Abstract
It has been recently demonstrated that endothelial progenitor cells (EPCs) have increasing potential for gene therapy or regenerative cell therapy for cardiovascular diseases and cancer. However, current therapies involving EPCs are inefficient because of the very low level of EPCs in the available sources, for example, in blood. One solution is to derive in vitro an expanded population of EPCs from circulation. In addition, EPCs like other progenitor cells have an intrinsic predisposition of differentiating into mature cell types, for example, mature endothelial cells; therefore, establishing a sufficient amount of EPCs alongside maintaining the EPC characteristic phenotype during genetic modification and long-term culture presents a significant challenge to the field of gene and cell therapies. In this study, we have systematically investigated EPCs from different sources and used multiple parameters, including cell surface markers and a tubule formation assay to identify factors that influence the establishment, characteristics, and vector transduction capability of EPCs. Our results show the considerable promise, as well as certain limitations in the establishment and manipulation of genetically modified EPCs for gene therapy. While obtaining high transduction efficiency and robust in vitro tubule formation of EPCs using lentiviral vectors, we also observed that lentiviral vector transduction significantly altered EPC phenotype as demonstrated by an increased percentage of CD34+ progenitor cells and increased expression of adhesion molecule CD144 (VE-cadherin). Taking account of the increased expression of CD144 reported in cancer patients, the altered expression of EPC-related markers, for example, VE-cadherin and the enrichment of CD34+ cells, after vector transduction indicates the importance of extensive characterization and vigorous safety control of genetically modified EPCs before they are accepted for clinical use.
Werling and colleagues systematically profile endothelial progenitor cells (EPC) from different sources in order to identify factors that influence EPC establishment and vector transduction capability. While lentiviral vector transduction resulted in high transduction efficiency and robust in vitro tubule formation of EPC, it also significantly altered EPC phenotype. Therefore, extensive characterization and vigorous safety control of genetically modified EPC is warranted before clinical use.
Introduction
Since their first identification in 1997 (Asahara et al., 1997), endothelial progenitor cells (EPCs) have been extensively studied for their potential as a vehicle for gene transfer of anti-tumorigenic or proangiogenic agents to patients and as a tool for regenerative cell therapy of cardiovascular diseases and musculoskeletal injuries (Werner et al., 2005; Briguori et al., 2010). Recent studies have shown that adenoviral vector–transduced EPCs, expressing vascular endothelial growth factor (VEGF) or hypoxia inducible factor 1α (HIF-1α), enhanced neovascularization and resulted in an significant reduction of limb necrosis in murine ischemia models (Iwaguro et al., 2002; Jiang et al., 2008). In combination with local administration of stromal cell–derived factor 1α (SDF-1α), retroviral-transduced EPCs expressing VEGF enhanced the incorporation of transplanted EPCs into the neovasculature (Yu et al., 2009). Unmodified EPCs have also been shown to improve neovascularization in rat and mouse ischemic models with the incorporation of injected cells into endothelial cell capillaries (Takahashi et al., 1999; Murohara et al., 2000). Because of the intrinsic ability of EPCs to home to vascular damage and tumor sites (Asahara et al., 1997; Lyden et al., 2001; Kaplan et al., 2005), EPCs are becoming a particularly attractive cell source in future therapies, including the use of genetically modified EPCs to deliver proangiogenic factors or cancer suicide genes to patients.
With their increasing potential as therapeutics comes the necessity to ensure that EPC isolation and culture procedures are well characterized and controlled, and that these procedures do not have adverse effects on the genetically modified cells before clinical use. So far, EPCs can be established in culture from a number of sources, including embryonic stem cells, umbilical cord blood, bone marrow, and most recently from peripheral blood (Shi et al., 1998; Ingram et al., 2004; Yoder et al., 2007; Rae et al., 2011). However, the presence of EPCs in the available sources, particularly in the circulation of normal adults, is very rare (Estes et al., 2010; Mariucci et al., 2010), which considerably limits their clinical use. Various factors have been shown to increase the mobilization of EPCs in the peripheral blood, including physical exercise, vascular damage, hypoxia, limb ischemia, and in response to VEGF, SDF-1α, and granulocyte/macrophage colony stimulating factor (Asahara et al., 1999; Takahashi et al., 1999; Hattori et al., 2001; Laufs et al., 2004). There are a number of in vitro methods published for the establishment of EPC in culture, employing various measures to enhance EPC cell growth, including the use of specific media, growth factors, cell enrichment via cell surface markers, adherence depletion, and choice of matrix for initial plating of isolated cells and subsequent cell passage. However, it has proved to be difficult to establish sufficient and characteristic EPCs in culture, which hinders the clinical application of EPCs.
Because of the lack of a specific EPC marker, EPC characterization relies on a combination of parameters, such as cell morphology and proliferative capacity, the expression of cell surface markers, and ability of the cells to generate vascular tubes in vitro (Hur et al., 2004; Yoder et al., 2007; Sieveking et al., 2008). Early EPCs, also called circulating endothelial progenitor and proangiogenic hematopoietic cells, are seen after 4–7 days in culture with a characteristic spindle-shaped morphology and are positive for common leukocyte antigen marker CD45 and other hematopoietic markers. Early EPCs have a low proliferative potential and do not generate vascular tubes in vitro. Late EPCs, also called endothelial outgrowth cells and endothelial colony-forming cells, appear after at least 7 days in culture forming a monolayer of cobblestone-like cells and are positive for CD31, CD34, CD144 (VE-Cadherin), and CD309 (KDR, VEGFR-2, and Flk-1) and negative for CD45. These cells have a high proliferative potential and generate vascular tubes in Matrigel in vitro. It is currently agreed that early EPCs originate from CD45+ hematopoietic lineage cells, and late EPCs originate from CD45−CD133−CD34+ cells (Asahara et al., 1997; Takahashi et al., 1999; Peichev et al., 2000; Ingram et al., 2004; Melero-Martin et al., 2007; Estes et al., 2010). Late EPCs also secrete less cytokines than mature endothelial cells and can be distinguished from the latter by several parameters, including in vivo angiogenic potency, resistance to oxidative stress, and urokinase expression (Dernbach et al., 2004; Basire et al., 2006). Nevertheless, most published data on EPC characterization have been so far based on one or two selected parameters at various single-time points, which makes direct comparison of these data particularly difficult. Moreover, there have been a number of studies demonstrating successful in vitro genetic modification of EPCs to express diverse transgenes, for example, VEGF and von Willebrand factor (Iwaguro et al., 2002; Griese et al., 2003; De Meyer et al., 2006; Yu et al., 2009; Sen et al., 2010), but little data have been published concerning the effects of vector transduction on the expression of EPC surface markers.
The ultimate goal of this study was to improve the understanding and control of EPC establishment in order to optimize the clinical use and control of EPCs. Therefore, we have systematically investigated EPCs from different sources and used multiple parameters, including cell surface markers to identify factors that affect the establishment, characteristics, and vector transduction capability of EPCs. Our results suggest that the cell source and manipulation significantly influence the numbers and types of EPCs established, and determine subsequent changes in EPC phenotypes. The implications of our findings in future clinical application of genetically modified EPCs are further discussed.
Materials and Methods
Human peripheral blood samples
Human adult peripheral blood samples were purchased as single-donor packs (National Blood Service, Colindale, UK). Whole blood (n=1), white blood cell (WBC) reduction filters (n=5), and buffy coat (n=14) products were tested. Blood was diluted 1:3 (whole blood and WBC filters) or 1:5 (buffy coat) with RPMI (Sigma Aldrich Ltd., Dorset, UK) containing 20 IU/ml heparin sodium (CP Pharmaceuticals Ltd., Wrexam, UK) and incubated at room temperature for 30 min, and then mononuclear cells (MNCs) were isolated using centrifugation with Ficoll-Paque PREMIUM (GE Healthcare UK Ltd., Bucks, UK). MNCs were either cultured directly or after being enriched for CD34+ cells using a human CD34 MicroBead Kit (Miltenyi Biotec Ltd., Surrey, UK) according to the manufacturer's protocols.
