Abstract
Background
Soluble adenylyl cyclase (sAC) is an enzyme that generates cyclic adenosine monophosphate (cAMP), a signaling molecule involved in regulating melanocyte differentiation, proliferation and melanogenesis. R21, a mouse monoclonal antibody against sAC, shows a pattern of pan nuclear staining in lentigo maligna showing possible utility for diagnosis and for margin assessment.
Design
31 re-excision specimens for lentigo maligna were evaluated for R21 expression using a previously published protocol. In addition, a prospective analysis of 123 cases including 41 lentigo malignas, 30 non-lentigo maligna type melanomas, 38 lentigos and 44 benign nevi were evaluated using a modified stringent protocol that eliminates non-melanocyte staining.
Results
The sensitivity of nuclear staining with R21 in lentigo maligna was 87.8%. Nuclear expression of sAC was observed in 40% of other melanomas and in only 2.3% of the benign nevi. R21 did not stain nuclei of resting melanocytes but nuclear staining was observed in 28.9% of melanocytic hyperplasias. R21 staining facilitated extent of the lesion in resection margins. In cases examined under the less stringent conditions interpretation can be facilitated by comparing R21 and Mart1/Melan A staining. Greater than 9 pan nuclear staining melanocytes within one high power field along with a pan nuclear sAC/melan A ratio >0.5 was consistent with a positive margin while five or less pan nuclear staining melanocytes along with a sAC/melan A ratio of <0.3 constituted a negative margin.
Conclusion
R21 is a useful diagnostic adjunct in the diagnosis of lentigo maligna and can facilitate the evaluation of margins in re-excisions.
INTRODUCTION
Soluble adenylyl cyclase (sAC) is a novel class of adenylyl cyclase, the enzyme responsible for generation of cAMP. cAMP is a key intracellular signaling molecule involved in regulation of melanocyte differentiation, proliferation and melanogenesis. sAC is ubiquitously expressed in many tissues.1–3 sAC is localized in different subcellular microdomains (cytoplasm, Golgi area, nucleus) in different tissues. sAC responds to both extracellular (TNF, Netrin, NGF) or intracellular (bicarbonate, pH, calcium) signals.2, 4–12
Magro et al. have recently reported expression of sAC in benign melanocytic proliferations and melanomas using a monoclonal antibody against sAC designated R21.13 This paper demonstrated that sAC expression in benign nevi is enriched to the perinuclear Golgi region. In contrast, many melanomas show apparent relocation of sAC to the nucleus frequently accompanied by loss of the perinuclear golgi staining pattern. In addition, different histological subsets of melanoma show distinct predominant patterns and intensity of staining with R21. The most striking reproducible pattern is strong pan-nuclear expression of sAC in lentigo maligna melanoma along with other melanomas exhibiting a lentiginous radial growth phase (i.e. acral lentiginous and mucosal lentiginous melanomas). Pan nuclear staining is also observed in a subset of neoplastic cells in superficial spreading melanomas and nodular melanoma but not to the extent seen in the setting of lentiginous melanomas. These results suggest that, in contrast to the currently available “first generation melanocytic markers” such as S100, microphthalmia transcription factor (MITF) or Mart1/Melan A,14–18 R21 immunohistochemistry can be used to distinguish melanoma from benign melanocytic proliferations and may be useful in the subclassification of melanoma.
In this report we share our experience with the utilization of the R21 antibody as a diagnostic adjunct both in the initial evaluation of lentigo maligna and in the assessment of margins.
MATERIALS AND METHODS
Two sets of cases and two staining protocols were examined. The initial set of cases was represented by 31 lentigo maligna re-excision specimens that XX (removed for blinded review) prospectively encountered in her routine clinical practice at the Weill Medical College of Cornell (New York City, NY) over a period of 6 months. In each case hematoxylin and eosin stained sections, deeper sections through relevant tissue blocks, and sAC immunohistochemical antibody stains were conducted. In certain cases a Melan A stain was also conducted on selected blocks. The details of the sAC analysis will be given below. The methodology for the stain has been previously published.3 10 control cases were examined, comprising re-excision specimens for nonmelanoma skin cancer associated with extensive chronic photoactivation of melanocytes.
In a parallel studies performed in Boston, a study independently examined 41 cases of classic lentigo maligna and 38 cases of reactive lentiginous melanocytic hyperplasia incidentally found on excision and/or biopsy specimens of nonmelanoma skin cancer during the period of 7/1/2011 to 10/15/2011 (see below). Using the same antibody, we established a distinct staining protocol designed to highlight only melanocyte pan nuclear staining, while eliminating all background staining. To validate the modified protocol, 44 benign nevi and 30 non-lentigo maligna melanomas were also examined.
