Abstract
Background.
Age-related muscle weakness due to atrophy and fatty infiltration in orofacial muscles may be related to swallowing deficits in older adults. An important component of safe swallowing is the geniohyoid (GH) muscle, which helps elevate and stabilize the hyoid bone, thus protecting the airway. This study aimed to explore whether aging and aspiration in older adults were related to GH muscle atrophy and fatty infiltration.
Method.
Eighty computed tomography scans of the head and neck from 40 healthy older (average age 78 years) and 40 younger adults (average age 32 years) were analyzed. Twenty aspirators and 20 nonaspirators from the 40 older adults had been identified previously. Two-dimensional views in the sagittal and coronal planes were used to measure the GH cross-sectional area and fatty infiltration.
Results.
GH cross-sectional area was larger in men than in women (p < .05). Decreased cross-sectional area was associated with aging (p < .05), and cross-sectional area was significantly smaller in aspirators compared with nonaspirators, but only among the older men (p < .01). Increasing fatty infiltration was associated with aging in the middle (p < .05) and posterior (p < .01) portions of the GH muscle. There was no significant difference in fatty infiltration of the GH muscle among aspirators and nonaspirators.
Conclusion.
GH muscle atrophy was associated with aging and aspiration. Fatty infiltration in the GH muscle was increased with aging but not related to aspiration status. These findings suggest that GH muscle atrophy may be a component of decreased swallowing safety and aspiration in older adults and warrants further investigation.
Key words: Atrophy, Geniohyoid muscle, Older adults, Fatty infiltration, Aspiration, Swallow, CT scans.
OROPHARYNGEAL aspiration increases in older adults (1), which can lead to lung infection and pneumonia (2). The latter is a major cause of morbidity/mortality and is a leading cause of death among elderly residents of nursing homes (3,4). More than 50% of healthy adults aspirate during sleep (5,6), and in a previous study, silent aspiration was demonstrated in 71% of patients with pneumonia compared with 10% in control participants (7). Our previous studies identified silent aspiration in 30% of community-dwelling healthy, awake, older adults (8,9) and determined that the etiology is multifactorial. Aspiration status in older adults was associated with decreased tongue strength and pharyngeal pressure (10,11), likely due to age-related atrophy of swallowing muscles. Muscle atrophy and associated functional decline with aging (sarcopenia) has been widely studied in muscles of the extremities. However, oropharyngeal muscle atrophy and loss of balance among different muscle groups during aging are poorly understood, which may explain the lack of effective treatment for oropharyngeal dysphagia, swallowing deficits with aging, and other age-related neurological diseases (4,12–14).
Decreased pharyngeal strength and decreased posterior tongue strength are associated with aspiration status in healthy older adults (10,15). However, aspiration status could also be associated with decreased pharyngeal closure, a process for airway protection in which the precise timing of the elevation of hyoid bone is important (16). A decreased range of motion and velocity of movement in the hyoid bone occurs in older adults compared with younger adults (17,18). Several suprahyoid muscles play a similar role in the raising, protracting, and stabilizing of the hyoid bone during swallowing. For example, the geniohyoid (GH) muscle connects the posterior aspect of the mandible in the midline and anterior surface of the body of the hyoid bone. The GH muscle’s contraction drives the hyoid bone upward and forward together with the mylohyoid, stylohyoid, and anterior belly of the digastric muscles (19). Sarcopenia of these muscles may play an important role in reducing hyoid bone movement during aging and the resultant increased risk of aspiration in older persons. In addition, age-related increase in fatty infiltration into the muscle is correlated with decreased muscle mass and strength in the elderly population (20,21).
In our preliminary studies in a nonhuman primate model, the GH muscle showed more obvious age-related muscle atrophy than other oropharyngeal muscles (unpublished data). Compared with the other suprahyoid muscles, the GH muscle is deeper; thus, its activity is difficult to record through noninvasive electromyographic techniques. However, GH muscle and fatty infiltration can be differentiated on head and neck computed tomography (CT) scans based on their relative densities (21,22) (fatty infiltration index). In addition, two-dimensional CT images in the sagittal and coronal planes allow measurement of the muscle cross-sectional area (CSA) to quantify atrophy. The purpose of this study was to compare atrophy of the GH muscle between younger and older adults and between aspirators and nonaspirators in the older adults. We tested the following two hypotheses: (a) age induces atrophy (decreased CSA) and increased intramuscular fat in the GH muscle and (b) aspirators have more muscle atrophy and intramuscular fat in the GH muscle.
