Abstract
Our aim was to investigate serotype-specific cell and tissue-transduction tropisms, transgene expression levels and longevity, and immunogenicity of candidate rAAV serotypes in rat osteochondral cells, tissues, and stifle joints. In vitro, we used six rAAV serotypes and two promoters to transduce synoviocytes and chondrocytes. Serotypes rAAV2/5 and 2/2 yielded the highest transduction efficiency 4 days after transduction. No differences were detected between cytomegalovirus and chicken β-actin promoters. In vivo, intra-articular injection was used to introduce four rAAV serotypes into 4-month-old rats in the left stifle joint. Eleven months later, serotype 2/5 vector, diluted with saline or surfactant, was injected into the right stifle joint of the same rats. Rats were analyzed up to 12 months after initial injection. Bioluminescence was detected at 7 days and all serotypes tested displayed bioluminescence above controls after 1 year in the left stifle. Gene expression was detected in the right stifle joints of all rats with the exception of rats previously injected with serotype 2/5. We observed no difference irrespective of whether the luciferin was injected subcutaneously or intraperitoneally. However, surfactant-diluted vectors led to increased gene expression compared with saline-diluted vectors. Cell- and tissue-specific transduction was observed in rat stifles injected with an nLacZ-containing rAAV. Transduction was greatest in stromal tissues and mesenchymal cell types. Exposure to a specific serotype did not inhibit subsequent transduction with a different serotype at a second vector injection. Including surfactant as a vector diluent increased gene expression within the stifle joint and should be considered for in vivo gene therapy applications.
Mason and colleagues investigate serotype-specific cell and tissue transduction tropisms, transgene expression levels, and longevity, as well as immunogenicity of candidate recombinant adenoassociated virus vectors (rAAV) in rat osteochondral cells, tissues, and stifle joints. Transduction was greatest in stromal tissues and mesenchymal cell types. Exposure to a specific serotype did not inhibit subsequent transduction with a different serotype. Including surfactant as a vector diluent increased gene expression within the stifle joint.
Introduction
Most osteoarthritis therapies treat symptoms of the disease and are not curative. Current therapies for osteoarthritis include the use of analgesics, nonsteroidal anti-inflammatory drugs, or intra-articular injections of hyaluronan or corticosteroids for temporary relief of pain and inflammation. Such treatments, however, can be associated with numerous side effects, including gastrointestinal erosion or hemorrhage, impairment of renal function, osteoporosis, and hypertension (Fraenkel et al., 2004). In patients who fail to respond to these conventional measures, surgical intervention is required to provide relief from pain and disability (Walker-Bone et al., 2000; Hunter and Felson, 2006).
Gene therapy is currently being investigated as an alternative approach to the treatment of arthritis. Osteoarthritis is an appropriate target for a gene therapy approach because it is common, poorly treatable, and a leading cause of disability. Gene therapy can be used in vivo to modify the intra-articular environment of joints without disrupting the native joint architecture. Despite the promise of gene therapy, there are three major disadvantages that must be overcome before these therapies could be clinically viable: limited transduction efficiency of dense articular tissues (cartilage, ligament), transient transgene expression, and elicitation of immunologic responses (Evans et al., 2006; Saraf and Mikos, 2006).
The use of an adeno-associated virus (AAV) to deliver the therapeutic gene may help us overcome these disadvantages. Limited transduction efficiency is often blamed on induction of a host immune response against the vector and/or viral proteins or on nonhomologous transgenes. AAVs are less immunogenic than commonly used adenoviral vectors, and most serotypes appear to have minimal immunogenicity in humans (Tenenbaum et al., 2003; Cottard et al., 2004; Wang et al., 2011).
Articular joint gene delivery strategies have traditionally targeted the synovium (Hung et al., 1994). A consensus of published studies has determined that transgene expression often persists for only 2–3 weeks after inoculation (Khoury et al., 2006). In these studies, the vast majority of transduced cells are CD90+ synovial fibroblasts. Although CD90+ synovial fibroblasts are highly vulnerable to genetic modification, this cell population is also transient, with an apparent half-life in normal joints of less than 1 month (Gouze et al., 2007). This half-life decreases further in diseased joints (Remmers et al., 1990; Kaklamanis, 1992; Pan et al., 1999). If the majority of vector-transduced cells in a target joint are transient, the long-term therapeutic effects in the joint will be based on the small remaining nontransient cell population. Small populations of nontransient cells do reside within synovial joints and provide alternative targets for transduction. Targeting cell types other than CD90+ synovial fibroblasts may provide a better opportunity for a long-term therapeutic effect. The different cell types present in articular joints may also respond differently to the introduction of a transgene. This observation points to the importance of establishing cell or tissue tropisms for the candidate vectors.
In the current experiment, we used six different rAAV serotypes (2/1, 2/2, 2/5, 2/6.2, 2/8, and 2/9) to transduce monolayer cells in vitro. In rat stifle joints in vivo, we used four different rAAV vector serotypes (2/2, 2/5, 2/8, and 2/9). Our aim was to investigate the serotype-specific cell and tissue-transduction tropisms, transgene expression levels and longevity, and immunogenicity of each serotype. Among the different serotypes, rAAV5 and rAAV2 have been reported to have superior transduction efficiency in rodent arthritic joints and rAAV2 has been used in rat in vivo studies (Payne et al., 2011). Serotypes rAAV2/8 and 2/9 have enhanced in vivo transduction properties in our laboratories despite their lackluster in vitro performance (Allocca et al., 2007; Keswani et al., 2012; Mason et al., 2012). In addition to serotype comparisons over a 1-year period, we investigated the use of an increased concentration of surfactant as a diluent for vector delivery. Finally, we examined the parameters required for accurate and consistent analysis of in vivo rAAV-transduced rat stifles.
Materials and Methods
Vector production
Recombinant AAV2-cytomegalovirus (CMV)–firefly luciferase (ffl), AAV2-CMV-nLacZ, AAV2-chicken β-actin (CB)–ffl, and the rAAV2/1, 2/2, 2/5, 2/6.2, 2/8, and 2/9 packaging constructs were generated, and vectors were produced by triple transfection and purified as previously described (Xiao et al., 1998; Auricchio et al., 2001; Hildinger et al., 2001; Gao et al., 2002). Briefly, a CMV- or CB-driven transgene cassette encoding ffl or nLacZ flanked by AAV2-inverted terminal repeats was co-transfected with a packaging plasmid encoding the rep and cap genes. Adenoviral helper function was delivered in trans from a third helper plasmid, pΔF6. Recombinant AAV vectors were purified as previously described (Gao et al., 2002). All vector preparations were banded on three, sequential CsCl density gradients. Vector titers were evaluated by TaqMan real-time polymerase chain reaction with primers and a probe specific for the polyadenylation signal in the vector transgene cassette (bGH forward, GCCAGCCATCTGTTGT; bGH reverse, GGAGTGGCACCTTCCA; bGH probe, 6FAM-TCC CCC GTG CCT TCC TTG ACC-TAMRA).