Culture of MNCs
Isolated MNCs were cultured in 10% endothelial cell growth medium-2 (EGM-2) containing EGM-2 BulletKit (Lonza Biologics Plc, Slough, UK), 10% fetal calf serum (Sera Laboratories International Ltd., West Sussex, UK), 10 units/ml penicillin (Sigma Aldrich), and 0.1 mg/ml streptomycin (Sigma Aldrich) on tissue culture plates coated with either 6 μg/cm2 type I collagen from rat tail (Sigma Aldrich) or 1 μg/cm2 fibronectin from bovine plasma (Sigma Aldrich). Nonadherent cells were removed after 24, 48, or 72 hr and the medium was replaced every 2–3 days. Enriched CD34+ cells were cultured in 10% EGM-2 on tissue culture plates coated with 6 μg/cm2 type I collagen. After late EPC colonies appeared, cells were harvested using the 0.05% trypsin–EDTA solution (Sigma Aldrich) either from the whole original culture plates, or as an isolated colony using a sterile cloning ring (Sigma Aldrich) and were then further cultured on 1 μg/cm2 fibronectin-coated plates.
Flow cytometry
Live cells used for the flow cytometry analysis were labeled with various antibodies, including mouse anti-human CD34 IgG2a R-phycoerythrin (PE) conjugated (Miltenyi Biotec), mouse anti-human CD34 IgG1 Pacific Blue conjugated (BioLegend, London, UK), mouse anti-human CD45 IgG2a fluorescein isothiocyanate (FITC) conjugated (Miltenyi Biotec), mouse anti-human CD45 IgG1 PE (BD Pharmingen, Oxford, UK), mouse anti-human CD31 IgG1 allophycocyanin (APC) conjugated (Biolegend), mouse anti-human CD31 IgG1 APC conjugated (Miltenyi Biotec), mouse anti-human CD144 IgG2a PE conjugated (BioLegend), and mouse anti-human CD309 IgG1 Peridinin chlorophyll protein Cyanine 5.5 (PerCP/Cy5.5) conjugated (BioLegend), and used according to the manufacturers' recommendations. Fresh peripheral blood mononuclear cells (PBMCs) and human umbilical endothelial cells were used as a positive control to validate antibody labeling. Negative controls included unlabeled cells and isotype-matched control antibodies; purified mouse IgG1, κ isotype control (BioLegend); and purified mouse IgG2a, κ isotype control (BioLegend) detected with anti-mouse IgG (whole molecule)-FITC (Sigma Aldrich).
Flow cytometry was performed on a BD FACSCanto II machine (BD Biosciences, Oxford, UK). Cells were gated based on forward angle light scatter and side angle light scatter characteristics and further analyzed using BD FACSDiva software version 6.1.1 (BD Biosciences).
Incorporation of DiI-AcLDL and lectin binding
Live cells were incubated in 10% EGM-2 for 4 hr at 37°C with the presence of 10 μg/ml acetylated low-density lipoprotein labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate (DiI-AcLDL; Biomedical Technologies Inc., Madrid, Spain), and then washed and fixed in 5% formaldehyde before incubation with 10 μg/ml FITC-UEA-1 (FITC-labeled Ulex europaeus agglutinin I; Vector Laboratories Ltd., Peterborough, UK) for 1 hr at 37°C. After a further incubation with 0.5 μg/ml Hoechst stain solution (Sigma Aldrich) cells were viewed under an Olympus IX51 microscope (Olympus Co., Tokyo, Japan) using a CPlanFl 10×/0.30 PhC∞/1 objective with appropriate filter sets.
In vitro tube formation assay
Cells were seeded at 5,000, 10,000, or 20,000 cells per well of a 96-well plate onto a thick gel layer of Cultrex Basement Membrane Extract (Trevigen Inc., Gaithersburg, MD) coated at 150 μl/cm2. Cells were incubated in 100 μl 10% EGM-2 at 37°C, 5% CO2, and observed over time under the microscope.
Lentiviral vector production and transduction
Four plasmids were used to produce HIV-1 lentiviral vector particles pseudotyped with the vesicular stomatitis virus G envelope protein and encoding the reporter gene green fluorescent protein (GFP). The plasmids used were pMD2.G (envelope, Addgene plasmid 12259), pRSVRev (rev, Addgene plasmid 12253), pMDLg/pRRE (packaging, Addgene plasmid 12251), and pRRLSIN.cppt.PGK-GFP.WPRE (vector, Addgene plasmid 12252), all of which were obtained from Didier Trono via Addgene (Cambridge, MA). Vector particles were produced by transient transfection of human embryonic kidney 293T cells (HEK293T, ATCC) with a weight ratio of 3:2:1:1 vector:packaging:rev:envelope plasmids using the calcium phosphate method (Kutner et al., 2009).
Vector particles were titrated on HEK293T cells. Briefly, HEK293T cells were infected with serial dilutions of vector and then cultured for 48 hr. Cells were harvested, washed, and analyzed by flow cytometry (BD FACSCanto II) to determine the percentage of GFP-expressing cells and calculate the transduction titer.
EPCs were transduced in serum-free EGM-2 with 8 μg/ml polybrene (Sigma Aldrich) for 6 hr. A volume of vector particles equivalent to a multiplicity of infection (MOI) ranging between 5 and 100 was used. Transduced cells were further cultured for 72 hr in 10% EGM-2. Cells were then harvested, washed, and either used for in vitro tube formation assays or analyzed by microscopy or flow cytometry.
Cell count imaging and analysis
Live cells were examined under an Olympus IX 51 microscope using both a UPlanFl 4×/0.13 PhL∞/− objective and a CPlanFl 10×/0.30 PhC∞/1 objective. Ten randomly chosen fields of view were recorded using the F-View Soft Imaging System and analySIS version 3.2 software (Olympus, Essex, UK). Images were analyzed using ImageJ 1.37a (National Institutes of Health, Bethesda, MD). Cells were counted manually using the ImageJ cell counter, and the number of cells per image were converted to number of cells per cm2.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA). Data were tested for normality using the Kolmogorov–Smirnov test and then analyzed using a one-way analysis of variance followed by Bonferroni's multiple comparison test or a Kruskal–Wallis test followed by Dunn's multiple comparison test. Differences were accepted to be statistically significant at p<0.05.
Results
Influence of cell sources and processing on EPC establishment
In this study, three different types of blood sources—whole peripheral blood, buffy coat blood, and WBC filters—were compared for the recovery of MNCs and the establishment of EPCs. The recovery of MNCs from WBC filters (Fig. 1A) was the lowest among the three blood sources with 4.9×108±1.99×108 cells per filter (p<0.05). Cell isolation from buffy coat (Fig. 1B) and whole peripheral blood provided comparable numbers of viable MNCs with around 1.4×109 cells per 500 ml blood pack.
FIG. 1.
Survival in culture of adherent cells over time. MNCs were isolated from five batches of WBC-filter blood (A) and 10 batches of buffy coats (B) and seeded at different cell densities at 1×106 (●), 1.5×106 (▲), 2×106 (■), 3×106 (♦), 6.5×106 (*), 8×106 (○), and 1×107 (□), cells per cm2. The total number of adherent cells was calculated by image analysis and was presented as a percentage of cells seeded on day 0. Data shown are mean±SEM. MNCs, mononuclear cells; SEM, standard error of the mean; WBC, white blood cell.
Seeding density and adherence enrichment of viable MNCs from WBC filters and buffy coats were tested for their influence on cell attachment and subsequent cell viability in culture. Seeding densities were calculated as the number of cells per cm2. Adherence enrichment was carried out by removal of nonadherent cells at one of three time-points, that is, 24, 48, or 72 hr, after seeding. MNCs from WBC filters were seeded at densities ranging from 3×106 to 1×107 cells per cm2 and nonadherent cells were removed 24 or 48 hr later. The number of adherent cells was significantly higher when the nonadherent cells were removed at 48 hr compared with at 24 hr after seeding (p<0.05). By day 8, the number of attached cells had reduced to less than 1% of the seeded MNCs in all samples, and by day 23 the cell numbers remained constant at 0.11% of the seeded population (data not shown).