Methods
Participants
A retrospective review of head and neck CT scans of 80 adults performed at Wake Forest Baptist Medical Center between 2006 and 2010 was performed. Forty healthy older adults (average age 78 years) comprised of 20 aspirators and 20 nonaspirators (identified via earlier flexible endoscopic evaluation of swallowing) were randomly selected from 73 older adults (10,23). As a control group, we selected at random CT scans from 40 younger adults (average age 32 years). These CT scans had been done to rule out brain infection, trauma, and intracranial bleeding in these patients. The medical records and notes for these participants were thoroughly reviewed and all selected participants met the following criteria: (a) no known head and neck cancer (or other cancer); (b) no known neurological or muscular diseases; (c) no known swallowing, speech, or respiratory diseases; and (d) no prior orofacial implant surgery or other head/neck surgery. The protocol was approved by the Wake Forest University Health Sciences Institutional Review Board.
Demographics
Demographic data collected for each participant included age, gender, height, and body weight. Body mass index (BMI) was calculated as follows: BMI (lbs/in2) = (weight in pounds × 703)/height in in2.
Image Analysis
For the older adults, noncontrast head and neck CT scan parameters were set at 120 KV, 280 mA, 0.8 seconds helical rotation time, 1.25mm slice thickness, 6.25 speed, 0.625 pitch, small focal spot, and 18cm display field view (GE Lightspeed Pro 16). CT scans were taken while each participant was in the supine position, with his or her head fixed in a padded head cradle with the chin slightly tilted toward the ceiling. Participants were instructed not to swallow or perform any oral movement during the CT scans. We could not document identical positioning among the younger adults, since their CT scans were done as part of clinical care. However, these patients’ heads would have been similarly positioned as part of the usual protocol for head and neck CT scans at our imaging center.
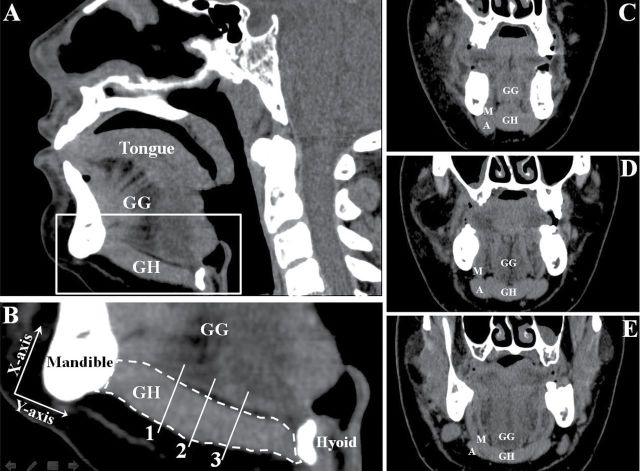
The scans were imported into analysis software loaded on a General Electric workstation, and two-dimensional images were reformatted and constructed in the sagittal and coronal planes. Relative to the coronal plane, the boundary of the GH muscle in the section of the midsagittal plane was identified and the CSA of the GH muscle was measured (mm2; Figure 1A). Then, the GH muscle in the midsagittal plane was divided into anterior (one third), middle, and posterior (two thirds) segments (Figure 1B). The CSA of anterior and middle GH muscle in the coronal plane was measured (Figure 1C and D). Finally, muscle fatty infiltration was measured in Hounsfield units (HU; [24]). The HU represents the different density levels of tissues. The density of pure water was arbitrarily set at 0 HU and that of air at −1000 HU (25). The higher the HU are the less the fatty infiltration is within the muscle and vice versa (26). Due to the regionalization of GH muscle activity (27,28), we analyzed the density at the three different sections (anterior, middle, and posterior) of the GH muscle in the coronal plane (Figure 1C–E). Tissue density was measured in a 5mm2 circular region of interest (29) in the central region of the muscle to assure that the region of interest (ROI) was intramuscular.
Figure 1.
Two-dimensional computed tomography (CT) scans used for measurements of the geniohyoid (GH) muscle. (A) is the section in midsagittal plane; (B) is an enlargement of the white square in (A). (B) also shows axis of the longitudinal GH muscle between the mandible and hyoid bone drawn at the one-third (1), middle (2), and two-thirds (3) portions of the GH muscle in the midsagittal plane. These represent the anterior (C), middle (D), and posterior (E) portions of the GH muscle in the coronal plane. Fatty infiltration index, in Hounsfield units (Hhs), was measured separately for three sections (C–E). GG = genioglossus muscle, A = anterior digastric muscle, and M = mylohyoid muscle.
Randomly selected images were reviewed by a radiologist and an otolaryngologist to establish standardized methods to determine muscle separation and measurements. Then, one investigator who was blinded to age groups and aspiration status performed all the measurements twice at different times. Intra-rater reliability was analyzed using the intraclass correlation coefficient (ICC) (30). ICC for all the measures were 0.92–0.97 (p < .001) suggesting a high intra-rater reliability. Values from each measurement were averaged and calculated for statistical analyses.
