Abstract
A wider application of living donor liver transplantation is limited by donor morbidity concerns. An observational cohort of 760 living donors accepted for surgery and enrolled in the Adult-to-Adult Living Donor Liver Transplantation cohort study provides a comprehensive assessment of incidence, severity and natural history of living liver donation (LLD) complications. Donor morbidity (assessed by 29 specific complications), predictors, time from donation to complications and time from complication onset to resolution were measured outcomes over a 12-year period. Out of the 760 donor procedures, 20 were aborted and 740 were completed. Forty percent of donors had complications (557 complications among 296 donors), mostly Clavien grades 1 and 2. Most severe counted by complication category; grade 1 (minor, n = 232); grade 2 (possibly life-threatening, n = 269); grade 3 (residual disability, n = 5) and grade 4 (leading to death, n = 3). Hernias (7%) and psychological complications (3%) occurred >1 year postdonation. Complications risk increased with transfusion requirement, intraoperative hypotension and predonation serum bilirubin, but did not decline with the increased center experience with LLD. The probability of complication resolution within 1 year was overall 95%, but only 75% for hernias and 42% for psychological complications. This report comprehensively quantifies LLD complication risk and should inform decision making by potential donors and their caregivers.
Keywords: Death risk, donation, donor outcomes, liver transplant, living donor
Introduction
Broader application of living donor liver transplants (LDLT), particularly for adult recipients, requires a comprehensive assessment of donor risk. One of the primary goals of the Adult-to-Adult living donor liver transplantation cohort study (A2ALL) was to analyze the incidence and significance of living liver donor (LLD) complications in a multicenter cohort of United States (US) transplant centers. It was felt that a comprehensive compilation of pre-, intra- and postdonation clinical parameters would allow for analyses that would inform an assessment of risk.
We previously published the complications in a retrospective A2ALL cohort of LLD (1). Prospective enrollment of an observational A2ALL cohort by the same participating centers is now complete, allowing us to answer several questions: (1) has the incidence and severity of LLD complications changed over time? (2) are there new or different risk factors associated with postdonation complications that were not previously identified? (3) should future data collection on the risk of LLD complications be more focused? and (4) does longer follow-up yield relevant data on late-onset LLD complications or insight into resolution of complications once they occur? The current study was designed to address each of these questions.
Methods
Study design and cohort era definitions
The A2ALL consortium includes nine US transplant centers, and collected data on subjects evaluated at these centers for LLD between 1/1/98 and 8/31/09, with follow-up extending through 8/31/10. Subjects included in this report were accepted for donation and went to the operating room with the intention to donate. Data on potential donors undergoing evaluation on or before 2/28/03 were collected retrospectively (retrospective era). At initiation of the prospective study, retrospective era potential or actual donors were invited to enroll for prospective follow-up from that point forward. For the subset that could not be contacted for consent, data were updated in 2008 under a waiver of consent. Among the retrospective era donors, 22% of the total postdonation follow-up time was prospective. Those who underwent evaluation for donation between 2/28/03 and the date of site initiation (bridge donors) were also invited to enroll in ongoing prospective follow-up, along with those who were evaluated after study site initiation (prospective donors); 23% of postdonation follow-up time in this cohort (bridge donors + prospective donors = prospective era) was retrospective, and 77% was prospective. Median postdonation follow-up was 3.4 years (retrospective era; range [0–10.4 years]) and 1.8 years (prospective era; range [0.01–6.9 years]). Data on 29 specific complications were collected for each donor. For each recorded complication, information required for Clavien grading (2-4) (Table 1) and the dates of onset and resolution were recorded. Previously published data from the retrospective era (1) were updated for complication occurrences, Clavien grade and time to resolution.
Table 1.
Clavien system for classification of negative outcomes in general surgery and solid organ transplantation (2–4)
| grade 1 | Any alteration from ideal postoperative course with complete recovery or which can be easily controlled and which fulfills the general characteristics:
|
| grade 2 | Any complication that is potentially life-threatening or results in ICU stay ≥5 days, hospital stay ≥4 weeks for the recipient or ≥2 weeks for the donor, but which does not result in residual disability or persistent diseases. |
| grade 3 | Any complication with residual or lasting functional disability or development of malignant disease. |
| grade 4 | Complications that lead to transplantation (grade 4a) or death (grade 4b) |
ICU = intensive care unit.
Grades were calculated by checking criteria from highest to lowest grade. Any reported complication that did not meet grade 2–4 criteria was considered to be grade 1. In particular, criteria 1(b) through 1(e) were not necessarily applied due to potential inconsistencies with criteria for higher grades. For example, in the A2ALL, median ICU stay was 1 day, and median hospital stay was 3 days; using criterion (e), an ICU stay of 4 days would exceed the criteria for grade 1 but not meet criteria for grade 2.