On the basis of the above data, MNCs from buffy coat blood were seeded at densities ranging from 1×106 to 6.5×106 cells per cm2, and nonadherent cells were removed only after 72 hr. By day 6, the attached cells from all samples had reduced to less than 4% of the seeded population, with the majority of samples retaining less than 1% of seeded cells adhered to the plates. By day 8 the mean percentage of adhered cells from the buffy coat blood was significantly higher than that from WBC filters, where nonadherent cells were removed 24 hr after seeding (p<0.01; Fig. 1A). In the buffy coat cultures, cell numbers in 2 out of 10 samples steadily declined over time, whereas the other 8 out of 10 samples showed that the adherent cell number increased during a 20-day culture period, indicating that low seeding density of MNCs resulted in a relatively high percentage of adherent cells sustained in culture (Fig. 1B).
Two days after seeding, the cultures from WBC filters had a high density of round, loosely adhered cells; by day 7, elongated cells were evident in three out of five cultures. From day 12, large colonies of cells had formed, but by day 16 many of the cells had detached and only two cultures contained numerous adherent spindle-shaped cells. These two cultures were observed for a further 40 days, but no late EPCs, identified by their characteristic cobblestone-like morphology and rapid proliferation, were observed from cultures of WBC filters at any time (data not shown).
The cell cultures from buffy coat blood showed numerous round adherent cells on day 3 (Fig. 2); by day 7, the first cell clusters were observed. By day 10, cell clusters became enlarged, giving rise to elongated cell types. In some cultures large multicellular bodies were evident in the center of these cell colonies, but by day 22 these large cell colonies had dispersed and the number of adherent cells had started to decline (data not shown).
FIG. 2.

Morphology of adherent MNCs. MNCs from buffy coats were seeded onto type I collagen and adherent cells were examined over time in culture. Scale bars are 200 μm.
Late EPC colonies with a characteristic cobblestone-like morphology were observed between 13 and 28 days after seeding of MNCs from buffy coat (Fig. 3). From around 35 days in culture, the majority of other cell types had died, and only late EPC colonies remained in culture. Late EPCs were established from 11 out of 14 buffy coat blood samples seeded on either type I collagen or fibronectin matrices. Once established, the EPC colonies proliferated rapidly as shown in Fig. 3 and could be subcultured for at least 160 days.
FIG. 3.

Morphology of late EPCs. Late EPCs from buffy coats formed colonies with a typical cobblestone-like cell morphology on day 21, 24, or 27 (left to right) after MNC seeding. Scale bars are 200 μm. EPCs, endothelial progenitor cells.
Although the recovery of MNCs from whole blood was comparable to that found for buffy coats, neither early nor late EPCs could be established from whole blood. As a result, whole blood samples were not further analyzed and discussed.
Phenotypic characterization of EPCs
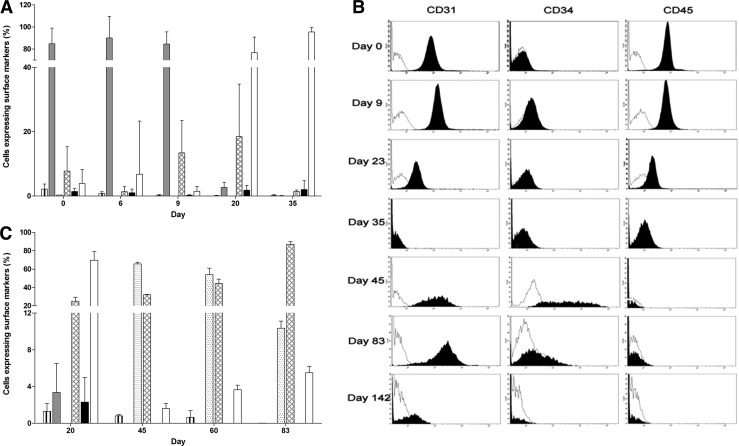
Viable MNCs from buffy coat blood were further investigated for the expression of EPC-related markers CD34, CD31, and CD45 in the initial cell population and marker changes in subsequent cultures and their influence on the establishment of EPCs. Up to 5.3% progenitor CD34+ cells were obtained from MNCs before seeding to culture plates (Fig. 4A, vertical striped bar); however, the percentage of this cell population declined in culture over time to less than 1.2% at day 20. More than 95% of the initial MNCs were CD34−CD31+CD45+; however, at day 20 (Fig. 4A, gray bar), only 2.6%±1.6% adherent MNCs retained the same marker profile and by day 35 over 95% of cells lost all EPC-related markers becoming CD34−CD31−CD45− (Fig. 4A, white bar).
FIG. 4.
Changes in the expression of EPC-related cell surface markers. CD34, CD31, and CD45 were measured by flow cytometry in (A) adherent MNCs in culture for up to 35 days, and (B and C) late EPCs established from different batches of buffy coats and in culture for up to 154 days. Vertical striped bar, CD34+CD31+CD45+; gray bar, CD34−CD31+CD45+; dotted bar, CD34+CD31+CD45−; hatched bar, CD34−CD31+CD45−; black bar, CD34−CD31−CD45+; white bar, cells negative for all three markers.
In addition to the marked changes in the expression of EPC-related molecules, the total number of adherent MNCs, including CD34−CD31−CD45− cell population, also declined over time to around 0.1% of adherent cells remaining in culture at day 28. Late EPC colonies emerged from adherent MNCs from day 13 and became well-established cell colonies around day 35 (Fig. 3). The expression of CD34, CD31, and CD45 in early adherent MNCs (up to day 9 in culture) was in stark contrast to the established late EPC colonies after 45 days in culture (Fig. 4B). At day 45, over 60% of late EPCs were CD34+CD31+CD45− (Fig. 4C, dotted bar) and majority of the remaining cells were CD34−CD31+CD45− (Fig. 4B, solid peak, and 4C, hatched bar). However, from day 70, the late EPCs started to lose the progenitor cell characteristics becoming CD34−CD31+CD45− (Fig. 4B, solid peak, and 4C, hatched bar) and from day 112 lost all markers becoming CD34−CD31−CD45− (Fig. 4B, solid peak, and 4C, white bar).
CD34 cell enrichments using CD34 antibody-labeled magnetic beads were performed in both the freshly isolated MNCs and the established late EPCs to assess whether a predominantly CD34+ cell population could improve the establishment of EPCs in culture. There were neither early nor late EPCs generated from CD34-enriched freshly isolated MNCs. However, CD34+ enrichment improved the establishment of the late EPCs in culture, with 50% more CD34+CD31+ cells obtained from the CD34+-enriched cell population at passage 3 compared with the nonenriched cell population (Table 1). These CD34+-enriched cells were subjected to further analysis using two additional endothelial markers, CD144 and CD309 (KDR). The expression of CD144 in the CD34+ enriched cells was comparable to that in the nonenriched late EPCs ranging from 0.3% to 4.7% (Tables 1 and 2, respectively). In contrast, the expression of CD309 (KDR) was greatly reduced in the CD34+-enriched cells with only 2.5% cells expressing the marker (Table 1), whereas 35.7%–41.5% of nonenriched cells were CD309+ (KDR) (Table 2).
Table 1.
