Abstract
Most breast cancer metastases in bone form osteolytic lesions, but the mechanisms of tumor-induced bone resorption and destruction are not fully understood. Although it is well recognized that Wnt/β-catenin signaling is important for breast cancer tumorigenesis, the role of this pathway in breast cancer bone metastasis is unclear. Dickkopf1 (Dkk1) is a secreted Wnt/β-catenin antagonist. In the present study, we demonstrated that activation of Wnt/β-catenin signaling enhanced Dkk1 expression in breast cancer cells and that Dkk1 over-expression is a frequent event in breast cancer. We also found that human breast cancer cell lines that preferentially form osteolytic bone metastases exhibited increased levels of Wnt/β-catenin signaling and Dkk1 expression. Moreover, we showed that breast cancer cell-produced Dkk1 blocked Wnt3A-induced osteoblastic differentiation and osteoprotegerin (OPG) expression of osteoblast precursor C2C12 cells, and that these effects could be neutralized by a specific anti-Dkk1 antibody. In addition, we found that breast cancer cell conditioned media were able to blocked Wnt3A-induced NF-kappaB ligand reduction in C2C12 Cells. Finally, we demonstrated that conditioned media from breast cancer cells in which Dkk1 expression had been silenced via RNAi were unable to block Wnt3A-induced C2C12 osteoblastic differentiation and OPG expression. Taken together, these results suggest that breast cancer-produced Dkk1 may be an important mechanistic link between primary breast tumors and secondary osteolytic bone metastases.
Keywords: Dkk1, Wnt signaling, osteoblast differentiation, OPG, bone metastasis
INTRODUCTION
Bone is an active tissue that is maintained by a balance of cellular activities carried out by specialized cell types. The osteoblasts are responsible for bone formation. Osteoblasts synthesize and secrete most proteins of the bone extracellular matrix (ECM) and express proteins that are both necessary and sufficient to induce mineralization of this specialized ECM. The osteoclasts are multinucleated cells responsible for bone resorption. Importantly, the differentiation of osteoclasts is regulated by osteoblasts.1 The receptor activator of NF-kappaB ligand (RANKL) is expressed by osteoblastic cells and promotes osteoclast differentiation and activity through interaction with its cognate signaling receptor RANK on the cell surface of hematopoietic cells.2,3 This process is regulated by osteoprotegerin (OPG), a secreted decoy receptor of RANKL that binds to and inhibits the activity of RANKL. The important roles that RANKL, RANK and OPG play in the control of osteoclast formation have been firmly established.4
The Wnt/β-catenin signaling pathway is involved in various differentiation events during embryonic development and, when aberrantly activated, can lead to tumor formation.5-9 In recent years, Wnt/β-catenin signaling has been shown to play a substantial role in the control of bone mass and is involved in many disorders of bone.9 Modulation of Wnt/β-catenin signaling in mesenchymal progenitors and osteoblasts has revealed that this pathway controls osteoblast differentiation and is critical for bone homeostasis during postnatal development.10-14 The Wnt target gene OPG is of particular interest in bone metabolism, as OPG expression was found to be upregulated by Wnt/β-catenin signaling in an in vitro screen for Wnt-regulated genes in a multipotenet mesenchymal cell line.15 Moreover, cellular and molecular studies demonstrated that OPG is a direct target gene of the β-catenin-TCF complex in osteoblasts.13
Bone metastasis is a frequent complication of cancer.16-18 In the case of breast cancer, up to 70% of patients with advanced disease develop osteolytic bone metastases, which are a common cause of morbidity and sometimes mortality. Recent studies from multiple myeloma and prostate cancer have implicated an important role of Wnt/β-catenin signaling in bone metastasis from these cancers.19-27 It has been reported that myeloma cells express the Wnt/β-catenin signaling antagonist Dickkopf1 (Dkk1), and that the presence of high levels of Dkk1 correlates with focal bone lesions in patients with myeloma.19 For prostate cancer, it has been demonstrated that tumor cell-produced Wnts act in a paracrine fashion to induce osteoblastic activity in prostate cancer bone metastasis.20 Although it is well recognized that Wnt/β-catenin signaling is important for breast cancer tumorigenesis,28-39 the role of this pathway in breast cancer bone metastasis has never been studied. In this report, we studied the expression of Dkk1 in human breast cancer tissues and cultured breast cancer cells, and examined the roles of breast cancer-produced Dkk1 in osteoblastic differentiation and OPG expression. Our data suggest that Dkk1 may be a critical contributor to the process of breast cancer osteolytic bone metastasis.
MATERIALS AND METHODS
Cell Culture and Conditioned Media
MCF-7 cells, C2C12 cells, Wnt3A-secreting L cells, and control L cells were obtained from American Type Culture Collection. MDA-MB-231/bone and the parent MDA-MB-231 cells have been described before.40 Wnt3A-secreting L cells and control L cells were cultured in Dulbecco’s minimum essential medium containing 10% fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin and 350 μg/ml G418, and maintained at 37°C in humidified air containing 5% CO2. MCF-7 cells, MDA-MB-231 cells, MDA-MB-231/bone cells, and L cells were cultured in Dulbecco’s minimum essential medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Wnt3A-conditioned medium (CM) and L cell control CM were prepared according to manufacturer’s specifications. For breast cancer cell CM, MCF-7 cells, MDA-MB-231 cells or MDA-MB-231/bone cells were cultured in 15 cm dishes. After the cells reached confluence, the media were changed to fresh Dulbecco’s minimum essential medium containing 10% fetal bovine serum for 24 h. After further 48 h incubation, the media were collected, centrifuged to remove cell debris, and stored at −80°C.
Knockdown of Dkk1 Expression
A vector-based short hairpin RNA (shRNA) method was used to generate MDA-MB-231 cells with inhibited Dkk1 expression. The preparation of Dkk1 shRNA and control vectors has been described before.20 Dkk1 shRNA and control were transfected into human MDA-MB-231 breast cancer cells using FuGENE 6 (Roche) according to manufacturer’s specifications. Individual clones were selected with 100 μg/ml of Zeocin (Invitrogen). The Dkk1 levels in cell lysates and cell culture CM were determined by Western blotting using a specific Dkk1 antibody.