Statistical methods
Donor and intraoperative characteristics are presented using descriptive statistics. Complications were tabulated by type and grade, using the highest grade for donors with multiple complications. The Kaplan–Meier method was used to estimate the unadjusted probability of complications by time since donation, and the probability of resolution of complications by time since complication onset, censored at the earliest of last known follow-up or death. The log-rank test was used to compare complication probabilities between those enrolled in the retrospective and prospective eras. Multivariable Cox regression models were fitted to identify significant predictors of complication risk and time to resolution; covariate effects are expressed as estimated hazard ratios (HR), and 95% confidence intervals (CI). Covariates tested (all measured at the time of donation) included donor age, sex, ethnicity, race, height, weight, BMI, relatedness to recipient, alkaline phosphatase level, total bilirubin level, donated lobe (left vs. right), remnant liver weight, ratio of remnant to original liver volume, amount of transfused blood, intraoperative hypotension, length of operation, year of donation and center-specific experience with living donation (defined as sequential donor case number at each center). In addition, three measures of liver regeneration by 3 months (absolute growth, percentage growth and percentage regeneration) were individually tested in Cox models as possible predictors for the following outcomes beyond 3 months: any complication, bile leak, infection and hernia. All analyses were carried out using SAS 9.2 statistical software (SAS Institute Inc., Cary, NC, USA).
Human subjects protection
The study was approved by the Institutional Review Boards of the University of Michigan Data Coordinating Center and each of the A2ALL programs.
Results
Baseline and intraoperative donor characteristics
Among the 1870 potential LLDs evaluated across the entire observational cohort (retrospective and prospective), 760 went to the operating room with an intention to donate, 740 underwent complete donation and 738/740 grafts were transplanted into the intended recipient. There were 707 right lobe and 33 full left lobe donations. Preoperative characteristics are shown in Table 2A for the 740 donors by era of donation (retrospective, n = 408; prospective, n = 352). Given the absence of any important differences between eras (only alkaline phosphatase levels were different [p < 0.0001] but 96% were below 117 IU/L), retrospective and prospective era study subjects were combined for some subsequent analyses. Out of the 20/760 (2.6%) aborted procedures, 12/408 (2.9%) occurred in the retrospective era and 8/352 (2.3%) in the prospective era. Reasons for aborting the procedure were similar in both eras and included unexpected observations during either donor or recipient procedures. One retrospective era aborted donor had a bile leak, bacterial infection and localized intraabdominal abscess, each of which was Clavien grade 2. The 20 aborted donors were not included in the subsequent analyses.
Table 2A.
Characteristics of adult LLD candidates who went to the operating room with the intention to donate
| Retrospective Era donors (N = 408) |
Prospective Era donors (N = 352) |
|||||
|---|---|---|---|---|---|---|
| Characteristic | N | Range | Mean (s.d.) or percent | N | Range | Mean (s.d.) or percent |
| Age at donor evaluation | 408 | 18–59 | 37(10) | 352 | 20–63 | 38(10) |
| Sex | ||||||
| Male | 227 | 56% | 173 | 49% | ||
| Female | 181 | 44% | 179 | 51% | ||
| Ethnicity | ||||||
| Hispanic/Latino | 76 | 18% | 47 | 13% | ||
| Nonhispanic/Nonlatino | 331 | 82% | 305 | 87% | ||
| Missing | 1 | 0 | ||||
| Race | ||||||
| White | 367 | 90% | 324 | 92% | ||
| African–American | 15 | 4% | 11 | 3% | ||
| Asian | 13 | 3% | 5 | 1% | ||
| Other | 12 | 3% | 12 | 3% | ||
| Missing | 1 | 0 | ||||
| Height (cm) | 402 | 150–203 | 172 (10) | 350 | 135–196 | 172 (10) |
| Weight (kg) | 407 | 43–141 | 78 (15) | 345 | 44–135 | 78 (16) |
| Body mass index (kg/m2) | 402 | 17–43 | 26 (4) | 344 | 16–42 | 26 (4) |
| BMI < 20 | 19 | 5% | 8 | 2% | ||
| 20 ≤ BMI < 25 | 136 | 33% | 130 | 38% | ||
| 25 ≤ BMI < 30 | 183 | 46% | 150 | 44% | ||
| BMI ≥30 | 64 | 16% | 54 | 16% | ||
| Missing | 6 | 8 | ||||
| Relatedness to recipient | ||||||
| Biologically related | ||||||
| Parent | 9 | 2% | 17 | 5% | ||
| Child | 141 | 35% | 121 | 35% | ||
| Sibling | 93 | 23% | 64 | 18% | ||
| Other biological | 34 | 8% | 27 | 8% | ||
| Not biologically related | ||||||
| Spouse | 52 | 13% | 28 | 8% | ||
| Other nonbiological | 78 | 19% | 92 | 26% | ||
| Unknown/Missing | 0 | 3 | ||||
| Alkaline phosphatase1 (IU/L) | 407 | 21–197 | 75 (26) | 350 | 15–179 | 65 (20) |
| Alkaline phosphatase ≤55 | 89 | 22% | 117 | 34% | ||
| 55< Alkaline phosphatase ≤65 | 73 | 18% | 77 | 22% | ||
| 65< Alkaline phosphatase ≤80 | 119 | 29% | 99 | 28% | ||
| 80< Alkaline phosphatase | 126 | 31% | 55 | 16% | ||
| Missing | 1 | 2 | ||||
| Bilirubin (mg/dL) | 407 | 0.1–2.8 | 0.7 (0.4) | 351 | 0–2.8 | 0.7 (0.4) |
| Bilirubin ≤ 0.5 | 144 | 35% | 114 | 32% | ||
| 0.5 < Bilirubin ≤ 0.7 | 120 | 29% | 94 | 27% | ||
| 0.7 < Bilirubin ≤ 0.9 | 69 | 17% | 73 | 21% | ||
| 0.9 < Bilirubin | 74 | 18% | 70 | 20% | ||
| Missing | 1 | 1 | ||||
Difference between retrospective and prospective era donors was significant only for alkaline phosphatase (p < 0.0001).
