INTRODUCTION
Like many viruses, the Human Immunodeficiency Virus (HIV) associates with the host cell cytoskeleton immediately after entry, and is dependent on it for the formation of subcellular complexes and trafficking during the early events of infection (Campbell and Hope, 2005; Greber and Way, 2006; Radtke et al., 2006). Consequently, microtubule integrity, functional cellular motors, and the reorganization of the actin network are necessary requirements for the successful cytoplasmic deposition of HIV cores and progression through the early events of infection (Bukrinskaya et al., 1998; McDonald et al., 2002; Naghavi and Goff, 2007).
As early as fusion, HIV depends on a reorganization of the actin cytoskeleton for deposition of the viral core into the cytoplasm. Several studies suggest that cortical actin plays an active role in the clustering of CD4 and CXCR4 receptors for successful fusion of viral and cellular membranes (Iyengar et al., 1998; Jimenez-Baranda et al., 2007; Pontow et al., 2004). This action is mediated by cellular factors such as cofilin and LIMK1, which are upregulated in response to CXCR4 engagement by viral glycoprotein gp120 (Bukrinskaya et al., 1998; Stolp et al., 2009; Yoder et al., 2008). Following fusion, the cortical actin represents first a physical obstacle (Campbell et al., 2004; Wu and Yoder, 2009; Yoder et al., 2008) that the released HIV core must overcome before progressing through to the cytoplasm.
After fusion, the viral core must navigate through the viscous cytoplasm to the nucleus where it can integrate into the host chromosomal DNA and establish infection. As with many viruses and macromolecular cellular cargoes, retrograde trafficking of HIV across the cytoplasm is accomplished on microtubules by the ATP-dependent molecular motor dynein (McDonald et al., 2002). As the viral cores navigate through the cytoplasm, they must successfully reverse transcribe their RNA genome into cDNA giving rise to a reverse transcription complex (RTC). During reverse transcription, the RTC must shed the p24 capsid (CA) shell that surrounds the viral core and generate the viral cDNA for integration (Hulme et al., 2011). Previous studies have utilized fluorescently labeled viral complexes to show that particles progress through uncoating during reverse transcription while trafficking on microtubules (Arhel et al., 2006; McDonald et al., 2002). These studies also demonstrated a relative accumulation of viral complexes proximal to the nucleus at two hours post-infection, while conversely demonstrating the accumulation of complexes at the cell periphery when the dynein motor complex is inhibited (McDonald et al., 2002). Together these studies highlight some of the dependencies that HIV has on the host cytoskeleton as it progresses through the early stages of infection.
While the dependency of HIV on the cell cytoskeleton and cellular motor proteins has been documented in multiple independent studies, the specific interactions between infecting viral cores and cytoskeletal proteins or components of motor complexes have not been characterized. Moreover, modulation of these interactions early in infection has primarily relied on broadly acting pharmaceutical agents such as nocodazole, vinblastine, cytochalasins, latrunculin B, or jasplakinolide, which disrupt or stabilize cytoskeleton networks (Bukrinskaya et al., 1998; Campbell et al., 2004; Jolly et al., 2007; Yoder et al., 2011). The broad effects of these drugs make it difficult to determine the effects that specific cytoskeletal components have on HIV reverse transcription, uncoating, and trafficking.
Recent genome-wide siRNA screens aimed at identifying HIV-1 dependency factors have uncovered hundreds of cellular factors that may be required for HIV infection at various stages of the life cycle (Brass et al., 2008; Bushman et al., 2009; Konig et al., 2008). Of particular interest are those factors that may facilitate the early events of infection, following fusion, but prior to integration, since these may highlight the specific cellular factors that interact with HIV cores to facilitate reverse transcription, uncoating, retrograde trafficking and nuclear translocation.
In an effort to identify factors that facilitate these early events in infection, we characterized a subset of screened cellular proteins with documented or predicted roles in cytoskeleton structure and function. Using siRNAs to these proteins, we were able to identify two proteins, Dynein Axonemal-Light Chain 1 (DNAL1) and Microtubule Associated Protein 4 (MAP4), which are necessary for infection independent of viral entry pathway. MAP4 has been characterized as a microtubule binding protein that is expressed in many types of tissues including muscle (Sato et al., 1997) and brain (Tokuraku et al., 2010). Overexpression of MAP4 results in over-decoration of microtubules. Several studies have documented inhibition of intracellular microtubule-based transport as a result of this over-decoration (Bulinski et al., 1997; Cheng et al., 2005; Nguyen et al., 1997). DNAL1 was previously identified as the human ortholog of the chlamydomonas outer dynein arm axonemal light chain 1 protein (Horvath et al., 2005). Recently, a homozygous mutation of the protein was determined to be one of the causes of primary ciliary dyskinesia (Mazor et al., 2011). In these studies, DNAL1 mRNA expression was documented in lymphoblastoid cells, making its expression relevant to HIV infection. Here we define the requirement of MAP4 and DNAL1 in the early events of HIV infection.
RESULTS
DNAL1 and MAP4 siRNA knockdowns inhibit HIV-1 infection
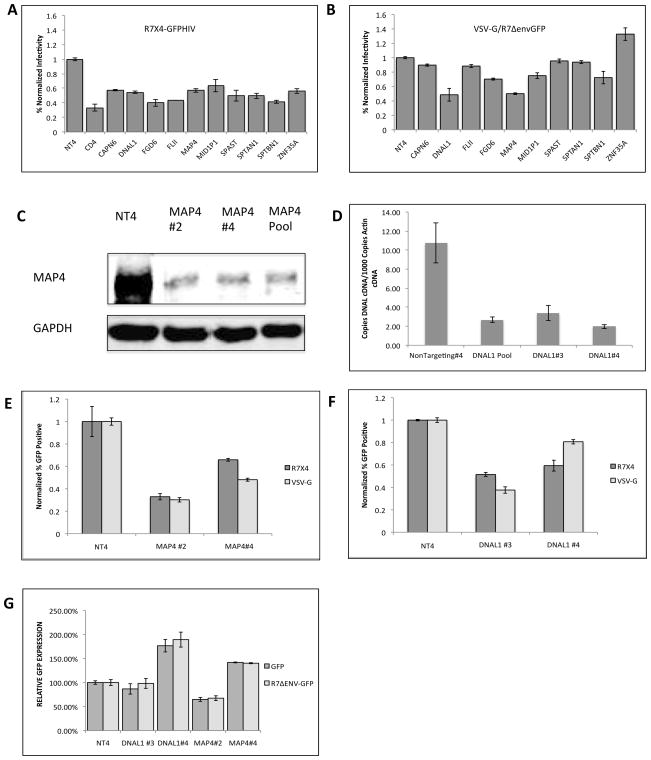
In an effort to identify cytoskeletal proteins that facilitate the early events of infection, we focused on 10 proteins that were initially identified as necessary for expression of viral proteins, following a single cycle of infection (Brass et al., 2008). These proteins include CAPN6, DNAL, FLII, FGD6, MAP4, MID1lP1, SPAST, SPTAN1, SPTBN, ZNF354A. To verify the viral requirement for each proposed HIV-1 dependency factor, HeLa P4.2 cells were transfected with pools of 4 siRNAs to each of the cytoskeleton protein for 48 hours. After transfection, the siRNA-treated cells were infected with either R7X4-GFPHIV (Figure 1A) or VSV-G pseudotyped R7ΔenvGFPHIV (Figure 1B). Infected cells were analyzed by flow cytometry and normalized to control cells transfected with the non-targeting siRNA control #4 (NT4). These results show that the envelope protein on the surface of the virus yielded different abilities to infect the siRNA-treated cells. While all 10 siRNAs consistently decreased R7X4-GFP infection by 40% or greater, only DNAL1 and MAP4 consistently inhibited infection of VSV-G pseudotyped virus to the same extent. Since DNAL1 and MAP4 siRNAs inhibit HIV infection to a similar extent in both X4 and VSV-G pseudotyped viruses, we hypothesized that DNAL1 and MAP4 function at a step after viral entry.
