Abstract
Lentiviral vectors, including double internal promoters, can be used to express two transgenes in a single vector construct; however, transcriptional activities from double internal promoters are often inhibited by promoter interference. To determine whether the chicken hypersensitivity site 4 insulator (cHS4) could block promoter interference, lentiviral vectors including an MSCV-U3 promoter (Mp) and an EF1α promoter (Ep) were generated, and transgene expression was evaluated among transduced cells. In the Ep-Mp configuration, transcriptional activity from Mp was much lower, while Mp-Ep had similar transcription levels from both promoters. The cHS4 core insulator increased expression levels from Mp in HeLa cells, hematopoietic cell lines, and mouse peripheral blood cells following hematopoietic stem cell transplantation transduced with the Mp-Ep configured vector. This blocking function was mainly mediated by barrier activity regions in the insulator but not by CCCTC-binding factor (CTCF) binding sites. Cytosine-phosphate-guanine (CpG) methylation did not contribute to this barrier activity. In summary, combining the cHS4 insulator in double promoter vectors can improve transgene expression levels in various cell lines and mouse hematopoietic repopulating cells. These findings are useful for developing hematopoietic stem cell gene therapy.
Uchida and colleagues demonstrate that the chicken hypersensitivity site 4 insulator (cHS4) is able to block promoter interference in lentiviral vectors containing double internal promoters. The cHS4 core insulator increased expression levels in HeLa cells, hematopoietic cell lines, and mouse peripheral blood cells following transplantation with lentivirus-transduced hematopoietic stem cells.
Introduction
Bicistronic vectors carrying the expression units of a therapeutic gene and a reporter gene are widely used, since reporter proteins such as enhanced green fluorescent protein (GFP) and DsRed are utilized for measuring the viral titers of vector stocks and for tracking genetically modified cells. Double internal promoters can allow expression of a therapeutic gene from a lineage-specific promoter and a reporter gene from a wide spectrum of promoters (Papapetrou et al., 2011). However, the transcriptional activities of double internal promoters in a bicistronic vector are generally inhibited by so-called “promoter interference” (Ginn et al., 2003; Yu et al., 2003; Ben-Dor et al., 2006; Curtin et al. 2008), and a distinct mechanism of the promoter interference has not been elucidated.
Internal ribosomal entry site (IRES) elements have been utilized to overcome this problem, which allow one internal promoter to express two transgenes, with an advantage of ensuring coexpression of the two genes. However, the expression level of a second gene downstream of IRES is sometimes low even with sensitive detection techniques, and this negative effect varies among target cell types and inserted genes (Yu et al., 2003; Dupuy et al., 2005). Alternatively, insertion of a 2A sequence between two genes in a single promoter vector leads to bicistronic mRNA and two protein products; however, the vector construction to express two transgenes, including the 2A sequence, is complicated, since the two genes must be in a single open reading frame (de Felipe et al., 1999). Additionally, the use of chimeric proteins is limited to circumstances in which the fusion should not reduce the function of each protein.
We hypothesized that the 5′-chicken hypersensitivity site 4 insulator (cHS4) elements could block the promoter interference in double promoter lentiviral vectors. The cHS4 has two distinct functions: enhancer-blocking activity and barrier activity (Villemure et al., 2001; Burgess-Beusse et al., 2002; Barkess et al., 2012). The enhancer-blocking activity interferes with the positive effects of an enhancer on a promoter when the insulator is placed between the enhancer and promoter. The barrier activity protects against gene silencing by preventing conversion of a euchromatic region (open) to a heterochromatic region (closed) when placed at the junction between the two. Heterochromatic regions are marked by high levels of cytosine-phosphate-guanine (CpG) methylation and a lack of acetylation at histone H3 Lys9 and Lys27. Recently, the cHS4 was inserted into the long terminal repeat (LTR) of γ-retroviral vectors and lentiviral vectors to improve the transgene expression by protecting these vectors from chromosomal position effects (Emery et al., 2000; Aker et al., 2007; Arumugam et al., 2007). The enhancer-blocking effects were thought mainly to contribute to shielding from the interference of the other promoter.
In this study, we sought to optimize bicistronic lentiviral vectors using double internal promoters to achieve high expression levels of both transgenes and evaluate the effects of cHS4 on the promoter interference in double internal promoters.