Cell Surface Marker Expression of Both Established Late EPCs, and Late EPCs Enriched for CD34+ Cells at Passage 2
| Sample | Passage | Day | CD31+ | CD45+ | CD34+ | CD144+ | CD309+ |
|---|---|---|---|---|---|---|---|
| Batch 1 CD34+ enriched | P3 | 111 | 97.7±1.3 | 1.3±0.2 | 26.8±1.0 | 1.6±0.1 | 2.5±0.1 |
| P4 | 149 | 55±28.4 | 2.1±0.1 | 8.5±0.9 | 1.0±0.1 | 1.1±0.1 | |
| Batch 1 | P3 | 83 | 95.1±1.4 | 0.9±0.5 | 12.3±0.3 | 4.7±0.7 | n.d. |
| P4 | 127 | 1.3±0.5 | 0.3±0.2 | 0.4±0.3 | 1.1±0.2 | 0.2±0.1 | |
| Batch 2 CD34+ enriched | P3 | 97 | 96.5±1.1 | 2.1±0.4 | 49.3±1.5 | 3.7±0.2 | 2.6±0.2 |
| P5 | 135 | 90.2±0.5 | 1.2±0.1 | 8.1±0.4 | 0.3±0.1 | 0.5±0.1 | |
| Batch 2 | P3 | 68 | 96.6±0.3 | 0.2±0.1 | 34±11.2 | 0.7±0.1 | n.d. |
| P7 | 112 | 2.0±0.9 | 0.7±0.5 | 1.0±0.4 | 1.5±1.0 | 0.1±0.1 |
Data shown are mean percentage±standard deviation of EPCs expressing CD markers at different passages in culture. n=3 for each buffy coat batch. EPCs, endothelial progenitor cells; n.d., not done.
Table 2.
Cell Surface Marker Expression of Established Late EPCs
| Sample | Passage | Day | CD31+ | CD34+ | CD144+ | CD309+ |
|---|---|---|---|---|---|---|
| Batch 1 | P5 | 60 | 99.1±0.1 | 2.4±0.1 | 2.5±0.8 | 41.5±3.9 |
| Batch 2 | P3 | 60 | 99.3±0.2 | 10.0±0.6 | 1.9±0.4 | 35.7±1.8 |
Data shown are mean percentage±standard deviation of late EPCs expressing CD markers at different passages in culture. n=3 for each buffy coat batch. EPCs, endothelial progenitor cells.
Nonadherent cells removed 72 hr after seeding MNCs from fresh buffy coats were re-plated and cultured for 35 days to assess whether EPCs could be established from nonadherent MNCs as previously reported by Yoder et al. (2007). Some of the cells adhered and started to spread out, but the majority of the re-plated cells remained in suspension. These cells showed many different cell morphologies (Fig. 5A); however, no early or late EPCs were observed in these nonadherent cell populations during the 35-day culture period. Changes in surface markers were also observed in the nonadherent MNCs, from 53% of nonadherent cells being CD34−CD31+CD45+ at day 3 to 99% of the cells CD34−CD31−CD45− by day 35 (Fig. 5B, white bar).
FIG. 5.
Morphology and cell surface marker expression of nonadherent cells. Nonadherent cells from buffy coats were re-plated 3 days after seeding and cultured for 20 (left) and 35 (right) days, showing (A) various cell morphologies (scale bars are 200 μm) and (B) percentage of cells expressing CD34, CD31, or CD45. Vertical striped bar, CD34+CD31+CD45+; gray bar, CD34−CD31+CD45+ cells; hatched bar, CD34−CD31+CD45−; black bar, CD34−CD31−CD45+; white bar, negative for all three markers.
Angiogenic properties of EPCs
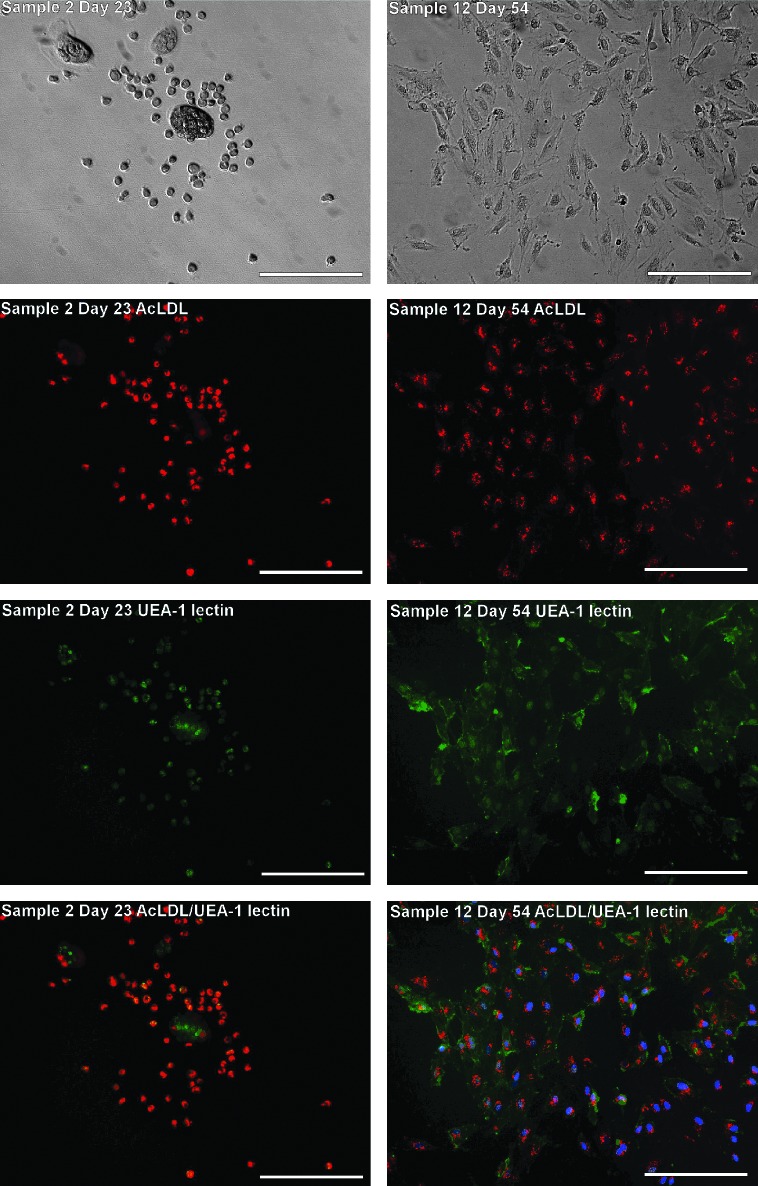
Because of the lack of an EPC-specific cell surface marker, the phenotype of adherent cells was further characterized using dual labeling of the cells with acetylated low-density lipoprotein (AcLDL) and UEA-1 lectin, an additional indication of EPC-like properties (Aoki et al., 2004). More than 85% of early EPCs (CD34+CD31+CD45+) from both WBC filters and buffy coat blood showed dual-labeling of AcLDL and UEA-1 lectin (Fig. 6, left panel), whereas a further 1%–3% or 2%–13% of the adherent cells were labeled for either UEA-1 lectin or AcLDL, respectively. In addition, all established late EPCs (CD34+CD31+CD45−) showed dual-labeling in culture, while the cells that only bound UEA-1 lectin tended to form multicellular clusters (Fig. 6, right panel).
FIG. 6.
Angiogenic properties of early and late EPCs. Early EPCs (left panel) from buffy coat blood showed dual-labeling for DiI-AcLDL (red) uptake and FITC-UEA1 lectin (green) binding on day 23. Some early EPCs formed large multicellular bodies (in the center of top left panel) and single staining for UEA1 lectin (green, in the third and fourth images of the left panel). All late EPCs (right panel) showed dual labeling for DiI-AcLDL uptake and FITC-UEA-1 lectin binding on day 54, that is, at passage 4. Cell nuclei of late EPC were also labeled with Hoechst 33258 (blue). Scale bars are 200 μm. DiI-AcLDL, acetylated low-density lipoprotein labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate; FITC, fluorescein isothiocyanate.