FIGURE 1.
SiRNA transfections targeting cytoskeletal proteins. (A and B) Percent infectivity of GFP reporter R7X4-GFPHIV (A) and VSV-G R7ΔenvGFPHIV (B) in HeLa P4.2 cells 48 hours post-infection. Infectivity is normalized to infection in control cells transfected with non-targeting siRNA #4(NT4). Experiments are representative of 4 independent experiments. Error bars represent standard deviation of triplicate samples within an experiment. (C) MAP4 western blot analysis on cellular extracts from HeLa P4.2 cells transfected with either pooled siRNAs or individual siRNAs to MAP4. (D) Reverse Transcription PCR analysis of DNAL1 expression in HeLa P-4.2 cells transfected with NT4, pooled siRNAs, or individual siRNAs to DNAL1. (E and F) R7X4-GFP and VSV-G GFP infectivity in HeLa P4.2 cells was assessed following 48-hour transfection with MAP4 siRNAs #2 and #4 (E), and DNAL1 siRNAs # 3 and #4 (F). Infectivity was normalized to siRNA transfection controls, transfected with NT4 (G) Protein expression levels in siRNA-transfected cells were assessed 48 hours after siRNA transfection by transfection with GFP or R7Δenv GFP expression plasmids. Cells were analyzed by flow cytometry. Graphs are representative of 3 independent experiments. Error bars represent the standard deviation of triplicate experiment samples within an experiment.
We next assessed the level of DNAL1 and MAP4 knockdown. For MAP4, P4.2 cells were transfected with the siRNA pool, to MAP4 for 48 hours. Cells were then lysed and analyzed by western blot for MAP4 expression levels (Figure 1C ). Relative to control cells, MAP4 siRNA transfected cells exhibited markedly lower expression of MAP4 protein. Previous studies demonstrated that DNAL1 was expressed in testicular tissue and lymphoblastoid cells, however, its expression in HeLa cells has not previously been documented (Campbell et al., 2004; Horvath et al., 2005; Mazor et al., 2011). Since DNAL1 monoclonal antibodies are not commercially available, we instead quantified DNAL1 mRNA in P4.2 cells that were transfected with DNAL1 siRNA (pool) or NT4 for 48 hours. Total mRNA was isolated, reverse transcribed, and subsequently amplified with DNAL1-specific primers via real-time PCR, to obtain relative levels of DNAL1 cDNA. Copies of β-actin were also determined via quantitative PCR, and the number of DNAL1 cDNA/1000copies of β-actin were calculated to yield relative expression levels in treated cells. The results demonstrate that DNAL1 mRNA is expressed in HeLa cells and that DNAL1 siRNA treatment was functional in decreasing mRNA expression.
Since all previous experiments relied on pools of 4 siRNAs, we next chose to use individual siRNAs to both minimize off-target effects, and to ensure that multiple siRNAs reproducibly inhibit HIV-1 infection. As before, we assessed protein expression of MAP4 or mRNA expression of DNAL1 after 48-hour siRNA transfections. In these cells, treatment with the individual MAP4 siRNAs #2 and #4 and DNAL1 siRNAs #3 and #4 resulted in similar levels of expression knockdown (Figure 1C and D), and HIV infectivity impairment (Figure 1 E and F). While all four of the siRNAs in the pool decreased expression to various extents, we chose to pursue experiments with these two, which yielded the strongest effects. To ensure that the siRNA effect was specific to infection and not a general effect on reporter expression, we transfected cells with GFP and R7ΔenvGFPHIV at 48 hours post siRNA transfection and assessed GFP expression via flow cytometry (Figure 1G). These results indicate that the defect in infectivity associated with the siRNAs is reproducible, specific to the siRNA treatment, and is not a defect in cellular transcription.
DNAL1 and MAP4 knockdowns function after HIV-1 fusion to inhibit reverse transcription and 2-LTR formation
We next sought to identify the stage of the viral life cycle in which DNAL1 and MAP4 exert their effects on infection. To accomplish this, we assayed the ability of viral complexes to progress through fusion, reverse transcription and nuclear translocation. Since DNAL1 and MAP4 may affect trafficking or cytoskeletal function, defects in progression towards the nucleus may be exposed through these assays.