Materials and Methods
Plasmid construction and lentiviral preparation
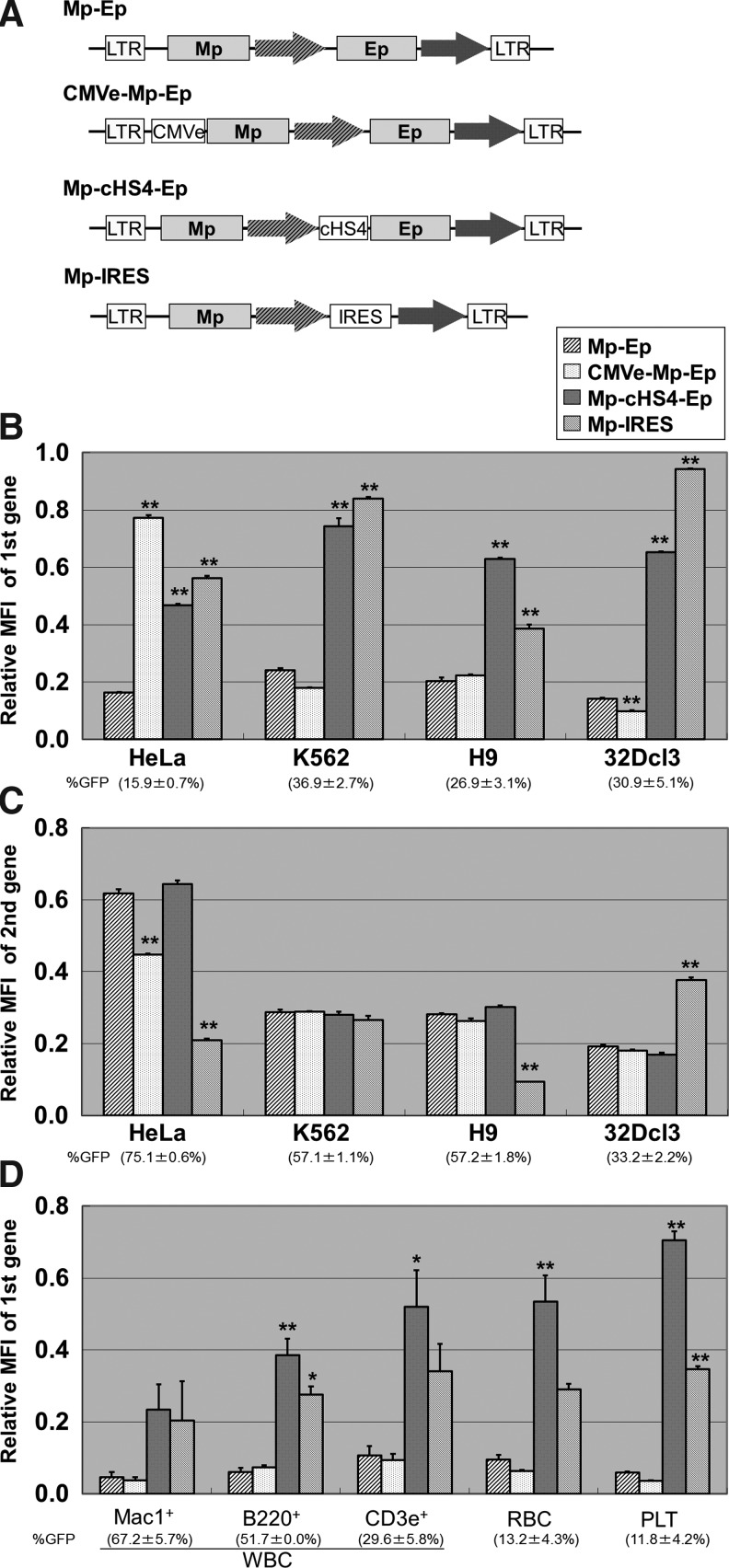
The various configurations of self-inactivating lentiviral vectors, including double internal promoters, were constructed from the murine stem cell virus (MSCV) U3 promoter (Mp, M17246), from the elongation factor 1α (EF1α) promoter without intron 1 (Ep, EF362804), from the GFP cDNA, and from the DsRed (for Northern blot analysis) or DsRed-Express (DsRedExp) (for flow cytometry analysis) cDNA (Fig. 1), using an SJ1 human immunodeficiency virus type 1 vector system, which was kindly provided by Dr. Arthur Nienhuis (St. Jude Children's Research Hospital, Memphis, TN) (Hanawa H et al., 2002). The lentiviral vectors were prepared with vesicular stomatitis virus glycoprotein envelope, as described previously (Uchida et al., 2009, 2010). We generated two types of double internal vector including Mp and Ep in a different order (Mp-Ep and Ep-Mp) (Fig. 1). The configurations of Mp-Ep were modified by adding a cytomegalovirus enhancer (CMVe, FW337461) upstream of Mp (Fig. 2A), inserting 250 bp cHS4 core insulator element (U78775) upstream of Ep (Fig. 2A), and deleting the site upstream of the TATA box EF1α promoter using polymerase chain reaction (PCR) with primers (mEF1a-f: 5′-GAC GAA TTC TAT ATA AGT GCA GTA GTC GCC GTG-3′, mEF1a-r: 5′-CGC ATG AAC TCC TTG ATG AC-3′). Additionally, we constructed an alternative bicistronic vector to express two transgenes that were linked by the IRES of the encephalomyocarditis virus and derived from a single promoter (Mp) (Fig. 2A).
FIG. 1.

Promoter interference in double internal promoters in lentiviral vectors. We evaluated the transcriptional activities in lentiviral vectors including double internal promoters; murine stem cell virus U3 promoter (Mp), and elongation factor 1α promoter (Ep), in which we generated two types of vectors in different orders (Mp-Ep and Ep-Mp). We extracted RNA from the transduced cells, and the RNA amounts derived from both Mp and Ep were detected by Northern blot analysis using enhanced green fluorescent protein (GFP) or DsRed probes. The RNA band densities were standardized by total RNA amounts (S28 densities) and the integrated vector amounts that were evaluated by Southern blot analysis. In the cells transduced with the Mp-Ep vector, Mp and Ep had similar transgene expression levels, which were approximately half of the transgene expression level from Mp in a single promoter vector (relative expression levels: Mp 0.44 and Ep 0.48). The Ep-Mp vector showed higher transgene expression level from Ep (0.75) but lower level from Mp (0.15). mRNA: messenger RNA.
FIG. 2.
Evaluation of transgene expression from double internal promoter vectors in various transduced cell lines and mouse hematopoietic repopulating cells. (A) To improve transgene expression from Mp in the Mp-Ep vector, we inserted the cytomegalovirus enhancer (CMVe) upstream of Mp (CMVe-Mp-Ep) or 250-bp core element of chicken hypersensitivity site 4 insulator (cHS4) between the two promoters (Mp-cHS4-Ep) and compared with a single promoter vector containing Mp and internal ribosomal entry site (IRES) (Mp-IRES). We transduced various cell lines including the HeLa cell line and blood cell–derived cell lines (K562, H9, and 32Dcl3) with the same multiplicity of infection (MOIs), and transgene expression levels were evaluated by mean fluorescent intensity (MFI) of GFP. (B) The CMVe increased transgene expression levels from Mp in HeLa cells (p<0.01) but not in blood cell lines (K562, H9, 32Dcl3). The cHS4 increased transgene expression levels from Mp in all cell lines (p<0.01), with comparable results to the Mp-IRES vector. (C) The transgene expression levels from the second promoter (Ep) were not increased by insertion of CMVe or cHS4, while the Mp-IRES vector had large variations in transgene expression levels from the downstream gene from Mp. (D) To evaluate transgene expression from Mp in hematopoietic repopulating cells, mouse bone marrow cells were transduced with these vectors and transplanted into lethally irradiated mice. The transgene (GFP) expression from Mp was evaluated by flow cytometry in the peripheral blood cells at 12 weeks after transplantation. The cHS4 increased transgene expression levels in Mac1+ granulocytes, B220+ B cells, CD3e+ T cells, red blood cells (RBC), and platelets (PLT), which were comparable to those in the Mp-IRES vector. However, the CMVe had no effect on transgene expression from Mp in the peripheral blood cells. WBC: white blood cells; %GFP: GFP-positive rates; *p<0.05, **p<0.01.
