Abstract
Background
Neuraxial anesthesia is utilized in children of all ages. Local anesthetics produce dose-dependent toxicity in certain adult models, but the developing spinal cord may also be susceptible to drug-induced apoptosis. In postnatal rodents, we examined effects of intrathecal levobupivacaine on neuropathology and long-term sensorimotor outcomes.
Methods
Postnatal day 3 (P3) or P7 rat pups received intrathecal levobupivacaine 2.5mg/kg (0.5%) or saline. Mechanical withdrawal thresholds and motor block were assessed. Spinal cord tissue analysis included: apoptosis counts (activated-caspase-3, Fluoro-Jade C) at 24 h; glial reactivity at 7 days; and histopathology in cord and cauda equina at 24 h and 7 days. Long-term spinal function in young adults (P35) was assessed by hindlimb withdrawal thresholds, electromyography responses to suprathreshold stimuli, and gait analysis.
Results
Intrathecal levobupivacaine produced spinal anesthesia at P3 and P7. No increase in apoptosis or histopathological change was seen in the cord or cauda equina. In the P3 saline group, activated-caspase-3 (mean±SEM per lumbar cord section 6.1±0.3) and Fluoro-Jade C (12.1±1.2) counts were higher than at P7, but were not altered by levobupivacaine (P=0.62 and P=0.11, two-tailed Mann-Whitney test). At P35, mechanical withdrawal thresholds, thermal withdrawal latency and electromyographic reflex responses did not differ across P3 or P7 levobupivacaine or saline groups (one way ANOVA with Bonferroni comparisons). Intrathecal bupivacaine at P3 did not alter gait.
Conclusion
Single dose intrathecal levobupivacaine 0.5% did not increase apoptosis or produce spinal toxicity in neonatal rat pups. This study provides preclinical safety data relevant to neonatal use of neuraxial local anesthesia.
Introduction
Spinal anesthesia in neonates and infants is well established in pediatric practice.1,2 Large case series report safe and effective anesthesia and analgesia,3–8 including use in high risk infants.9,10 Neuraxial anesthesia may have particular advantages in preterm-born neonates who are susceptible to postoperative apnoea or have co-existing respiratory disease.1,11,12
Persistent neurological deficits are rare following pediatric regional anesthesia,13 but in fact there has been limited detailed follow-up in this patient group.1,14,15 Transient neurological symptoms following spinal anesthesia have been reported in adults16,17 and in children,2 but neonates and infants are unable to report these symptoms. Models have been established for evaluating local anesthetic toxicity in adult animals,18 and the importance of also evaluating nerve root histopathology in developmental studies has been emphasized.19 A recent study reported no histopathologic change in white matter tracts within lumbar spinal cord sections following intrathecal racemic bupivacaine at postnatal day (P)7,14 or 21.20 As in-vivo studies in adult animals show variable neurotoxicity with different local anesthetics,21–23 further preclinical evaluation of different preparations during early development is also needed.
Neuraxial anesthesia and analgesia in neonates and infants avoid or reduce exposure to general anesthetic agents, that have been reported to increase neuronal apoptosis in the developing rodent and primate brain, with associated adverse long-term cognitive outcomes.24,25 Apoptosis in the central nervous system has a temporal profile that varies across brain regions, with different time windows for increased susceptibility to the pro-apoptotic effects of certain drugs.26 Prolonged general anesthesia, but not intrathecal bupivacaine, at P7 increases apoptosis in both cortex and spinal cord, but neither intervention produced long-term motor deficits.20 As our previous work demonstrated higher baseline apoptosis in the cord at P3/4, predominantly in the dorsal horn,27–29 we have included a younger age group and evaluation of sensory outcomes in our toxicicty studies. As different local anesthetics produce varying degrees of apoptosis in neuronal cell cultures,30,31 evaluation of additional preparations in in-vivo developmental models will further define the safety profile of spinal anesthesia in early life.
Levobupivacaine, the S (-)-enantiomer of bupivacaine, produces reliable and effective spinal anesthesia in neonates32,33 and children,34 and is also widely used for single-shot caudal blocks35,36 and perioperative epidural infusions37,38 in pediatric practice. Compared with the same dose of racemic bupivacaine, levobupivacaine has an improved systemic toxicity profile after intravenous administration39 and reduced neurotoxicity following intrathecal administration in some,23 but not all,40 adult animal studies. Our developmental model of spinal toxicity had sufficient sensitivity to demonstrate a relatively wide safety margin following morphine27 and clonidine,29 but adverse outcomes following ketamine,28 and will now be the basis for further evaluation of local anesthetic toxicity in the neonatal spinal cord.
The current study evaluates spinal toxicity following intrathecal levobupivacaine in neonatal (P3 and P7) rat pups. Evaluation includes: behavioral analysis; quantification of apoptosis; and histopathological evaluation of spinal cord and cauda equina. Functional outcomes in early adulthood (P35) included sensory withdrawal thresholds; electromyographic responses to suprathreshold stimuli during anesthesia. Gait analysis following neonatal intrathecal bupivacaine is also presented.
Materials and Methods
Animals
Experiments were carried out according to protocols approved by IACUC (Institutional Animal Care and Use Committee) of University of California, San Diego, La Jolla, California. Pregnant Holtzman Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed in accordance with the National Institute of Health guidelines in a 12 h light-dark cycle and with free access to standard food and water. Dams were monitored, and the day of birth of pups noted. Pups were randomly assigned to treatment groups containing equal numbers of males and females at postnatal day 3 (P3) or day 7 (P7). Body weights of the rat pups were between 8–11 g at P3 and 12–18 g at P7.
Additional experiments evaluating long-term spinal reflex function were performed in the United Kingdom under personal (SMW) and project licences in accordance with the requirements of the United Kingdom Animal (Scientific Procedures) Act 1986. Sprague-Dawley dams and litters were bred in-house in the Biological Services Unit University College London, and were maintained on a 12-h light/dark cycle at constant ambient temperature with free access to food and water. Pups were randomly assigned to treatment groups at P3 or P7. Litters were restricted to a maximum of 12, with pups weaned into same-sex cages at P21, and maintained until P35.
For all experimental interventions, rat pups were kept on a heating pad to maintain body temperature. Care was taken to minimize the duration of maternal separation and handling of pups, and this was the same for both control and treatment groups. Due to the need to confirm correct intrathecal placement and motor block, experimenters were aware of treatment allocation during initial behavioral testing. Animals for long-term evaluation of spinal reflex function were coded following injections to ensure that the investigator was blinded to initial treatment allocation during testing at P35. Similarly, tissue sections were coded and the experimenter was unaware of treatment allocation at the time of analysis.
Intrathecal injections and solutions
Percutaneous intrathecal injections were performed as previously described.27 Under brief isoflurane anesthesia (3%) with oxygen and room air via a nose cone, 0.5% levobupivacaine hydrochloride (Chirocaine 50mg/10ml; Abbott Laboratories Limited, Maidenhead, Berkshire, United Kingdom) or sterile saline was injected intrathecally at the L4-L5 or L5-L6 intervertebral space using a 30 gauge needle connected to a micro-injector and Hamilton syringe. The volume of the injectate was 0.5µl/g body weight, which produces spread over low thoracic and lumbar segments in rat pups.27 Our pilot experiments confirmed that this volume of local anesthetic produced reliable motor block of the hindlimbs. Dose escalation was precluded as we restricted analysis to a clinically-available 0.5% preparation, and higher volumes (1µl/g) were associated with impaired respiration and increased mortality, consistent with previous reports following 0.5% bupivacaine.20,27
Behavioral testing and assessment of spinal reflex function
Acute effects
In rat pups, baseline mechanical withdrawal threshold was determined using calibrated von Frey filaments which apply logarithmically increasing pressure (0.4 to 15g). Each von Frey filament was applied five times with 1 sec intervals to the dorsal surface of the hindpaw41. The number of evoked withdrawal responses to each stimulus of increasing intensity was recorded until a given stimulus evoked five responses, or a supra-threshold cut-off pressure was reached (10grams at P3; 15grams at P7). Following injection and recovery from anesthesia, pups were assessed for visible motor block (failure of hip flexion, dragging of hindlimbs, and no response to a suprathreshold mechanical stimulus). Animals were only retained for further analysis if dense motor block was apparent following levobupivacaine, and motor function was normal following saline. Motor block scores (0 = no movement; 1=partial block, 2=full movement) for the left and right hindlimbs were added to give a total score between 0 and 4 for each animal20. Mechanical withdrawal thresholds were measured in both hindlimbs at 15, 30, 45 and 60 minutes following injection, and at 24 hours or 7 days prior to tissue analysis.
Spinal reflex function at P35
Separate groups of P3 and P7 animals received intrathecal 0.5% levobupivacaine 0.5mcl/g or saline as described above. Spinal cord function was assessed at 5 weeks of age (P35) by evaluating hindlimb withdrawal reflex responses to mechanical and thermal stimuli, with the experimenter blinded to initial treatment group.29 Following habituation on an elevated mesh platform, the mechanical stimulus (electronic von Frey device; Dynamic Plantar Aesthesiometer, Ugo Basile, Comerio, Italy) was applied to the mid-plantar surface of the hindpaw. The threshold was averaged from 3 measures of the force (0 to 50 grams; ramp 20 grams/sec) required to produce hind-limb withdrawal. Thermal withdrawal latency was determined using a modified Hargreaves Box (University Anesthesia Research and Development Group, University of California San Diego, La Jolla, California) with a glass surface (maintained at 30 °C) on which the rats were placed in individual Plexiglas cubicles. The thermal nociceptive stimulus from a focused projection bulb positioned below the glass surface was directed to the mid-plantar hindpaw. Latency was defined as the time required for the paw to show a brisk withdrawal as detected by photodiode motion sensors that stopped the timer and terminated the stimulus. In the absence of a response within 20 seconds, the stimulus was terminated (cut-off time). Thermal latency was the average of three measures from each hindpaw.