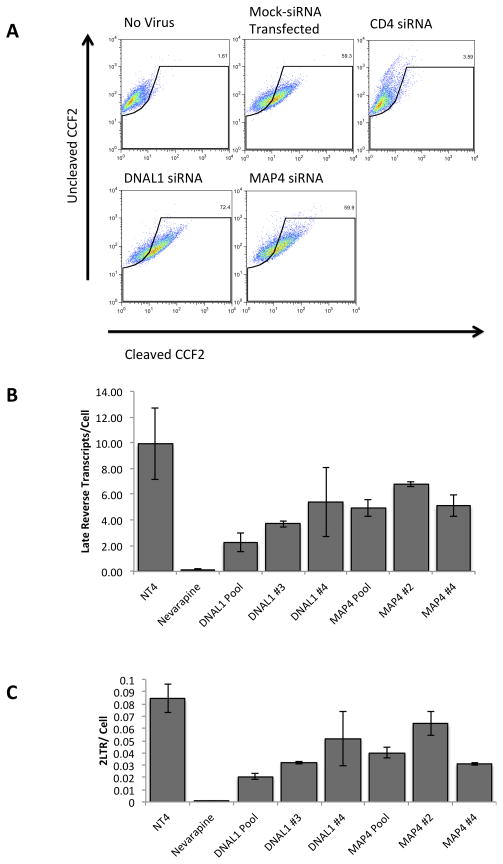
To further exclude the possibility that DNAL1 and MAP4 function at the entry step of the HIV life cycle we assayed knocked-down cells for defects in fusion. For this experiment we employed a previously described Beta-Lactamase (BLam) Fusion Assay (Cavrois et al., 2004). In this assay, cells are infected with a virus containing the beta-lactamase protein, then loaded with a fluorescent substrate to the enzyme. Conversion of the substrate from green to blue is only accomplished after fusion of the virions, and cytoplasmic release of the enzyme. This color conversion is subsequently analyzed by flow cytometry. For these experiments, HeLa P4.2 cells were transfected with siRNAs to CD4, DNAL1 or MAP4. At 48 hours after transfection, cells were infected with R7X4-GFPHIV/Vpr- BLam virus by spinoculation at 16°C, to synchronize infection, for 2 hours and then incubated at 37°C for 3 hours. Cells were then loaded with CCF2-AM for 14 hours and analyzed by flow cytometry. Relative to cells that were mock transfected, DNAL1 and MAP4 did not impact HIV-1 fusion. Contrarily, CD4-siRNA transfected cells showed a >90% decrease in fusion, when compared to the negative control (Figure 2A). These results agree with the infectivity studies, which suggest that DNAL1 and MAP4 act at a stage following viral entry.
Figure 2.
Fusion, Reverse Transcription and 2-LTR formation in MAP4 and DNAL1 silenced cells. (A) A representative fusion assay (n=3) in DNAL1 and MAP4 siRNA knocked down cells. HeLa P4.2 cells were transfected with listed siRNAs for 48 hours then infected with R7X4-GFPHIV/Vpr-BLam, loaded with CCF2, and assessed for CCF2 cleavage to determine relative levels of fusion. (B and C) Late reverse transcripts (B) and 2-LTR circles (C) were assessed at 24 hours after infection, following a 48-hour siRNA transfection, by quantitative-PCR. Copies of late reverse transcripts and 2-LTR circles were normalized to copies of β-actin to yield relative copies per cell. Error bars indicate the standard deviation within triplicate samples. Results are representative of 5 independent experiments.
The impact of siRNA transfection on late reverse transcript formation and 2-LTR circle formation was also assessed in HeLa P4.2 cells. The siRNA-transfected cells were infected by synchronized infection with R7ΔenvGFPHIV, via a 2-hour spinoculation at 16°C. Unbound virus was washed and infection was allowed to proceed for 24 hours. Cells were harvested, and total genomic DNA was isolated. The number of late reverse transcripts and 2-LTR circles was then determined by quantitative PCR analysis and normalized to copies of β-actin. Reverse transcription was inhibited by approximately 50% in DNAL1 and MAP4 siRNA-treated cells, with DNAL1 siRNA #3 and MAP4 siRNA #4 having the greatest effect (Figure 2B). The formation of 2-LTR products was also inhibited in DNAL1 and MAP4 siRNA-transfected cells by approximately 50% (Figure 2C).
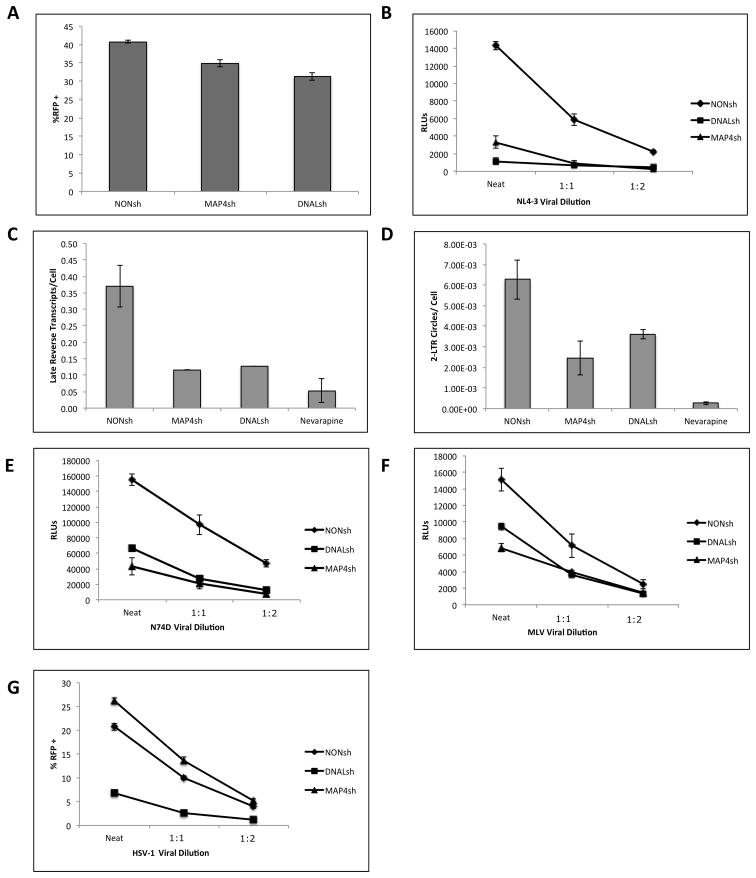
We next generated monoclonal cell lines expressing shRNAs to either DNAL1 shRNA (DNALsh) or MAP4 shRNA (MAP4sh), along with a TurboGFP reporter. As a result, transduced cells are all GFP-positive. First, knockdown of both proteins was assessed by western blot or RT-PCR, as in the siRNA-treated cells, and the results were similar to those in Figure 1 (Data not shown). Next, the shRNA cell lines were assessed for general defects in protein expression by transfection with an RFP expression vector. Both MAP4sh and DNAL1sh shRNA lines were within 15–20% of the levels of RFP expression when compared to the Non-Targeting shRNA control cell line (NONsh) (Figure 3A).
Figure 3.
