Abstract
Conditionally replicating adenoviruses (CRAds) utilize tissue specific promoters to control the expression of the early genes, E1A and E1B, to preferentially replicate and lyse tumor cells (oncolysis). Previous CRAds used in prostate cancer gene therapy require androgens to activate prostate specific promoters and induce viral replication. Unfortunately, these CRAds have reduced activity in patients on androgen suppressive therapy. We describe a novel prostate specific CRAd generated by fusing the E1A gene to the androgen receptor (AR) cDNA with a point mutation in codon 685 (C685Y). The E1A-AR fusion neutralizes the previously described mutual inhibition of E1A & AR, and the C685Y point mutation alters specificity of steroid ligand binding to the AR, such that both androgens and non-steroidal anti-androgens can activate viral replication. We demonstrate that the mutated E1A-AR retained the ability to function in regulating AR responsive genes and E1A responsive viral genes. In combination therapy of virus, bicalutamide (anti-androgen) and radiation, a profound impact on cell death by viral oncolysis was seen both in vitro and tumor xenografts. To our knowledge, this is the first gene therapy engineered to be enhanced by anti-androgens, and a particularly attractive adjuvant strategy for intensity modulated radiation therapy (IMRT) of high-risk prostate cancers.
Keywords: Adenovirus, CRAd, Fiber-IRES-GFP, Radiation, E1A-ARC685Y, Anti-Androgen
Introduction
Despite intense research, advanced prostate cancer (PCa) remains an incurable disease. Traditional chemotherapeutic strategies have only modestly extended life expectancy by a few months in hormone refractory disease1–3. This drug resistance can be partially attributed to the unusually slow growth rate seen in prostate cancer compared to other cancers4, 5. Prostate specific conditionally replicating adenoviral gene therapy offers an alternative approach to traditional therapy due to the ability to infect quiescent and dividing cells6, 7. Several groups have developed gene therapy vectors for prostate cancer treatment, however for a variety of reasons only a few have been translated clinically8–10.
Prostate-specific conditionally replicating adenoviruses (CRAds) work by placing the replication control genes under the control of a prostate specific promoter, resulting in a selectively replication competent virus. Early clinical experience has demonstrated that CRAds offer a safe, auxiliary platform for treating prostate cancer; however, monotherapy with these agents has been associated with only modest clinical activity. Our group has been integrally involved in the development of prostate specific CRAds for clinical translation11, 12. In order to optimize the oncolytic activity of these agents, we have focused on understanding the biology of action in prostate cancer cells. Our analyses reveal that the early adenoviral genes (E1A) interact with the androgen receptor in prostate cancer cells, limiting the activity of both E1A and AR. This mutual inhibition led to decreased potency of the adenoviral vectors. Recently, we were able to overcome this deficiency by fusing E1A with AR, such that the chimeric fusion now allows augmentation of activity, rather than inhibition13.
Since the standard of care for high risk patients (Gleason 7 or higher, T2 or higher) is to combine androgen suppressive hormone therapy with radiation therapy14, 15, we sought to develop a virus which could be activated by anti-androgens. By incorporating a single point mutation in codon 685 changing the amino acid from cysteine to tyrosine (C→Y) in the androgen receptor ligand binding domain16 of the E1A-AR fusion, we were able to construct a virus that is activated for replication by both androgens and non-steroidal anti-androgens. This novel virus is an ideal construct for combination with IMRT external beam radiation therapy for the treatment of high risk prostate cancer.
Materials and Methods
Cell Culture & Reagents
LNCaP, DU145 and OVCAR3 cancer cells were obtained from American Type Culture Collection (Manassas, VA) and cultured as per the supplier’s protocol. C4-2 was obtained from Leland Chung, who derived the cell line from LNCaP. They were cultured in RPMI (Cellgro Mediatech, Herndon, VA) supplemented with 10% FBS, and maintained at passages 50 to 60. Virus packaging cell line DPL-S11 described previously was maintained in DMEM (Cellgro Mediatech, Herndon, VA) with 5% FBS and 200µg/ml G418. The 293HEK cell line (Quantum Biotech) was used for viral titration. It was maintained in DMEM with 10% FBS. All media were supplemented with Ciprofloxacin Hydrochloride 5 µg/ml (US Biological, Swampscott, MA) and Gentamicin 50 µg/ml (Quality Biological Inc., Gaithersburg, MD). All cells were maintained at 37°C in an atmosphere containing 5% CO2. All the restriction enzymes used are from NEB (New England Biolabs, MA). Primary mouse monoclonal antibodies for androgen receptor (AR 441) and E1A (M73) were bought from Santa Cruz Biotech (USA). Adenoviral DNA binding protein (DBP) Mouse monoclonal B6-8 was a kind gift from Dr. Arnold J. Levine (Cancer Institute of New Jersey). Most of the chemicals and reagents used in this study were ordered from Sigma-Aldrich unless otherwise specified.
Generation of Recombinant Adenoviruses
Ad5 PSE/PBN E1A-AR and Ad5 PSE/PBN E1A-ARC685Y were generated using Ad-Easy system (Stratagene, CA). The E1A-AR chimera includes wild-type AR while the E1A-ARC685Y includes a mutated AR in the ligand binding domain (C685Y) introduced by site directed mutagenesis, and fused with the C-terminus of adenovirus E1A gene. Briefly shuttle plasmids RpsToad-PSE/PBN-E1A-AR or RpsToad-PSE/PBN-E1A-ARC685Y that carries prostate-specific enhancer and rat probasin promoter driving E1A-AR or E1A-ARC685Y were linearized with Pme1 restriction endonuclease. After gel purification the linearized vectors were separately transformed into the electro-competent AdEasier-1 (BJ5183-AD-1) cells for homologous recombination. The desired clones (pAd5-PSE/PBN- E1A-AR and pAd5-PSE/PBN- E1A-ARC685Y) after screening were transformed into DH10B cells for large-scale DNA amplification. For viral propagation the recombinant plasmids (pAd5-PSE/PBN- E1A-AR and pAd5-PSE/PBN- E1A-ARC685Y) were linearized with EcoR1 and transfected into adenovirus packaging cell line DPL-S11 to generate recombinant adenoviruses.
