Abstract
Background
Children with cerebral palsy (CP) are prone to secondary complications related to physical inactivity and poor cardiorespiratory capacity. This problem could be greatly attenuated through the use of video games that incorporate physical activity for 2 reasons: Video games already represent an important component of leisure time in younger people, and such games can lead to a high level of exercise intensity in people who are healthy.
Objective
The study objective was to evaluate exercise intensity in children with spastic diplegic CP and children who were typically developing while playing with an active video game console.
Design
This was a cross-sectional study.
Methods
Ten children (7–12 years old) with spastic diplegic CP (Gross Motor Function Classification System level I or II) and 10 children who were age matched and typically developing were evaluated in a movement analysis laboratory. Four games were played with the active video game console (jogging, bicycling, snowboarding, and skiing) for 40 minutes. Heart rate was recorded during the entire playing period with a heart rate belt monitor. Exercise intensity was defined as the percentage of heart rate reserve (HRR). In addition, lower extremity motion analysis was carried out during the final minute of the playing period for the jogging and bicycling games.
Results
No difference between groups was observed for any variables. A main effect of games was observed for the amount of time spent at an intensity greater than 40% of HRR. Specifically, more than 50% of the playing time for the jogging game and more than 30% of the playing time for the bicycling game were spent at an intensity greater than 40% of HRR. In addition, the jogging game produced a larger range of motion than the bicycling game.
Limitations
A limitation of this study was the relatively small and heterogeneous sample.
Conclusions
For all 4 games, similar exercise intensity levels were observed for children who were typically developing and children with CP, suggesting that children with CP could obtain exercise-related benefits similar to those obtained by children without CP while playing with an active video game console.
In children with cerebral palsy (CP), reduced levels of physical activity increase the occurrence of secondary conditions (eg, poor bone density, fatigue, and chronic pain) as they age and can affect functional mobility and gait.1 In this population, the intensity of daily activities is usually too low to significantly improve physical fitness.2 The American Physical Therapy Association has emphasized the importance of identifying and promoting accessible physical exercise for children with CP with the goals of reversing deconditioning secondary to impaired mobility and optimizing motor functions. However, various financial and societal barriers, such as a lack of equipment, a lack of availability of exercise instructors, and a lack of access to adapted transportation, greatly limit accessible physical activity for children with disabilities.3 In this context, physical activity performed at home and independently by children with CP may be a suitable and pragmatic approach.
Video games represent an important part of leisure time in younger people.4 In the last decade, new types of consoles, namely, active video game consoles (AVGCs), have provided an opportunity to transform what has traditionally been sedentary screen time into a period of physical activity. Active video game consoles are based on virtual reality concepts and involve interactive physical activity.5 One commercially available AVGC, the Nintendo Wii (Nintendo, Redmond, Washington), allows people to interact with a virtual environment and play a variety of sports games through the use of a handheld motion sensor (remote control) or an instrumented platform (Wii Fit). The player approximately reproduces movements similar to those performed in real life. For example, the player can control the direction of a virtual skier by changing the distribution of weight between his feet. The Wii can provide many task repetitions, real-time feedback, a safe environment, and a high level of motivation, which are among the key factors for successful rehabilitation.6 This inexpensive and commercially available technology has generated tremendous interest among physical therapists worldwide.