HIV, MLV, and HSV-1 infectivity in DNAL1 and MAP4 shRNA-expressing cell lines. (A) Control plasmid expression levels were assessed by 24-hour transfection of an RFP expression plasmid, and analyzed by flow cytometry. (B) VSV-G pseudotyped NL4-3-Luciferase infectivity in monoclonal cell lines was assessed by 48 hour infection (n=7). For all luciferase infectivity assays, a representative experiment is shown over three dilutions within the linear range. Error bars represent standard error of the mean(SEM) amongst triplicate experiment samples. Luciferase activity was normalized by cell count. (C and D) Late reverse transcripts(C) and 2-LTR circles/cell (D) were assessed following a 24-hour infection with NL4-3-Luc. Error bars represent SD within triplicate samples. (E) VSV-G pseudotyped N74D HIV-Luciferase infectivity in monoclonal cell lines (n=4). (F) VSV-G pseudotyped MLV Luciferase infectivity in monoclonal cell lines (n=7). (G) Susceptibility to HSV-1 infection was assessed by a 48-hour infection with RFP-HSV-1 over three dilutions in the linear range (n=3). Cells were analyzed by flow cytometry analysis of the RFP+ population of cells.
We next verified that the knockdown cell lines were capable of inhibiting infection as well as late reverse transcript formation and 2-LTR circle formation, as documented in siRNA transfections. Both MAP4sh and DNAL1sh cell lines inhibited HIV infection, as measured by luciferase activity of cells infected with NL4-3-Luciferase (Figure 3B). Over 7 independent experiments, MAP4sh decreased HIV-1 infection by an averaged 78% (+/− SEM 2.7%, p<.001) while DNALsh decreased infection by 85% (+/− SEM 2.4%, p<.001). Similarly, late reverse transcripts/cell and 2-LTR circles/cell were decreased by about 50% in both DNAL1sh and MAP4sh cell lines (Figure 3C and 3D).
In previously published studies, the N74D CA mutant has been demonstrated to overcome HIV’s dependence on the cellular protein TNPO3 for nuclear translocation, instead entering the nucleus via a different cellular pathway(Lee et al., 2010). To test if this mutant could also over come the requirement for MAP4 and DNAL1, we infected the cell lines with N74D-Luciferase virus. Infection levels were also decreased by approximately 66% (+/− SEM 4.6%, p<.01) and 62%(+/− SEM 4.8%, p<.01) for MAP4sh and DNAL1sh respectively (n=4) (Figure 3E). Together with the quantitative PCR data this data argues that DNAL1 and MAP4 likely do not mediate nuclear translocation, and do not exert variable effects on capsid mutant viruses.
An inhibition of 2-LTR circle formation, with little or no impact to reverse transcription is believed to identify factors required for nuclear translocation, since reverse transcription takes place in the cytoplasm, while 2-LTR circle formation takes place in the nucleus. (Christ et al., 2008). However, since both DNAL1 and MAP4 siRNAs inhibit 2-LTR circle formation to levels similar to the late reverse transcript formation, DNAL1 and MAP4 likely do not function in nuclear translocation. Instead, the observed decreases in 2-LTR circles and late reverse transcripts indicate a potential requirement for DNAL1 and MAP4 during reverse transcription.
Infection of shRNA-expressing cell lines with HIV, MLV, and HSV-1 demonstrates retroviral specificity for MAP4
We next sought to identify if the effects of MAP4 and DNAL1 knockdown were specific to HIV, or similarly exerted on distinct viruses. For these experiments we tested the ability of two distinct viruses with variable life cycles, MLV and HSV-1, to infect DNAL1 and MAP4 shRNA-expressing cell lines. As a retrovirus that is incapable of infecting dividing cells, MLV provides further opportunity to determine if MAP4 and DNAL1 function at nuclear translocation, while also revealing the level of specificity these proteins have to lentiviral versus retroviral life cycles. As a structurally distinct virus, which also relies on microtubule trafficking, HSV-1 provides the opportunity to further assess the viral specificity of these proteins.
While our results indicate that DNAL1 and MAP4 are required for HIV infection, we chose to further explore the impact of these proteins on other viruses, with the aim of revealing any potential viral specificity. We first infected DNAL1sh and MAP4sh lines with MLV-Luciferase (Figure 3F). The results demonstrated an MLV dependency on both DNAL1 and MAP4, although the effect was weaker than observed with HIV, with a 41% (+/− SEM 5.7%, p<.01) and 40% (+/− SEM 6.1%, p<.01) decrease in infectivity for MAP4sh and DNAL1sh respectively (n=7). Since DNAL1 and MAP4 knockdowns similarly impact lentiviruses and retroviruses in general, we next tested their requirement in the cellular infection of HSV-1, an unrelated virus. HSV-1 is an enveloped linear double-stranded DNA virus, with an icosahedral capsid. For these experiments we infected shRNA cell lines with an mRFP-reporter HSV-1 (n=3) (Figure 3G). Unlike wildtype CA HIV, N74D HIV and MLV, the MAP4sh line did not alter HSV-1 infection. Over three independent experiments MAP4sh showed an averaged 114% (+/− SEM 6.0%) infection rate relative to the NONsh control cell line. However, the DNAL1sh line did inhibit HSV-1 infection by approximately 58% (+/− SEM 6.0%) relative to controls. These results indicate that DNAL1 is required for optimal infection with HIV, as well as retroviruses such as MLV, and HSV-1. Together, these results suggest that the role of DNAL1 in the viral life cycle of HIV may be the result of a general effect on microtubule-dependent trafficking. On the other hand, MAP4 appears to be necessary for HIV and MLV, but not HSV-1, indicating that it exhibits some functional specificity in retroviral life cycles.