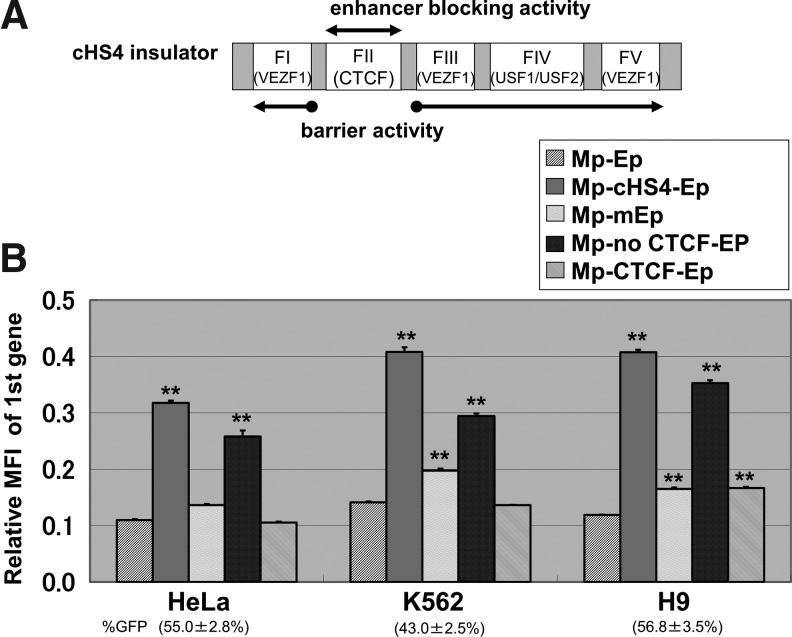
The cHS4 core element was divided into the CTCF binding site and other sites (Fig. 3A and Supplementary Fig. 1; Supplementary Data available online at www.liebertonline.com). The CTCF binding site was produced by artificial oligonucleotides (FII-f: 5′-AAT TCC AGG GAT GTA ATT ACG TCC CTC CCC CGC TAG GGG GCA GCA G-3′, FII-r: 5′-AAT TCT GCT GCC CCC TAG CGG GGG AGG GAC GTA ATT ACA TCC CTG G-3′), while the cHS4 without the CTCF binding site was constructed by PCR using primers (FII(-)IR-f: 5′-GAC GAA TTC GGG GAC AGC CCC CCC CCA AAG CCC GCG AGC CGC CCG GGG CTC CGC TCC-3′, FII(-)IR-r: 5′-CCT TCT CTA GGC ACC GGT TC-3′).
FIG. 3.
The CCCTC-binding factor (CTCF) binding site is not essential to block promoter interference in double promoter vectors. (A) The core element of chicken hypersensitivity site 4 insulator (cHS4) is composed of five footprints and it has two functions: (1) enhancer-blocking activity mediated by the CTCF binding site and (2) barrier activity mediated by the other four footprints (one USF1/USF2 binding site and three VEZF1 binding sites). (B) To evaluate whether enhancer-blocking activity or barrier activity of cHS4 contributed to blocking promoter interference, we evaluated transgene expression from Mp in the cell lines (HeLa, K562, and H9) that were transduced with various derivative Mp-Ep vectors: (1) deletion of enhancer region in Ep (Mp-mEp), (2) insertion of the minimum CTCF binding site of cHS4 (Mp-CTCF-Ep), and (3) insertion of cHS4 without the CTCF binding site (Mp-no CTCF-Ep). Insertion of cHS4 without the CTCF binding site lead to 2.1- to 2.9-fold higher transgene expression level from Mp, while it was minimally increased by deletion from the Ep enhancer (1.3- to 1.4-fold) and insertion of the CTCF site (1.0- to 1.4-fold). %GFP: GFP-positive rates; **p<0.01.
Lentiviral transduction and plasmid transfection for cell lines
A total 5×104 cells were cultured for 24 hr and then transduced with the lentiviral vectors at a multiplicity of infection (MOI) of 1 in the presence of 8 μg/ml polybrene, while a total 1×105 of K562, H9, and 32Dcl3 cells were transduced for 24 hr at MOI 11. After an additional 3 days in culture, GFP and DsRedExp expression among the transduced cell lines were analyzed by flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ).
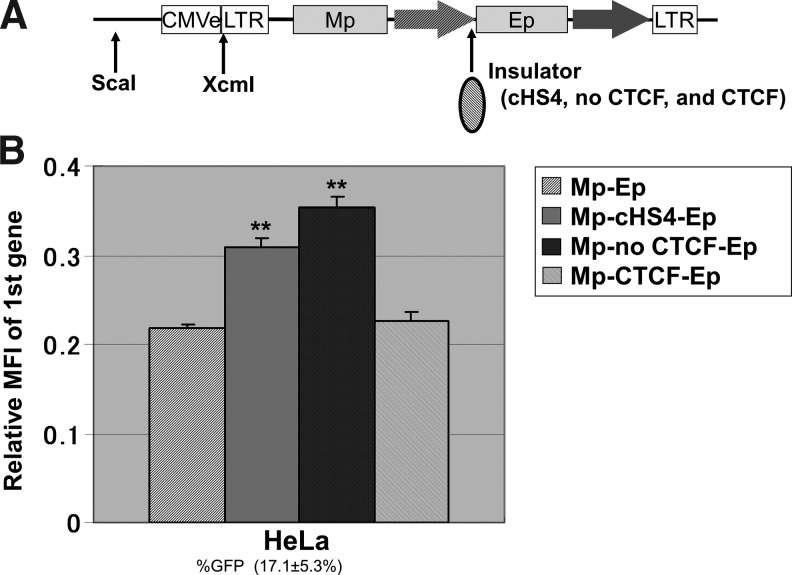
The plasmids, including double internal promoters for transient expression, were digested by the restriction enzymes ScaI (TaKaRa, Otsu, Japan) and XcmI (New England Bio Labs, Beverly, MA) to divide the enhancer region from the 5′ LTR (Fig. 4A). These plasmids were transfected into HeLa cells using Lipofectamine2000 (Invitrogen, Carlsbad, CA). Sixteen hours following the transfection, GFP and DsRedExp expression were analyzed by flow cytometry (FACSCalibur).