The intensity of exercise while playing with an AVGC is a key factor in determining its relevance in the context of a physical training or rehabilitation program. Exercise intensity corresponds to the degree of difficulty or effort associated with an exercise and can be classified as mild, moderate, or vigorous.7 The intensity and duration of a given exercise influence the activity-based energy expenditure, that is, the energetic cost required to perform the exercise. According to the American College of Sports Medicine (ACSM), exercise at moderate intensity (between 40% and 70% of heart rate reserve [HRR]) is needed to ensure the maintenance or improvement of cardiorespiratory fitness in people with chronic disease and disabilities,8 whereas an exercise intensity greater than 50% of HRR is needed in people who are healthy.7
Several studies have shown that using AVGCs can lead to positive changes in the aerobic capacities of various asymptomatic and symptomatic populations,9–12 including adults with CP.13 Some studies have shown that in children who are typically developing, playing with AVGCs results in an intensity exceeding the minimal exercise requirement for improving aerobic capacity in this population.10,12 Other studies have shown that exercise intensity is insufficient to meet the recommended requirement.14,15 The types of games chosen could explain some of the discrepancies among these studies. Miyachi et al16 evaluated exercise intensity levels in 12 adults who were healthy while playing 68 games and reported intensity levels equivalent to or greater than those of moderate exercise for 22 of the 68 games. Most of those 22 games (17/22) involved lower limb or full body movements.16 In a recent review, Biddiss and Irwin17 proposed that games involving lower body or full body movements lead to higher energy expenditure than games soliciting mostly upper limb movements.
The fact that movements in lower limbs are affected in children with spastic diplegic CP18 could limit the benefits obtained by using the Wii to improve their physical fitness. One recent study of children with hemiplegic CP showed that a moderate level of exercise intensity could be achieved with Wii games soliciting mostly upper limb movements.19 However, to our knowledge, exercise intensity levels in children with spastic diplegic CP while playing with the Wii have never been evaluated. The primary goal of this study was to compare exercise intensity levels in children with spastic diplegic CP and children who were typically developing while playing Wii games mainly soliciting lower limb movements. The secondary goal was to explore whether motor limitations associated with spastic diplegic CP (spasticity, limited range of motion, and lower strength) influenced exercise intensity levels.
Method
Participants
Ten children who were 7 to 12 years old (4 boys, 6 girls; mean age=9.1 years, SD=2.02) and had spastic diplegic CP (Gross Motor Function Classification System [GMFCS] level I or II) were compared with 10 children who were age matched (7–12 years old; 5 boys, 5 girls; mean age=9.4 years, SD=1.78) and typically developing (without CP). Inclusion criteria for children with CP were the ability to follow simple verbal instructions, the ability to maintain a standing position without support for at least 10 minutes, and normal or corrected-to-normal vision. Exclusion criteria were the inability to provide parental consent or participant assent, surgical procedures or botulinum toxin type A (Botox, Allergan Inc, Irvine, California) injection in the preceding 3 months, and other known neurological problems (eg, epilepsy). All parents and participants provided written informed consent or assent.
Measurements
Before the experiment, several measurements were collected, in the following order: resting heart rate, range of motion, spasticity, and maximal strength. The participants were then asked to complete various Wii tests that provide information to players on how to use the Wii Fit and the remote control. For calibration of the Wii Fit according to the weight of the participants, the participants were asked to perform a balance test. Finally, the participants played 4 games (skiing, jogging, snowboarding, and bicycling) for 10 minutes each in a random order with a 5-minute rest period between games. Each game was played for 10 minutes to obtain a valid measure of the heart rate response and to avoid excessive fatigue (for a similar procedure, see Worley et al20 and Lannigham-Foster et al21). The jogging game had to be restarted a maximum of 4 times (eg, at the end of a level), but restarting could be done very quickly (<5 seconds). The games were chosen because they involve mostly lower limbs movements. Preliminary testing (unpublished data) had shown that the jogging and bicycling games led to moderate to vigorous levels of exercise intensity in participants who were healthy.
For the jogging game, a player followed a virtual guide by stepping in place with the remote control in his or her pocket. During the bicycling game, a player controlled the direction and speed of the bicycle by tilting the remote control and by stepping in place on the Wii Fit platform. The jogging and bicycling games were performed at a self-selected speed with a relatively steady effort and therefore did not require short, intense bursts of effort. Games requiring lower exercise intensity (skiing and snowboarding) were chosen to maintain participants' motivation by allowing them to alternate between demanding games and less demanding games. These games required a player to produce an anteroposterior (snowboarding game) or mediolateral (skiing game) weight transfer to control the displacement of an onscreen avatar.
