Abstract
Pou5f1/Oct4, a member of the POU transcription factor family, is exclusively expressed in embryonic stem cells, which are involved in self-renewal and maintaining pluripotency. In the present study, we report on the establishment of a monoclonal antibody that is specific for Oct4 using the rat medial iliac lymph node method. In an immunoblotting analysis, our antibody detected endogenous Oct4. In addition, immunocytochemical staining using the antibody revealed the nuclear localization of Oct4. This monoclonal antibody has the potential for use in the further analysis of Oct4 function in stem cells.
Introduction
Amember of POU transcription factor family, Pou5f1/Oct4 contains a POU domain.(1) The POU domain, which is located in the center of Oct4, consists of two structurally independent subdomains: a 75 amino acid amino-terminal POU-specific (POUs) region and a 60 amino acid carboxyl-terminal homeodomain (POUh).(2) The POU family is divided into five classes based on the pairwise comparison of all POU homeodomain and POU subdomain sequences (the POUs domain contains subdomains A and B).(2) Oct4 belongs to class V, which activates the expression of their target genes through the binding of an octameric sequence motif of an AGTCAAAT consensus sequence.(3)
Novel ES-like stem cells have recently been generated from adult mouse and human tissues by reprogramming; these are referred to as induced pluripotent stem cells (iPSCs). It is known that Oct4 functions as a core transcription factor in the generation of iPSCs,(4) and is specifically expressed in ESCs, which are involved in self-renewal and maintaining pluripotency(5–7); however a complete understanding of their involvement in this phenomenon has not been developed. In the present study, we established a monoclonal antibody (MAb) against Oct4 using the rat medial iliac lymph node method. This MAb promises to be useful in immunoblotting and immunostaining of ES cells.
Materials and Methods
Cell culture
Mouse embryonic stem cells were cultured in Dulbecco's modified Eagle's medium supplemented with 15% fetal bovine serum (FBS), LIF, penicillin (100 U/mL), streptomycin (μg/mL), 1x non-essential amino acids (Gibco, Grand Island, NY), 1x Glutamax (Life Technologies Invitrogen, San Diego, CA), and 2-mercaptoethanol (1 μM/mL) under a humidified atmosphere with 5% CO2 at 37°C.
Design of peptide
The Oct4 peptide CKKKKPSVPVTALGSPMHSN was synthesized by Sigma-Aldrich (Tokyo, Japan). This peptide corresponds to the C-terminal 15 amino acids of mouse Oct4 (338–352 aa) and the five amino acids (CKKKK) that were added to the N-terminal site as a hydrophilic linker. The peptide was coupled to keyhole limpet hemocyanin (KLH) or BSA using 3-maleimidobenzoic acid N-hydroxysuccinimide ester (MBS, Sigma, St. Louis, MO).
Rat immunization and monoclonal antibody production
The anti-Oct4 rat monoclonal antibody was generated based on the rat lymph node method established by Sado and colleagues.(8–10) An 8-week-old female WKY/Izm rat (SLC, Shizuoka, Japan) was injected via the hind footpads with 150 μL of an emulsion containing 125 μg of Oct4 peptide-KLH and Freund's complete adjuvant. After 18 days, the cells from the medial iliac lymph nodes of a rat immunized with an antigen were fused with mouse myeloma SP2 cells at a ratio of 5:1 in a 50% polyethyleneglycol (PEG 1500, Roche, Mannheim, Germany) solution. The resulting hybridoma cells were placed on 96-well plates and cultured in HAT selection medium (Hybridoma-SFM [Invitrogen, Carlsbad, CA]; 10% FBS; 10% BM-condimed H1 [Roche, Indianapolis, IN]; 100 μM hypoxathine; 0.4 μM aminopterin; 16 μM thymidine). At 7 days post-fusion, the hybridoma supernatants were screened by means of an enzyme-linked immunoadsorbent assay (ELISA) against the Oct4 peptide-BSA. Positive clones were subcloned and rescreened by ELISA, immunoblotting, and immunocytochemistry.
Enzyme-linked immunoadsorbent assay
Oct4 peptide-BSA (0.1 μg/mL) in ELISA buffer (10 mM sodium phosphate [pH 7.0]) was adsorbed on the surface of Serocluster 96-well U bottom plates (Corning Inc., Corning, NY) by means of an overnight incubation at 4°C. To avoid non-specific binding, the plates were blocked with 1% BSA in PBS. Hybridoma supernatants were incubated for 1 h at room temperature and then washed three times with TBS-T. The plates were incubated for 30 min at room temperature with alkaline phosphatase-conjugated anti-rat IgG antibody (Sigma) at a dilution of 1:20,000. After washing with TBS-T, immunoreactivity was visualized by means of a pNPP phosphatase substrate system (KPL, Gaithersburg, MD).(11)
Immunoblotting
Mouse embryonic stem cells (mESCs) were washed twice with phosphate-buffered saline (PBS) and lysed in 1x SDS-PAGE sample buffer. The samples were separated by 10% SDS-PAGE, and electrophoretically transferred to nitrocellulose membranes. The membranes were blocked for 1 h at room temperature with a blocking solution containing 3% skim milk (Nacalai tasque, Kyoto, Japan) in TBS-T (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, and 0.05% Tween-20), and then incubated for 1 h at 4°C with anti-Oct4 rat MAb 1C10 diluted in Immunoreaction Enhancer Solution 1 (Toyobo, Osaka, Japan). After washing with TBS-T, the membrane was incubated for 30 min at room temperature with alkaline phosphatase-conjugated anti-rat IgG Ab (GE Healthcare, Buckinghamshire, United Kingdom). After washing with TBS-T, the membrane was developed by treatment with nitroblue tetrazolium (NBT) and bromo-chloro-indolylphosphate (BCIP).
