Abstract
Background
Pasireotide (SOM230), a long acting somatostatin analogue (LAR) has improved agonist activity at somatostatin receptors. We tested the effect of SOM230 on insulin secretion, glucose levels, tumor growth, and survival using an MEN1 transgenic mouse model.
Methods
Eight 12 month-old conditional Men1 knockout mice with insulinoma were assessed. The treatment (N=4) and control groups (N=4) received monthly subcutaneous injections of SOM230 or PBS. Serum insulin and glucose levels were determined by ELISA and enzymatic colorimetric assay, respectively. Tumor activity, growth, and apoptosis were determined by microPET/CT scan and histological analysis.
Results
On day 7, there was a significant decrease in serum insulin from 1.060μg/L±0.2769 to 0.3653μg/L±0.1676 (p=0.0128) and a significant increase in serum glucose from 4.246mmol/L±0.4536 to 7.122mmol/L±1.058 (p=0.0075) in the treatment group, but no change in the control group. Tumor size was significantly smaller in the treatment group (2098μm2±388) compared with the control group (7067μm2±955) (p=0.0024). Furthermore, apoptosis was significantly increased in the treatment group (6.92%±1.23) compared with the control group (0.299%±0.103).
Conclusions
SOM230 demonstrates antisecretory, antiproliferative, and proapoptotic activity in our MEN1 model of insulinoma. Further studies of the effects of SOM230 in PNET patients with MEN1 mutations are warranted.
Keywords: SOM230, PNETs, Apoptosis, conditional knockout mice
BACKGROUND
The incidence of pancreatic neuroendocrine tumors (PNETs) in the general population is between 1.4 – 3.1% 1. However, this may be an underestimate as many databases do not include benign PNETs, which may or may not produce symptoms, and autopsy reports demonstrate an incidence as high as 10%2. A retrospective epidemiological study of neuroendocrine tumors, using the SEER database, demonstrated a 5-fold increase in the annual age-adjusted incidence rate between the years 1973-20043. Within the neuroendocrine tumors, PNETs have an annual age-adjusted incidence rate of 0.32/100,000 from 2000 to 2004 with a median survival of 136 months for localized PNETs, 77 months for regional PNETs, and 24 months for metastatic PNETs3. PNETs can be classified by hormonal production, with insulinomas being the most common (17%), followed by gastrinomas (15%), VIPomas (2%), and glucagonomas (1%) while the remaining 65% have no detectable hormonal production4.
The treatment of insulinomas can be divided into two complementary therapeutic paradigms: surgical therapy and/or medical therapy. Surgical therapy is a curable treatment for insulinomas if and only if the neoplasm can be precisely pin-pointed (via modalities such as: helical CT, endoscopic ultrasonography, laprascopic ultrasound [intraoperative], as well as calcium stimulated arteriography), enucleated or excised, and metastases can likewise be resected. If metastases develop or surgical resection is incomplete, medical therapy is utilized to diminish symptoms. Patients with unresectable metastases are educated to prevent episodes of hypoglycemia by avoiding extended periods of fasting by encouraging frequent snacking5. In conjunction with dietary modification, specific medical therapy is initiated to mitigate the symptoms of metastatic disease.
Diazoxide, a benzothiadiazine derivative, is a commonly used medication to treat insulinomas due to its hyperglycemic effects, which arise via its activation of an ATP sensitive K+ channel, resulting in hyperpolarization of the β-cell which subsequently inactivates a voltage gated Ca2+ channel that is requisite for insulin release6. Restoration of normal glucose levels with diazoxide has been reported to be as low as 59% with side-effects experienced by 47% of patients, which include: fluid retention, hirsutism, hypotension and one reported case of Stevens-Johnson syndrome7-9. Alternatively, a long-acting somatostatin analogue can be used to inhibit the release of insulin.
Octreotide, a somatostatin analogue, has the ability to inhibit somatostatin receptor (sstr) 2 and sstr510. To date, there are 5 main sstr subtypes (sstr1-5) described in human physiology with sstr2 and sstr5 expressed in approximately 70% of insulinomas; however, 30% of insulinomas express some other sstr subtype, which is of particular clinical significance11. For instance, if the insulinoma expresses sstr2, then the action of octreotide will tend to inhibit the release of insulin, thus restoring glucose to optimal levels. However, if the insulinoma lacks sstr2, octreotide can actually exacerbate hypoglycemia via its inhibition of glucagon and growth hormone secretion10. Consequently, it is of paramount importance to discover alternative treatments that lack the uncertainty of diazoxide and octreotide and may be more effective in the treatment of PNETs. To that end, a novel long-acting release somatostatin analogue, pasireotide (SOM230), has been developed that demonstrates improved agonist activity at multiple sstr subtypes.
SOM230 (Novartis) is a cyclohexapeptide somatostatin analogue with improved agonist activity at sstr1, 2, 3, and 5 with the greatest activity at sstr5, 2, 3, and 1, respectively12-13. In pre-clinical studies, SOM230 has demonstrated the ability to reduce tumor volumes in prolactinomas and decrease the secretion of growth hormone and insulin-like growth factor (IGF-1)14-16. Consequently, our study sought to investigate the effects of SOM230 on the secretion of insulin as well as its effect on tumor volume and survival in a mouse model of insulinoma.