CN702 is a wildtype E3-deleted serotype 5 recombinant Adenovirus described previously (12). Adeno-X-LacZ (Clontech, Mountain View, CA) was used as a control virus. All viruses were used at 2 multiplicities of infection (MOI). FFIG, a replication-defective reporter virus that encodes GFP under the control of the major late promoter (MLP) (10) was used at 30 MOI. Large-scale viral purification was performed using either CsCl2 gradient ultracentrifugation or commercial adenovirus purification kit (Adenopure; Puresyn, PA) and kept in dialysis buffer containing 15 mM Tris (pH 7.8), 2 mM MgCl2 and 5% sucrose. The titer of the viral stocks was determined using the Adeno-XTM Rapid Titer Kit (BD Biosciences, CA) and 293 cells. All viral stocks were tested for wild-type replication competent adenovirus (RCA) background generated by homologous recombination, using quantitative PCR with primers set spanning wild-type E1A promoter and E1A gene. The RCA content of all the viruses amplified in DPL-S11 cells were undetectable.
Western Blot Analysis
Cells were washed with 1 × PBS and re-suspended with five volumes of cold lysis buffer (50 mM Tris-HCl, pH 7.5, 250 mM NaCl, 5 mM EDTA, 50 mM NaF, 0.5% NP-40) supplemented with protease inhibitor cocktail (Roche, Indianapolis, IN). The cell lysate was incubated on ice for 30 min and then centrifuged for 10 min at 4 °C. Equal amounts of proteins were separated by SDS-PAGE, and the resolved proteins were then transferred to a nitrocellulose membrane. After blocking with 5% nonfat milk in TBST overnight at 4 °C, the blot was incubated with primary antibody at 1 h at room temperature. The membrane was then probed with HRP-conjugated secondary antibody for 1 h and developed (ECL-Plus system, Amersham Pharmacia, Piscataway, NJ) using the manufacturer's protocol.
Reporter-based Viral Replication Assay
A reporter FFIG virus was utilized for viral replication assay. As described previously, FFIG is a replication incompetent reporter virus that was made by linking green fluorescent protein (GFP) to the viral major late fiber gene through an Internal Ribosome Entry Site (IRES). It expresses GFP in a replication-dependent manner when co-infected with a replicating adenovirus (10). Cancer cells plated at the density of 2 ×104 cells per well in 48-well plates were infected with 2 MOI of virus (Ad5 PSE/PBN E1A-ARC685Y, Ad5 PSE/PBN E1A-AR, CN702 or Adeno-LacZ) together with 30 MOI of reporter FFIG virus. Viral replication was monitored by GFP expression using fluorescence microscopy. Each saved image from the fluorescence microscope was blindly scored by two individuals. Data from these experiments were plotted as an average number of GFP cells per field.
In Vitro Radiation & Cell viability
Acute single HDR radiation was performed 24 hours prior to viral infection at a dose 6 Gy (0.67 Gy/min) (Gammacell 40 137Cs irradiator, Atomic Energy Commission of Canada, Ltd); non-irradiated control cells were seeded and infected at the same time. Cell viability was measured using MTT assay kit (ATCC, Manassas, VA) over a period of nine or twelve days post-treatment. Briefly, growth media was removed and replaced with MTT solution 10 fold diluted with fresh media; after incubation for 3 hours at 37°C, detergent reagent provided in the kit was added to lyse the purple precipitates. At each time point, percentage of cell survival after respective treatment was calculated by normalizing to the growth of untreated cells.
Luciferase Assay
For luciferase assay PC-3 cells plated at the density of 1 × 104 cells/ well in 96-well plate were transfected with 100ng of the AR reporter pBK-PSE-PBN-Luc plasmid, together with equal molar concentrations of either pBK-CMV-AR or pBK-CMV-E1A-AR or pBK-CMV-E1A-ARC685Y using Lipofectamine Plus Reagent (Invitrogen, CA). The transfection media was replaced with media containing 10% dextran-charcoal-stripped serum in the presence or absence of 5 nM synthetic androgen R1881 (Methyltrienolone, Sigma) and 5 uM to 40 uM Bicalutamide (Sigma Aldrich USA) after 4h post-transfection. Luciferase activity was measured 24h post-transfection using the dual-luciferase reporter system (Promega). pRL-CMV (10ng/well) was used as an internal control in all wells. All transfection assays were performed in triplicate and normalized to internal control pRL-CMV reporter. Luciferase activity is reported as relative light forming units to reflect the renilla normalization.
BALB/c-nude Mice and Tumor Implantations
Four to six-week-old athymic BALB/c-nude male mice, weighing approximately 20–24 g were obtained from Harlan (Indianapolis, IN). Mice were quarantined for a minimum of 5 days in the SPF Grade Animal House under 12 h light/dark cycles at 24–25 °C with a relative humidity of 50–55%. Institutional guidelines were followed in handling the animals. Tumors were established by subcutaneous (s.c.) injection with C4-2 cells (1 × 106) resuspended in 1× phosphate-buffered saline (Ph 7.4; BioSource, Rockville, MD) mixed 1 × with Matrigel (BD Biosciences, Palo Alto, CA), in right dorsal flanks of the animals. Once tumors were established, animals were randomized into three groups (virus alone, Radiation alone and virus plus radiation). Each group consists of six animals.
Statistical Analysis
Statistical analysis was performed on Graph Pad Prism 5.0, running on an IBM compatible computer, using the Windows operating system. Comparisons for paired data were analyzed using the Student’s t-test. Statistical significance was defined as a p-value < 0.05 and was denoted in each of the figures by an asterisk.