The selected games were chosen because they were easy to understand, appeared to be appropriate for children with CP, and were associated with a high level of motivation. The order of presentation of the games was stratified and randomized so that games expected to generate a high exercise intensity (bicycling and jogging) were alternated with games expected to produce a low exercise intensity (skiing and snowboarding). Before each game, the participants received standardized instructions on how to play, and the experimenter made sure that the task was well understood.
Exercise intensity level was the primary outcome measure and was defined as the percentage of HRR (HRR = maximum heart rate − resting heart rate). The HRR has been used in other studies of children with CP, notably to monitor exercise intensity during an intervention.22,23 Resting heart rate was evaluated after 10 minutes in a lying position with a heart rate belt monitor (Polar RS400; Polar, Kempele, Finland) and was defined as the minimum value recorded by the monitor. This monitor samples the heart rate every 5 seconds through a chest belt and transmits the data to a watch. Maximum heart rate was first calculated with the following formula: 208 − (years of age × 0.7).24,25 This formula has been shown to be valid for children and adolescents.25 Maximal heart rate also was estimated in children with CP using the value of 194 in accordance with the recommendation of Verschuren et al.26 However, no significant difference between the formulas was observed. Therefore, the formula 208 − (years of age × 0.7) was used for both groups.
Heart rate was recorded during the entire playing period for all games. Heart rate measures have been used to determine the intensity of exercise in children and adults playing AVGCs.10,12,15 Heart rate monitor devices do not restrict movements,27 are less intimidating for children than indirect calorimetry, provide precise values, are field-based measures that are more readily available and easier for clinicians to use in the context of a rehabilitation program, and have been extensively validated in other studies (for a review, see Achten and Jeukendrup28).
For each 10-minute game period, the percentage of time spent at an intensity greater than 40% of HRR (ie, moderate to vigorous intensity or greater than 3 metabolic equivalents [1 MET=3.5 mL O2·kg−1·min−1]) was determined (for a similar procedure, see Koopman et al29). This threshold was chosen in accordance with ACSM's suggestion that physical activity needs to be performed at an intensity greater than 40% of HRR to provide significant benefits to the cardiorespiratory system in people with motor limitations or disabilities.8,29,30
Secondary measures were collected to control for the possible influence of motor limitations on exercise intensity levels. Quadriceps muscle spasticity was evaluated with the Modified Ashworth Scale at the knee articulation (flexion and extension).31,32 The Modified Ashworth Scale is a 6-point scale (0, 1, 1+, 2, 3, and 4); lower values are associated with a lower level of spasticity. Spasticity can affect exercise intensity levels by restraining movement, causing compensatory movements, or both.33 The passive flexion and extension range of motion of the hip, knee, and ankle articulations was measured with a goniometer. Limitations in joint range of motion can lead to smaller movements and, therefore, reduce exercise intensity levels.34
The maximal flexion and extension isometric strength of the flexor and extensor muscles at the hip, knee, and ankle joints was measured with a handheld dynamometer (Lafayette Instrument Co, Lafayette, Indiana).35 A reduction in strength is associated with an increase in intensity for various exercises, such as walking.36 Handheld dynamometers have been validated for measuring maximal isometric strength in children with CP.37 In the present study, the examiner held the device rigidly in place while the participant was encouraged (with standardized verbal encouragement) to push “as hard as possible” for 4 seconds. Once familiar with the task, each participant performed 3 maximal exertions for each muscle with at least a 30-second rest period between exertions. Peak force was recorded with the dynamometer, and the 2 highest values were retained. The values then were averaged and normalized with respect to body weight and lower limb length (N·m/kg).38 Values obtained from the right and left sides were combined into 1 measure for each participant.