Experiment one
All experiments were conducted in accordance with protocols and procedures approved by the Institutional Animal Care and Use Committee of The University of Pennsylvania. Cartilage and synovial tissues were collected aseptically from the stifle joints of four 3-month-old male Sprague-Dawley rats. Cartilage was collected from the tibial plateau and femoral condyle and consisted of cartilage tissue from the tangential layer to the tidemark. Synovial tissue was collected from the areas surrounding the patella and articular surfaces and consisted of synovial, adipose, and fibrous capsular tissue. Samples were collected within 30 min after euthanasia. The tissue was placed in 50 ml centrifuge tubes with PBS+(PBS with CA2+ and Mg2+) that contained a 2× antimicrobial solution and was maintained at room temperature (21°C) until all tissues were collected. Cells were isolated from explant tissue and cultured as described elsewhere (Mason et al., 2012). Cells were plated at 3.3×103 cells/cm2 in 150 mm tissue culture-treated dishes and cultured with DMEM/F12 culture medium with 10% fetal bovine serum. An additional 50 μg of ascorbate-2-phosphate/ml was added to chondrocyte cultures. All cells were used at passage three for transduction assays. Nontransduced control cells were cultured and assayed in parallel with experimental groups. For in vitro transduction experiments, 1.0×103 mitomycin-C-inactivated cells were plated directly in 96-well, opaque-walled assay plates (day 0) with and without surfactant (0.01% final surfactant concentration [Pluronic F68]) and transduction was initiated the following day (day 1).
Monolayer-cultured chondrocytes and synoviocytes were transduced with six rAAV serotypes (2/1, 2/2, 2/5, 2/6.2, 2/8, and 2/9) and two promoters (CMV and CB) with ffl as the marker gene. Cells were transduced at 1.0×102 genome copies (GCs)/cell in 100 μl of a basal medium consisting of DMEM/F12 with no protein added. One hour after initiation of transduction, an additional 100 μl of a serum-containing culture medium was added to each well. Two hours after initiation of transduction, cells were rinsed with PBS+ solution and the wells were filled with 200 μl of the serum-containing culture medium and replaced in the incubator (37°C at 5% CO2).
Four days after initiation of transduction, the cells were lysed and bioluminescence was measured 2 sec after addition of 100 μl of luciferase assay substrate (Promega) in a bioluminescence-imaging plate reader and recorded as relative light units per well. Transduction efficiency for monolayer-cultured cells was defined as bioluminescence per cell (BPC). Corrections were not necessary for cell growth over the 4-day culture period between vector inoculation and the assessment of gene expression since the cells were mitotically inactivated before plating and transduction.
Experiment two
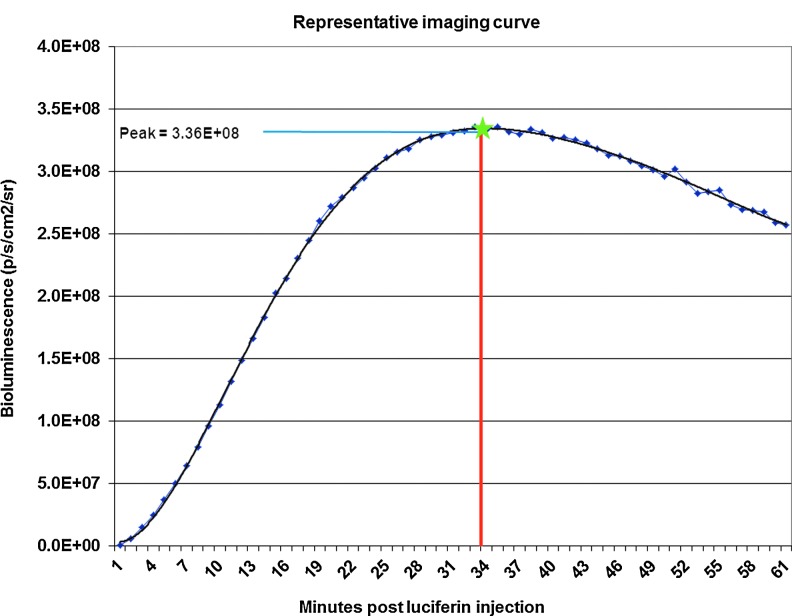
An intra-articular injection was used to introduce rAAVs to the left stifle joint of 16 four-month-old male Sprague-Dawley rats. Rats were anesthetized by intraperitoneal (IP) injection of ketamine (30 mg/kg) and xylazine (10 mg/kg). The injection site was clipped and aseptically prepared, and 1.0×1010 GCs of vector that contained an ffl marker gene diluted to a 50 μl volume with saline was injected intra-articularly into the left stifle joint using a Hamilton syringe with a 30-guage needle. A 50 μl volume of saline was injected intra-articularly into the right stifle joint as an injection control. As in the in vitro experiments, we used ffl as our marker gene. Live animal bioluminescence imaging was used to determine the extent of gene expression of reporter genes in the injected joints. To collect quality data from our in vivo bioluminescence imaging experiment, the parameters for collection of data were closely defined for treatment or experimental group. We used a Xenogen VivoVision IVIS Lumina optical imaging system. Animals were anesthetized and injected with luciferin (150 mg/kg) through either a subcutaneous (SC) or IP route. Image acquisition began 1 min after luciferin injection and continued for 61 min. Data were collected as bioluminescence (p/sec/cm2/sr) in a designated region of interest (ROI) with a unique ROI for each stifle joint and for control regions used for normalization of bioluminescence values. Bioluminescence data were also plotted against time. Peak and time-to-peak bioluminescence values were extrapolated from the plots (Fig. 1). Imaging began at 7 days after vector injection and continued at increasing intervals throughout 1 year.
FIG. 1.
Representative imaging curve. Bioluminescence readings at 1 min intervals were plotted for each animal beginning at the time of luciferin injection.
Experiment three
Eleven months after the initial vector injection in the left stifle, all the rats used for in vivo bioluminescence imaging in experiment two were reinjected in the right stifle with 1.0×1010 GCs of an ffl-containing serotype 2/5 vector. For this experiment, the injected vector was diluted with either saline or surfactant (0.01%). At the end of the experiment, rats were anesthetized injected with luciferin as previously described, and both stifles were imaged. After both stifles were imaged in the live rats, the rats were euthanized and the stifles were further imaged during dissection.
Experiment four
Naïve rats were injected in both left and right stifles with 1.0×1010 GCs of an nLacZ-containing serotype 2/9 vector. The vector was again diluted with either saline or surfactant as in experiment three. Three weeks after vector injection, confirmatory tissue sections from sacrificed animals were analyzed for depth of vector penetration. Stifle joints were removed and were stained for 48 hr for beta-galactosidase (β-gal) expression. After staining for β-gal expression, the rat stifle joints were fixed in 10% neutral buffered formalin (NBF) for 48 hr. The joints were subsequently decalcified. Briefly, the joints were washed free of NBF with double-deionized (DDI) water and placed in 5% formic acid that was changed daily. After 5 days in formic acid, the joints were washed with DDI and soaked in DDI overnight, after which they were immersed in 70% ETOH and submitted for histology. The joints were bisected coronally at the mid-point of the joint. The cranial section containing the patella was subsequently bisected in a sagittal orientation. The sections were routinely processed and embedded for analysis.