FIG. 4.
The promoter interference in double promoter vector constructs in transient plasmid transfection. (A) To evaluate whether gene silencing mediated by CpG methylation contributes to promoter interference, we evaluated transgene expression from Mp in transfected plasmid DNAs, which were theoretically independent of CpG methylation. The double promoter vector plasmids were digested by restriction enzymes (XcmI and ScaI), which deleted the enhancer region (CMVe) from upstream of 5′LTR to decrease the transcriptional activity and evaluate gene expression from internal promoters. (B) The transient expression from Mp had a similar pattern as in Figure 3: increased by the core element of chicken hypersensitivity site 4 insulator (cHS4) and cHS4 without the CTCF binding site (no CTCF) but not increased by the CTCF binding site alone. %GFP, GFP-positive rates; ** p<0.01.
Transplantation of mouse bone marrow cells transduced with lentiviral vectors
All procedures were carried out in accordance with the animal experimentation guidelines of Nippon Medical School. We harvested bone marrow (BM) cells from C57BL/6J donor mice (Saitama Experimental Animals Supply, Saitama, Japan), and lineage marker-positive cells from the BM cells were depleted using the Mouse Hematopoietic Progenitor Cell Enrichment Set-DM (Pharmingen, San Diego, CA). Lineage negative BM cells were transduced with the bicistronic lentiviral vectors at MOI 25 (no prestimulation) at a concentration of 6×105 cells/ml in serum-free medium (StemSpan™ SFEM; Stem Cell Technologies, Vancouver, Canada) containing 50 ng/ml mouse stem cell factor (mSCF) (Kirin Brewery, Tokyo, Japan) and 8 μg/ml polybrene on fibronectin CH296 (RetroNectin™; TaKaRa) coated plates. After a 12-hr transduction, the transduced cells (2×105 cells/mouse) were injected into lethally irradiated (10 Gy) C57BL/6J mice. GFP expression among peripheral blood cells was evaluated 12 weeks after transplantation by flow cytometry (FACSCalibur) with lineage-specific monoclonal antibodies (B220- Allophycocyanin [APC], Mac1- APC, CD3e-APC; BD Biosciences, Franklin Lakes, NJ).
Northern blot analysis
Total RNAs were extracted after 6 days of culture following transduction of 293T cells at MOI 50, and the RNAs were analyzed by electrophoresis, transferred, and hybridized with a GFP probe (728-bp fragment digested by BamHI and NotI) and a DsRed probe (711bp fragment digested by SmaI and ClaI). The specific signals were detected by an Alkphos Direct Labeling Kit (Amersham Biosciences, Little Chalfont Buckinghamshire, United Kingdom). The band densities were normalized against the densities of 28S and the integrated vector copy numbers, which were evaluated by Southern blot analysis. The relative expression levels from each promoter in double promoter vectors were compared to a single promoter vector including Mp, which were detected by the DsRed probe except for the expression level from Ep in Mp-Ep vector. The relative expression level from Ep in Mp-Ep vector was calculated by multiplying the relative expression level from Ep in the Mp-Ep vector compared to Mp in the Mp-Ep vector (evaluated by a GFP probe) by the relative expression level from Mp in the Mp-Ep vector compared to Mp in the Mp vector (evaluated by a DsRed probe).
Southern blot analysis using CpG methylation-sensitive restriction enzyme
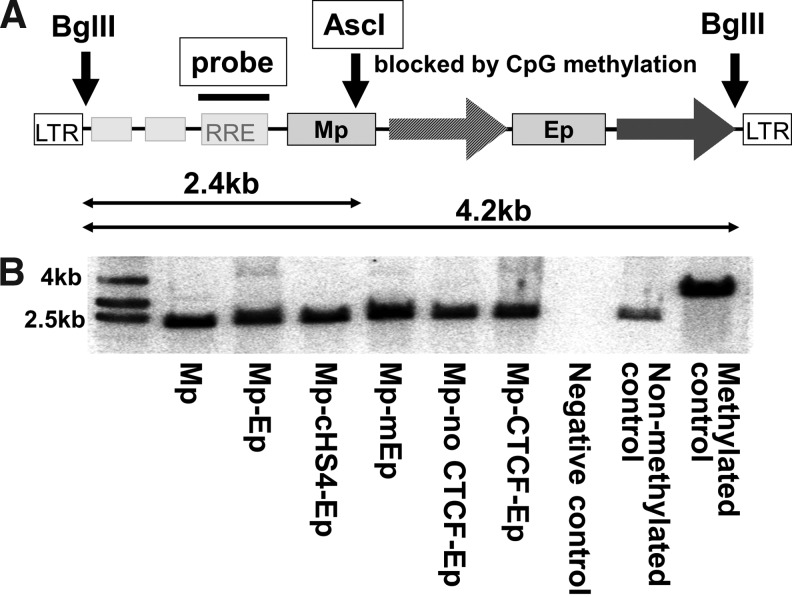
The CpG methylation status within Mp was evaluated by sensitivity to the AscI restriction enzyme (New England Bio Labs), which is inhibited by CpG methylation of the recognized sequences (Fig. 5A). HeLa cells were transduced with various types of double internal promoter vectors at MOI 10, and 10 days after transduction, the genomic DNAs were extracted from the transduced cells. Ten μg of DNA were digested by AscI and BglII (New England Bio Labs) (Fig. 5A), and the sizes of the digested DNAs were determined by Southern blot analysis using an RRE probe (761-bp fragment digested by MfeI and BstBI), in which we evaluated whether the AscI enzyme could cut the specific site within Mp. As a nonmethylated control, we used a mixture of a single promoter vector plasmid (Mp) and genomic DNA of HeLa cells, while as a methylated control, the DNA extracted from the transduce HeLa cells was treated by the SssI CpG methylase (New England Bio Labs).
