Abstract
Living donor liver transplantation (LDLT) may have better immunological outcomes compared to deceased donor liver transplantation (DDLT). The aim of this study was to analyze the incidence of acute cellular rejection (ACR) after LDLT and DDLT.
Data from the Adult-to-Adult Living Donor Liver Transplantation (A2ALL) Retrospective Cohort Study on 593 liver transplants done between May 1998 and March 2004 were studied (380 LDLT; 213 DDLT). Median LDLT and DDLT follow-up was 778 and 713 days, respectively. Rates of clinically treated and biopsy-proven ACR were compared.
There were 174 (46%) LDLT and 80 (38%) DDLT recipients with ≥1 clinically treated episodes of ACR, whereas 103 (27%) LDLT and 58 (27%) DDLT recipients had ≥1 biopsy-proven ACR episode. A higher proportion of LDLT recipients had clinically treated ACR (P=0.052), but this difference was largely attributable to one center. There were similar proportions of biopsy-proven rejection (P=0.97) and graft loss due to rejection (P=0.16). Longer cold ischemia time was associated with a higher rate of ACR in both groups despite much shorter median cold ischemia time in LDLT.
These data do not show an immunological advantage for LDLT, and therefore do not support the application of unique post-transplant immunosuppression protocols for LDLT recipients.
INTRODUCTION
The favorable long term graft function and excellent graft survival in the setting of living donor kidney transplantation is attributed, in part, to selection of transplant candidates, utilization of organs from pristine donors, recovery of donor organs that have not been exposed to the stress associated with brain death, and minimization of injuries related to more prolonged cold ischemia time (1-3). In addition, there might be an immunological advantage due to HLA matching among biologically related individuals. The end result is that living donor kidney recipients experience a lower incidence of acute and chronic allograft rejection compared to recipients of deceased donor kidneys (4, 5). It is logical to hypothesize that the variables affecting kidney graft survival are attributable in part to a reduction in the intensity of the early proinflammatory response, and contribute to better immunological acceptance of the organ. In theory, such an advantage should extend to the setting of LDLT, in which an excellent liver lobe taken from a stable donor is exposed to a short period of cold ischemia, and may be placed in selected recipients who may be genetically related. An additional and potentially important variable in the LDLT setting is the induction and progression of liver regeneration that occurs in the immediate post-transplant period. Molecular pathways associated with regeneration may regulate proinflammation, and consequently, may play a role in the development of the alloimmune response (6, 7). The sum of these variables may affect alloimmunity, an outcome that may be measured clinically by the frequency and severity of episodes of acute rejection.
Limited information is available regarding the frequency and severity of acute rejection episodes in the setting of LDLT. Previous clinical observations are limited to analysis of the Scientific Registry of Transplant Registry (SRTR) database, which lacks detailed clinical information about rejection and single center experiences with relatively small numbers of recipients (8-10). Our prior analysis of the SRTR database suggested a lower rate of rejection in recipients of LDLT when compared to deceased donor liver transplant (DDLT) recipients (8). However, a recent validation study comparing A2ALL and SRTR data demonstrated discrepancies representing missed reporting of LDLT rejection in the SRTR as reported by centers (11). Other reports indicated a lower rate of rejection in a small number of LDLT recipients after relatively short-term follow-up (9, 10).
The relevance of this information to the clinical management of this population is clear: a differential pattern of acute cellular rejection (ACR) in LDLT vs. DDLT that is determined by suppressed or enhanced alloimmune response in one or the other setting may suggest procedure-specific immunosuppression management. The aim of the retrospective study reported here was to determine the incidence of rejection in recipients undergoing LDLT or DDLT, to examine the rate of recurrent rejection, to determine whether the etiology of the primary liver disease or other recipient factors were associated with early and/or long-term rates of acute rejection, and to examine whether rejection was differentially affected by the use of antibody induction therapy in the two groups.
METHODS
Data for this study were derived from the A2ALL Retrospective Cohort Study. Information was collected from extensive chart reviews, supplemented by data from the SRTR made available through a data use agreement. The study included 819 subjects who had a potential living donor evaluated between January 1, 1998 and February 28, 2003 at nine U.S. transplant centers. Those analyzed relate to the 593 patients who received a transplant: 380 LDLT and 213 DDLT. Potential recipients whose procedures were aborted were not included. Recipients of domino transplants (n=2) were included in DDLT group. Median post-transplant follow-up was 778 days for LDLT and 713 days for DDLT recipients, respectively. There was a range of LDLT and DDLT recipients from A2ALL participating centers; all centers performed at least 20 LDLT. The use of induction therapy, maintenance of immunosuppression, and treatment modalities for ACR were not uniform within the participating centers.
The database included extensive information that documented the time to the first episode of rejection and recurrent rejection, whether the diagnosis was confirmed by liver biopsy, and what type of anti-rejection treatment was given. The following rejection definitions were used. The term clinically treated rejection was used when the diagnosis of rejection was suspected, and was accompanied by anti-rejection treatment. Such recipients were treated for rejection with or without confirmation of the diagnosis by liver biopsy. The treatment for rejection included any of the following options: steroid bolus and/or taper, with or without administration of antibody therapy. In a very small number of cases (n=8), rejection was treated with a switch or addition to baseline immunosuppressive drugs. Biopsy-proven rejection was used when the diagnosis of rejection was suspected, the presence of rejection was confirmed by liver biopsy, and treatment for rejection was given. The diagnosis of ACR was confirmed by the local pathologist, and there was no central reading of biopsy slides in this study.