Intraoperative donor characteristics are shown in Table 2B for the 740 individuals who underwent a completed donor lobectomy. Differences between the retrospective and prospective cohorts were significant for remnant liver weight (on average 28 g higher in the retrospective era; p = 0.015), units of transfused blood (0.2 units more in the retrospective era; p < 0.0001), intraoperative hypotension (more common in the prospective era; p < 0.0001), operative time (97 min longer for retrospective era donors; p < 0.0001) and donor lobe resected (more left lobe donors in the prospective era; p = 0.002).
Table 2B.
Intraoperative characteristics of adult living liver donors who underwent graft resection1
| Retrospective Era donors (N = 396) |
Prospective Era donors (N = 344 ) |
|||||
|---|---|---|---|---|---|---|
| Characteristic | N | Range | Mean (SD) or percent | N | Range | Mean (SD) or percent |
| Remnant liver weight2,3 (grams) | 393 | 169–1152 | 576 (156) | 344 | 195–1605 | 548 (152) |
| Weight ≤ 470 | 99 | 25% | 110 | 32% | ||
| 470 < Weight ≤ 570 | 87 | 22% | 91 | 26% | ||
| 570 < Weight ≤ 660 | 96 | 24% | 78 | 23% | ||
| 660 < Weight | 111 | 28% | 63 | 18% | ||
| Missing | 3 | 0 | ||||
| Units of transfused blood3 | 392 | 0–4 | 0.4 (0.8) | 339 | 0-6 | 0.2 (0.6) |
| 0 | 272 | 69% | 284 | 84% | ||
| 1 | 84 | 21% | 48 | 14% | ||
| 2 | 26 | 7% | 3 | <1% | ||
| 3 | 6 | 2% | 3 | <1% | ||
| 4 | 4 | 1% | 0 | 0% | ||
| 6 | 0 | 0% | 1 | <1% | ||
| Missing | 4 | 5 | ||||
| Hypotension3 (< 100 mmHg systolic) | ||||||
| Yes | 89 | 23% | 162 | 48% | ||
| Total duration (minutes) | 3-455 | 62 (68) | 5-420 | 73 (77) | ||
| No | 291 | 77% | 180 | 52% | ||
| Missing | 16 | 2 | ||||
| Operative time3 (minutes) | 333 | 236-930 | 457 (133) | 340 | 185-770 | 360 (111) |
| Time ≤ 320 | 44 | 13% | 137 | 40% | ||
| 320 < Time ≤ 380 | 76 | 23% | 96 | 28% | ||
| 380 < Time ≤ 480 | 82 | 25% | 57 | 17% | ||
| 480 < Time | 131 | 39% | 50 | 15% | ||
| Missing | 63 | 4 | ||||
| Donor lobe resected3 | ||||||
| Right | 387 | 98% | 320 | 93% | ||
| Left | 9 | 2% | 24 | 7% | ||
Donors who successfully donated (Retrospective Era: N = 395, Prospective Era: N = 343) and those with graft resected but not transplanted into the intended recipient (Retrospective Era: N = 1, Prospective Era: N = 1).
Remnant liver weight was obtained from imaging at evaluation (Retrospective Era: 69%, Prospective Era: 98%) or 0.4 × donor Standard Liver Volume (Retrospective Era: 30%, Prospective Era: 2%).
Differences between Retrospective and Prospective Era donors were significant for remnant liver weight (p = 0.015), units of transfused blood (p < 0.0001), hypotension (p < 0.0001), operative time (p < 0.0001) and donor lobe resected (p = 0.002).
Number of complications and severity grading of complications
Complication type, number and highest Clavien grade (among those with multiple complications of a given type) are delineated in Table 3 for the entire observational cohort. One or more complications were experienced by 296 donors (40%), resulting in an aggregate of 557 recorded complications; 140 donors (19%) experienced multiple complications. For donors who experienced more than one occurrence of a given complication, the highest Clavien grade of that complication type was used.
Table 3.
