Abstract
SPOR domains are present in thousands of bacterial proteins and probably bind septal peptidoglycan (PG), but the details of the SPOR:PG interaction have yet to be elucidated. Here we characterize the structure and function of the SPOR domain for an Escherichia coli division protein named DamX. NMR revealed the domain comprises a 4-stranded antiparallel β-sheet buttressed on one side by two α helices. A third helix, designated α3, associates with the other face of the β sheet but this helix is relatively mobile. Site-directed mutagenesis revealed the face of the β-sheet that interacts with α3 is important for septal localization and binding to PG sacculi. The position and mobility of α3 suggest it might regulate PG binding, but although α3 deletion mutants still localized to the septal ring, they were too unstable to use in a PG binding assay. Finally, to assess the importance of the SPOR domain in DamX function, we constructed and characterized E. coli mutants that produced DamX proteins with SPOR domain point mutations or SPOR domain deletions. These studies revealed the SPOR domain is important for multiple activities associated with DamX—targeting the protein to the division site, conferring full resistance to the bile salt deoxycholate, improving the efficiency of cell division when DamX is produced at normal levels, and inhibiting cell division when DamX is overproduced.
Bacterial cell division has been studied most intensively in Escherichia coli, where this process is mediated by a collection of about 30 proteins that localize to a structure at the mid-cell called the “septal ring” or “divisome” (reviewed in (1-3)). Recently, several labs have shown that SPOR domain proteins are prominent components of the septal ring in a wide range of bacteria (4-6). SPOR domains (Pfam 05036) are about 75 a.a. long and occur in over 5000 bacterial proteins in release 26 of the Pfam database (7). These domains are called SPOR domains because the founding member of this family, a Bacillus subtilis cell wall amidase named CwlC, is a sporulation protein (8). The available evidence suggests most SPOR domain proteins are involved in cell division.
SPOR domains have at least two noteworthy biological functions. The first is that they bind peptidoglycan (PG) (4, 6, 9). The second is that SPOR domains localize to the septal ring, even when artificially produced in heterologous bacteria (4-6). Taken together, these observations imply SPOR domains localize to the division site by binding preferentially to septal PG. This would be a new mode of septal targeting as most bacterial division proteins are recruited to the septal ring via interactions with other division proteins. Although the chemical features of PG recognized by SPOR domains are still under investigation, mounting evidence suggests SPOR domains target “naked” glycan strands generated by the action of amidases that remove stem peptides during biogenesis of PG cell wall in the division septum (5, 9, 10). Nevertheless, septal PG has not been shown to contain this or any other novel structure that would distinguish it from PG in the lateral wall or at the poles (11-14). These considerations suggest that a molecular understanding of how SPOR domains bind (septal) PG will lead to new insights into PG metabolism during cell division.
Two SPOR domain structures have been published already, one from the E. coli cell division protein FtsN and the other from the B. subtilis sporulation protein CwlC (15, 16). These domains adopt a similar fold despite being only ∼18% identical at the amino acid level. Each domain comprises a βαββαβ secondary structure that assembles into a 4-stranded antiparallel β-sheet buttressed on one side by two alpha-helices. This fold places SPOR domains in the ribonucleoprotein (RNP) fold superfamily, so-called because the first examples were RNA- binding domains from eukaryotic proteins involved in processing of mRNAs (17-19). In the meantime, RNP folds have been identified in a wide range of proteins and shown to bind a wide range of ligands. As one example, the E. coli cell division protein ZipA uses a modified RNP-fold to bind the C-terminus of FtsZ (20). In many RNP-fold domains, including those from ZipA and the human RNA-binding protein U1A, the β-sheet serves as the primary binding surface for ligands (17, 20-23). How SPOR domains interact with PG is not known, although it has been suggested that the SPOR domain from CwlC binds glycan strands using two symmetrical sites comprising residues near the beginning of α1 and α2, respectively (15).
We would like to know more about the SPOR:PG interaction and what role this plays in the biological function of SPOR domain proteins. Here we report the solution structure and some functional characterization of the SPOR domain from the DamX cell division protein of E. coli. Our choice of DamX reflected several considerations, starting with the fact that we expected to observe some new structural features because this SPOR domain has less than 20% identity to the SPOR domains from CwlC or FtsN. But DamX is interesting from a biological perspective too. Although DamX clearly localizes to the septal ring, damX null mutants have no obvious division defect (4, 5). Nevertheless, deleting damX exacerbates the mild filamentation and chaining phenotypes caused by loss of another septal ring protein with a SPOR domain, DedD (4, 5). Conversely, deleting damX rescues growth of an ftsQ1(Ts) mutant at the non-permissive temperature (4), which is unexpected because combining mutations in septal ring proteins typically exacerbates division defects. Another noteworthy property of DamX is that, unlike most E. coli division proteins, it inhibits cell division when overproduced (24). Finally, damX mutants are sensitive to bile salts, defective in invasion of host cells and attenuated for induction of the MarA regulon needed for multidrug resistance (4, 25-27). These phenotypes suggest DamX may contribute to cell envelope biogenesis or integrity in ways that are distinct from the protein's role in cell division.
Experimental Procedures
Media
For routine maintenance, cloning and genetic analysis, E. coli strains were grown in Luria-Bertani medium with 10 g NaCl per liter. When NaCl was omitted the medium is referred to as LB0N. Ampicillin and kanamycin were used at 200 μg/ml and 40 μg/ml, respectively. Spectinomycin was used at 100 μg/ml for plasmids, 35 μg/ml for chromosomal alleles. Isopropyl β-D-1-thiogalactopyranoside (IPTG) was used to induce gene expression at various concentrations as indicated in each experiment. To prepare isotopically-labeled protein for NMR, cultures were grown in M9 minimal media supplemented with B vitamins (1 mg/L), U- 13C-glucose (4 g/L) and 15NH4Cl (1 g/L). 15NH4Cl and U- 13C glucose were obtained from Sigma.
Molecular Biology
Detailed descriptions of plasmid construction and mutagenesis are in the supplemental information, as are tables listing strains, plasmids and primers (Supplemental Tables S1-3). PCR was done with VENT DNA polymerase (New England Biolabs) and regions of plasmids derived by PCR were sequenced to verify their integrity. Localization studies of isolated SPOR domains were done using derivatives of the Tat-targeted GFP (TTGFP) fusion vector pDSW962 (28). Expression plasmids for protein purification were derivatives of pQE80L (Qiagen). For some experiments GFP fusions were introduced into the chromosome at the Φ80 attachment site using the CRIM vector system as described (29).
Protein purification
Hexahistidine-tagged (His6) DamX SPOR domain for PG binding assays and His6-tagged DamX periplasmic domain for raising antibody were purified from cultures grown in LB essentially as described (4). This procedure was modified to obtain samples for NMR as follows. A 10 ml overnight culture of BL21(DE3) carrying a His6-DamXSPOR overproduction plasmid was grown in M9 containing glucose and ampicillin. The entire 10 ml were used to inoculate 1 L M9 Amp containing 15NH4Cl and glucose (either unlabeled or 13C-labeled, as appropriate). The 1 L culture was grown at 30°C for ∼12 h until the OD600 reached 0.5, then protein production was induced by addition of IPTG to 1 mM final concentration and the cultures were allowed to grow until the following morning (∼12 hr). Cells were harvested and protein purified as described for inductions performed in LB except that the yield was only ∼2 mg protein per liter of culture (4).
Typically, NMR spectra were collected using 0.7 mM protein in NMR buffer (50 mM potassium phosphate, 50 mM KCl, pH 6.5, 90% H2O, 10% D2O). To prepare samples, purified protein was dialyzed into 1.1× NMR buffer (55 mM potassium phosphate, 55 mM KCl, pH 6.5), concentrated using a Microcon Centrifugal Filter device with 3000 MW cut-off (Millipore), and then diluted 10% by adding one-tenth volume 99.9% D2O. For side chain and NOESY experiments not requiring detection of amide protons, an otherwise identical sample was exchanged into NMR buffer containing 99.9% D2O using a Microcon Centrifugal Filter device as above. Samples of wild-type SPOR domain proved to be stable for roughly 2 weeks at which point fresh samples needed to be prepared.