It has been reported that synoviocytes may produce significant endogenous levels of lysosomal galactosidase, an enzyme that may react with X-Gal during the β-gal staining procedure and produce false-positive signals (Roessler et al., 1993). We used a nuclear-targeted nLacZ gene to more easily identify β-gal staining that was caused by vector injection in contrast to endogenous β-gal-positive signals from lysosomal galactosidase in synoviocytes. Nontransduced tissues were included during the staining procedures as negative controls.
Statistical analysis
Experimental data were analyzed preliminarily by one-way ANOVA and linear regression analysis. Significance tests for each predictor in the multiple linear regression model were implemented with the Student's t-test, two-tailed, unequal distribution of variance assumed and Chi-square (χ2) analysis where appropriate. Test results were considered significant for P-values P<0.05.
Results
Transduction of monolayer cell cultures
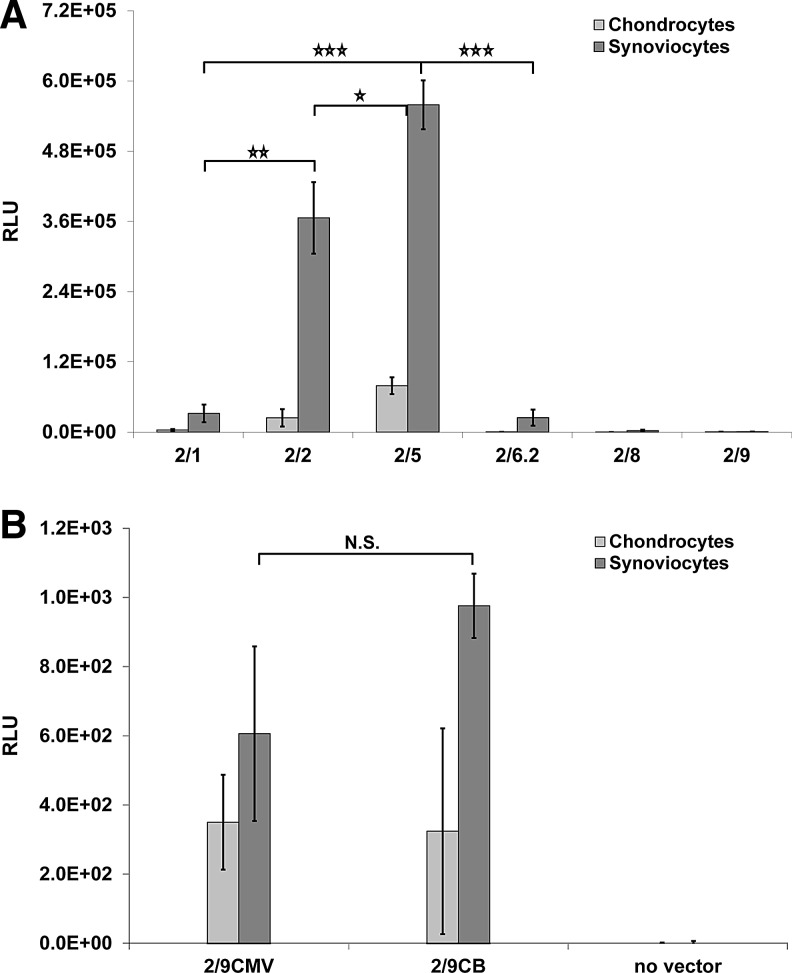
Cells were isolated from the tissues, plated in monolayer cultures, and transduced with six different rAAV vector serotypes. Four days after initiation of transduction, the highest ranking serotypes were clearly separated from the lowest ranking serotypes on the basis of a considerably higher BPC. Cells transfected with serotypes rAAV2/5 and 2/2 displayed the greatest BPC in chondrocyte and synoviocyte cultures (Fig. 2A). In all serotypes tested, synoviocytes were more readily transduced than chondrocytes. No differences were detected between cells transduced with vectors containing a CB promoter, compared with a CMV promoter (Fig. 2B), or in cell cultures with or without surfactant (data not shown).
FIG. 2.
Mean RLU results for serotype transduction in control and vector-inoculated monolayer-cultured cells. (A) Four days after vector inoculation at 1.0×102 GCs/cell in monolayer-cultured synoviocytes and chondrocytes, serotype rAAV2/5 displayed the highest BPC, followed by serotypes rAAV2/2 and rAAV2/1. (B) No difference was detected between cells transduced with CMV or CB promoter-containing vectors. Error bars represent SE (*p<0.1, **p<0.05, ***p<0.01). BPC, bioluminescence per cell; CB, chicken β-actin; CMV, cytomegalovirus; GCs, genome copies; N.S., not significant; RLU, relative light units; SE, standard error.
In vivo subcutaneous versus intraperitoneal luciferin delivery
Because of the possibility that repeated intra-articular injection of luciferin over the period of 1 year might alter normal stifle joint physiology and pathology, we opted for an indirect luciferin delivery method. We compared both SC and IP luciferin delivery and found that luciferin effectively reached the joint capsule with both delivery methods.
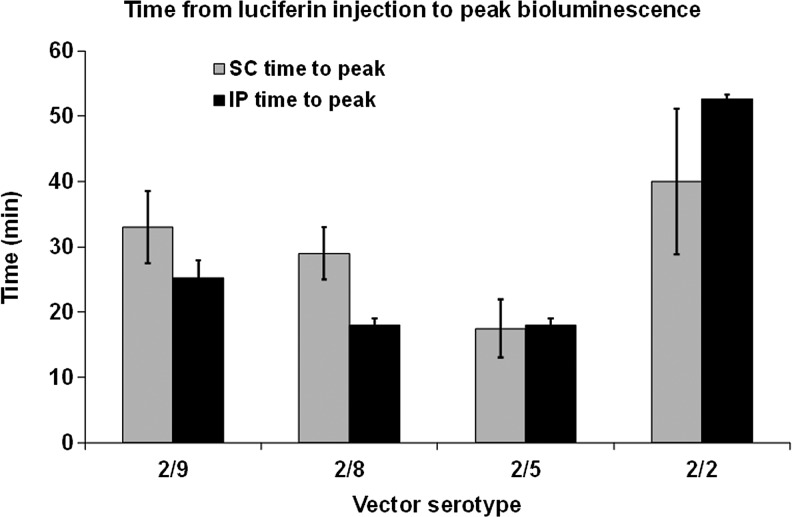
Very little difference in bioluminescence signal was observed irrespective of whether the luciferin was injected SC or IP. However, the dynamics of luciferin-induced bioluminescence was significantly different between vector serotypes (Fig. 3). Serotype 2/5 reached its peak level of expression sooner than the other serotypes, and serotype 2/2 took almost twice as long as the other serotypes to reach its peak expression level. This trend was maintained whether the luciferin was injected SC or IP.