Loss of heterozygosity of the MEN1 gene leads to the multiple endocrine neoplasia type 1 (MEN1) syndrome seen in humans, which is characterized by anterior pituitary adenoma, parathyroid hyperplasia, and enteropancreatic endocrine tumors17. In order to recapitulate the human disease in a mouse model, our lab developed a transgenic mouse line which utilizes a pancreas specific promoter, Pdx1, in combination with the Cre-Lox system to conditionally knockout Men1 in the pancreata of mice (Pdx1-Cre:Men1 floxed/floxed). As discussed at length elsewhere, this mouse model leads to the selective development of a single insulinoma18. Consequently, with the development of insulinoma in our mouse model, a parallel of one phenotypic expression seen in MEN1, our lab sought to investigate the effects of SOM230 on insulin and glucose levels, apoptosis, tumor size, and overall survival.
METHODS
Animals
Eight 12-month old mice carrying the Men1 allele flanked by loxP sites with Cre recombinase under the promoter of the pancreas specific transcription factor Pdx1 (Pdx1-Cre:Men1 floxed/floxed) were used in this study. Moreover, as demonstrated by our lab previously, these mice have already developed an insulinoma as evidenced by increased serum levels of insulin and by microPET/CT analysis18. All animal experiments were conducted under an Albert Einstein College of Medicine (AECOM) approved animal protocol.
SOM230 and PBS Administration
Mice were anesthetized using halothane and then shaved on their flank for subcutaneous injection of either phosphate buffered saline (PBS) buffer or SOM230 at a concentration of 160mg/Kg/month (64mg/ml) every month for 4 months.
Glucose and Insulin Levels
The mice underwent a 24-h fast prior to collecting whole blood via a retro-orbital bleeding technique weekly at pre- and post-treatments. Serum glucose was measured by enzymatic colorimetric assay using a GM7 Analyzer (Analox Instruments, Lundeburg, MA). Serum insulin was measured by enzyme-linked immunosorbent assay (ELISA) with the UltrasensitiveMouse Insulin ELISA kit (Mercodia, Inc) according to the manufacturer’s instructions.
Survival and Toxicity
Mice were weighed every week for 4 months as an indicator of toxicity. If a death occurred, the pancreas was collected and fixed for further analysis.
Positron emission tomography (PET)/Computerized tomography (CT) scans
All mice were imaged after 3 hours of fasting, breathing 1.5% isoflurane-oxygen mixture anesthesia to continue through the imaging procedure. A heating pad, before and during scanning, maintained normal body temperature. 300-400 uCi (12-15 MBq) in 0.1 ml normal saline [18F] fluoro-2-deoxyglucose (FDG) was administered intravenously via tail vein followed immediately by CT (80 kV, 0.5 mA x-ray gun) on an Inveon Multimodality scanner (Siemens). Subsequent PET imaging was performed (approximately one hour after the FDG injection) by transferring the mouse on the gantry into the PET device. List mode data acquisition was corrected for deadtime counting losses, random coincidences and the measured nonuniformity of detector response (i.e., normalized) but not for attenuation or scatter. After both acquisitions, CT images were co-registered with PET images using a previously stored linear transformation matrix.
SUV analysis for tumor activity
Analysis was performed using either ASIPRO or IRW (both Siemens) dedicated software. All image studies are displayed visually in a rotating 3D projection to examine for interpretability and artifact. Manual regions of interest (ROI) are defined around pathologic uptake areas and compared with co-registered CT image data. Successive scrolling through 2 dimensional slices (each 1.2 mm thick in the axial images) permits measurement of radioactivity within defined volumes. The activity concentration within this volume divided by the activity per gram of body mass of total injected radioactivity determines the standardized-uptake value (SUV). The SUVmax is the maximum SUV value within each tumor volume. SUVmax has been validated in numerous animal and human models as a reproducible and robust measure of radioactivity in longitudinal studies. Furthermore, the glucose levels in our mouse model do not impact the SUV calculation.
H&E staining
Mice were sacrificed after 4 months, and whole pancreata were dissected, formalin-fixed with paraffin embedding (FFPE), and sectioned (5 μm) on a rotating microtome (Microm, Walldorf, Germany). Hematoxylin and eosin staining was performed according to standard procedures. For morphometric analysis, three 100μm sections were digitalized and pancreas cross-sectional area in the section with maximal tumor diameter was determined by computerized pixel counting.