A kinematic analysis with an 8-camera motion capture system recording at 60 Hz (Vicon 512, Oxford Metrics, Oxford, United Kingdom) also was performed during the jogging and bicycling games. These 2 games were expected to be associated with higher exercise intensity levels than the skiing and snowboarding games. They also required lifting the feet from the ground. The analysis was performed to evaluate whether larger ranges of motion would be associated with higher exercise intensity levels. Sixteen reflective markers were placed on the lower limbs at the following anatomic landmarks: anterior superior iliac spines, posterior superior iliac spines, lateral aspect of the knee joints, lateral malleoli, heels, second metatarsals, and lateral aspects of the thigh and calf segments. The last 30 seconds of each game were recorded (for a similar procedure, see Berry et al39). Because of their potential impact on exercise intensity levels,34 the following parameters were measured: hip flexion, hip extension, knee flexion, knee extension, ankle dorsiflexion, and ankle plantar flexion.
Finally, after each game, participants completed the Borg Scale to quantify the perceived exertion on a scale from 6 (no exertion) to 20 (maximal exertion).10 For facilitating the interpretation of each level of the Borg Scale, standardized pictograms representing each level of perceived effort were shown. The Borg Scale has been shown to have good validity and reliability for children who are healthy,40 and the measure was used in a previous study with the Wii.10 The Borg Scale also was used to evaluate perceived exertion in children with CP.41,42 The participants also reported their degree of interest in each game on a numeric scale from 1 (not motivated) to 10 (very motivated).
Data Analysis
The normality of the distributions was determined with the Kolmogorov-Smirnov test. To examine differences between groups and games, we submitted the main outcome measure to a 2 (groups) × 4 (games) analysis of variance with repeated measurements on the last factor. Secondary measures were submitted independently to a 2 (groups) × 2 (games: jogging and bicycling) analysis of variance with repeated measurements on the last factor. Effect size was calculated by dividing the difference of the means for the outcome variables by the pooled standard deviations and was interpreted in accordance with Cohen guidelines: 0.20 as small, 0.50 as moderate, and 0.80 as large.43 To determine the variables predicting exercise intensity, we implemented a linear regression model with a forward stepwise model selection procedure. This supplementary analysis was used to predict exercise intensity with the secondary measures as independent variables. The variables were entered into the model if the F probability was inferior to .05 and were removed from the model if the F probability was superior to .1. All statistical analyses were performed with SPSS (version 17.0, SPSS Inc, Chicago, Illinois).
Role of the Funding Source
Mr Robert was supported by a Fonds de Recherche du Québec-Santé (FRQS) master's training award, and Dr Ballaz was supported by CIHR-MENTOR postdoctoral training fellowship.
Results
The characteristics of the participants are shown in Table 1. There was no significant difference between groups for age, height, weight, sex, or body mass index (P>.05). Dorsiflexion strength and plantar-flexion strength were lower in children with CP than in children without CP (P≤.05).
Table 1.