Statistical Analysis
The results are presented as means ± standard deviation. An analysis of variance was used to examine differences in muscle volume and fatty infiltration as a function of age, gender, and aspiration status. Pearson correlation coefficients were calculated between muscle size/fatty infiltration and BMI. Independent t tests and Wilcoxon’s rank-sum tests were done to analyze the differences among age, gender, and aspiration status groups, respectively. Significance level was set at .05. All analyses were performed using SAS 9.2 (Cary, NC).
Results
Demographic Data
The demographic data are shown in Table 1. No significant differences in BMI were found across age and gender groups (p > .05).
Table 1.
Demographic Data
| Younger Men | Younger Women | Older Men | Older Women | |||
|---|---|---|---|---|---|---|
| Nonaspirator | Aspirator | Nonaspirator | Aspirator | |||
| Number of participants | 20 | 20 | 9 (37.5%) | 15 (62.5%) | 11 (68.5%) | 5 (31.5%) |
| Age (Y) | 30.6±6.1 | 32.6±6.1 | 76.9±4.2 | 77.7±6.1 | 76.6±8.7 | 79.4±7.8 |
| BMI | 25.1±4.5 | 31.2±9.0 | 28.7±4.5 | 28.6±7.8 | 28.1±5.7 | 28.1±6.1 |
Note: Values are means ± standard deviation; BMI = Body mass index.
Correlation Between GH Muscle CSA/Fatty Infiltration and BMI
No significant correlations were found between BMI and CSA of the GH muscle in the sagittal (r = .24, p = .16) and coronal planes (anterior: r = .03, p = .79; middle: r = .01, p = .90). Fatty infiltration of the GH was not related to BMI in any portion of the muscle (anterior: r = .04, p = .76; middle: r = .08, p = .48; and posterior: r = .12, p = .29).
CSA of the GH Muscle
CSA of the GH muscle was decreased in the older adults compared with the younger adults in both men and women, in both the midsagittal (p < .01; Figure 2A) and coronal planes (p < .01; Figure 2B and C). Among older adults, CSA of the GH muscle was larger in men than in women in the midsagittal plane and anterior coronal planes (p < .01; Figures 2A and B). The men had larger CSAs for the middle GH muscle in the coronal plane than women, across age groups (p < .05; Figure 2C). Among the older adults who were aspirators, CSA of the GH muscle in the midsagittal plane was smaller than in the nonaspirators, but only in the men (p < .01; Figure 3).
Figure 2.
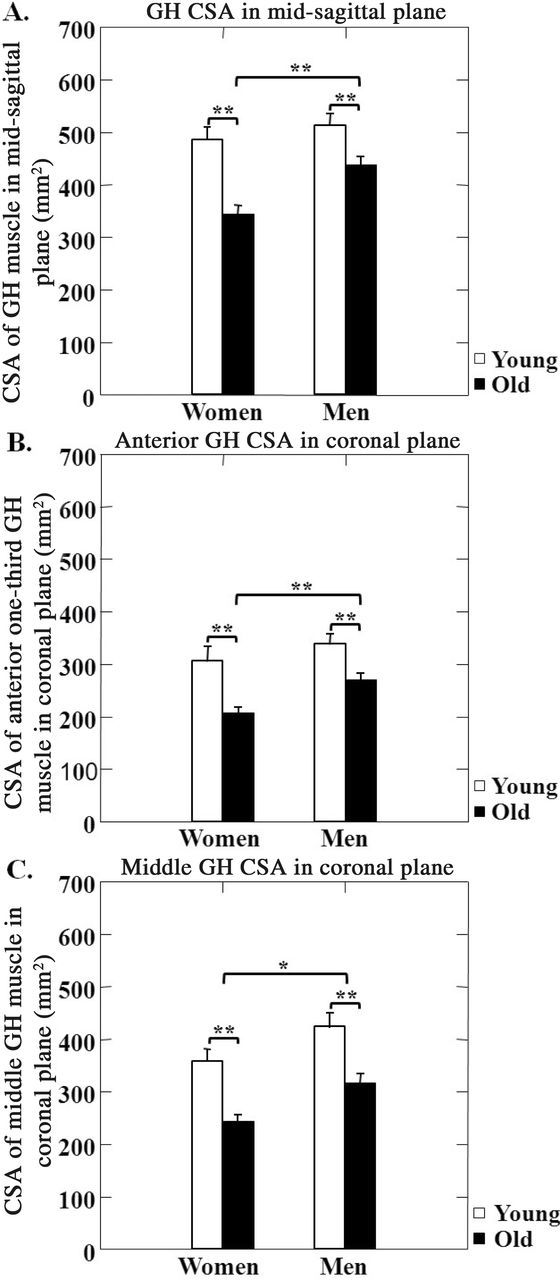
Changes in geniohyoid (GH) muscle size associated with aging. Cross-sectional area (CSA) of GH muscle in older adults compared with younger adults in both men and women in the midsagittal (A) or anterior (B) or middle (C) sections of the coronal planes. *—indicates p < .05 and **— indicates p < .01.