Statistical Methods
Two-sample t-tests and chi-square tests were used to compare LDLT and DDLT recipients with respect to baseline characteristics. Chi-square tests were also used to compare the transplant centers for the proportion of their patients with any clinically treated rejection and any biopsy-proven rejection. Chi-square tests were used to compare differences between LDLT and DDLT for the proportion with any clinically treated rejection and any biopsy-proven rejection. Mantel-Haenszel trend tests were used to compare LDLT vs. DDLT with respect to the number of clinically treated and biopsy-proven rejection episodes (0, 1, 2, and more than 2). Time to the first clinically treated rejection and time to the first biopsy-proven rejection were evaluated by the Kaplan-Meier (KM) method. Unadjusted comparisons between LDLT and DDLT were made using the log-rank test. Many of these comparisons were performed both with and without Center A, which had an extreme value for the proportion with treated rejection.
Cox regression models were used to investigate predictors of time from transplant to the first biopsy-proven rejection. Covariate effects were presented as adjusted hazard ratios (HR) with 95% confidence intervals (CI). Analyses of variables associated with biopsy-proven rejection were not controlled for center effect. Cumulative probabilities of graft failure or death over time were estimated by the KM method. P-values < 0.05 were considered to be statistically significant. All analyses were carried out using SAS 9.1 statistical software (SAS/STAT 9.1 User's Guide, SAS Publishing, Cary, NC: SAS Institute Inc., 2004).
Human Subjects Protection
The study was approved by the Institutional Review Boards and Privacy Boards of the University of Michigan Data Coordinating Center and each of the nine participating transplant centers.
RESULTS
Patient characteristics
Patient characteristics, particularly those previously described to affect the incidence and severities of ACR, are presented in Table 1. The distributions of recipient age, gender, and race were similar in the LDLT and DDLT groups. Importantly, proportions with immune-related disease etiologies known to be associated with post-transplant rejection were similar in the LDLT and DDLT recipients. Finally, the frequency of antibody induction therapy and/or maintenance immunosuppression was similar between the groups.
Table 1.
Recipient Characteristics
| LDLT(N=380) Mean (SD) or N (%) | DDLT(N=213) Mean (SD) or N (%) | P-value* | |
|---|---|---|---|
| Recipient Age | 49.3 (10.7) | 50.9 (9.8) | 0.06 |
| Recipient Gender (% Male) | 219 (58%) | 127 (60%) | 0.64 |
| Recipient Race (% White) | 344 (91%) | 187 (88%) | 0.59 |
| Recipient Ethnicity (% Non-Hispanic) | 306 (81%) | 173 (81%) | 0.84 |
| Diagnosis at Listing and Enrollment (more than one diagnosis per patient possible) | |||
| Hepatitis C virus-related (HCV) cirrhosis | 181 (48%) | 99 (46%) | 0.79 |
| Hepatocellular carcinoma (HCC) | 56 (15%) | 33 (15%) | 0.80 |
| Alcoholic cirrhosis | 52 (14%) | 31 (15%) | 0.77 |
| Cholestatic liver disease | 70 (18%) | 39 (18%) | 0.97 |
| Non-cholestatic cirrhosis (other than HCV and alcoholic cirrhosis) | 80 (21%) | 48 (23%) | 0.67 |
| Metabolic disease | 11 (3%) | 7 (3%) | 0.79 |
| Biliary atresia | 3 (1%) | 0 (0%) | 0.56 |
| Malignancy other than HCC | 11 (3%) | 5 (2 %) | 0.69 |
| Autoimmune liver disease | 19 (5%) | 11 (5%) | 0.93 |
| Other | 10 (3%) | 5 (2%) | 0.83 |
| Immunosuppressant Medications | |||
| Antibody induction | 52 (14%) | 26 (12%) | 0.61 |
| Steroids | 371 (98%) | 207 (97%) | 0.74 |
| Calcineurin Inhibitor | 365 (96%) | 202 (95%) | 0.49 |
| Cyclosporine | 61 (16%) | 34 (16%) | 0.98 |
| Tacrolimus | 308 (81%) | 174 (82%) | 0.85 |
| Third agent | 275 (72%) | 143 (67%) | 0.18 |
P-values are based on two-sample t-tests for continuous variables, and from chi-square tests for dichotomous variables.
Center-specific incidence of rejection
Clinical practices varied by center. In some centers in this retrospective study, confirmatory biopsy was obtained in most treated recipients, while empirical treatment without biopsy was more common in others (Table 2). At one site (Center A), LDLT recipients were routinely given anti-rejection treatment in the early post-transplant phase with minimal indications. The center-specific incidence of clinically treated and biopsy-proven rejection demonstrated significant differences for LDLT and DDLT recipients within the same center, and between centers (Table 2). These center differences were also significant when Center A was excluded.
Table 2.