Type and severity of complications (intraoperative/postoperative) of donors with nonaborted procedure according to highest Clavien grade (n = 740)1
| Highest Clavien grade |
||||||
|---|---|---|---|---|---|---|
| Complication | Number of complications | Number of donors | 1 | 2 | 3 | 4 |
| Intraoperative | ||||||
| Intraoperative injury1 | 4 | 4 | ||||
| Intraoperative other complications2 | 11 | 11 | ||||
| Biliary | ||||||
| Bile leak/biloma | 62 | 60 | 25 | 35 | ||
| Biliary stricture | 5 | 5 | 1 | 4 | ||
| Abdominal | ||||||
| Intraabdominal bleeding | 7 | 7 | 2 | 5 | ||
| Upper/lower GI bleeding | 2 | 2 | 2 | |||
| Intraabdominal abscesses | 9 | 9 | 1 | 7 | 1 | |
| Ileus | 25 | 25 | 13 | 12 | ||
| Bowel obstruction | 13 | 12 | 3 | 8 | 1 | |
| Incisional hernia | 54 | 49 | 10 | 37 | 2 | |
| Wound dehiscence | 6 | 6 | 3 | 2 | 1 | |
| Unplanned reexploration | 20 | 20 | 1 | 19 | ||
| Clostridium difficile colitis | 2 | 2 | 2 | |||
| Cardiopulmonary | ||||||
| Pneumothorax | 6 | 6 | 5 | 1 | ||
| Pleural effusion | 83 | 81 | 64 | 17 | ||
| Pulmonary edema | 15 | 15 | 12 | 3 | ||
| Respiratory arrest | 1 | 1 | 1 | |||
| Aspiration | 2 | 2 | 1 | 1 | ||
| Pulmonary embolism | 7 | 7 | 2 | 5 | ||
| Hepatic | ||||||
| Encephalopathy/hepatic coma | 2 | 2 | 1 | 1 | ||
| Ascites | 21 | 21 | 17 | 4 | ||
| Liver failure | 0 | 0 | ||||
| Hepatic artery thrombosis | 0 | 0 | ||||
| Portal vein thrombosis | 4 | 4 | 2 | 2 | ||
| Inferior vena cava thrombosis | 3 | 3 | 3 | |||
| Other | ||||||
| Deep vein thrombosis | 6 | 6 | 6 | |||
| Neuropraxia | 24 | 24 | 19 | 5 | ||
| Infections2 | 112 | 98 | 25 | 72 | 1 | |
| Psychological difficulties2 | 51 | 42 | 23 | 17 | 2 | |
| Total | 557 | 2963 | 232 | 269 | 5 | 3 |
Donors who successfully donated (Retrospective: N = 395, Prospective: N = 343) plus those with graft resected but not transplanted into the intended recipient (Retrospective: N = 1, Prospective: N = 1).
Some or all instances of this complication type were not graded individually.
Total is less than sum of individual rows because 140 donors had more than 1 complication.
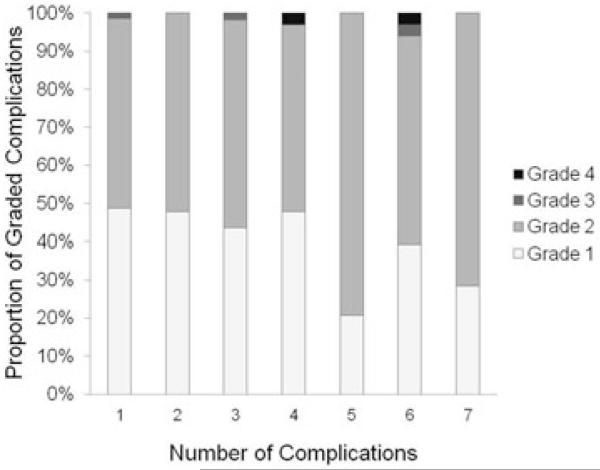
Among retrospective era donors, there were four Clavien grade 3 complications, one each with intraabdominal abscess and bowel obstruction and two with complex hernias. In addition, there were three donor deaths (grade 4). One donor died as a result of sepsis and multiorgan failure in the perioperative period and two donors died relatively late following donation (1.8 and 1.9 years) from psychological complications (one accidental drug overdose and one suicide) (5). Among prospective era donors, there was one Clavien grade 3 complication (wound dehiscence) and no Clavien grade 4 complications. There was no significant association between the distribution of Clavien grades and the number of complications each donor experienced, although it was notable that grade 4 complications occurred only in donors with >4 complications (Figure 1).
Figure 1. Distribution of Clavien grades by number of complications per person.
Probability of donor complications
Unadjusted probabilities of selected short-term (by 90 days) and long-term (by 6 years) complications are reported by study era (Table 4). Aside from pleural effusions, other short-term complications, including infection, bile leak, neuropraxia, reexploration and prolonged ileus, all occurred with similar probabilities in the two eras. Pleural effusion may have been more completely ascertained among prospective era donations. The probabilities of long-term complications (hernia, psychological complications and bowel obstruction) were also similar in the two eras. The overall probabilities of any complication by 6 years were 0.45 for retrospective era donors and 0.51 for prospective era donors (p = 0.84).
Table 4.