Figure 3.
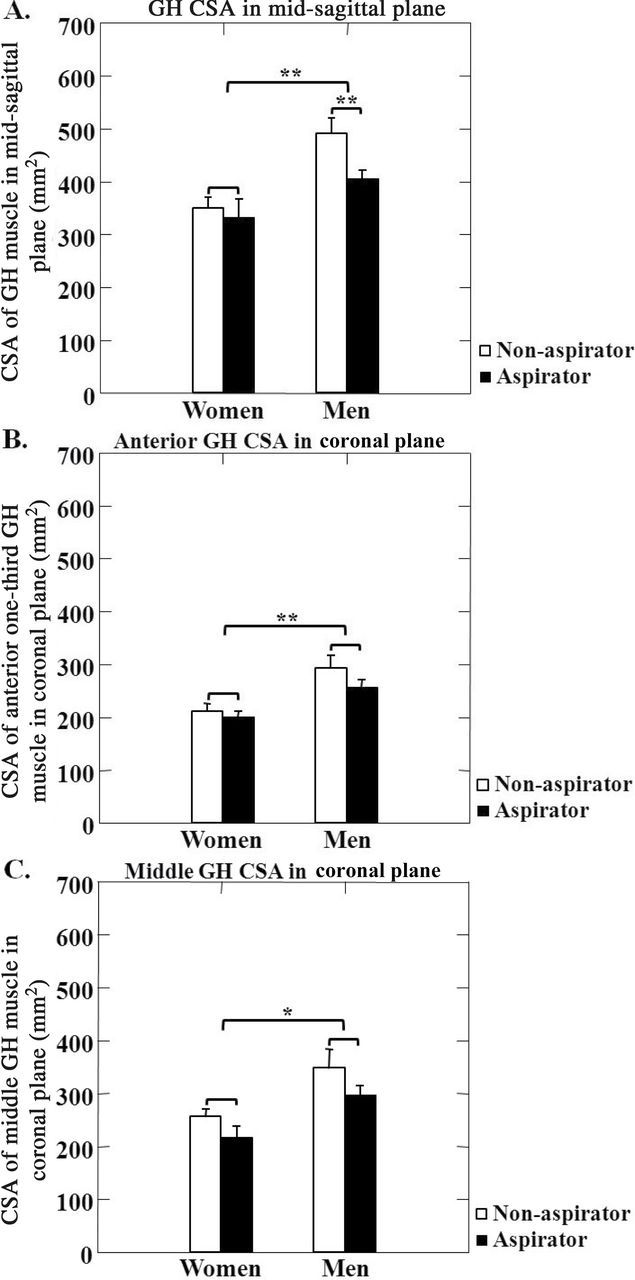
Geniohyoid (GH) muscle size among older adults who were aspirators (black bars) vs nonaspirators (white bars), showing cross-sectional areas (CSA) of the GH muscle in the midsagittal plane (A) and anterior and middle portions of the coronal planes in (B) and (C). *—indicates p < .05 and **—indicates p < .01.
Fatty Infiltration of GH Muscle
No effects of gender on fatty infiltration index were found in either younger or older adults (p > .05); thus, data from men and women were combined for analysis. The fatty infiltration index was bigger in the anterior compared with the middle and posterior GH muscle across age groups (p < .01, anterior vs middle, anterior vs posterior, middle vs posterior; Figure 4A and B). Different portions of the GH muscle were affected differently by aging, relative to fatty infiltration. In the middle and posterior GH muscle, the fatty infiltration index was smaller in the older adults compared with the younger adults (p < .05 and p < .01, respectively; Figure 4A). However, no significant differences were found in the anterior GH muscle between the older and younger adults (p > .05, Figure 4A). Aspirators and nonaspirators showed no significant differences in the fatty infiltration index in the anterior, middle, and posterior GH muscle (p > .05 for all; Figure 4B).
Figure 4.