Branching characteristics and formation of tube-like structures on Cultrex Basement Membrane Extract have been commonly used as an indication of EPC-like properties and angiogenic potential (Hur et al., 2004). Established late EPCs were seeded onto a thick gel layer of membrane extract at three different densities with 5,000, 10,000, and 20,000 cells per well of a 96-well plate. Before tube-forming assays, cells retained the typical cobblestone-like morphology before seeding (Fig. 7, top panel) and 69.5% of the cells were expressing late EPC-related markers (CD31+CD34+ and CD45−) from flow cytometry analysis (data not shown). Within 1 hr after seeding, the cells had started to migrate and within 2 hr tube formation was evident (Fig. 7, middle panel). After 6 hr in culture, the tube structures had formed an extensive cell network spanning the entire well. The tube network could remain intact for as long as 120 hr in some of samples, while some of the tube structures started to contract causing the cells to clump together (Fig. 7, bottom panel). Although only data from cells seeded at 20,000 cells/well were presented, no significant difference was observed using other seeding densities or from cryopreserved cells.
FIG. 7.
In vitro tubule formation assay. Top left panel shows late EPCs cultured in a normal TC plate before being seeded at 20,000 cells per well on to Cultrex Basement Membrane Extract. The rest of the figures show the time course of cell migration and tube formation within 2 hr (middle left), extensive cell networks formed within 5 hr (middle right and bottom left), and the tube structures contracted within 48 hr (bottom right) causing the cells to clump together. The asterisk locates the same position over time in each image. Scale bars are 500 μm.
Influence of vector transduction on EPC phenotype and angiogenic properties
To determine the efficiency of blood-derived EPCs as target cells for gene therapy, early and late EPCs were transduced using a lentiviral vector expressing, under the control of CMV promoter, a reporter gene encoding enhanced green fluorescence protein. Early EPCs were transduced at MOI 5 on day 10 (n=7), 17 (n=2), or 24 (n=4) after initial MNC isolation. After being exposed to the vector for 6 hr, both the mock (without vector) and transduced (with vector) cells showed a spindle-shaped morphology, indicating a stress response from early EPCs to serum deprivation. Using flow cytometry, the expression of the reporter gene GFP was undetectable in transduced early EPCs. Vector transduction significantly increased cell detachment and death when compared with mock transduction (p<0.001) (Fig. 8A). Vector transduction also led to a marked decrease (over 60%, p<0.01) in the number of cells expressing CD31 or CD45, while the number of CD34+ cells remained unchanged after transduction (Fig. 8B).
FIG. 8.
Survival in culture (A) and changes in cell surface marker expression (B) of early EPCs. (A) The reduction in total cell numbers over time from the control population (white bar) and the vector-transduced cell population (gray bar). (B) The percentage of cells expressing CD34, CD31, or CD45 in the control and transduced cell populations at 0 and 72 hr after transduction. Data shown are mean±SD. **p<0.01; ***p<0.001. SD, standard deviation.
In contrast to the early EPCs, serum deprivation did not alter late EPC morphology or cell growth (data not shown); as a result, late EPCs were subjected to three consecutive transductions at MOI 5 from 30 to 120 days after initial MNC isolation. Flow cytometry analysis showed that the number of GFP cells increased by approximately 10% after each additional transduction, resulting in a total of 52% cells expressing GFP after three consecutive transductions. After vector transductions, late EPCs continued proliferating and expressing GFP for at least 17 days. Vector transduction was also performed at an early stage on the established late EPC colonies that had cell numbers ranging from 10 cells to 750 cells per colony. In a single transduction at MOI 5, the percentage of GFP-expressing cells was significantly higher in small colonies (<50 cells) with 62.9%±15.7% cells GFP+ than that in large colonies (>250 cells) with 20.9%±7.8% GFP+ cells (p<0.01, data not shown).
The expression profiles of EPC-related cell surface markers were further investigated in the mock and transduced cells to assess whether vector transduction led to phenotypic changes in the transduced cells. Late EPCs remained CD45− in both the mock- and vector-transduced cells before and after transduction. The percentage of late EPCs expressing the endothelial cell marker CD31 was also comparable between mock- and vector-transduced cells and remained unchanged before and after multiple transductions.
The number of cells expressing the progenitor cell marker CD34 declined significantly over time in the control cell population. Initially, 7.2% cells were CD34+ and reduced to only 0.3% CD34+ cells after a further 8 days in culture (Fig. 9A, gray bar). In contrast, the total number of CD34+ cells in the transduced cell population remained unchanged after two rounds of vector transduction, with 35% and 31% of CD34+ cells expressing GFP after the first and second transduction, respectively (Fig. 9A, white bar). Reduction of CD34+ cells was observed after the third transduction, with a significant decrease of CD34+ cells in GFP+ cells (Fig. 9A, gray bar), indicating that vector transduction prolonged the survival of CD34+ progenitor cells. The number of control cells expressing the endothelial cell marker CD144 also declined markedly from 12.2% to 0.1% 8 days later (Fig. 9B, gray bar); however, in contrast to CD34 expression, vector transduction significantly increased the percentage of cells expressing the endothelial cell marker CD144 from 12.2% to 52.2% after first transduction (Fig. 9B). Additional vector transductions did not increase the CD144+ cell population; however, the number of cells expressing CD144 was 10% higher in vector-transduced cells than in mock-transduced cells (Fig. 9B).
FIG. 9.
Changes of cell surface marker expression of transduced late EPCs on days 121, 125, and 129 after MNC seeding. The percentage of cells expressing CD34 (A) and CD144 (B) was measured in GFP+ (white bar) and GFP− (gray bar) cells by flow cytometry. Control: mock transduction without vectors; transduced: vector transduction at MOI 5. MOI, multiplicity of infection.
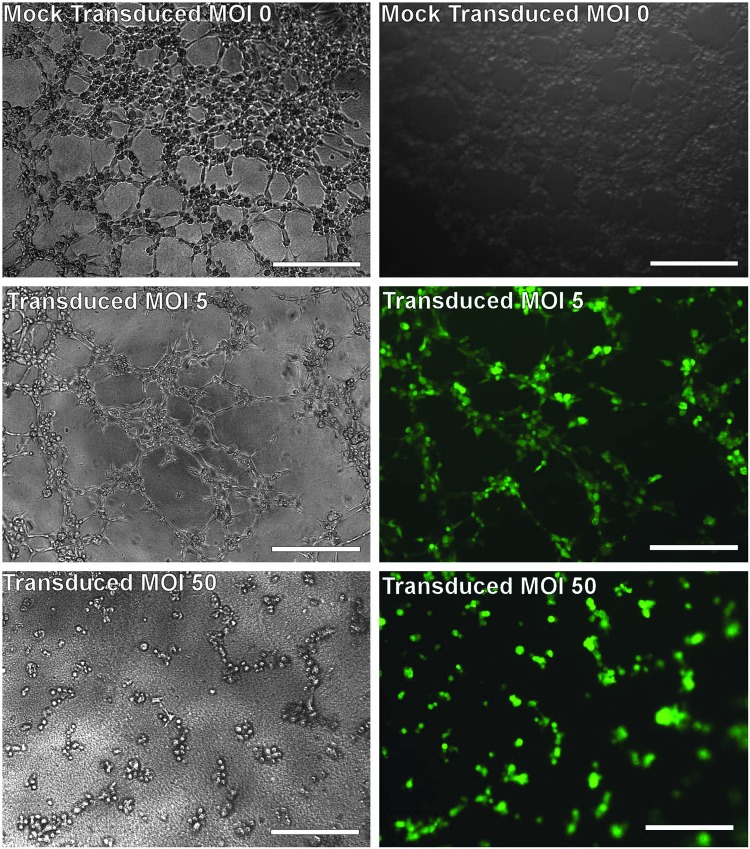
The intrinsic angiogenic properties of EPCs, including neovascularization, have made them an attractive candidate for gene or cell therapies of vascular diseases; therefore, retaining the angiogenic potential can be a key for a successful gene or cell therapy using genetically modified EPCs. In this study, the angiogenic properties of vector-transduced EPCs were investigated based on a tube formation assay. Established late EPCs were transduced for 72 hr at MOIs of 0, 5, 10, 25, 50, and 100, and were then seeded onto a thick gel layer of the Cultrex Basement Membrane Extract at 20,000 cells per well of a 96-well plate. At the time of performing the tube formation assay, all vector-transduced EPCs were expressing the reporter gene GFP. In the mock-transduced (control) cell population, numerous cell tubes were formed within 2 hr after cell seeding and an extensive cell network was observed within 4 hr as observed earlier (Fig. 7). The vector-transduced cells at MOIs 5 and 10 appeared to behave similarly to the mock-transduced cells; however, the formation of a cell network appeared at a slower rate. After 5 hr, a cell network was well established (Fig. 10). In contrast, vector-transduced cells at a higher MOI of 25 developed only a few tube structures with no cell network formation after 6 hr. Increasing the MOI to 50 slowed both the cell migration and tube formation, with only few tubes observed between 4 and 5 hr. Vector-transduced cells at the highest MOI of 100 tested were slow to migrate and failed to form a tube-like structure (data not shown). At the end of a tube formation assay, all vector-transduced cells remained GFP+.