NMR Spectroscopy
All NMR spectra were collected at 25° C on a 4 channel Varian UnityInova NMR spectrometer operating at 600 MHz and equipped with a triple resonance PFG probe. Assignments were made using standard protein NMR methodology. Backbone resonances were assigned from HNCA, HN(CO)CA, HNCACB, CBCA(CO)NH, HNCO, and HN(CA)CO experiments. The side chain resonances were assigned using HCCH-TOCSY, C(CO)NH-TOCSY, H(CCO)NH-TOCSY, HBHA(CO)NH, and 3D-15N-TOCSY. Aromatic side chains were assigned using 2D NOESY, TOCSY, and COSY experiments and connected to the rest of the molecule using (HB)CB(CGCD)HD and (HB)CB(CGCDCE)HE. Distance restraints were obtained from 3D 13C-NOESY (140 and 200 ms) and 15N-NOESY (100 and 200 ms) experiments. RDCs were measured for the backbone amides using an IPAP-15N-HSQC experiment (30); for the oriented sample, alignment was obtained using 5% PEG(C12E5):hexanol bicelles (31) that gave a deuterium quadrupolar splitting of the HOD resonance of 25.2 Hz. Finally, backbone 15N T1, 15N T2, and {1H}-15N NOE relaxation parameters were measured (32). Mixing times were T1: 0, 22.1, 66.3, 132.6, 221.0(x2), 442.0, 773.4, 1215.4, and 1767.9 ms; T2: 12.9, 25.8, 51.6, 77.4(x2), 103.2, 129.0, 154.8, 206.4, and 232.2 ms; and NOE: 0 and 3.5 s. All spectra were processed using NMRPipe (33). RDC data and spectral overlays were analyzed using tools present in NMRPipe, relaxation rates were extracted using the program CurveFit (34), and backbone and NOESY assignments were made using CCPN's Analysis software (35). Order parameters were calculated and motional models for the SPOR domain residues were determined according to the model free formalism (36, 37) using the program Fast-Modelfree (38) to run the program Modelfree 4.20 (34, 39).
Structure Calculations
All structure calculations were carried out using the program XPLOR-NIH (40, 41). Visualization and analysis of structures, as well as generation of figures, was done using both PyMOL (42) and UCSF Chimera (43). Structure calculations began from an extended structure of the DamX construct. Initial structures were calculated using only those distance restraints that could be uniquely and unambiguously assigned, and the resulting structures were used to assign additional NOEs in an iterative manner. Dihedral angle restraints were obtained for backbone phi and psi torsion angles using the program TALOS+ (44) and assigned chemical shifts; flexible N-terminal residues as determined from relaxation analysis were excluded. Finally, RDC restraints were measured and implemented into later stage structure calculations using the TENSOR module in XPLOR-NIH (45); only those residues for which the RDCs could be unambiguously assigned, with minimal spectral overlap, and not in the flexible N-terminus were included. Force constants were ramped up during the structure calculations to the following final values: k(vdW) = 4.0, k(angles) = 1.0, k(impr) = 1.0, k(noe) = 20.0, k(dihedral) = 200.0, and k(RDC) = 1.0. A final ensemble of 250 structures was calculated, with the 25 lowest energy structures accepted. NMR assignments and final coordinates were submitted to BMRB and RCSB, respectively (BMRB accession number 17783, PDB ID 2LFV).
Peptidoglycan binding assays
Binding of His6-DamX SPOR domain to PG sacculi was assayed by co-sedimentation in an ultracentrifuge as described (4). Whole PG sacculi for this assay were isolated and quantified by amino sugar analysis as described (4). Routine PG binding assays used sacculi from wild type strain EC251 harvested during exponential growth; the cells in such cultures are in various stages of the division cycle.
Localization of GFP fusion proteins by fluorescence microscopy
Live cells in exponential growth were transferred to an agarose pad and visualized by fluorescence and phase contrast microscopy as described (4). For TTGFP-DamXSPOR constructs produced from plasmids, cultures grown overnight in LB medium with 200 μg/ml ampicillin, then diluted 1:100 in the same medium and grown for about 4 hrs at 30°C to reach an OD600 of 0.5. The experiments were also conducted at 21 °C for some strains. GFP-DamX proteins produced from the chromosome were analyzed similarly except overnight cultures were diluted 1:500, antibiotic was omitted, and IPTG was used to induce expression (at the concentration indicated in each experiment).
Generation of antibody against DamX and Western blotting
Polyclonal antiserum against the periplasmic domain of DamX was raised in New Zealand White Rabbits. The protein antigen was raised against His6-DamX (residues 122-428) overproduced in BL21 from pDSW1133 and purified by cobalt affinity chromatography. To reduce undesired cross-reactivity, the antiserum was cleaned up prior to use by pre-absorption against an extract from a ΔdamX strain, EC1926. The pre-absorbed antiserum was used at a dilution of 1:5000 for Western blotting, essentially as described (4).
Deoxycholate sensitivity
To assay for deoxycholate sensitivity, overnight cultures grown in LB were adjusted to an OD600 of 0.01 in LB0N medium and then five-fold serial dilutions were made and 3 μl aliquots were spotted onto an LB0N agar plate containing 0, 25, or 100 μM IPTG (isopropyl-β-D-thiogalactopyranoside) and 0.1% deoxycholate. Plates were incubated for 18 h at 30 °C, and photographed after keeping plates at 4°C overnight.
Cell division phenotypes of damX SPOR mutants in a damX dedD double mutant background
IPTG-inducible gfp-damX fusions (either wild-type or damX mutants) were integrated into the chromosome of EC1926 (dedD damX<>kan) in single copy at the Φ80 attachment site using spectinomycin-resistant CRIM vectors as described (4, 29). Strains were grown overnight at 30°C in LB medium containing spectinomycin at 25 μg/ml. In the morning, cultures were diluted 1:500 in LB without antibiotic but containing 0 to 2 mM IPTG. These cultures were grown for about 4 hrs at 30°C to an OD600 of 0.5, at which point samples were taken for Western blotting, phase contrast and fluorescence (GFP) microscopy. A subset of mutant proteins was also tested without the GFP tag. For these experiments damX variants were cloned into pDSW204, which allows for the IPTG-inducible expression of genes under control of a modified Trc promoter. Plasmids were transformed in EC1926. Transformants were grown and analyzed as for the chromosomal integrants, except that the medium always contained ampicillin at 200 μg/ml to maintain plasmids and cells were not photographed under fluorescence microscopy.
Results
Structure determination
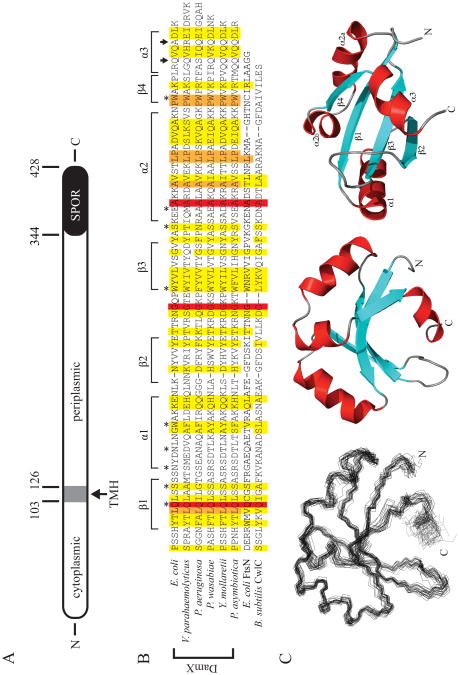
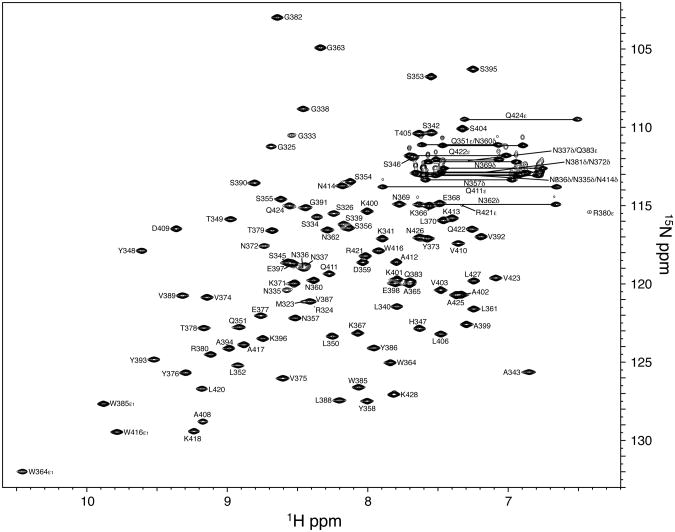
DamX is a bitopic membrane protein with a C-terminal SPOR domain (Figure 1A). To determine the structure of the SPOR domain, we cloned residues 338-428 into an expression vector that provided an N-terminal His6 purification tag. Isotopically labeled His6-SPOR domain was overproduced in E. coli and purified by cobalt-affinity chromatography. The purified protein was soluble at up to 80 mg/ml and bound PG (4). Backbone resonances were assigned using the standard technique of a backbone walk with no difficulties. An assigned 1H-15N HSQC spectrum is shown in Figure 2. Aside from His6-tag resonances, which could not be unambiguously assigned, the only unassigned amide resonance corresponds to asparagine 381, which lies in the short loop between the second and third β strands. Given the high quality of the data, one can only speculate that this resonance is 100% degenerate with another amide.
Figure 1.