To assess sensorimotor reflex function to both threshold and suprathreshold stimuli, flexor reflex electromyography recordings from the biceps femoris muscle were performed as previously described42,43. In brief, animals were anesthetised with halothane (2–4%) in oxygen and a tracheal tube was inserted to facilitate mechanical ventilation (Small Animal Ventilator, Harvard Apparatus Ltd). Halothane was reduced to 0.9% in oxygen for 30 minutes to ensure equilibration to a stable plane of anesthesia, and maintained at this concentration during electromyography recordings. Animals were placed in a spinal frame with the left hindpaw secured on a fixed platform. Heart rate was continuously monitored and body temperature was monitored with a rectal probe and maintained with a thermostatically controlled heat source. Bipolar electromyography electrodes (Ainsworks, London, United Kingdom) comprising stainless steel 30G needles with a central copper wire core were placed through a small skin incision into the belly of the biceps femoris muscle. Electromyography responses to mechanical hindpaw stimuli were processed (Neurolog System, Digitimer Ltd, Welwyn Garden City, United Kingdom) and recorded in 12-second epochs (PowerLab 4S, AD Instruments, Castle Hill, Australia). Von Frey hairs were sequentially applied to the plantar surface of the hindpaw up to a maximum of hair number 20 (180g) to quantify the response to suprathreshold stimuli.
Gait analysis at P35
In separate experiments, gait analysis was performed at P35 following intrathecal injection of 0.5% bupivacaine at P3. These experiments were performed in conjunction with our previous experiments evaluating effects of intrathecal morphine27 and ketamine28, but the local anesthetic results have not been previously reported. Gait analysis was performed as the animal crossed the glass runway of the CatWalk® system (Noldus Information Technology, Wageningen, The Netherlands). Animals commenced a daily training paradigm at P22–25, with runway crossings toward food rewards at the farther end. At P35, runway crossings were recorded and included in analysis if the maximal time for crossing the 60-cm-long section of the runway used for gait recording was ≤2 seconds, and there were no intermediate stops during the crossing. Three crossings per animal were analyzed using the CatWalk® 7.1.6 software (Noldus Information Technology, Wageningen, The Netherlands).
Spinal cord tissue preparation and staining
Tissue analysis was performed either 24 hours or 7 days following injection. Rat pups were terminally anesthetized with intraperitoneal injection of 100mg/kg pentobarbital and transcardially perfused with saline followed by 4% paraformaldehyde. Following laminectomy, the spinal cord and cauda equina were dissected, post-fixed in 4% paraformaldehyde overnight, then transferred to 30% sucrose and stored at +4°C. Transverse sections of lumbosacral spinal cord (7 and 14 µm) were cut using a cryostat (Leica CM 1800, San Marcos, CA), mounted on slides (Fisher Superfrost Plus, Fisher Scientific, Houston, TX), and stored at −30°C. Using our previously described protocols,27–29 we assessed: histopathology with hematoxylin and eosin staining 24hrs and 7 days post-injection; apoptosis with activated caspase-3 immunohistochemistry and Fluoro-Jade C staining at 24 hours; and glial reactivity by staining with microglial (ionized calcium binding adapter molecule 1; Iba-1) and astrocytic (glial fibrillary acidic protein) markers 7 days following injection. For histopathological evaluations of the nerve roots, cauda equina was cut from the spinal cord 24 hours and 7 days following injection and transferred into 2.5% glutaraldehyde in 0.1M PB.
Activated caspase-3
Immunohistochemistry with an antibody to activated caspase-3, the final member of an intracellular cascade activated during programmed cell death, was performed on tissue 24 hours post-injection. Tris-Buffered Saline was used for initial washes and between steps. Slides were incubated in 3% peroxidase in methanol for 10 min, blocked with 0.3% Triton X-100 and 5% normal goat serum in Tris-Buffered Saline for one hour at room temperature, then incubated overnight at 4°C with rabbit monoclonal anti-activated caspase 3 (1:100; Cell Signaling, Danvers, MA). Biotinylated goat anti-rabbit secondary antibody (Vector, Burlingame, CA) was applied at 1:250 for 30 min at room temperature, followed by avidin-biotin-peroxidase complex (ABC reagent; Vector Laboratories, Burlingame, CA) for 30 min. Staining was developed with 3,3’-diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA) for 8 min and then slides were counterstained with hematoxylin, dehydrated and coverslipped (Permount, Fischer SP15, Fair Lawn, NJ). At least four sections from each animal were counted for caspase-3 immunoreactive cells under the light microscope.
Fluoro-Jade C
Fluoro-Jade C staining was performed in 14µm sections of spinal cord 24 hours following injection using an established protocol44. Slides were immersed in 1% sodium hydroxide in 80% ethanol, rinsed with 70% ethanol, then incubated in 0.06% potassium permanganate. Sections were stained with 0.0002% Fluoro-Jade C (Millipore, Temecula, CA) and 0.01% 4,6-diamidino-2-phenylindole (DAPI; Molecular Probes, Eugene, OR) dissolved in 0.1% acetic acid, cleared with Citrisolve and coverslipped. Fluoro-Jade C immunfluorescent positive cells in at least four sections from each animal were counted under the appropriate wavelength fluorescent microscopy.
Glial fibrillary acidic protein and ionized calcium binding adapter molecule 1 (Iba1)
Spinal cords sections (14 µm thick) 7 days following injection were evaluated for glial reactivity with astrocyte (glial fibrillary acidic protein) and microglial (Iba1) markers. Following washes with Triton X-100 0.1% in phosphate buffered saline (used for rinses throughout), slides were incubated in 5% goat blocking serum at room temperature for one hour, followed by mouse anti-glial fibrillary acidic protein (1:500; Chemicon, Temecula, CA) and rabbit anti-Iba1 (1:1000; WAKO, Richmond, VA) for 48 hours at 4°C, and then fluorescent secondary antibodies for 2 hours (1:250 goat anti-mouse Alexa 555 and 1:250 goat anti-rabbit Alexa 488; Molecular Probes, Eugene, OR). Slides were cover-slipped with Prolong Gold antifade mounting media with DAPI (Molecular Probes, Eugene, OR). At least four sections from each animal were imaged using standardized exposures and an Olympus BX51 microscope with appropriate wavelength fluorescence illuminator (Olympus America, Inc., Center Valley, PA) equipped with a digital camera and image-capture software. The mean intensity of immunofluorescence within a fixed size region of interest in the dorsal horn and background intensity was calculated using Image Pro Plus software (Media Cybernatics Inc., Silver Spring, MD).29
Hematoxylin and Eosin
Seven-micron sections of spinal cord from 24 hour and 7 day survival groups were stained with Hematoxylin (Gill No.2; Sigma Aldrich, St Louis, MO) and Eosin (Eosin Y solution Alcoholic; Sigma Aldrich, St Louis, MO). At least 4 non-consecutive sections per animals were evaluated for histopathological changes (degenerating neurons, tissue necrosis, inflammation or other changes) by a neuropathologist experienced in the evaluation of neonatal neurological injury and spinal toxicity (MG).27–29,45,46
Nerve root histology
Neurotoxicologic evaluation of nerve roots of the cauda equina was performed 24 hours or 7 days after saline or levobupivacaine admnistration in P3 and P7 pups (n=4 per group, total=32 animals). Cauda equina was cut from the spinal cord and transferred into 2.5% glutaraldehyde in 0.1M PB. The nerve roots were rinsed with 0.1M PB, postfixed in 1% osmium tetroxide, dehydrated in serial concentrations of alcohol, and embedded in araldite resin according to the recommended procedure47. Transverse, 1-µm-thick sections were cut on an automated Leica RM2065 microtome and stained with methylene blue, azure II for light microscopy. Images were taken using Openlab 4.04 software (Improvision, Waltham, MA) and examined for pathological change by an investigator experienced in nerve pathology (VS).48
Statistical Analysis
For determination of mechanical withdrawal thresholds in rat pups, the number of withdrawal responses was plotted against the mechanical stimulus (force expressed as grams on log10 scale). A sigmoidal stimulus-response curve with nonvariable slope was constructed using non-linear regression curve fit, and the mid-point of the curve (50% effective force; EF50) was designated as the mechanical withdrawal threshold, as previously described.26,27 For graphical display, data within the group were pooled for evaluation of EF50 at 15 minutes as motor block resulted in either no response to the maximum mechanical threshold or a sub-maximal responses (i.e. less than 5/5 responses to the maximum mechanical stimulus)(Fig. 1 and 2). To allow analysis of the effect of time and treatment, individual threshold values for each animal were calculated at baseline, 30, 45, 60 minutes and 24 hours, and the maximum applied force was designated the threshold if motor block was present at 15 minutes. Repeated measures two-way ANOVA with Bonferroni’s post hoc test was used to evaluate differences between levobupivacaine and saline groups. Thresholds seven days following injection of levobupivacaine or saline at P3 or P7 were compared with unpaired two-tailed Student’s t-test. In P35 rats, mechanical withdrawal thresholds and thermal latencies were the mean of 3 values for each hindpaw. As data were obtained at the same age, and were normally distributed (Kolmogorov-Smirnov test) continuous variables, all treament groups compared with one-way ANOVA with Bonferroni’s post hoc comparisons. In addition, thresholds and latencies were compared with two-way ANOVA with sex and treatment as variables, and age at time of injection and treatment as variables. The duration of the electromyography response was outlined from the display of the raw data and the integral of the root mean square (RMS) of the signal was calculated (EMG response)(Chart, Powerlab AD Instruments, Castle Hill, Australia). The electromyography response was plotted against the von Frey hair number (mechanical stimulus) and the area under the stimulus-response curve (AUC) calculated to quantify the overall “reflex response”.42,42 For tissue sections measures from at least 4 non-consecutive sections were averaged for each animal, with analysis based on n=number of animals and comparison with two-tailed Mann-Whitney test. Statistical analysis was performed using Prism Version 5.0 (GraphPad, San Diego, CA) and P < 0.05 was considered statistically significant.
