration of DCS augments fear extinction in humans. Specifically, we randomized 29 individuals with height phobia to receive 2 sessions of VRE plus either placebo or 50 mg of DCS administered post-session, in contrast to the Ressler et al. (2004) in which DCS was administered pre-session. We hypothesized that those in the DCS condition would demonstrate greater reductions in acrophobia symptoms and severity compared to those in the PBO condition. Our results indicated that participants in both groups improved significantly on all measures of acrophobia severity, but participants receiving DCS did not outperform participants receiving PBO.
Our findings reflect a failure to translate results from animal studies showing that post-extinction training administration of DCS facilitates extinction learning (Ledgerwood et al., 2003; Parnas et al., 2005). Pre- rather than post-session administration of DCS in humans was first adopted because of the time taken between oral administration and bioavailability, with peak plasma levels at approximately 90–120 min following oral administration (D’Souza et al., 2000; van Berckel et al., 1997). In animal studies investigating post-extinction administration of DCS, DCS has been administered subcutaneously (Ledgerwood et al., 2003; Parnas et al., 2005), which results in peak plasma levels within as little as 15 min (Wlaz et al., 1994). As such, post-session oral administration of DCS may simply fail to arrive at the salient site of action in a time course necessary for therapeutic effect.
Nonetheless, there are a number of other important considerations for interpreting the null findings in our study. First, the failure of DCS to augment exposure treatment with post-session DCS administration may be specific to phobic samples that are not severely disabled (i.e., adults with a diagnosis of acrophobia). As suggested by Grillon (2009), individuals with “lower” levels of fear may improve in exposure-based protocols via more high-level cognitive processing (i.e., cognitive restructuring) instead of amygdala-mediated circuits (i.e., NMD-Amediated), while those with greater levels of fear may improve in exposure therapy protocols via more automatic learning processes (i.e., habituation) that are driven by amygdala-mediated circuits, thus supporting the indication of DCS-augmented treatment for more severely disabled patients. Evidence consistent with this hypothesis comes from the failure to observe augmentation effects with pre-session DCS dosing in samples with “low-level” fears actively recruited from the community (Guastella et al., 2007a, 2007b) and a recent report showing stronger augmentation effects of pre-session DCS administration among severe relative to less severe patients with post-traumatic stress-disorder (de Kleine et al., 2012). In terms of our purpose of replicating Ressler et al. (2004), albeit with post-session dosing, the mean severity scores for the DCS and PBO groups on the AAQ measure were 48.27 (SD = 20.98) and 58.36 (SD = 25.71), respectively, in the present study vs. 65.8 (SD = 6.2) and 73.4 (SD=5.6), respectively, in the Ressler et al. (2004) study. Accordingly, it is possible that the absent DCS augmentation effect in the present study was linked to recruitment of participants with lower baseline height phobia severity than those in the Ressler et al. (2004) study.
A third possible explanation for the null findings is that, despite providing suboptimal dosing of VRE, many patients responded very well to treatment with VR and placebo, thus perhaps creating a ceiling effect that left little room for the demonstration of additional improvement (with DCS). As indicated by data from the CGI, almost 3 out of 4 participants in the present trial were rated by a clinician as “much improved” or better at post-treatment and 1-month follow-up. Interestingly, using the same manualized VRE protocol and dosing, Ressler et al. (2004) appeared to have observed weaker overall treatment effects, with only approximately 1 of 4 participants rated, by self-report in this study, as “much improved” at post-treatment and 3-month follow-up, thereby perhaps leaving the room necessary for augmentation to exert additional positive effects.
The present study has a number of limitations that deserve mention. First, we did not include a pre-session pill administration manipulation. Had we included this, we would have been able to test some of the hypotheses for our null findings and demonstrate whether the failure to replicate the Ressler et al. (2004) study was specific to a post-session administration of DCS. Second, our CGI ratings were completed by a clinician who was, although blind to group assignment, not an independent evaluator. While this methodological feature cannot explain the absence of between-group differences, it may have introduced a bias resulting in larger overall clinician-rated improvement rates. Third, we only examined one dose of DCS (50 mg).We selected this dose because it had shown efficacy in the Ressler et al. (2004) trial and subsequent trials by our group (Hofmann et al., 2006; Otto et al., 2010), but it is possible that positive effects of post-session DCS dosing would have been evident had we used a different dose. Finally, unlike Ressler et al. (2004), we did not include a physiological measure of fear, and can therefore make no inferences with respect to the effects of post-session DCS administration on objective measures of fear.