All four authors reviewed both sets of data to establish a consensus regarding staining results using both protocols.
R21 immunohistochemistry
R21 is a mouse monoclonal antibody directed against amino acids 203–216 of human sAC protein.3 Application of R21 immunohistochemistry for evaluation of sAC expression in melanocytic proliferations was recently described in great detail by Magro et al.13 In this study, results were obtained as previously described13 or using a modified protocol with a higher dilution of R21 antibody (1:1200–2000). The modified protocol takes advantage of the high level of sAC expression in lentigo maligna and achieves conditions which preserve the pan-nuclear staining in lentigo maligna but minimize staining in benign melanocytes and other cell types. This allows for a focused evaluation of sAC nuclear staining. R21 staining was performed using Leica, DAKO or Ventana automatic stainers.
Assessment of sAC Staining
Using conditions described in our previous paper13 the main staining pattern which defined chronic photoactivation of melanocytes is one of discrete dot like Golgi staining oftentimes accompanied by nucleolar staining. In contrast, in lentigo maligna, a striking pattern of pan nuclear staining highlighting melanocytes typically larger than the adjacent keratinocyte nucleus is characteristic. In each case the number of cells exhibiting a pan nuclear staining pattern within one high power field (40X) of the inked margin was determined. Melanocytes that either did not stain or showed other staining patterns (i.e. Golgi and incomplete granular nuclear staining) were not deemed as positive cells. A similar count was given for Melan A positive staining melanocytes. In cases where both stains were done a pan nuclear sAC/Melan A ratio was determined.
Using the modified technique developed by one of the authors, the antibody was applied at a dilution designed to highlight only pan nuclear staining. A lesion was considered positive if at least 50% of evaluable melanocytes in at least one 20× magnification field showed unequivocal nuclear staining. Most cases showed uniform staining throughout the entire lesion. In some cases areas of both positive and negative staining were observed. Resting melanocytes present in sections were also scrutinized for R21 staining.
Other methods
As part of routine work up some lesions were also stained with S100, Mart1/Melan A or microophthalmia transcription factor (MITF) antibodies according to routine laboratory procedures.
Statistical methods
Descriptive statistics included estimations such as mean, standard deviation, and medians. Chi-square statistics (i.e. Fisher exact test) were used, where appropriate, as tests of association and the weighted kappa statistics provided us with diagnostic agreement. Significance level was set a α=0.05.
Results
Utility of sAC in the evaluation of lentigo maligna margins
Using the previously published protocol, we examined a total of 31 excision specimens to determine the presence of residual lentigo maligna and investigate adequacy of excision. In 27 of the 31 cases, there was evidence of residual lentigo maligna while in 4 of the cases a residual atypical intraepidermal melanocytic proliferation was identified although not diagnostic of fully evolved lentigo maligna. In 17 of 31 cases associated with a positive margin the mean number of pan nuclear staining melanocytes within one high power field was 15.88 (standard deviation of 6.77 with a median value of 13.0). The average pan nuclear sAC/melan A ratio was 0.72 (standard deviation of 0.24; median of 0.725). A representative case with positive margin is illustrated in Figure 1. When the margin was negative (n=42), there was an average of 2.24 pan nuclear staining melanocytes within one high power field (median of 2.0; standard deviation of 2.80). (Figure 2) The pan nuclear sAC/melan A ratio was 0.22 (median 0.155; standard deviation of 0.304). Thus, greater than 9 pan nuclear staining melanocytes within one high power field and/or a sAC/Melan A ratio > 0.5 was associated with a positive margin. Five or less pan nuclear staining melanocytes and/or a sAC/Melan A ratio < 0.3 was associated with a negative margin. (<0.0001, weighted kappa statistic of 0.625). This value of kappa indicates substantial agreement with margin results. All non-melanoma skin cancer re-excision specimens showed a benign pattern of sAC staining as revealed by a dot like Golgi staining pattern with variable nucleolar staining. The overall density within one high power field of the lateral margin ranged from 0 to 4 cells per high power field. The sAC/Melan A ratio was less than 0.1.