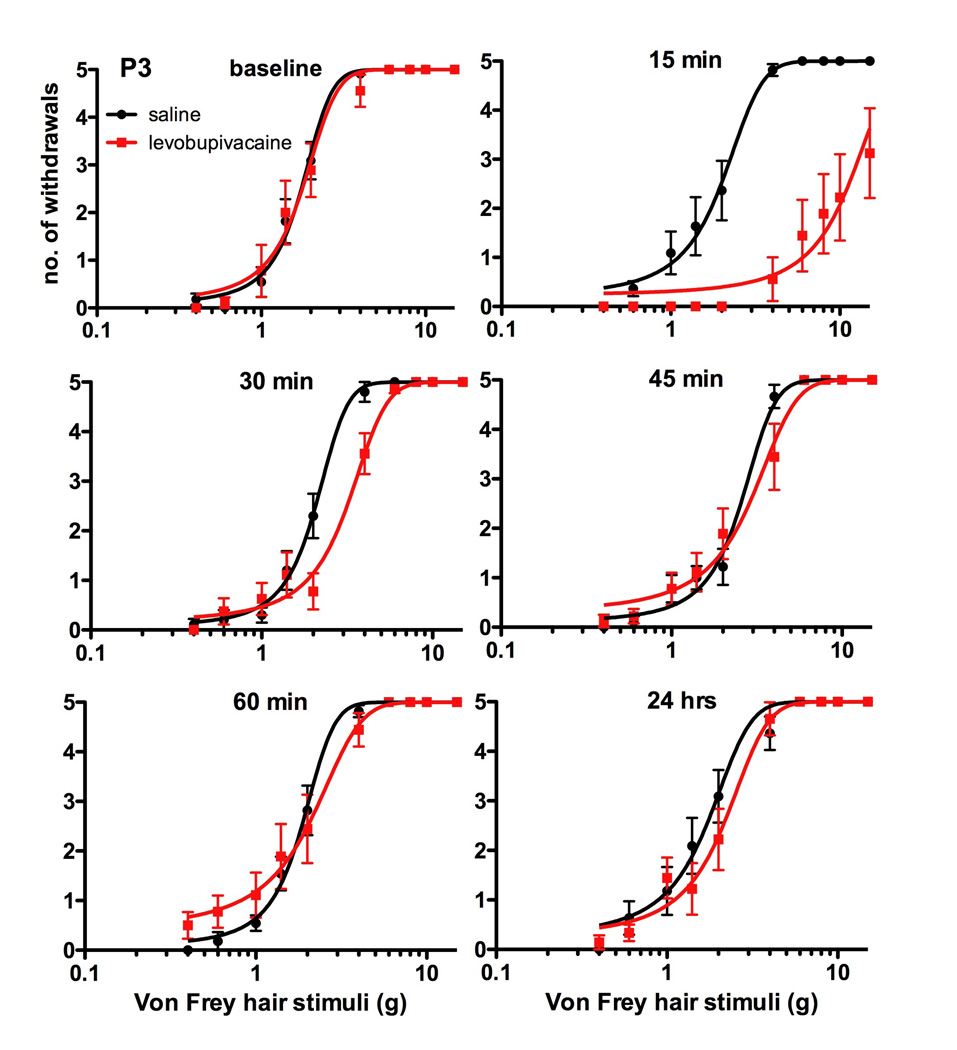
Figure 1.
Mechanical withdrawal thresholds of postnatal day (P)3 rat pups prior to (baseline) and at intervals (15, 30, 45, 60mins and 24hrs) following intrathecal injection of saline or 0.5mcl/g of 0.5% levobupivacaine. Graphs represent the log10 stimulus-response relationship between number of hindlimb withdrawal responses (0-5) and mechanical stimulus (grams, g) applied with graded von Frey hairs. At 15 minutes, thresholds were higher in the levobupivacaine (log EC50 11.3g; 95%CI: 9.2-13.5) than saline (log EC50 2.0; 95%CI 1.7 to 2.3) group. Data points = pooled mean ± SEM for saline (n=11) and levobupivacaine (n=9) groups.
Figure 2.
Mechanical withdrawal thresholds of postnatal day (P)7 rat pups prior to (baseline) and at intervals (15, 30, 45, 60mins and 24hrs) following intrathecal injection of saline or 0.5mcl/g of 0.5% levobupivacaine. Graphs represent the log10 stimulus-response relationship between number of hindlimb withdrawal responses (0-5) and mechanical stimulus (grams, g) applied with graded von Frey hairs. Thresholds were higher in the levobupivacaine versus saline group at 15 minutes (log EC50 14.9g 95%CI: 11.8-18.0 versus 2.4g; 95%CI 2.0-2.9) and to a lesser degree at 30 minutes (log EC50 5.1g 95%CI: 4.3-6.0 versus 2.5g; 95%CI 2.1-3.0) . Data points = pooled mean ± SEM for saline (n=9) and levobupivacaine (n=9) groups.
Results
Intrathecal levobupivacaine produces sensory and motor blockade in rat pups
Body weights of rat pups were between 8–11 g (mean± SEM 9.75 ± 0.24) at P3, and 12–18 g (15.74 ± 0.39) at P7. Baseline mechanical withdrawal thresholds were lower at P3 than P7 (1.9±0.2g, n=20 vs 2.7±0.3, n=19; P<0.05 unpaired 2-tailed t-test) but did not differ significantly between saline versus levobupivacaine groups within age groups (1.9±0.2 vs 2.0±0.3, P=0.66 at P3; 2.5±0.3 vs 2.8±0.4, P=0.41 at P7). Dense motor block of the hindlimbs and failure of hip flexion was apparent 5 minutes following intrathecal levobupivacaine, when animals had recovered from anesthesia. Fifteen minutes following injection, bilateral block (motor score 0/4) was apparent in 4/9 P3 pups and 6/10 P7 pups; unilateral block (motor score 1/4) in 4/9 P3 and 3/10 P7 pups; and one animal at each age had partial motor response to a suprathreshold mechanical stimulus (motor score 2/4). Animals in which correct intrathecal placement could not be confirmed by early dense motor block, or that had a motor score of 3 or 4 at 15 minutes, were precluded from further analysis. Motor deficits were not apparent in any saline treated animals.
Fifteen minutes following levobupivacaine in P3 (Fig. 1) and P7 pups (Fig. 2), the mechanical stimulus-response curve was shifted to the right and the maximal response was reduced due to complete motor block in the majority of animals. Within group pooled thresholds were higher in levobupivacaine versus saline groups 15 minutes following intrathecal injection at both P3 (11.3±1.1 vs 2.0±0.1g, P<0.001, two-tailed unpaired two-tailed Student’s t-test) and P7 (14.9±1.6g vs 2.4 ± 0.2g, P<0.001). Analysis of individual values with time, similarly demonstrated significant differences at 15 minutes (P<0.001 in P3 and P7 animals; two way repeated measures ANOVA with Bonferroni post-test). Values did not differ between saline and levobupivacaine groups at other time points to 24 hours in P3 pups, but sensory block was more prolonged in P7 pups with higher mechanical withdrawal thresholds at 30 minutes in the levobupivacaine versus saline group (5.3±1.1 vs 2.8±0.7g, P<0.05, two way repeated measures ANOVA with Bonferroni post-test). Withdrawal thresholds increased with age, but there were no statistically significant differences between groups 7 days following injection of levobupivacaine or saline at P3 (8.4±1.4 vs 5.8±0.6; P=0.11) or P7 (15.1±2.4 vs. 19.0±3.6g; P=0.38; unpaired two-tailed Student’s t-test).
Intrathecal levobupivacaine, apoptosis and glial reactivity
The number of activated caspase-3 positive cells in the lumbar spinal cord (Fig. 3A) did not differ between saline and levobupivacaine groups 24 hours following injection at P3 or P7 (Fig. 3B). Similarly, numbers of Fluoro-Jade C positive cell counts was not altered by intrathecal levobupivacaine (Fig. 3C). Consistent with our previous studies, levels of apoptosis where higher at P3 than P7, and were predominantly distributed in the dorsal horn (Fig. 3B,C).
Figure 3.
Levels of spinal cord apoptosis are influenced by postnatal age but not by intrathecal levobupivacaine.