Real-time RT-PCR
TissueScan Breast Cancer Tissue qPCR Array I (BCRT501) was purchased from Origene. The product contains first-strand cDNAs prepared from 48 human breast tissues including both malignant and normal controls. These 48 cDNAs have been normalized against β-actin by RT-PCR, and arrayed onto PCR plates. Human Dkk1 real-time primer set (PPG01752B) was from SuperArray. Dkk1 expression was quantitatively measured by real-time PCR using SYBR Green (Invitrogen) in a total volume of 30 μl over 42 two-step cycles using the following temperature protocol: 95°C for 15 s and 55°C for 60 s. For analysis of RANKL expression in C2C12 cells, total RNA was isolated using RNA-Bee reagent (Tel-Test, Inc.), first-strand cDNA synthesis was performed using ProSTARTM Ultro HF RT-PCR Kit (Strategene) primed with oligo(dT) primer in a 10 μl reaction mixture containing 0.5 μg total RNA, and real-time RT-PCR for RANKL mRNA was performed as described in.41
Western Blotting
Cells in 6-well plates were lysed in 0.5 ml of lysis buffer (phosphate-buffered saline containing 1% Triton X-100 and 1 mM PMSF) at 4°C for 30 min. Equal quantities of protein were subjected to SDS-PAGE under reducing conditions. Following the transfer to immobilon-P transfer membrane, successive incubations with either anti-Dkk1 (R&D Systems), anti-β-catenin (BD Biosciences), anti-OPG (R&D systems), anti-Axin2 (Cell Signaling), or anti-actin (Sigma), and horseradish peroxidase-conjugated secondary antibody were carried out for 60-120 min at room temperature. The immunoreactive proteins were then detected using the ECL system. Films showing immunoreactive bands were scanned by Kodak Digital Science DC120 Zoom Digital Camera and analyzed with Kodak Digital Science1D Image Analysis Software.
GST-E-cadherin Binding Assay
Plasmid pGST-E-cadherin was provided by Dr. Gail Johnson (University of Alabama at Birmingham, Alabama). The GST-E-cadherin binding assay was carried out exactly as previously described.42-44 Uncomplexed β-catenin present in 100 μg of total cell lysate was subjected to SDS-PAGE and detected using a monoclonal antibody to β-catenin.
Alkaline Phosphatase Activity Assay
C2C12 cells in 12-well plates were treated with Wnt3A CM, breast cancer cell CM, and anti-Dkk1 IgG (R&D Systems), as described in each figure legend. Cells were harvested 48 h later for assay of alkaline phosphatase (ALP) activity (Pierce) by determining the amount of p-nitrophenol synthesized from p-nitrophenylphosphate according to the manufacturer’s specifications. Cell lysates were analyzed for protein content using a Bio-Rad protein assay kit, and ALP activity was normalized to total protein content in each well.
Cell proliferation assay
Cells were seeded into 96-well tissue culture microtiter plates at a density of 5000 cells/well. After 24h incubation, cells were treated with 25% of breast cancer CM for 72 h. Cell proliferation was measured by the MTT assay kit (Promega).
RESULTS
Dkk1 Is Frequently Up-regulated in Human Breast Malignant Tissues
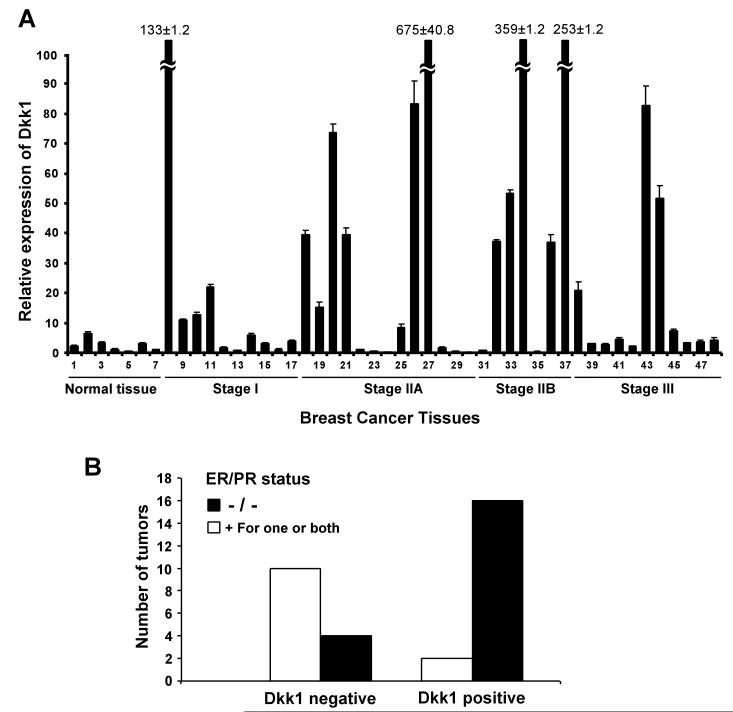
To examine whether Dkk1 over-expression is a frequent event in human breast cancer, we analyzed Dkk1 expression in breast cancer tissues by quantitative real-time RT-PCR, using a Breast Cancer TissueScan Real-Time qPCR Arrays (Origene). This array contains 7 normal control breast tissues and 41 breast cancer tissues representing different clinical stages. All the samples were from female patients with ages ranging from 31 to 75. Pathological information including hormone receptor status is provided for each sample. As seen in Fig. 1A, we found that Dkk1 expression was low in all 7 control samples, whereas about 50% of the breast cancer tissues exhibited high levels of Dkk1. It is interesting to note that high levels of Dkk1 expression were over-represented in estrogen receptor (ER)/progesterone receptor (PR)-double negative breast tumors (Fig. 1B), suggesting that Dkk1 is preferentially expressed in hormone-resistant breast tumors, which typically have poorer prognosis. Together, these results indicate that Dkk1 over-expression is a frequent event in human breast cancer.
Fig. 1.
Dkk1 is frequently up-regulated in human breast cancer. A. Breast cancer TissueScan Real-Time qPCR array was analyzed for Dkk1 expression by real-time PCR. Averages of relative Dkk1 expression from three independent plates are plotted with clinical status indicated. Four samples with extremely high Dkk1 levels are marked with numbers. B. Breast tumors with relative Dkk1 expression that is lower (Dkk1-) or higher (Dkk1+) than 10 in (A) were analyzed against estrogen receptor (ER) and progesterone receptor (PR) status. Note high levels of Dkk1 expression are over-represented in ER/PR-double negative breast tumors.