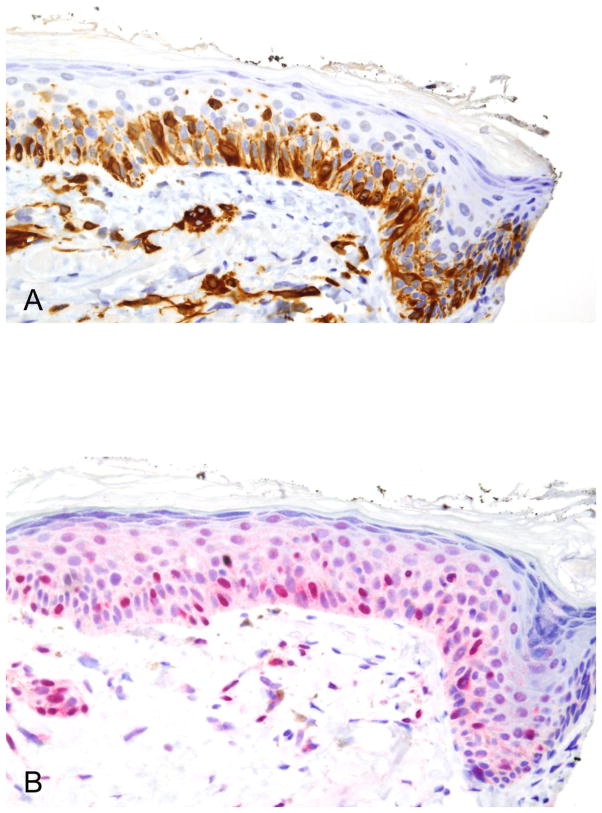
Figure 1.
Positive lentigo maligna surgical margin. (A) This 40× view shows 24 positive staining cells for melan A. Only cells exhibiting an intact nucleus are countered. (B) The R21 stain shows pan nuclear staining in 26 melanocytes. The melan A/R21 stain is close one to one.
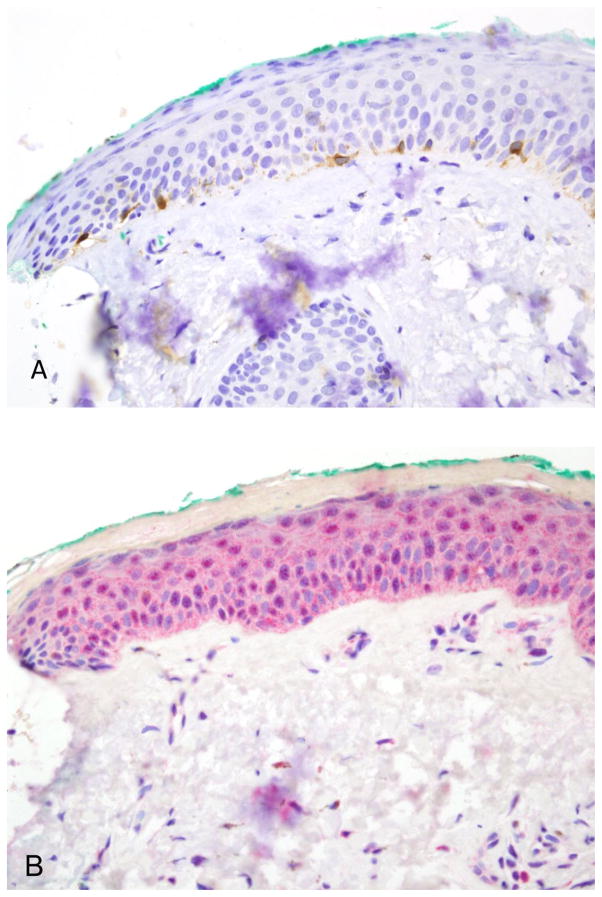
Figure 2.
Negative lentigo maligna surgical margin. (A) This 40× view shows 7 melan A positive cells. Only cells exhibiting an intact nucleus are counted. (B) The R21 preparation is essentially negative. The sAC to melan A ratio is < 0.1.