Despite these shortcomings, this study provides a meaningful contribution to the growing literature on DCS augmentation of exposure treatment. Our findings indicate that post-session oral DCS administration of 50 mg may not be effective for augmenting the efficacy of exposure-based treatments; however, these initial negative findings should be re-examined with multiple treatment-seeking samples that span a greater range of anxiety severity.
Acknowledgments
This report was supported by Diversity Supplement to R01MH075889 from the National Institute of Mental Health (NIMH). We appreciate the support of the NIMH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the National Institutes of Health.
Footnotes
Given the trend for an interaction effect, we conducted additional analyses. The findings revealed that DCS outperformed PBO at some time points (post-treatment) but underperformed it at others (session 2). Also, the interaction effect remained non-significant when we removed the 1-month follow-up assessment from the analyses.
Contributors
C.D.T and J.A.J.S. designed the study, conducted the literature searches, participated in the data analyses, and wrote a first draft of the manuscript. P.R.H., L.B.D. M.H.P., S.G.H., M.B.P. and M.W.O. assisted with the design and implementation of the study, interpretation of the data and the writing of the manuscript. D.R. participated in the data analyses strategies, interpretation of data and writing of the manuscript. All authors contributed to and approved the final manuscript.
Conflict of interest
Dr. Tart, Ms. Handelsman, Ms. DeBoer, Dr. Rosenfield and Dr. Powers report no financial relationships with commercial interests. Dr. Pollack’s disclosures over the last three years include: Advisory Boards and Consultation: Eli Lilly, Medavante, Otsuka, Targia Pharmaceuticals Research Grants: Bristol Myers Squibb, Euthymics, Forest Laboratories, GlaxoSmithKline, NCCAM, NIDA, NIMH Equity: Medavante, Mensante Corporation, Mindsite, Targia Pharmaceutical Royalty/patent: SIGH-A, SAFER interviews. Dr. Hofmann has served as a consultant for Merck and Schering-Plough and receives book royalties from various book publishers for work unrelated to this study. Dr. Otto serves as consultant for MicroTransponder, Inc. and receives royalties from various book publishers for work unrelated to this study. Dr. Smits receives royalties from various book publishers for work unrelated to this study.
References
- Abelson JL, Curtis GC. Cardiac and neuroendocrine responses to exposure therapy in height phobics: desynchrony within the ‘physiological response system’. Behaviour Research and Therapy. 1989;27:561–567. doi: 10.1016/0005-7967(89)90091-0. [DOI] [PubMed] [Google Scholar]
- Anderson KC, Insel TR. The promise of extinction research for the prevention and treatment of anxiety disorders. Biological Psychiatry. 2006;60:319–321. doi: 10.1016/j.biopsych.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BL, Cohen DC, Saunders JT. Self-directed desensitization for acrophobia. Behaviour Research and Therapy. 1973;11:79–89. doi: 10.1016/0005-7967(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behavioral Neuroscience. 1996;110:618–620. doi: 10.1037//0735-7044.110.3.618. [DOI] [PubMed] [Google Scholar]
- Coelho C, Silva C, Santos J, Tichon J, Wallis G. Contrasting the effectiveness and efficiency of virtual reality and real environments in the treatment of acrophobia. PsychNology Journal. 2008;6:203–216. [Google Scholar]
- Cohen D. Comparison of self-report and overt-behavioral procedures for assessing acrophobia. Behavior Therapy. 1977;8:17–23. [Google Scholar]
- D’Souza DC, Gil R, Cassello K, Morrissey K, Abi-Saab D, White J, et al. IV glycine and oral D-cycloserine effects on plasma and CSF amino acids in healthy humans. Biological Psychiatry. 2000;47:450–462. doi: 10.1016/s0006-3223(99)00133-x. [DOI] [PubMed] [Google Scholar]
- Davis M, Myers KM. The role of glutamate and gamma-aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biological Psychiatry. 2002;52:998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biological Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- de Kleine RA, Hendriks GJ, Kusters WJ, Broekman TG, van Minnen A. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biological Psychiatry. 2012;71:962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. Journal of Neuroscience. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Clinician version. Arlington, VA: American Psychiatric Publishing, Inc; 2001. Structured clinical interview for DSM-IV axis-I disorders. [Google Scholar]