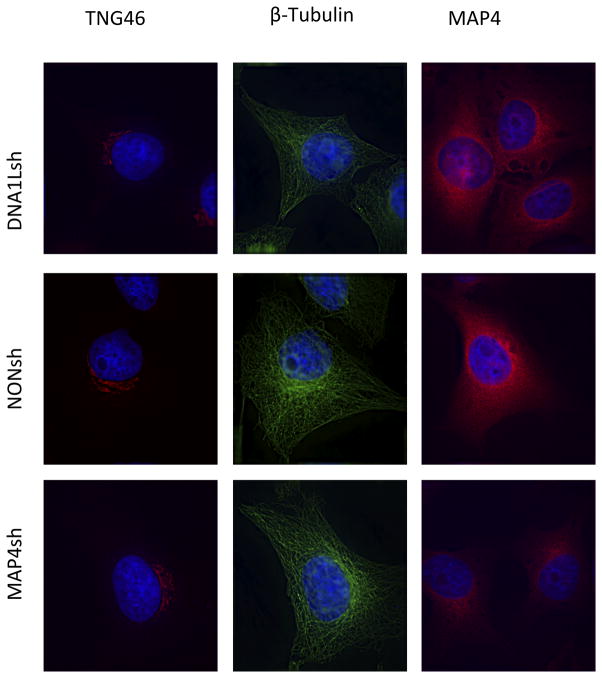
DNAL1 and MAP4 knockdown does not disrupt the cytoskeleton
Since DNAL1 and MAP4 function in the early events of infection, after fusion but prior to integration, it is possible that they mediate their effects on the viral life cycle by interaction with the cytoskeleton. In the absence of DNAL1 monoclonal antibodies, we assessed cytoskeletal and trafficking function by immunofluorescent staining of DNALsh and MAP4sh knockdown cells. DNAL1 and MAP4 shRNA cell lines were stained for β-Tubulin, MAP4 and the trans-golgi network protein 46 (TGN46) (Figure 4). When compared to NONsh cells, DNALsh and MAP4sh did not show microtubule morphological defects (Figure 4). When microtubule-dependent trafficking is perturbed, the perinuclear localization of TGN-46 is disrupted, yielding a dispersed cytoplasmic localization (Cobbold et al., 2004). Neither MAP4sh nor DNAL1sh showed this disrupted localization. DNALsh and MAP4sh cells were also stained for MAP4. DNAL1 knockdown did not affect the localization of this microtubule associated protein, however MAP4sh shows a clear decrease in MAP4 expression relative to NONsh. These results indicate that neither DNAL1 nor MAP4 knockdown results in disruption of the cytoskeleton, or impairment of cell-wide trafficking.
Figure 4.
Microtubule integrity and intracellular trafficking in DNAL1 and MAP4 shRNA-expressing cell lines. Defects in cytoskeletal morphology were analyzed by immunofluorescence staining for β-Tubulin and MAP4. Defects in trafficking were assessed by staining for the trans-golgi network protein 46. Fluorescent intensities were normalized to control cells.
DISCUSSION
The early events of HIV-1 infection, consisting of viral fusion, capsid uncoating, reverse transcription, retrograde trafficking of viral complexes, and nuclear translocation, are a continuing focus of HIV-1 characterization studies. Recent advances in the field have highlighted the role of cellular factors at every step of the early events of infection. However the role that the cytoskeleton plays in uncoating and in the formation of reverse transcription complexes is still not clear. Although previous studies document a defect in infection when the cytoskeleton is entirely disrupted, the specific cytoskeletal proteins that facilitate the early infection events, possibly via direct interaction with viral proteins, remain undefined.
New approaches to studying the early events of infection via siRNA-mediated knockdown of cellular proteins have identified and confirmed the requirement of a multitude of cellular factors that are necessary for fusion and nuclear translocation (Brass et al., 2008; Konig et al., 2008). Moreover, similar studies highlight the ability of certain viral mutants to overcome the observed requirement for cellular proteins in siRNA-treated cells (Lee et al., 2010). DNAL1 was originally documented as the chlamydomonas outer arm dynein axonemal light chain 1(Horvath et al., 2005). However, its expression in HeLa cells had not previously been documented. MAP4 is a widely characterized protein with distinct functions in neuronal and cardiac tissues.
Here we show that DNAL1 and MAP4 are expressed in HeLa cells, and that their expression can be silenced by transfection of either siRNAs or shRNAs. SiRNA-mediated knockdown of MAP4 and DNAL1 inhibits HIV-1 infection by more than 50% relative to controls. Based on the results from our fusion assays, quantitative PCR experiments, and N74D infectivity, this inhibition maps to a step after fusion, but prior to nuclear translocation. Specifically, the ability of HIV to reverse-transcribe its RNA genome is inhibited by more than 50% after knockdown, as is 2-LTR formation. This indicates that dependency on DNAL1 and MAP4 occurs during, or prior to, reverse transcription.
Recent studies have suggested the use of different HIV-1 infection pathways when required cellular factors are silenced with siRNAs. One such example shows that TNPO3 is required for nuclear translocation, but its absence in siRNA-transfected cells can be overcome by a single amino acid mutation at position 74 of capsid (N74D). Similarly to previous experiments on TNPO3 dependency, we infected DNAL1 and MAP4 shRNA lines with N74D, and WT CA HIV, but both were inhibited by a lack of DNAL1 and MAP4, indicating that DNAL1 and MAP4 do not exert variable effects on CA mutant viruses. To further confirm this, we also tested other previously characterized HIV CA mutants (E45A, N74D, Q63A, T54A/N57A, G89V, P90A, G94D, A92E) in siRNA-treated cells, but no mutants were able to overcome the dependency on DNAL1 and MAP4 for infection, since infection was inhibited by more than 50% in all cases (Data not shown).
As a lentivirus, HIV is capable of infecting non-dividing cells. By testing the dependence of MLV, a retrovirus that cannot infect non-dividing cells, on MAP4 and DNAL1 we show that both proteins are generally required for retroviral infection. A small difference in viral susceptibility was observed between HIV and MLV infection assays. This difference may be the result of structural differences in the capsid core or RTC, differences in the process of uncoating, or variations in their relative requirements for microtubule association. The disparate ability of DNAL1 knockdown to inhibit HSV-1 infection, where MAP4 knockdown fails to inhibit it, demonstrates that there is specificity in the viral requirement for MAP4. As a structurally different virus, which does not undergo reverse transcription, HSV-1 shows no dependence on MAP4, instead demonstrating a slight increase in infection in the absence of MAP4. HSV-1 however does demonstrate a dependence on DNAL1. RFP transfection experiments and fluorescence imaging exclude the possibility that these results are the outcome of general effects on proteins expression and cell toxicity respectively.
Altogether, these results help demonstrate an siRNA-specific and reproducible, post-fusion, and pre-nuclear translocation impairment of HIV-1 infection in tested cells. While DNAL1 had previously been described as having a function in ciliary cells, and more recently as a facilitator of dynein heavy chain and tubulin binding (Mazor et al., 2011), its requirement in the intracellular events of HIV have not previously been described. Similarly, MAP4 is often associated with pathologies when it is upregulated or overexpressed, however, there have not been studies on the cellular effects of its down-regulation.