Apoptosis analysis
Apoptotic status in endocrine tumor tissues was measured in control and treated mice by Terminal deoxynucleotidyl transferase dUTP Nick End-Labeling (TUNEL) assay. For quantification of apoptosis, the TUNEL assay was performed according to the manufacturer on paraffin-embedded sections with an In Situ Cell Death Detection Kit (Roche Diagnostic). The tissue sections were deparaffinized and treated with proteinase K (10 μg/ml) for 20 min. The sections were then washed twice with PBS, labeled and stained with the TUNEL reaction mixture (label plus enzyme solutions) for 60 min at 37°C, and washed twice with PBS in the dark. The slides were mounted in Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, Calif). The apoptotic fluorescent cells were counted under a fluorescent microscope and the numbers were expressed as the percentage of total cells ± standard deviation (SD). A negative control without enzyme treatment and a positive control with DNase I treatment were also performed.
SSTR detection
The detection of sstr1-5 in pancreatic endocrine tumor tissue was measured in our mouse model. Immunofluorescent (IF) staining for sstr1-5 on sections was performed using rabbit or goat anti-sstr1, sstr2, sstr3, sstr4, and sstr5 antibodies (Abs) (BD Biosciences) and were incubated at a 1:50 dilution overnight at 4° C, respectively. Pig anti-insulin Ab was also used for identification of β-cells. Sections were then washed with PBS and were incubated with a 1:200 dilution of anti-rabbit or anti-goat Alexa Fluor 488 and anti-pig Alexa Fluor 647 secondary antibodies for 45 min in the dark, respectively. A negative control without primary antibody was also performed.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism v.5.0a in conjunction with Microsoft Excel 2010. An unpaired t-test with a Welch correction for nonequal variance was used for image data quantification and a one-way repeated ANOVA was used to analyze serum insulin levels and serum glucose levels. A P value of < 0.05 was considered statistically significant.
Results
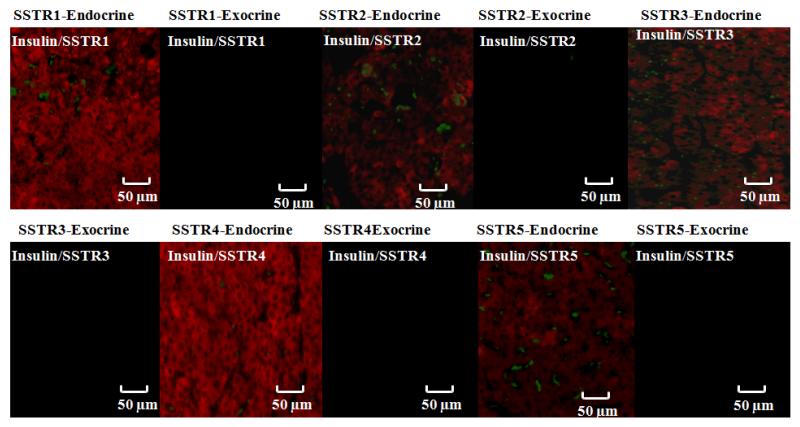
Pdx1-Cre;Men1 floxed/floxed conditional knockout mice express somatostatin receptor subtypes 1-5 (sstr1-5) (Figure 1)
Fig 1.
Evaluation of somatostatin receptor subtypes (sstr1-5) in representative insulinomas from Pdx1-Cre:Men1 floxed/floxed conditional knockout mice by immunofluorescence. Islets of Langerhans were visualized with anti-insulin antibody and corresponding secondary antibody (red), and sstr1-5 were identified with anti-sstr1, - sstr2, -sstr3, -sstr4, or -sstr5 antibodies along with corresponding secondary antibodies (green). All 5 sstr were identified in this mouse model.
In order for SOM230 to be a feasible treatment in our Pdx1-Cre;Men1 floxed/floxed conditional knockout mouse model, we first had to identify the presence of sstrs. Our results demonstrated that sstr1-5 were expressed in the pancreatic neuroendocrine tumor (PNET) tissues of mouse model used in this study. The presence of sstr1-5 in the PNETs of our mouse model further supports the proposed mechanism of action of SOM230; namely, its activity as a somatostatin analogue.
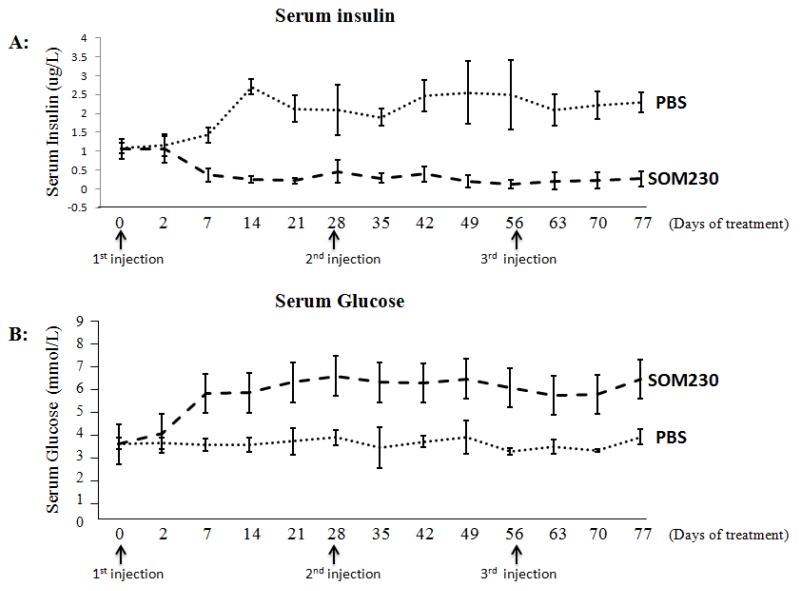
Subcutaneous (SC) injection of SOM230 decreases serum insulin levels and increases serum glucose levels in Pdx1-Cre; Men1 floxed/floxed conditional knockout mice (Figure 2)
Fig 2.