Number and Percent of Patients with Clinically Treated and Biopsy-Proven Rejection Episodes, by Transplant Center.
| Number of Transplants | Clinically Treated Rejection Episodes | Biopsy-Proven Rejection Episodes | ||||
|---|---|---|---|---|---|---|
| Transplant Center | LDLT | DDLT | LDLT (N=380) | DDLT (N=213) | LDLT (N=380) | DDLT (N=213) |
| A | 70 | 40 | 64 (91%) | 20 (50%) | 21 (30%) | 7 (18%) |
| B | 25 | 10 | 19 (76%) | 6 (60%) | 9 (36%) | 3 (30%) |
| C | 28 | 42 | 18 (64%) | 8 (19%) | 9 (32%) | 7 (17%) |
| D | 20 | 10 | 9 (47%) | 3 (30%) | 7 (37%) | 3 (30%) |
| E | 24 | 29 | 10 (42%) | 7 (24%) | 10 (42%) | 7 (24%) |
| F | 59 | 25 | 20 (34%) | 17 (68%) | 19 (33%) | 17 (68%) |
| G | 63 | 23 | 20 (32%) | 9 (39%) | 20 (32%) | 8 (35%) |
| H | 63 | 18 | 10 (16%) | 7 (39%) | 6 (10%) | 5 (28%) |
| I | 31 | 16 | 4 (13%) | 3 (19%) | 2 (7%) | 1 (6%) |
| Chi-square Test for Centers | P<0.0001 | P=0.0012 | P=0.0034 | P=0.0002 | ||
| Chi-square Test for Centers excluding Center A | P<0.0001 | P=0.0015 | P=0.0017 | P=0.0005 | ||
Incidence of clinically treated rejection
A total of 174 (46%) LDLT recipients had at least one clinically treated rejection episode, compared to 80 (38%) DDLT recipients with at least one clinically treated rejection episode (P=0.052) (Table 3). A total of 271 clinically treated rejection episodes were reported in LDLT recipients vs. 105 clinically treated rejection episodes in DDLT recipients. The majority of these episodes were confirmed by liver biopsy (vide infra). However, some recipients were clinically treated for the diagnosis of rejection without biopsy. Excluding center A, the respective numbers (%) of clinically treated rejection episodes were 110/310 (35%) for LDLT and 60/173 (35%) for DDLT (chi-square p=0.86 and Mantel-Haenszel trend test p=0.63).
Table 3.
Number of Patients by Number of Clinically Treated or Biopsy-Proven Rejection Episodes
| Clinically Treated | Biopsy-Proven | |||
|---|---|---|---|---|
| # Rejection Episodes | LDLT (N=380) | DDLT (N=213) | LDLT (N=380) | DDLT (N=213) |
| 0 | 206 (54%) | 133 (62%) | 277 (73%) | 153 (72%) |
| 1 | 105 (28%) | 59 (28%) | 72 (19%) | 44 (14%) |
| 2 | 53 (14%) | 18 (8%) | 25 (7%) | 13 (6%) |
| ≥ 3 | 16 (4%) | 3 (1%) | 6 (2%) | 1 (0.5%) |
| Total # with Any Rejection* | 174 (46%) | 80 (38%) | 103 (27%) | 58 (27%) |
| Chi-square Test for % with Any Rejection in LDLT vs. DDLT | P=0.052 | P=0.97 | ||
| Mantel-Haenszel Trend Test | P=0.006 | P=0.65 | ||
Excluding Center A, the respective numbers (%) of clinically treated rejection episodes were 110/310 (35%) for LDLT and 60/173 (35%) for DDLT (chi-square p=0.86 and Mantel-Haenszel trend test p=0.63). Excluding Center A had no effect on the statistical comparisons between LDLT and DDLT for biopsy-proven rejection episodes: the respective numbers (%) of biopsy-proven rejection episodes were 82/310 (26%) for LDLT and 51/173 (29%) for DDLT (chi-square p=0.48 and Mantel-Haenszel trend test p=0.78).
Incidence of biopsy-proven rejection
A total of 103 (27%) LDLT recipients had at least one biopsy-proven rejection episode and 58 (27%) DDLT recipients had at least one biopsy-proven rejection episode (P=0.97) (Table 3). A total of 143 biopsy-proven rejection episodes were reported in LDLT recipients vs. 73 biopsy-proven rejection episodes in DDLT recipients. These results demonstrated a similar rate of biopsy-proven rejection in the LDLT and DDLT groups, with or without Center A.
Incidence of first vs. recurrent episodes of clinically treated and/or biopsy-proven rejection
LDLT and DDLT recipients experienced a similar incidence of initial episodes of biopsy-proven rejection (Table 3). Interestingly, there was a similar proportion of recipients who were clinically treated for a single episode of rejection (with or without confirmation by liver biopsy) in LDLT and DDLT recipients. However, more LDLT recipients were clinically treated for second and third episodes of rejection when compared to the DDLT group (Table 3, P=0.006). Taken together, these data suggest that there was a lower threshold for empirical treatment of clinically suspected recurrent rejection in LDLT recipients. However, after excluding Center A, LDLT recipients were not significantly more likely to be clinically treated for recurrent rejection (P=0.63). The data do not provide an identifiable reason or other relevant information to explain and/or justify treatment without biopsy.