Number of donors with selected complications and associated Kaplan-Meier estimates of the probabilities of occurrence by 90 days (short-term) or 6 years (long-term) in the retrospective and prospective eras
| Retrospective Era (n = 396) |
Prospective Era (n = 344) |
||||
|---|---|---|---|---|---|
| Complication | N | Probability at 90 days | N | Probability at 90 days | Log-rank p-value |
| Short-term (within 90 days) | |||||
| Infection | 55 | 0.15 | 43 | 0.13 | 0.45 |
| Bile leak/Biloma | 36 | 0.09 | 24 | 0.07 | 0.25 |
| Pleural effusion | 24 | 0.06 | 57 | 0.17 | <0.001 |
| Neuropraxia | 17 | 0.04 | 7 | 0.02 | 0.08 |
| Reexploration | 13 | 0.03 | 7 | 0.02 | 0.28 |
| Prolonged ileus | 11 | 0.03 | 14 | 0.04 | 0.34 |
|
| |||||
| N | Probability at 6 years | N | Probability at 6 years | ||
|
| |||||
| Long-term (within 6 years) | |||||
| Any complication | 158 | 0.45 | 138 | 0.51 | 0.84 |
| Hernia | 26 | 0.11 | 23 | 0.16 | 0.56 |
| Psychological | 23 | 0.09 | 19 | 0.07 | 0.71 |
| Bowel obstruction | 6 | 0.02 | 6 | 0.03 | 0.55 |
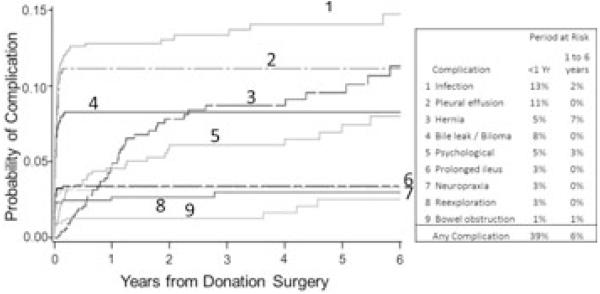
Among the combined cohort of retrospective and prospective era subjects, the majority of first occurrences of infections, pleural effusions, bile leaks, neuropraxia, reex-plorations and prolonged ileus were within the first few weeks after donation (Figure 2). However, a number of complications first occurred many months or even years after donation. Hernia, bowel obstruction and psychological complications first developed as late as 5 to 6 years following donation. Up to 7% of donors first experienced individual types of complications more than 1 year after donation.
Figure 2. Cumulative probability of selected complication after hepatic lobe donation.
Independent predictors of donor complications
Multivariable Cox regression models were fitted to test potential predictors of a first complication of any type, and also specifically of bile leak, hernia and infection. There was a significant association of transfusion requirement with the development of a first complication of any type (HR = 1.38 per unit; p < 0.0001) and specifically with the occurrence of bile leak (HR = 1.55; p < 0.0001) and infection (HR = 1.40; p = 0.0011) (Table 5). In addition to transfusion, intraoperative hypotension was associated with a 48% higher risk of any complication (p = 0.0013), and higher predonation serum bilirubin was associated with a lower risk of any complication (HR = 0.59 per mg/dL; p = 0.0061). The only additional significant predictor of bile leak was body weight (HR = 1.22 per 10 kg; p = 0.0192). Older age, male gender and higher BMI were independently significant predictors of hernia formation. No other covariates among those tested (listed in methods) were significant predictors of these outcomes. In particular, when added to the multivariable models, neither center experience nor year of donation (continuous or by trend test using 3-year intervals) were significantly associated with the risk of complications.
Table 5.
Multivariable Cox models testing predictors of any complication, bile leak, hernia, and infection. Time to first complication was modeled1
| Outcome | Predictors | HR | 95% CI | p-value |
|---|---|---|---|---|
| Any | Bilirubin (per mg/dL) | 0.59 | 0.41-0.86 | 0.006 |
| Complication | Units of transfused blood (per unit) | 1.38 | 1.21-1.61 | <0.001 |
| Intraoperative hypotension | 1.48 | 1.17-1.88 | 0.001 | |
| Bile leak | Weight (per 10 kg) | 1.22 | 1.03-1.43 | 0.019 |
| Units of transfused blood (per unit) | 1.55 | 1.25-1.92 | <0.001 | |
| Hernia | Age at donor evaluation (per 10 yrs) | 1.41 | 1.08-1.92 | 0.01 |
| Sex: male vs. female | 1.82 | 1.00-3.33 | 0.05 | |
| Body mass index (per 5 kg/m2) | 1.82 | 1.27-2.61 | 0.001 | |
| Infection | Units of transfused blood (per unit) | 1.40 | 1.15-1.72 | 0.001 |
HR = hazard ratio; CI = confidence interval
The complete list of variables tested in each of the models is detailed in the Methods section.
A test of the statistical interaction between units of transfused blood and intraoperative hypotension was not significant in the model for any complications. Thus, hypotension did not appear to potentiate the adverse effect of transfused blood, or vice versa. We also tested the potential interaction of gender and BMI in the time-to-hernia model; this interaction was not significant.
The number of left lobe donors (n = 33) precluded exhaustive covariate examination. Nonetheless, when tested in a single-variable Cox model, the unadjusted risk of any complication was significantly higher for left lobe donors (HR = 1.60; 95% CI 0.99–2.56; p = 0.05). When added to the final multivariable Cox model, left lobe donation was unexpectedly associated with a higher risk of complications (HR = 1.55; 95% CI 0.96-2.51; p = 0.08), but this result did not reach the traditional level of statistical significance.