FIG. 10.
In vitro tubule formation assays of transduced late EPCs. Late EPCs were transduced with a lentiviral vector encoding GFP at an MOI of 0 (mock transduction, top panel), 5 (middle panel), or 50 (bottom panel) for 72 hr. The transduced cells were then seeded at 20,000 cells per well onto a thick gel layer of Cultrex Basement Membrane Extract. The cells transduced at MOI 5 show extensive formation of cell networks, while the cells transduced at MOI 50 had limited tube formation.
Discussion
In this study, we describe the establishment in culture of EPCs including early and late EPCs from three sources of human peripheral blood cells. Our results demonstrate that MNCs from buffy coats are the best source for both early and late EPCs. Although the use of whole peripheral blood resulted in a great number of viable MNCs, no EPCs were generated from this MNC population. Late EPCs could only be established from buffy coat, whereas early EPCs could be established from both WBC filters and buffy coat with the latter giving rise to a higher yield of early EPCs. We were unable to confirm the early reports from other groups that early EPCs (Teleron et al., 2005) and late EPCs (Ingram et al., 2004; Yoder et al., 2007; Estes et al., 2010; Kolbe et al., 2010) could be established from whole blood. This discrepancy may be caused by the difference in the source and status of the whole blood used. The majority of previously reported studies used fresh whole blood from donors onsite, while our whole blood samples were obtained from a commercial source that might have lost some viable progenitor cells during storage and transportation, which underscores the importance of blood cell status for the establishment of EPCs in culture.
In addition, the number of EPC colonies established from buffy coat blood in this study was also lower than that reported in previous studies using fresh whole blood (Ingram et al., 2004; Yoder et al., 2007; Estes et al., 2010; Kolbe et al., 2010). The low recovery of EPCs may be because buffy coat blood used in this study was platelet depleted. Recent studies have indicated that platelets play a role in EPC recruitment and migration to sites of vascular injury (de Boer et al., 2006; Langer et al., 2006) and promote EPC maturation and differentiation to endothelial cells (Daub et al., 2006). The presence of platelets in a coculture system resulted in a higher number of EPC colonies in vitro (Leshem-Lev et al., 2010) and the addition of platelet-released growth factors to CD34+CD133+ selected cells led to increased cell proliferation in early culture (Lippross et al., 2011). Density-gradient separation of cells from buffy coat blood used in this study could result in purified MNCs with a further reduction of platelets that were potentially required for EPC colony formation (Lad et al., 1988; Prokopi et al., 2009). The presence of platelets, or more specifically platelet microparticles, may lead to miscounting and misidentification of EPCs (Prokopi et al., 2009; Prokopi and Mayr, 2011); however, it has been suggested that platelets could be important in providing the right signals for EPC maturation and that the stimulus from platelets may increase the number of early and late EPC colonies generated in cultures. Our results also showed that adherence enrichment for 72 hr instead of 24 hr after MNC seeding significantly improved the establishment of both early and late EPCs from buffy coat MNCs. Hur et al. (2004) have previously shown that prolonged adherence enrichment, for example, removal of nonadherent cells 6 days after MNC seeding, improved the generation of both early and late EPCs. It has been suggested that early adherence enrichment results in the removal of monocytes, hematopoietic progenitors, and circulating endothelial cells in the nonadherent cell population that are generally believed to enhance EPC development; therefore, early removal of nonadherent cells should be avoided whenever possible.
Our results showed that neither early nor late EPCs could be established from CD34+-enriched MNCs, which is in agreement with the report by Case et al. (2007), reinstating the important role of platelets and possibly other blood cells, in supporting the growth of EPCs (Lad et al., 1988; Prokopi et al., 2009). In contrast, CD34+ enrichment has been well documented to enhance EPC generation from cord blood cells (Asahara et al., 1997; Melero-Martin et al., 2007), demonstrating intrinsic differences between PBMC-derived EPCs and cord blood EPCs. Cord blood EPCs have been reported to possess longer telomeres and larger capacity to form colonies (Ingram et al., 2004), which may account for their independent growth without the presence of other blood cells, for example, platelets. Our results also showed that CD34+ enrichment of established late EPCs resulted in a greater number of late EPCs maintaining CD34+ progenitor phenotype for a longer period in culture, which may be particularly beneficial for therapies requiring substantial numbers of EPCs.
During EPC isolation and subculture procedure, there were significant changes observed in cell morphology and cell surface marker phenotypes. Candidate EPCs that are generally believed to be CD34+CD31+CD45+/− (Asahara et al., 1997; Shi et al., 1998; Takahashi et al., 1999; Peichev et al., 2000; Ingram et al., 2004; Case et al., 2007; Melero-Martin et al., 2007; Estes et al., 2010) were present at 0.01%–5.5% of the total MNC population. However, only a very small number of these progenitor cells, for example, up to 3.4 EPC colonies for every 109 MNCs, were able to persist in culture, indicating that currently available culture conditions are suboptimal.
Early EPC colonies that were CD34+, CD31+, CD45+, CD144+, and CD309+ (Asahara et al., 1997; Shi et al., 1998; Takahashi et al., 1999; Peichev et al., 2000) emerged as early as 7 days after MNC seeding with characteristic spindle-shaped cell morphology. Early EPCs showed limited proliferation and vector transduction efficiency. On the contrary, late EPCs that were CD34+, CD31+ CD144+, and CD309+ and CD45− (Case et al., 2007; Melero-Martin et al., 2007; Zhang et al., 2009; Estes et al., 2010) showed exponential proliferation and maintained their cobblestone and progenitor cell morphology for more than 140 days in culture. However, the number of cells expressing the EPC-related cell surface markers CD34+ and CD31+ declined over time; in particular, the number of cells maintaining CD34+ progenitor phenotype reduced to less than 1% after 70 days in culture. Loss of CD34+ progenitor phenotype has been considered a result of unfavorable culture conditions (Müller et al., 2002). Long-term culture of EPCs has been proposed to cause chromosomal aberrations, which in turn reduce the proliferation capability of the cells (Corselli et al., 2008). In comparison, changes in the expression of the endothelial cell marker CD31 were less significant with more than 70% of cells still CD31+ after 100 days in culture. Moreover, the established late EPC colonies showed uptake of AcLDL and binding of UEA-lectin and formed tube-like structures in basement membrane gel assays and expressed two further EPC markers, CD144 and CD309 (Asahara et al., 1997; Shi et al., 1998; Takahashi et al., 1999; Peichev et al., 2000). Our results demonstrate that, in addition to optimizing current culture conditions, repeated cell enrichments, for example, CD34+ cell enrichment, may be beneficial of providing a substantial number of EPCs that maintain both progenitor and endothelial cell characteristics.