FIG. 3.
Time from luciferin injection to peak bioluminescence. Animals were anesthetized and injected with luciferin through either an SC or IP route, and images were collected beginning at 1 min after luciferin injection and imaging continued for 61 min. Bioluminescence is plotted against time. Peak and time-to-peak values were extrapolated from the plots. Error bars represent SE. IP, intraperitoneal; SC, subcutaneous.
In vivo bioluminescence is often measured shortly after luciferin injection. We measured the slope of increasing bioluminescence from 1 to 10 min after luciferin injection to capture the linear range of the bioluminescence curve in all animals imaged. The ranking of the slopes of increasing bioluminescence did not correlate well with the ranking of the peak bioluminescence values (no correlation between slope and peak in 17 of the 28 curves, 61%).
Temporal gene expression for a 1-year period
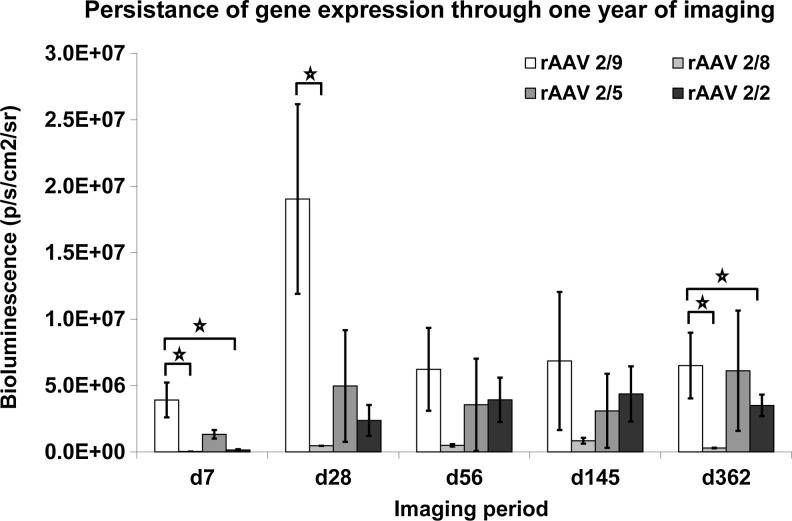
In vivo bioluminescence persisted for 1 year after initial vector inoculation. Bioluminescence was detected at the first imaging time point of 7 days and all four vector serotypes tested displayed bioluminescence levels above controls at 1 year after inoculation. Vector serotype 2/9 was initially the highest expressing serotype and remained the highest expressing serotype throughout the experiment (Fig. 4). Serotype 2/9 was also the only vector serotype to display a significant decrease in gene expression after the 1-month imaging time point.
FIG. 4.
Persistence of gene expression through 1 year. Imaging began at 7 days after vector injection and continued at increasing intervals through 1 year. We used a Xenogen VivoVision IVIS Lumina optical imaging system to detect bioluminescence from an ROI encompassing the vector-injected stifle joint. Data are presented as mean bioluminescence (p/sec/cm2/sr) from designated ROIs (normalized with ROIs from nontransduced tissues). Error bars represent SE (*p<0.1). ROI, region of interest.
Localization of gene expression
During the year-long experiment, marker gene expression was never detected outside of the injected stifle joint capsule using in vivo bioluminescence imaging. At euthanasia, the stifle joints were imaged during dissection to localize bioluminescence within the dissected joint tissues. Bioluminescence was detected in most tissues surrounding the joint, but was never detected on intact cartilage surfaces (Fig. 5).
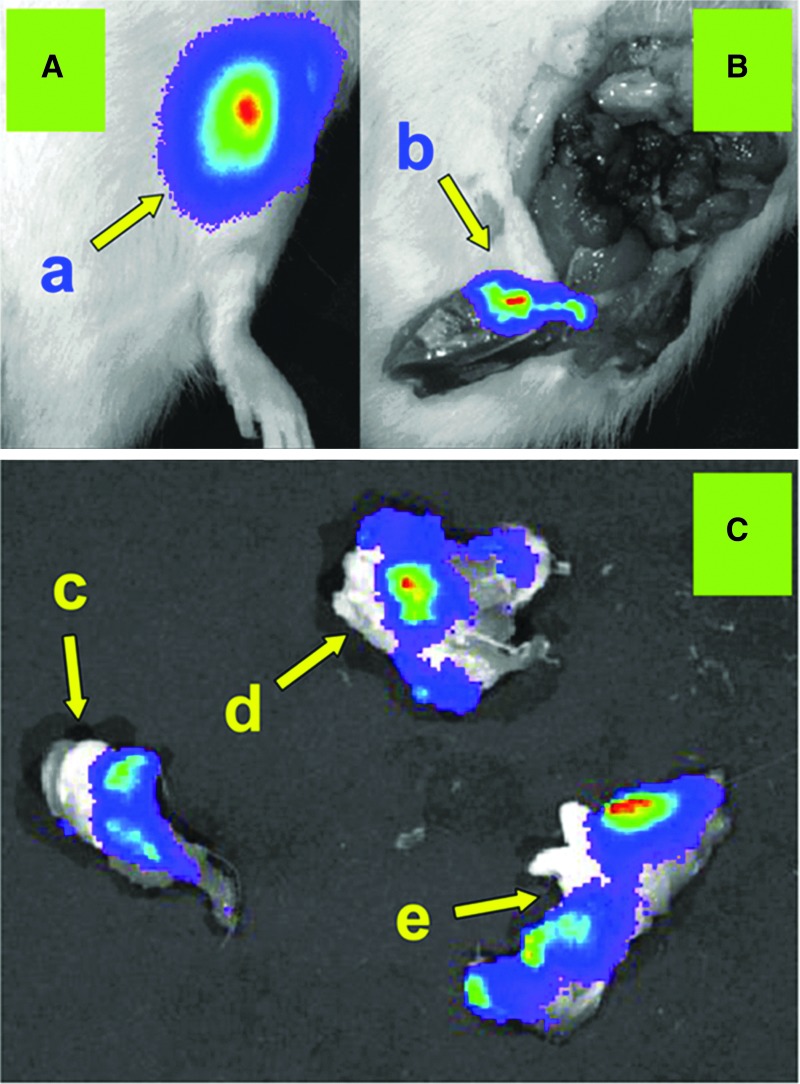
FIG. 5.
In vivo bioluminescence produced from luciferase gene expression within the vector-injected stifle joint. (A) In vivo bioluminescence emanating from the ffl vector-injected left stifle joint. (a) Representative in vivo luciferin-injected stifle before dissection. (B) Postdissection bioluminescence after removal of the stifle joint. (b) Bioluminescence from skin and connective tissue that previously covered the patellar region. (C) Bioluminescence was detected abundantly within joint tissues with the exception of the cartilage surfaces. Cartilaginous surfaces with no detectable bioluminescence signal included (c) femoral condyles, (d) tibial plateau, and (e) patellar cartilage surface.