Dkk1 Is Up-regulated in Human Breast Bone Metastatic Cancer Cells
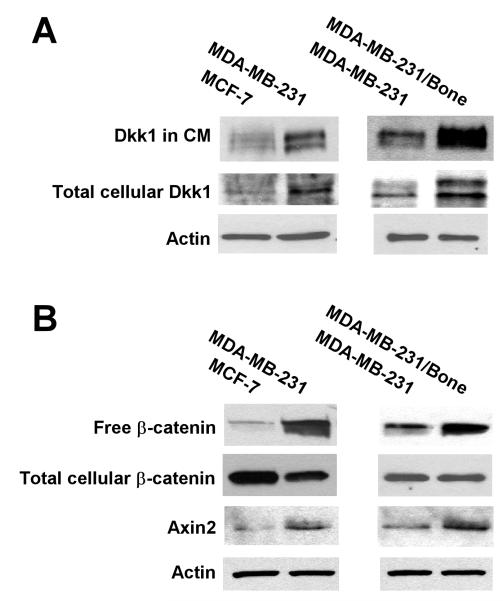
Breast cancer MDA-MB-231 cells form typical osteolytic bone metastases when inoculated into the arterial circulation of mice.40,45-47 MDA-MB-231/bone is a subpopulation of MDA-MB-231 that was isolated by in vivo selection.48 MDA-MB-231/bone cells exclusively metastasize to bone with larger osteolytic lesions than the parent MDA-MB-231 cells.48 To explore the role of Dkk1 in breast cancer bone metastases, we examined Dkk1 expression in MDA-MB-231 and MDA-MB-231/bone cells. We found that MDA-MB-231/bone cells exhibited higher levels of Dkk1 expression and Dkk1 secretion into the conditioned media than the parent MDA-MB-231 cells (Fig. 2A). Quantification of the Western blot signals revealed that the levels of Dkk1 in CM and total cellular Dkk1 in MDA-MB-231/bone cells were 4.5 and 3.1 fold higher than those in the parent MDA-MB-231 cells, respectively. MCF-7 is another breast cancer cell line that is commonly used for bone metastasis studies. However, MCF-7 cells display lower metastatic activity and form smaller bone osteolytic lesions than MDA-MB-231 cells.49-52 Interestingly, we also found that MCF-7 cells displayed lower levels of Dkk1 expression and Dkk1 secretion than MDA-MB-231 cells (Fig. 2A). Quantification of the Western blot signals revealed that the levels of Dkk1 in CM and total cellular Dkk1 in MDA-MB-231 cells were 3.3 and 2.7 fold higher than those in MCF-7 cells, respectively. Together, our results suggest that breast cancer cells with high levels of metastatic activity exhibit high levels of Dkk1 expression and secretion.
Fig. 2.
Wnt/β-catenin signaling activation and Dkk1 expression in human breast cancer cells in culture. A. Wnt/β-catenin signaling in human breast cancer cells. Cytosolic free β-catenin from MCF-7, MDA-MB-231 and MDA-MB-231/bone cells was pulled down from 100 μg of cell lysate using pGST-E-Cadherin, and then examined by Western blotting with a specific β-catenin antibody. The levels of total cellular β-catenin and Axin2 were analyzed by Western blotting with a specific β-catenin antibody or Axin2 antibody, and the samples were also probed with an anti-actin antibody to verify equal loading. B. Dkk1 expression in human breast cancer cells. The level of total cellular Dkk1 from MCF-7, MDA-MB-231 and MDA-MB-231/bone cells were analyzed by Western blotting with a specific Dkk1 antibody, and the samples were also probed with an anti-actin antibody to verify equal loading. Dkk1 in 24h serum free conditioned media (CM) was normalized by protein concentrations, and examined by Western blotting.
Induction of Dkk1 Expression by Activation of Wnt/β-catenin signaling in Breast Cancer Cells
It has been recently demonstrated that Dkk1 is a direct downstream target of Wnt/β-catenin signaling in several cell line models.53-55 Wnt/β-catenin signaling is overactivated in breast cancer.28-39 At the heart of the Wnt/β-catenin pathway is the stabilization of cytosolic β-catenin, which binds to transcription factors of the T-cell factor/lymphoid enhancing factor (TCF/LEF) family, leading to the transcription of Wnt/β-catenin target genes. Using the GST-E-cadherin binding assay and subsequent Western blotting,42-44 we examined cytosolic free β-catenin levels as a measure of Wnt/β-catenin signaling activation. We found that MDA-MB-231/bone cells exhibited the highest level of uncomplexed cytosolic β-catenin (free β-catenin), while MCF-7 cells displayed the lowest level of free β-catenin (Fig. 2B). Quantification of the Western blot signals revealed that the levels of free β-catenin in MDA-MB-231/bone cells were 31 and 4.4 fold higher than those in MCF-7 and MDA-MB-231 cells, respectively.
Axin2 is a specific transcriptional target of the Wnt/β-catenin signaling pathway. It is well recognized that the expression level of Axin2 is a signature of the activation of the Wnt/β-catenin signaling pathway.56-59 To further examine the activation of Wnt/β-catenin signaling in breast cancer cells, we studied Axin2 expression by Western blotting. As expected, MDA-MB-231/bone cells exhibited the highest level of Axin2 expression, while MCF-7 cells displayed the lowest level of Axin2 expression (Fig. 2B). Quantification of the Western blot signals revealed that the levels of Axin2 in MDA-MB-231/bone cells were 6.5 and 3.2 fold higher than those in MCF-7 and MDA-MB-231 cells, respectively.