Effect of pasireotide (SOM230) on serum insulin (A) and glucose levels (B) in Pdx1-Cre:Men1 floxed/floxed conditional knockout mice with monthly subcutaneous injections of SOM230 (160mg/Kg/month [64mg/ml]) or PBS for four months.
*One-way repeated ANOVA was performed on data as long as there were at least 3 mice in each group (i.e., up to and including day 70)
†Indicates the occurrence of death (days 68, 74, and 82)
In order to study the antisecretory effects of SOM230, baseline serum insulin levels in the control and treatment groups were measured with no significant difference between the two groups: 1.078μg/L±0.13 and 1.060μg/L±0.2769, respectively (p=0.9096). By day 7 after administration of PBS or SOM230, there was a significant difference in serum insulin levels between the control and treatment groups: 1.427μg/L±0.1946 and 0.3652μg/L±0.167, respectively (p=0.0004). Furthermore, with monthly SC injections, the ability of SOM230 to decrease insulin levels, compared to SC PBS injections, remained significant over the four months of treatment (p<0.0001). The baseline serum glucose levels measured in the control and treatment groups, prior to treatment initiation, were not significantly different: 4.289mmol/L±0.305 and 4.246mmol/L±0.4536, respectively (p=0.8811). However, 7 days following SC injection of SOM230, serum glucose levels in the treatment group were significantly higher than those in the control group: 7.122mmol/L±1.058 and 4.208mmol/L±0.3507, respectively (p=0.0136). The increase in serum glucose levels was sustained over the four month experiment in the group receiving monthly SC injections of SOM230 as compared to monthly PBS injections (p<0.0001)
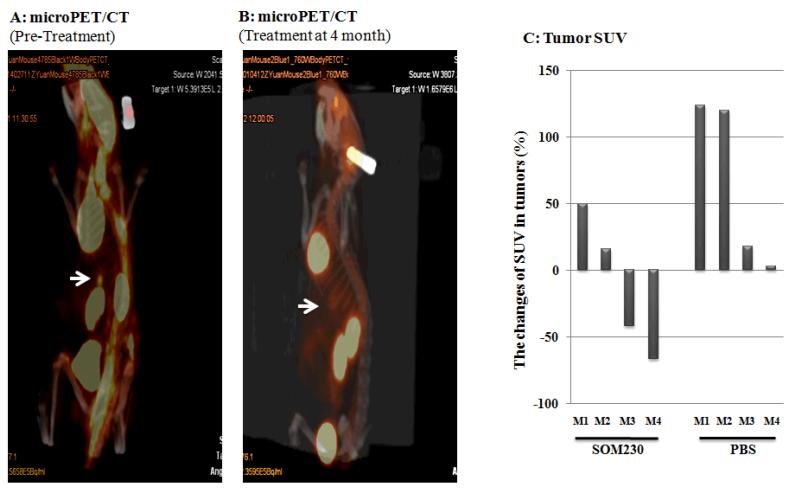
Subcutaneous injection (SC) of SOM230 demonstrated a reduction in tumor activity on PET/CT scan in Pdx1-Cre; Men1 floxed/floxed conditional knockout mice (Figure 3)
Fig 3.
microPET/CT scan and standardized-uptake value (SUV) analysis as a means of visualizing insulinoma responsiveness to monthly subcutaneous injections of pasireotide (SOM230) (160mg/Kg/month [64mg/ml]) or PBS over four months. (A) microPET/CT scan demonstrating the presence of increased metabolism/excretion by normal organs (brain, heart, kidneys, and bladder) and an insulinoma prior to treatment with SOM230 (arrow). (B) microPET/CT scan demonstrating the presence of increased metabolism/excretion by normal organs with decreased activity in the insulinoma following four months treatment with SOM230. (C) SUV measurements for each mouse represented as a percent change in activity beginning from pre-treatment until either death or four months treatment with either SOM230 or PBS (100 × [SUVfinal - SUVpre-treatment]/SUVpre-treatment)
*Mice M2, M3, and M4 of the PBS group expired prior to completion of the study (days 68, 74, and 82, respectively)
Over the four month study period, 50% of the mice in the treatment group had a reduction in SUV compared to baseline by as much as 67.8%. The remaining 50% had an increase of 43.6% and 16.7%. In contrast, the only control mouse to survive the study had an increase in tumor activity of 124%. Of the three control mice that did not survive (death on days 68, 74, and 82) the increase in tumor activity was 2.9%, 16.7%, and 125%, respectively. Of note, the two control mice that died early in the study had a minimal change in tumor activity due to the fact that they did not survive long enough for a change to be observed. In contrast, the other two control mice survived long enough for the serial microPET/CT scans to identify a change in tumor activity, approximately 3-7 fold of that seen in the two mice in the treatment group with increased tumor activity. This suggests that SOM230 may also reduce tumor activity via an antiproliferative and/or proapoptotic mechanism. In contrast, none of the mice in the control group had a reduction in SUV compared to baseline.