Previously published results indicate that successful formation of an RTC and generation of late reverse transcription products were confined to cytoskeletal and nuclear fractions, indicating a role of cytoskeletal association in RTC formation (Bukrinskaya et al., 1998). Those studies relied primarily on cytochalasins, inhibitors of actin polymerization, and thus argued that actin microfilaments were required for RTC formation and RT. Our results show that generation of late reverse transcription products may involve cytoskeletal components other than actin. DNAL1 and MAP4 are both documented microtubule associated proteins. The ability of MAP4 knockdown to inhibit infection of HIV and MLV further argues that MAP4 may have a specific interaction with RTC components of retroviruses, while different levels of dependency may be the result of differences in cellular interactions that result in variations in the early events of infection. DNAL1 on the other hand, also impacted reverse transcription of HIV-1, but was not specific to retroviruses, since HSV-1 was also dependent on the protein. DNAL1 has been documented to link components of the cytoskeleton with the molecular motor dynein, perhaps acting as a scaffold for larger functional structures. Both DNAL1 and MAP4 may be impacting reverse transcription by affecting the formation of RTCs on microtubules or by impacting RTC association with microtubules. However, further studies are needed to document direct interactions between DNAL1 and MAP4 with HIV-1. Recently published studies document an interdependence between reverse transcription and uncoating (Hulme et al., 2011). Future studies on HIV’s requirement for DNAL1 and MAP4 in uncoating may provide further insight into their roles in reverse transcription.
Materials and Methods
Cell Culture and siRNA transfections
293T cells, HeLa cells, HeLa P-4.2 cells and shRNA stably transduced cell lines were cultured at 37°C and 5% CO2 in DMEM (HyClone) supplemented with 10% FBS, 100units/ml penicillin, 100μg streptomycin, 292μg/ml L-glutamine. Additionally shRNA stably transduced cell lines were kept under 5 μg/ml puromycin (Sigma) selection.
Virus production
Virus was produced in 10-cm dishes of 293T cells as previously described (Anderson et. al. 2006). Briefly, for HIV viruses, cells were transfected with 12ug of viral cDNA expression plasmids: R7ΔenvGFPHIV, R7X4-GFPHIV (Hulme et al., 2011), NL4-3Δenv Luciferase (Lee et. al., 2010), N74D-Luciferase, and 4μg of VSV-G expression plasmid (for proviral plasmids lacking envelope) and using 35μL of 10ug/ml polyethylenimine (PEI) (molecular weight, 25,000; Polysciences). The NL4-3 Luciferase plasmid was a gift from Dr. Vineet N. KewalRamani (NCI Frederick National Cancer Institute). For BLam assays, virus was produced by transfection with 12μg R7X4-GFPHIV and 2ug Vpr-BLam (Cavrois et al., 2004), For MLV-Luciferase reporter virus 293T cells were transfected with 7ug FB-Luc (Stratagene), 7ug of pCG Gag-Pol (Shun et. al, 2007), and 4 ug VSV-G. The pCG Gag-Pol plasmid was a gift from A. Engleman (Dana-Farber Cancer Institute, Harvard University, Boston, MA). Virus was harvested as previously described (Anderson et al., 2006, Hulme et al. 2011). HSV-1 was produced by infecting a confluent 10 cm plate of Vero cells with Pseudorabies Virus-psuedotyped HSV-1 that contains an mRFP1-VP26 fusion. Produced virus was harvested at approximately 2 days after infection, following cell rounding. HSV-1 mRFP-VP26 was a generous gift from Greg Smith (Feinberg School of Medicine, Northwestern University, Chicago, IL)
siRNA transfections and infections
All siRNA transfections were carried out in HeLa P4.2 cells, which express the CD4 and CXCR4 cellular receptors (NIH AIDS Research and Reference Reagent Program). HeLa P4.2 cells were seeded at a density of 50,000 cells/well in a 24-well dish. Cells were transfected with each of the indicated siRNAs at a final concentration of 10μM. After 24 hours of transfection cells were then re-seeded in 96-well dishes at a density of 10,000/well for infectivity assays. At 48 hours after siRNA transfection cells were then infected with either R7X4-GFPHIV reporter virus or VSV-G- pseudotyped R7Δenv-GFP HIV reporter virus. To assess virus infectivity, HeLa P4.2 were cells were infected by serial dilutions of viral stocks. For all subsequent experiments, viral infections were analyzed using amounts of virus resulting in the linear infection range. GFP expression was determined at 48 hours after infection by using an Accuri C6 96-well flow cytometer.
Creation of shRNA cell lines
HeLa cells were transduced with lentiviral vectors expressing shRNAs to either MAP4 or DNAL1. The shRNA constructs targeting MAP4 (V2LHS_151564) and DNAL1 (V2LHS_118241) sequences were obtained from Open Biosystems. Lentiviral vector was produced in 293T cells by co-transfecting 7μg each of the shRNA plasmids, VSV-G plasmid and nrf (Xu et al., 2001), using 35 μl of 10ug/ml of PEI into a 10 cm plate. Media was changed at 24 hours post transfection and media containing lentiviral vector was harvested at 48 hours post transfection. Forty-eight hours after transducing, cells were aliquoted into 96 well plates by limiting dilution to ensure the generation of monoclonal populations. Cells were allowed to expand and then characterized for a decrease in mRNA expression in the case of DNAL1, or a decrease in protein expression in the case of MAP4.
DNAL1 cDNA quantification
HeLa cells were transfected with siRNAs for 48 hours. Cells were then harvested and total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen). One μg of total RNA was reverse transcribed using the Quanta qScript cDNA SuperMix. 3μg of the total reaction was then analyzed by quantitative real-time PCR using iCycler iQ real-time PCR detection system (BioRad) with the following primers specific to DNAL1: 5′ CCTGGTAAAAGACTG GGCTG 3′ and 5′GGCACTCTCTTGGTTGCTTC 3′, and previously published β-actin primers (Anderson et al., 2006). The relative number of β-actin cDNA copies and DNAL1 cDNA copies was determined with standard curves.
HIV fusion assay
The Fusion assay was carried out in HeLa P4.2 cells expressing CXCR4 and CD4, as previously described (Cavrois et al., 2004). Briefly, HeLa P4.2 cells were transfected with siRNAs to CD4, DNAL1 or MAP4. At 48 hours after transfection, cells were infected with R7X4-GFPHIV/Vpr-BLam virus by spinoculation at 16°C for 2 hours and then incubated at 37°C for 3 hours. Cells were then loaded with CCF2-AM for 14 hours. Cells were then trypsinized, fixed, and analyzed by flow cytometry.