Time to first rejection
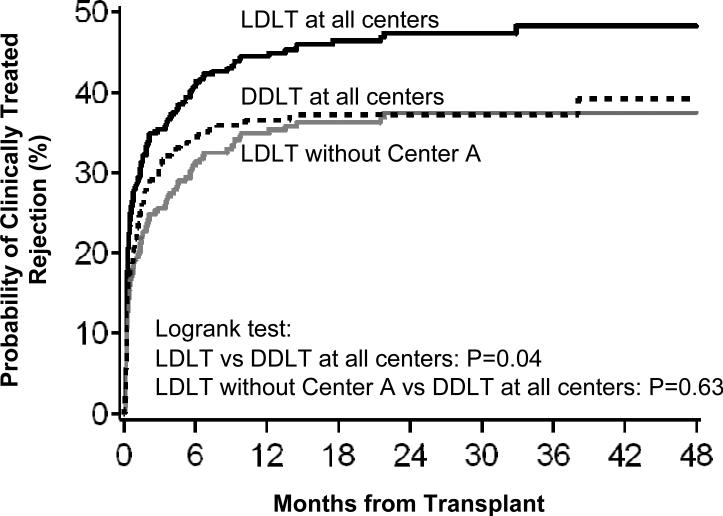
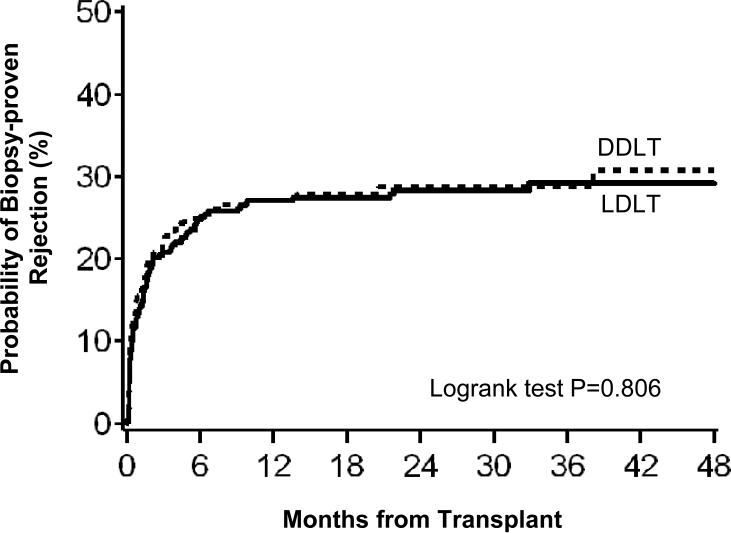
The majority of first acute rejection episodes were diagnosed in the first year after transplantation (Figures 1 and 2). Consistent with the analyses above, the time to first clinically treated rejection (Figure 1) was shorter in LDLT than DDLT recipients when all nine sites were included (P=0.044), but not when Center A was excluded from LDLT group (P=0.63). For biopsy-proven rejection (Figure 2), the time from transplant to first occurrence was similar for LDLT and DDLT recipients (P=0.81), whether or not Center A was included (P=0.71).
Figure 1.
Time interval from transplant to first clinically treated rejection for LDLT (with and without Center A) and DDLT recipients.
Figure 2.
Time interval from transplant to first biopsy-proven rejection for LDLT and DDLT recipients.
Variables associated with the incidence of biopsy-proven rejection
In a multivariable Cox regression model of time to biopsy-proven rejection, we observed a higher relative risk of rejection for patients with autoimmune liver disease (HR=1.87, P=0.038) and for patients with HCV diagnosis (HR=1.53, P=0.011). Hispanic patients had a higher risk of rejection compared to non-Hispanic patients although the difference was not statistically significant (HR=1.41, P=0.066) (Table 4 Model A). Similar to the Kaplan-Meier analysis in Figure 2, no significant difference was found in the overall adjusted risk of biopsy-proven rejection between LDLT and DDLT in Model B (P=0.75). In addition, the lack of a significant interaction (P=0.77) between LDLT and autoimmune disease suggested that there were no alloimmune-related benefits of LDLT compared to DDLT. Antibody induction therapy in the immediate post-transplant period was not associated with biopsy-proven rejection (P=0.20) or a differential immunological advantage in LDLT vs. DDLT recipients (interaction P=0.38).
Table 4.
Multivariable Cox Regression Models of Time to Biopsy-Proven Rejection*
| Hazard Ratio | 95% Confidence Limits | P-Value | ||
|---|---|---|---|---|
| Model A: | ||||
| Autoimmune liver disease | 1.87 | 1.04 | 3.39 | 0.038 |
| HCV diagnosis | 1.53 | 1.10 | 2.13 | 0.011 |
| Hispanic | 1.41 | 0.98 | 2.02 | 0.066 |
|
Model B (includes all covariates in Model A, plus LDLT vs. DDLT): | ||||
| LDLT | 0.95 | 0.69 | 1.31 | 0.752 |
|
Model C (includes all covariates in Model A, plus cold ischemia time): | ||||
| Cold ischemia time (per hour) | 1.04 | 1.00 | 1.09 | 0.050 |
|
Model D (includes all covariates in Model A, plus LDLT vs. DDLT and cold ischemia time): | ||||
| LDLT | 1.63 | 0.90 | 2.95 | 0.105 |
| Cold ischemia time (per hour) | 1.10 | 1.02 | 1.17 | 0.010 |
Variables tested but not significant included recipient age, gender, race, diagnosis of hepatocellular carcinoma and cholestatic liver disease, transplant year, and antibody induction, and donor age.