Assessment of sAC using the modified stringent protocol
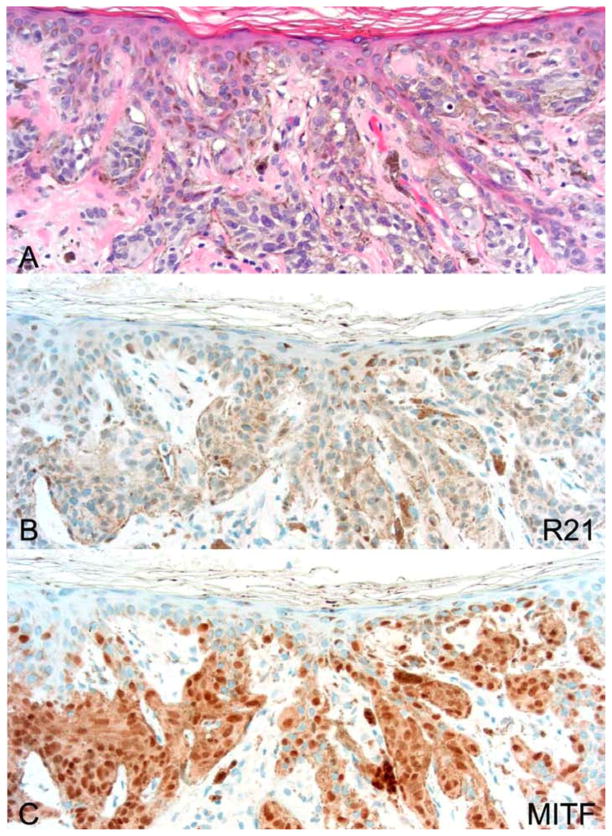
Following our initial assessment of lentigo maligna margins with R21 and Melan A (Figures 1–2), we felt this immunostain held promise as tool to both diagnose lentigo maligna and assess surgical margins. However because of the occasional keratinocyte staining of this ubiquitous protein, we wanted to establish whether a more stringent immunostaining protocol could allow for a focused evaluation of nuclear sAC staining exclusively in melanocytes. Our hope was such a protocol would allow for a more simplified evaluation of melanoma. Results of staining using this protocol are summarized in Table 1. Indeed, using a modified protocol utilizing 50% less antibody, nuclear expression of sAC was observed in 87.8 % of lentigo malignas. Figure 3 illustrates a representative case showing strong nuclear staining with R21 of both in situ and invasive lentigo maligna melanoma. Focal areas with negative R21 staining were observed in 2 of 35 R21 positive cases. Figure 4 shows the peripheral margin of a representative resection of lentigo maligna melanoma. It demonstrates that the R21 stain allows precise determination of the limit of peripheral spread of lentigo maligna.
TABLE 1.
Expression of sAC staining in melanomas, melanocytic hyperplasias, and nevi.
| NUMBER | POSITIVE | PERCENT POSITIVE | |
|---|---|---|---|
| MELANOMAS | |||
| Lentigo Maligna | 41 | 36 | 87.8 |
| Other Melanomas | |||
| Total | 30 | 12 | 40.0 |
| Melanoma in-situ | 2 | 2 | 100 |
| Superficial Spreading | 17 | 6 | 35.3 |
| Unclassified | 9 | 3 | 33.3 |
| Acral Lentiginous | 1 | 0 | 0 |
| Mucosal Lentiginous | 1 | 1 | 100 |
| LENTIGO/MELANOCYTIC HYPERPLASIA | |||
| Total | 38 | 11 | 28.9 |
| With Atypia | 23 | 6 | 26.1 |
| Without Atypia | 15 | 5 | 33.3 |
| NEVI | |||
| Total | 44 | 1 | 2.3 |
| Compound | 3 | 0 | 0 |
| Dermal | 6 | 0 | 0 |
| Lentiginous Junctional | 8 | 0 | 0 |
| Lentiginous Junctional with Atypia | 12 | 1 | 0 |
| Lentiginous Compound Dysplastic | 8 | 0 | 0 |
| Spitz | 7 | 0 | 0 |
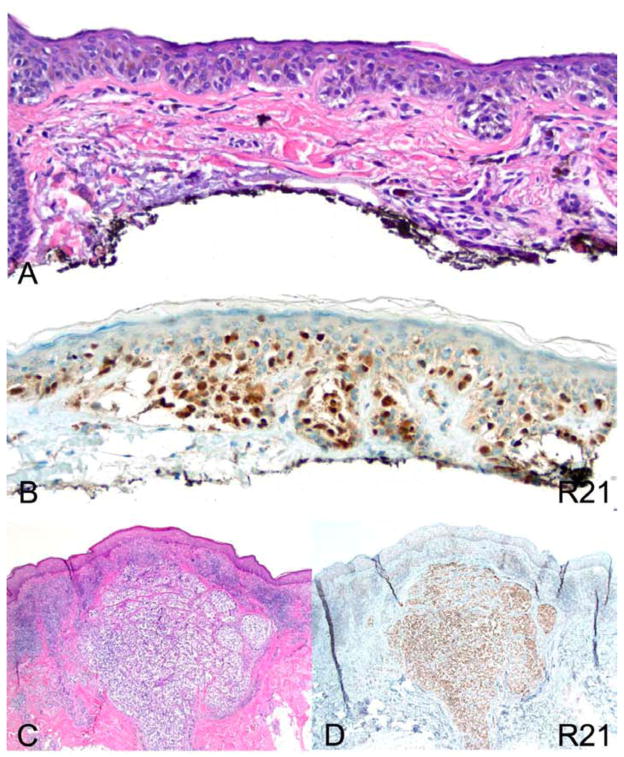
Figure 3.