(A) Representative section of dorsal horn demonstrating activated caspase-3 positive cells 24 hours following saline at postnatal day (P)3 (scale bar=100mm).
(B) Activated caspase-3 positive (AC-3+ve) cell counts 24 hours after intrathecal injection of saline or 0.5% levobupivacaine (l-bupi) in P3 and P7 rat pups. Bars represent mean ± SEM, n=4-5 per group. There were no significant differences between saline versus levobupivacaine groups in the number of positive cells in the total section (P=0.62) or dorsal horn (P=0.17) at P3 or at P7 (P=0.88 and P=0.66 for total and dorsal horn cells respectively; two-tailed Mann-Whitney test).
(C) Fluoro-Jade C positive (FJ-C+ve) cell counts 24 hours after intrathecal injection of saline or 0.5% levobupivacaine in P3 and P7 rat pups. Total numbers in the transverse spinal sections and the proportion distributed in the dorsal horn are shown. Bars represent mean ± SEM, n=4-5 per group. There were no significant differences between saline versus levobupivacaine groups in the number of positive cells in the total section (P=0.11) or dorsal horn (P=0.10) at P3 or at P7 (P=0.56 and P=0.89 for total and dorsal horn cells respectively; two-tailed Mann-Whitney test).
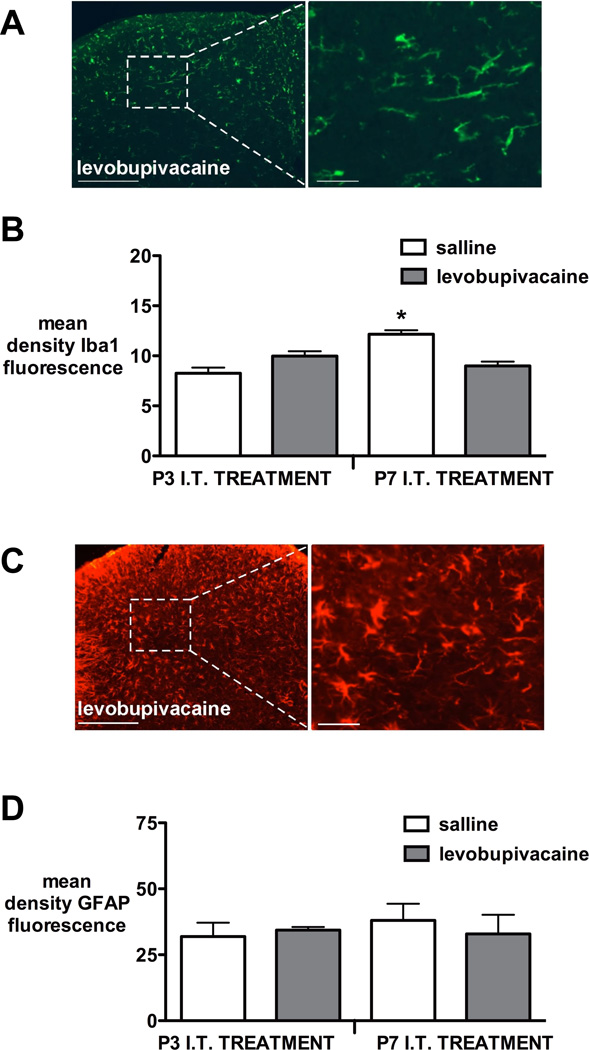
Microglial Iba1 staining 7 days after injection was higher in the P7 saline versus levobupivacaine group (P<0.05, two tailed Mann Whitney test), but P7 levobupivacaine did not differ from animals receiving saline or levobupivacaine at P3 (P=0.22, Kruskal Wallis test with Dunn’s multiple comparisons)(Fig. 4A,B). Intrathecal levobupivacaine at either P3 or P7 did not alter the intensity of staining with the astrocytic marker glial fibrillary acidic protein 7 days after injection when compared with saline control groups (Fig. 4C,D).
Figure 4.
Glial reactivity 7 days following intrathecal injection of saline or levobupivacaine at postnatal day (P)3 or P7.
(A) Representative ionized calcium binding adapter molecule 1 (Iba1) immunoreactivity in spinal cord dorsal horn 7 days after intrathecal (I.T.) levobupivacaine at P3. Scale bars = 200 µm in low and 40 µm in high magnification image.
(B) Quantification of mean density of Iba1 immunoreactivity in dorsal horn revealed no difference between P3 saline and levobupivacaine (P=0.24), but increased staining in the P7 intrathecal saline vs levobupivacaine group (*P<lt;0.05, two-tailed Mann Whitney test). Bars = mean±SEM, n=4-5 per group.
(C) Representative glial fibrillary acidic protein (GFAP) immunoreactivity in spinal cord dorsal horn 7 days after I.T. (intrathecal) levobupivacaine at P7. Scale bars = 200 µm in low and 40 µm in high magnification image.
(D) Quantification of GFAP immunoreactivity in the dorsal horn was not significantly different following injection at P3 (P=1.0) or P7 (P=0.55; two-tailed Mann Whitney test). Bars=mean±SEM, n=4-5 per group.
Histopathological evaluation in the spinal cord and cauda equina
There was no evidence of necrosis, gliosis, or inflammation in saline or levobupivacine groups at any time point. Scattered apoptotic cells were seen in all animals. These were present in greatest numbers in the youngest age group (ie. P3 animals with 24 hour survival) but did not differ between saline and levobupivacaine groups (5.7±0.6 vs 7.2±1.8 per section respectively, P=0.6, Mann Whitney two-tailed test). At all older ages and time points (P8, P7 plus 24 hours; P10, P3 plus 7 days; P14, P7 plus 7 days), the mean number of apototic cells was less than 2 per section in both saline and levobupivacaine groups.
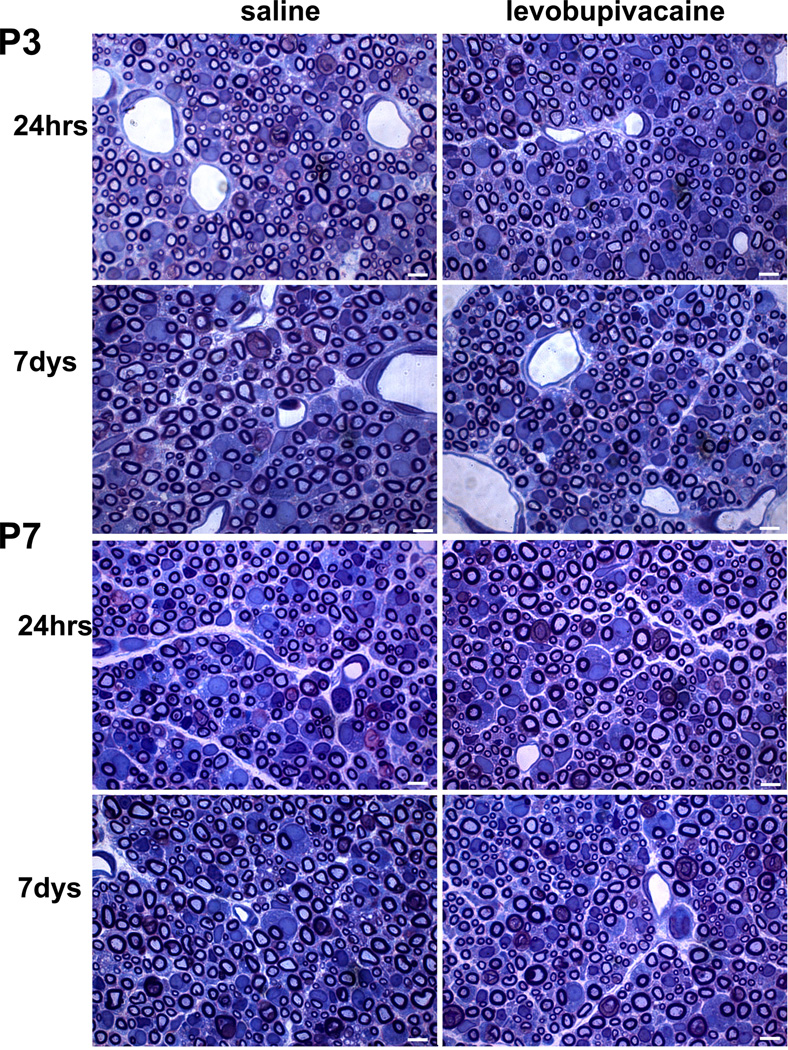
Multiple intact myelinated axons were observed in the endoneurium of the cauda equina in both levobupivacaine and saline treatment groups in postnatal age P3 and P7 day pups (Fig. 5). Mild endoneurial edema, particularly in the subperineurial and perivascular spaces was observed in all groups. Enlarged cytoplasm of activated Schwann cells, characteristic of their morphology during developmental remodeling of myelin in the nerves of P3 and P7 rat pups49, was observed in both treatment groups. There were no apparent differences between saline and levobupivacaine groups in either P3 or P7 pups.
Figure 5.

Histologic sections of cauda equina nerves 24 hrs or 7 days after intrathecal injection of saline or levobupivacaine in postnatal day (P)3 and P7 pups. Transverse, 1-µm-thick, plastic-embedded sections stained with methylene blue Azure II at x1400 magnification. Intact myelinated axons, mild endoneurial edema and activated Schwann cells characteristic of an age-appropriate proliferative phenotype were observed in all sections.