The structure of DamX and its SPOR domain. (A) Cartoon of DamX from E. coli. DamX is predicted to comprise a 103 a.a. cytoplasmic domain, a 23 a.a. transmembrane helix (TMH), and a 302 a.a. periplasmic domain, of which the last 84 a.a. constitute the SPOR domain. (B) Sequence alignment of SPOR domains of several DamX's with the SPOR domains from CwlC and FtsN. The secondary structure elements determined for the SPOR domain of DamX from E. coli are shown above the alignment. Asterisks denote residues mutated in this study. Arrows indicate the site of C-terminal truncations. The alignment was obtained using CLUSTAL W (54) with the following sequences: E. coli DamX residues 344-428; Vibrio parahaemolyticus DamX residues 417-505; Pseudomonas aeruginosa DamX residues 464-551; Pectobacterium wasabaie WPP163 residues 251-336; Yersinia mollaretii DamX residues 249-333; Photorhabdus asymbiotica DamX residues 232-316; E. coli FtsN residues 244-319; and B. subtilis CwlC residues 182-255. (C) Solution structure of the SPOR domain (DamX residues 344-428). (Left) Side-on view of a backbone superposition of the 25 final structures. The average pairwise RSMD for residues is 0.795 Å. (Middle) Side-on and (Right) bottom-up views of a ribbon diagram of the domain, with β-strands in blue, α-helices in red, and loops in gray. Note that α2 is interrupted by a proline.
Figure 2.
Assigned 1H-15N HSQC spectrum of DamX SPOR domain. The spectrum was obtained with 0.7 mM DamXSPOR in 50 mM potassium phosphate buffer (pH 6.5), 50 mM KCl at 25°C. Backbone amide groups and nitrogen-containing sidechains (Asn/Gln NH2, Trp ε NH and aliased Arg ε NH) are labeled.
Complete but non-stereospecific side chain assignments (Supplemental Table S4) were made mostly from H(CCO)NH-, C(CO)NH-, and HCCH-TOCSY experiments. An initial set of NOEs was identified for which both protons could be unambiguously assigned and used to calculate an initial family of structures. This initial fold was used to assign further NOEs unambiguously, and the list of distance restraints was further refined during subsequent rounds of structure calculations in an iterative manner. The final ensemble of structures for the DamX SPOR domain was generated by taking the 25 lowest energy structures from an ensemble of 250 calculated structures. None of the structures had any experimental restraint violations in excess of 0.5 Å (NOEs), 5° (dihedral angles), or 2 Hz (RDCs). All statistics and discussion will focus on the actual SPOR domain (residues 344-428 in DamX); a summary of structural statistics can be found in Table 1. In all figures, the first 21 residues of the construct have been omitted because they are essentially unstructured (data not shown). The omitted amino acids comprise 10 from the His6-tag, 5 from a synthetic linker, and 6 DamX-derived residues that presumably precede the start of the SPOR domain per se.
Table 1. Structural statistics for the final ensemble of DamX (25 structures).
| Experimental restraints | |
| Short range NOES (|i-j| ≤ 1) | 898 |
| Medium range NOEs (1 < |i-j| < 5) | 253 |
| Long range NOEs (|i-j| ≥ 5) | 450 |
| Total unambiguous NOEs | 1,601 |
| NOEs with multiple assignments | 361 |
| Total NOEs | 1,962 |
| Distance restraints per residue | 23.3 |
| φ/ψ angles | 162 |
| backbone amide RDCs | 62 |
| Restraint violations | |
| NOE distances violated > 0.5 Å | 0 |
| dihedral angles violated > 5° | 0 |
| RDCs violated > 2 Hz | 0 |
| RMSDs from experimental restraints | |
| NOE (Å) | 0.0123 ± 0.0016 |
| Dihedrals (°) | 0.139 ± 0.040 |
| RDCs (Hz) | 0.149 ± 0.037 |
| XPLOR energies (kcal/mol) | |
| average overall energy | 139.61 ± 4.14 |
| RMSD from idealized geometry | |
| Bond lengths (Å) | 0.0022 ± 0.0001 |
| Bond angles (°) | 0.423 ± 0.005 |
| Impropers (°) | 0.243 ± 0.004 |
| Structure Z-scores | |
| Ramachandran plot quality | -3.4 |
| 1st generation packing quality | -1.6 |
| 2nd generation packing quality | 3.3 |
| χ1/χ2 rotamer normality | -4.9 |
| backbone conformation | -1.7 |
| Ramachandran plot statistics (%) | |
| Most favored regions | 70.4 |
| Additionally allowed regions | 27.9 |
| Generously allowed regions | 1.7 |
| Disallowed regions | 0.0 |
| Coordinate RMSD (residues 344-428, average difference to mean, Å) | |
| Backbone atoms | 0.795 |
| Heavy atoms | 1.296 |
All statistics are for the actual SPOR domain (residues 344-428) in all 25 structures except restraint violations, RMSDs, and energies are for all 106 residues in the construct. Structural statistics are taken from the output of XPLOR-NIH (40, 41) with the exception of the Ramachandran statistics which come from PROCHECK-NMR (46). The Ramachandran plot quality value is from the PSVS server (http://psvs-1_4-dev.nesg.org/); the remaining Z-scores were generated using the iCING server (http://nmr.cmbi.ru.nl/cing/iCing.html).
Figure 1C shows a backbone trace (Cα, CO, and N) through all 25 members of the final ensemble and a ribbon representation of the molecule in both a side on and bottom up orientation. The backbone RMSD for this ensemble is 0.795 Å, but improves to 0.663 Å if the final α helix (starting at residue 420) is excluded; this helix is the least well defined part of the SPOR domain. The structure also has good Ramachandran characteristics: as determined by PROCHECK-NMR (46), 98.3% of residues fall in the most favorable (70.4%) or allowed regions (27.9%). None of the amino acids analyzed (glycines and prolines are excluded) for the ensemble of 25 structures have phi/psi angles that fall in the disallowed region of Ramachandran space.
The DamX SPOR domain belongs to the RNP fold family
In terms of secondary structure, the domain contains four β strands and three α helices, which are arranged in a βαββαβα topology. The four β strands comprise a concave antiparallel β-sheet on one face of the molecule, arbitrarily shown as the bottom face in Figure 1C. Helices α1 and α2 pack along the top of the β-sheet; α2 is discontinuous owing to the presence of a proline residue (P407). The C-terminal helix, α3, is short and packs against the bottom of the β-sheet, partially occluding this face of the molecule.
Overall, the structure of DamX's SPOR domain looks like an RNP domain, a very common fold characterized by 2 α helices flanking a single four-stranded β sheet. RNP stands for ribonucleoprotein and refers to the fact that the first examples of this fold were RNA binding domains (19). This fold has since been found in many proteins and is no longer considered to be associated with a particular class of ligand. Yang et al. (2004) noted that the SPOR domain from FtsN adopts an RNP fold. A DALI search using the lowest energy structure for DamX's SPOR domain returned many statistically significant matches, including the SPOR domain from Cw1C (Z = 3.6; RSMD = 3.7 Å, PDB code 1×60-A), a PII-like domain from SA1388, a Staphylococcus aureus protein of unknown function (Z = 3.4, RMSD = 6.8 Å; PDB code 2nyd-B) and over a dozen eukaryotic RNA-binding proteins, such as Splicing Factor 45 (Z = 3.3, RMSD = 3.8 Å; PDB code 2peh-A). Interestingly, the SPOR domain from FtsN was not among the statistically significant matches.
Backbone dynamics
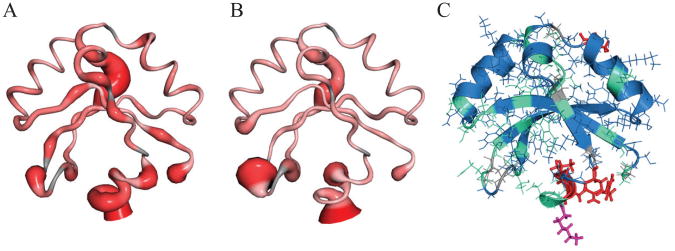
Measured NMR relaxation parameters were analyzed according to the model free formalism (36, 37) using the programs Fast-Modelfree and ModelFree 4.20 (34, 38, 39). Regions of the domain with high mobility are illustrated in Figure 3. An overall rotational correlation time, τm, of 6.98 ns suggests that the DamX SPOR domain tumbles in solution as a well-structured monomer. However, several areas of increased flexibility are indicated by reduced values for both order parameters (S2) and {1H}-15N NOE values. One is the α1/β2 loop at the top rear of the structure as depicted in Figure 3. Two other regions exhibiting increased motion are the β2/β3 loop at the lower left as well as α3 through the C-terminus at the bottom center of the figure.
Figure 3.
NMR relaxation dynamics of the DamX SPOR domain. (A,B) Sausage figures depicting 15N-{1H} NOE and S2 values, respectively. Increasing flexibility (smaller values) is indicated by both increasing width and deeper red. (C) Structure colored according to motional models as determined by the program ModelFree 4.2. Blue = model 1 (S2; global tumbling only), green = model 2 (S2 + fast internal motions), red = model 4 (S2 + fast internal motion + Rex/chemical exchange), and magenta = model 5 (S2 + both fast and slow internal motions). No residues are best described by model 3.