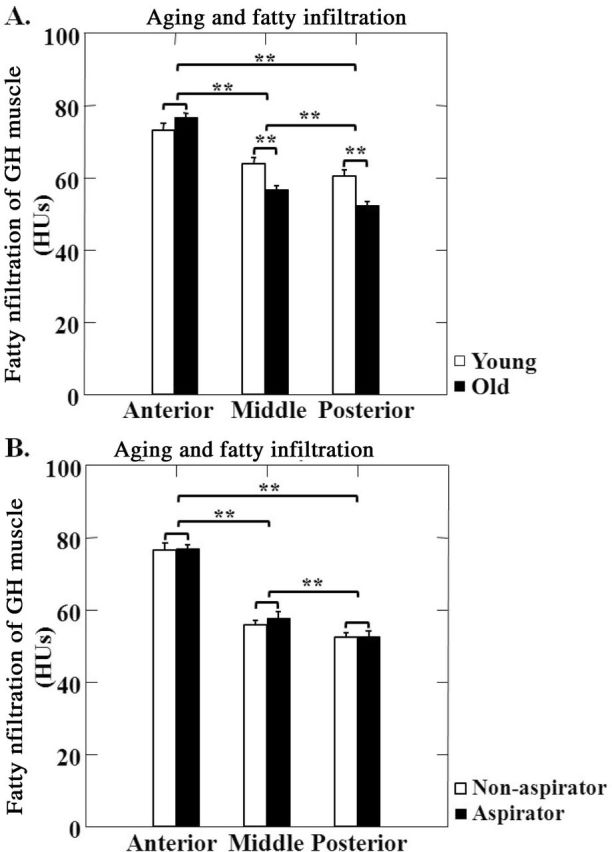
Fatty infiltration of the anterior, middle, and posterior portions of the geniohyoid (GH) muscle. (A) Effect of aging on these associations in older (black bars) vs younger (white bars) participants. (B) Effects of aspiration on these associations in aspirators (black bars) vs nonaspirators (white bars). Fatty infiltration measured in Hounsfield units (HU). **—indicates p < .01.
Discussion
To our knowledge, this is the first comparison of GH atrophy and fatty infiltration among younger and older adults, with additional comparisons among aspirators and nonaspirators in the older adults. The main findings in this study were as follows:(a) GH muscle atrophy was associated with aging, and aspirators had more atrophy compared with the nonaspirators, but only among the older men and (b) intramuscular fatty infiltration increases with aging but was not related to aspiration status in the older adults.
This pilot study used head and neck CT scans to measure the size and fatty infiltration of GH muscle as a factor of gender, age, and aspiration status. The CT system used allowed us to view the same anatomic structure in three different planes (sagittal, coronal, and axial) at the same time. This allowed us analyze the muscle of interest in each plane relative to the other two planes at the same time. Thus, we could exclude other adherent muscles in the analysis.
The midsagittal plane was the section where the mylohyoid muscle could be best distinguished in the anterior and middle portions of the GH muscle. The posterior portion of the GH muscle in the midsagittal section might include some of the mylohyoid muscle during measurements; however, it is a small percentage compared with the whole GH muscle area. We standardized measurements in each participant to try to minimize this bias. We also analyzed the anterior and middle portions of the GH muscle in the coronal plane to strengthen our measurements, since these portions of the GH muscle could be more easily distinguished from the genioglossus and mylohyoid muscles based on the muscle outline.
The CT imaging technology did have limitations in measuring total volume of the GH muscle. Other techniques, such as MRI, might be better to visualize and separate the muscular structure in the submental area and provide more detailed information regarding muscle volume measurements (31,32). These preliminary findings from our current database of head and neck CT scans could help determine which imaging modalities are better for future mechanistic studies (eg, MRI and ultrasonography [31,33]). In the future, we may also use histological analyses from autopsied tissues to verify our results.
Clinical Implications
In this study, CSA of GH muscle from head and neck CT scans decreased in older adults, regardless of gender. More importantly, aspiration status was linked to age-related decrease in CSA of GH muscle in the older men, indicating that our hypothesis that GH atrophy may play an important role in age-related aspiration is worthy of study.
Currently, many clinical strategies for swallowing rehabilitation focus on enhancing tongue strength (34). Targeting and improving GH muscle activity during swallowing rehabilitation has not been previously addressed. In addition, whether the activity of GH muscle can be efficiently improved by swallowing exercises that aim to strengthen all swallowing muscles has not been determined. GH muscle activity with different swallowing exercises is difficult to estimate by electromyography using surface electrodes in the submental area because the GH muscle is in a deeper position (35). Although submental transcutaneous electrical stimulation (36) has had beneficial effects in treating oropharyngeal dysphagia (29), this intervention inconsistently elevates the hyolaryngeal complex (37), which limits its beneficial effects in airway protection. Our pilot findings from the CT images suggest that it has value to verify (a) risk of loss of muscle mass in the whole GH muscle and (b) GH muscle functional decline associated with aging that could predispose to aspiration. GH muscle mass can be measured by MRI, and GH muscle contraction can be evaluated by ultrasound (31,38). These studies will help elucidate the mechanisms of GH muscle sarcopenia underlying aging-related aspiration and help to develop efficient strategies to improve airway protection and avoid aspiration.
GH and Fat Infiltration
Sarcopenia in the elderly population occurs from age-related loss of muscle mass, strength, and coordination (39). In addition, age-related changes in body composition, such as increased body fat mass, play important roles in functional impairment in the elderly population because high fat mass is associated with lower muscle quality and accelerated loss of lean mass (40,41). This body fat is distributed in the visceral, subcutaneous, and intermuscular adipose tissues outside the skeletal muscle (40). Increased intramuscular fatty infiltration is also related to muscle strength in the elderly popualtion. For example, attenuation values of limb muscle in CT scans of older persons account for differences in muscle strength (21). These findings provided the rationale for this study to examine effects of aging and aspiration on fatty infiltration within the GH muscle. The average BMI of the participants in this study was 28±7 (considered overweight but not obese). Although intramuscular lipid content is increased in obesity (42), we found no significant differences in BMI across age, gender, or aspiration status. In addition, no correlation was found between the BMI and the fatty infiltration index of the GH muscle.