Intrathecal levobupivacaine and spinal reflex function in early adulthood
Spinal reflex responses at P35 were not altered by neonatal spinal anesthesia. Mechanical withdrawal threshold (Fig. 6A) and thermal withdrawal latency (Fig. 6B) did not differ significantly between groups receiving intrathecal saline or levobupivacaine at either P3 or P7 (P=0.1 and 0.051 respectively, one way ANOVA with Bonferroni’s post-hoc comparisons).
Figure 6.
Spinal reflex responses to hindpaw stimuli in young adulthood are not altered by prior intrathecal levobupivacaine.
(A) Mechanical withdrawal threshold at postnatal day (P)35 following intrathecal (IT) injection on postnatal day 3 (P3 IT) or 7 (P7 IT) of saline or 0.5% levobupivacaine (l-bupi). Data points = box and whiskers (5-95 percentile); n=6-8 all groups. Values did not differ significantly across treatment groups (P=0.10 one way ANOVA).
(B) Thermal withdrawal latency at P35 following IT saline or levobupivacaine at P3 or P7. Data points = box and whiskers (5-95 percentile); n=6-8 all groups. (P=0.051 one way ANOVA).
(C) (i): Example of electromyography (EMG) recordings in biceps femoris at P35 in response to increasing mechanical stimuli (von Frey hair, vFh 17-20), and (ii) plot of stimulus-response relationship for quantification of area under the curve (AUC) to threshold (vFh 18) and suprathreshold (vFh 19 and 20) stimuli. (D) The quantified reflex response (AUC EMG) did not differ between animals treated with saline or levobupivacaine at P3 or P7 (P=0.87 one way ANOVA). Data points = mean±SEM; n=6 per group.
At P35, males weighed more than females (131 ± 4.5 g vs 116 ± 4.1g), and there was a significant main effect of sex (F1,11=6.2, P=0.03), but not of treatment (F1,11=2.38, P=0.15; two-way ANOVA with sex and treatment as variables) on body weight. There was no main effect of intrathecal treatment (saline or levobupivacaine) (F1,11=0.64, P=0.44) or sex (F1,11=0.05, P=0.83) on mechanical withdrawal threshold. Similarly, there was no main effect of treatment (F1,11=0.39, P=0.54) or sex (F1,11=0.51, P=0.49) on thermal withdrawal latency at P35 (two-way ANOVA with treatment and sex as variables). Age at time of injection (P3 or P7) did not influence mechanical withdrawal threshold (F1,28=6.6, P=016) or thermal withdrawal latency (F1,28=3.4, P=0.08) in early adulthood (two-way ANOVA with age at time of injection and treatment as variables).
Spinal reflex sensitivity to suprathreshold mechanical stimuli, quantified from the area under the mechanical stimulus versus electromyography response relationship (Fig. 6C), did not differ between animals receiving intrathecal levobupivacaine or saline at P3 or P7 (P=0.87, one way ANOVA with Bonferroni’s post-hoc comparisons; Fig. 6D).
Gait analysis using the CatWalk® runway system at P35 was not altered by prior intrathecal injection of 0.5% bupivacaine at P3. Static (paw print area) and dynamic gait parameters (regularity index, duty cycle, stride length, stability of gait) did not differ from animals that received intrathecal saline at P3 (Table 1) or age-matched naïve controls (as previously reported27).
Table 1.
Gait Parameters at Postnatal Day (P)35 following Intrathecal Treatment at P3
| Treatment | Print Area | Regularity Index |
Duty Cycle | Stride Length |
Base of Support |
|---|---|---|---|---|---|
| saline* | 40.3 ± 3.5 | 99.8 ± 0.2 | 54.1 ± 1.4 | 107.5 ± 2.1 | 27.6 ± 0.7 |
| bupivacaine | 40.5 ± 1.9 | 99.7 ± 0.3 | 57.7 ± 1.0 | 99.6 ± 3.5 | 31.0 ± 0.93 |
Legend: P = postnatal day; Print area = surface area of floor contacted by hindpaw; Regularity index = index for degree of interlimb co-ordination during gait; Duty cycle = ratio between stance duration and full stepcycle duration [stance phase duration / (stance + swing phase duration)]; Stride length = distance between placement of hindpaw and subsequent placement of same paw; Base of Support = distance between two hindpaws measured perpendicular to walking direction;
saline data previously reported26.
Discussion
Single dose intrathecal levobupivacaine produced reliable sensory and motor blockade in neonatal rat pups at postnatal day 3 (P3) and P7. Intrathecal levobupivacaine did not increase apoptosis in the spinal cord, or produce histopathological change in the cord or cauda equina. Neonatal spinal anesthesia did not adversely affect spinal cord function in young adulthood (P35), as sensory withdrawal reflex thresholds, electromyographic responses to suprathreshold hindpaw stimuli, and gait did not differ from litter-mate controls receiving intrathecal saline.
Neuraxial local anesthetics can be used as an alternative to general anesthesia, or as a supplemental technique for peri-operative analgesia, in children of all ages, and case series in neonates and infants have recently been summarised.1 Excessive absorption or inadvertent vascular injection can result in systemic toxicity and neurological and cardiovascular complications. As a result, there is increasing use of the stereoisomers levobupivacaine or ropivacaine, which have wider therapeutic windows than racemic bupivacaine.50,51 Levobupivacaine 0.5% produced reliable motor and sensory blockade in P3 and P7 pups. Duration of sensory block was shorter in younger animals (P3<P7), as seen following bupivacaine (P7<P21).20 Similarly, duration of intrathecal local anesthetic block in infants is less than adults, and postulated to be due to age-dependent differences in relative volume and more rapid turn-over of cerebrospinal fluid.52
Serious neurological complications following neuraxial techniques in children are rare,1,53 but complication rates are higher in neonates.7,54 Although technical issues in small patients may be a factor, specific evaluation of the relative safety of neuraxial drugs in early development is essential. Use of spinal analgesia in neonates is increasing in some centres7,8 and may be further encouraged to reduce exposure to general anesthetics and the potential risk of increased neuronal apoptosis in the developing brain.24,55 However, the developing spinal cord is also susceptible to apoptosis, which is increased following prolonged general anesthesia at P720,56 and analgesic doses of intrathecal ketamine at P3.28 By contrast, intrathecal bupivacaine 3.5mg/kg did not increase neuronal apoptosis or produce histopathological change in spinal cord white matter following injection at P7, P14 or P21.20 Similarly, intrathecal levobupivacaine 2.5mg/kg did not increase apoptosis at P7, but additionally we found no adverse effect at the younger age of P3, when baseline apoptosis is higher in spinal cord.28,29 This was confirmed using 3 different but complementary methods: antibodies to activated caspase-3 (an enzyme in the apoptotic cascade expressed once the neuron is committed to cell death)57; Fluoro-Jade C staining (which labels degenerating neurons)44; and identification of apoptotic cells under high power microscopy.
Clinical concerns regarding local anesthetic toxicity followed reports of cauda equina syndrome with continuous spinal anesthesia and high local concentrations of lidocaine or tetracaine.58,59 Transient neurological symptoms (pain in gluteal region and radiating to legs which usually resolves by the 5th postoperative day) may occur following spinal anesthesia; most commonly following lidocaine (relative risk about seven times higher than bupivacaine), but there is insufficient data to compare rates associated with levobupivacaine or ropivacaine.16 Although there has been limited detailed evaluation, transient neurological symptoms (tingling in the feet) have been reported following spinal anesthesia in children.2 Such symptoms and subtle motor deficits cannot be detected in neonates and infants. This emphasizes the need to evaluate comparative local anesthetic toxicity in developmental models.
Local anesthetic toxicity has been evaluated using in vivo and in vitro models. In cell cultures, including adult dorsal root ganglion neurons from rodents and human neuroblastoma cell lines, concentration-dependent toxicity has consistently been shown. Local anesthetics produce mitochondrial injury, caspase activation, apoptosis, increased calcium influx60 and necrosis at higher concentrations.61 Although selective sensitivity to lidocaine has been reported,31 cellular toxicity has been shown following most local anesthetics, and apoptotic potency correlated with lipid solubility rather than chemical structure.30 However, in vitro models may over-estimate neurotoxicity as isolated cells in culture are more vulnerable (no diffusion barriers and no vascular clearance of drug), and duration of exposure to high concentrations is often more prolonged.30,62 Local anesthetics impaired outgrowth of developing neurites (increased growth cone collapse) in cultured dorsal root ganglion neurons from chick embryo,63 but there has been limited evaluation in developmental models.