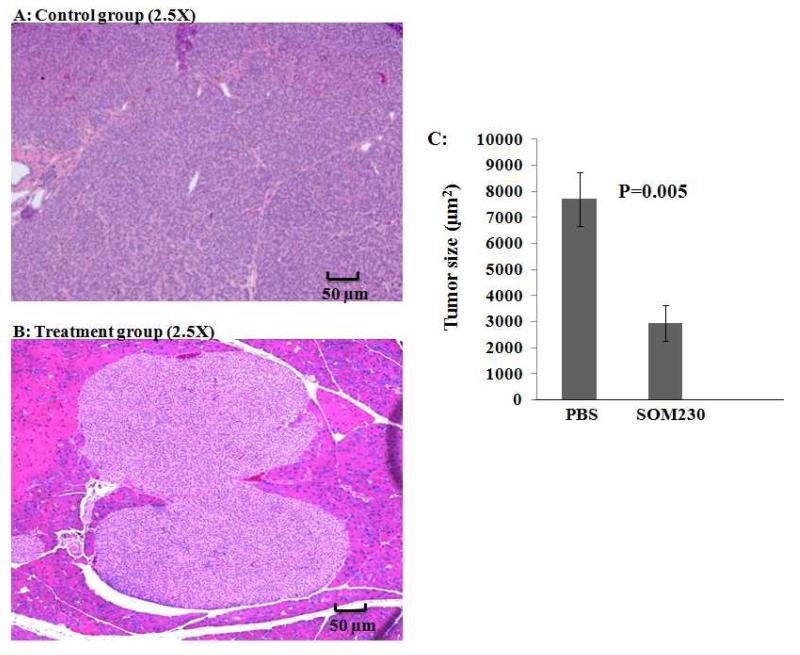
Subcutaneous injection (SC) of SOM230 reduced PNET tumor size in Pdx1-Cre; Men1 floxed/floxed conditional knockout mice compared to PBS treated mice (Figure 4)
Fig 4.
Histological analysis of pancreata from Pdx1-Cre:Men1 floxed/floxed conditional knockout mice with monthly subcutaneous (SC) injections of PBS or pasireotide (SOM230) (160mg/Kg/month [64mg/ml]) for four months at same magnification demonstrating a significant difference in tumor size. (A) Hematoxylin and eosin (H&E) staining of representative pancreas with insulinoma from the control group receiving monthly SC injections of PBS at the end of four months at 2.5× magnification. (B) H&E staining of representative pancreas with insulinoma from the treatment group receiving monthly SC injections of SOM230 at the end of four months at 2.5× magnification. (C) Mean insulinoma size in control and treatment group after 4 months.
At the completion of the study, all remaining mice were sacrificed by CO2 inhalation. Pancreata from all mice (including those that died during the study) were collected, sectioned (3 sections/mouse), and prepared for microscopic analysis by hematoxylin and eosin (H&E) staining to identify the size of the insulinoma from each mouse. The control group had a significantly larger average tumor size of 7067μm2±955 compared to the treatment group with an average tumor size of 2098μm2±388 (p=0.0024) as seen at the same magnification of 2.5×. The reduction in measured tumor size between the two groups is consistent with the results of the microPET scans and SUV calculations.
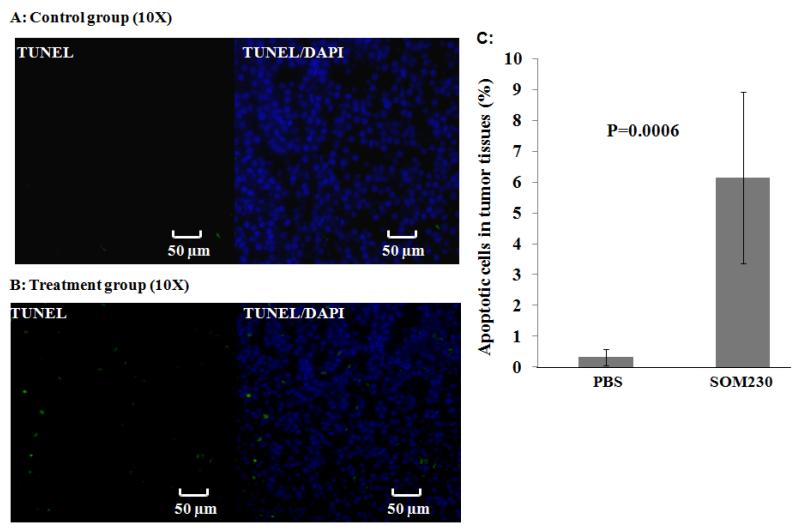
Subcutaneous (SC) injection of SOM230 increases apoptosis in PNETs in Pdx1-Cre; Men1 floxed/floxed conditional knockout mice (Figure 5)
Fig 5.