FIG. 5.
Evaluation of CpG methylation around murine stem cell virus U3 promoter (Mp). (A) We extracted genomic DNAs from the transduced HeLa cells and digested with CpG methylation-sensitive restriction endonuclease AscI targeting the Mp sequence. Vector sequences were cut out by BglII, and the DNA signals were detected by an RRE probe using Southern blot analysis. If the AscI site within Mp had CpG methylation, it could not be digested (4 kb), while nonmethylated DNA could be digested by AscI (2.4 kb). As a positive control, we used methylated DNA, which was treated by SssI CpG methylase. (B) Although low densities of nondigested 4-kb bands were observed in Mp-Ep (8.5%), Mp-mEp (3.1%), and Mp-CTCF-Ep (5.9%), but not in others (<1%: Mp-cHS4-Ep and Mp-no CTCF-Ep), almost all genomic DNAs (>90%) of HeLa cells transduced with various double promoter vectors were digested by AscI to 2.4-kb fragments. SssI-treated DNA was not digested.
Results
mRNA expression levels in double internal promoters in lentiviral vectors
To evaluate whether the transcriptional activities are inhibited in a lentiviral vector including double internal promoters, both MSCV-U3 promoter (Mp) and EF1α promoter (Ep) were inserted into a lentiviral vector construct in forward orientation (Fig. 1), in which we generated two types of double promoter vectors in different orders (Mp-Ep and Ep-Mp). We transduced 293T cells with these double promoter vectors at the same MOIs and compared transgene expression levels from both promoters by Northern blot analysis to that of a single promoter vector including Mp. The Mp-Ep vector had similar transgene expression levels between two internal promoters, and both expression levels were approximately half (relative expression levels in Mp: 0.44 and Ep: 0.48) (Fig. 1). On the other hand, the Ep-Mp vector produced relatively higher expression from Ep (0.75) and lower expression from Mp (0.15) (Fig. 1). These data suggest that when Mp was placed in the second position, transcriptional activity from Mp was inhibited in double promoter vectors. The transcriptional activity of Ep was relatively stable irrespective of its position.
Optimization of the Mp-Ep vector in vitro and in vivo
Although both Mp and Ep have similar transcriptional activity in the Mp-Ep vector (Fig. 1), the Mp still had much lower transcriptional activity compared to the single promoter vector. Thus, we sought to improve Mp gene expression by inserting CMVe upstream of Mp (CMVe-Mp-Ep), or the 250-bp core element of cHS4 between the two promoters (Mp-cHS4-Ep), and compared a single promoter vector (Mp) with an IRES to express two transgenes (Mp-IRES; Fig. 2A). We transduced various cell lines including the HeLa cell line and blood cell–derived cell lines (K562, H9, and 32Dcl3) with the same MOIs, and transgene expression levels from the first promoter (Mp) were evaluated by mean fluorescent intensity (MFI) of GFP in the GFP-positive gate. The CMVe increased transgene expression levels from Mp in HeLa cells (p<0.01) but not in blood-derived cell lines (K562: ns; H9: ns; 32Dcl3: p<0.01 with decrease) (Fig. 2B). The cHS4 increased transgene expression levels from Mp in all cell lines (all: p<0.01), with comparable results from the Mp-IRES vector (Figure 2B).
With regard to the activity of the second promoter (Ep), the insertion of CMVe and cHS4 did not increase transgene expression levels (ns, except for CMVe in HeLa cells: p<0.01 with decrease) (Fig. 2C), whereas the transgene expression levels of the downstream gene from Mp in the Mp-IRES vector showed large variations (HeLa: p<0.01 with decrease; K562: ns; H9: p<0.01 with decrease; 32Dcl3: p<0.01 with increase).
Mouse bone marrow cells were then transduced with the double promoter vectors and transplanted into lethally irradiated mice. Three months after transplantation, transgene expression levels from Mp were evaluated by MFIs of GFP in the GFP-positive gate. GFP expression was confirmed in all lineage blood cells in the transplanted mice (Table 1). The cHS4 increased gene expression from the Mp among peripheral blood cells (Mac1+ granulocytes: ns; B220+ B-cells: p<0.01; CD3e+ T-cells: p<0.05; red blood cells: p<0.01; and platelets: p<0.01), which were 1.2- to 2.0-fold higher compared to those from Mp in the Mp-IRES vector (Fig. 2D). As expected, the CMVe did not increase transgene expression levels from Mp in the peripheral blood cells (all lineage cells: ns). Additionally, second gene expression (DsRedExp) was too low in peripheral blood cells to evaluate expression levels. These data suggest that the cHS4 improved transcriptional activity of the first promoter, with results comparable to IRES in hematopoietic cells in vitro and in vivo.
Table 1.