Our results showed that fatty infiltration was not evenly distributed within the GH muscle. The fatty infiltration index averaged 75, 64, and 60 HU at the anterior, middle, and posterior regions of the GH muscle, respectively, suggesting a gradual increase of fatty infiltration in the GH muscle from anterior to posterior sections. Fatty infiltration increased with aging only in the middle and posterior areas, not in the anterior portion of the GH muscle. This finding suggests more severe muscle tissue loss in the middle and posterior portions of the GH muscle, consistent with earlier reports of a 13%–26% loss of fiber CSA in muscles of older individuals (43–45).
Unexpectedly, we found no difference in GH fatty infiltration between aspirators and nonaspirators among older adults. A previous study found that isometric strength of the lower posterior tongue was associated with greater posterior tongue adiposity but was not related to aspiration in healthy older adults (46). Thus, these data suggest that fatty infiltration in the GH muscle may not be a direct biomarker for aspiration in such individuals.
GH Atrophy and Possible Mechanisms
Decreased range of motion and velocity of the hyoid bone (17,18) predisposes older adults to aspiration. Our findings strongly indicate that loss of GH volume in the elderly population may play an important role in this process. Considering that the etiology of muscle weakness during aging is multifactorial, which may include age-related muscle atrophy, increased inter- and intramuscular fatty infiltration, denervation/reinnervation, inflammation, and changes in circulating hormones (47), further studies are needed to investigate the underlying mechanisms that contribute to the decreased CSA of the GH muscle during aging. Our pilot studies in a nonhuman primate model (unpublished data) showed a similar age-related GH atrophy, possibly due to a decline in myonuclear function, since changes in nuclear morphology and distribution are highly related to reduced nuclear function with aging (30,48). Other factors such as decreased satellite cells and increased myonuclear apoptosis (49,50) could also contribute to GH atrophy. Because of the difficulty of obtaining oropharyngeal muscle samples from humans, an appropriate animal model is helpful for mechanistic studies.
The GH muscle plays a key role in hyoid bone movement in the anterior direction for airway protection (19). However, swallowing is a biomechanical process that needs a balanced output from multiple skeletal muscles in the oral, pharyngeal, and esophageal compartments. If the swallowing muscles are disproportionately affected by aging, the output of the skeletal muscular balance will be interrupted and result in swallowing disorders. This study is an initial attempt at quantification of the GH muscle involved in aging and aspiration. In future studies, we plan to examine the effects of aging and aspiration on other swallowing muscles, in the oropharyngeal and bulbar regions using MRI or other imaging modalities for more detailed and precise measurements. In addition, future biomechanical studies to analyze the functional contribution of each swallowing muscle and how they are affected by aging will be valuable to illuminate the mechanisms underlying swallowing dysfunction that occurs during normal aging.
A limitation of this study is the lack of information on aspiration status in the younger participants. However, previously, we found a 0% incidence of aspiration in young adults during a flexible endoscopic evaluation of swallowing study (8). Thus, it is unlikely that the presence of aspiration in the younger adults we studied was significant. In addition, our recruitment criteria reduced the possibility of including aspirators among the younger adults. Future studies in larger cohorts that include aspiration status will provide more information on the relationship of GH and other swallowing muscles to aspiration status.
Conclusions
In summary, using head and neck CT scans, we found that decreased CSA of GH muscle was associated with aging and was greater in aspirators compared with nonaspirators in older men. However, fatty infiltration of the GH muscle was correlated only with aging. These findings may indicate an association between GH muscle atrophy and decreased swallowing safety and aspiration in older adults and warrants further investigation. Future physiological studies will be valuable to elucidate the relationship between the functional decline of the GH muscle and aspiration and will be beneficial in the development of targeted rehabilitation strategies for treating aspiration associated with dysphagia.
Funding
This work was supported by the Wake Forest School of Medicine Claude D. Pepper Older Americans Independence Center (P30 AG21332), the GCRC grant of Wake Forest University Baptist Medical Center (M01-RR07122), and NIDCD R03 DC009875 to Susan Butler.
Acknowledgments
The authors thank Caresse Hightower and Chris O’Rourke (Department of Radiology) for their assistance with CT acquisition and imaging analysis, Greg Russell (Department of Biostatistics) for his help with the data analysis, and Karen Potvin Klein, MA, ELS (Research Support Core, Wake Forest University Health Sciences), for her editorial contributions to this manuscript.