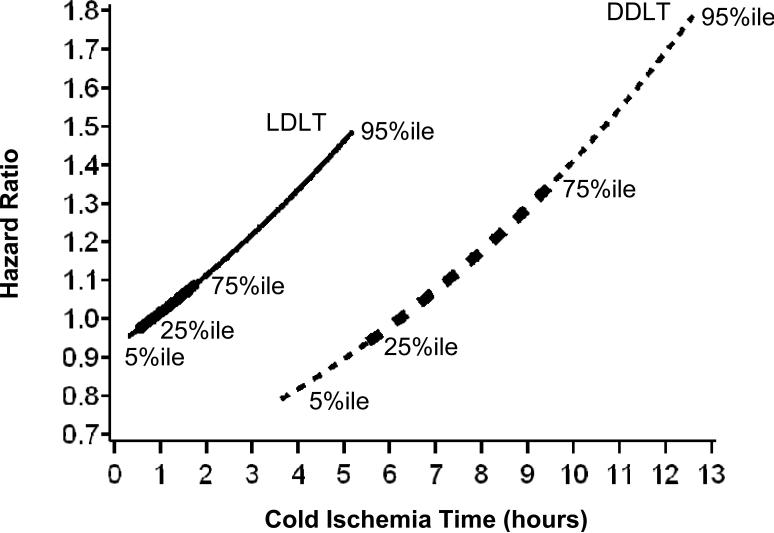
When cold ischemia time was added to the Cox regression model, we observed that the risk of biopsy proven rejection for all liver transplant recipients was higher with longer cold ischemia time (HR=1.04 per hour, P=0.050) (Table 4 Model C). When cold ischemia time was added to the model, for a given cold ischemia time, LDLT was associated with a higher risk of rejection than DDLT, although the difference did not meet the traditional level of statistical significance (HR=1.63, P=0.10). Because cold ischemia time is generally much longer for DDLT than for LDLT recipients (DDLT: median 465 minutes, range 75 to 1230 minutes; LDLT: median 53 minutes, range 10 to 840 minutes; P<0.001), the cold ischemia time and LDLT effects must be interpreted simultaneously to estimate the risk of rejection for recipients of the average LDLT compared to the average DDLT. Figure 3 shows this relationship by plotting the relative risk of rejection for LDLT and DDLT transplants by cold ischemia time. For example, the risk of rejection for an LDLT with 50 minutes of cold ischemia time is similar to that of a DDLT with 380 minutes of cold ischemia time. After adjusting for transplant type (LDLT vs. DDLT), longer cold ischemia time was associated with increased risk of rejection (HR=1.10; P=0.01) (Table 4 Model D).
Figure 3.
Relative risk of biopsy-proven rejection for LDLT and DDLT transplants by cold ischemia time. The reference (hazard ratio = 1.0) is LDLT with 50 minutes of cold ischemia time. The hazard ratios are shown for the 5th to 95th percentiles of cold ischemia time within LDLT and DDLT. In addition, the lines are thicker in the regions of the 25th to 75th percentiles of cold ischemia time.
Rejection as a cause of graft loss or death
Of the 380 LDLTs, 117 experienced graft failure (KM probabilities: 20% by one year and 26% by three years). Six of 117 (5.1%) were reported to be associated with acute rejection and one was due to chronic rejection. Of the 213 DDLTs, 55 experienced graft failure (KM probabilities: 13% by one year and 25% by three years). None was reported to be associated with acute rejection and one of 55 (1.8%) was due to chronic rejection. Unadjusted rates of graft failure due to rejection were not significantly different between LDLT and DDLT grafts (P=0.16).
DISCUSSION
We hypothesized that LDLT, when compared to DDLT, presents a unique inflammatory and immunological setting that may affect immediate and long term alloimmune response. This hypothesis was based on observations seen after living donor kidney transplantation in which the live donor kidney is associated with favorable immunological and function outcomes in the short and long term. Experimental and clinical observations suggest that these advantages are attributable to donor quality, a relatively short period of cold ischemia resulting in less injury from proinflammatory mediators, and to shared HLA haplotypes in the majority of biologically related living donor kidney transplants (12-14). These variables, in aggregate, contribute to better outcomes of kidney transplants performed using living donors.
Similar issues may affect outcomes after transplantation of living donor liver allografts, with the addition of a few important processes that are unique to the regenerating liver lobe. In this context, we have identified four principal differences between LDLT and DDLT, including those related to donor quality, the proinflammatory response affected by cold ischemia, upregulated genomic activity as a result of liver regeneration, and better HLA matching between donors and recipients. The aims of the current study were to explore, in aggregate, the effects of these considerations on immune-related injury and survival of the allograft in the short and long term after transplantation. We expected that these injuries would be expressed in the clinical setting by the rates of acute rejection and/or graft loss related to rejection. It is important to recognize that whereas these clinical outcomes would not be attributable to a particular pre-transplant liver diagnosis, observation of a significant difference in the rejection pattern between LDLT vs. DDLT would suggest that immunosuppression protocols should be individualized in each setting to reduce the rates of rejection and consequent graft loss. Better understanding of mechanisms and reducing episodes of rejection are beneficial to patients since the treatment options for ACR are associated with harmful outcomes that are related to the side effects of higher doses of calcineurin inhibitors, steroids, and/or antibody therapy.