Transgene Expression Rates in Peripheral Blood Cells in Transplanted Mice (3 Months After Transplantation)
| |
WBC |
|
|
||
|---|---|---|---|---|---|
| Mac1+ | B220+ | CD3e+ | RBC | PLT | |
| Mp | 88.9±1.2 | 51.7±5.1 | 53.9±6.9 | 32.5±9.2 | 29.8±5.0 |
| Mp-Ep | 48.9±18.5 | 20.1±1.1 | 20.1±2.1 | 9.1±2.3 | 12.5±7.5 |
| CMVe-Mp-Ep | 66.2±4.4 | 20.2±5.3 | 24.6±3.7 | 9.5±2.4 | 4.6±0.5 |
| Mp-cHS4-Ep | 67.0±7.0 | 21.5±2.6 | 30.9±4.1 | 7.5±1.9 | 6.1±2.6 |
| Mp-IRES | 65.1±15.6 | 18.6±4.1 | 18.6±2.9 | 7.2±3.9 | 5.8±3.6 |
The transgene expression rates were evaluated by green fluorescent protein (GFP)-positive rates (%).
WBC, white blood cells; RBC, red blood cells; PLT, platelets.; Mp, MSCV-U3 promoter; Ep, EF1α promoter; CMVe, cytomegalovirus enhancer; cHS4, chicken hypersensitivity site 4 insulator; IRES, internal ribosomal entry site.
Evaluation of cHS4 effects on the Ep-Mp vector
To evaluate effects of the 250-bp core element of cHS4 on transgene expression in the Ep-Mp vector, we inserted the cHS4 between the two promoters (Ep-cHS4-Mp) and compared them with a single promoter vector including Mp. Additionally, we evaluated transgene expression in a single promoter vector including Ep (Ep). We transduced various cell lines including 293T, HeLa, and H9 cell lines with the same MOIs, and transgene expression levels were evaluated by MFI of GFP (Supplementary Fig. 2). The transgene expression levels from the Ep vector were 0.6–0.8 fold lower in all cell lines, compared to the Mp vector. The cHS4 increased transgene expression levels from Ep in HeLa and H9 cell lines (p<0.01 in HeLa and p<0.05 in H9), while the transgene expression levels from second promoter (Mp) were not increased by insertion of cHS4. These data corroborate that the cHS4 improved transcriptional activity of the first promoter.
Effects of cHS4 on promoter interference
The cHS4 core element is comprised of five footprints and is thought to have two functions: enhancer-blocking activity or barrier activity. The enhancer-blocking activity is mediated by the CTCF binding site, which blocks the action of enhancers on promoters, while barrier activity results from the combined effects of the other four footprints to prevent gene silencing (Fig. 3A and Supplementary Fig. 1). To investigate the enhancer-blocking or barrier activity in cHS4, we evaluated transgene expression levels from Mp among the cell lines (HeLa, K562, and H9), which were transduced with various derivative Mp-Ep vectors: deletion of enhancer region in Ep (Mp-mEp), insertion of the minimum CTCF binding site of cHS4 (Mp-CTCF-Ep), and insertion of cHS4 without the CTCF binding site (Mp-no CTCF-Ep) (Fig. 3B). Mp-no CTCF-Ep vector produced 2.1- to 2.9-fold higher transgene expression levels from Mp (all cell lines: p<0.01), while these expression levels were minimally increased in Mp-mEp vector (1.3- to 1.4-fold; HeLa: ns; K562: p<0.01; and H9: p<0.01) or Mp-CTCF-Ep vector (1.0- to 1.4-fold, ns except for H9: p<0.01) (Fig. 3B). Additionally, We confirmed that the expression levels from Ep (DsRedExp) were decreased in Mp-mEp vector, compared to Mp-Ep vector (all cell lines: p<0.01) (Supplementary Fig. 3). These data demonstrate that the increase in Mp activity is derived mainly by the cHS4 components other than the CTCF binding site.
Evaluation of CpG methylation in double internal promoter vectors
Since the barrier activity is related to protection from gene silencing, we investigated CpG methylation status with respect to transgene expression levels in transfected plasmid DNAs, which were theoretically independent of CpG methylation. The transient expression in plasmid transfection was similar to transduction by the double promoter vectors (Fig. 4 and Supplementary Fig. 4). The cHS4 (p<0.01) and cHS4 without the CTCF binding site (p<0.01) increased the transgene expression levels from Mp.
Next, to evaluate CpG methylation status within Mp in double promoter vectors, genomic DNAs from the transduced HeLa cells were digested by CpG methylation-sensitive restriction endonuclease AscI targeting the Mp sequence. A methylated DNA control was used, which was treated by SssI CpG methylase. If the AscI site within Mp was methylated, it could not be digested (yielding a 4-kb band), while nonmethylated DNA could be digested (2.4-kb band). Although low densities of nondigested 4-kb bands were observed in Mp-Ep (8.5%), Mp-mEp (3.1%), and Mp-CTCF-Ep (5.9%), but not in others (<1%: Mp-cHS4-Ep and Mp-no CTCF-Ep), almost all genomic DNAs (>90%) of HeLa cells transduced with various double promoter vectors were digested by AscI to 2.4-kb fragments (Fig. 5A, B). SssI-treated DNA was not digested. These data suggest that promoter interference was independent of CpG methylation.
Discussion
Lentiviral vectors including double internal promoters that can express two transgenes from different promoters have obvious advantages for gene therapy applications. For example, a double internal promoter vector including both a therapeutic gene (from a lineage-specific promoter) and GFP (from a wide spectrum of promoters) allows us not only to deliver a gene of interest, but also to calculate vector titer in common cell lines and evaluate the distribution of gene-modified cells in all lineage cells (Papapetrou et al., 2011). Moreover, with the addition of a drug-resistance gene (from a wide spectrum of promoters) with a therapeutic gene, the gene-modified stem cells can be positively selected by a specific anti-cancer drug (Papapetrou et al., 2011). However, a major limitation of double internal promoter vectors is that the transgene expression levels are generally lower due to promoter interference (Fig. 1) (Ben-Dor et al., 2006). In this study, we demonstrated that the cHS4 could improve transgene expression from Mp (first gene) in the double promoter vector, when inserted between the two promoters (Fig. 2). This improvement was seen in HeLa cells, blood cell–derived cell lines (K562, H9, and 32Dcl3), and mouse peripheral blood cells after bone marrow transplantation (Fig. 2B). Additionally, the higher expression levels in the first gene even remained when the order of the promoters was changed (Supplementary Fig. 2).