Assessment of apoptosis in representative pancreata from Pdx1-Cre:Men1 floxed/floxed conditional knockout mice following monthly subcutaneous (SC) injections of PBS or pasireotide (SOM230) (160mg/Kg/month [64mg/ml]) for four months by Terminal deoxynucleotidyl transferase dUTP Nick End-Labeling (TUNEL) assay. (A) Immunofluorescent staining of representative pancreas with insulinoma from the control group receiving monthly SC injections of PBS at the end of four months. Nuclei were visualized via DAPI staining (blue), whereas apoptotic foci were identified by terminal deoxynucleotidyl transferase-mediated addition of fluorescein-dUTP (green). (B) Immunofluorescent staining of representative pancreas with insulinoma from the treatment group receiving monthly SC injections of SOM230 at the end of four months. (C) Quantification was achieved by counting the apoptotic fluorescent cells under a fluorescent microscope (expressed as the percentage of total cells ± standard deviation [SD]).
Sections of pancreata (3 sections/mouse) from the treatment and control groups were evaluated for the presence of apoptotic cells via a terminal deoxynucleotidyl transferase 0 dUTP nick end labeling (TUNEL) assay. The proportion of apoptotic PNET cells in the control groups (0.299%±0.103) was significantly lower than in the treatment group (6.92%±1.23) (p=0.002).
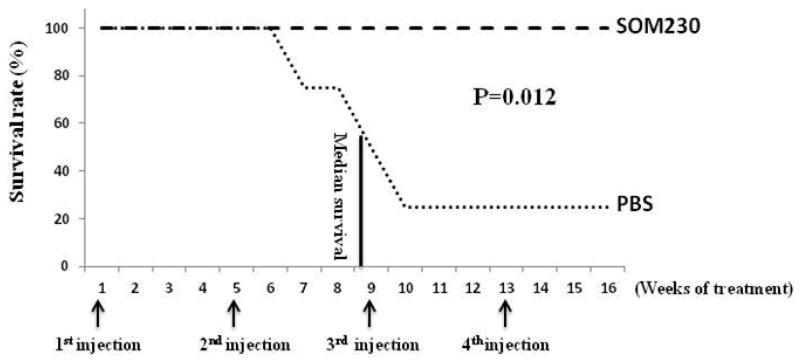
Subcutaneous injection (SC) of SOM230 improved survival in Pdx1-Cre; Men1 floxed/floxed conditional knockout mice (Figure 6)
Fig 6.
Effect of pasireotide (SOM230) on survival in Pdx1-Cre:Men1 floxed/floxed conditional knockout mice with monthly subcutaneous injections of SOM230 (160mg/Kg/month [64mg/ml]) or PBS for four months.
Within the treatment group (n=4), there was no mortality and all mice were alive at the end of the four month study period. However, in the control group, only 1 of the 4 mice was alive at the conclusion of the experiment with the cause of death believed to be associated with metabolic dysfunction due to chronic hypoglycemia. The overall survival at four months was improved in the SOM230 treatment group (4/4, 100%) compared with the control group (1/4, 25%), but due to the small sample size, statistical analysis was not performed.
Discussion
Although surgery remains the primary treatment of PNETs, it is important to consider those patients not amenable to a surgical resection due to the presence of unresectable primary or metastatic lesions. Indeed, medical therapy has developed over the past 60 years in an attempt to ameliorate the symptoms produced by functional PNETs such as insulinomas. However, even with the use of benzothiadiazine derivatives, such as diazoxide, and narrow-spectrum somatostatin analogues, such as octreotide, there remains a group of individuals whose symptoms are not alleviated by these medications. The aim of this study was to determine if a long acting release somatostatin analogue, pasireotide (SOM230), with broadened sstr subtype activity can be an effective antisecretory, antiproliferative and proapoptotic therapy in a mouse model of insulinoma.
This Pdx1-Cre: Men1 floxed/floxed mouse model of insulinoma recapitulates the human phenotype as evidenced by the markedly elevated basal insulin levels measured by ELISA in mice prior to treatment. Furthermore, the elevated insulin levels led to markedly depressed serum glucose levels, a finding consistent with the pathophysiology of human insulinomas. The use of microPET/CT allowed quantification of the PNET’s metabolic activity as well as localization of the insulinoma in the pancreas of the mouse model. Finally, histology of the collected pancreata demonstrated an expansion of the islet of Langerhans, visualized with H&E staining, and immunofluorescence confirmed insulin secretion as well as characterizing the sstr subtypes found in the PNETs of this mouse model.