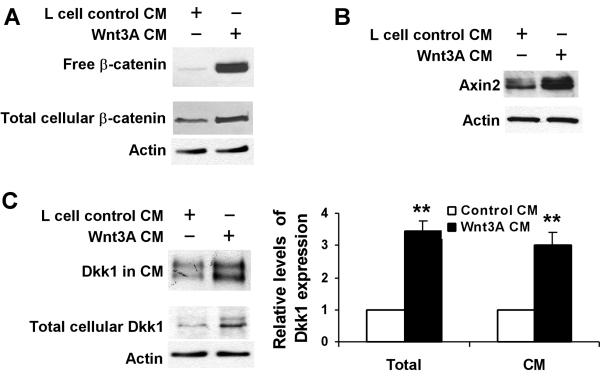
Previous studies have shown that Wnt3A is a canonical Wnt ligand that binds to the low density lipoprotein receptor-related proteins (LRP) and frizzled receptors, leading to the activation of Wnt/β-catenin signaling.60 To confirm that Dkk1 expression is upregulated via Wnt/β-catenin signaling in human breast cancer cells, we treated MDA-MB-231 cells with either L cell Wnt3A CM or control CM. As shown in Fig. 3A &3B, treatment of MDA-MB-231 cells with Wnt3A CM significantly increased free β-catenin level and Axin2 expression. Quantification of the Western blot signals of free β-catenin and Axin2 revealed 18 and 3.9 fold increases when compared to control cells, respectively. Consistent with the activation of Wnt/β-catenin signaling by Wnt3A, the levels of endogenous Dkk1 in total cellular lysates and secreted into the conditioned media were significantly increased (Fig. 3C).
Fig. 3.
Induction of Dkk1 expression by Wnt3A in MDA-MB-231 cells. A. Wnt3A stabilized cytosolic free β-catenin in MDA-MB-231 cells. MDA-MB-231 cells in 6 well plates were incubated with 25% of either L cell control CM or Wnt3A CM for 3h. The level of total cellular β-catenin was analyzed by Western blotting, and the samples were also probed with an anti-actin antibody to verify equal loading. Cytosolic free β-catenin was pulled down from 100 μg of cell lysate using pGST-E-Cadherin, and then examined by Western blotting. B. Wnt3A induced Axin2 expression in MDA-MB-231 cells. MDA-MB-231 cells in 6 well plates were incubated with 25% of either L cell control CM or Wnt3A CM for 48h. The level of total cellular Axin2 was analyzed by Western blotting. C. Wnt3A induced Dkk1 expression and secretion in MDA-MB-231 cells. MDA-MB-231 cells in 6 well plates were incubated with 25% of either L cell control CM or Wnt3A CM. After 36h incubation, the cells were cultured in serum free medium for 8h. The level of total cellular Dkk1 was analyzed by Western blotting. Level of Dkk1 in 8h serum free CM was normalized by protein concentrations, and then analyzed by Western blotting. Right panel, quantification of the Western blot signals of Dkk1 in CM and total cellular Dkk1 expression, which was normalized to the actin level, from three determinations. **P<0.01 indicates a significant difference compared to cells incubated with L cell control CM.
Effects of Breast Cancer Cell CM on C2C12 Cell Proliferation
C2C12 cells are uncommitted mesenchymal progenitor cells that can be differentiated into osteoblasts upon activation of Wnt/β-catenin signaling.19 We employed C2C12 cells to examine the effects of breast cancer-produced Dkk1 on osteoblast proliferation, differentiation and function. As shown in Fig. 4, breast cancer cell CM slightly decreased the growth of C2C12 cells at 72 h treatment. Furthermore, there was no significant difference of C2C12 cell proliferation after the cells were treated with different breast cancer cell conditioned media.
Fig. 4.
Effects of breast cancer cell CM on C2C12 cell proliferation. C2C12 cells in 96-well plates were treated with 25% of breast cancer CM for 72 h, and cell growth was assessed using the MTT assay. Values are the average of triple determinations with the s.d. indicated by error bars.
Breast Cancer Cell-produced Dkk1 Blocks Wnt3A-induced Osteoblastic Differentiation of C2C12 Cells
Wnt3A can induce differentiation of uncommitted mesenchymal progenitor-cells into osteoblasts through the activation of Wnt/β-catenin signaling.19 Wnt3A are expressed in osteoblasts and several breast cancer cell lines.14,43 To determine whether breast cancer cell-produced Dkk1 affects Wnt3A-induced osteoblastic differentiation, we examined the activity of alkaline phosphatase (ALP), a specific marker of osteoblast differentiation, in C2C12 cells. It was found that only small amounts of ALP were detectable in C2C12 cells without Wnt3A stimulation, and that breast cancer cell CM alone had no effects on basal level of ALP activity in C2C12 cells (Fig. 5A). On the other hand, treatment of C2C12 cells with media containing Wnt3A CM for two days greatly induced ALP expression, which was blocked by recombinant Dkk1 protein (Fig. 5A). Interestingly, conditioned media from MDA-MB-231 cells or MDA-MB-231/bone cells, but not from MCF-7 cells, inhibited the Wnt3A-induced ALP production in C2C12 cells (Fig. 5B). More importantly, this effect on ALP production was neutralized by a polyclonal anti-Dkk1 antibody but not by a nonspecific polyclonal goat IgG (Fig. 5C & 5D).
Fig. 5.
Breast cancer cell-produced Dkk1 blocks Wnt3A-induced osteoblastic differentiation of C2C12 cells. A. Dkk1 blocked Wnt3A-induced ALP in C2C12 cells. C2C12 cells in 12-well plates were incubated breast cancer CM (25%), Wnt3A (20%) and Dkk1 (300 ng/ml) as indicated for 48 h. ALP activity was measured as described under “Materials and Methods”. B. Conditioned media from breast cancer cells blocked Wnt3A-induced ALP in C2C12 cells. C2C12 cells in 12 well plates were incubated with Wnt3A CM in the presence or absence breast cancer CM as indicated. Cells were harvested 48h later and assayed for ALP activity. C-D. Anti-Dkk1 antibody reversed the effect of breast cancer cell CM on Wnt3A-induced ALP production in C2C12 cells. C2C12 cells in 12 well plates were incubated with Wnt3A CM, breast cancer CM and a polyclonal anti-Dkk1 antibody as indicated. Cells were harvested 48h later for assay for ALP activity. In (C), MDA-MB-231 CM was used. In (D), MDA-MB-231/bone CM was used. All the values are the average of triple determinations with the s.d. indicated by error bars. *P<0.05 indicates a significant difference compared to cells incubated with Wnt3A CM along with L cell control CM. **P<0.01 indicates a significant difference compared to cells incubated with Wnt3A CM along with L cell control CM, or cells incubated with Wnt3A CM along with breast cancer CM and anti-Dkk1 antibody.