Characteristics of Participantsa
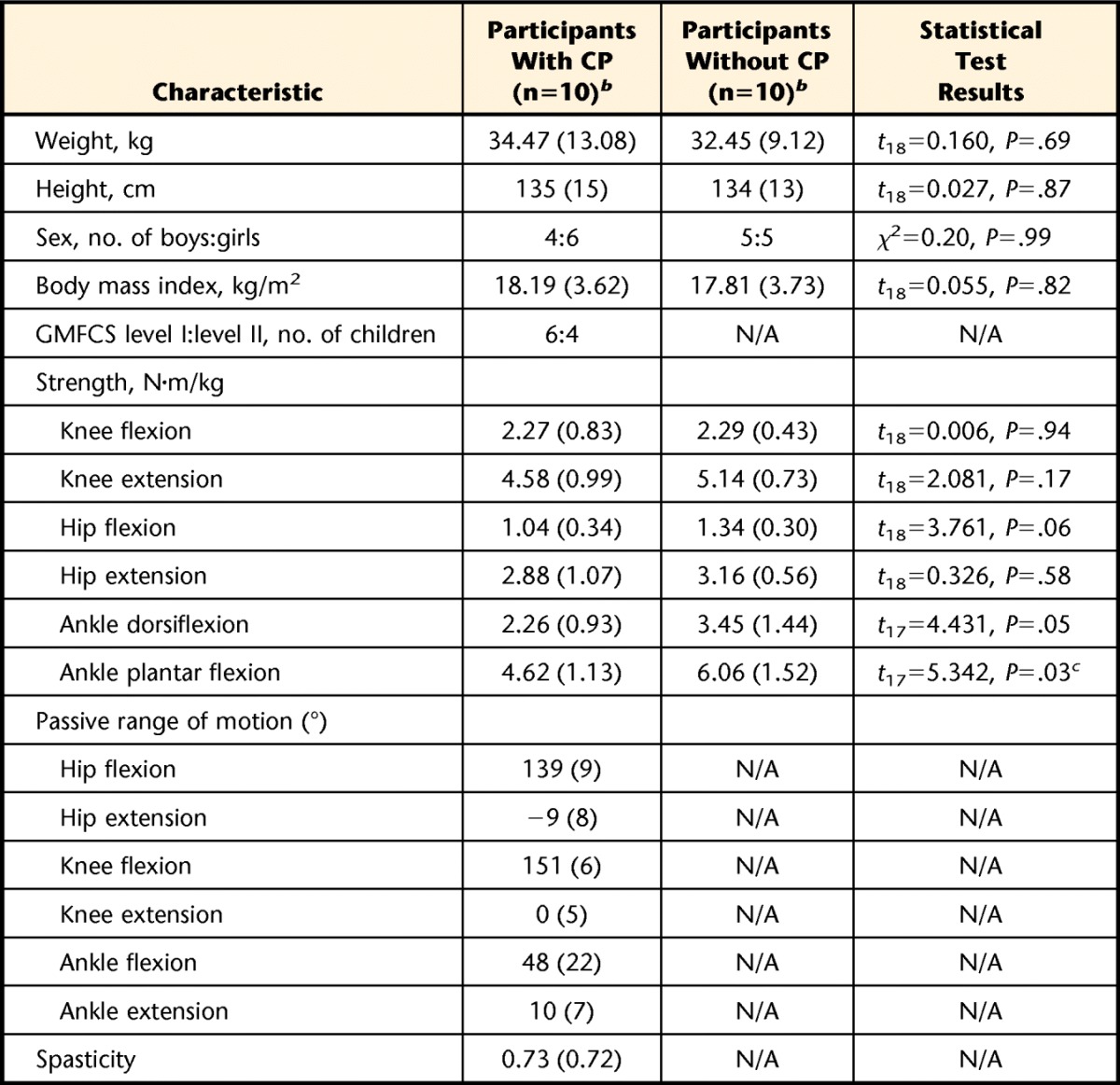
CP=cerebral palsy, GMFCS=Gross Motor Function Classification System, N/A=not applicable.
b Data are reported as mean (standard deviation) unless indicated otherwise.
c Significant difference between the groups.
Resting heart rate in children with CP was between 58 and 93 bpm, with an average of 74 bpm (SD=10). Resting heart rate in children without CP was between 53 and 81 bpm, with an average of 68 bpm (SD=7). Working heart rate in children with CP was between 133 and 199 bpm, with an average of 168 bpm (SD=23). Working heart rate in children without CP was between 119 and 197 bpm, with an average of 158 bpm (SD=30). No significant difference was observed between groups for resting heart rate and working heart rate (P>.05).
No significant difference was observed between groups for the percentage of time spent at an intensity greater than 40% of HRR (P>.05) (Figure). However, a main effect of games was observed for the percentage of time spent at an intensity greater than 40% of HRR (F=16.538; df=1,18; P=.001; effect size=0.970). Participants spent more time at an intensity greater than 40% of the HRR in the jogging game than in any of the other games. In addition, the bicycling game was significantly more demanding than the snowboarding game (Figure).
Figure.