To increase the precision of the localization of vector transduction within the injected stifle joints over what was capable with in vivo bioluminescence imaging, localization was additionally observed in histological sections from rats injected with an nLacZ-containing rAAV.
Gene expression 3 weeks after vector inoculation revealed that transduction was greatest in stromal tissues and mesenchymal cell types (Fig. 6). In these representative sections, transduced cell types included, but were not limited to, osteoblasts, osteoclasts, adipocytes, chondrocytes, muscle cells, synovial fibroblasts, and macrophages. Transduction appeared to favor more primitive cell types.
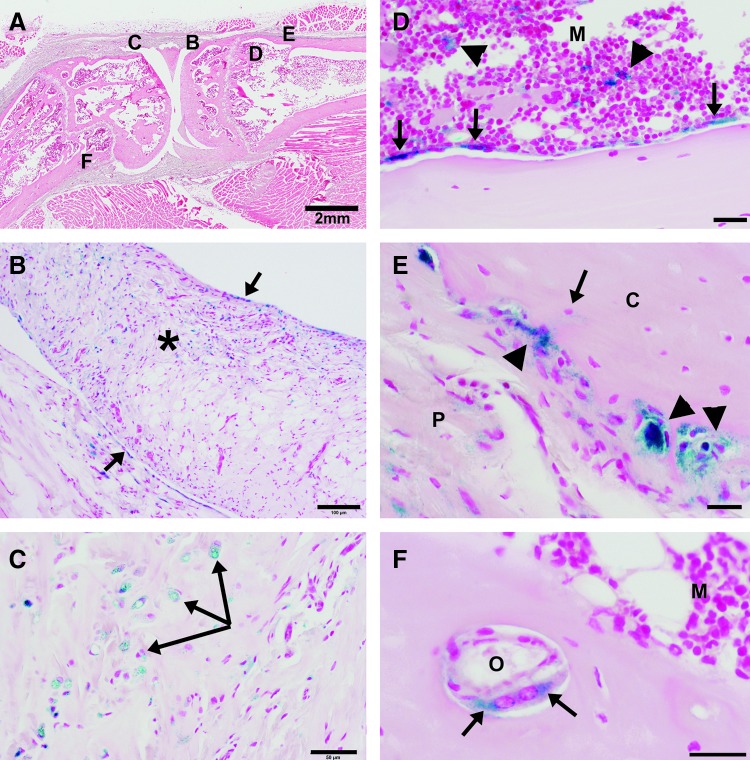
FIG. 6.
Representative beta-galactosidase staining of stifle joint tissues injected in vivo with an nLacZ marker-containing vector. (A) Low-magnification (scale bar=2 mm), hematoxylin & eosin–stained photomicrograph of a sagittal section of the femorotibial joint (left, distal femur; right, proximal tibia). (B–F) Regions of the section highlighted in the remaining panels in this composite figure. (B) Higher magnification (scale bar=100 μm) of region B in panel A shows β-gal stain within cells lining the synovial membrane (arrows) and within cells of the subsynovial fibroadipose tissue (asterisk). (C) Higher magnification (scale bar=50 μm) of region C in panel A shows β-gal stain within chondrocytes (arrows) and spindle cells of the fibrocartilage within the patellar tendon. (D) Higher magnification (scale bar=25 μm) of region D in panel A shows β-gal stain within bone-lining cells along the endosteal surface (arrows) and within cells of the bone marrow (M). (E) Higher magnification (scale bar=20 μm) of region E in panel A of the periosteum from the proximal cranial tibial cortex. C indicates β-gal stain within cells lining the bone (morphology consistent with osteoblasts and multinucleate osteoclasts; arrow heads), an ostocyte (arrow), and within spindle cells of the periosteum (P). (F) Higher magnification (scale bar=20 μm) of region F in panel A of bone from the distal caudal femoral cortex shows β-gal stain within spindle cells (arrows) lining the endoseum of an osteonal canal (O) in proximity to the medullary cavity (M).
Reinjection of AAV vector
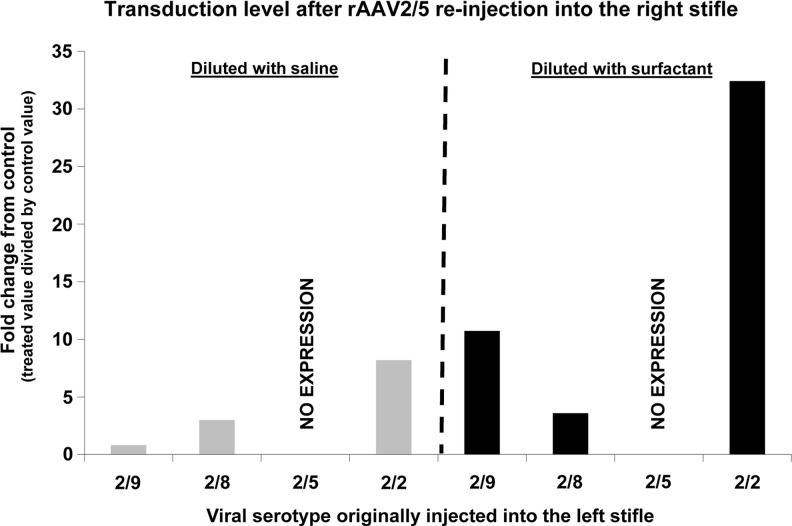
Eleven months after the initial marker gene vector injection in the left stifle, the same rats used for the initial in vivo bioluminescence imaging were reinjected with vector serotype 2/5 in the right stifle with and without surfactant. All rats displayed detectable gene expression above background in the right stifle by 14 days after injection (Fig. 7). The one exception to this was the rats previously injected in the left stifle using serotype 2/5. None of these previously 2/5-serotype, left-stifle-injected rats displayed any detectable gene expression in the 2/5-injected right stifle. However, all of these rats did maintain gene expression in the left stifle previously injected with the 2/5 vector. The efficiency of rAAV2/5 vector for right-stifle-directed gene transfer of the luciferase marker gene was not impacted by preimmunization with the other rAAV serotypes tested in the left stifle. At the end of the study, all rats tested displayed neutralizing antibodies (Nab) to the vector serotypes they had been injected with, but not to any of the other serotypes we used. Rats injected twice with the 2/5 serotype, once in each stifle, had no greater 2/5 Nab load than rats injected only once with this serotype.
FIG. 7.