In addition to the general flexibility described by the order parameters and NOE values, the ModelFree formalism attempts to categorize the motions of each residue into various models based on their timescales. The majority of residues in the DamX SPOR domain fit into model 1 (global tumbling only, S2) or model 2 (S2 + fast internal motion). No amino acids in this protein are well fit by model 3 (S2 + chemical exchange, Rex). Several residues are more dynamic; T405, R421, V423, and Q424 are best described by model 4 (S2 + fast internal motion + Rex), and K428 is best described by model 5 (S2 + both fast and slower internal motions). The chemical exchange of T405 may simply arise from being located near the proline kink that breaks up helix 2. The chemical exchange of R421, V423, and Q424, as well as the more complex motions of K428 at the C-terminus, are consistent with enhanced overall motion of α3.
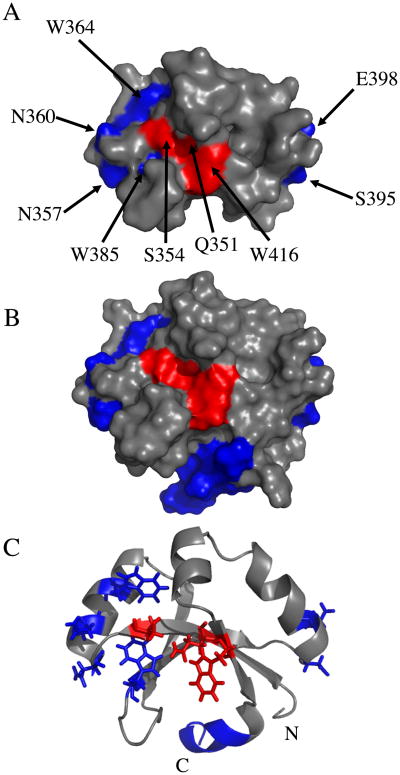
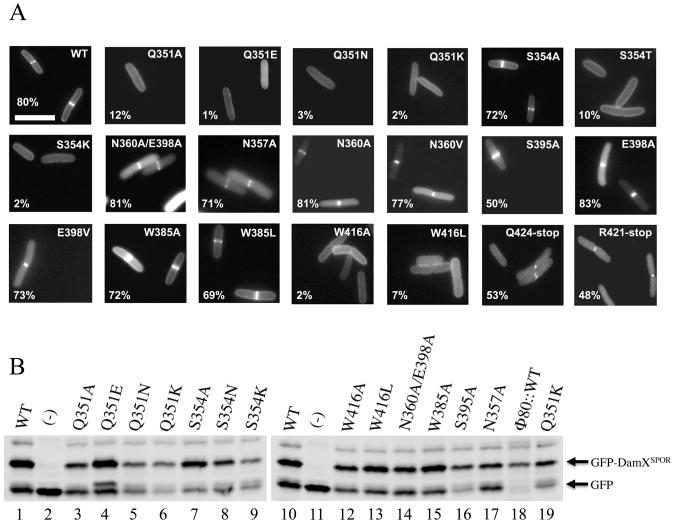
Identification of 3 amino acids important for septal localization
We targeted nine amino acids for mutagenesis (Figure 1B, Figure 4). Two of these, Gln 351 and Ser 354, were targeted because they are the most highly conserved surface-exposed residues as revealed by an alignment of 132 SPOR domains that was mapped onto the surface of the SPOR domains of CwlC and DamX using Consurf (Supplemental Figure S1) (47). Four amino acids were targeted because they correspond to residues suggested by Mishima et al. (15) to interact with PG in CwlC: Asn 357, Asn 360, Ser 395 and Glu 398. Finally, three tryptophans (residues 364, 385 and 416) were targeted because their side-chains exhibited a modest NMR chemical shift when DamX SPOR domain was incubated with muropeptides derived by digesting B. subtilis PG to completion with mutanolysin (Supplemental Figure S2). [Whether muropeptides are physiologically relevant ligands is not known. We used muropeptides from B. subtilis rather than E. coli because they were already on-hand from an unrelated experiment, the two organisms have a very similar muropeptide profile, and a B. subtilis SPOR domain has been shown to localize to the midcell when produced in E. coli(5, 48-50).]
Figure 4.
Amino acids targeted for mutagenesis. Residues found to be important for septal localization are red, while residues found to be unimportant in blue. (A) Space-filling model of the SPOR domain from DamX with the final helix, α3, omitted for clarity. (B) As in (A) but α3 is included. (C) Ribbon diagram of SPOR domain with targeted residues illustrated as stick models, except for the portion of α3 that was deleted.
The mutant SPOR domains were fused to Tat-targeted GFP (TTGFP) to direct their export to the periplasm via the Tat system and produced from plasmids in a wild-type E. coli background. Septal localization was assayed by fluorescence microscopy of live cells. The results are summarized in Figure 5A and Supplemental Table S5 (including exposure times, which varied because some fusion proteins were brighter than others). The wild-type TTGFP-DamX SPOR domain fusion protein appeared as a bright band of fluorescence at the mid-cell in about 80% of the cells, similar to our previous report (4). Comparable frequencies of septal localization were observed for proteins with substitutions at Asn 357, Asn 360, Ser 395 or Glu 398. These lesions should have impaired the previously proposed PG binding sites near the start of α1 and α2 (15). Even a double mutant protein with (N360A, E398A) with substitutions in both proposed sites localized as efficiently as wild-type. Essentially wild-type localization was also observed when Trp 385 was changed to either Ala or Leu. Multiple substitutions at Trp 364, whose side-chain is buried in the domain, resulted in the production of non-fluorescent GFP fusion proteins (not shown), so we assume this residue is important for folding.
Figure 5.
(A) Septal localization activities of several mutant SPOR domains. The images are fluorescence micrographs of live cells producing TTGFP-DamXSPOR proteins, either wild-type (WT) or with the indicated amino acid substitution or deletion. Numbers in the lower left of each image are the % of cells in the population scored as having septal localization. Size bar = 2 μm. (B) Western blot showing abundance of several TTGFP-DamXSPOR proteins, as indicated [(-) is a TTGFP vector control]. Proteins shown on both blots come from independent samples and illustrate day-to-day variation (e.g., Q351K).
Substitutions at Q351, S354 or W416—all of which are in the β-sheet—reduced septal localization to <10% of the cells in the population. The poorly-localizing proteins often appeared as fluorescent halos illuminating the outline of the cells, suggesting the proteins either diffuse freely in the periplasm or associate with PG in a relatively non-specific manner. An interesting exception was the S354A mutant protein, which localized about as well as wild-type. This finding is consistent with SPOR domain alignments showing that Ser and Ala occur with roughly equal frequency at this position (Figure 1B and Supplemental Figure S1). Apparently the size of the residue at position 354 is important but the hydroxyl moiety is not.
Evaluating expression and folding of the non-localizing SPOR domain mutants
Western blotting revealed that the mutant TTGFP-DamXSPOR proteins were not all produced at the same level (Figure 5B), consistent with the variations in photographic exposure times needed to visualize the various proteins in vivo (Supplemental Table S5). The different expression levels raised two related concerns about interpretation of the localization experiments. One is whether low abundance per se prevents detection of septal localization. The other is whether the mutant proteins have folding or stability defects that render the localization phenotypes meaningless.
Regarding the impact of TTGFP-DamXSPOR abundance on scoring of septal localization, the fact that we could detect the low-abundance mutants like Q351K as fluorescent halos argues we would have seen a band at the mid-cell had there been one. Moreover, some of the mutant proteins that localized poorly were produced at levels similar to wild-type (e.g., Q351E and W416L in lanes 4 and 13 of Figure 5B). Conversely, some mutant proteins localized well despite being of low abundance (e.g., compare the localizers S395A and N357A to the non-localizer Q351K in lanes 16-19 of Figure 5B). Finally, to make a direct assessment of how TTGFP-DamXSPOR abundance affects scoring of septal localization, we lowered production of the wild-type construct by integrating it into the chromosome in single copy at the Φ80 attachment site. When this strain was grown without IPTG, levels of the GFP fusion protein were as low as any of the localization-defective mutants (Figure 5B, lane 18). Nevertheless, we detected septal localization in ∼55% of the cells and elevating expression by inducing with IPTG had a more profound effect on the brightness of the septal bands than on their frequency (Supplemental Figure S3).
We also verified that the localization-defective mutant proteins were for the most part well folded by purifying them and collecting HSQC spectra. The spectra for the Q351A, Q351E, Q351N, S354T and W416A proteins were very similar to wild-type (Supplemental Figure S4). However, the HSQC spectrum of the Q351K mutant revealed some extra peaks, suggesting this protein interconverts between two or more conformations and is not as close to the native structure as the other mutants (Supplemental Figure S4C).