Lentigo maligna type melanoma exhibits strong pan-nuclear R21 staining. (A) hematoxylin and eosin (H&E) 200×. (B) R21 immunohistochemistry of an in situ melanoma 200×. (C) H&E of an invasive melanoma lentigo maligna type 40×. (D) R21 immunohistochemistry of the melanoma depicted in (C) 40×.
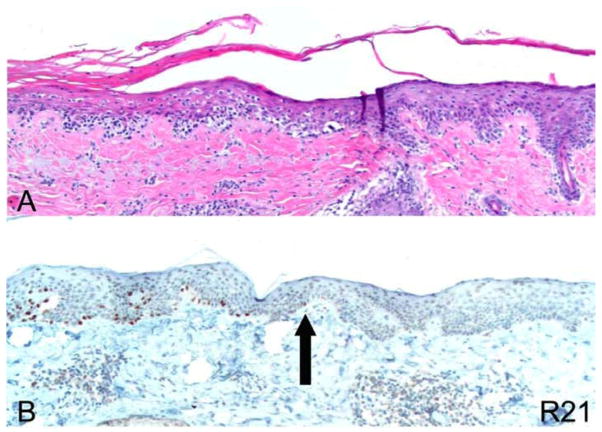
Figure 4.
Use of R21 immunohistochemical stain to assess margins of excision with stringent protocol. (A) H&E). (B) R21 immunohistochemistry 10×. The arrow indicates the precise margin of the lesion pointing to the last cell showing nuclear expression of sAC.
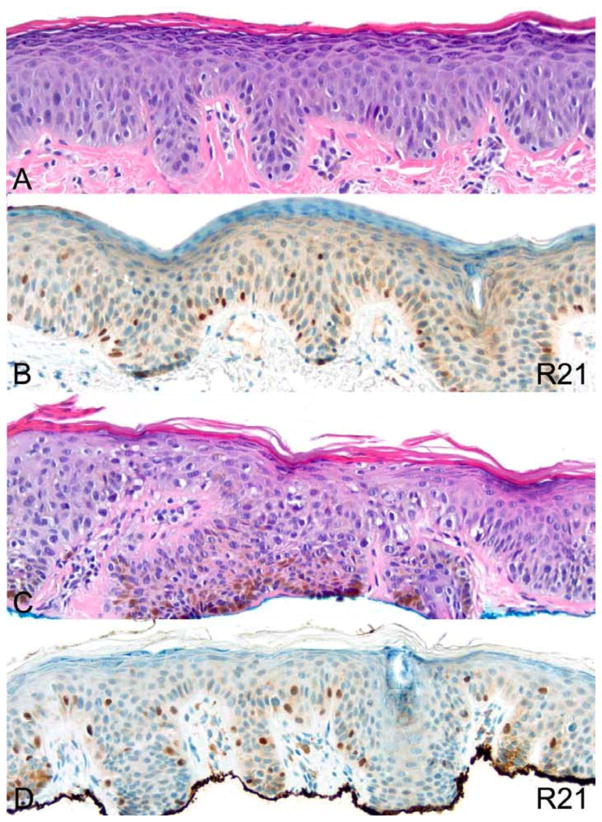
Various benign melanocytic nevi were examined including dermal, compound, junctional, dysplastic and Spitz nevi (Table 1). All cases, except for one, were devoid of all R21 nuclear staining (Figure 5). The one case in which nuclear expression was observed was reported as a junctional dysplastic nevus (data not shown).
Figure 5.
A representative example of a lentiginous compound dysplastic nevus. (A) H&E. (B) R21 immunohistochemistry. (C) MITF immunohistochemistry. All photomicrographs taken at 20×. There is no nuclear expression of sAC in the nevus, while MITF offers positive control.
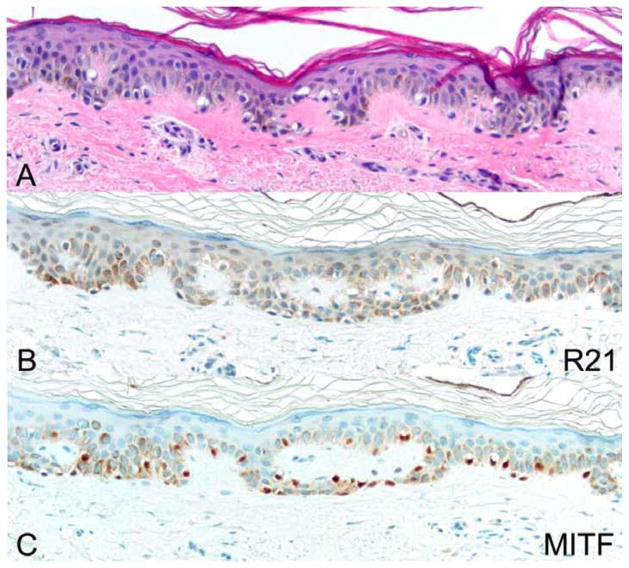
Most lesions diagnosed as lentiginous junctional hyperplasias showed no nuclear staining with R21 (Figure 6, Table 1); 28.9% of lesions were positive (Figure 7). R21 staining in lentigo maligna versus melanocytic hyperplasia was statistically significant by Fisher exact test (p<0.0001). R21-positive melanocytic hyperplasias were scrutinized for presence of atypical melanocytes. Occasional enlarged or hyperchromatic melanocytes could be identified in 6 of 11 lesions. However, no atypical features were observed in 5 of 11 R21-positive melanocytic hyperplasias. In these cases all the melanocytes were small and inconspicuous in routine sections.