Results
Functional Evaluation of an Activating Mutation of Androgen Receptor ARC685Y in the E1A-ARC685Y chimera and Construction of a Novel CRAd
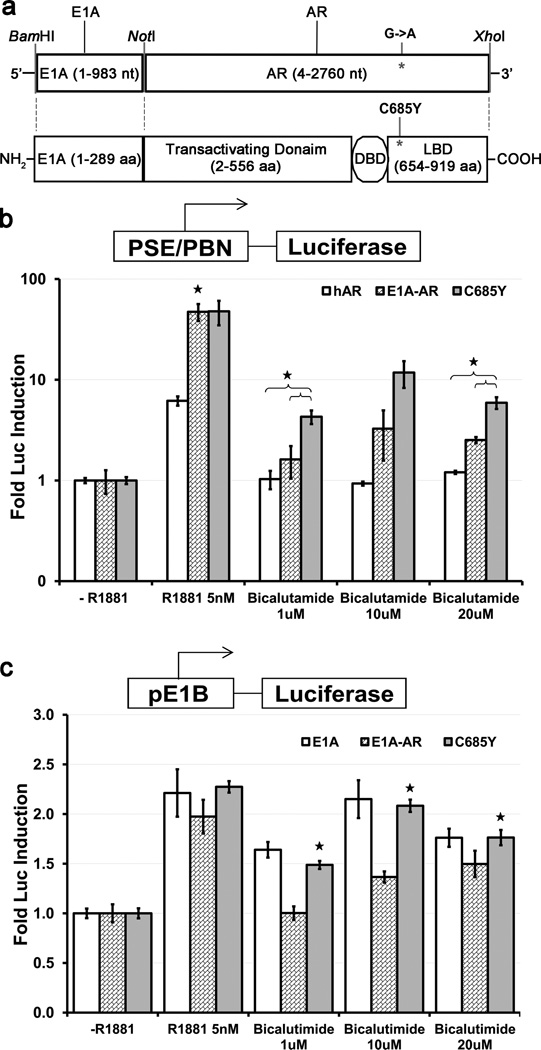
Mutation from Cysteine to Tyrosine in the Ligand Binding Domain (LBD) of Androgen receptor at codon 685 (C685Y) induces a significant conformational change16 resulting in the ability of the androgen receptor to be activated by non-steroidal anti-androgens such as hydroxy-flutamide and bicalutamide. We introduced the C685Y gene mutation into an E1A-AR gene fusion product to produce the E1A-ARC685Y chimera (Fig.1a). In order to confirm whether the mutant C685Y AR was transcriptionally active in the E1A-ARC685Y chimeric fusion, a reporter assay was performed as previously described using the AR dependent prostate specific enhancer and probasin promoter (PSE/PBN) driving the firefly luciferase gene17. AR negative prostate cancer cells (e.g. are unable to activate the prostate specific promoters utilized in our constructs as they all require active AR for maximal activity. However, reconstitution of AR transiently does allow full activation of our prostate specific constructs. Hence, PC3 cells (AR negative) co-transfected with the dual luciferase reporter plasmids (pBK-PSE/PBN-F.luc and pBK-CMV-R.luc) together with the AR-expression plasmids pcDNA3.1-hAR, pBK-CMV-E1A-AR, or pBK-CMV-E1A-ARC685Y induced expression of luciferase in the presence of the synthetic androgen R1881 (5nM) when compared to charcoal stripped media (Fig. 1b). However, in the presence of anti-androgen (bicalutamide) the reporter expression was induced to a higher extent by the chimeric mutant E1A-ARC685Y compared to E1A-AR and AR at various concentrations of bicalutamide (Fig. 1b). To confirm that viral E1A is functional in the E1A-ARC685Y construct, we looked at the ability of the E1A-ARC685Y chimera, to activate the transcription of the immediate down-stream E1B gene of adenovirus. Using a reporter plasmid of the E1B promoter driving firefly luciferase, PC3 cells were transfected with equal amount of plasmids (pBK-CMV-E1A-ARC685Y or pBK-CMV-E1A-AR or pUC19 and pBK-CMV-R.Luc for normalization). As shown in Figure 1C, the induction of the E1B promoter by E1A was not compromised by the addition of the mutant ARC685Y and was induced to higher levels with different concentrations of bicalutamide similar to E1A alone and was statistically higher compared to E1A-AR(Fig. 1c), consistent with an augmentation of activity by the mutated AR.
Figure 1. E1A-ARC685Y activates the expression of an androgen receptor and viral promoter reporter.
Schematic of E1A/AR fusion construct. The large (13S) E1A protein is fused on the C terminal end to the amino acid 2 of the Androgen Receptor (DBD=DNA Binding Domain, LBD=Ligand Binding Domain). A single point mutation was introduced to convert Cysteine 685 to Tyrosine in the LBD of AR (a). Firefly luciferase assay was performed using the AR reporter pBK-PSE/PBN-F.Luc co-transfected with a renilla luciferase transfection control (pRL-CMV) along with expression plasmids; AR, E1A-AR or E1A-ARC685Y. Transfections were performed in PC3 (AR negative) cell lines in the presence of 5nm R1881, 1uM, 10uM, or 20uM bicalutamide. Significant difference between E1A-AR and AR in R1881 and E1A-ARC685Y vs. AR and E1A-AR in bicalutamide represented by *(p<0.05) (b). Similarly, the E1A reporter plasmid pE1B-F.Luc was co-transfected with expression plasmids encoding E1A, E1A-AR, and E1A-ARC685Y or vector alone (pUC19) in PC3 cells in the presence of 5nm R1881, 1uM, 10uM, or 20uM bicalutamide. The addition of the point mutation to E1A-ARC685Y did not hamper E1A's ability to activate transcription of the viral promoter E1B in the presence of R1881 and in the presence of bicalutamide. Significant difference of E1A and E1A-ARC685Y vs. E1A-AR at all concentrations of bicalutamide represented by *(p<0.05) (c). Error bars represent means ± SE.
Next, the overall expression of the E1A-ARC685Y fusion construct was assessed. Lysates collected from the PC3 cells transfected with the plasmids pBK-CMV-E1A-ARC685Y or pcDNA-E1A were subjected to western blot analysis. Fusion E1A-ARC685Y protein was detected with two different antibodies (anti-AR and anti-E1A) and was expressed as a single intact protein at the predicted size of approximately 155 kDa (45 kDa for E1A and 110 kDa for AR) compared to the pcDNA3.1 transfected PC3 cells (Supplementary Fig. 1). Based on these results, we concluded that both E1A and ARC685Y components of the fusion were able to express and function as transcriptional activators in the presence of androgen or anti-androgens (bicalutamide) and as a result, are suitable for use in the construction of prostate specific CRAd for high risk prostate cancer.