Monthly SC injections of SOM230 led to a sustained decrease in serum insulin levels, a finding that is consistent with both the mechanism of action of somatostatin analogues as well as the specific binding affinity of SOM230 for sstr1, 2, 3, 5. Importantly, SOM230’s half maximal inhibitory concentration (IC50) for sstr5 and sstr2 are reported to be 0.1nM and 1.0nM, respectively, while octreotide’s IC50 for sstr5 and sstr2 are reported to be 7nM and 0.6nM, respectively13. This is of particular clinical relevance as approximately 70% of human insulinomas are estimated to express sstr5 or sstr211 and these are the main sstr subtypes that, when activated, are associated with a decrease in hormone secretion, with sstr2 being the primary receptor involved19. Moreover, an mRNA expression study concluded that there is an association of sstr5 with insulinoma aggressiveness, an expression profile which tends to be less responsive to octreotide administration20. As such, this study is the first to investigate the use of a somatostatin analog, with enhanced sstr5 activity without compromising sstr2 activity, to restore serum insulin to physiologically compatible levels and may be useful for the treatment of malignant insulinomas.
In addition to the antisecretory effects of somatostatin analogues, primarily through interaction with sstr2, an antiproliferative effect has been associated with activity of both sstr5 and sstr219. A recent study of the effects of SOM230 on pituitary adenomas demonstrated a decrease in tumor volume as compared to treatment with octreotide, a finding consistent with the sstr binding spectrum of SOM23014. To that end, our study also demonstrates a significant reduction in tumor volume in the treatment group compared to the control group as measured by morphometric analysis with standard H&E staining as well as by microPET/CT scan analysis, utilizing SUV values measured prior and post treatment acquired monthly.
Furthermore, the various sstr subtypes are not limited to antisecretory and antiproliferative effects but also include apoptotic induction. A recent study demonstrated that SOM230 has a proapoptotic effect on rat mammary gland hyperplastic cells, mediated through sstr321. Analogously, our study demonstrated a significant increase in apoptotic events as evidenced by a TUNEL assay, which demonstrated increased DNA fragmentation in the pancreata of mice treated with SOM230 vs. the pancreata of mice treated with PBS. This is of particular clinical relevance as SOM230 may actually not only alleviate symptoms in patients with insulinomas, but it might actually lead to regression of the tumors.
Finally, an overall increased survival in the treatment group compared to the control group was observed at the end of this study. Due to the small sample size that is often seen in transgenic mouse studies, this increase could not be analyzed with appropriate statistics. However, the overall magnitude of increased survival in the treatment group compared to the control group, 4 to 1, is suggestive of a protective mechanism and ought to be further investigated. Indeed, the 50% mortality seen in this mouse model is approximately 13-14 months with almost all mice dying by 16-18 months18. As a result, we specifically chose 12-month old mice for this 4-month study in order to investigate a protective role of monthly SC injections of SOM230.
In summary, this study is the first of its kind to demonstrate the antisecretory, antiproliferative and proapoptotic actions of the novel long acting release somatostatin analogue SOM230 in a mouse model of insulinomas with improved overall survival. The enhanced spectrum of activity of SOM230 is a result of its enhanced activity at 4 of the 5 sstr subtypes: sstr5, sstr2, sstr3, and sstr1. This is of particular clinical importance in unresectable or metastatic insulinomas that are relatively unresponsive to traditional therapy with octreotide and/or diazoxide. Treatment with SOM230 may facilitate symptomatic relief and result in tumor regression. We believe that this novel strategy of targeted therapy with SOM230 will be of benefit to patients with PNETs. While these data strongly support the effects of pasireotide in this model, we acknowledge that further studies with larger sample sizes are warranted to advance this novel approach toward clinical trials.
ACKNOWLEDGEMENTS
We would like to thank Novartis Pharmaceuticals for providing SOM230 for this study. This work was supported in part through a generous gift from Linda and Earle Altman.
Footnotes
DISCLOSURES
The authors state that they have no conflict of interest.