Breast Cancer Cell-produced Dkk1 Blocks Wnt3A-induced OPG Expression in C2C12 Cells
Recent studies have demonstrated that OPG is a direct target gene of Wnt/β-catenin signaling in osteoblasts, and that Wnt/β-catenin signaling controls bone resorption by directly regulating RANKL/RANK/OPG signaling activity.10-14 To determine whether breast cancer cell-produced Dkk1 affects Wnt3A-induced OPG expression in osteoblasts, we examined OPG expression in C2C12 cells. It was found that OPG was nearly undetectable in C2C12 cells without Wnt3A stimulation, and that breast cancer cell CM alone had no significant effect on basal level of OPG expression in C2C12 cells (Fig. 6A). On the other hand, treatment of C2C12 cells with Wnt3A CM for two days greatly induced OPG expression, which was completely blocked by recombinant Dkk1 protein (Fig. 6A). As expected, conditioned medium from MDA-MB-231 cells or MDA-MB-231/bone cells, but not from MCF-7 cells, inhibited Wnt3A-induced OPG expression in C2C12 cells (Fig. 6B). Quantification of the Western blot signals revealed that OPG expression was reduced to 25 and 12% after C2C12 cells were treated with MDA-MB-231 CM and MDA-MB-231 CM, respectively. Furthermore, this effect on OPG expression was neutralized by a polyclonal anti-Dkk1 antibody but not by a nonspecific polyclonal goat IgG (Fig. 6C).
Fig. 6.
Breast cancer cell-produced Dkk1 blocked Wnt3A-induced OPG expression in C2C12 Cells. A. C2C12 cells in 6-well plates were incubated with breast cancer CM (25%), Wnt3A (20%) and Dkk1 (300 ng/ml) as indicated for 48 h, and OPG expression was examined by Western blotting. B. Conditioned media from breast cancer cells block Wnt3A-induced OPG expression in C2C12 cells. C2C12 cells in 6 well plates were incubated with Wnt3A CM in the presence or absence breast cancer CM as indicated. Cells were harvested 48h later for Western blotting of OPG expression. C. Anti-Dkk1 antibody reversed the effect of breast cancer cell CM on Wnt3A-induced OPG expression in C2C12 cells. C2C12 cells in 6 well plates were incubated with Wnt3A CM, breast cancer CM and a polyclonal anti-Dkk1 antibody as indicated. Cells were harvested 48h later for Western blotting for OPG with a specific anti-OPG antibody. Right panel, quantification of the Western blot signals of OPG expression, which was normalized to actin level, from three independent experiments. *P<0.05 indicates a significant difference compared to cells incubated with Wnt3A CM along with L cell control CM, or cells incubated with Wnt3A CM along with breast cancer CM and anti-Dkk1 antibody.
Breast Cancer Cell CM Blocked Wnt3A-induced RANKL Reduction in C2C12 Cells
Recent studies have demonstrated that expression of RANKL, another key player of the RANK/RANKL/OPG signaling pathway, is also regulated by Wnt/β-catenin signaling in osteoblasts.12,14,41 To determine whether breast cancer cell CM affects RANKL expression in osteoblasts, we examined RANKL mRNA by real-time RT-PCR in C2C12 cells. As shown in Fig. 7A, breast cancer cell CM alone had no significant effect on basal level of RANKL expression in C2C12 cells. Treatment of C2C12 cells with Wnt3A CM for three days resulted in a significant decrease in RANKL expression, which was blocked by recombinant Dkk1 protein (Fig. 7A). Importantly, conditioned media from MDA-MB-231/bone cells were also able to block the Wnt3A-induced RANKL reduction in C2C12 cells, although conditioned media from MDA-MB-231 cells only partially blocked the Wnt3A-induced RANKL reduction (Fig. 7B).
Fig. 7.
Breast cancer cell CM blocked Wnt3A-induced RANKL reduction in C2C12 cells. C2C12 cells in 6-well plates were incubated with breast cancer CM (25%), Wnt3A (20%) and Dkk1 (300 ng/ml) as indicated for 72 h. Cells were harvested and RANKL mRNA was determined by real-time RT-PCR. All the values are the average of triple determinations with the s.d. indicated by error bars. *P<0.05 indicates a significant difference compared to cells incubated with Wnt3A CM plus Dkk1, or cells without Wnt3A treatment. #P<0.05 indicates a significant difference compared to cells incubated Wnt3A CM along with L cell control CM.
MDA-MB-231 Cells with Dkk1 Knockdown Are Unable to Block Wnt3A-induced C2C12 Cell Osteoblastic Differentiation and OPG Expression
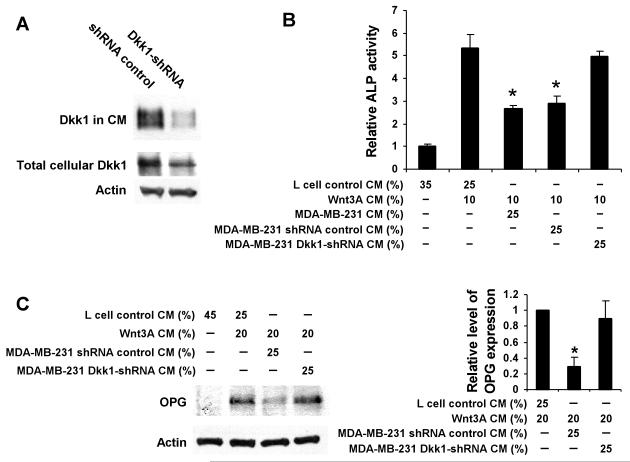
To further define the roles of breast cancer-produced Dkk1 in osteoblast differentiation and OPG expression, we stably expressed a Dkk1 shRNA20 in MDA-MB-231 cells. Fig. 8A shows that a single MDA-MB-231 clone stably transfected with Dkk1-shRNA exhibited significant down-regulation of the Dkk1 protein in the conditioned media. Quantification of the Western blot signals revealed that Dkk1 in CM and total cellular Dkk1 in MDA-MB-231 Dkk1-shRNA cells were reduced to 8 and 17% than those in control cells, respectively. Moreover, conditioned media from MDA-MB-231 Dkk1-shRNA cells failed to block Wnt3A-induced ALP production (Fig. 8B) and OPG expression (Fig. 8C). Taken together, these results show that reducing the expression of the Wnt/β-catenin signaling inhibitor Dkk1 unmasked an osteoinductive effect in osteolytic MDA-MB-231 cells.