Ad5-PSE/PBN-E1A-ARC685Y Retains Androgen Requirement and AR Specificity for replication
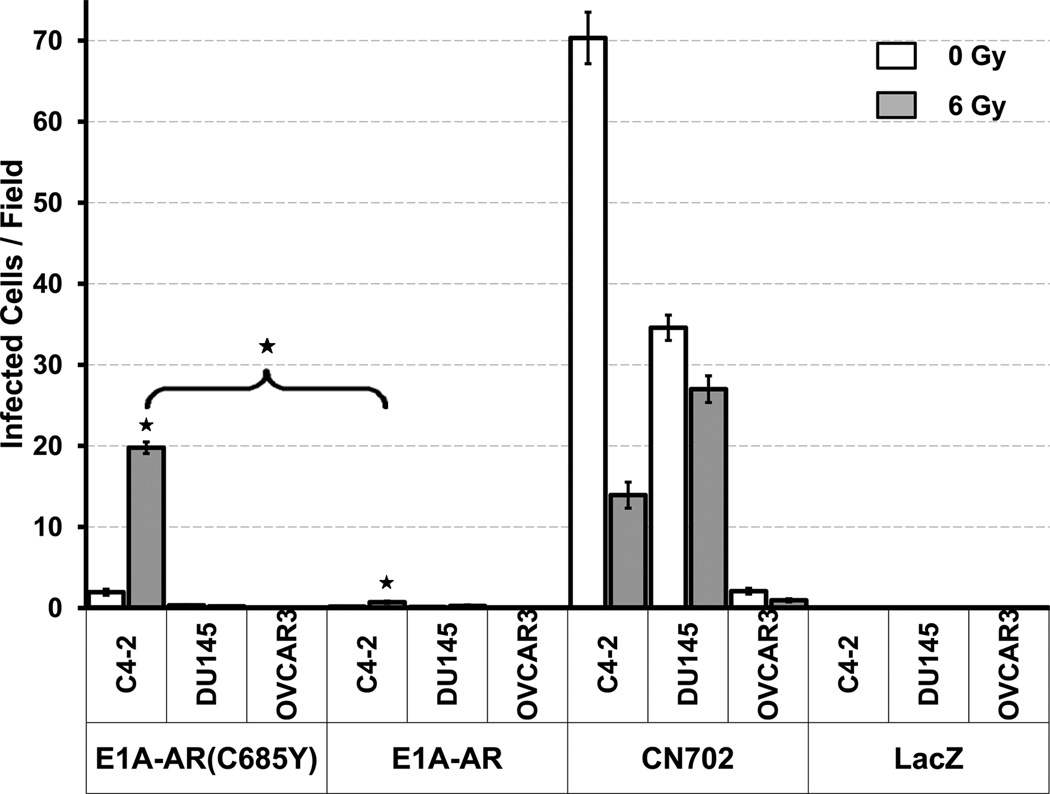
We have previously reported that the replication of the parental virus Ad5-PSE/PBN-E1A-AR was strictly dependent on the presence of androgen and was highly specific to AR positive prostate cell lines13. The addition of the point mutation to E1A-ARC685Y within the context of the intact CRAd was assessed to ensure that Ad5-PSE/PBN-E1A-ARC685Y virus retains the same features. A replication-incompetent reporter virus (FFIG) was used to follow viral replication by linking expression of GFP to the major late promoter, which is only active during the terminal phases of viral replication as previously described. Cells (LNCaP, C4-2, DU145 and OVCAR3) were co-infected with 2 MOI of the Ad5-PSE/PBN E1A-ARC685Y, Ad5-PSE/PBN-E1A-AR, CN702 (E3-deleted WT Ad5 virus, positive control) or Ad-LacZ (replication-deficient virus, negative control) virus together with FFIG (30 MOI). All cells were incubated in media without FBS during infection. Two hours post infection (p.i.), the media was supplemented with 5nM R1881 in 10% charcoal-stripped FBS. Ad5-PSE/PBN-E1A-ARC685Y successfully replicated in LNCaP and C4-2 cells (both AR positive prostate cell lines) and had significantly higher amounts of GFP infected cells compared to AR negative control DU145 and non prostate control OVCAR3 (ovarian cancer cell line) (Fig. 2a and Supplementary Fig. 2). CN702 infected all cell types which demonstrate the specificity of the prostate specific CRAds.
Figure 2. Comparing replication and cytotoxicity of parental Ad5-PSE/PBN-E1A-AR and Ad5-PSE/PBN-E1A-ARC685Y virus in the presence of Androgens.
2 MOI of Parental strain Ad5-PSE/PBN-E1A-AR, Ad5-PSE/PBN-E1A-ARC685Y, CN702 (WT Ad5), or Ad-Lac-Z(replication deficient) was co-infected with replication reporter virus FFIG (30MOI) in LNCaP, C4-2, DU145 and OVCAR3 in the presence of R1881 with or without pretreatment of 6 gy of acute single dose ionizing radiation. Seven days p.i. GFP fluorescence was visualized by microscopy (Fig. S2) and images were scored for GFP positive cells. The average number of GFP positive cells per field was plotted. Higher replication seen in Ad5-PSE/PBN-E1A-ARC685Y and Ad5-PSE/PBN-E1A-AR infected cells with radiation (vs. non-radiation treated; p<0.05) in C4-2 and LNCaP cells. Both CRAds do not replicate in the negative control DU145 and OVCAR cells (C4-2 and LNCaP vs DU145 and OVCAR; p<0.01) but CN702 (wild type Ad5) does, which shows the specificity of these viruses for AR positive prostate cells. Error bars represent mean ± SE (n = 20 fields per virus) (a). Cytotoxicity of the same viruses in the presence of R1881 were assessed by MTS assay at 12 days post treatment in the presence and absence of 6 gy of acute single dose ionizing in LNCaP (b) C4-2 (c) DU145 (d) cell lines and plotted as fold cell viability. The radiation treated cells had significantly higher cell kill compared to non-radiation treated cells in all occurrences (except for CN702 infected C4-2 cells) (p<0.05). Error bars represent means ± SE.
Previously we demonstrated that pre-treatment of cells with radiation enhances viral replication and cytotoxicity18. The addition of radiation to Ad5-PSE/PBN E1A-ARC685Y infection was assessed to evaluate whether any benefit to viral replication also occurs with Ad5-PSE/PBN E1A-ARC685Y. Various cell lines (LNCaP, C4-2, DU145 and OVCAR3) were pre-treated with 6 Gy of acute single dose ionizing radiation followed by co-infection with the viruses; Ad5-PSE/PBN E1A-ARC685Y, Ad5-PSE/PBN-E1A-AR, CN702(E3 deleted Ad5 wildtype control)19 & reporter virus FFIG (which turns cells green in proportion to viral replication). The replication of Ad5-PSE/PBN E1A-ARC685Y Ad5-PSE/PBN-E1A-AR was enhanced by radiation in LNCaP and C4-2 cells as significantly higher amounts of GFP infected cells were seen in radiation treated cells compared to non-irradiated cells (Fig. 2a). Although it appears there is a decrease in CN702 infected C4-2 cells with radiation compared to non-radiation (Fig. 2a), there is more cell kill seen in the MTT cytotoxicity assay when irradiated cells are compared to nonirradiated cells (Fig. 2b), therefore this decrease in GFP infected cells is likely to be due to viral mediated cell lysis. To further evaluate the effect of radiation on viral oncolysis, we pre-treated the cells (LNCaP, C4-2, and DU145) with 6 Gy of acute single dose ionizing radiation followed by viral infection (2 MOI). Cell viability was observed over a period of 12 days using MTT assay. Pre-treatment of radiation significantly enhanced viral kill compared to the virus alone in both AR positive prostate cancer cell lines (Fig. 2b,c). Interestingly, in C4-2 cells in the absence of radiation both viruses exerted little to no cytotoxicity, however, in combination with radiation, the Ad5-PSE/PBN-E1A-ARC685Y had killed over 78% of the C4-2 cells and Ad5-PSE/PBN-E1A-AR had 68% cell kill at the end of 12 days (Fig. 2c). As expected, no viral-derived cytotoxicity was observed in the control cell line DU145 (Fig. 2d). Therefore, Ad5-PSE/PBN-E1A-ARC685Y retains the ability to cause tissue-specific cytotoxicity which is enhanced by radiation in hormone sensitive and AR positive prostate cancer cells similar to the parental virus.