The C-terminal α-helix is dispensable for SPOR domain localization
The data presented so far argue the β-sheet of DamX is important for septal localization. The simplest interpretation is that the β-sheet binds septal PG, but a short C-terminal α-helix designated α3 appears to limit access to the β sheet (Figure 1C). SPOR domain alignments indicate α3 is conserved in DamX proteins but absent from CwlC and FtsN, suggesting it has an important function special to DamX (Figure 1B). We therefore constructed TTGFP fusions to two derivatives of the SPOR domain in which α3 was removed by truncating at R421 and Q424, respectively. Our prediction was that removing α3 would increase both septal localization and PG binding, but technical issues prevented us from making much headway with these issues. Cells producing TTGPF-DamXSPOR α3 deletion mutants were dim and Western blotting revealed the mutant proteins were produced at about one-third the level of WT (Supplemental Figure S5). Nevertheless, both truncated proteins localized to the septal ring in 50% of the cells in the population (Figure 5A and Supplemental Table S5). We purified the R421 deletion protein with the intent of assaying it for binding to PG, but HSQC spectra of the 15N-labeled protein revealed spectral collapse, indicative of loss of 2° structure (Supplemental Figure 4G). Regarding the apparent contradiction that the truncated protein localizes relatively well despite having a serious folding or stability defect, it must be noted that NMR is done using SPOR domain that has been out of the cell for at least 2 days.
PG binding assays
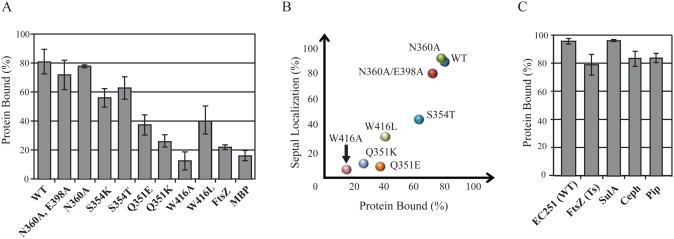
If septal localization is driven by binding of SPOR domains to septal PG, then the localization-defective mutants should bind poorly. Unfortunately, isolated septal PG is not available and the features of PG recognized by SPOR domains have yet to be defined. As a surrogate ligand, we used PG sacculi isolated from E. coli cells in exponential growth; sacculi from such cultures are predicted to contain septal PG since many of the cells are dividing at the time of harvest (but see below). In the binding assay, a mixture of SPOR domain protein and PG sacculi is subjected to centrifugation, which causes the sacculi to sediment. Samples of the pellet and supernatant are then analyzed by SDS-PAGE to determine how much SPOR domain protein is in each fraction. As a control, SPOR domains are also centrifuged in the absence of sacculi to verify that they do not simply sediment on their own. Previously, we reported that ∼80% of the DamX SPOR domain co-sedimented with PG, as compared to only ∼20% of two negative control proteins, the cytoplasmic GTPase FtsZ and the periplasmic maltose transport protein MBP (4).
We tested 6 localization-defective DamX SPOR mutants in the co-sedimentation assay: Q351E, Q351K, S354T, S354K, W416A and W416L. We also tested wild-type and two mutants that localized well, N360A and the double mutant N360A/E398A, which were chosen because they have lesions in the previously proposed PG binding sites in CwlC (15).
The results of the binding assay were for the most part consistent with expectations (Figure 6A). Wild-type and the two localization proficient mutant proteins bound PG well, whereas four of the localization-defective mutant proteins did not. But two mutant proteins behaved anomalously—S354T and S354K bound PG almost as well as wild-type despite exhibiting a profound localization defect. In thinking about potential explanations for the discrepancy, we realized binding was assayed at 4°C but localization was assayed using cells grown at 30°C. The subset of TTGFP-DamXSPOR constructs used in the PG-binding assay was therefore tested for localization in cells grown at 21°C. The S354K mutant protein was too dark to visualize, presumably owing to a folding defect at the lower temperature, and had to be excluded from the analysis. All of the other proteins localized better at 21°C. In most cases the improvement was small, but the S354T mutant protein localized much better. When the localization data from 21°C are used as the basis of comparison, there is a good correlation between localization and PG binding (Figure 6B).
Figure 6.
Peptidoglycan binding assay. (A) PG-binding activities of SPOR domains with the indicated amino acid substitutions as determined by the fraction of input protein that co-sedimented with isolated PG sacculi upon ultracentrifugation at 4°C. Bars indicate the average and standard deviation from at least three experiments. FtsZ and MBP (maltose binding protein) were assayed as negative controls. (B) Scatter plot showing correlation of septal localization assayed in live cells grown at 21°C with PG-binding activity data from part (A). (C) Binding of wild-type SPOR domain to different PG preparations. EC251(WT) refers to sacculi from wild-type cells harvested in exponential growth. FtsZ(Ts) and SulA refer to sacculi from filamentous cells in which division was blocked by interfering with FtsZ function. Ceph and Pip refer to sacculi from filamentous cells in which division was blocked by interfering with FtsI function using the β-lactams cephalexin or piperacillin.
We also investigated whether the pull-down assay faithfully reflects binding to septal PG, as reported previously for the periplasmic domain of FtsN (9). We isolated sacculi from filamentous cells obtained after blocking cell division in four ways: shifting an ftsZ(Ts) mutant to 42°C, inducing the FtsZ inhibitor sulA, or inactivating the septal PG synthase FtsI with cephalexin or piperacillin. In all cases the average cell length was >20 μm at the time of harvest. When the amount of PG from the various preparations was normalized according to N-acetylglucosamine content, the SPOR domain from DamX bound as well to “filamentous” sacculi as to sacculi from a culture that was dividing normally (Figure 6C). This result is clearly different from what was reported for FtsN and might mean that, at least for the SPOR domain from DamX, the co-sedimentation assay measures a general affinity of PG rather than site-specific binding (see Discussion).
The SPOR domain is required for proper function of damX in a variety of biological assays
The experiments described so far were done with isolated SPOR domains. But we were also interested in whether DamX's SPOR domain has much relevance for DamX function in vivo. This question may seem like a straw man, but three studies have addressed this issue in the case of FtsN, which unlike DamX is essential for cell division (51). All three studies concluded that the SPOR domain makes little or no contribution to the ability of FtsN to support cell division, although the two studies that looked specifically at septal localization agreed the SPOR domain is very important for this activity (5, 6, 9).
We constructed a set of IPTG-inducible gfp-damX fusions with deleterious amino acid substitutions in the SPOR domain: Q351K, S354K, or W416L. We also constructed two SPOR domain deletion mutants, which were truncated after S345 and T349, respectively. These residues are 2 and 5 amino acids into the SPOR domain according to the alignment shown in Figure 1B. As a positive control we used a gfp fusion to full-length wild-type damX and as a negative control we used gfp alone. The gfp fusion constructs were integrated into the chromosome at the Φ80 attachment site using CRIM vector technology (29). To assess septal localization and deoxycholate sensitivity we integrated the constructs into a damX null mutant. To assess the ability of these constructs to promote and inhibit cell division, we integrated them into a damX dedD double mutant. The double mutant background was employed because a simple damX null is not filamentous, but combining a damX null mutation with a dedD null mutation exacerbates the mild filamentation and chaining defect caused by deleting dedD(4, 5).
First we assayed production of the various fusions by Western blotting. When the wild-type gfp-damX fusion was induced with 10 μM IPTG, the amount of GFP-DamX in the cells was comparable to the amount of DamX normally present in E. coli grown under the same conditions (Supplemental Figure S6A). Inducing with 100 μM IPTG resulted in 4- to 8-fold overproduction, depending on the day (Supplemental Figure S6A). Finally, the wild-type GFP-DamX fusion was consistently about twice as abundant as the mutant fusion proteins when assayed in a damX mutant background, but these proteins were all produced at similar levels in the damX dedD double mutant background (Supplemental Figure S6B and C). Thus, the Q351K and S354K substitutions had little or no effect on expression of full-length gfp-damX fusions even though they significantly reduced abundance of the corresponding TTGFP-DamX SPOR constructs.
We then tested the relevance of the SPOR domain for DamX function in a damX mutant background. Deletion of the SPOR domain from DamX essentially eliminated septal localization, as did the Q351K substitution (Table 2). Interestingly, the S354K and W416L substitutions also impaired localization, but these lesions were not nearly as deleterious in the context of full-length DamX as in the context of the isolated SPOR domain. Results from the deoxycholate sensitivity test paralleled the localization results. Thus, the SPOR domain deletion constructs and the Q351K mutant protein did not confer any resistance, whereas the S354K and W416L mutant proteins conferred some resistance if they were overproduced (Table 2, Supplemental Figure S7).