Neutralizing antibodies and surfactant dilution. Eleven months after the initial marker gene injection with various serotypes in the left stifle (previously injected serotype along the x-axis), the rats used for in vivo bioluminescence imaging were reinjected with 1.0×1010 genome copies of vector serotype 2/5 in the right stifle. For this experiment, the injected vector was diluted with either saline or surfactant (0.01% final surfactant [F68] concentration). Unfortunately, not all animals initially injected with the various serotypes were available for the second injection 11 months later. For serotype 2/9 animals, three were reinjected with serotype 2/5 vector diluted with saline and two with vector diluted with surfactant. For serotype 2/8 animals, one was reinjected with vector diluted with saline and two with vector diluted with surfactant. For serotype 2/5 animals, two were reinjected with vector diluted with saline and one with vector diluted with surfactant, and for serotype 2/2 animals, one was reinjected with vector diluted with saline and two with vector diluted with surfactant. These results reflect what has been observed when using surfactant supplementation in other animal models, and the use of surfactant supplementation has become standard practice in our laboratory. No transduction in the right stifle was observed in rats previously injected in the left stifle with serotype 2/5.
Surfactant as a vector diluent
The use of a surfactant as the vector diluent instead of saline increased gene expression in the stifle joint as measured with in vivo bioluminescence imaging. All rat stifle joints that received vector diluted with surfactant displayed greater bioluminescence than joints that received vector diluted in saline (Fig. 7).
Discussion
In the current in vivo experiments, we used rAAV vector serotypes 2/2, 2/5, 2/8, and 2/9. Serotypes 2/2 and 2/5 have been used previously to transduce articular joints in vivo and are consistently top-performing in vitro serotypes when using articular joint cells and tissues. Serotypes 2/8 and 2/9 are often poor in vitro transducers, but were chosen for this experiment because of their past performance with in vivo experiments in our laboratory. At 7 days after vector injection, the level of gene expression detected for serotype 2/9-injected rats was fourfold above compared with that for serotype 2/5-injected rats, the only two detectable expressing serotypes above control levels of bioluminescence at 7 days after vector inoculation. This is in contrast to previous reports where bioluminescence in the rat stifle joint was first reported 14 days after vector injection (Payne et al., 2011). However, in the Payne et al.'s study, serotype 2/2 was utilized and their results were similar to ours for this serotype. Serotype 2/2-injected animals in our study also did not display detectable gene expression until 14 days after injection. After 4 weeks, serotypes 2/5 and 2/2 both displayed stable gene expression well above control levels and just below the levels displayed by 2/9-injected rats. These levels of expression persisted throughout the remainder of the experiment, suggesting that similar cell types were transduced by these vector serotypes and that they were not transient cell populations, but more stable, resident cell populations.
Vector serotype 2/9 displayed a significant decrease in gene expression between 4 and 8 weeks after vector injection. This observed decrease may have been caused by initial high transduction of a transient cell population, such as CD90+ synovial fibroblasts, which have a high turnover rate and may have contributed to the high initial rate of gene expression seen with serotype 2/9 (Gouze et al., 2007). In previous reports (Goater et al., 2000), transgene expression within the joint was lost within 3 weeks, suggesting that the vectors used in that study elicited a strong immune response or that the vectors transduced only a transient cell population.
Serotype 2/9 has previously displayed long-term gene expression in many tissue types, including cardiac tissue (Fechner et al., 2008; Jiang et al., 2009), nervous system (Rahim et al., 2011; Mattar et al., 2012), vascular tissue (Kotchey et al., 2011), lung (Bell et al., 2011; Pfeifer et al., 2011), liver (Chen et al., 2009; Luo et al., 2011), and skeletal muscle (Koo et al., 2011; Pulicherla et al., 2011; Wang et al., 2011). When delivered systemically (tail vein injection), the viral genome clearance rate from the blood was slower for serotype 2/9 than any other serotype tested (Zincarelli et al., 2008; Kotchey et al., 2011).
The superiority of serotype 2/9 in vivo is in contrast with our current in vitro observations and in contrast with previous observations using articular joint cells and tissues (Mason et al., 2012). In the current study, serotype 2/9 displayed the lowest in vitro gene expression among all serotypes tested. As seen in previous work, synoviocytes displayed superior in vitro gene expression levels compared with chondrocytes.
In vivo bioluminescence is frequently measured soon after luciferin injection, often within 5–10 min. In the current experiment, values from this early, linear portion of the bioluminescence curve correlated with the ultimate peak bioluminescence value in only 11 of 28 curves plotted (39%). This observation suggests that peak bioluminescence may be a better measure of differences in gene expression between treatments than an earlier reading taken during the linear portion of the curve.
SC and IP luciferin delivery allowed us to capture the peak bioluminescence from the joint because of the time delay during diffusion of luciferin from the site of injection into the joint. In vivo SC and IP luciferin delivery was technically less demanding than intra-articular delivery and eliminated any chance of the injection needle causing joint damage after repeated intra-articular injections over the course of the experiment. Additionally, because luciferin was distributed throughout the body, we would have detected any extra-articular bioluminescence, which would be difficult to detect using intra-articular luciferin delivery. Previous reports have addressed the issue of SC versus IP luciferin injection and the issue of the timing of in vivo bioluminescence imaging, but the issue of luciferin delivery within the stifle joint of mature male rats had not been addressed (Inoue et al., 2009, 2010). In our rat stifle joints, very little difference in bioluminescence signal was detected whether luciferin was injected through an SC or an IP route. By injecting luciferin IP or SC, rather than intra-articularly, we were able to confidently scan the entire animal for bioluminescence every time we imaged.
Marker gene bioluminescence was never detected outside of the injected stifle joint capsule using in vivo bioluminescence imaging. Our rats were imaged in three distinct orientations to detect bioluminescence from any region of the animal, including, but not limited to, the contralateral stifle, IP luciferin injection site, SC luciferin injection site, abdomen (liver region), head, and testis. Bioluminescence was occasionally detected from the metallic ear tags used to identify each animal. These observations suggest limited systemic exposure and limited gene transfer outside the joint capsule, which may potentially help to reduce an animal's total viral load. The volume of luciferin we used was calculated from literature values for similar studies and from previous in vivo imaging in our laboratory. The concentration and/or volume of luciferin used can affect imaging parameters, but should not have had an effect on the vector serotype hierarchy we observed in time-to-peak bioluminescence.
While we noticed very little difference in bioluminescence caused by the luciferin injection route, we did discover a significant difference in the timing of the response to luciferin depending on the vector serotype used. Vector serotype 2/5 reached peak bioluminescence earlier than any of the other serotypes, and serotype 2/2 took roughly twice as long as the other serotypes to reach peak bioluminescence. These differences in the timing of peak bioluminescence likely reflected the specific cell types transduced and/or differences in systemic circulation patterns dictating access of luciferin to the specific cells and tissues transduced by each serotype.
Various AAV vector serotypes are known to possess specific cell and tissue tropisms or the ability to transduce different populations of cells or tissue types with a range of efficiencies. In vivo bioluminescence is very good at providing a qualitative assessment of gene expression for an ROI in an animal, but to determine specific cell and tissue types transduced, we used basic histology of tissue sections from rats injected with an nLacZ-containing rAAV to acquire a more precise depiction of transduction effectiveness within the stifle joint. Gene expression 3 weeks after vector inoculation revealed that transduction was greatest in stromal tissues and mesenchymal cell types and appeared to favor more primitive cell types. This may be one explanation for the longevity of gene expression observed in our vector-injected stifle joints. However, this 3-week time point was before the post-4-week drop in gene expression detected with serotype 2/9 in the year-long in vivo bioluminescence experiments and was therefore likely to include transient as well as stably transduced cell populations.