Figure 6.
An example of a lentiginous melanocytic hyperplasia without R21 nuclear staining. (A) H&E. (B) R21 immunohistochemistry. (C) MITF immunohistochemistry. All photomicrographs taken at 20×. There is no nuclear expression of sAC in melanocytes, while MITF offers positive control.
Figure 7.
Two examples of lentiginous melanocytic hyperplasia with R21 nuclear staining. H&E (A) and R21 immunohistochemistry (B) show a lesion with no significant melanocytic atypia. H&E (C) and R21 immunohistochemistry (D) show a lesion with moderate atypia evident by increased nuclear size. All photomicrographs taken at 20×
We have also examined 30 non-lentigo maligna melanomas using the stringent protocol. As expected, we confirmed the findings of Magro et al.,13 and found nuclear expression of sAC in 40% of non-lentigo maligna melanomas.
DISCUSSION
Lentigo maligna is one of the most common forms of malignant melanoma. Recent insights into the molecular pathogenesis of melanoma revealed that melanomas occurring on the sun-exposed skin show a distinct pattern of chromosomal instability and are less frequently associated with BRAF mutations, which preferentially occur in melanomas occurring on intermittently sun-exposed skin.19, 20 Despite these advancements in the molecular understanding of lentigo maligna, the histological diagnosis of lentigo maligna remains problematic. The classic morphologic depiction of incipient lentigo maligna is one characterized by single cell lentiginous proliferation at the dermoepidermal junction of atypical melanocytes manifesting large angulated hyperchromatic nuclei with an irregular pattern of melanization. With progression of the in situ lesion, pagetoid spread and prominent junctional nest formation occurs. It is well known that benign reactive melanocytic hyperplasias in sun-exposed areas or in the vicinity of surgical scars can exhibit histological patterns not dissimilar to early lentigo maligna. In particular the classic morphologic depiction of chronic photoactivation of melanocytes includes lentiginous (single cell) melanocytic proliferation with cellular enlargement, the potential for nuclear hyperchromasia, bi- and multi-nucleation and low-level pagetoid ascent.
In this regard, developing an antibody that reliably distinguishes between chronic photoactivation, paracicaticial melanocytic hyperplasia, junctional nevus and lentigo maligna would be a valuable diagnostic adjunct. The first generation melanocytic markers such as S100, microophthalmia transcription factor (MITF) or Mart1/Melan A, do not distinguish between benign and malignant melanocytes. The most common adjunctive immunohistochemical stain in the evaluation of lentigo maligna margins is Mart 1/Melan A.21 However in our experience we find its sensitivity in highlighting benign melanocytes in zones of chronic photoactivation renders the stain suboptimal in the accurate assessment of lentigo maligna margins. In our study, a relatively high-density pattern of proliferation revealed by Melan A stain could be seen at seemingly negative margins. Excessive pagetoid ascent and/or junctional nest formation along the dermal epidermal junction could be deemed a pattern indicative of residual lentigo maligna in some cases. However, our experience confirmed by a recent study suggests that the aforesaid features can be observed on Melan A stains procured from normal skin chronically exposed to sun.22 In the same study, the Mart 1/Melan A stained section showed stacking of melanocytes, confluence of melanocytes or suprabasilar spread and adnexal extension in >95% of cases. Almost 20% of negative control specimens showed at least 4 of the criteria held to be suspicious for lentigo maligna, defining a pattern closely mimicking lentigo maligna/lentigo maligna melanoma and up to 33% of normal skin samples could show at least one suspicious feature.22 In another study melanocyte confluence was observed in as much as 45% of Melan A-stained excisions of basal cell carcinoma on sun-exposed skin; while cytological atypia and pagetoid spread can be seen in 19% and 3% of cases, respectively.23
We have presented our experience with the utilization of anti-sAC antibodies (R21) in the evaluation of lentigo maligna including the utilization of this stain to evaluate margins. We provide evidence that R21 a “second generation” immunohistochemical marker. It is the first stain, which can discriminate between benign and malignant melanocytic proliferations.