Replication and Cytotoxicity of Ad5-PSE/PBN-E1A-ARC685Y in Combination with Radiation in the Presence of Anti-Androgen (Bicalutamide)
Testosterone suppression through androgen deprivation therapy (ADT) is standard treatment for patients presenting with advanced prostate cancer20. However, a large fraction of patients treated with ADT develop androgen resistant cancer which is exemplified by the C4-2 cell line model21. This cell line was chosen as a model of androgen independent disease to test the potency of Ad5-PSE/PBN-E1A-ARC68Y in the presence of the non-steroidal anti-androgen bicalutamide. First, replication of Ad5-PSE/PBN-E1A-ARC68Y in C4-2 was monitored by FFIG assay as previously described13. C4-2 cells plated in charcoal stripped media were irradiated (6 Gy) 24 hours prior to Ad5-PSE/PBN-E1A-ARC685Y viral infection (2 MOI) together with FFIG (30 MOI) in the presence of bicalutamide (5 µM to 40 µM). Viral replication was monitored using fluorescent microscopy (through GFP expression in the FFIG reporter virus) and quantitated by scoring of GFP positive infected cells. Ad5-PSE/PBN-E1A-ARC685Y was able to replicate in the presence of bicalutamide in irradiated C4-2 cells, furthermore, there was a dose dependent response of the Ad5-PSE/PBN-E1A-ARC685Y replication with increasing concentration of bicalutamide (Fig. 3a and Supplementary Fig. 3).
Figure 3. Ad5-PSE/PBN-E1A-ARC685Y replication in bicalutamide combined with radiation increases in a bicalutamide dose-dependent manner.
Viral replication FFIG reporter assay was performed in C4-2 cells pre-treated with 6 Gy of ionizing radiation and infected with 2 MOI of Ad5-PSE/PBN-E1A-ARC685Y and 30 MOI FFIG in increasing concentration of Bicalutamide 24 hours post radiation. GFP fluorescence was visualized by microscopy 12 days after treatment (Fig. S3) and the average number of GFP positive fields for each concentration was counted and plotted. Error bars represent means ± SE (n = 20 fields per virus). Higher Ad5-PSE/PBN-E1A-ARC685Y replication in 20uM and 40uM Bicalutamide (vs 5uM and 10uM; p<0.05) (a). Growth inhibition and cytotoxicity of Ad5-PSE/PBN-E1A-ARC685Y was assessed by MTT assay 12 days after treatment in the presence and absence of 6 Gy of ionizing radiation and increasing concentrations of bicalutamide from 5uM to 40uM. Significant higher cell kill in radiation treated cells vs mock treated cells in all concentrations of Bicalutamide (p<0.001) (b).Error bars represent means ± SE.
Next, we looked at replication mediated cytotoxicity of Ad5-PSE/PBN E1A-ARC685Y in the presence of radiation and bicalutamide. C4-2 cells were irradiated with 6 Gy of acute single dose radiation, then infected with the Ad5-PSE/PBN-E1A-ARC685Y or mock treated in the presence of bicalutamide ranging from 5 µM to 40 µM. Cell viability was observed by MTT over a period of 12 days. The virus caused minimal cell death in non-irradiated cells in the presence of bicalutamide, however, when both treatments were combined together the viability of the C4-2 cells significantly decreased in a dose dependent manner (Fig. 3b). These results demonstrate that Ad5-PSE/PBN-E1A-ARC685Y virus is not only able to replicate in the presence of the bicalutamide in androgen independent cells, but it can also markedly increase the therapeutic impact of neo-adjuvant radiation therapy.
Comparison of the Effect of Bicalutamide and Radiation on Viral Replication in vitro
Although Ad5-PSE/PBN E1A-ARC685Y virus in combination with radiation and 40 µM bicalutamide caused the greatest extent of viral-derived cytotoxicity, we chose to focus on 10 µM bicalutamide in the following experiments since this is the calculated expected concentration corresponding to a 70-kg patient taking 100–150 mg bicalutamide orally per day. Next, we expanded the cell lines tested in order to ensure that the specificity of Ad5-PSE/PBN E1A-ARC685Y replication in the presence of bicalutamide is limited to AR positive prostate cancer cells. C4-2, DU145, and OVCAR3 cells were first pre-treated with 6 Gy of acute single dose ionizing radiation, followed by co-infections of Ad5-PSE/PBN-E1A-ARC685Y or Ad5-PSE-PBN-E1A-AR or CN702 (positive control) or Ad-Lac-Z (negative control) with reporter FFIG virus in the presence of 10 µM bicalutamide and GFP positive cells were scored. The presence of bicalutamide severely hampered the ability of the parental Ad5-PSE-PBN-E1A-AR virus to replicate due to the androgen blockade as very low levels of GFP infected cells were seen (Fig. 4 and Supplementary Fig. 4). In comparison, Ad5-PSE/PBN-E1A-ARC685Y replication was restricted to androgen insensitive C4-2 prostate cancer cells in the presence of bicalutamide and replication was significantly induced when combined with a single acute dose of irradiation (6 Gy).
Figure 4. Ad5-PSE/PBN-E1A-ARC685Y replication is specific and higher in C4-2 cells in the presence of bicalutamide combined with radiation.
Visualization of replication by GFP Fluorescence in the presence of bicalutamide and radiation was done by pre-treating C4-2, DU145 and OVCAR cell lines with or without 6 Gy of acute single dose ionizing radiation. Co-infection of 2 MOI Ad5-PSE/PBN-E1A-AR, Ad5-PSE/PBN-E1A-ARC685Y, CN702, or Ad-Lac-Z with 30 MOI of FFIG in LNCaP, C4-2, DU145 and OVCAR3 in the presence of 1uM bicalutamide was done 24 hours post-radiation. 12 days after infection, GFP fluorescence microscopy was performed to visualize the amount of GFP within the infected cells and images (Fig. S4) were scored for GFP positive cells. The average number of GFP positive cells per field was plotted. Error bars represent means ± SE (n = 20 fields per virus). Higher replication of Ad5-PSE/PBN-E1A-ARC685Y in radiation treated C4-2 cells in bicalutamide (vs. Ad5-PSE/PBN-E1A-ARC685Y non-radiation; p<0.001 and vs Ad5-PSE/PBN-E1A-AR C4-2 ± radiation; p<0.001).