Resolution of complications
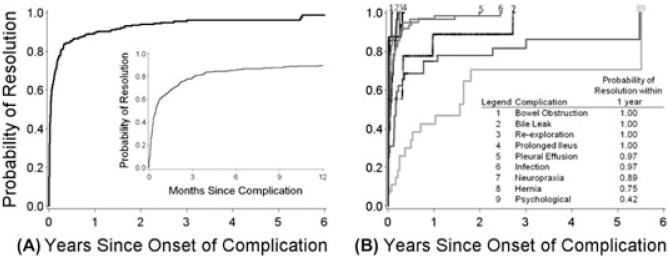
Analysis of the course of complications from onset to resolution is depicted in Figure 3. Over a follow-up period up to 9 years, 95% of complications were resolved and the vast majority were resolved by 3 months postdonation (Figure 3A). Complication types that presented later postdonation (e.g., psychological and hernia) also tended to take longer to resolve (Figure 3B). Several cases of neuropraxia took more than 1 year to resolve. The probability of complete resolution within 1 year after diagnosis was only 75% for hernia and 42% for psychological complications, although both probabilities increased with continued follow-up time.
Figure 3.
(A) Time to resolution of all complications. [Inset in A – magnification of first 12 months]. (B) Time to resolution for specific complications.
Discussion
In 2008, we reported that in a retrospective cohort of 396 LLD (387 right and 9 full left lobes), 38% developed at least one complication, the vast majority within the first year (1). In 2010, the Toronto group reported that in a retrospective single center experience of 202 right lobe donors with a minimum follow-up of 12 months, 40% developed complications within the first year (6). Also in 2010, the Kyoto group updated their previously published experience with LLD complications (7). A retrospective single-center review of 500 right lobe donors with a median follow-up period of 36.5 months revealed that 44% experienced at least one complication within the follow-up period. In the current study, we have markedly extended the duration of follow-up in the retrospective era donors and include an additional 352 donors whose procedures occurred in the prospective era. In total, this represents the complete experience with LLD at these nine centers over a nearly 12-year period. We now report that among 740 LLD (707 right lobes), 39% developed at least one complication in the first year, an incidence strikingly similar to and confirming our prior experience and that of others. We have also confirmed that increasing center experience is not associated with a reduction in donor complications. Therefore, we propose that 40% can be considered a fairly definitive assessment of the risk of complications in the first year following live donor right lobectomy. Regarding the severity of these complications, we had previously reported that 2.8% of patients had Clavien grade 3 or 4 complications (1). Using a slight modification of the Clavien classification in which grade 4 included residual disability or death, the Toronto group (6) reported that out of 202 donors, 15.8% of patients had a grade 3 and none had a grade 4 complication. The Kyoto group (7), using a 5-tier modification of the Clavien system, reported that in 500 LLD (right lobe), 17% developed >grade 3 complications (consistent over 3 eras) with 1/500 grade 5 (death). Using the same grading system, the Pittsburgh group (8) reported that among the 121 LLD (right lobectomy), 10.7% of patients developed >grade 3 complications (no grade 5). Our current combined A2ALL study shows that 8/740 LLD (1.1%) developed a grade 3 (residual disability, n = 5) or grade 4 (liver failure or death, n = 3), 7/396 (1.8%) in the retrospective era and 1/344 (0.3%) in the prospective era. The Kyoto and Pittsburgh groups used versions of the Clavien system that did not specifically capture residual disabilities. Based on the Toronto and A2ALL experience, the combined risk of residual disability, liver failure or death following LLD (0/202 and 8/740, respectively), is approximately 1%. We have previously defended our use of the 4-tier Clavien system (9). Our current study also demonstrates that 20/760 (2.6%) procedures were aborted intraoperatively (2.9% and 2.3% in retrospective and prospective cohorts prospectively). The Toronto group has reported 4.9% (12/252) aborted procedures (10). Thus, we consider that 2%–5% represents the risk of aborted LLD.
A major aim of our study was to evaluate predictors associated with postdonation complications. The Pittsburgh group identified BMI ≥ 30 and macrovesicular steatosis as significant risk factors for the development of grade 4a complications (8). The Kyoto group found donor age and prolonged operative time to be associated with any complication and with biliary complications, but gender, BMI, and blood loss were not significant risk factors in a limited analysis (7). We now confirm our previous observation, that blood transfusion is associated with a significantly higher risk of complication, and specifically of bile leaks and infection (1). In our current study, each unit of transfused blood was associated with a 38% to 55% higher risk of complication. Intraoperative hypotension (systolic blood pressure <100 mm Hg) was associated with an overall higher risk of complications, independent of the requirement for blood transfusions. Donors with higher body weight were at significantly higher risk of bile leak; each increment of 10 kg in body weight increased the risk by 22%. Risk factors for incisional hernia formation included older age, male gender and higher BMI, each of which has been observed in populations of general surgical patients undergoing open abdominal procedures (11). Baseline predonation laboratory studies have had inconsistent associations with donor complications. In the current study, we failed to confirm our prior finding that higher levels of alkaline phosphatase were associated with a higher risk of complications. However, higher predonation serum bilirubin was surprisingly associated with a lower risk of development of complications. As with alkaline phosphatase, the vast majority of serum bilirubin levels fell within the normal range. A biological basis for these associations is not obvious.