Table 2. Characterization of DamX SPOR domain mutants (fused to GFP).
| Map of GFP-DamX constructsa | ΔdamX backgroundb | ΔdamXdedD backgrounde | |||
|---|---|---|---|---|---|
|
|
|
||||
| Septal Localization (%)c | DC resistanced | Rescue divisionf | Inhibit divisionf | ||
|
|
GFP-DamX(WT) | 71 ±4 | +++ | +++ | +++ |
|
|
GFP | 0 | - | - | - |
|
|
GFP-DamX(Q351K) | 8±1 | - | - | - |
|
|
GFP-DamX(S354K) | 26 ±6 | +/- | - | - |
|
|
GFP-DamX(W416L) | 40 ±7 | + | - | - |
|
|
GFP-DamX(A350-428) | 2±1 | - | - | - |
|
|
GFP-DamX(A346-428) | 2±1 | - | - | - |
For clarity, domains not drawn to scale. Cyto, cytoplasmic domain; TM, transmembrane helix; Linker, putative unstructured linker region; SPOR, SPOR domain.
The following derivatives of EC1910 were used: EC2882, EC2884, EC2886, EC2888, EC2965, EC2966, and EC2967.
The percentage of cells in the population judged to have a fluorescent band at the division site. Values are the average ± S.D. of 2-6 separate assays, with at least 100 cells scored each time.
Growth in the presence of 0.1 % deoxycholate. See supplemental figure S7 for the original data.
The following derivatives of EC1926 were used: EC2312, EC2313, EC2314, EC2315, EC2316, EC2785, and EC2786.
Cell length during growth in the presence of various concentrations of IPTG. See supplemental figure S8 for the data.
Next, we examined the ability of the gfp-damX constructs to improve or inhibit cell division in a damX dedD background. This proved to be much more difficult than we had anticipated. Two previous studies, including one from our lab, reported the double mutant had an average length of ∼15 ± 13 μm, while the dedD single mutant was ∼6 ± 8 μm (4, 5). These means are sufficiently different that determining whether introduction of a gfp-damX fusion reverts the double mutant to the phenotype of the single mutant seemed like it should be straightforward, but the relatively large standard deviations testify to a problem— populations of these mutants consist of many cells that are only slightly elongated and occasional cells that are much longer than normal. This makes estimates of the mean length very sensitive to sampling procedures. In the present study we were more careful to sample (photograph) cells at random and we report geometric means, which are better measures of central tendency than arithmetic means when lengths do not follow a normal distribution. Finally, we tested the effect of gfp-damX fusions at multiple IPTG concentrations because for the wild-type construct low levels rescue division but high levels inhibit division. Our findings are summarized in Table 2 and presented in more detail in Supplmental Figures S8 and S9.
The damX dedD double mutant had a length of ∼ 11 μm, which shortened to ∼ 8 μm when gfp-damX was induced with 10 μM IPTG and increased to ∼20 μm at 100 μM IPTG. None of the gfp-damX SPOR domain mutant constructs convincingly rescued or inhibited division at IPTG concentrations ranging from 10 μM to 2 mM, although close inspection of Supplemental Figure S8 suggests that perhaps S354K rescued slightly at high IPTG while W416L rescued slightly at moderate IPTG but then started to inhibit as IPTG was increased. Localization of the GFP-DamX SPOR domain constructs in the damX dedD double mutant paralleled results obtained in the damX single mutant. To wit, both deletion constructs and the Q351K protein exhibited little or no localization to potential division sites, the S354K protein localized sporadically, and the W416L protein localized moderately well (Supplemental Figure S9).
To address the possibility that the complementation defect was an artifact of including a GFP-tag in our constructs, we verified that DamX(Q351K) and DamXΔ(349-428) mutant proteins failed to rescue division when produced from plasmids without the tag (Supplemental Figure S10). We also verified expression of these constructs by Western blotting with anti-DamX antibody (Supplemental Figure S10).
Discussion
Comparison of SPOR domains from DamX, CwlC and FtsN
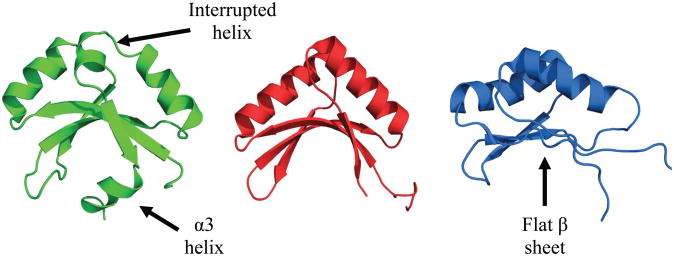
Figure 7 compares the solution structures reported for the SPOR domains from DamX, CwlC and FtsN. Note that all three SPOR domains adopt a similar fold despite sharing less than 20% amino acid identity in pairwise comparisons (Figure 1B). This fold is known as an RNP (ribonucleoprotein) fold, also called an RBD (ribonucleotide binding domain) or RRM (RNA recognition motif). The fold was originally identified as a single-stranded RNA binding motif, but some members of this structural superfamily bind to protein, DNA and, in the case of the SPOR domains, PG. RNP domains are characterized by a βαββαβ secondary structure wherein the four β strands comprise an antiparallel β-sheet buttressed on one side by the two α-helices.
Figure 7.
Comparison of the solution structures of the SPOR domains from DamX (green, this study), CwlC (red, PBB: 1X60) and FtsN (blue, PDB: 1UTA). Structures were rendered using PyMOL (42).
Despite the overall similarity among the three SPOR domain structures, there are a few noteworthy differences. First, in DamX α2 is interrupted by a proline, resulting in a sharp kink in that feature. While the proline is conserved in all DamX sequences, it is not present in CwlC or FtsN, so α2 is longer in those proteins and the loop connecting α2 with β4 is longer as well. Whether the kink has any biological relevance is not known. Second, DamX and CwlC have a concave β-sheet that forms a cleft, whereas the β-sheet of FtsN is rather flat. These differences are intriguing because our working hypothesis is that the β-sheet is the PG binding site. If this is true, we would expect the β-sheet has to adopt a very similar overall conformation in all three domains when they are bound to PG. Perhaps the different conformations represent “closed” and “open” states sampled by all three domains. The third major difference is the extra helix, designated α3, located at the C-terminus of DamX. This difference is also interesting because, assuming PG binds to the β-sheet, α3 might have to move out of the way and could regulate binding.
The β-sheet is important for septal localization and possibly for PG binding too
The β-sheet is the primary ligand binding surface in many members of the RNP fold superfamily (17, 19-22, 52, 53). Consistent with this theme, we found that mutations at Q351, S354 and W416— all of which are in the β-sheet and have solvent-exposed side-chains—impair septal localization and PG binding (Figures 5 and 7). Moreover, Q351 and S354 correspond to the most highly conserved surface-exposed residues in SPOR domains (Supplemental Figure S1). But the binding results must be considered tentative because our assay used PG sacculi rather than isolated “septal PG,” by which we mean the still undefined form of PG to which SPOR domains are thought to bind.
Until now, it has been thought that the co-sedimentation assay using purified PG sacculi reflected specific binding of the SPOR domain to septal PG. This inference stems from an earlier report showing the periplasmic domain from FtsN bound better to sacculi from dividing cells than to sacculi from cells that were not dividing (9). We performed an analogous experiment using the SPOR domain from DamX but observed equivalent binding when input PG was matched by N-acetylglucosamine content (Figure 6C). We do not know whether the discrepancy between the two reports reflects a real difference between the proteins assayed or a technical problem. It is worth noting, however, that the experimental design requires accurately matching the amount of PG used in each trial, which is challenging because PG is insoluble and difficult to aliquot reproducibly. Regardless of the origin of the discrepancy, it is clearly a high priority to develop better binding assays that use chemically-defined, physiologically relevant PG fragments.
The indifference of DamX's SPOR domain to whether the sacculi were obtained from dividing or filamentous cells raises questions about what the SPOR domain is actually binding to in the co-sedimentation assay. One possibility is that septal PG is lost from the sacculi during the purification procedure, which involves harsh processes like boiling in SDS. If septal PG is lost, then the assay might reflect a general propensity of SPOR domains to bind bulk PG. Non-localizing mutant proteins might bind poorly because the β-sheet is used for both site-specific and non-specific binding to PG. This idea is analogous to many site-specific DNA binding proteins, which typically have a general affinity for DNA and a higher affinity for a specific DNA site. Another possibility is that the SPOR domain is binding septal PG in the co-sedimentation assay, but this structure is not unique to the septum. For example, amidases probably contribute to PG turnover during elongation, so glycan strands that lack stem peptides could be found in both the septum and the cell cylinder. If these glycan strands are not firmly anchored to the sacculus, the ones that remain after sacculi have been purified might be relatively uniformly distributed. We considered the possibility that SPOR domains might be excluded from the cell cylinder by Lpp and thus accumulate at the midcell where there is a lot of new PG that has yet to acquire Lpp. Because one of the steps in the procedure used to purify sacculi involves treatment with a protease to remove Lpp, the purification process might alter the distribution of SPOR domain binding sites. However, this does not appear to be a viable hypothesis because SPOR domains localize sharply to the midcell in an Lpp null mutant (A. Yahashiri and D. Weiss, unpublished).