Transduction of early EPCs with a lentiviral vector was unsuccessful in this study, which might largely be caused by a compound effect of serum deprivation and vector toxicity to early EPC cells. There is one study in the literature that reported a successful transduction of early EPCs 2 days after their isolation from peripheral blood (Liu et al., 2006); however, the expression of the EPC-related markers was not studied and thus the percentage of true early EPCs in this report was unclear. Early EPCs, although with a lower in vitro proliferation potential (Yoder et al., 2007), are thought to play an important role in augmenting angiogenesis and arteriogenesis via a paracrine role (Yoon et al., 2005; Sieveking et al., 2008). Therefore, successful gene modification of early EPCs would be particularly valuable in the context of enhancing angiogenesis for the treatment of myocardial and limb ischemia. Methods to optimize the culture and transduction of early EPCs require further development.
Late EPCs demonstrated high transduction efficiency, with 31% cells transduced after one exposure to vector particles at MOI 5 and increased to 52% after three transductions. De Meyer et al. (2006) achieved a 45% transduction efficiency using a comparable HIV vector carrying an internal CMV promoter at MOI 6. Increasing MOI to 10 and 60 resulted in 80% and 88% transduction efficiency, respectively (De Meyer et al., 2006; van den Biggelaar et al., 2009). The transduction efficiency could be improved to as high as 96% when using a lentiviral vector LeGo with an SFFV-U3-derived internal promoter (Stockschlaeder et al., 2010), demonstrating that transduction efficiencies may vary significantly depending on the particular viral vector, internal promoter, and particularly the MOI used. In addition, we found that transduction efficiency could also be improved from 47% to 79% when transducing EPC colonies containing fewer cells, suggesting that transduction of isolated EPC colonies as early as possible could be an improved way to obtain a large population of transduced EPCs.
In this study, we, for the first time, systematically investigated the influence of vector transduction on the angiogenic properties of EPCs. Our results showed that lentiviral vector transduction at an MOI of up to 10 did not significantly alter the ability of EPCs forming tube-like structure in vitro, an important angiogenic property, indicating the robustness of EPCs to lentiviral vector transduction. Only when vectors transduction was performed at an exceedingly high MOI of 50 and 100, was tubule formation of EPCs inhibited. An imbalanced number of EPCs in circulation are associated with a range of pathological conditions, suggesting a natural therapeutic role for EPC in angiogenesis. Upholding the angiogenic potential of genetically modified EPCs is particularly beneficial for angiogenic therapies of vascular disease or anti-angiogenic therapies of cancer (Lyden et al., 2001; Stroncek et al., 2011).
Consecutive vector transductions for up to three cycles did not alter the morphology and proliferative ability of late EPCs. However, vector transduction led to significant changes in the expression of EPC-related cell surface molecules. We observed that the percentage of CD34+ cells in the control population declined significantly, which has also reported by other groups (Suga et al., 2009) and may be related to the physiological changes during culture and/or cell differentiation from progenic status into specific cell lineages. In contrast, the percentage of cells expressing CD34 remained unchanged in the vector-transduced cells, indicating that vector transduction prevented the decline or augmented the proliferation of CD34+ progenitor cells. Other groups (Lin et al., 2002; Herder et al., 2003) reported that the expression of CD34 remained unchanged in lentiviral-vector-transduced or a plasmid-DNA-transduced EPCs for several weeks in culture; however, they did not report the expression of CD34 in the nontransduced cells. Although maintaining the progenitor phenotype of EPCs is a key requirement during EPC establishment, it is unknown whether the vector integration, particularly the unwanted integration and thus oncogenic potential of lentiviral vectors, played a role in augmenting the long-term proliferation of transduced CD34+ cells, particularly of the GFP+ cell population. This may impact on the safe use of such transduced EPCs.
Vector transduction markedly increased the percentage of EPCs expressing CD144 in both GFP+ and GFP− populations when compared with a rapid reduction of CD144+ cells in the control population, which is particularly evident after the first and second transductions. It has been suggested that during in vitro EPC transplantation, the expression of CD144 increased between 60% and 80% cell confluence, consistent with the adherent function of CD144 in vascularization (Rae et al., 2011). However, we did not observe any correlation between CD144 expression and cell confluency, implicating the induction of CD144 expression by vector transduction. A study by Herder et al. (2003) has showed that the total number of CD144+ cells was similar to that of untransduced cells; however, the percentage of CD144+ cells was not stated and CD144 expression was not measured until several weeks after transduction. Lin et al. (2002) reported a high percentage of CD144+ cells after transduction, yet again, no direct comparison was given between transduced and untransduced cells. The signaling mediated via CD144 controls endothelial cell survival and maturation (Carmeliet et al., 1999; Dejana et al., 2008); therefore, a significant increase in CD144+ cells after vector transduction might indicate possible EPC transformation and a loss of EPC advantageous properties, for example, homing to vasculatures, for clinical use of these cells.
In summary, this study shows the considerable promise as well as certain limitations of genetically modified EPCs for gene therapy. We have accomplished the establishment of EPCs in culture and obtained high transduction efficiency and robust in vitro tubule formation of transduced EPCs using lentiviral vectors. However, vector transduction significantly altered the EPC phenotype as demonstrated by an increased percentage of CD34+ progenitor cells and an increased expression of adhesion molecule CD144. Taking account of the increased expression of CD144 reported in cancer patients (Rabascio et al., 2004; Dome et al., 2006; Strijbos et al., 2008), the altered expression of CD144 and the enrichment of CD34+ cells after vector transduction demonstrate the importance of extensive characterization and vigorous safety control of genetically modified EPCs before they are accepted for clinical use.
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- Aoki M. Yasutake M. Murohara T. Derivation of functional endothelial progenitor cells from human umbilical cord blood mononuclear cells isolated by a novel cell filtration device. Stem Cells. 2004;22:994–1002. doi: 10.1634/stemcells.22-6-994. [DOI] [PubMed] [Google Scholar]
- Asahara T. Murohara T. Sullivan A., et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Asahara T. Takahashi T. Masuda H., et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basire A. Sabatier F. Ravet S., et al. High urokinase expression contributes to the angiogenic properties of endothelial cells derived from circulating progenitors. Thromb. Haemost. 2006;95:678–688. [PubMed] [Google Scholar]
- Briguori C. Testa U. Riccioni R., et al. Correlations between progression of coronary artery disease and circulating endothelial progenitor cells. FASEB J. 2010;24:1981–1988. doi: 10.1096/fj.09-138198. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Lampugnani M.G. Moons L., et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Case J. Mead L.E. Bessler W.K., et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp. Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Corselli M. Parodi A. Mogni M., et al. Clinical scale ex vivo expansion of cord blood-derived outgrowth endothelial progenitor cells is associated with high incidence of karyotype aberrations. Exp. Hematol. 2008;36:340–349. doi: 10.1016/j.exphem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Daub K. Langer H. Seizer P., et al. Platelets induce differentiation of human CD34+ progenitor cells into foam cells and endothelial cells. FASEB J. 2006;20:2559–2561. doi: 10.1096/fj.06-6265fje. [DOI] [PubMed] [Google Scholar]
- de Boer H.C. Verseyden C. Ulfman L.H., et al. Fibrin and activated platelets cooperatively guide stem cells to a vascular injury and promote differentiation towards an endothelial cell phenotype. Arterioscler. Thromb. Vasc. Biol. 2006;26:1653–1659. doi: 10.1161/01.ATV.0000222982.55731.f1. [DOI] [PubMed] [Google Scholar]