The CMVe and IRES have traditionally been used to increase expression of transgenes. In these experiments, CMVe showed little effect on transgene expression levels in blood cells, and the second gene (downstream of IRES) typically yielded lower expression levels compared to the first gene (Fig. 2B and C). When comparing expression levels of the second gene in vectors including IRES vs. double promoters, the latter vector system yielded higher expression levels in two of four cell lines (Fig. 2C). These data show that the cHS4 with double internal promoter vectors is a better combination for developing hematopoietic stem-cell-targeted gene therapy.
The mechanism of promoter interference was thought to be through negative enhancing activity of other promoters (Proudfoot, 1986; Steinwaerder et al., 2000; Villemure et al., 2001; Ben-Dor et al., 2006). Here we demonstrated that cHS4 exerted its effects through the barrier activity regions; insertion of only the CTCF binding site was not sufficient (Fig. 3) (Villemure et al., 2001). Additionally, deletion of the enhancer element in Ep had little effects on the transcriptional activity from Mp (Fig. 3), even though the enhancer-deleted Ep (second gene) showed lower levels of transgene expression (Supplementary Fig. 3).
To further understand the barrier activity of cHS4, we then evaluated transient gene expression of plasmid DNAs from transient transfections (Fig. 4 and Supplementary Fig. 4). We found the barrier activity regions of cHS4 increased transgene expression levels from the plasmids alone, similar to that previously shown in transduced cell lines and peripheral blood cells after mouse bone marrow cell transplantation with lentiviral transduction. Since there should be little or no CpG methylation in the plasmid transfection experiments, the results reaffirmed that promoter interference is not modified by CpG methylation. We then directly evaluated CpG methylation status of the Mp promoter in the next set of experiments (Fig. 5). Low levels of methylation within Mp were observed in some but not others, consistent with a model where the presence of footprints I, III, IV, and V in the cHS4 prevents methylation. Another interpretation could be that these low levels of methylation are insufficient for explaining promoter interference in double internal promoter vectors. Further experiments are needed to work out other mechanisms of barrier activity.
There are several mechanisms of promoter interference. Our results showing the preferential expression of the first over the second promoter (Fig. 2 and Supplementary Fig. 2) is also termed transcriptional interference and could be due to read-through transcription of the first gene. Adding a transcriptional termination signal between the promoters has been previously shown to reduce the interference of the second promoter (Proudfoot, 1986; Villemure et al., 2001). However, a termination signal could disturb transcription of full-length viral genome, thus its use in our vector systems is likely limited. In our study, insertion of cHS4 did not change transgene expression levels from Mp in the second promoter (Fig. 2C), while it slightly decreased the expression levels from Ep in the second promoter (Supplementary Fig. 2C). The cHS4 effects on internal promoters might be associated with configurations in double promoter vectors.
Suppression of the first promoter by the second promoter, termed promoter suppression, is another mechanism of promoter interference. In lentiviral vectors that contain double internal promoters of erythroid-specific ankyrin promoter and retroviral-derived myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted (MND) promoter (Robert-Richard et al., 2007), erythroid specificity of the ankyrin promoter (first position) was lost due to the MND promoter. Insertion of cHS4 partially restored erythroid-specific expression from the ankyrin promoter. Similarly in our study, insertion of cHS4 increased transgene expression levels from Mp in double promoter vectors in all lineages of mouse peripheral blood cells (Fig. 2) (Robert-Richard et al., 2007).
In another experimental system, stable cell lines were transfected with plasmids including two expression cassettes of EF1α promoter, cDNA, and polyadenylation signal (Yahata et al., 2007). Insertion of tandem cHS4 between two expression cassettes increased gene expression from both promoters, and the cHS4 effects were dependent on CTCF protein (required for enhancer blocking activity) or USF1 protein (required for barrier activity). These data suggested that CTCF and USF1 cooperatively reduced promoter interference. However, in their experiment the polyadenylation signals were in both promoters, whereas in our vector system the signal was only in the 3′LTR downstream of the second promoter. Thus the role of the polyadenylation signal in reducing promoter interference could not be fully tested in our experiments.
While increased transgene expression is desired for therapeutic effects in hematopoietic stem-cell-targeted gene therapy, recent trials reported monoclonal cell expansion and leukemia development caused by insertional mutagenesis (Ott et al., 2006; Hacein-Bey-Abina et al., 2008; Cavazzana-Calvo et al., 2010). This process is thought to be induced by activation of leukemia-related genes from enhancer activity in vector sequences. Our double promoter vectors including the cHS4 thus has the benefit for increased transgene expression levels of the first gene, and the potential for increasing the risk of insertional mutagenesis. Insertional mutagenesis was reportedly reduced by insertion of cHS4 insulator into LTR (Ryu et al., 2008). Since the vector sequence contains two internal promoters, a single cHS4 core element might not be sufficient to reduce insertional mutagenesis. Additional safety testing is likely needed in double internal promoter vectors.
In summary, the cHS4 fragment could block the promoter interference in double internal promoter vectors to improve transgene expression levels from Mp (first transgene) in various cell lines (including hematopoietic cell lines) and hematopoietic repopulating cells in transplanted mice, and this blocking function was mediated by the barrier activity regions in cHS4 but not the CTCF binding site. The transgene expression levels from Ep in the double promoter vectors had less variation in various cells compared to the second gene expression in a bicistronic vector using IRES. These findings should be utilized to develop strategies for gene therapy, especially for hematopoietic stem-cell-targeted gene therapy.
Supplementary Material
Acknowledgments
We thank Drs. John Tisdale and Matthew Hsieh in Molecular and Clinical Hematology Branch, National Heart Lung and Blood Institute (NHLBI)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health for the preparation and editing of our manuscript. This work was supported in part by the intramural programs of NHLBI/NIDDK.
Author Disclosure Statement
No competing financial interests exist.