References
- 1.Key C. Cancer of the pancreas. In: Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. Patient and Tumor Characteristics. National Cancer Institute; Bethesda, MD: 2007. Chapter 7. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001. SEER Program, NIH Pub. No. 07-6215. [Google Scholar]
- 2.Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases. Dig Dis Sci. 1991;36:933–942. doi: 10.1007/BF01297144. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 4.Ehehalt F, Saeger H, Schmidt C, Grützmann R. Neuroendocrine tumors of the pancreas. Oncologist. 2009;12:456–67. doi: 10.1634/theoncologist.2008-0259. [DOI] [PubMed] [Google Scholar]
- 5.de Herder WW, Niederle B, Scoazec JY, Pauwels S, Kloppel G, Falconi M, et al. Frascati Consensus Conference; European Neuroendocrine Tumor Society. Well-differentiated pancreatic tumor/carcinoma: insulinoma. Neuroendocrinology. 2006;84(3):183–8. doi: 10.1159/000098010. Epub 2007 Feb 20. [DOI] [PubMed] [Google Scholar]
- 6.Sturgess NC, Kozlowski RZ, Carrington CA, Hales CN, Ashford ML. Effects of sulphonylureas and diazoxide on insulin secretion and nucleotide-sensitive channels in an insulin secreting cell line. Br J Pharmacol. 1988 Sep;95(1):83–94. doi: 10.1111/j.1476-5381.1988.tb16551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry RR, Vinik AI. Clinical review 72: diagnosis and management of functioning islet cell tumors. J Clin Endocrinol Metab. 1995 Aug;80(8):2273–8. doi: 10.1210/jcem.80.8.7629220. [DOI] [PubMed] [Google Scholar]
- 8.Goode PN, Farndon JR, Anderson J, Johnston ID, Morte JA. Diazoxide in the management of patients with insulinoma. World J Surg. 1986 Aug;10(4):586–92. doi: 10.1007/BF01655532. [DOI] [PubMed] [Google Scholar]
- 9.Gill GV, Rauf O, MacFarlane IA. Diazoxide treatment for insulinoma: a national UK survey. Postgrad Med J. 1997 Oct;73(864):640–641. doi: 10.1136/pgmj.73.864.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Usukura M, Yoneda T, Oda N, Yamamoto Y, Takata H, Hasatani K, et al. Medical treatment of benign insulinoma using octreotide LAR: a case report. Endocr J. 2007 Feb;54(1):95–101. doi: 10.1507/endocrj.k05-157. Epub 2006 Nov 24. [DOI] [PubMed] [Google Scholar]
- 11.Bertherat J, Tenenbaum F, Perlemoine K, Videau C, Alberini JL, Richard B, Dousset B, et al. Somatostatin receptors 2 and 5 are the major somatostatin receptors in insulinomas: an in vivo and in vitro study. J Clin Endocrinol Metab. 2003 Nov;88(11):5353–60. doi: 10.1210/jc.2002-021895. [DOI] [PubMed] [Google Scholar]
- 12.Bauer W, Briner U, Doepfner W, Haller R, Huguenin R, Marbach P, et al. SMS 201-995: a very potent and selective octapeptide analog of somatostatin with prolonged action. Life Sci. 1982;31:1133–1140. doi: 10.1016/0024-3205(82)90087-x. [DOI] [PubMed] [Google Scholar]
- 13.Pawlikowski M, Melen-Mucha G. Somatostatin analogs – from new molecules to new applications. Curr Opin Pharmacol. 2004;4(6):608–13. doi: 10.1016/j.coph.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Fedele M, De Martino I, Pivonello R, Ciarmiello A, Del Basso De Caro ML, Visone R, et al. SOM230, a new somatostatin analogue, is highly effective in the therapy of growth hormone/prolactin-secreting pituitary adenomas. Clin Cancer Res. 2007;13:2738–2744. doi: 10.1158/1078-0432.CCR-06-2505. [DOI] [PubMed] [Google Scholar]
- 15.van der Hoek J, de Herder WW, Feelders RA, van der Lely AJ, Uitterlinden P, Boerlin V, et al. A single-dose comparison of the acute effects between the new somatostatin analog SOM230 and octreotide in acromegalic patients. J Clin Endocrinol Metab. 2004;89(2):638–645. doi: 10.1210/jc.2003-031052. [DOI] [PubMed] [Google Scholar]
- 16.Petersenn S, Schopohl J, Barkan A, Mohideen P, Colao A, Abs R, et al. Pasireotide Acromegaly Study Group Pasireotide (SOM230) demonstrates efficacy and safety in patients with acromegaly: a randomized, multicenter, phase II trial. J Clin Endocrinol Metab. 2010 Jun;95(6):2781–9. doi: 10.1210/jc.2009-2272. Epub 2010 Apr 21. [DOI] [PubMed] [Google Scholar]
- 17.Powell AC, Libutti SK. Multiple endocrine neoplasia type 1: clinical manifestations and management. Treat Res. 2010;153:287–302. doi: 10.1007/978-1-4419-0857-5_16. [DOI] [PubMed] [Google Scholar]
- 18.Shen HC, He M, Powell A, Adem A, Lorang D, Heller C, et al. Recapitulation of pancreatic neuroendocrine tumors in human multiple endocrine neoplasia type I syndrome via Pdx1-directed inactivation of Men1. Cancer Res. 2009 Mar 1;69(5):1858–66. doi: 10.1158/0008-5472.CAN-08-3662. Epub 2009 Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asa SL. Pancreatic endocrine tumors. Mod Pathol. 2011 Apr;24(Suppl 2):S66–77. doi: 10.1038/modpathol.2010.127. [DOI] [PubMed] [Google Scholar]
- 20.de Sá SV, Corrêa-Giannella ML, Machado MC, de Souza JJ, Pereira MA, Patzina RA, et al. Somatostatin receptor subtype 5 (SSTR5) mRNA expression is related to histopathological features of cell proliferation in insulinomas. Endocr Relat Cancer. 2006 Mar;13(1):69–78. doi: 10.1677/erc.1.00962. [DOI] [PubMed] [Google Scholar]
- 21.Kleinberg DL, Ameri P, Singh B. Pasireotide, an IGF-I action inhibitor, prevents growth hormone and estradiol-induced mammary hyperplasia. Pituitary. 2011 Mar;14(1):44–52. doi: 10.1007/s11102-010-0257-0. [DOI] [PubMed] [Google Scholar]