In lentigo maligna, we observe a strong pan-nuclear staining of atypical enlarged junctional and pagetoid melanocytes. With invasion, one can see a similar pattern of pan nuclear staining. In most cases regardless of the protocol used one can easily determine the margins of the lesion, often to the level of a single cell by direct observation. In lesions stained by the original protocol which also highights sAC expression in keratinocytes, examination of margins can be facilitated by comparison between Melan A and R21 stains. If one examines the number of pan nuclear staining melanocytes within one high power field of an inked margin, histologically positive margins were associated with a number of positive staining cells typically in excess of 9 and/or a sAC/melan A ratio approximating 1 while negative margins consistently gave results of less than 5 cells manifesting the pan nuclear staining pattern and/or a sAC/melan A ratio approaching 0. In contrast, sAC expression in nevi and amid chronically photoactivated melanocytes is almost exclusively restricted to the Golgi area and nucleoli.13
We optimized the R21 staining protocol by titrating the antibody to minimize staining of resting melanocytes, keratinocytes, cutaneous adnexae and inflammatory cells. Using this modified technique, the interpretation of a positive result depends exclusively on the presence or absence of pan nuclear staining. Our results with the modified protocol estimate sensitivity of R21 staining in lentigo maligna at 87.8 %. The R21 stain can precisely determine the extent of involvement by lentigo maligna in some cases up to the level of a single cell. False-negative cases were otherwise histologically and clinically classic lentigo malignas that did not express sufficient levels of sAC to be detected by R21 in our more stringent protocol.
Further studies are needed to better understand why some “benign” melanocytes exhibit a pan nuclear pattern of sAC expression. Do these melanocytes contain genetic aberrations? Do they represent transitional lesions between frank benignity and cancer? We hope that ongoing basic research into the biology of sAC will offer explanations. An important parallel is drawn with the common V600E BRAF mutations driving melanoma but also commonly present in benign nevi.
Regardless of the antibody dilution used, authors had qualitatively similar results to published reports.13 Pathologists who are interested in examining both pan nuclear and golgi staining as XX (removed for blinded review) does in routine practice will prefer a more concentrated dilution of the antibody, while those who prefer to focus exclusively on the pan nuclear staining pattern will prefer a more diluted stain.
In summary, unequivocal cases of lentigo maligna are defined by pan nuclear expression of sAC amidst the dominant intraepidermal melanocyte population; conversely rare cells with pan nuclear staining are likely not significant especially if the cell is not enlarged (i.e. morphologically abnormal). That said one must always correlate R21 staining with routine morphologic assessment. As with any ancillary technique, a diagnostic interpretation based purely on immunohistochemical studies is not advised. The sAC immunostain especially when coupled with Melan A (or MITF) to define a sAC/Melan A (or sAC/MITF) ratio is a very simple but discriminatory diagnostic test that allows a quick and reliable assessment of lentigo maligna margins. The exact titration of sAC needed to achieve optimal results will likely vary depending on the goal of the pathologist and immunostaining protocols of the lab.
Contributor Information
Cynthia M. Magro, Professor of Pathology and Laboratory Medicine, Director of Dermatopathology, Weill Medical College of Cornell University, 1300 York Avenue, New York, New York.
Sung-Eun Yang, Department of Pathology and Laboratory Medicine, Tufts Medical Center, 800 Washington Street, Boston, Massachusetts.
Jonathan H. Zippin, Assistant Professor of Dermatology, Weill Medical College of Cornell University, 1300 York Avenue, New York, New York.
Artur Zembowicz, Email: Artur.Zembowicz@Lahey.org, Associated Professor of Pathology, Medical Director www.DermatopathologyConsultations.com, Senior Consultant, Lahey Clinic, Department of Pathology, Lahey Clinic, 41 Mall Street, Burlington, Massachusetts.