Fig. 8.
MDA-MB-231 cells with Dkk1 knockdown were unable to block Wnt3A-induced osteoblastic differentiation and OPG expression. A. Reduction of Dkk1 protein expression in MDA-MB-231 cells by shRNA. MDA-MB-231 cells stably transfected with Dkk1-shRNA expression vector or control shRNA vector were cultured in 6 well plates. Cell lysates and 24h serum free CM were prepared. The level of total cellular Dkk1 was analyzed by Western blotting, and the samples were also probed with an anti-actin antibody to verify equal loading. Level of Dkk1 in 24h serum free CM was normalized by protein concentrations, and then analyzed by Western blotting. B. MDA-MB-231 cells with Dkk1 knockdown were unable to block Wnt3A-induced C2C12 cell osteoblastic differentiation. C2C12 cells in 12 well plates were incubated with Wnt3A CM in the presence or absence breast cancer CM as indicated. Cells were harvested 48h later for assay for ALP activity. Values are the average of triple determinations with the s.d. indicated by error bars. *P<0.01 indicates a significant difference compared to cells incubated with Wnt3A CM along with L cell control CM, or cells incubated with Wnt3A CM along with CM from breast cancer MDA-MB-231 Dkk1-shRNA. C. MDA-MB-231 cells with Dkk1 knockdown were unable to block Wnt3A-induced OPG expression in C2C12 cells. C2C12 cells in 6 well plates were incubated with Wnt3A CM in the presence or absence breast cancer CM as indicated. Cells were harvested 48h later for Western blotting for OPG with a specific anti-OPG antibody. Right panel, quantification of the Western blot signals of OPG expression, which was normalized to the actin level, from three independent experiments. *P<0.05 indicates a significant difference compared to cells incubated to Wnt3A CM along with L cell control CM, or cells incubated with Wnt3A CM along with breast cancer CM and anti-Dkk1 antibody.
DISCUSSION
Dkk1 is a secreted protein that negatively modulates the Wnt/β-catenin pathway. In contrast to other Wnt/β-catenin signaling antagonists, Dkk1 is overexpressed in many malignant tissues including breast cancer,61 lung cancer,62 esophageal carcinomas,62 multiple myeloma,19 ovarian endometrioid adenocarcinomas,55 hepatoblastomas and Wilms’ tumors.63 In the case of breast cancer, it has been reported that Dkk1 is preferentially expressed in ER and PR-negative tumors and in tumors from women with a family history of breast cancer.61 Furthermore, Dkk1 is a potential prognostic and diagnostic marker for cohorts of breast cancer patients with poor prognosis.61 In the present study, by using a Breast Cancer TissueScan Real-Time qPCR Arrays, we also found that ~50% of the breast cancer tissues exhibited high levels of Dkk1, and that high levels of Dkk1 expression were over-represented in ER/PR-double negative breast tumors. All together, these studies suggest that Dkk1 is frequently overexpressed in breast malignant tissues.
Recent studies have demonstrated that Dkk1 is not only a key inhibitor but also a direct downstream target of Wnt/β-catenin signaling. Activation of Wnt/β-catenin signaling by Wnt1 or ectopic expression of active β-catenin, TCF4 or LRP6 mutants induces transcription of the human Dkk1 gene in several cell line models in vitro.53-55 Multiple β-catenin/TCF4 binding sites in the Dkk1 gene promoter region allow for this activation.53-55 In the present study, we demonstrate that Wnt3A activates Wnt/β-catenin signaling and enhances Dkk1 expression in breast cancer MDA-MB-231 cells. Although genetic mutations of APC or β-catenin are rarely observed in breast cancer, compelling evidence has implicated abnormal regulation of Wnt/β-catenin signaling in tumorigenic program of breast cancer. For example, Wnt1, the founding member of the Wnt gene family, was initially identified as a mammary oncogene insertionally activated by mouse mammary tumor virus.28-30 Overexpression of several Wnts has been reported in breast cancer.31-33,39 Secreted Frizzled-related protein1 (sFRP1), a member of the secreted Wnt antagonist family, is down-regulated in breast cancers.34 Up-regulation of β-catenin mRNA levels was detected by microarray analysis in human breast cancer.35 More importantly, it has been reported that β-catenin protein levels are significantly upregulated in human breast cancer tissues and correlate with poor prognosis, acting as a strong and independent prognostic factor in human breast cancer patients.36-38 Thus, Dkk1 up-regulation is likely a consequence of overactivation of Wnt/β-catenin signaling in human breast cancer. Further studies will be required to define whether Dkk1 expression is correlated with the activation of Wnt/β-catenin signaling in human breast cancer tissues. As Dkk1 is a major antagonist of Wnt/β-catenin signaling, it will be also interesting to explore the mechanism employed by human breast cancer cells that are able to escape Dkk1 inhibition.
Studies in the past several years have established that Wnt/β-catenin signaling plays a critical role in the regulation of bone mass and is a causative factor for many disorders of the bone. Osteoblast differentiation is the primary event of bone formation, characterized by the synthesis, deposition and mineralization of the extracellular matrix. One of the mechanisms whereby Wnt/β-catenin signaling increases bone formation is via stimulation of the development of osteoblasts.9 In the present study, we demonstrate that human breast cancer cells with a predisposition toward the formation of osteolytic bone metastases exhibit increased levels of Dkk1 expression, and that breast cancer cell-produced Dkk1 inhibits the Wnt3A-induced osteoblastic differentiation of osteoblast precursor C2C12 cells. These results suggest that breast cancer-produced Dkk1 is involved in breast cancer-derived osteolytic metastases.
It has been demonstrated that Wnt/β-catenin signaling in osteoblasts is able to coordinate postnatal bone acquisition by controlling the differentiation and activity of osteoclasts. OPG is a direct target gene of the β-catenin-TCF complex in osteoblasts,13,15 and acts as a decoy receptor that blocks the binding of RANKL to its cognate signaling receptor RANK on hematopoietic cells, thereby inhibiting osteoclast formation and activity.2-4 In the present study, we found that breast cancer cell-produced Dkk1 inhibited Wnt3A-induced OPG expression and RANKL reduction in osteoblast precursor C2C12 cells, strengthening the notion that breast cancer-produced Dkk1 could be a critical modulator for breast cancer osteolytic metastases. In the future, we should examine whether Dkk1 is frequently up-regulated in human breast cancer patient serums and malignant tissues with osteolytic bone metastases.