The host immune response to AAV capsid can be species specific and is mediated principally by circulating antibodies, which may prevent initial or repeated AAV administrations (Manno et al., 2006). Among individuals that are seropositive for AAV9, most present with low titers (Boutin et al., 2010). To test if our vectors injected into the left stifle joint and expressing only in the left stifle joint had produced a systemic immune response great enough to prevent repeat AAV administration with other serotypes, 11 months after the initial marker gene vector injection, the same rats previously injected with various serotypes in the left stifle were reinjected with vector serotype 2/5 in the right stifle. All rats displayed detectable gene expression above background by 14 days after injection with the exception of rats previously injected in the left stifle with serotype 2/5. None of these previously 2/5-injected rats displayed any detectable gene expression in the right stifle, but did maintain gene expression in the previously injected left stifle. Preimmunization with serotypes other than 2/5 did not impact the efficiency of stifle-directed gene transfer of the luciferase marker gene by a serotype 2/5 vector.
Delivery of AAV vector in vitro or in vivo is often compromised by fractional loss of the vector to the devices used to handle and/or deliver the vector. In a previous study (Bennicelli et al., 2008), vector recovery was assessed in vitro in the presence or absence of surfactant after vector dilution and passage through injection devices. In this study, recovery of the vector approximated 100% in the presence of surfactant and ranged from 24% to 51% without the use of surfactant. When used in vivo, surfactant treatment significantly increased β-gal expression in mouse adipose tissue (Zhang et al., 2011). In vitro, we found no difference in gene expression when cells were transduced in the presence of surfactant compared with saline. However, in vivo with our 11-month reinjection experiment, all rats that received vector diluted with surfactant displayed greater bioluminescence than rats that received vector diluted in saline. In addition, rat joints injected with an nLacZ-marker gene that was diluted with surfactant subjectively appeared to display a superior range of tissue gene expression within the joint compared with saline-diluted vector. This surfactant effect is further supported by results from the use of surfactant dilution in other animal models of in vivo transduction in our laboratory. The use of a surfactant as the vector diluent instead of saline significantly increased the gene expression in the stifle joint as measured with in vivo bioluminescence imaging. The appearance of the β-gal-stained tissues appeared to suggest that surfactant may have increased the invasiveness of the viral particles, but further experiments will be required to determine if the increase in observed gene expression with surfactant was caused by a superior range of tissues transduced or simply a decrease in vector loss at delivery, which resulted in a greater dose of vector being delivered to the tissue.
In summary, differences in the dynamics of in vivo bioluminescence were detected between the various vector serotypes tested. This suggests that the serotype used should be considered during collection of in vivo bioluminescence data. No differences were detected irrespective of whether luciferin was injected using an IP or SC luciferin injection route. This observation presents the option to choose from multiple methods of luciferin delivery based on specific experimental requirements. All serotypes tested displayed year-long persistence of gene expression, which was restricted to the injected stifle joint, and serotype 2/9 displayed the highest gene expression of all serotypes tested. However, gene expression was not detected at the cartilage surfaces, but tended to favor more primitive cell types within the joint. Exposure to a specific serotype did not inhibit subsequent transduction with an alternate serotype at a second vector injection. Successive rAAV treatments can be a complication for some in vivo rAAV vector delivery schemes. Multiple treatments may be successful if alternate vector serotypes are utilized. Including surfactant as a vector diluent can increase measureable gene expression within the stifle joint and should be considered for in vivo gene therapy applications.
Acknowledgments
We gratefully acknowledge Drs. Peter Bell, affiliated with Gene Therapy Program, and Steven Berkowitz, associated with Unionville Equine Associates, Oxford, PA, 19363; Ms. Deirdre McMenamin and Ms. Christine Draper, affiliated with Gene Therapy Program, and Mr. Ryan Delaney, affiliated with Department of Clinical Studies, for their intellectual support and technical assistance. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number 1F32AR056936-01A2. Research was also supported by The University of Pennsylvania Vector Core Facility and a grant to J.M.W. (NIH Grant 2-P30-DK047757-16). Recombinant AAV preparations were provided by The University of Pennsylvania Vector Core Facility (Philadelphia, PA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- Allocca M. Mussolino C. Garcia-Hoyos M., et al. Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J. Virol. 2007;81:11372–11380. doi: 10.1128/JVI.01327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auricchio A. Hildinger M. O'Connor E., et al. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum. Gene Ther. 2001;12:71–76. doi: 10.1089/104303401450988. [DOI] [PubMed] [Google Scholar]
- Bell C.L. Vandenberghe L.H. Bell P., et al. The AAV9 receptor and its modification to improve in vivo lung gene transfer in mice. J. Clin. Invest. 2011;121:2427–2435. doi: 10.1172/JCI57367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennicelli J. Wright J.F. Komaromy A., et al. Reversal of blindness in animal models of Leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol. Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin S. Monteilhet V. Veron P., et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- Chen C.C. Sun C.P. Ma H.I., et al. Comparative study of anti-hepatitis B virus RNA interference by double-stranded adeno-associated virus serotypes 7, 8, and 9. Mol. Ther. 2009;17:352–359. doi: 10.1038/mt.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottard V. Valvason C. Falgarone G., et al. Immune response against gene therapy vectors: influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J. Clin. Immunol. 2004;24:162–169. doi: 10.1023/B:JOCI.0000019781.64421.5c. [DOI] [PubMed] [Google Scholar]
- Evans C.H. Gouze E. Gouze J.N., et al. Gene therapeutic approaches-transfer in vivo. Adv. Drug Deliv. Rev. 2006;58:243–258. doi: 10.1016/j.addr.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Fechner H. Sipo I. Westermann D., et al. Cardiac-targeted RNA interference mediated by an AAV9 vector improves cardiac function in coxsackievirus B3 cardiomyopathy. J. Mol. Med. 2008;86:987–997. doi: 10.1007/s00109-008-0363-x. [DOI] [PubMed] [Google Scholar]