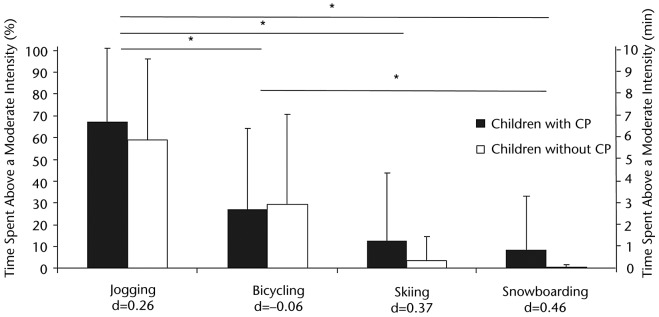
Comparison of the relative time (%) (left axis) and actual time (minutes) (right axis) spent at an intensity greater than 40% of heart rate reserve for groups and games. Effect sizes (d) are shown for each game. CP=cerebral palsy. Asterisk indicates P<.05.
The analysis of variance revealed no significant difference between groups (P>.05) for the secondary measures obtained during game play. The range of motion for lower limb articulation was larger in the jogging game than in the bicycling game (P<0.05) (Tab. 2). The children's degree of interest in the different games did not vary (P>.05); however, the perceived exertion, as measured with the Borg Scale, tended to differ among the games (P=.058) (Tab. 2). Concerning the linear regression analysis, no variable was entered in the model because the conditions were not met. Therefore, exercise intensity could not be predicted by any of the secondary measures.
Table 2.
Measures During Game Playa
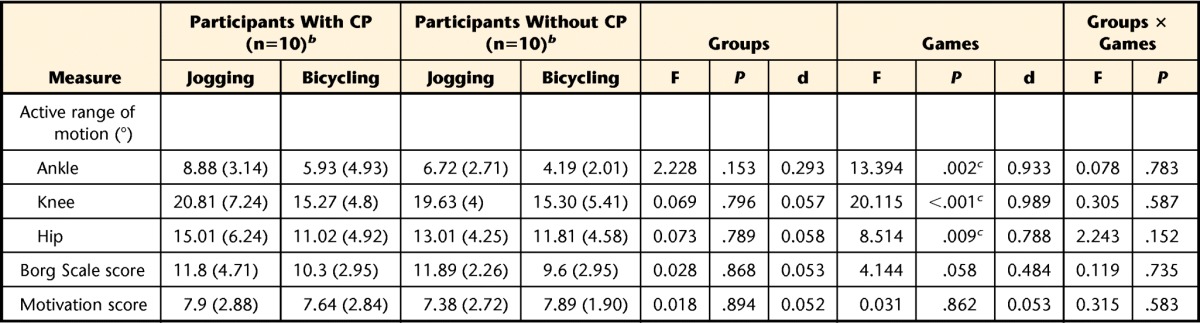
CP=cerebral palsy, F=F statistic, d=effect size.
b Data are reported as mean (standard deviation).
c Values were significantly different (P<.05).
Discussion
Previous studies showed that exercise intensity levels while playing the Wii can be sufficiently high in children who are typically developing to benefit the cardiorespiratory system, especially if the lower limbs are involved.44 However, it was not known whether children with CP could similarly benefit from this system. The present study showed similar exercise intensity levels in children with CP and children without CP for all tested games. This finding suggests that AVGC systems such as the Wii could be used as an adjunct therapeutic tool to increase the amount of physical activity in children with CP, at least for children who are ambulatory without devices.
The present study also showed that children with CP played in a fashion similar to that of their counterparts who were healthy, as shown by their similar ranges of motion for lower limb articulation. All other secondary measures were similar between the groups, with the exception of the level of strength at the ankle, which was lower in children with CP. Mockford and Caulton45 also showed that children with CP had a lower level of ankle strength. This reduction in strength, however, did not affect exercise intensity levels. It should be noted that only children who scored at level I or II on the GMFCS participated in the present study. These children had minor motor dysfunctions that did not seem to interfere with exercise intensity.