References
- Aker M. Tubb J. Groth A.C., et al. Extended core sequences from the cHS4 insulator are necessary for protecting retroviral vectors from silencing position effects. Hum. Gene Ther. 2007;18:333–343. doi: 10.1089/hum.2007.021. [DOI] [PubMed] [Google Scholar]
- Arumugam P.I. Scholes J. Perelman N., et al. Improved human beta-globin expression from self-inactivating lentiviral vectors carrying the chicken hypersensitive site-4 (cHS4) insulator element. Mol. Ther. 2007;15:1863–1871. doi: 10.1038/sj.mt.6300259. [DOI] [PubMed] [Google Scholar]
- Barkess G. West A.G. Chromatin insulator elements: establishing barriers to set heterochromatin boundaries. Epigenomics. 2012;4:67–80. doi: 10.2217/epi.11.112. [DOI] [PubMed] [Google Scholar]
- Ben-Dor I. Itsykson P. Goldenberg D., et al. Lentiviral vectors harboring a dual-gene system allow high and homogeneous transgene expression in selected polyclonal human embryonic stem cells. Mol. Ther. 2006;14:255–267. doi: 10.1016/j.ymthe.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Burgess-Beusse B. Farrell C. Gaszner M., et al. The insulation of genes from external enhancers and silencing chromatin. Proc. Natl. Acad. Sci. U.S.A. 2002;99(Suppl 4):16433–16437. doi: 10.1073/pnas.162342499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzana-Calvo M. Payen E. Negre O., et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin J.A. Dane A.P. Swanson A., et al. Bidirectional promoter interference between two widely used internal heterologous promoters in a late-generation lentiviral construct. Gene Ther. 2008;15:384–390. doi: 10.1038/sj.gt.3303105. [DOI] [PubMed] [Google Scholar]
- de Felipe P. Martin V. Cortes M.L., et al. Use of the 2A sequence from foot-and-mouth disease virus in the generation of retroviral vectors for gene therapy. Gene Ther. 1999;6:198–208. doi: 10.1038/sj.gt.3300811. [DOI] [PubMed] [Google Scholar]
- Dupuy F.P. Mouly E. Mesel-Lemoine M., et al. Lentiviral transduction of human hematopoietic cells by HIV-1- and SIV-based vectors containing a bicistronic cassette driven by various internal promoters. J. Gene Med. 2005;7:1158–1171. doi: 10.1002/jgm.769. [DOI] [PubMed] [Google Scholar]
- Emery D.W. Yannaki E. Tubb J., et al. A chromatin insulator protects retrovirus vectors from chromosomal position effects. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9150–9155. doi: 10.1073/pnas.160159597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginn S.L. Fleming J. Rowe P.B., et al. Promoter interference mediated by the U3 region in early-generation HIV-1-derived lentivirus vectors can influence detection of transgene expression in a cell-type and species-specific manner. Hum. Gene Ther. 2003;14:1127–1137. doi: 10.1089/104303403322167975. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Garrigue A. Wang G.P., et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa H. Kelly P.F. Nathwani A.C., et al. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol Ther. 2002;5:242–251. doi: 10.1006/mthe.2002.0549. [DOI] [PubMed] [Google Scholar]
- Ott M.G. Schmidt M. Schwarzwaelder K., et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- Papapetrou E.P. Lee G. Malani N., et al. Genomic safe harbors permit high beta-globin transgene expression in thalassemia induced pluripotent stem cells. Nat. Biotechnol. 2011;29:73–78. doi: 10.1038/nbt.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N.J. Transcriptional interference and termination between duplicated alpha-globin gene constructs suggests a novel mechanism for gene regulation. Nature. 1986;322:562–565. doi: 10.1038/322562a0. [DOI] [PubMed] [Google Scholar]
- Robert-Richard E. Richard E. Malik P., et al. Murine retroviral but not human cellular promoters induce in vivo erythroid-specific deregulation that can be partially prevented by insulators. Mol. Ther. 2007;15:173–182. doi: 10.1038/sj.mt.6300030. [DOI] [PubMed] [Google Scholar]
- Ryu B.Y. Evans-Galea M.V. Gray J.T., et al. An experimental system for the evaluation of retroviral vector design to diminish the risk for proto-oncogene activation. Blood. 2008;111:1866–1875. doi: 10.1182/blood-2007-04-085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinwaerder D.S. Lieber A. Insulation from viral transcriptional regulatory elements improves inducible transgene expression from adenovirus vectors in vitro and in vivo. Gene Ther. 2000;7:556–567. doi: 10.1038/sj.gt.3301139. [DOI] [PubMed] [Google Scholar]
- Uchida N. Washington K.N. Hayakawa J., et al. Development of a human immunodeficiency virus type 1-based lentiviral vector that allows efficient transduction of both human and rhesus blood cells. J. Virol. 2009;83:9854–9862. doi: 10.1128/JVI.00357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N. Washington K.N. Lap C.J., et al. Chicken HS4 insulators have minimal barrier function among progeny of human hematopoietic cells transduced with an HIV1-based lentiviral vector. Mol. Ther. 2011;19:133–139. doi: 10.1038/mt.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemure J.F. Savard N. Belmaaza A. Promoter suppression in cultured mammalian cells can be blocked by the chicken beta-globin chromatin insulator 5'HS4 and matrix/scaffold attachment regions. J Mol. Biol. 2001;312:963–974. doi: 10.1006/jmbi.2001.5015. [DOI] [PubMed] [Google Scholar]
- Yahata K. Maeshima K. Sone T., et al. cHS4 insulator-mediated alleviation of promoter interference during cell-based expression of tandemly associated transgenes. J. Mol. Biol. 2007;374:580–590. doi: 10.1016/j.jmb.2007.09.054. [DOI] [PubMed] [Google Scholar]
- Yu X. Zhan X. D'Costa J., et al. Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol. Ther. 2003;7:827–838. doi: 10.1016/s1525-0016(03)00104-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.