DNAL1 and MAP4 knockdown analysis of late reverse transcripts and 2-LTR circles
HeLa P4.2 cells were seeded at a density of 40,000 cells/well in 24-well dishes. Cells were transfected with Dharmacon siRNA pools to listed proteins or non-targeting, using RNAiMAX lipofectamine (Invitrogen) to a final siRNA concentration of 10μM. After 24 hours, cells were re-seeded into 24-well plates at a density of 50,000 cells/well. VSV-G-pseudotyped R7ΔEnvGFPHIV was pretreated with 20 units/ml DNaseI (Roche) in 10 mM MgCl2 for 60 min at room temperature before addition to cell monolayers with drug if appropriate. At 48 hours of siRNA transfection, cells were infected with VSV-G pseudotyped R7Δenv-GFPHIV reporter virus. At 24 hours post infection, cells were trypsinized, and total cellular DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen). One μg of total cellular DNA was then digested with DpnI for 4 hours at 37°C. Late reverse transcripts and 2-LTR circles were assessed using iCycler iQ real-time PCR detection system (BioRad) with previously published late reverse transcription product primers (Dismuke and Aiken 2006, Butler et al., 2001). The number of cells in each sample was determined by quantitative PCR amplification of β-actin DNA using previously published primers (Anderson et al., 2006). The amount of each cDNA product or genomic DNA was quantified using a standard curve generated from 10-fold dilutions of proviral plasmid.
Protein analysis and western blotting
For Western blot analysis, HeLa cells were transfected with siRNAs to MAP4 for 48 hours. Cells were trypsinized, washed with PBS, and resuspended in TEN buffer, then lysed with 2X TNEN buffer and loaded in laemmli buffer. Proteins were resolved by SDS-PAGE, transferred to a nitrocellulose membrane (Whatman) and probed with anti-MAP4 monoclonal antibody (Sigma), or anti-GAPDH monoclonal antibody (Chemicon), followed by goat-anti-rabbit 680nm (Odyssey) or goat anti-mouse 800nm (Odyssey) secondary antibodies. Western blots were imaged on an infrared imager.
Research Highlight.
DNAL1 and MAP4 play a role in post-fusion events of HIV-1 infection
DNAL1 and MAP4 knockdown impairs HIV- 1 reverse transcription.
DNAL1 and MAP4 do not mediate nuclear translocation.
DNAL1 and MAP4 knockdown does not disrupt cytoskeletal organization.
Acknowledgments
We would like to thank the Northwestern University Flow Cytometry Core Facility for providing assistance with flow cytometry experiments. Fb-Luc, and pCG-Gag-Pol plasmids were kind gifts from Dr. Alan Engleman at the Dana Farber Cancer Institute. The N74D NL4-3-Luciferase plasmid was kindly provided by Dr. Vineet N. KewalRamani at NCI Frederick National Cancer Institute. We would also like to thank Dr. Greg Smith at Northwestern University Feinberg School of Medicine for providing mRFP-VP26 labeled HSV-1. This study was supported by the NIH R01 AI047770 (with associated diversity supplement), P50 GM082545, and the James B Pendleton Charitable Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daniel E. Gallo, Email: d-gallo@northwestern.edu, Feinberg School of Medicine, Northwestern University, Lurie 9-290, 303 E. Superior Avenue, Chicago, IL 60611, Phone: (312) 503-1142
Thomas J. Hope, Email: thope@northwestern.edu, Department of Cell and Molecular Biology, Feinberg School of Medicine, Northwestern University, Lurie 9-290, 303 E. Superior Avenue, Chicago, IL 60611, Phone: (312) 503-1360, Fax: (312) 503-2696.
References
- Anderson JL, Campbell EM, Wu X, Vandegraaff N, Engelman A, Hope TJ. Proteasome inhibition reveals that a functional preintegration complex intermediate can be generated during restriction by diverse TRIM5 proteins. J Virol. 2006;80:9754–9760. doi: 10.1128/JVI.01052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arhel N, Genovesio A, Kim KA, Miko S, Perret E, Olivo-Marin JC, Shorte S, Charneau P. Quantitative four-dimensional tracking of cytoplasmic and nuclear HIV-1 complexes. Nat Methods. 2006;3:817–824. doi: 10.1038/nmeth928. [DOI] [PubMed] [Google Scholar]
- Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, Lieberman J, Elledge SJ. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- Bukrinskaya A, Brichacek B, Mann A, Stevenson M. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J Exp Med. 1998;188:2113–2125. doi: 10.1084/jem.188.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski JC, McGraw TE, Gruber D, Nguyen HL, Sheetz MP. Overexpression of MAP4 inhibits organelle motility and trafficking in vivo. J Cell Sci. 1997;110 ( Pt 24):3055–3064. doi: 10.1242/jcs.110.24.3055. [DOI] [PubMed] [Google Scholar]
- Bushman FD, Malani N, Fernandes J, D’so I, Cagney G, Diamond TL, Zhou H, Hazuda DJ, Espeseth AS, Konig R, Bandyopadhyay S, Ideker T, Goff SP, Krogan NJ, Frankel AD, Young JA, Chanda SK. Host cell factors in HIV replication: meta-analysis of genome-wide studies. PLoS Pathog. 2009;5:e1000437. doi: 10.1371/journal.ppat.1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SL, Hansen MS, Bushman FD. A quantitative assay for HIV DNA integration in vivo. Nat Med. 2001;7:631–634. doi: 10.1038/87979. [DOI] [PubMed] [Google Scholar]
- Greber UF, Way M. A superhighway to virus infection. Cell. 2006;124:741–754. doi: 10.1016/j.cell.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Campbell EM, Hope TJ. Gene therapy progress and prospects: viral trafficking during infection. Gene Ther. 2005;12:1353–1359. doi: 10.1038/sj.gt.3302585. [DOI] [PubMed] [Google Scholar]
- Campbell EM, Nunez R, Hope TJ. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J Virol. 2004;78:5745–5755. doi: 10.1128/JVI.78.11.5745-5755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrois M, Neidleman J, Yonemoto W, Fenard D, Greene WC. HIV-1 virion fusion assay: uncoating not required and no effect of Nef on fusion. Virology. 2004;328:36–44. doi: 10.1016/j.virol.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Cheng G, Qiao F, Gallien TN, Kuppuswamy D, Cooper Gt. Inhibition of beta-adrenergic receptor trafficking in adult cardiocytes by MAP4 decoration of microtubules. Am J Physiol Heart Circ Physiol. 2005;288:H1193–1202. doi: 10.1152/ajpheart.00109.2004. [DOI] [PubMed] [Google Scholar]