In adult recipients, LDLT is done under elective circumstances in which a healthy individual undergoes removal of a lobe (usually the right lobe) of the liver. This is very different than the brain dead donor that has experienced massive physiological and hormonal shifts, all of which may be harmful to the liver (15). In addition, the living donor procedure is well coordinated with the recipient operation, resulting in short cold ischemia time (16). Preliminary genomic studies on liver biopsies taken from brain dead and living donor liver donors demonstrate differences in regulation of proinflammatory response genes, which may reflect the differential degree of liver injury or relate to the duration of cold ischemia (17, 18). Lessons learned in the deceased donor kidney transplant setting demonstrate that donor-related injury and prolonged cold ischemia time are associated with a higher rate of delayed graft function, and a subsequent increase in the frequency of ACR (19). This may be different for recipients of kidney from a living donor, in whom cold ischemia and delayed graft function may not expose the organ to a higher rate of ACR (20).
The similar rate of biopsy-proven rejection in our large groups of LDLT and DDLT recipients suggest that the impact of the type of allograft on the frequency of ACR is relatively minimal. Proinflammatory pathways may be activated by brain death, cold ischemia, or during induction of liver regeneration. Whereas the clinical outcomes appear to be the same, we are able to clearly demonstrate in both groups an important association between the length of cold ischemia and the frequency of biopsy proven ACR. However, it appears that the living donor liver is far more susceptible to prolonged cold ischemia than the deceased donor allograft. We have given the example that a LDLT allograft with only 50 minutes of cold ischemia has the same risk of ACR as a DDLT allograft with 380 minutes of cold ischemia time. The potential for development of ACR increases in both groups as organs are kept under cold preservation. It was expected that exposure to a shorter cold ischemia time in the LDLT setting would be associated with decreased cold ischemia-mediated injury, manifested via differential regulation of proinflammatory pathways. In theory, decreased proinflammation may affect the pattern of alloimmune response. However, other variables must be considered including the initiation of regenerative pathways in the LDLT allograft, which may also have some impact on alloimmunity. We suggest that the net result of these processes should be taken into consideration when correlating cold ischemia time and ACR in the LDLT and DDLT settings. A practical interpretation of these clinical data strongly suggests that all liver allografts should be exposed to the shortest possible period of cold ischemia, which may in turn be associated with a reduced incidence of ACR, and avoid potential harmful immune and non-immune consequences such as chronic rejection and recurrence of the primary liver disease.
It has been well established that liver regeneration starts within minutes after partial hepatectomy and is associated with upregulation of cell cycle genes (21, 22). Recent genomic analysis of deceased donor and living donor liver biopsy done within one hour after reperfusion demonstrated additional differential regulation of proinflammatory and immune response gene expression (6, 18). Microarray analysis revealed significant changes in the activation and deactivation of multiple chemokine and interleukin genes, adhesion molecules, and antigen processing genes. While similarities existed between DDLT and LDLT, each group also expressed unique patterns of up- and down-regulation of sets of proinflammatory and immunoregulatory genes. Intuitively, the differences in proinflammatory and immune gene activation in LDLT vs. DDLT should have been associated with a differential activation of alloimmune response, and consequently, a difference in the pattern and rate of rejection. In the clinical setting, however, these differences were not seen either in the early post-transplant period or at one and three years. It appears that the immunosuppression strategies employed were similarly effective in preventing biopsy-proven rejection in the two groups.
The potential beneficial effect of more similar HLA identity in the living donor setting has not been proven for adult or pediatric patients receiving partial liver allografts from blood relatives (23, 24). Similarly, we are unable to comment whether this variable has any short or long term impact in living donor liver recipients, and are cognizant that such data may require analysis of larger cohorts of patients.
Analysis of our data reaffirmed previous observations related to the impact of the etiology of the underlying liver disease on biopsy-proven rejection with respect to autoimmune disease. In addition, we now show that hepatitis C virus infection as a cause of liver failure is a significant predictor of rejection.
Our study revealed interesting findings related to the practice of treating rejection in the participating centers. The gold standard for the diagnosis of rejection requires biopsy confirmation. However, we observed that 19% and 11% of LDLT and DDLT recipients, respectively, were treated for rejection based on clinical judgment alone by the transplant team without a confirmatory biopsy. Even excluding one site where 91% of LDLT recipients had clinically treated rejection (Center A), 9% and 6%, respectively, were treated without biopsy.
Overall, a significantly higher proportion of LDLT (vs. DDLT) recipients had clinically treated rejection, but this difference also varied greatly among centers. When we excluded Center A, the proportion of LDLT recipients with clinically treated rejection was identical to that for DDLT (35%). Similarly, the times to first clinically treated rejection were the same for LDLT and DDLT when Center A was excluded. Thus, at some centers, there may be a lower threshold for treating LDLT recipients for rejection in response to fluctuating elevated liver enzymes, and/or reluctance to perform a biopsy in the setting of a partial allograft. We conclude that these represent center-specific practices. It remains to be seen whether such divergences in practice will be observed in the prospective A2ALL cohort study, in which immunosuppression is more protocol-driven than in the current retrospective study.