References
- 1. Rofes L, Arreola V, Romea M, et al. Pathophysiology of oropharyngeal dysphagia in the frail elderly. Neurogastroenterol Motil. 2010;22:851–858, e230 [DOI] [PubMed] [Google Scholar]
- 2. Vergis EN, Brennen C, Wagener M, Muder RR. Pneumonia in long-term care: a prospective case-control study of risk factors and impact on survival. Arch Intern Med. 2001;161:2378–2381 [DOI] [PubMed] [Google Scholar]
- 3. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 4. Cabre M, Serra-Prat M, Palomera E, Almirall J, Pallares R, Clavé P. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39:39–45 [DOI] [PubMed] [Google Scholar]
- 5. Huxley EJ, Viroslav J, Gray WR, Pierce AK. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978;64:564–568 [DOI] [PubMed] [Google Scholar]
- 6. Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest. 1997;111:1266–1272 [DOI] [PubMed] [Google Scholar]
- 7. Kikuchi R, Watabe N, Konno T, Mishina N, Sekizawa K, Sasaki H. High incidence of silent aspiration in elderly patients with community-acquired pneumonia. Am J Respir Crit Care Med. 1994;150:251–253 [DOI] [PubMed] [Google Scholar]
- 8. Butler SG, Stuart A, Kemp S. Flexible endoscopic evaluation of swallowing in healthy young and older adults. Ann Otol Rhinol Laryngol. 2009;118:99–106 [DOI] [PubMed] [Google Scholar]
- 9. Butler SG, Stuart A, Markley L, Rees C. Penetration and aspiration in healthy older adults as assessed during endoscopic evaluation of swallowing. Ann Otol Rhinol Laryngol. 2009;118:190–198 [DOI] [PubMed] [Google Scholar]
- 10. Butler SG, Stuart A, Leng X, et al. The relationship of aspiration status with tongue and handgrip strength in healthy older adults. J Gerontol A Biol Sci Med Sci. 2011;66:452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Butler SG, Stuart A, Wilhelm E, Rees C, Williamson J, Kritchevsky S. The effects of aspiration status, liquid type, and bolus volume on pharyngeal peak pressure in healthy older adults. Dysphagia. 2011;26:225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serra-Prat M, Hinojosa G, López D, et al. Prevalence of oropharyngeal dysphagia and impaired safety and efficacy of swallow in independently living older persons. J Am Geriatr Soc. 2011;59:186–187 [DOI] [PubMed] [Google Scholar]
- 13. Kalia M. Dysphagia and aspiration pneumonia in patients with Alzheimer’s disease. Metabolism. 2003;52(10 Suppl 2):36–38 [DOI] [PubMed] [Google Scholar]
- 14. Robbins J, Butler SG, Daniels SK, et al. Swallowing and dysphagia rehabilitation: translating principles of neural plasticity into clinically oriented evidence. J Speech Lang Hear Res. 2008;51:S276–S300 [DOI] [PubMed] [Google Scholar]
- 15. Butler SG, Stuart A, Castell D, Russell GB, Koch K, Kemp S. Effects of age, gender, bolus condition, viscosity, and volume on pharyngeal and upper esophageal sphincter pressure and temporal measurements during swallowing. J Speech Lang Hear Res. 2009;52:240–253 [DOI] [PubMed] [Google Scholar]
- 16. Steele CM, Bailey GL, Chau T, et al. The relationship between hyoid and laryngeal displacement and swallowing impairment. Clin Otolaryngol. 2011;36:30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Lang Hear Res. 2000;43:1264–1274 [DOI] [PubMed] [Google Scholar]
- 18. Kang BS, Oh BM, Kim IS, Chung SG, Kim SJ, Han TR. Influence of aging on movement of the hyoid bone and epiglottis during normal swallowing: a motion analysis. Gerontology. 2010;56:474–482 [DOI] [PubMed] [Google Scholar]
- 19. Pearson WG, Jr, Langmore SE, Zumwalt AC. Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia. 2011;26:345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333 [DOI] [PubMed] [Google Scholar]
- 21. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165 [DOI] [PubMed] [Google Scholar]
- 22. Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892 [DOI] [PubMed] [Google Scholar]
- 23. Butler SG, Stuart A, Leng X, Rees C, Williamson J, Kritchevsky SB. Factors influencing aspiration during swallowing in healthy older adults. Laryngoscope. 2010;120:2147–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barkdull GC, Kohl CA, Patel M, Davidson TM. Computed tomography imaging of patients with obstructive sleep apnea. Laryngoscope. 2008;118:1486–1492 [DOI] [PubMed] [Google Scholar]
- 25. Hofer M. CT Teaching Manual. Stuttgart, New York: Thieme; 2000. [Google Scholar]
- 26. Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54:509–515 [DOI] [PubMed] [Google Scholar]