Previous studies have focused principally on the development of complications within the first year following donation. We had previously reported that 46% of all complications occurred during the initial hospitalization (1). The Toronto group focused their analyses on complications within 30 days of donation and between 30 days and 1-year postdonation (6), but noted one keloid, one incisional hernia and one bowel obstruction that presented between 1 and 5 years postdonation. The Pittsburgh group did not report long-term complications (8). The Kyoto report spanned three empirical eras but made little attempt to analyze long-term complications (7). Our current study demonstrates that certain complications (hernia, bowel obstruction and psychological complications) may develop as late as 5 or more years after donation. A novel feature of the current report is the analysis of the time to resolution of postdonation complications, starting from their time of onset. Nearly 80% of complications resolved by 3 months. However, some complications took many years to resolve or remained unresolved as long as 3 to 5 years after presentation. The probabilities of resolution by 1 year after diagnosis were only 75% and 42% for hernias and psychological complications, respectively. This is especially important since these same types of complications tended to occur later postdonation. In contrast, neuropraxia, which developed immediately after donation, took as long as 3 years to resolve. Of interest, with longer follow-up, some complications initially defined as Clavien grade 3 (residual disability at the time of recording) eventually resolved and were downgraded to 2, explaining the discrepancy in the number of grade 3 complications between our previous and current reports. This was true for several cases of neuropraxia. The exact proportion of grade 3 complications that eventually resolve over time and are therefore downgraded to grade 2 is unclear, and while this may add confusion to the assessment of donor morbidity, it highlights the need for longer term follow-up and underlines the significance of the current study. We did not observe any examples of upgrading over time.
We should note that since the current analysis, two well publicized donor (right lobectomy) deaths have occurred in the US. One of these occurred at a center that participated in the A2ALL study, on which we currently report, but the study was closed for enrollment at the time of donor death and therefore it is not included in the analysis. The other donor death occurred at a nonparticipating center. Both centers were among the most experienced adult LLD in the US at the time of these events. Therefore, although not part of our analysis, we believe that these deaths warrant mention to prevent an erroneous interpretation of a decrease in grades 3 and 4 complications between the retrospective and prospective cohorts.
Limitations of our study include limited data for some donors who may have sought treatment elsewhere, and the possibility of under-ascertainment of retrospectively assessed complications. Although we collected data on 29 specific complications generated by consensus among the study investigators, we did not include an ‘other’ category for rare or unexpected complications. All centers were monitored annually, but only approximately 10% of records were audited for accuracy of reporting. Over the course of the study, these audits uncovered a few new complications, suggesting that complications may be slightly underestimated. In addition, the exact nature of donor work up was not prescribed by the study and it is certainly possible that participating centers used varying inclusion and exclusion criteria for donors, and that these may have changed over the period of study. Finally, our observations regarding the relative risk of complications for left versus right lobe donation are inconsistent with that of others (7), most likely as a result of the small number of left lobe donors in our cohort. We believe that all the limitations described above could have only a modest effect on our results.
From this longitudinal observational cohort of 740 completed hepatic lobe donations in the multicenter A2ALL consortium, we can draw several important conclusions: (1) the incidence and severity of LLD complications are well defined and do not appear to change over time (40% overall incidence of complications, 1% incidence of residual disability, liver failure or death and 2%–5% risk of aborted donation); (2) there do not appear to be any significant risks associated with LLD complications not previously identified; (3) the impetus to further compile and register LLD complications specifically for the purpose of assessing general donor risk should take into consideration our first two conclusions. We believe that any further investigation should focus on assessing the relative risk of evolving LLD approaches that may alter the risk profile and (4) we have found that longer follow-up (>1 year) allows for a better understanding of the natural history of LLD complications, including late complications and time to resolution. Therefore, we believe that the current study will serve as a comprehensive report to inform decision making by potential donors and their caregivers, and as a benchmark against which the effectiveness of future interventions or strategies to reduce donor complications can be measured.
Acknowledgments
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases through cooperative agreements (listed in parentheses). Additional support was provided by the Health Resources and Services Administration and the American Society of Transplant Surgeons.
The following individuals were instrumental in the planning, conduct, and/or care of patients enrolled in this study at each of the participating institutions:
Columbia University Health Sciences, New York, NY (DK62483): principal investigator, Jean C. Emond, M.D.; coprincipal investigator, Robert S. Brown Jr, M.D., M.P.H.; study coordinators, Scott Heese, B.A. and Jonah S. Zaretsky, B.A.
Northwestern University, Chicago, IL (DK62467): principal investigator, Michael M. I. Abecassis, M.D., M.B.A.; coprincipal investigator, Laura M. Kulik, M.D.; study coordinator, Patrice Al-Saden, R.N., C.C.R.C.
University of Pennsylvania Health System, Philadelphia, PA (DK62494): principal investigator, Abraham Shaked, M.D., Ph.D.; coprincipal investigator, Kim M. Olthoff, M.D.; study coordinators, Brian Conboy, P.A., M.B.A., and Mary Shaw, R.N., B.B.A.