In adult rabbits and rodents, intrathecal local anesthetics can produce signs of spinal toxicity, but results vary with dose and drug. Spinal histopathology (increased macrophage infiltration, axonal degeneration and myelin changes) followed intrathecal 10% lidocaine21 but not equi-effective bupivacaine.64 Levobupivacaine 5% 0.12mcl/g (approximately 0.6mg/kg) did not produce histology in adult rats,65 but a higher dose (approximately 2.8mg/kg) produced white matter injury that was similar to procaine but greater than following ropivacaine.23 Vacuolization and degeneration of neurons in the gray matter has also been demonstrated following 10% lidocaine,21 and to a lesser degree following tetracaine, 2% bupivacaine and 2% ropivacaine.22 In cauda equina, histopathology in adult rodents was greater following 6.9mg/kg lidocaine (10%) than after equi-effective 1.5mg/kg bupivacaine (~2.1%).64 Similar injury scores were reported following high dose bupivacaine or levobupivacaine (approximately 10mg/kg total dose).40 The current study found no differences in spinal cord or cauda equina histology between intrathecal levobupivacaine and saline groups administered at either P3 or P7. However, rat pups received a lower dose per body weight (2.5mg/kg), and were exposed to a lower drug concentration as a consequence of both the injectate concentration (0.5%) and further dilution in the relatively greater cerebrospinal fluid volume in pups (8.8mcl/g at P5, 4mcl/g at P30).66 Intrathecal injectates of 0.5mcl/g produce spread over thoracic and lumbar segments in rat pups,27 and higher volumes of local anesthetic can produce significant respiratory compromise.20 Consistent with a previous evaluation of intrathecal bupivacaine at P7, P14 and P21,20 the maximum dose of levobupivacaine at P3 or P7 did not increase apoptosis or produce histopathology. As spinal catheters and prolonged infusions are not practical in these small pups, we have suggested intrathecal dose escalation and calculation of a therapeutic index (maximum tolerated or minimum toxic dose / analgesic dose) to evaluate comparative spinal toxicity in developmental models.1 The current dose of levobupivacaine produced reversible motor and sensory block without toxicity, but dose escalation was limited by side-effects. Therefore, the therapeutic index is greater than 1, but we cannot exclude toxicity at higher concentrations or doses of levobupivacaine.
Assessing effects of neonatal exposure on long-term spinal cord function can include several parameters. Prolonged general anesthesia at P7 increased apoptosis in the spinal cord, but did not impair motor performance at P30 (assessed by time on the Rotarod apparatus).20,56 However, as apoptosis in the post-natal cord is greatest in the dorsal horn,67 increased apoptosis may also influence sensory function. Intrathecal ketamine at P3 increased apoptosis, reduced mechanical withdrawal threshold, and altered static, but not dynamic, gait parameters at P35.28 Local anesthetics may have additional toxic effects unrelated to developmental apoptosis, that influence both motor and sensory outcomes. In adult animals, changes in thermal tail flick latency, mechanical paw pressure withdrawal threshold, and motor function have been shown 4 to 7 days following doses of local anesthetic that produce tissue histopathology.21,22,40,64,68 Here, single intrathecal doses of 2.5mg/kg levobupivacaine at either P3 or P7 did not produce persistent changes in spinal reflex withdrawal to mechanical or thermal stimuli, and did not alter quantified electromyography responses to suprathreshold mechanical stimuli. Intrathecal bupivacaine 3.75mg/kg at P7 did not to alter motor function (time on the Rotarod) at P30.20 The Catwalk® system allows analysis of sensorimotor co-ordination and both static and dynamic components of gait. Following intrathecal bupivacaine at P3, gait parameters at P35 did not differ from our previously reported values in age-matched naïve or P3 intrathecal saline control animals.27
Local anesthetics and spinal analgesics are commonly co-administered to improve analgesia or reduce local anesthetic requirements,1,69 but some analgesics enhance local anesthetic toxicity in cell culture models. Ropivacaine-induced decreases in neuronal viability (adult dorsal root ganglion cell culture) were potentiated by midazolam, but not clonidine or buprenorphine.62 Midazolam and ketamine both increased lidocaine toxicity (human neuroblastoma and rat astrocyte cultures), but sufentanil, clonidine, epinephrine and neostigmine had no effect.70 Our in vivo studies demonstrated increased neuronal apoptosis in the neonatal cord following ketamine,28 but not clonidine29 or morphine27. Further in vivo developmental studies evaluating age- and dose-dependent analgesic efficacy, spinal cord and nerve root histology, and long-term function following combinations of spinal analgesics and local anesthetic would allow further comparison of the safety profile of current clinical regimens.
In conclusion, single doses of 0.5% intrathecal levobupivacaine produce reliable spinal anesthesia in P3 and P7 neonatal rat pups, but do not increase apoptosis or produce histopathological changes in the spinal cord or cauda equina. This study provides further preclinical safety data relevant to the use of spinal anesthesia in neonates, and supports the use of neuraxial anesthesia as a comparison group71 for evaluating outcomes following surgery and anesthesia in early life. Further, these results add to a growing body of data that validate the neonatal rat as a robust model for assessing the pathogenic propensity of neuraxial agents throughout postnatal development, and the requirement for preclinical evaluation of toxicity as part of the rational development of neonatal neuraxial therapeutics.1
Summary Statement.
Spinal cord toxicity and neuronal apoptosis was evaluated following intrathecal levobupivacaine in neonatal rats. Single dose intrathecal levobupivacaine had no adverse effects on spinal cord or nerve root histology or on longterm spinal reflex function.
Final Box Summary.
What we already know about this topic
Spinally injected local anesthetics can cause neurotoxicity and general anesthesia during infancy enhances apoptosis in rodents
Safety regarding these potential toxicities of spinal local anesthetics in infant animals has had limited testing
What this article tells us that is new
In 3 and 7 day old rats, intrathecal injection of 0.5% levobupivacaine produced temporary spinal anesthesia, but did not increase apoptosis, or result in histologic or behavioral neurotoxicity
Acknowledgements
Funding Support:
This research was supported by funding from:
National Institutes of Health: NIH-DA02110 and NIH-DA15353, Bethesda, MD, USA (T Yaksh); British Journal of Anaesthesia/Royal College of Anaesthetists Project Grant, London, United Kingdom (S Walker); The Council of Higher Education, Bilkent, Ankara, Turkey (E Hamurtekin).
We would like to thank Jennifer Dolkas, Senior Technician, Peripheral Nerve Research Group Laboratory, Department of Anesthesiology, University of California San Diego, La Jolla, California, USA for technical assistance with plastic nerve section preparation, and Noldus Information Technology, Wageningen, Gelderland, The Netherlands for the loan of a CatWalk® system.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Emre Hamurtekin, Research Fellow, Department of Anesthesiology, University of California San Diego, La Jolla, California, USA and Assistant Professor of Pharmacology, Kafkas University School of Medicine, Department of Pharmacology, Kars, Kars Province, Turkey.
Bethany L. Fitzsimmons, Staff Research Associate III, Department of Anesthesiology, University of California San Diego, La Jolla, California, USA.
Veronica I. Shubayev, Associate Professor of Anesthesiology, Department of Anesthesiology, University of California San Diego, La Jolla, California, USA.
Marjorie R. Grafe, Professor of Neuropathology, Oregon Health and Science University, Portland, Oregon, USA.
Ronald Deumens, Research Fellow, Department of Anesthesiology, University of California San Diego, La Jolla, California, USA.
Tony L. Yaksh, Professor of Anesthesiology and Pharmacology, Department of Anesthesiology, University of California San Diego, La Jolla, California, USA.
Suellen M. Walker, Senior Clinical Lecturer and Consultant in Paediatric Anaesthesia and Pain Medicine, UCL Institute of Child Health and Great Ormond St Hospital for Children NHS Foundation Trust, London, United Kingdom.