An unexpected finding was that amino acid substitutions at Q351, S354 and W416 had a more dramatic effect on septal localization of isolated SPOR domains than on septal localization of full-length DamX proteins. We can envision several potential explanations for this discrepancy. First, parts of DamX outside of the SPOR domain might interact with other septal ring proteins and thus contribute directly to septal localization. In support of this idea, DamX interacts with several septal ring proteins in a bacterial two-hybrid system (4). But we did not detect septal localization of DamX when the SPOR domain was deleted, so any targeting domains other than the SPOR domain would have to be rather weak. Second, whereas TTGFP-DamXSPOR proteins are free to diffuse throughout the periplasm, in the context of the complete DamX protein the SPOR domain is tethered to the inner membrane and may thus be maintained in relatively high local concentration with respect to the PG layer. Finally, NMR and dynamic light scattering indicate the isolated DamX SPOR domain is monomeric (not shown), but DamX interacts with itself in a bacterial two-hybrid system, suggesting the full length protein is a dimer, which could have a much higher affinity for PG and might better tolerate mutations that reduce affinity.
α3 is not required for septal localization but whether it regulates localization needs further study
If the β-sheet of the SPOR domain binds PG, or any other ligand for that matter, α3 probably has to move out of the way. Consistent with this possibility, NMR relaxation data revealed α3 is one of the most dynamic parts of the DamX SPOR domain. We therefore predicted that removing α3 would improve both septal localization and binding to PG. Unfortunately, the deletion proteins we constructed to test this notion were not very stable, so the only solid conclusion that can be drawn at this time is that α3 is not required for septal localization. Efforts to circumvent the folding issues by substituting individual amino acids in α3 also resulted in unstable proteins (A. Yahashiri and D. Weiss, unpublished). We therefore doubt that genetic approaches have much potential to resolve the question of whether α3 moves to allow ligand binding. It will probably be more fruitful to solve the structure of the SPOR domain in complex with a PG fragment.
DamX requires an intact SPOR domain for proper function in vivo
The fact that so many bacterial proteins contain SPOR domains argues for their importance. Here we showed that DamX requires an intact SPOR domain to localize to the septal ring, similar to previous reports for FtsN (5, 9). Thus, the SPOR domain of DamX is not only sufficient for septal localization (4, 5), it is also necessary, and there are no other potent septal targeting domains in the protein. Although FtsN proteins that lack the SPOR domain still support reasonably efficient cell division (5, 6, 9), this is not true of DamX, which requires an intact SPOR domain for every activity we assayed—to correct the division defect of a damX dedD double mutant, to confer full resistance to deoxycholate and to inhibit cell division when DamX is overproduced. Even the W416L mutant protein was defective in these functions despite the fact that this lesion impaired septal localization only modestly. Our finding that DamX must have an intact SPOR domain to inhibit division complements previous reports that overproduction of isolated SPOR domains impairs division (4, 5). Perhaps too much SPOR domain displaces FtsN or blocks the access of other proteins to septal PG. It will be interesting to explore whether the SPOR domain from DamX can be functionally replaced with SPOR domains from unrelated proteins, as this experiment should give some insight into whether the SPOR domain is important only because it delivers DamX to the septal ring or whether it has a more sophisticated function.
Supplementary Material
Acknowledgments
We thank Tammi Duncan, Matthew Jorgenson, Linda McCarter and Tim Yahr for helpful discussions. We are grateful to David Bentley, Matthew Jorgenson, Chun-Sing Huang and Jenny Merryfield for technical assistance.
Funding statement: This research was supported by National Institutes of Health Grant GM083975 to D.S.W. The DNA facility is supported through the Holden Comprehensive Cancer Center's cancer center support grant 2 P30 CA086862 from the National Cancer Institute/NIH and the Carver College of Medicine, The University of Iowa.
Footnotes
Abbreviations: PG, peptidoglycan; RNP, ribonucleoprotein; TTGFP, Tat-targeted green fluorescent protein; CRIM plasmids, conditional-replication, integration and modular plasmids; Δ, deletion; NOE, nuclear Overhauser effect, NOESY, NOE spectroscopy; TOCSY, total correlation spectroscopy; HSQC, heteronuclear single-quantum correlation spectroscopy, rms, root-mean square.
Supporting Information Available: Supplemental materials may be accessed free of charge online at http://pubs.acs.org. The following is available: a description of strain and plasmid construction, a strain table, a plasmid table, a primer table, a table of assigned chemical shifts, a table quantifying localization of mutant SPOR domains, a plot of surface conservation on the SPOR domains from CwlC and DamX, an alignment of 132 SPOR domains, a plot of chemical shifts induced by incubating PG fragments with DamX SPOR domain, a figure documenting the effect of TTGFP-DamX(SPOR) abundance on scoring of septal localization, comparisons of the HSQC spectra of wild-type and mutant DamX SPOR domains, a Western blot documenting expression of DamX SPOR domains with deletions of α3, Western blots documenting expression of gfp-damX fusions used in this study, photographs of plates used to score growth in the presence of deoxycholate, bar graph depicting effect of GFP-DamX proteins on cell division, phase contrast and fluorescence images documenting localization and division phenotypes associated with these GFP-DamX proteins, and a figure documenting the effect of DamX proteins (without a GFP tag) on cell division.
References
- 1.de Boer PA. Advances in understanding E. coli cell fission. Curr Opin Microbiol. 2010;13:730–737. doi: 10.1016/j.mib.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutkenhaus J, Pichoff S, Du S. Bacterial cytokinesis: From Z ring to divisome. Cytoskeleton (Hoboken) 2012;69:778–790. doi: 10.1002/cm.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Typas A, Banzhaf M, Gross CA, Vollmer W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol. 2012;10:123–136. doi: 10.1038/nrmicro2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arends SJ, Williams K, Scott RJ, Rolong S, Popham DL, Weiss DS. Discovery and characterization of three new Escherichia coli septal ring proteins that contain a SPOR domain: DamX, DedD, and RlpA. J Bacteriol. 2010;192:242–255. doi: 10.1128/JB.01244-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerding MA, Liu B, Bendezu FO, Hale CA, Bernhardt TG, de Boer PA. Self-enhanced accumulation of FtsN at division sites and roles for other proteins with a SPOR domain (DamX, DedD, and RlpA) in Escherichia coli cell constriction. J Bacteriol. 2009;191:7383–7401. doi: 10.1128/JB.00811-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moll A, Thanbichler M. FtsN-like proteins are conserved components of the cell division machinery in proteobacteria. Mol Microbiol. 2009;72:1037–1053. doi: 10.1111/j.1365-2958.2009.06706.x. [DOI] [PubMed] [Google Scholar]
- 7.Finn RD, Mistry J, Tate J, Coggill P, Heger A, Pollington JE, Gavin OL, Gunasekaran P, Ceric G, Forslund K, Holm L, Sonnhammer EL, Eddy SR, Bateman A. The Pfam protein families database. Nucleic Acids Res. 2010;38:D211–222. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith TJ, Foster SJ. Characterization of the involvement of two compensatory autolysins in mother cell lysis during sporulation of Bacillus subtilis 168. J Bacteriol. 1995;177:3855–3862. doi: 10.1128/jb.177.13.3855-3862.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ursinus A, van den Ent F, Brechtel S, de Pedro M, Holtje JV, Lowe J, Vollmer W. Murein (peptidoglycan) binding property of the essential cell division protein FtsN from Escherichia coli. J Bacteriol. 2004;186:6728–6737. doi: 10.1128/JB.186.20.6728-6737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lupoli TJ, Taniguchi T, Wang TS, Perlstein DL, Walker S, Kahne DE. Studying a cell division amidase using defined peptidoglycan substrates. J Am Chem Soc. 2009;131:18230–18231. doi: 10.1021/ja908916z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Pedro MA, Quintela JC, Holtje JV, Schwarz H. Murein segregation in Escherichia coli. J Bacteriol. 1997;179:2823–2834. doi: 10.1128/jb.179.9.2823-2834.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishidate K, Ursinus A, Holtje JV, Rothfield L. Analysis of the length distribution of murein glycan strands in ftsZ and ftsI mutants of E. coli. FEMS Microbiol Lett. 1998;168:71–75. doi: 10.1111/j.1574-6968.1998.tb13257.x. [DOI] [PubMed] [Google Scholar]