- 27. Wentzel SE, Konow N, German RZ. Regional differences in hyoid muscle activity and length dynamics during mammalian head shaking. J Exp Zool A Ecol Genet Physiol. 2011;315:111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holman SD, Konow N, L Lukasik S, German RZ. Regional variation in geniohyoid muscle strain during suckling in the infant pig. J Exp Zool A Ecol Genet Physiol. 2012;317:359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verin E, Maltete D, Ouahchi Y, et al. Submental sensitive transcutaneous electrical stimulation (SSTES) at home in neurogenic oropharyngeal dysphagia: a pilot study. Ann Phys Rehabil Med. 2011;54:366–375 [DOI] [PubMed] [Google Scholar]
- 30. Malatesta M, Perdoni F, Muller S, Zancanaro C, Pellicciari C. Nuclei of aged myofibres undergo structural and functional changes suggesting impairment in RNA processing. Eur J Histochem. 2009;53:97–106 [DOI] [PubMed] [Google Scholar]
- 31. Otonari-Yamamoto M, Nakajima K, Tsuji Y, et al. Imaging of the mylohyoid muscle: separation of submandibular and sublingual spaces. Am J Roentgenol. 2010;194:W431–W438 [DOI] [PubMed] [Google Scholar]
- 32. La’porte SJ, Juttla JK, Lingam RK. Imaging the floor of the mouth and the sublingual space. Radiographics. 2011;31:1215–1230 [DOI] [PubMed] [Google Scholar]
- 33. Jain P. High-resolution sonography of sublingual space. J Med Imaging Radiat Oncol. 2008;52:101–108 [DOI] [PubMed] [Google Scholar]
- 34. Clark HM, Henson PA, Barber WD, Stierwalt JA, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. Am J Speech Lang Pathol. 2003;12:40–50 [DOI] [PubMed] [Google Scholar]
- 35. Perlman AL, Palmer PM, McCulloch TM, Vandaele DJ. Electromyographic activity from human laryngeal, pharyngeal, and submental muscles during swallowing. J Appl Physiol. 1999;86:1663–1669 [DOI] [PubMed] [Google Scholar]
- 36. Kellermeier JD, Tittor AW, Brooks JC, et al. Effects of zilpaterol hydrochloride with or without an estrogen-trenbolone acetate terminal implant on carcass traits, retail cutout, tenderness, and muscle fiber diameter in finishing steers. J Anim Sci. 2009;87:3702–3711 [DOI] [PubMed] [Google Scholar]
- 37. Ludlow CL. Electrical neuromuscular stimulation in dysphagia: current status. Curr Opin Otolaryngol Head Neck Surg. 2010;18:159–164 [DOI] [PubMed] [Google Scholar]
- 38. Yabunaka K, Konishi H, Nakagami G, et al. Ultrasonographic evaluation of geniohyoid muscle movement during swallowing: a study on healthy adults of various ages. Radiol Phys Technol. 2012;5:34–39 [DOI] [PubMed] [Google Scholar]
- 39. Katsiaras A, Newman AB, Kriska A, et al. Skeletal muscle fatigue, strength, and quality in the elderly: the Health ABC Study. J Appl Physiol. 2005;99:210–216 [DOI] [PubMed] [Google Scholar]
- 40. Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79:874–880 [DOI] [PubMed] [Google Scholar]
- 41. Koster A, Ding J, Stenholm S, et al. Health ABC study Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011;66:888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metab Clin Exp. 2000;49:467–472 [DOI] [PubMed] [Google Scholar]
- 43. Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294 [DOI] [PubMed] [Google Scholar]
- 44. Coggan AR, Spina RJ, King DS, et al. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol. 1992;47:B71–B76 [DOI] [PubMed] [Google Scholar]
- 45. Aniansson A, Hedberg M, Henning GB, Grimby G. Muscle morphology, enzymatic activity, and muscle strength in elderly men: a follow-up study. Muscle Nerve. 1986;9:585–591 [DOI] [PubMed] [Google Scholar]
- 46. Butler SG, Lintzenich CR, Leng X, et al. Tongue adiposity and strength in healthy older adults. Laryngoscope. 2012;122:1600–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–824 [DOI] [PubMed] [Google Scholar]
- 48. Cristea A, Qaisar R, Edlund PK, Lindblad J, Bengtsson E, Larsson L. Effects of aging and gender on the spatial organization of nuclei in single human skeletal muscle cells. Aging Cell. 2010;9:685–697 [DOI] [PubMed] [Google Scholar]
- 49. Alway SE, Siu PM. Nuclear apoptosis contributes to sarcopenia. Exerc Sport Sci Rev. 2008;36:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jang YC, Sinha M, Cerletti M, Dall’Osso C, Wagers AJ. Skeletal muscle stem cells: effects of aging and metabolism on muscle regenerative function. Cold Spring Harb Symp Quant Biol. 2011;76:101–111 [DOI] [PubMed] [Google Scholar]