References
- 1.Walker SM, Yaksh TL. Review article: Neuraxial analgesia in neonates and infants: a review of clinical and preclinical strategies for the development of safety and efficacy data. Anesth Analg. 2012;115:638–662. doi: 10.1213/ANE.0b013e31826253f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kokki H. Spinal blocks. Paediatr Anaesth. 2012;22:56–64. doi: 10.1111/j.1460-9592.2011.03693.x. [DOI] [PubMed] [Google Scholar]
- 3.Kachko L, Simhi E, Tzeitlin E, Efrat R, Tarabikin E, Peled E, Metzner I, Katz J. Spinal anesthesia in neonates and infants - A single-center experience of 505 cases. Paediatr Anaesth. 2007;17:647–653. doi: 10.1111/j.1460-9592.2007.02194.x. [DOI] [PubMed] [Google Scholar]
- 4.Williams RK, Adams DC, Aladjem EV, Kreutz JM, Sartorelli KH, Vane DW, Abajian JC. The safety and efficacy of spinal anesthesia for surgery in infants: The Vermont Infant Spinal Registry. Anesth Analg. 2006;102:67–71. doi: 10.1213/01.ANE.0000159162.86033.21. [DOI] [PubMed] [Google Scholar]
- 5.Imbelloni LE, Vieira EM, Sperni F, Guizellini RH, Tolentino AP. Spinal anesthesia in children with isobaric local anesthetics: Report on 307 patients under 13 years of age. Paediatr Anaesth. 2006;16:43–48. doi: 10.1111/j.1460-9592.2005.01680.x. [DOI] [PubMed] [Google Scholar]
- 6.Kokki H, Tuovinen K, Hendolin H. Spinal anaesthesia for paediatric day-case surgery: A double-blind, randomized, parallel group, prospective comparison of isobaric and hyperbaric bupivacaine. Br J Anaesth. 1998;81:502–506. doi: 10.1093/bja/81.4.502. [DOI] [PubMed] [Google Scholar]
- 7.Ecoffey C, Lacroix F, Giaufre E, Orliaguet G, Courreges P. Epidemiology and morbidity of regional anesthesia in children: A follow-up one-year prospective survey of the French-Language Society of Paediatric Anaesthesiologists (ADARPEF) Paediatr Anaesth. 2010;20:1061–1069. doi: 10.1111/j.1460-9592.2010.03448.x. [DOI] [PubMed] [Google Scholar]
- 8.Rochette A, Dadure C, Raux O, Troncin R, Mailhee P, Capdevila X. A review of pediatric regional anesthesia practice during a 17-year period in a single institution. Paediatr Anaesth. 2007;17:874–880. doi: 10.1111/j.1460-9592.2007.02217.x. [DOI] [PubMed] [Google Scholar]
- 9.Kachko L, Birk E, Simhi E, Tzeitlin E, Freud E, Katz J. Spinal anesthesia for noncardiac surgery in infants with congenital heart diseases. Paediatr Anaesth. 2012;22:647–653. [PubMed] [Google Scholar]
- 10.Shenkman Z, Johnson VM, Zurakowski D, Arnon S, Sethna NF. Hemodynamic changes during spinal anesthesia in premature infants with congenital heart disease undergoing inguinal hernia correction. Paediatr Anaesth. 2012;22:865–870. doi: 10.1111/j.1460-9592.2012.03873.x. [DOI] [PubMed] [Google Scholar]
- 11.Craven PD, Badawi N, Henderson-Smart DJ, O'Brien M. Regional (spinal, epidural, caudal) versus general anaesthesia in preterm infants undergoing inguinal herniorrhaphy in early infancy. Cochrane Database Syst Rev. 2003:CD003669. doi: 10.1002/14651858.CD003669. [DOI] [PubMed] [Google Scholar]
- 12.Brouwers M, Driessen J, Severijnen R. Clinical letter: Epidural analgesia in a newborn with Hirschsprung's disease, associated with congenital central hypoventilation syndrome. Eur J Anaesthesiol. 2000;17:751–753. doi: 10.1046/j.0265-0215.2000.00771.x. [DOI] [PubMed] [Google Scholar]
- 13.Ecoffey C. Safety in pediatric regional anesthesia. Paediatr Anaesth. 2012;22:25–30. doi: 10.1111/j.1460-9592.2011.03705.x. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix F. Epidemiology and morbidity of regional anaesthesia in children. Curr Opin Anaesthesiol. 2008;21:345–349. doi: 10.1097/ACO.0b013e3282ffabc5. [DOI] [PubMed] [Google Scholar]
- 15.Valois T, Otis A, Ranger M, Muir JG. Incidence of self-limiting back pain in children following caudal blockade: An exploratory study. Paediatr Anaesth. 2010;20:844–850. doi: 10.1111/j.1460-9592.2010.03365.x. [DOI] [PubMed] [Google Scholar]
- 16.Zaric D, Pace NL. Transient neurologic symptoms (TNS) following spinal anaesthesia with lidocaine versus other local anaesthetics. Cochrane Database Syst Rev. 2009:CD003006. doi: 10.1002/14651858.CD003006.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Hampl KF, Schneider MC, Drasner K. Toxicity of spinal local anaesthetics. Curr Opin Anaesthesiol. 1999;12:559–564. doi: 10.1097/00001503-199910000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Drasner K. Models for local anesthetic toxicity from continuous spinal anesthesia. Reg Anesth. 1993;18:434–438. [PubMed] [Google Scholar]
- 19.Drasner K. Anesthetic Effects on the Developing Nervous System: If You Aren't Concerned, You Haven't Been Paying Attention. Anesthesiology. 2010;113:10–12. doi: 10.1097/ALN.0b013e3181dcd8b3. [DOI] [PubMed] [Google Scholar]
- 20.Yahalom B, Athiraman U, Soriano SG, Zurakowski D, Carpino EA, Corfas G, Berde CB. Spinal anesthesia in infant rats: Development of a model and assessment of neurologic outcomes. Anesthesiology. 2011;114:1325–1335. doi: 10.1097/ALN.0b013e31821b5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirihara Y, Saito Y, Sakura S, Hashimoto K, Kishimoto T, Yasui Y. Comparative neurotoxicity of intrathecal and epidural lidocaine in rats. Anesthesiology. 2003;99:961–968. doi: 10.1097/00000542-200310000-00032. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita A, Matsumoto M, Matsumoto S, Itoh M, Kawai K, Sakabe T. A comparison of the neurotoxic effects on the spinal cord of tetracaine, lidocaine, bupivacaine, and ropivacaine administered intrathecally in rabbits. Anesth Analg. 2003;97:512–519. doi: 10.1213/01.ANE.0000068885.78816.5B. [DOI] [PubMed] [Google Scholar]
- 23.Takenami T, Wang G, Nara Y, Fukushima S, Yagishita S, Hiruma H, Kawakami T, Okamoto H. Intrathecally administered ropivacaine is less neurotoxic than procaine, bupivacaine, and levobupivacaine in a rat spinal model. Can J Anaesth. 2012;59:456–465. doi: 10.1007/s12630-012-9685-9. [DOI] [PubMed] [Google Scholar]
- 24.Stratmann G. Review article: Neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg. 2011;113:1170–1179. doi: 10.1213/ANE.0b013e318232066c. [DOI] [PubMed] [Google Scholar]
- 25.McCann ME, Soriano SG. General anesthetics in pediatric anesthesia: Influences on the developing brain. Curr Drug Targets. 2012;13:944–951. doi: 10.2174/138945012800675768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- 27.Westin BD, Walker SM, Deumens R, Grafe M, Yaksh TL. Validation of a Preclinical Spinal Safety Model: Effects of Intrathecal Morphine in the Neonatal Rat. Anesthesiology. 2010;113:183–199. doi: 10.1097/ALN.0b013e3181dcd6ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker SM, Westin BD, Deumens R, Grafe M, Yaksh TL. Effects of Intrathecal Ketamine in the Neonatal Rat: Evaluation of Apoptosis and Long-term Functional Outcome. Anesthesiology. 2010;113:147–159. doi: 10.1097/ALN.0b013e3181dcd71c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker SM, Grafe M, Yaksh TL. Intrathecal clonidine in the neonatal rat: Dose-dependent analgesia and evaluation of spinal apoptosis and toxicity. Anesth Analg. 2012;115:450–460. doi: 10.1213/ANE.0b013e3182501a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Werdehausen R, Fazeli S, Braun S, Hermanns H, Essmann F, Hollmann MW, Bauer I, Stevens MF. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br J Anaesth. 2009;103:711–718. doi: 10.1093/bja/aep236. [DOI] [PubMed] [Google Scholar]
- 31.Boselli E, Duflo F, Debon R, Allaouchiche B, Chassard D, Thomas L, Portoukalian J. The induction of apoptosis by local anesthetics: A comparison between lidocaine and ropivacaine. Anesth Analg. 2003;96:755–756. doi: 10.1213/01.ANE.0000047201.85815.9D. [DOI] [PubMed] [Google Scholar]
- 32.Frawley GP, Farrell T, Smith S. Levobupivacaine spinal anesthesia in neonates: A dose range finding study. Paediatr Anaesth. 2004;14:838–844. doi: 10.1111/j.1460-9592.2004.01364.x. [DOI] [PubMed] [Google Scholar]
- 33.Frawley G, Smith KR, Ingelmo P. Relative potencies of bupivacaine, levobupivacaine, and ropivacaine for neonatal spinal anaesthesia. Br J Anaesth. 2009;103:731–738. doi: 10.1093/bja/aep259. [DOI] [PubMed] [Google Scholar]
- 34.Kokki H, Ylonen P, Heikkinen M, Reinikainen M. Levobupivacaine for pediatric spinal anesthesia. Anesth Analg. 2004;98:64–67. doi: 10.1213/01.ANE.0000093309.75358.30. [DOI] [PubMed] [Google Scholar]
- 35.Chalkiadis GA, Anderson BJ, Tay M, Bjorksten A, Kelly JJ. Pharmacokinetics of levobupivacaine after caudal epidural administration in infants less than 3 months of age. Br J Anaesth. 2005;95:524–529. doi: 10.1093/bja/aei218. [DOI] [PubMed] [Google Scholar]
- 36.Taylor R, Eyres R, Chalkiadis GA, Austin S. Efficacy and safety of caudal injection of levobupivacaine, 0.25%, in children under 2 years of age undergoing inguinal hernia repair, circumcision or orchidopexy. Paediatr Anaesth. 2003;13:114–121. doi: 10.1046/j.1460-9592.2003.01036.x. [DOI] [PubMed] [Google Scholar]