Immunocytochemistry
mESCs grown on coverslips were washed in PBS at 37°C and fixed by treatment with 3.7% formaldehyde for 15 min at room temperature. After washing with PBS, cells were permeabilized for 5 min in 0.5% Triton X-100 in PBS and then incubated in blocking buffer as described previously.(12) Oct4 was detected with 1C10, followed by an Alexa 488-conjugated anti-rat IgG (Invitrogen) for 1 h at room temperature. Coverslips were mounted with Prolong antifade reagent (Molecular Probes, Carlsbad, CA).(13) Oct4-expressing cells were visualized using a fluorescent microscopy (Olympus, Tokyo, Japan).
Results and Discussion
Oct4 possesses a full-length 352 amino acid sequence. To establish a MAb specific for Oct4, we utilized a 20-amino acid synthetic peptide, CKKKKPSVPVTALGSPMHSN, which corresponds to the C-terminal region of mouse Oct4 as the antigen. A Blast analysis indicated that this region is highly conserved among species but has no homology with other proteins. The peptide-conjugated KLH was immunized via the hind footpads of the rat with only a single injection. At 18 days post-immunization, lymphocytes were collected from the enlarged lymph nodes of the rat. The hybridomas, obtained after fusing the lymphocytes with mouse myeloma SP2 cells, were tested for the production of monoclonal antibodies that react with Oct4 peptide-conjugated BSA by ELISA. Some positive clones that were identified by ELISA were examined by immunoblotting for specificity to total extracts of mouse ES cells. One clone, designated as MAb 1C10, yielded a strong signal in immunoblotting using the mouse ES cell extracts (Fig. 1). The specific immunoglobulin class of the MAb was determined using a rat isotyping kit. The analysis indicated that MAb 1C10 is rat IgG2a (λ).
FIG. 1.
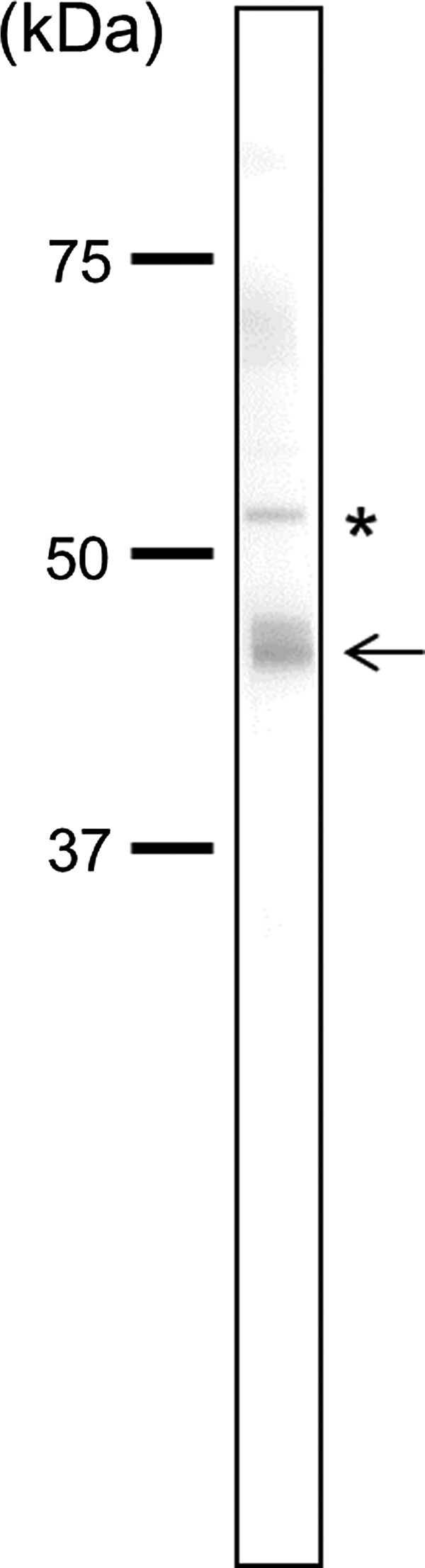
Specificity of anti-Oct4 monoclonal antibody 1C10. Immunoblotting analysis of total cell extracts from mouse ES cells using MAb 1C10. Molecular mass markers in kDa are indicated at left. Asterisk indicates a splicing variant of Oct4.