Next, we compared the cytotoxic effect of the parental Ad5-PSE-PBN-E1A-AR virus to Ad5-PSE/PBN-E1A-ARC685Y virus. The triple therapy of 2 MOI of virus, 6 Gy of radiation, and 10uM bicalutamide was tested on C4-2, DU145, and OVCAR3 cells, and the resulting cytotoxicity was measured using MTT assay over 12 days. The viruses were not cytotoxic to any of the bicalutamide treated cell lines in the absence of radiation (Fig. 5). However, when radiation (6 Gy) was combined together with viruses and bicalutamide (10 µM) a significant therapeutic effect was observed in Ad5-PSE/PBN-E1A-ARC685Y infected C4-2 cells. Approximately 71% (p < 0.05) of cells were killed when compared to 39% in parental Ad5-PSE/PBN-E1A-AR infected C4-2 cells (Fig. 5a). This finding of radiation and bicalutamide induced viral cell death was not observed in the other two control cell lines (DU145 and OVCAR3). These in vitro results demonstrate the utility of Ad5-PSE/PBN-E1A-ARC685Y in gene therapy of advance prostate disease in combination with radiation.
Figure 5. Comparison of cytotoxicity of bicalutamide and radiation treated cells in combination with virus infection.
Cytotoxicity of C4-2, DU145, and OVCAR cells pre-treated with or without 6 gy of acute single dose ionizing radiation and infected Ad5-PSE/PBN-E1A-AR, Ad5-PSE/PBN-E1A-ARC685Y , Ad-Lac-Z or mock infected were assessed by MTS assay at different time points (0–12 days) and plotted as fold cell viability. All cells were infected in serum free media which was supplemented with 10uM bicalutamide in 10% charcoal-stripped FBS 2 hour p.i. In C4-2 cells Ad5-PSE/PBN-E1A-ARC685Y with radiation has a statistically significant higher cell kill than Ad5-PSE/PBN-E1A-AR (p<0.05) (a). In DU145 and OVCAR cells no measurable cell kill was detectable (b,c). Error bars represent means ± SE.
Antitumor effect of Ad5-PSE/PBN-E1A-ARC685Y in Combination with Radiation in Androgen ablated C4-2 Xenograft Mouse Model
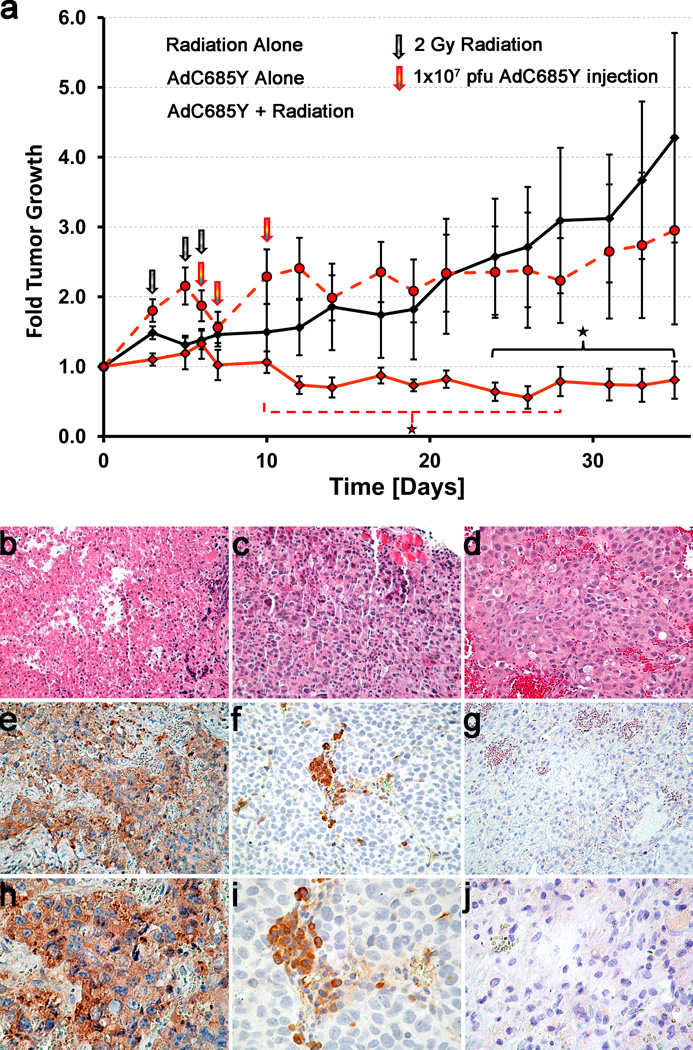
Lastly, the therapeutic efficacy of Ad5-PSE/PBN-E1A-ARC685Y virus in combination with radiation and bicalutamide was tested in vivo. Androgen independent C4-2 xenograft tumors were established in nude mice. Once the tumor volume reached to approximately 220 mm3, animals were randomized into three different groups (virus alone, radiation alone and virus plus radiation). One week prior to radiation and viral infection all mice were started on the bicalutamide regimen (20mg/kg) given i.p. three times a week throughout the duration of the study. The treatment schedule consisted of 3 separate doses of 2 Gy of local radiation for a total of 6 Gy of radiation and subsequently followed by 3 separate intratumoral injections of 1×107 PFU of Ad5-PSE/PBN-E1A-ARC685Y in the virus plus radiation group. We selected large tumor burden in these experiments in order to maximize the chance of demonstrating an additive effect between the two therapies which may have been missed due to eradication of the tumors by either virus or radiation alone. Due to the variation in size of the tumors in each group the fold tumor growth averages were plotted.
Fold growth of the tumor volume of the virus plus radiation group was statistically significantly lower than the virus alone group between 10 to 28 days post start of treatment. The virus plus radiation group compared to radiation alone was statistically significantly lower from 24 days post start of treatment through the duration of the study (Fig. 6a). Residual tumor cells that have recovered from radiation treatment and begin to proliferate would cause the increase in fold tumor growth seen in the radiation alone group towards the end of the study. In the virus plus radiation treated tumors the growth stays approximately the same as the radiation alone tumors increase and this appears to be a long lasting virus effect against tumor growth. This may be why this group becomes significantly lower than radiation at these later time points, but loses the earlier seen significance compared to the virus alone group. Although the low amount of virus used was not able to overcome the tumor burden used in the study, it is possible that given at a much higher M.O.I., with the addition of radiation and the presence of bicalutamide, the combination may have been able to eradicate these tumors completely. These results indicate that even at a low dosage of virus, the cumulative effective of radiation and virotherapy was able to keep the tumor size much smaller compared to single treatment groups.