- 37.Lerman J, Nolan J, Eyres R, Schily M, Stoddart P, Bolton CM, Mazzeo F, Wolf AR. Efficacy, safety, and pharmacokinetics of levobupivacaine with and without fentanyl after continuous epidural infusion in children: A multicenter trial. Anesthesiology. 2003;99:1166–1174. doi: 10.1097/00000542-200311000-00025. [DOI] [PubMed] [Google Scholar]
- 38.De Negri P, Ivani G, Tirri T, Modano P, Reato C, Eksborg S, Lonnqvist PA. A comparison of epidural bupivacaine, levobupivacaine, and ropivacaine on postoperative analgesia and motor blockade. Anesth Analg. 2004;99:45–48. doi: 10.1213/01.ANE.0000120162.42025.D0. [DOI] [PubMed] [Google Scholar]
- 39.Mather LE, Copeland SE, Ladd LA. Acute toxicity of local anesthetics: Underlying pharmacokinetic and pharmacodynamic concepts. Reg Anesth Pain Med. 2005;30:553–566. doi: 10.1016/j.rapm.2005.07.186. [DOI] [PubMed] [Google Scholar]
- 40.Muguruma T, Sakura S, Kirihara Y, Saito Y. Comparative somatic and visceral antinociception and neurotoxicity of intrathecal bupivacaine, levobupivacaine, and dextrobupivacaine in rats. Anesthesiology. 2006;104:1249–1256. doi: 10.1097/00000542-200606000-00021. [DOI] [PubMed] [Google Scholar]
- 41.Walker SM, Howard RF, Keay KA, Fitzgerald M. Developmental age influences the effect of epidural dexmedetomidine on inflammatory hyperalgesia in rat pups. Anesthesiology. 2005;102:1226–1234. doi: 10.1097/00000542-200506000-00024. [DOI] [PubMed] [Google Scholar]
- 42.Walker SM, Meredith-Middleton J, Lickiss T, Moss A, Fitzgerald M. Primary and secondary hyperalgesia can be differentiated by postnatal age and ERK activation in the spinal dorsal horn of the rat pup. Pain. 2007;128:157–168. doi: 10.1016/j.pain.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Walker SM, Tochiki KK, Fitzgerald M. Hindpaw incision in early life increases the hyperalgesic response to repeat surgical injury: Critical period and dependence on initial afferent activity. Pain. 2009;147:99–106. doi: 10.1016/j.pain.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 44.Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 45.Grafe MR, Woodworth KN, Noppens K, Perez-Polo JR. Long-term histological outcome after post-hypoxic treatment with 100% or 40% oxygen in a model of perinatal hypoxic-ischemic brain injury. Int J Dev Neurosci. 2008;26:119–124. doi: 10.1016/j.ijdevneu.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang S, Abrahams MS, Hurn PD, Grafe MR, Kirsch JR. Local anesthetic Schwann cell toxicity is time and concentration dependent. Reg Anesth Pain Med. 2011;36:444–451. doi: 10.1097/AAP.0b013e318228c835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sekiguchi M, Kikuchi S, Myers RR. Experimental spinal stenosis: Relationship between degree of cauda equina compression, neuropathology, and pain. Spine (Phila Pa 1976) 2004;29:1105–1111. doi: 10.1097/00007632-200405150-00011. [DOI] [PubMed] [Google Scholar]
- 48.Myers RR, Shubayev VI. The ology of neuropathy: An integrative review of the role of neuroinflammation and TNF-alpha axonal transport in neuropathic pain. J Peripher Nerv Syst. 2011;16:277–286. doi: 10.1111/j.1529-8027.2011.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webster HD. In: Development of peripheral nerves, Peripheral Neuropathy. Dyck PJ, Thomas PK, editors. Philadelphia: W.B. Saunders Co.; 1993. pp. 243–266. [Google Scholar]
- 50.Gunter J. Benefit and risks of local anesthetics in infants and children. Paediatr Drugs. 2002;4:649–672. doi: 10.2165/00128072-200204100-00003. [DOI] [PubMed] [Google Scholar]
- 51.Ivani G, Mossetti V. Continuous central and perineural infusions for postoperative pain control in children. Curr Opin Anaesthesiol. 2010;23:637–642. doi: 10.1097/ACO.0b013e32833d4f81. [DOI] [PubMed] [Google Scholar]
- 52.Dalens BJ, Truchon R. In: Neural blockade for pediatric surgery, Neural Blockade in Clinical Anesthesia and Pain Medicine. 4th edition. Cousins MJ, Bridenbaugh PO, Carr D, Horlocker T, editors. Lippincott: Williams & Wilkins; 2009. pp. 595–629. [Google Scholar]
- 53.Polaner DM, Taenzer AH, Walker BJ, Bosenberg A, Krane EJ, Suresh S, Wolf C, Martin LD. Pediatric Regional Anesthesia Network (PRAN): A Multi-Institutional Study of the Use and Incidence of Complications of Pediatric Regional Anesthesia. Anesth Analg. 2012;115:1353–1364. doi: 10.1213/ANE.0b013e31825d9f4b. [DOI] [PubMed] [Google Scholar]
- 54.Llewellyn N, Moriarty A. The national pediatric epidural audit. Paediatr Anaesth. 2007;17:520–533. doi: 10.1111/j.1460-9592.2007.02230.x. [DOI] [PubMed] [Google Scholar]
- 55.McGowan FX, Jr, Davis PJ. Anesthetic-related neurotoxicity in the developing infant: Of mice, rats, monkeys and, possibly, humans. Anesth Analg. 2008;106:1599–1602. doi: 10.1213/ane.0b013e31817330cf. [DOI] [PubMed] [Google Scholar]
- 56.Sanders RD, Xu J, Shu Y, Fidalgo A, Ma D, Maze M. General anesthetics induce apoptotic neurodegeneration in the neonatal rat spinal cord. Anesth Analg. 2008;106:1708–1711. doi: 10.1213/ane.0b013e3181733fdb. [DOI] [PubMed] [Google Scholar]
- 57.Jevtovic-Todorovic V, Olney JW. PRO: Anesthesia-induced developmental neuroapoptosis: Status of the evidence. Anesth Analg. 2008;106:1659–1663. doi: 10.1213/ane.0b013e3181731ff2. [DOI] [PubMed] [Google Scholar]
- 58.Rigler ML, Drasner K, Krejcie TC, Yelich SJ, Scholnick FT, DeFontes J, Bohner D. Cauda equina syndrome after continuous spinal anesthesia. Anesth Analg. 1991;72:275–281. doi: 10.1213/00000539-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 59.Drasner K. Local anesthetic neurotoxicity: Clinical injury and strategies that may minimize risk. Reg Anesth Pain Med. 2002;27:576–580. doi: 10.1053/rapm.2002.37410. [DOI] [PubMed] [Google Scholar]
- 60.Gold MS, Reichling DB, Hampl KF, Drasner K, Levine JD. Lidocaine toxicity in primary afferent neurons from the rat. J Pharmacol Exp Ther. 1998;285:413–421. [PubMed] [Google Scholar]
- 61.Friederich P, Schmitz TP. Lidocaine-induced cell death in a human model of neuronal apoptosis. Eur J Anaesthesiol. 2002;19:564–570. doi: 10.1017/s0265021502000911. [DOI] [PubMed] [Google Scholar]
- 62.Williams BA, Hough KA, Tsui BY, Ibinson JW, Gold MS, Gebhart GF. Neurotoxicity of adjuvants used in perineural anesthesia and analgesia in comparison with ropivacaine. Reg Anesth Pain Med. 2011;36:225–230. doi: 10.1097/AAP.0b013e3182176f70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Radwan IA, Saito S, Goto F. The neurotoxicity of local anesthetics on growing neurons: A comparative study of lidocaine, bupivacaine, mepivacaine, and ropivacaine. Anesth Analg. 2002;94:319–324. doi: 10.1097/00000539-200202000-00016. [DOI] [PubMed] [Google Scholar]
- 64.Sakura S, Kirihara Y, Muguruma T, Kishimoto T, Saito Y. The comparative neurotoxicity of intrathecal lidocaine and bupivacaine in rats. Anesth Analg. 2005;101:541–547. doi: 10.1213/01.ANE.0000155960.61157.12. [DOI] [PubMed] [Google Scholar]
- 65.Takenami T, Yagishita S, Murase S, Hiruma H, Kawakami T, Hoka S. Neurotoxicity of intrathecally administered bupivacaine involves the posterior roots/posterior white matter and is milder than lidocaine in rats. Reg Anesth Pain Med. 2005;30:464–472. doi: 10.1016/j.rapm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 66.Bass NH, Lundborg P. Postnatal development of bulk flow in the cerebrospinal fluid system of the albino rat: clearance of carboxyl-( 14 C)inulin after intrathecal infusion. Brain Res. 1973;52:323–332. doi: 10.1016/0006-8993(73)90668-9. [DOI] [PubMed] [Google Scholar]
- 67.Lawson SJ, Davies HJ, Bennett JP, Lowrie MB. Evidence that spinal interneurons undergo programmed cell death postnatally in the rat. Eur J Neurosci. 1997;9:794–799. doi: 10.1111/j.1460-9568.1997.tb01428.x. [DOI] [PubMed] [Google Scholar]
- 68.Kishimoto T, Bollen AW, Drasner K. Comparative spinal neurotoxicity of prilocaine and lidocaine. Anesthesiology. 2002;97:1250–1253. doi: 10.1097/00000542-200211000-00031. [DOI] [PubMed] [Google Scholar]
- 69.Walker SM, Goudas LC, Cousins MJ, Carr DB. Combination spinal analgesic chemotherapy: a systematic review. Anesth Analg. 2002;95:674–715. doi: 10.1097/00000539-200209000-00033. [DOI] [PubMed] [Google Scholar]
- 70.Werdehausen R, Braun S, Hermanns H, Kremer D, Kury P, Hollmann MW, Bauer I, Stevens MF. The influence of adjuvants used in regional anesthesia on lidocaine-induced neurotoxicity in vitro. Reg Anesth Pain Med. 2011;36:436–443. doi: 10.1097/AAP.0b013e318226ba62. [DOI] [PubMed] [Google Scholar]
- 71.Davidson AJ, McCann ME, Morton NS, Myles PS. Anesthesia and outcome after neonatal surgery: The role for randomized trials. Anesthesiology. 2008;109:941–944. doi: 10.1097/ALN.0b013e31818e3f79. [DOI] [PubMed] [Google Scholar]