- Christ F, Thys W, De Rijck J, Gijsbers R, Albanese A, Arosio D, Emiliani S, Rain JC, Benarous R, Cereseto A, Debyser Z. Transportin-SR2 imports HIV into the nucleus. Curr Biol. 2008;18:1192–1202. doi: 10.1016/j.cub.2008.07.079. [DOI] [PubMed] [Google Scholar]
- Cobbold C, Coventry J, Ponnambalam S, Monaco AP. Actin and microtubule regulation of trans-Golgi network architecture, and copper-dependent protein transport to the cell surface. Mol Membr Biol. 2004;21:59–66. doi: 10.1080/096870310001607350. [DOI] [PubMed] [Google Scholar]
- Dismuke DJ, Aiken C. Evidence for a functional link between uncoating of the human immunodeficiency virus type 1 core and nuclear import of the viral preintegration complex. J Virol. 2006;80:3712–3720. doi: 10.1128/JVI.80.8.3712-3720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath J, Fliegauf M, Olbrich H, Kispert A, King SM, Mitchison H, Zariwala MA, Knowles MR, Sudbrak R, Fekete G, Neesen J, Reinhardt R, Omran H. Identification and analysis of axonemal dynein light chain 1 in primary ciliary dyskinesia patients. Am J Respir Cell Mol Biol. 2005;33:41–47. doi: 10.1165/rcmb.2004-0335OC. [DOI] [PubMed] [Google Scholar]
- Hulme AE, Perez O, Hope TJ. Complementary assays reveal a relationship between HIV–1 uncoating and reverse transcription. Proc Natl Acad Sci U S A. 2011;108:9975–9980. doi: 10.1073/pnas.1014522108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Hildreth JE, Schwartz DH. Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J Virol. 1998;72:5251–5255. doi: 10.1128/jvi.72.6.5251-5255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Baranda S, Gomez-Mouton C, Rojas A, Martinez-Prats L, Mira E, Ana Lacalle R, Valencia A, Dimitrov DS, Viola A, Delgado R, Martinez AC, Manes S. Filamin-A regulates actin-dependent clustering of HIV receptors. Nat Cell Biol. 2007;9:838–846. doi: 10.1038/ncb1610. [DOI] [PubMed] [Google Scholar]
- Jolly C, Mitar I, Sattentau QJ. Requirement for an intact T-cell actin and tubulin cytoskeleton for efficient assembly and spread of human immunodeficiency virus type 1. J Virol. 2007;81:5547–5560. doi: 10.1128/JVI.01469-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig R, Zhou Y, Elleder D, Diamond TL, Bonamy GM, Irelan JT, Chiang CY, Tu BP, De Jesus PD, Lilley CE, Seidel S, Opaluch AM, Caldwell JS, Weitzman MD, Kuhen KL, Bandyopadhyay S, Ideker T, Orth AP, Miraglia LJ, Bushman FD, Young JA, Chanda SK. Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell. 2008;135:49–60. doi: 10.1016/j.cell.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Ambrose Z, Martin TD, Oztop I, Mulky A, Julias JG, Vandegraaff N, Baumann JG, Wang R, Yuen W, Takemura T, Shelton K, Taniuchi I, Li Y, Sodroski J, Littman DR, Coffin JM, Hughes SH, Unutmaz D, Engelman A, KewalRamani VN. Flexible use of nuclear import pathways by HIV-1. Cell Host Microbe. 2010;7:221–233. doi: 10.1016/j.chom.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazor M, Alkrinawi S, Chalifa-Caspi V, Manor E, Sheffield VC, Aviram M, Parvari R. Primary Ciliary Dyskinesia Caused by Homozygous Mutation in DNAL1, Encoding Dynein Light Chain 1. Am J Hum Genet. 2011;88:599–607. doi: 10.1016/j.ajhg.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Vodicka MA, Lucero G, Svitkina TM, Borisy GG, Emerman M, Hope TJ. Visualization of the intracellular behavior of HIV in living cells. J Cell Biol. 2002;159:441–452. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghavi MH, Goff SP. Retroviral proteins that interact with the host cell cytoskeleton. Curr Opin Immunol. 2007;19:402–407. doi: 10.1016/j.coi.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HL, Chari S, Gruber D, Lue CM, Chapin SJ, Bulinski JC. Overexpression of full- or partial-length MAP4 stabilizes microtubules and alters cell growth. J Cell Sci. 1997;110 ( Pt 2):281–294. doi: 10.1242/jcs.110.2.281. [DOI] [PubMed] [Google Scholar]
- Pontow SE, Heyden NV, Wei S, Ratner L. Actin cytoskeletal reorganizations and coreceptor-mediated activation of rac during human immunodeficiency virus-induced cell fusion. J Virol. 2004;78:7138–7147. doi: 10.1128/JVI.78.13.7138-7147.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke K, Dohner K, Sodeik B. Viral interactions with the cytoskeleton: a hitchhiker’ guide to the cell. Cell Microbiol. 2006;8:387–400. doi: 10.1111/j.1462-5822.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- Sato H, Nagai T, Kuppuswamy D, Narishige T, Koide M, Menick DR, Cooper Gt. Microtubule stabilization in pressure overload cardiac hypertrophy. J Cell Biol. 1997;39:963–973. doi: 10.1083/jcb.139.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shun MC, Daigle JE, Vandegraaff N, Engelman A. Wild–type levels of human immunodeficiency virus type 1 infectivity in the absence of cellular emerin protein. J Virol. 2007;81:166–172. doi: 10.1128/JVI.01953-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp B, Reichman–Fried M, Abraham L, Pan X, Giese SI, Hannemann S, Goulimari P, Raz E, Grosse R, Fackler OT. HIV-1 Nef interferes with host cell motility by deregulation of Cofilin. Cell Host Microbe. 2009;6:174–186. doi: 10.1016/j.chom.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Tokuraku K, Okuyama S, Matsushima K, Ikezu T, Kotani S. Distinct neuronal localization of microtubule-associated protein 4 in the mammalian brain. Neurosci Lett. 2010;484:143–147. doi: 10.1016/j.neulet.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Wu Y, Yoder A. Chemokine coreceptor signaling in HIV-1 infection and pathogenesis. PLoS Pathog. 2009;5:e1000520. doi: 10.1371/journal.ppat.1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder A, Guo J, Yu D, Cui Z, Zhang XE, Wu Y. Effects of microtubule modulators on HIV-1 infection of transformed and resting CD4 T cells. J Virol. 2011;85:3020–3024. doi: 10.1128/JVI.02462-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shun MC, Daigle JE, Vandegraaff N, Engelman A. Wild-type levels of human immunodeficiency virus type 1 infectivity in the absence of cellular emerin protein. J Virol. 2007;81:166–172. doi: 10.1128/JVI.01953-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder A, Yu D, Dong L, Iyer SR, Xu X, Kelly J, Liu J, Wang W, Vorster PJ, Agulto L, Stephany DA, Cooper JN, Marsh JW, Wu Y. HIV envelope- CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell. 2008;134:782–792. doi: 10.1016/j.cell.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]