- 13.Obermann W, Holtje JV. Alterations of murein structure and of penicillin-binding proteins in minicells from Escherichia coli. Microbiology. 1994;140(Pt 1):79–87. doi: 10.1099/13500872-140-1-79. [DOI] [PubMed] [Google Scholar]
- 14.Romeis T, Kohlrausch U, Burgdorf K, Holtje JV. Murein chemistry of cell division in Escherichia coli. Res Microbiol. 1991;142:325–332. doi: 10.1016/0923-2508(91)90048-f. [DOI] [PubMed] [Google Scholar]
- 15.Mishima M, Shida T, Yabuki K, Kato K, Sekiguchi J, Kojima C. Solution structure of the peptidoglycan binding domain of Bacillus subtilis cell wall lytic enzyme CwlC: characterization of the sporulation-related repeats by NMR. Biochemistry. 2005;44:10153–10163. doi: 10.1021/bi050624n. [DOI] [PubMed] [Google Scholar]
- 16.Yang JC, Van Den Ent F, Neuhaus D, Brevier J, Lowe J. Solution structure and domain architecture of the divisome protein FtsN. Mol Microbiol. 2004;52:651–660. doi: 10.1111/j.1365-2958.2004.03991.x. [DOI] [PubMed] [Google Scholar]
- 17.Allain FH, Howe PW, Neuhaus D, Varani G. Structural basis of the RNA-binding specificity of human U1A protein. EMBO J. 1997;16:5764–5772. doi: 10.1093/emboj/16.18.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birney E, Kumar S, Krainer AR. Analysis of the RNA-recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res. 1993;21:5803–5816. doi: 10.1093/nar/21.25.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagai K, Oubridge C, Jessen TH, Li J, Evans PR. Crystal structure of the RNA-binding domain of the U1 small nuclear ribonucleoprotein A. Nature. 1990;348:515–520. doi: 10.1038/348515a0. [DOI] [PubMed] [Google Scholar]
- 20.Mosyak L, Zhang Y, Glasfeld E, Haney S, Stahl M, Seehra J, Somers WS. The bacterial cell-division protein ZipA and its interaction with an FtsZ fragment revealed by X-ray crystallography. EMBO J. 2000;19:3179–3191. doi: 10.1093/emboj/19.13.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fribourg S, Gatfield D, Izaurralde E, Conti E. A novel mode of RBD-protein recognition in the Y14-Mago complex. Nat Struct Biol. 2003;10:433–439. doi: 10.1038/nsb926. [DOI] [PubMed] [Google Scholar]
- 22.Kadlec J, Izaurralde E, Cusack S. The structural basis for the interaction between nonsense-mediated mRNA decay factors UPF2 and UPF3. Nat Struct Mol Biol. 2004;11:330–337. doi: 10.1038/nsmb741. [DOI] [PubMed] [Google Scholar]
- 23.Varani G, Nagai K. RNA recognition by RNP proteins during RNA processing. Annu Rev Biophys Biomol Struct. 1998;27:407–445. doi: 10.1146/annurev.biophys.27.1.407. [DOI] [PubMed] [Google Scholar]
- 24.Lyngstadaas A, Lobner-Olesen A, Boye E. Characterization of three genes in the dam-containing operon of Escherichia coli. Mol Gen Genet. 1995;247:546–554. doi: 10.1007/BF00290345. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Garrido J, Cheng N, Garcia-Quintanilla F, Garcia-del Portillo F, Casadesus J. Identification of the Salmonella enterica damX gene product, an inner membrane protein involved in bile resistance. J Bacteriol. 2010;192:893–895. doi: 10.1128/JB.01220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leclerc GJ, Tartera C, Metcalf ES. Environmental regulation of Salmonella typhi invasion-defective mutants. Infect Immun. 1998;66:682–691. doi: 10.1128/iai.66.2.682-691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz C, Levy SB. Many chromosomal genes modulate MarA-mediated multidrug resistance in Escherichia coli. Antimicrob Agents Chemother. 2010;54:2125–2134. doi: 10.1128/AAC.01420-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarry M, Arends SJ, Roversi P, Piette E, Sargent F, Berks BC, Weiss DS, Lea SM. The Escherichia coli cell division protein and model Tat substrate SufI (FtsP) localizes to the septal ring and has a multicopper oxidase-like structure. J Mol Biol. 2009;386:504–519. doi: 10.1016/j.jmb.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haldimann A, Wanner BL. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J Bacteriol. 2001;183:6384–6393. doi: 10.1128/JB.183.21.6384-6393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ottiger M, Delaglio F, Bax A. Measurement of J and dipolar couplings from simplified two-dimensional NMR spectra. J Magn Reson. 1998;131:373–378. doi: 10.1006/jmre.1998.1361. [DOI] [PubMed] [Google Scholar]
- 31.Ruckert M, Otting G. Alignment of biological macromolecules in novel nonionic liquid crystalline media for NMR experiments. J Am Chem Soc. 2000;122:7793–7797. [Google Scholar]
- 32.Farrow NA, Muhandiram R, Singer AU, Pascal SM, Kay CM, Gish G, Shoelson SE, Pawson T, Formankay JD, Kay LE. Backbone dynamics of a free and a phosphopeptide-complexed Src homology-2 domain studied by 15N NMR relaxation. Biochemistry. 1994;33:5984–6003. doi: 10.1021/bi00185a040. [DOI] [PubMed] [Google Scholar]
- 33.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 34.Palmer AG, Rance M, Wright PE. Intramolecular motions of a zinc finger DNA-binding domain from Xfin characterized by proton-detected natural abundance 13C heteronuclear NMR spectroscopy. J Am Chem Soc. 1991;113:4371–4380. [Google Scholar]
- 35.Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas P, Ulrich EL, Markley JL, Ionides J, Laue ED. The CCPN data model for NMR spectroscopy: Development of a software pipeline. Proteins. 2005;59:687–696. doi: 10.1002/prot.20449. [DOI] [PubMed] [Google Scholar]
- 36.Lipari G, Szabo A. Model-free approach to the interpretation of nuclear magnetic-resonance relaxation in macromolecules .1. Theory and range of validity. J Am Chem Soc. 1982;104:4546–4559. [Google Scholar]
- 37.Lipari G, Szabo A. Model-free approach to the interpretation of nuclear magnetic-resonance relaxation in macromolecules .2. Analysis of experimental results. J Am Chem Soc. 1982;104:4559–4570. [Google Scholar]
- 38.Loria JP, Cole R. FAST-Modelfree: a program for rapid automated analysis of solution NMR spin-relaxation data. Journal of Biomolecular NMR. 2003;26:203–213. doi: 10.1023/a:1023808801134. [DOI] [PubMed] [Google Scholar]
- 39.Mandel AM, Akke M, Palmer AG., 3rd Backbone dynamics of Escherichia coli ribonuclease HI: correlations with structure and function in an active enzyme. Journal of Molecular Biology. 1995;246:144–163. doi: 10.1006/jmbi.1994.0073. [DOI] [PubMed] [Google Scholar]
- 40.Schwieters CD, Kuszewski JJ, Clore GM. Using Xplor-NIH for NMR molecular structure determination. Prog Nucl Magn Reson Spectr. 2006;48:47–62. doi: 10.1016/s1090-7807(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 41.Schwieters CD, Kuszewski JJ, Tjandra N, Clore GM. The Xplor-NIH NMR molecular structure determination package. J Magn Reson. 2003;160:65–73. doi: 10.1016/s1090-7807(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 42.DeLano WL. The PyMOL Molecular Graphics System. 1.1. DeLano Scientific; San Carlos, CA: 2008. [Google Scholar]
- 43.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera - A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 44.Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS plus: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR. 2009;44:213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sass HJ, Musco G, Stahl SJ, Wingfield PT, Grzesiek S. An easy way to include weak alignment constraints into NMR structure calculations. J Biomol NMR. 2001;21:275–280. doi: 10.1023/a:1012998006281. [DOI] [PubMed] [Google Scholar]
- 46.Laskowski RA, Rullmann JAC, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 47.Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010;38:W529–533. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schleifer KH, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972;36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glauner B, Holtje JV, Schwarz U. The composition of the murein of Escherichia coli. J Biol Chem. 1988;263:10088–10095. [PubMed] [Google Scholar]
- 50.Atrih A, Bacher G, Allmaier G, Williamson MP, Foster SJ. Analysis of peptidoglycan structure from vegetative cells of Bacillus subtilis 168 and role of PBP 5 in peptidoglycan maturation. J Bacteriol. 1999;181:3956–3966. doi: 10.1128/jb.181.13.3956-3966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dai K, Xu Y, Lutkenhaus J. Cloning and characterization of ftsN, an essential cell division gene in Escherichia coli isolated as a multicopy suppressor of ftsA12(Ts) J Bacteriol. 1993;175:3790–3797. doi: 10.1128/jb.175.12.3790-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oubridge C, Ito N, Evans PR, Teo CH, Nagai K. Crystal structure at 1.92 A resolution of the RNA-binding domain of the U1A spliceosomal protein complexed with an RNA hairpin. Nature. 1994;372:432–438. doi: 10.1038/372432a0. [DOI] [PubMed] [Google Scholar]
- 53.Varani L, Gunderson SI, Mattaj IW, Kay LE, Neuhaus D, Varani G. The NMR structure of the 38 kDa U1A protein - PIE RNA complex reveals the basis of cooperativity in regulation of polyadenylation by human U1A protein. Nat Struct Biol. 2000;7:329–335. doi: 10.1038/74101. [DOI] [PubMed] [Google Scholar]
- 54.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.