Figure 6. Oncolytic activity of Ad5-PSE/PBN-E1A-ARC685Y in combination with radiation and bicalutamide treatment in vivo tumor xenograft model.
C4-2 prostate cancer xenografts were established by injecting 1 × 106 cells into the dorsal rear flank region of athymic nude mice (n = 6 per group) to examine the antitumor activity of Ad5-PSE/PBN-E1A-ARC685Y virus. All animals were put on bicalutamide therapy of 20mg/kg given 3 times a week I.P. Radiation group tumors were pre-treated with 2 Gy of ionizing radiation given on 3 separate days for a total of 6 Gy prior to virus infection. Ad5-PSE/PBN-E1A-ARC685Y virus was injected 3 times intratumorally at 1 × 107 plaque forming units on the day of the last dose of radiation, day 1 post radiation, and day 4 post radiation, and tumors were measured 3 times a week along with bicalutamide treatment for the duration of the study. Average fold tumor growth was plotted for each animal group. There was a long lasting antitumor growth activity seen in Ad5-PSE/PBN-E1A-ARC685Y plus radiation group that was significant compared to virus treatment alone from 10–28 days post treatment (p<0.05). Ad5-PSE/PBN-E1A-ARC685Y treated tumors compared to radiation alone wer significantly lower starting 24 days post treatment and continued throughout the study (p<0.05). Error bars represent fold means ± SE. (a). After 35 days mice were euthanized and tumors harvested from all groups for IHC were sectioned and stained with anti-Adenoviral DNA binding protein (DBP) to assess active viral replication. H&E stain of Radiation + AdC685Y (b), Radiation Alone(c), and AdC685Y Alone (d). IHC of Adenovirus 72K DNA Binding Protein 20× magnification Radiation + AdC685Y (e), Radiation Alone (f), and AdC685Y Alone (g). IHC of Adenovirus 72K DNA Binding Protein 40× magnification Radiation + AdC685Y (h), Radiation Alone (i), and AdC685Y Alone (J).
To further confirm that the differential therapeutic effect observed in the virus plus radiation group was due to active viral replication and oncolysis, tumors harvested at the end of the study from each treatment group were subjected to immunohistochemistry (IHC). We used the Adenovirus E2 72k DNA binding protein (DBP) for IHC staining because it has been previously shown as a marker for viral replication and is expressed in abundance during viral infection22. By IHC, we were able to detect the expression of DBP in both Ad5-PSE/PBN-E1A-ARC685Y alone and the Ad5-PSE/PBN-E1A-ARC685Y plus radiation groups, however, the amount of expression of the DBP was significantly higher in the virus plus radiation group indicating that the active replicating virus still persisted long after (35 days P.I.) the initial infection and contributed to the therapeutic effect of the tumors seen in these animals (Fig. 6b). This data demonstrates the persistence of Ad5-PSE/PBN-E1A-ARC685Y virus long after initial virus injection, and has the potential to enhance both the therapeutic efficacy of radiation and viral replication in the presence of hormone therapy to be used for the treatment of high-risk and androgen-refractory prostate cancers.
Discussion
Early clinical experience has demonstrated that CRAds offer a safe, auxiliary platform for treating prostate cancer; however, monotherapy with these agents has been associated with only modest clinical activity12. More recently, we have found that the combination of these adenoviral vectors with chemotherapy or radiation therapy results in significant enhancement of activity18–23. In this study, we sought to augment the activity of radiation therapy by the incorporation of a conditionally replication competent adenovirus, designed specifically for the intermediate to high risk prostate cancer patient, who normally would receive neo-adjuvant androgen suppression.
Patients with high grade cancer (Gleason 7 or higher) are typically treated with either radiation therapy or radical surgery. Which approach is used depends on the clinical situation, the patient's preference and the likelihood of success. Patients who ultimately elect to proceed forward with radiation therapy most typically are treated with a combination of hormone therapy (androgen suppression) and IMRT, particularly if the cancer is Gleason 8 or higher and the disease is palpable. Unfortunately, these relatively high risk patients have a significant treatment failure rate (in excess of 20% at 5 years)24 despite advanced imaging technology and escalating radiation doses (IMRT). Hence, there is a necessity for improvement in outcomes for this group. Using an anti-androgen inducible prostate specific CRAd in combination with radiation therapy, we were able to engineer a gene therapy vector specifically for these high risk prostate patients.
Mutations in androgen receptor, especially in the LBD, are one of the mechanisms proposed to explain how advanced prostate cancer may escape androgen deprivation25. These mutations expand the specificity and affinity of the AR to other hormones, resulting in inappropriate receptor activation26. In order to use these mutations in prostate adenoviral gene therapy to activate the vectors for therapeutic applications, we fused the N-terminus of an AR-C685Y mutant which has been shown to become activated by non-steroidal anti-androgens. Studying the biology of this point mutation we found that the mutated E1A-AR685Y fusion retained ability to function both in regulating AR responsive genes (e.g. the probasin promoter we use to direct specificity of the virus) and in viral genes. The activity was best augmented by the use of bicalutamide (Fig. 1). Based on this fusion, we constructed the prostate specific CRAd and found that it replicated more slowly than our previous constructs (data not shown) and had minimal activity in terms of killing prostate cancer cells (Fig. 5a). However, when this virus was combined with increasing concentration of bicalutamide and high dose rate radiation, it exerts a profound impact on viral replication which was shown to be bicalutamide dose dependent. The replication of Ad5 PSE/PBN-E1A-ARC685Y virus in the presence of androgens and anti-androgens (bicalutamide) was shown in the context of the replication deficient reporter virus (FFIG), for which green fluorescent protein (GFP) expression is linked to the viral major late promoter and can only replicate when co-infected with replication competent adenovirus (Fig. 2, 3). We believe the therapeutic effect observed in C4-2 cells is because of the viral oncolysis that was induced by bicalutamide and radiation treatment as no such effect was observed in AR negative prostate DU145 or control OVCAR3 ovarian cancer cell lines (Fig. 4). To further support our in vitro results we measured the activity of this CRAd in the in vivo androgen insensitive C4-2 tumors xenograft model which have been treated with radiation alone, virus alone or in combination with radiation and virus in the presence of bicalutamide (20mg/kg). As expected, virus in combination with radiation was able to significantly reduce the size of the established tumors compared to the virus or radiation alone. We also evaluated and compared the presence of replication foci by IHC in virus-treated tumors versus tumors treated with virus irradiation. Tumors harvested at the end of the experiment were stained with Adenoviral DNA binding protein (DBP). A robust staining pattern with larger foci was only observed in the virus and radiation combination group compared to the virus alone.