In summary, we propose that Dkk1 expression in breast cancer cells contributes to the development and progression of breast osteolytic bone metastases. Breast cancer cells with overactivated Wnt/β-catenin signaling produce high levels of Dkk1, which blocks osteoblast differentiation, OPG expression and RANKL reduction, and consequently stimulates osteoclastic bone resorption. Thus, Dkk1 is a potential therapeutic target in designing pharmacologic interventions for bone metastases in breast cancer.
Novelty and impact: the present study demonstrates for the first time that breast cancer-produced Dkk1 could be a critical modulator of breast cancer osteolytic metastases.
Acknowledgments
This work was supported in part by a grant from the American Heart Association (0330118N) to Y. L. and grants from the National Institutes of Health to E.T.K. (P01 CA093900) and to G.B. (RO1 CA100520). We are grateful to Dr. Gail Johnson for providing the cDNA for E-cadherin and Dr. Jane Knisely for critical reading of the manuscript.
Abbreviations
- ALP
alkaline phosphatase
- CM
conditioned medium
- Dkk1
Dickkopf1
- ECG
extracellular matrix
- ER
oestrogen receptor
- Fz
frizzled
- HBM
high bone mass
- LRP
the low density lipoprotein receptor-related protein
- OPG
osteoprotegerin
- PR
progesterone receptor
- RANKL
receptor activator of NF-kappaB ligand
- shRNA
short hairpin RNA
- sFRP1
secreted Frizzled-related protein1
- TCF/LEF
T-cell factor/lymphoid enhancing factor
REFERENCES
- 1.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 2.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 3.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 6.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 7.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development. 2004;131:1663–77. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 8.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and β-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–9. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–50. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–38. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL, Williams BO. Essential role of β-catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–8. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 13.Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–64. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006;119:1283–96. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- 15.Jackson A, Vayssière B, Garcia T, Newell W, Baron R, Roman-Roman S, Rawadi G. Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone. 2005;36:585–98. doi: 10.1016/j.bone.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 17.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 18.Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10:169–80. doi: 10.1007/s10911-005-5399-8. [DOI] [PubMed] [Google Scholar]
- 19.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–94. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 20.Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65:7554–60. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- 21.Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–91. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 22.Politou MC, Heath DJ, Rahemtulla A, Szydlo R, Anagnostopoulos A, Dimopoulos MA, Croucher PI, Terpos E. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer. 2006;119:1728–31. doi: 10.1002/ijc.22033. [DOI] [PubMed] [Google Scholar]
- 23.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–11. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clines GA, Mohammad KS, Bao Y, Stephens OW, Suva LJ, Shaughnessy JD, Jr, Fox JW, Chirgwin JM, Guise TA. Dickkopf homolog 1 mediates endothelin-1-stimulated new bone formation. Mol Endocrinol. 2007;21:486–98. doi: 10.1210/me.2006-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terpos E, Heath DJ, Rahemtulla A, Zervas K, Chantry A, Anagnostopoulos A, Pouli A, Katodritou E, Verrou E, Vervessou EC, Dimopoulos MA, Croucher PI. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol. 2006;135:688–92. doi: 10.1111/j.1365-2141.2006.06356.x. [DOI] [PubMed] [Google Scholar]
- 26.Colla S, Zhan F, Xiong W, Wu X, Xu H, Stephens O, Yaccoby S, Epstein J, Barlogie B, Shaughnessy JD., Jr. The oxidative stress response regulates DKK1 expression through the JNK signaling cascade in multiple myeloma plasma cells. Blood. 2007;109:4470–7. doi: 10.1182/blood-2006-11-056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwaninger R, Rentsch CA, Wetterwald A, van der Horst G, van Bezooijen RL, van der Pluijm G, Lowik CW, Ackermann K, Pyerin W, Hamdy FC, Thalmann GN, Cecchini MG. Lack of noggin expression by cancer cells is a determinant of the osteoblast response in bone metastases. Am J Pathol. 2007;170:160–75. doi: 10.2353/ajpath.2007.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 29.Peters G, Brookes S, Smith R, Dickson C. Tumorigenesis by mouse mammary tumor virus: evidence for a common region for provirus integration in mammary tumors. Cell. 1983;33:369–77. doi: 10.1016/0092-8674(83)90418-x. [DOI] [PubMed] [Google Scholar]
- 30.Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984;307:131–6. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- 31.Dale TC, Weber-Hall SJ, Smith K, Huguet EL, Jayatilake H, Gusterson BA, Shuttleworth G, O’Hare M, Harris AL. Compartment switching of WNT-2 expression in human breast tumors. Cancer Res. 1996;56:4320–3. [PubMed] [Google Scholar]
- 32.Huguet EL, McMahon JA, McMahon AP, Bicknell R, Harris AL. Differential expression of human Wnt genes 2, 3, 4, and 7B in human breast cell lines and normal and disease states of human breast tissue. Cancer Res. 1994;54:2615–21. [PubMed] [Google Scholar]
- 33.Bui TD, Zhang L, Rees MC, Bicknell R, Harris AL. Expression and hormone regulation of Wnt2, 3, 4, 5a, 7a, 7b and 10b in normal human endometrium and endometrial carcinoma. Br J Cancer. 1997;75:1131–6. doi: 10.1038/bjc.1997.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ugolini F, Charafe-Jauffret E, Bardou VJ, Geneix J, Adélaïde J, Labat-Moleur F, Penault-Llorca F, Longy M, Jacquemier J, Birnbaum D, Pébusque MJ. WNT pathway and mammary carcinogenesis: loss of expression of candidate tumor suppressor gene SFRP1 in most invasive carcinomas except of the medullary type. Oncogene. 2001;20:5810–7. doi: 10.1038/sj.onc.1204706. [DOI] [PubMed] [Google Scholar]