There are some limitations of this retrospective observational cohort study. We did not have detailed information (e.g., drug dosages and blood levels) regarding baseline immunosuppression practices beyond the drug regimens used at discharge from the transplant hospitalization and those used for maintenance immunosuppression at the time of rejection episodes. We did not have information on the specific indications for treatment of clinically suspected rejection, such as laboratory values or exclusionary diagnostic studies that were obtained prior to initiation of anti-rejection therapy. Since there was no central pathology reading, there may have been differences in histological interpretations among centers. Despite these shortcomings, our results present a picture of clinical practice on the basis of information available to treating physicians.
Based on the results reported here, we conclude that there is no demonstrable immunological advantage under current standard immunosuppression and clinical practices for recipients who undergo LDLT. The current results do not support the use of different immunosuppression protocols for LDLT vs. DDLT recipients. In either setting, a beneficial impact on immune related outcomes may be achieved by minimizing the duration of cold ischemia of the allograft.
ACKNOWLEDGMENTS
This study was supported by National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (listed below). Additional support was provided by Health Resources and Services Administration (HRSA), and the American Society of Transplant Surgeons (ASTS).
The following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
Columbia University Health Sciences, New York, NY (DK62483): PI: Jean C. Emond, MD; Co-PI: Robert S. Brown, Jr., MD, MPH; Study Coordinators: Rudina Odeh-Ramadan, PharmD; Scott Heese, BA
Northwestern University, Chicago, IL (DK62467): PI: Michael M.I. Abecassis, MD, MBA; Co-PI: Andreas Blei, MD; Study Coordinator: Patrice Al-Saden, RN, CTCC
University of Pennsylvania Health System, Philadelphia, PA (DK62494): PI: Abraham Shaked, MD, PhD; Co-PI: Kim M. Olthoff, MD; Study Coordinators: Mary Kaminski, PA-C; Mary Shaw, RN, BBA
University of Colorado Health Sciences Center, Denver, CO (DK62536): PI: James F. Trotter, MD; Co-PI: Igal Kam, MD; Study Coordinators: Carlos Garcia, BS
University of California Los Angeles, Los Angeles, CA (DK62496): PI: Ronald W. Busuttil, MD, PhD; Co-PI: Sammy Saab, MD; Study Coordinator: Janet Mooney, RN, BSN
University of California San Francisco, San Francisco, CA (DK62444): PI: Chris E. Freise, MD, FACS; Co-PI: Norah A. Terrault, MD; Study Coordinator: Dulce MacLeod, RN
University of Michigan Medical Center, Ann Arbor, MI (DK62498): PI: Robert M. Merion, MD; DCC Staff: Anna S.F. Lok, MD; Akinlolu O. Ojo, MD, PhD; Brenda W. Gillespie, PhD; Margaret Hill-Callahan, BS, LSW; Terese Howell, BS; Lan Tong, MS; Tempie H. Shearon, MS; Karen A. Wisniewski, MPH; Monique Lowe, BS
University of North Carolina, Chapel Hill, NC (DK62505): PI: Paul H. Hayashi, MD; Study Coordinator: Carrie A. Nielsen, MA
University of Virginia (DK62484): PI: Carl L. Berg, MD; Co-PI: Timothy L. Pruett, MD; Study Coordinator: Jaye Davis, RN
Medical College of Virginia Hospitals, Virginia Commonwealth University, Richmond, VA (DK62531): PI: Robert A. Fisher, MD, FACS; Co-PI: Mitchell L. Shiffman, MD; Study Coordinators: Ede Fenick, RN; April Ashworth, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: James E. Everhart, MD; Leonard B. Seeff, MD; Patricia R. Robuck, PhD; Jay H. Hoofnagle, MD
Funding Sources: Supported in part by the National Institutes of Health (NIDDK grant numbers U01-DK62536, U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531), the American Society of Transplant Surgeons, and the U.S. Department of Health and Human Services, Health Resources and Services Administration.
Footnotes
Presented in part at the World Transplant Congress, Boston, MA, July, 2006.
Supported in part by the National Institutes of Health (NIDDK grant numbers U01-DK62536, U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531), the American Society of Transplant Surgeons, and the U.S. Department of Health and Human Services, Health Resources and Services Administration.
This is publication number 10 of the Adult-to-Adult Living Donor Liver Transplantation Cohort Study.