In summary, we describe a novel prostate specific CRAd engineered to replicate specifically in patients receiving hormone therapy via non-steroidal anti-androgens. These findings highlight a novel therapeutic strategy for augmenting radiation therapy in high-risk prostate cancers.
Supplementary Material
Acknowledgments
This study was supported in part by grants from Flight Attendant Medical Research Institute (FAMRI) to Naser Uddin Hoti and by the PCW and RO1 (R01CA121153-01A2) award to Dr Ronald Rodriguez.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Petrylak DP. Chemotherapy for advanced hormone refractory prostate cancer. Urology. 1999;54:30–35. doi: 10.1016/s0090-4295(99)00452-5. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Kantoff P. Recent progress in management of advanced prostate cancer. Oncology (Williston Park) 2005;19:631–636. [PubMed] [Google Scholar]
- 4.Berges RR, Vukanovic J, Epstein JI, CarMichel M, Cisek L, Johnson DE, et al. Implication of cell kinetic changes during the progression of human prostatic cancer. Clin Cancer Res. 1995;1:473–480. [PMC free article] [PubMed] [Google Scholar]
- 5.Visakorpi T, Kallioniemi OP, Paronen IY, Isola JJ, Heikkinen AIKoivula TA. Flow cytometric analysis of DNA ploidy and S-phase fraction from prostatic carcinomas: implications for prognosis and response to endocrine therapy. Br J Cancer. 1991;64:578–582. doi: 10.1038/bjc.1991.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spindler KR, Eng CYBerk AJ. An adenovirus early region 1A protein is required for maximal viral DNA replication in growth-arrested human cells. J Virol. 1985;53:742–750. doi: 10.1128/jvi.53.3.742-750.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greber UF, Willetts M, Webster PHelenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 8.Gurnani M, Lipari P, Dell J, Shi BNielsen LL. Adenovirus-mediated p53 gene therapy has greater efficacy when combined with chemotherapy against human head and neck, ovarian, prostate, and breast cancer. Cancer Chemother Pharmacol. 1999;44:143–151. doi: 10.1007/s002800050959. [DOI] [PubMed] [Google Scholar]
- 9.Shalev M, Kadmon D, Teh BS, Butler EB, Aguilar-Cordova E, Thompson TC, et al. Suicide gene therapy toxicity after multiple and repeat injections in patients with localized prostate cancer. J Urol. 2000;163:1747–1750. [PubMed] [Google Scholar]
- 10.Freytag SO, Khil M, Stricker H, Peabody J, Menon M, DePeralta-Venturina M, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 2002;62:4968–4976. [PubMed] [Google Scholar]
- 11.Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L, et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14:107–117. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 12.DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. [PubMed] [Google Scholar]
- 13.Hoti N, Li Y, Chen CL, Chowdhury WH, Johns DC, Xia Q, et al. Androgen receptor attenuation of Ad5 replication: implications for the development of conditionally replication competent adenoviruses. Mol Ther. 2007;15:1495–1503. doi: 10.1038/sj.mt.6300223. [DOI] [PubMed] [Google Scholar]
- 14.D'Amico AV, Schultz D, Loffredo M, Dugal R, Hurwitz M, Kaplan I, et al. Biochemical outcome following external beam radiation therapy with or without androgen suppression therapy for clinically localized prostate cancer. JAMA. 2000;284:1280–1283. doi: 10.1001/jama.284.10.1280. [DOI] [PubMed] [Google Scholar]
- 15.Zapatero A, Garcia-Vicente F, Martin de Vidales C, Cruz Conde A, Ibanez Y, Fernandez I, et al. Long-term results after high-dose radiotherapy and adjuvant hormones in prostate cancer: how curable is high-risk disease? Int J Radiat Oncol Biol Phys. 2011;81:1279–1285. doi: 10.1016/j.ijrobp.2010.07.1975. [DOI] [PubMed] [Google Scholar]
- 16.Ceraline J, Erdmann E, Erbs P, Deslandres-Cruchant M, Jacqmin D, Duclos B, et al. A yeast-based functional assay for the detection of the mutant androgen receptor in prostate cancer. Eur J Endocrinol. 2003;148:99–110. doi: 10.1530/eje.0.1480099. [DOI] [PubMed] [Google Scholar]
- 17.van der Poel HG, McCadden J, Verhaegh GW, Kruszewski M, Ferrer F, Schalken JA, et al. A novel method for the determination of basal gene expression of tissue-specific promoters: an analysis of prostate-specific promoters. Cancer Gene Ther. 2001;8:927–935. doi: 10.1038/sj.cgt.7700385. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Zhang Y, Liu MM, Zhou H, Chowdhury W, Lupold SE, et al. Evaluation of continuous low dose rate versus acute single high dose rate radiation combined with oncolytic viral therapy for prostate cancer. Int J Radiat Biol. 2010;86:220–229. doi: 10.3109/09553000903419338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez R, Schuur ER, Lim HY, Henderson GA, Simons JWHenderson DR. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57:2559–2563. [PubMed] [Google Scholar]
- 20.Ryan CJSmall EJ. The selection of hormonal therapy in prostate cancer: who, when, and for how long? J Natl Compr Canc Netw. 2004;2:261–268. doi: 10.6004/jnccn.2004.0023. [DOI] [PubMed] [Google Scholar]
- 21.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak SChung LW. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994;57:406–412. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 22.Reich NC, Sarnow P, Duprey ELevine AJ. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology. 1983;128:480–484. doi: 10.1016/0042-6822(83)90274-x. [DOI] [PubMed] [Google Scholar]
- 23.Hoti N, Chowdhury WH, Mustafa S, Ribas J, Castanares M, Johnson T, et al. Armoring CRAds with p21/Waf-1 shRNAs: the next generation of oncolytic adenoviruses. Cancer Gene Ther. 2010;17:585–597. doi: 10.1038/cgt.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolla M, Fourneret P, Beneyton V, Tessier A, Jover FVerry C. Combination of external irradiation and androgen suppression for prostate cancer: facts and questions. Cancer Radiother. 2010;14:510–514. doi: 10.1016/j.canrad.2010.07.226. [DOI] [PubMed] [Google Scholar]
- 25.Han G, Buchanan G, Ittmann M, Harris JM, Yu X, Demayo FJ, et al. Mutation of the androgen receptor causes oncogenic transformation of the prostate. Proc Natl Acad Sci U S A. 2005;102:1151–1156. doi: 10.1073/pnas.0408925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waltering KK, Urbanucci AVisakorpi T. Androgen receptor (AR) aberrations in castration-resistant prostate cancer. Mol Cell Endocrinol. 2012;360:38–43. doi: 10.1016/j.mce.2011.12.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.