The total relative amounts of time spent above a moderate level of exercise intensity (above 40% of HRR) differed greatly between the 2 games expected to produce a high level of exercise intensity (jogging and bicycling). Accordingly, the perception of exertion, as measured with the Borg Scale, was significantly higher for jogging than for bicycling. This difference could be explained by the observation of a greater range of motion in lower limb articulation for the jogging game than for the bicycling game, confirming previous observations that larger movements elicit greater exercise intensities.46 Another explanation could be related to the different levels of complexity of the games. The bicycling game involved dual tasks (moving the legs while operating the remote control). According to Baranowski et al,47 a more complex game could reduce exercise intensity levels in children.
Despite the fact that the jogging game was much more demanding than the bicycling game, the levels of interest in the games were similar. This finding demonstrates that a game can be both strenuous and motivating at the same time; these factors are important in successful rehabilitation48 and for participation in physical activity.12,49,50
The ACSM recommends that children who are healthy should participate in a minimum of 60 minutes of moderate to intense physical activity daily. To meet that recommendation, children would have to play the equivalent of 95 minutes of the jogging game or 210 minutes of the bicycling game. It is clearly unrealistic to expect children to achieve the recommendations of the ACSM solely by using the Wii. It should be noted, however, that boys and girls already spend averages of 59 and 23 minutes, respectively, playing passive video games each day (for a review, see Marshall et al4). This sedentary playing time could be converted to active playing time and complemented with other physical activities. Because children with CP often have poor cardiorespiratory capacities, a smaller amount of physical activity is required to observe a positive adaptation in their cardiorespiratory systems.51
A limitation of the present study was the relatively small and heterogeneous sample; the results must be confirmed with a larger sample. However, the results confirmed the findings of previous studies showing that playing the Wii can result in adequate exercise intensity in children without CP.10,12 More importantly, the results clearly showed that playing the Wii Fit jogging and bicycling games increased exercise intensity as much as moderate to vigorous exercises in children with CP (at GMFCS level I or II).
Conclusion
The regular use of the Wii bicycling and jogging games could increase the amounts of physical activity in children with CP. This system can be considered a low-cost, safe, readily available, and efficient tool that can be used at home to improve the health of children with motor limitations such as CP. With proper supervision, this tool also could complement the effort of clinicians to increase daily physical activity levels in their patients. Further studies should examine the effects of long-term AVGC training in children with CP. It also would be interesting to evaluate the benefits of using the Wii for children with CP at GMFCS level III or higher.
The Bottom Line
What do we already know about this topic?
In the past few years, several studies have shown that commercially available active video game consoles (AVGCs) can improve the fitness of children who are typically developing. Despite the fact that AVGCs such as the Wii are currently used in several rehabilitation centers, very few studies to date have evaluated exercise intensity in children with spastic diplegic cerebral palsy (CP) during game play.
What new information does this study offer?
This study showed that exercise intensity while playing Wii games was similar between children with and without CP.
If you're a patient, what might these findings mean for you?
Active video game consoles are an affordable, safe, and playful approach to improve aerobic capacity in children with CP.
Footnotes
Mr Robert, Dr Ballaz, and Dr Lemay provided concept/idea/research design. All authors provided writing and data collection and analysis. Dr Lemay provided project management and fund procurement. Dr Ballaz and Mr Hart provided consultation (including review of manuscript before submission). The authors thank the children who participated in this study, their parents, and the Programme des Déficits Moteurs Cérébraux du Centre de Réadaptation Marie Enfant for their collaboration. The authors report no conflict of interest, and they alone are responsible for the content and writing of the article.
The study was approved by the Ethics Committee of the Sainte-Justine University Hospital Research Center.
Mr Robert was supported by a Fonds de Recherche du Québec-Santé (FRQS) master's training award, and Dr Ballaz was supported by CIHR-MENTOR postdoctoral training fellowship.