- Fraenkel L. Bogardus S.T. Concato J. Wittink D.R. Treatment options in stifle osteoarthritis—the patient's perspective. Arch. Intern. Med. 2004;164:1299–1304. doi: 10.1001/archinte.164.12.1299. [DOI] [PubMed] [Google Scholar]
- Gao G.P. Alvira M.R. Wang L.L., et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goater J. Muller R. Kollias G., et al. Empirical advantages of adeno associated viral vectors for in vivo gene therapy for arthritis. J. Rheumatol. 2000;27:983–989. [PubMed] [Google Scholar]
- Gouze E. Gouze J.N. Palmer G.D., et al. Transgene persistence and cell turnover in the diarthrodial joint: implications for gene therapy of chronic joint diseases. Mol. Ther. 2007;15:1114–1120. doi: 10.1038/sj.mt.6300151. [DOI] [PubMed] [Google Scholar]
- Hildinger M. Auricchio A. Gao G., et al. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle- directed gene transfer. J. Virol. 2001;75:6199–6203. doi: 10.1128/JVI.75.13.6199-6203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung G.L. Galealauri J. Mueller G.M., et al. Suppression of intraarticular responses to interleukin-1 by transfer of the interleukin-1 receptor antagonist gene to synovium. Gene Ther. 1994;1:64–69. [PubMed] [Google Scholar]
- Hunter D.J. Felson D.T. Osteoarthritis. Br. Med. J. 2006;332:639–642B. doi: 10.1136/bmj.332.7542.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y. Kiryu S. Izawa K., et al. Comparison of subcutaneous and intraperitoneal injection of d-luciferin for in vivo bioluminescence imaging. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:771–779. doi: 10.1007/s00259-008-1022-8. [DOI] [PubMed] [Google Scholar]
- Inoue Y. Kiryu S. Watanabe M., et al. Timing of imaging after d-luciferin injection affects the longitudinal assessment of tumor growth using in vivo bioluminescence imaging. Int. J. Biomed. Imaging. 2010;8:1–6. doi: 10.1155/2010/471408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X.F. Burdorf L. Hinkel R., et al. Optimization of delivery of adeno-associated virus mediated gene transfer to a transplanted heart in a rat model. Exp. Clin. Transplant. 2009;7:184–187. [PubMed] [Google Scholar]
- Kaklamanis P.M. Experimental animal models resembling rheumatoid arthritis. Clin. Rheumatol. 1992;11:41–47. doi: 10.1007/BF02207082. [DOI] [PubMed] [Google Scholar]
- Keswani S.G. Balaji S. Le L., et al. Pseudotyped adeno-associated viral vector tropism and transduction efficiencies in murine wound healing. Wound Repair Regen. 2012;20:592–600. doi: 10.1111/j.1524-475X.2012.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury M. Bigey P. Louis-Plence P., et al. A comparative study on intra-articular versus systemic gene electrotransfer in experimental arthritis. J. Gene Med. 2006;8:1027–1036. doi: 10.1002/jgm.922. [DOI] [PubMed] [Google Scholar]
- Koo T. Malerba A. Athanasopoulos T., et al. Delivery of AAV2/9-microdystrophin genes incorporating helix 1 of the coiled-coil motif in the C-terminal domain of dystrophin improves muscle pathology and restores the level of alpha 1-syntrophin and alpha-dystrobrevin in skeletal muscles of mdx mice. Hum. Gene Ther. 2011;22:1379–1388. doi: 10.1089/hum.2011.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotchey N.M. Adachi K. Zahid M., et al. A potential role of distinctively delayed blood clearance of recombinant adeno-associated virus serotype 9 in robust cardiac transduction. Mol. Ther. 2011;19:1079–1089. doi: 10.1038/mt.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X.Y. Hall G. Li S.T., et al. Hepatorenal correction in murine glycogen storage disease type i with a double-stranded adeno-associated virus vector. Mol. Ther. 2011;19:1961–1970. doi: 10.1038/mt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C.S. Arruda V.R. Pierce G.F., et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mason J.B. Vandenberghe L.H. Xiao R., et al. Transduction efficiency of recombinant adeno-associated viral vectors is differentially influenced by vector serotype and tissue composition in equine synovial joint tissues. Am. J. Vet. Res. 2012;73:1178–1185. doi: 10.2460/ajvr.73.8.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar C.N. Nathwani A. Rosales C., et al. Early AAV-mediated intrauterine gene therapy at 0.4G in non-human primates. Mol. Ther. 2012;20:S252–S253. [Google Scholar]
- Pan R.Y. Xiao X. Chen S.L., et al. Disease-inducible transgene expression from a recombinant adeno- associated virus vector in a rat arthritis model. J. Virol. 1999;73:3410–3417. doi: 10.1128/jvi.73.4.3410-3417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne K.A. Lee H.H. Haleem A.M., et al. Single intra-articular injection of adeno-associated virus results in stable and controllable in vivo transgene expression in normal rat stifles. Osteoarthritis Cartilage. 2011;19:1058–1065. doi: 10.1016/j.joca.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer C. Aneja M.K. Hasenpusch G., et al. Adeno-associated virus serotype 9-mediated pulmonary transgene expression: effect of mouse strain, animal gender and lung inflammation. Gene Ther. 2011;18:1034–1042. doi: 10.1038/gt.2011.42. [DOI] [PubMed] [Google Scholar]
- Pulicherla N. Shen S. Yadav S., et al. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol. Ther. 2011;19:1070–1078. doi: 10.1038/mt.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim A.A. Wong A.M.S. Hoefer K., et al. Intravenous administration of AAV2/9 to the fetal and neonatal mouse leads to differential targeting of CNS cell types and extensive transduction of the nervous system. FASEB J. 2011;25:3505–3518. doi: 10.1096/fj.11-182311. [DOI] [PubMed] [Google Scholar]
- Remmers E.F. Lafyatis R. Kumkumian G.K., et al. Cytokines and growth regulation of synoviocytes from patients with rheumatoid arthritis and rats with streptococcal cell wall arthritis. Growth Factors. 1990;2:179–188. [PubMed] [Google Scholar]
- Roessler B.J. Davidson B.L. Genetic modification of synoviocytes in vivo using recombinant adenoviral vectors. Clin. Res. 1993;41:A171. [Google Scholar]
- Saraf A. Mikos A.G. Gene delivery strategies for cartilage tissue engineering. Adv. Drug Deliv. Rev. 2006;58:592–603. doi: 10.1016/j.addr.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum L. Lehtonen E. Monahan P.E. Evaluation of risks related to the use of adeno-associated virus-based vectors. Curr. Gene Ther. 2003;3:545–565. doi: 10.2174/1566523034578131. [DOI] [PubMed] [Google Scholar]
- Walker-Bone K. Javaid K. Arden N. Cooper C. Regular review—medical management of osteoarthritis. Br. Med. J. 2000;321:936–940. doi: 10.1136/bmj.321.7266.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Louboutin J.P. Bell P., et al. Muscle-directed gene therapy for hemophilia B with more efficient and less immunogenic AAV vectors. J. Thromb. Haemost. 2011;9:2009–2019. doi: 10.1111/j.1538-7836.2011.04491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X. Li J. Samulski R.J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.L. Jia S.Q. Zheng S.P., et al. Celastrol enhances AAV1-mediated gene expression in mice adipose tissues. Gene Ther. 2011;18:128–134. doi: 10.1038/gt.2010.120. [DOI] [PubMed] [Google Scholar]
- Zincarelli C. Soltys S. Rengo G. Rabinowitz J.E. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]