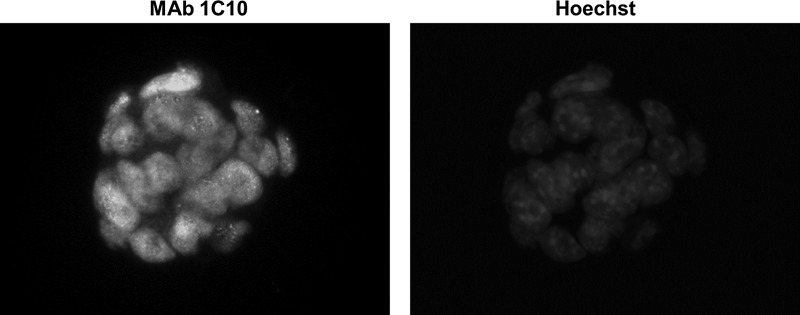
This antibody was next characterized by the immunofluorescence staining of mouse ES cells. The cells were fixed with formaldehyde and permeabilized with Triton X-100. After treatment with blocking solution, the cells were probed with MAb 1C10 against Oct4 followed by staining with Alexa 488-conjugated secondary antibody. As shown in Figure 2, endogenous Oct4 was observed to be localized in the nucleus of mouse ES cells but not in other cells (e.g., L929 and NIH3T3 cells that do not express endogenous Oct4; data not shown). From these findings, it was concluded that this MAb specifically recognizes endogenous Oct4. The use of this antibody will allow for the further elucidation of the role of Oct4 in reprogramming and maintaining the pluripotency of stem cells.
FIG. 2.
Indirect immunofluorescence using anti-Oct4 MAb 1C10. Mouse ES cells were immunostained with MAb 1C10. MAb 1C10 was detected by an Alexa 488-conjugated anti-rat IgG. The cells were counterstained with Hoechst dye.
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Scholer H. Ruppert S. Suzuki N. Chowdhury K. Gruss P. New type of POU domain in germ line-specific protein Oct4. Nature. 1990;344:435–439. doi: 10.1038/344435a0. [DOI] [PubMed] [Google Scholar]
- 2.Scholer H. Octamania: the POU factors in murine development. Trends Genet. 1991;7:323–329. doi: 10.1016/0168-9525(91)90422-m. [DOI] [PubMed] [Google Scholar]
- 3.Pesce M. Scholer H. Oct4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19:271–278. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Pesce M. Scholer H. Oct4: control of totipotency and germline determination. Mol Rep. 2000;55:452–457. doi: 10.1002/(SICI)1098-2795(200004)55:4<452::AID-MRD14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Nichols J. Zevnik B. Anastassiadis K. Niwa H. KleweNebenius D. Chambers I. Scholer H. Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 7.Niwa H. Molecular mechanism to maintain stem cell renewal of ES cells. Cell Struct Func. 2001;26:137–148. doi: 10.1247/csf.26.137. [DOI] [PubMed] [Google Scholar]
- 8.Kishiro Y. Kagawa M. Naito I. Sado Y. A novel method of preparing rat monoclonal antibody-producing hybridomas by using rat medial iliac lymph-node cells. Cell Struct Func. 1995;20:151–156. doi: 10.1247/csf.20.151. [DOI] [PubMed] [Google Scholar]
- 9.Sado Y. Kagawa M. Kishiro Y. Sugihara K. Naito I. Seyer JM. Sugimoto M. Oohashi T. Ninomiya Y. Establishment by the rat lymph-node method of epitope-defined monoclonal antibodies recognizing the 6 different alpha chains of human type-Iv collagen. Histochem Cell Biol. 1995;104:267–275. doi: 10.1007/BF01464322. [DOI] [PubMed] [Google Scholar]
- 10.Harada A. Okada S. Odawara J. Kumamaru H. Saiwai H. Aoki M. Nakamura M. Nishiyama Y. Ohkawa Y. Production of a rat monoclonal antibody specific for Myf5. Hybridoma. 2010;29:59–62. doi: 10.1089/hyb.2009.0066. [DOI] [PubMed] [Google Scholar]
- 11.Ohkawa Y. Harada A. Nakamura M. Yoshimura S. Tachibana T. Production of a rat monoclonal antibody against Brg1. Hybridoma. 2009;28:463–466. doi: 10.1089/hyb.2009.0041. [DOI] [PubMed] [Google Scholar]
- 12.Molkentin JD. Black BL. Martin JF. Olson EN. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 13.Okada S. Harada A. Saiwai H. Nakamura M. Ohkawa Y. Generation of a rat monoclonal antibody specific for Brm. Hybridoma. 2009;28:455–458. doi: 10.1089/hyb.2009.0044. [DOI] [PubMed] [Google Scholar]