- 35.Roh MS, Hong SH, Jeong JS, Kwon HC, Kim MC, Cho SH, Yoon JH, Hwang TH. Gene expression profiling of breast cancers with emphasis of β-catenin regulation. J Korean Med Sci. 2004;19:275–82. doi: 10.3346/jkms.2004.19.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC. β-Catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA. 2000;97:4262–6. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim SC, Lee MS. Significance of E-cadherin/β-catenin complex and cyclin D1 in breast cancer. Oncol Rep. 2002;9:915–28. [PubMed] [Google Scholar]
- 38.Chung GG, Zerkowski MP, Ocal IT, Dolled-Filhart M, Kang JY, Psyrri A, Camp RL, Rimm DL. β-Catenin and p53 analyses of a breast carcinoma tissue microarray. Cancer. 2004;100:2084–92. doi: 10.1002/cncr.20232. [DOI] [PubMed] [Google Scholar]
- 39.Ayyanan A, Civenni G, Ciarloni L, Morel C, Mueller N, Lefort K, Mandinova A, Raffoul W, Fiche M, Dotto GP, Brisken C. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl. Acad Sci USA. 2006;103:3799–804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mbalaviele G, Dunstan CR, Sasaki A, Williams PJ, Mundy GR, Yoneda T. E-cadherin expression in human breast cancer cells suppresses the development of osteolytic bone metastases in an experimental metastasis model. Cancer Res. 1996;56:4063–70. [PubMed] [Google Scholar]
- 41.Fujita K, Janz S. Attenuation of WNT signaling by DKK-1 and -2 regulates BMP2-induced osteoblast differentiation and expression of OPG, RANKL and M-CSF. Mol Cancer. 2007;6:71. doi: 10.1186/1476-4598-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bafico A, Gazit A, Wu-Morgan SS, Yaniv A, Aaronson SA. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene. 1998;16:2819–25. doi: 10.1038/sj.onc.1201797. [DOI] [PubMed] [Google Scholar]
- 43.Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 44.Mi K, Johnson GV. Role of the intracellular domains of LRP5 and LRP6 in activating the Wnt canonical pathway. J Cell Biochem. 2005;95:328–38. doi: 10.1002/jcb.20400. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki A, Boyce BF, Story B, Wright KR, Chapman M, Boyce R, Mundy GR, Yoneda T. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Res. 1995;55:3551–7. [PubMed] [Google Scholar]
- 46.Yoneda T, Sasaki A, Dunstan C, Williams PJ, Bauss F, De Clerck YA, Mundy GR. Inhibition of osteolytic bone metastasis of breast cancer by combined treatment with the bisphosphonate ibandronate and tissue inhibitor of the matrix metalloproteinase-2. J Clin Invest. 1997;99:2509–17. doi: 10.1172/JCI119435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, Guise TA, Massagué J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–49. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 48.Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res. 2001;16:1486–95. doi: 10.1359/jbmr.2001.16.8.1486. [DOI] [PubMed] [Google Scholar]
- 49.Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, Gillespie MT. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999;140:4451–8. doi: 10.1210/endo.140.10.7037. [DOI] [PubMed] [Google Scholar]
- 50.Silva J, Beckedorf A, Bieberich E. Osteoblast-derived oxysterol is a migration-inducing factor for human breast cancer cells. J Biol Chem. 2003;278:25376–85. doi: 10.1074/jbc.M301233200. [DOI] [PubMed] [Google Scholar]
- 51.Bendre MS, Margulies AG, Walser B, Akel NS, Bhattacharrya S, Skinner RA, Swain F, Ramani V, Mohammad KS, Wessner LL, Martinez A, Guise TA, et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res. 2005;65:11001–9. doi: 10.1158/0008-5472.CAN-05-2630. [DOI] [PubMed] [Google Scholar]
- 52.Fisher JL, Thomas-Mudge RJ, Elliott J, Hards DK, Sims NA, Slavin J, Martin TJ, Gillespie MT. Osteoprotegerin overexpression by breast cancer cells enhances orthotopic and osseous tumor growth and contrasts with that delivered therapeutically. Cancer Res. 2006;66:3620–8. doi: 10.1158/0008-5472.CAN-05-3119. [DOI] [PubMed] [Google Scholar]
- 53.Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the β-catenin/TCF pathway. Oncogene. 2004;23:8520–6. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- 54.González-Sancho JM, Aguilera O, García JM, Pendás-Franco N, Peña C, Cal S, García de Herreros A, Bonilla F, Muñoz A. The Wnt antagonist DICKKOPF-1 gene is a downstream target of β-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005;24:1098–103. doi: 10.1038/sj.onc.1208303. [DOI] [PubMed] [Google Scholar]
- 55.Chamorro MN, Schwartz DR, Vonica A, Brivanlou AH, Cho KR, Varmus HE. FGF-20 and DKK1 are transcriptional targets of β-catenin and FGF-20 is implicated in cancer and development. EMBO J. 2005;24:73–84. doi: 10.1038/sj.emboj.7600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, Sakamoto D, Caothien RH, Fuller JH, Reinhard C, Garcia PD, Randazzo FM, et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/β-catenin signaling is activated in human colon tumors. Proc Natl Acad Sci USA. 2001;98:14973–8. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–93. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Activation of AXIN2 expression by β-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–65. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- 60.Liu G, Bafico A, Harris VK, Aaronson SA. A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Mol Cell Biol. 2003;23:5825–35. doi: 10.1128/MCB.23.16.5825-5835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forget MA, Turcotte S, Beauseigle D, Godin-Ethier J, Pelletier S, Martin J, Tanguay S, Lapointe R. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br J Cancer. 2007;96:646–53. doi: 10.1038/sj.bjc.6603579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamabuki T, Takano A, Hayama S, Ishikawa N, Kato T, Miyamoto M, Ito T, Ito H, Miyagi Y, Nakayama H, Fujita M, Hosokawa M, et al. Dikkopf-1 as a novel serologic and prognostic biomarker for lung and esophageal carcinomas. Cancer Res. 2007;67:2517–25. doi: 10.1158/0008-5472.CAN-06-3369. [DOI] [PubMed] [Google Scholar]
- 63.Wirths O, Waha A, Weggen S, Schirmacher P, Kühne T, Goodyer CG, Albrecht S, Von Schweinitz D, Pietsch T. Overexpression of human Dickkopf-1, an antagonist of wingless/WNT signaling, in human hepatoblastomas and Wilms tumors. Lab Invest. 2003;83:429–34. doi: 10.1097/01.lab.0000059926.66359.bd. [DOI] [PubMed] [Google Scholar]