Reference List
- 1.Park HY, Gilchrest BA. Signaling pathways mediating melanogenesis. [Review] [76 refs] Cell Mol Biol (Noisy -le-grand) 1999 Nov;45(7):919–30. [PubMed] [Google Scholar]
- 2.Tresguerres M, Levin LR, Buck J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int. 2011 Jun;79(12):1277–88. doi: 10.1038/ki.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zippin JH, Chadwick PA, Levin LR, Buck J, Magro CM. Soluble adenylyl cyclase defines a nuclear cAMP microdomain in keratinocyte hyperproliferative skin diseases. J Invest Dermatol. 2010 May;130(5):1279–87. doi: 10.1038/jid.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper DM, Mons N, Karpen JW. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature. 1995 Mar 30;374(6521):421–4. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- 5.Bundey RA, Insel PA. Discrete intracellular signaling domains of soluble adenylyl cyclase: camps of cAMP? Sci STKE. 2004 May 4;2004(231):e19. doi: 10.1126/stke.2312004pe19. [DOI] [PubMed] [Google Scholar]
- 6.Feng QP, Zuo J, Meng Y, Fang FD. Nuclear localization region in soluble adenylyl cyclase. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2005 Jun;27(3):280–4. [PubMed] [Google Scholar]
- 7.Feng Q, Zhang Y, Li Y, Liu Z, Zuo J, Fang F. Two domains are critical for the nuclear localization of soluble adenylyl cyclase. Biochimie. 2006 Mar;88(3–4):319–28. doi: 10.1016/j.biochi.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Krupinski J. The adenylyl cyclase family. Mol Cell Biochem. 1991 May 29;104(1–2):73–9. doi: 10.1007/BF00229806. [DOI] [PubMed] [Google Scholar]
- 9.Stessin AM, Zippin JH, Kamenetsky M, Hess KC, Buck J, Levin LR. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J Biol Chem. 2006 Jun 23;281(25):17253–8. doi: 10.1074/jbc.M603500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hess KC, Jones BH, Marquez B, et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005 Aug;9(2):249–59. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zippin JH, Chen Y, Nahirney P, et al. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003 Jan;17(1):82–4. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- 12.Zippin JH, Farrell J, Huron D, et al. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J Cell Biol. 2004 Feb 16;164(4):527–34. doi: 10.1083/jcb.200311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magro CM, Crowson AN, Desmaze C, Zippin JH. The sAC Antibody Profile as a Diagnostic Adjunct in the Assessment of Pigmented Lesions. Arch Dermatol. 2011 doi: 10.1001/archdermatol.2011.338. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busam KJ, Chen YT, Old LJ, et al. Expression of melan-A (MART1) in benign melanocytic nevi and primary cutaneous malignant melanoma. Am J Surg Pathol. 1998 Aug;22(8):976–82. doi: 10.1097/00000478-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 15.King R, Googe PB, Weilbaecher KN, Mihm MCJ, Fisher DE. Microphthalmia transcription factor expression in cutaneous benign, malignant melanocytic, and nonmelanocytic tumors. Am J Surg Pathol. 2001 Jan;25(1):51–7. doi: 10.1097/00000478-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 16.King R, Weilbaecher KN, McGill G, et al. Microphthalmia transcription factor. A sensitive and specific melanocyte marker for MelanomaDiagnosis. Am J Pathol. 1999 Sep;155(3):731–8. doi: 10.1016/S0002-9440(10)65172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prieto VG, Shea CR. Immunohistochemistry of melanocytic proliferations. Arch Pathol Lab Med. 2011 Jul;135(7):853–9. doi: 10.5858/2009-0717-RAR.1. [DOI] [PubMed] [Google Scholar]
- 18.Black WH, Thareja SK, Blake BP, Chen R, Cherpelis BS, Glass LF. Distinction of melanoma in situ from solar lentigo on sun-damaged skin using morphometrics and MITF immunohistochemistry. Am J Dermatopathol. 2011 Aug;33(6):573–8. doi: 10.1097/DAD.0b013e3182093b13. [DOI] [PubMed] [Google Scholar]
- 19.Bastian BC, Olshen AB, LeBoit PE, et al. Classifying melanocytic tumors based on DNA copy number changes. Am J Pathol. 2003 Nov;163(5):1765–70. doi: 10.1016/S0002-9440(10)63536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005 Nov 17;353(20):2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 21.Kelley LC, Starkus L. Immunohistochemical staining of lentigo maligna during Mohs micrographic surgery using MART-1. J Am Acad Dermatol. 2002 Jan;46(1):78–84. doi: 10.1067/mjd.2002.119197. [DOI] [PubMed] [Google Scholar]
- 22.Bowen AR, Thacker BN, Goldgar DE, Bowen GM. Immunohistochemical staining with Melan-A of uninvolved sun-damaged skin shows features characteristic of lentigo maligna. Dermatol Surg. 2011 May;37(5):657–63. doi: 10.1111/j.1524-4725.2011.01946.x. [DOI] [PubMed] [Google Scholar]
- 23.Hendi A, Wada DA, Jacobs MA, et al. Melanocytes in nonlesional sun-exposed skin: A multicenter comparative study. J Am Acad Dermatol. 2011 Jun 16; doi: 10.1016/j.jaad.2010.10.039. [DOI] [PubMed] [Google Scholar]