- Dejana E. Orsenigo F. Lampugnani M.G. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- De Meyer S.F. Vanhoorelbeke K. Chuah M.K., et al. Phenotypic correction of von Willebrand disease type 3 blood-derived endothelial cells with lentiviral vectors expressing von Willebrand factor. Blood. 2006;107:4728–4736. doi: 10.1182/blood-2005-09-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernbach E. Urbich C. Brandes R.P., et al. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- Dome B. Timar J. Dobos J., et al. Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res. 2006;66:7341–7347. doi: 10.1158/0008-5472.CAN-05-4654. [DOI] [PubMed] [Google Scholar]
- Estes M.L. Mund J.A. Mead L.E., et al. Application of polychromatic flow cytometry to identify novel subsets of circulating cells with angiogenic potential. Cytometry A. 2010;77:831–839. doi: 10.1002/cyto.a.20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griese D.P. Achatz S. Batzlsperger C.A., et al. Vascular gene delivery of anticoagulants by transplantation of retrovirally-transduced endothelial progenitor cells. Cardiovasc. Res. 2003;58:469–477. doi: 10.1016/s0008-6363(03)00266-9. [DOI] [PubMed] [Google Scholar]
- Hattori K. Dias S. Heissig B., et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J. Exp. Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herder C. Tonn T. Oostendorp R., et al. Sustained expansion and transgene expression of coagulation factor VIII-transduced cord blood-derived endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2003;23:2266–2272. doi: 10.1161/01.ATV.0000100403.78731.9F. [DOI] [PubMed] [Google Scholar]
- Hur J. Yoon C.H. Kim H.S., et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- Ingram D. Mead L. Tanaka H., et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- Iwaguro H. Yamaguchi J. Kalka C., et al. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- Jiang M. Wang B. Wang C., et al. In vivo enhancement of angiogenesis by adenoviral transfer of HIF-1alpha-modified endothelial progenitor cells (Ad-HIF-1alpha-modified EPC for angiogenesis) Int. J. Biochem. Cell Biol. 2008;40:2284–2295. doi: 10.1016/j.biocel.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Kaplan R.N. Riba R.D. Zacharoulis S., et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbe M. Dohle E. Katerla D., et al. Enrichment of outgrowth endothelial cells in high and low colony-forming cultures from peripheral blood progenitors. Tissue Eng. Part C Methods. 2010;16:877–886. doi: 10.1089/ten.tec.2009.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner R.H. Zhang X.Y. Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 2009;4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- Lad P.M. Easton J. Niedzin H., et al. A method for the preparation of mononuclear cells devoid of platelet contamination and its application to the evaluation of putative alpha-receptors in normal and asthmatic subjects. J. Immunol. Methods. 1988;110:193–202. doi: 10.1016/0022-1759(88)90103-2. [DOI] [PubMed] [Google Scholar]
- Langer H. May A.E. Daub K., et al. Adherent platelets recruit and induce differentiation of murine embryonic endothelial progenitor cells to mature endothelial cells in vitro. Circ. Res. 2006;98:e2–e10. doi: 10.1161/01.RES.0000201285.87524.9e. [DOI] [PubMed] [Google Scholar]
- Laufs U. Werner N. Link A., et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- Leshem-Lev D. Omelchenko A. Perl L., et al. Exposure to platelets promotes functional properties of endothelial progenitor cells. J. Thromb. Thrombolysis. 2010;30:398–403. doi: 10.1007/s11239-010-0514-0. [DOI] [PubMed] [Google Scholar]
- Lin Y. Chang L. Solovey A., et al. Use of blood outgrowth endothelial cells for gene therapy for hemophilia A. Blood. 2002;99:457–462. doi: 10.1182/blood.v99.2.457. [DOI] [PubMed] [Google Scholar]
- Lippross S. Loibl M. Hoppe S., et al. Platelet released growth factors boost expansion of bone marrow derived CD34(+) and CD133(+) endothelial progenitor cells for autologous grafting. Platelets. 2011;22:422–432. doi: 10.3109/09537104.2011.559559. [DOI] [PubMed] [Google Scholar]
- Liu J.W. Pernod G. Dunoyer-Geindre S., et al. Promoter dependence of transgene expression by lentivirus-transduced human blood-derived endothelial progenitor cells. Stem Cells. 2006;24:199–208. doi: 10.1634/stemcells.2004-0364. [DOI] [PubMed] [Google Scholar]
- Lyden D. Hattori K. Dias S., et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Mariucci S. Rovati B. Bencardino K., et al. Flow cytometric detection of circulating endothelial cells and endothelial progenitor cells in healthy subjects. Int. J. Lab. Hematol. 2010;32:e40–e48. doi: 10.1111/j.1751-553X.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- Melero-Martin J. Khan Z. Picard A., et al. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- Müller A.M. Hermanns M.I. Skrzynski C., et al. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp. Mol. Pathol. 2002;72:221–229. doi: 10.1006/exmp.2002.2424. [DOI] [PubMed] [Google Scholar]
- Murohara T. Ikeda H. Duan J., et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J. Clin. Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichev M. Naiyer A.J. Pereira D., et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- Prokopi M. Mayr M. Proteomics: a reality-check for putative stem cells. Circ. Res. 2011;108:499–511. doi: 10.1161/CIRCRESAHA.110.226902. [DOI] [PubMed] [Google Scholar]
- Prokopi M. Pula G. Mayr U., et al. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–732. doi: 10.1182/blood-2009-02-205930. [DOI] [PubMed] [Google Scholar]
- Rabascio C. Muratori E. Mancuso P., et al. Assessing tumor angiogenesis: increased circulating VE-cadherin RNA in patients with cancer indicates viability of circulating endothelial cells. Cancer Res. 2004;64:4373–4377. doi: 10.1158/0008-5472.CAN-04-0265. [DOI] [PubMed] [Google Scholar]
- Rae P.C. Kelly R.D. Egginton S. St. John J.C. Angiogenic potential of endothelial progenitor cells and embryonic stem cells. Vasc. Cell. 2011;3:11. doi: 10.1186/2045-824X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S. Merchan J. Dean J., et al. Autologous transplantation of endothelial progenitor cells genetically modified by adeno-associated viral vector delivering insulin-like growth factor-1 gene after myocardial infarction. Hum. Gene Ther. 2010;21:1327–1334. doi: 10.1089/hum.2010.006. [DOI] [PubMed] [Google Scholar]
- Shi Q. Rafii S. Wu M.H., et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- Sieveking D. Buckle A. Celermajer D. Ng M. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J. Am. Coll. Cardiol. 2008;51:660–668. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- Stockschlaeder M. Shardakova O. Weber K., et al. Highly efficient lentiviral transduction of phenotypically and genotypically characterized endothelial progenitor cells from adult peripheral blood. Blood Coagul. Fibrinolysis. 2010;21:464–473. doi: 10.1097/MBC.0b013e328339cc1c. [DOI] [PubMed] [Google Scholar]
- Strijbos M.H. Gratama J.W. Kraan J., et al. Circulating endothelial cells in oncology: pitfalls and promises. Br. J. Cancer. 2008;98:1731–1735. doi: 10.1038/sj.bjc.6604383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroncek J.D. Xue Y. Haque N., et al. In vitro functional testing of endothelial progenitor cells that overexpress thrombomodulin. Tissue Eng. Part A. 2011;17:2091–2100. doi: 10.1089/ten.tea.2010.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga H. Matsumoto D. Eto H., et al. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18:1201–1210. doi: 10.1089/scd.2009.0003. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Kalka C. Masuda H., et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Teleron A.A. Carlson B. Young P.P. Blood donor white blood cell reduction filters as a source of human peripheral blood-derived endothelial progenitor cells. Transfusion. 2005;45:21–25. doi: 10.1111/j.1537-2995.2005.04191.x. [DOI] [PubMed] [Google Scholar]
- van den Biggelaar M. Bouwens E.A. Kootstra N.A., et al. Storage and regulated secretion of factor VIII in blood outgrowth endothelial cells. Haematologica. 2009;94:670–678. doi: 10.3324/haematol.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner N. Kosiol S. Schiegl T., et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- Yoder M. Mead L. Prater D., et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C.H. Hur J. Park K.W., et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- Yu J.X. Huang X.F. Lv W.M., et al. Combination of stromal-derived factor-1alpha and vascular endothelial growth factor gene-modified endothelial progenitor cells is more effective for ischemic neovascularization. J. Vasc. Surg. 2009;50:608–616. doi: 10.1016/j.jvs.2009.05.049. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Fisher N. Newey S.E., et al. The impact of proliferative potential of umbilical cord-derived endothelial progenitor cells and hypoxia on vascular tubule formation in vitro. Stem Cells Dev. 2009;18:359–375. doi: 10.1089/scd.2008.0071. [DOI] [PubMed] [Google Scholar]