Supplemental data included here have been supplied by the Arbor Research Collaborative for Health as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
REFERENCES
- 1.Kim MS, Kim DK, Myoung SM, et al. Chronologically different impacts of immunologic and non-immunologic risk factors on renal allograft function. Clin Transplant. 2005;19(6):742–50. doi: 10.1111/j.1399-0012.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- 2.van der Hoeven JA, Molema G, Ter Horst GJ, et al. Relationship between duration of brain death and hemodynamic instability on progressive dysfunction and increased immunologic activation of donor kidneys. Kidney Int. 2003;64(5):1874–82. doi: 10.1046/j.1523-1755.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqi N, McBride MA, Hariharan S. Similar risk profiles for post-transplant renal dysfunction and long-term graft failure: UNOS/OPTN database analysis. Kidney Int. 2004;65(5):1906–13. doi: 10.1111/j.1523-1755.2004.00589.x. [DOI] [PubMed] [Google Scholar]
- 4.Krieger NR, Becker BN, Heisey DM, et al. Chronic allograft nephropathy uniformly affects recipients of cadaveric, nonidentical living-related, and living-unrelated grafts. Transplantation. 2003;75(10):1677–82. doi: 10.1097/01.TP.0000063830.60937.06. [DOI] [PubMed] [Google Scholar]
- 5.Cosio FG, Grande JP, Larson TS, et al. Kidney allograft fibrosis and atrophy early after living donor transplantation. Am J Transplantation. 2005;5(5):1130–6. doi: 10.1111/j.1600-6143.2005.00811.x. [DOI] [PubMed] [Google Scholar]
- 6.Borozan I, Chen L, Sun J, et al. Gene expression profiling of acute liver stress during living donor liver transplantation. Am J Transplantation. 2006;6(4):806–24. doi: 10.1111/j.1600-6143.2006.01254.x. [DOI] [PubMed] [Google Scholar]
- 7.Debonera F, Wang G, Xie J, et al. Severe preservation injury induces Il-6/STAT3 activation with lack of cell cycle progression after partial liver graft transplantation. Am J Transplantation. 2004;4(12):1964–71. doi: 10.1111/j.1600-6143.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- 8.Shaked A, Hulbert-Shearon TE, Everhart J, Merion RM, the A2ALL Investigator Study Group Rejection is decreased among adult recipients of living donor vs. cadaveric liver allografts. Am J Transplantation. 2004;4(Suppl 8):268. [Google Scholar]
- 9.Maluf DG, Stravitz RT, Cotterell AH, et al. Adult living donor versus deceased donor liver transplantation: a 6-year single center experience. Am J Transplantation. 2005;5(1):149–56. doi: 10.1111/j.1600-6143.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu LU, Bodian CA, Gondolesi GE, et al. Marked differences in acute cellular rejection rates between living-donor and deceased-donor liver transplant recipients. Transplantation. 2005;80(8):1072–80. doi: 10.1097/01.tp.0000176483.52769.5a. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie BW, Merion RM, Ojo AO, et al. Comparison of patient information in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) and the Scientific Registry of Transplant Recipients (SRTR) database. Am J Transplantation. 2008;8(Suppl 2):289. [Google Scholar]
- 12.Schuurs TA, Morariu AM, Ottens PJ, et al. Time-dependent changes in donor brain death related processes. Am J Transplantation. 2006;6(12):2903–11. doi: 10.1111/j.1600-6143.2006.01547.x. [DOI] [PubMed] [Google Scholar]
- 13.Coulson MT, Jablonski P, Howden BO, Thomson NM, Stein AN. Beyond operational tolerance: effect of ischemic injury on development of chronic damage in renal grafts. Transplantation. 2005;80(3):353–61. doi: 10.1097/01.tp.0000168214.84417.7d. [DOI] [PubMed] [Google Scholar]
- 14.Opelz G, Wujciak T, Döhler B, Scherer S, Mytilineos J. HLA compatibility and organ transplant survival. Collaborative Transplant Study. Rev Immunogenet. 1999;1(3):334–42. [PubMed] [Google Scholar]
- 15.Kutsogiannis DJ, Pagliarello G, Doig C, Ross H, Shemie SD. Medical management to optimize donor organ potential: review of the literature. Can J Anaesth. 2006;53(8):820–30. doi: 10.1007/BF03022800. [DOI] [PubMed] [Google Scholar]
- 16.Olthoff KM, Merion RM, Ghobrial RM, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL consortium. Ann Surg. 2005;242(3):314–23. doi: 10.1097/01.sla.0000179646.37145.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss S, Kotsch K, Francuski M, et al. Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. Am J Transplantation. 2007;7(6):1584–93. doi: 10.1111/j.1600-6143.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 18.De Jonge J, Kurian S, Shaked A, Salomon DR, Olthoff KM. Gene expression profiles of hepatic regeneration and metabolism in human living and deceased donor liver transplants (submitted)
- 19.Quiroga I, McShane P, Koo DD, et al. Major effects of delayed graft function and cold ischaemia time on renal allograft survival. Nephrol Dial Transplant. 2006;21(6):1689–96. doi: 10.1093/ndt/gfl042. [DOI] [PubMed] [Google Scholar]
- 20.Simpkins CE, Montgomery RA, Hawxby AM, et al. Cold ischemia time and allograft outcomes in live donor renal transplantation: is live donor organ transport feasible? Am J Transplantation. 2007;7(1):99–107. doi: 10.1111/j.1600-6143.2006.01597.x. [DOI] [PubMed] [Google Scholar]
- 21.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5(10):836–47. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 22.Debonera F, Krasinkas AM, Gelman AE, et al. Dexamethasone inhibits early regenerative response of rat liver after cold preservation and transplantation. Hepatology. 2003;38(6):1563–72. doi: 10.1016/j.hep.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 23.Navarro V, Herrine S, Katopes C, Colombe B, Spain CV. The effect of HLA class I (A and B) and class II (DR) compatibility on liver transplantation outcomes: an analysis of the OPTN database. Liver Transplantation. 2006;12(4):652–8. doi: 10.1002/lt.20680. [DOI] [PubMed] [Google Scholar]
- 24.Sieders E, Hepkema BG, Peeters PM, et al. The effect of HLA mismatches, shared cross-reactive antigen groups, and shared HLA-DR antigens on the outcome after pediatric liver transplantation. Liver Transplantation. 2005;11(12):1541–9. doi: 10.1002/lt.20521. [DOI] [PubMed] [Google Scholar]