University of Colorado Health Sciences Center, Denver, CO (DK62536): principal investigator, Gregory T. Everson, M.D.; coprincipal investigator, Igal Kam, M.D.; study coordinators, Carlos Garcia, B.S., and Anastasia Krajec, R.N.
University of California Los Angeles, Los Angeles, CA (DK62496): principal investigator, Johnny C. Hong, M.D.; coprincipal investigator, Ronald W. Busuttil, M.D., Ph.D.; study coordinator, Janet Mooney, R.N., B.S.N.
University of California San Francisco, San Francisco, CA (DK62444): principal investigator, Chris E. Freise, M.D., F.A.C.S.; coprincipal investigator, Norah A. Terrault, M.D.; study coordinator, Dulce MacLeod, R.N.
University of Michigan Medical Center, Ann Arbor, MI (DK62498): principal investigator, Robert M. Merion, M.D.; data coordinating center staff, Anna S. F. Lok, M.D., Akinlolu O. Ojo, M.D., Ph.D., Brenda W. Gillespie, Ph.D., Margaret Hill-Callahan, B.S., L.S.W., Terese Howell, B.S., A.C.R.P., Lisa Holloway, B.S., A.C.R.P., Monique Lowe, B.S., Abby Smith, B.A. and Abby Brithinee, B.A.
University of North Carolina, Chapel Hill, NC (DK62505): principal investigator, Paul H. Hayashi, M.D., M.P.H.; study coordinator, Tracy Russell, M.A.
University of Virginia (DK62484): principal investigator, Carl L. Berg, M.D.; study coordinator, Jaye Davis, R.N.
Medical College of Virginia Hospitals, Virginia Commonwealth University, Richmond, VA (DK62531): principal investigator, Robert A. Fisher, M.D., F.A.C.S.; coprincipal investigator, R. Todd Stravitz, M.D.; study coordinators, April Ashworth, R.N. and Andrea Lassiter, B.S.
Division of Digestive Diseases and Nutrition, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, M.: James E. Everhart, M.D., M.P.H., Leonard B. Seeff, M.D., Patricia R. Robuck, Ph.D. and Jay H. Hoofnagle, M.D.
Editorial assistance was provided by Shauna A. Leighton, a medical editor at Arbor Research Collaborative for Health.
Abbreviations
- A2ALL
adult-to-adult living donor liver transplantation cohort study
- CI
confidence intervals
- HR
hazard ratio
- LDLT
living donor liver transplants
- LLD
living liver donor
- US
United States
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Supported in part by the National Institutes of Health (NIDDK grant numbers U01-DK62536, U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531), the American Society of Transplant Surgeons, and the U.S. Department of Health and Human Services, Health Resources and Services Administration.
References
- 1.Ghobrial RM, Freise CE, Trotter JF, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology. 2008;135:468–476. doi: 10.1053/j.gastro.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111:518–526. [PubMed] [Google Scholar]
- 3.Clavien PA, Sanabria JR, Mentha G, et al. Recent results of elective open cholecystectomy in a North American and a European center. Comparison of complications and risk factors. Ann Surg. 1992;216:618–626. doi: 10.1097/00000658-199212000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clavien PA, Camargo CA, Jr., Croxford R, Langer B, Levy GA, Greig PD. Definition and classification of negative outcomes in solid organ transplantation. Application in liver transplantation. Ann Surg. 1994;220:109–120. doi: 10.1097/00000658-199408000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trotter JF, Hill-Callahan MM, Gillespie BW, et al. Severe psychiatric problems in right hepatic lobe donors for living donor liver transplantation. Transplantation. 2007;83:1506–1508. doi: 10.1097/01.tp.0000263343.21714.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adcock L, Macleod C, Dubay D, et al. Adult living liver donors have excellent long-term medical outcomes: The University of Toronto liver transplant experience. Am J Transplant. 2010;10:364–371. doi: 10.1111/j.1600-6143.2009.02950.x. [DOI] [PubMed] [Google Scholar]
- 7.Iida T, Ogura Y, Oike F, et al. Surgery-related morbidity in living donors for liver transplantation. Transplantation. 2010;89:1276–1282. doi: 10.1097/TP.0b013e3181d66c55. [DOI] [PubMed] [Google Scholar]
- 8.Marsh JW, Gray E, Ness R, Starzl TE. Complications of right lobe living donor liver transplantation. J Hepatol. 2009;51:715–724. doi: 10.1016/j.jhep.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freise C, Ghobrial M, A2ALL Study Group Response to letter “Systematic grading of morbidity after living donation for liver transplantation”. Gastroenterology. 2009;137:1855–1858. doi: 10.1053/j.gastro.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 10.Guba M, Adcock L, MacLeod C, et al. Intraoperative ‘no go’ donor hepatectomies in living donor liver transplantation. Am J Transplant. 2010;10:612–618. doi: 10.1111/j.1600-6143.2009.02979.x. [DOI] [PubMed] [Google Scholar]
- 11.Yahchouchy-Chouillard E, Aura T, Picone O, Etienne JC, Fingerhut A. Incisional hernias. I. Related risk factors. Dig Surg. 2003;20:3–9. doi: 10.1159/000068850. [DOI] [PubMed] [Google Scholar]