- Grillon C. D-cycloserine facilitation of fear extinction and exposure-based therapy might rely on lower-level, automatic mechanisms. Biological Psychiatry. 2009;66:636–641. doi: 10.1016/j.biopsych.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on exposure therapy for spider fear. Journal of Psychiatric Research. 2007a;41:466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behaviour Research and Therapy. 2007b;45:663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Guy W. Clinical global impression. Manual for the ECDEU assessment battery. 1970 [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Archives of General Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA, Asnaani A, Gutner CA, Otto MW. Cognitive enhancers for anxiety disorders. Pharmacology Biochemistry and Behavior. 2011;99:275–284. doi: 10.1016/j.pbb.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biological Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behavioral Neuroscience. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. Journal of Neuroscience. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL. Memory reconsolidation mediates the strengthening of memories by additional learning. Nature Neuroscience. 2008;11:1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. Journal of Neuroscience. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litz BT, Salters-Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, et al. A randomized placebo-controlled trial of d-cycloserine and exposure therapy for posttraumatic stress disorder. Journal of Psychiatric Research. 2012;46:1184–1190. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Liu S, Rovine MJ, Molenaar PC. Selecting a linear mixed model for longitudinal data: repeated measures analysis of variance, covariance pattern model, and growth curve approaches. Psychological Methods. 2012;17:15–30. doi: 10.1037/a0026971. [DOI] [PubMed] [Google Scholar]
- Lopes T, Neubauer P, Boje KM. Chronic administration of NMDA glycine partial agonists induces tolerance in the Porsolt swim test. Pharmacology Biochemistry and Behavior. 1997;58:1059–1064. doi: 10.1016/s0091-3057(97)00302-x. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nature Reviews Neuroscience. 2000;1:216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biological Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Otto MW, Basden SL, McHugh RK, Kantak KM, Deckersbach T, Cather C, et al. Effects of D-cycloserine administration on weekly nonemotional memory tasks in healthy participants. Psychotherapy and Psychosomatics. 2009;78:49–54. doi: 10.1159/000172620. [DOI] [PubMed] [Google Scholar]
- Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, et al. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biological Psychiatry. 2010;67:365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- Parnas AS, Weber M, Richardson R. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiology of Learning and Memory. 2005;83:224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH. Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. European Journal of Pharmacology. 1994;257:7–12. doi: 10.1016/0014-2999(94)90687-4. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Archives of General Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Rothbaum Effectiveness of computer-generated (virtual reality) graded exposure in the treatment of acrophobia. The American Journal of Psychiatry. 1995;152:626–628. doi: 10.1176/ajp.152.4.626. [DOI] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials. a review. Controlled Clinical Trials. 2002;23:662–674. doi: 10.1016/s0197-2456(02)00242-8. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: methods for studying change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- van Berckel BN, Lipsch C, Timp S, Gispen-de Wied C, Wynne H, et al. Behavioral and neuroendocrine effects of the partial NMDA agonist D-cycloserine in healthy subjects. Neuropsychopharmacology. 1997;16:317–324. doi: 10.1016/S0893-133X(96)00196-0. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. Journal of Neuroscience. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. American Journal of Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. [DOI] [PubMed] [Google Scholar]
- Wlaz P, Baran H, Loscher W. Effect of the glycine/NMDA receptor partial agonist, D-cycloserine, on seizure threshold and some pharmacodynamic effects of MK-801 in mice. European Journal of Pharmacology. 1994;257:217–225. doi: 10.1016/0014-2999(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Wolpe J. Psychotherapy by reciprocal inhibition. Palo Alto, CA: Stanford; 1958. [Google Scholar]