References
- 1. Rose J, Gamble JG, Burgos A, et al. Energy expenditure index of walking for normal children and for children with cerebral palsy. Dev Med Child Neurol. 1990;32:333–340 [DOI] [PubMed] [Google Scholar]
- 2. van den Berg-Emons HJ, Saris WH, de Barbanson DC, et al. Daily physical activity of schoolchildren with spastic diplegia and of healthy control subjects. J Pediatr. 1995;127:578–584 [DOI] [PubMed] [Google Scholar]
- 3. Fowler EG, Kolobe TH, Damiano DL, et al. Promotion of physical fitness and prevention of secondary conditions for children with cerebral palsy: Section on Pediatrics research summit proceedings. Phys Ther. 2007;87:1495–1510 [DOI] [PubMed] [Google Scholar]
- 4. Marshall SJ, Gorely T, Biddle SJ. A descriptive epidemiology of screen-based media use in youth: a review and critique. J Adolesc. 2006;29:333–349 [DOI] [PubMed] [Google Scholar]
- 5. LaViola JJ., Jr Bringing VR and spatial 3D interaction to the masses through video games. IEEE Comput Graph Appl. 2008;28:10–15 [DOI] [PubMed] [Google Scholar]
- 6. Snider L, Majnemer A. Virtual reality: we are virtually there. Phys Occup Ther Pediatr. 2010;30:1–3 [DOI] [PubMed] [Google Scholar]
- 7. American College of Sports Medicine American College of Sports Medicine position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–991 [DOI] [PubMed] [Google Scholar]
- 8. Dirienzo LN, Dirienzo LT, Baceski DA. Heart rate response to therapeutic riding in children with cerebral palsy: an exploratory study. Pediatr Phys Ther. 2007;19:160–165 [DOI] [PubMed] [Google Scholar]
- 9. Graves LE, Ridgers ND, Williams K, et al. The physiological cost and enjoyment of Wii Fit in adolescents, young adults, and older adults. J Phys Act Health. 2010;7:393–401 [DOI] [PubMed] [Google Scholar]
- 10. Graf DL, Pratt LV, Hester CN, Short KR. Playing active video games increases energy expenditure in children. Pediatrics. 2009;124:534–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deutsch JE, Borbely M, Filler J, et al. Use of a low-cost, commercially available gaming console (Wii) for rehabilitation of an adolescent with cerebral palsy. Phys Ther. 2008;88:1196–1207 [DOI] [PubMed] [Google Scholar]
- 12. Penko AL, Barkley JE. Motivation and physiologic responses of playing a physically interactive video game relative to a sedentary alternative in children. Ann Behav Med. 2010;39:162–169 [DOI] [PubMed] [Google Scholar]
- 13. Hurkmans HL, van den Berg-Emons RJ, Stam HJ. Energy expenditure in adults with cerebral palsy playing Wii Sports. Arch Phys Med Rehabil. 2010;91:1577–1581 [DOI] [PubMed] [Google Scholar]
- 14. Graves L, Stratton G, Ridgers ND, Cable NT. Comparison of energy expenditure in adolescents when playing new generation and sedentary computer games: cross sectional study. BMJ. 2007;335:1282–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White K, Schofield G, Kilding AE. Energy expended by boys playing active video games. J Sci Med Sport. 2011;14:130–134 [DOI] [PubMed] [Google Scholar]
- 16. Miyachi M, Yamamoto K, Ohkawara K, Tanaka S. METs in adults while playing active video games: a metabolic chamber study. Med Sci Sports Exerc. 2010;42:1149–1153 [DOI] [PubMed] [Google Scholar]
- 17. Biddiss E, Irwin J. Active video games to promote physical activity in children and youth: a systematic review. Arch Pediatr Adolesc Med. 2010;164:664–672 [DOI] [PubMed] [Google Scholar]
- 18. Shortland A. Muscle deficits in cerebral palsy and early loss of mobility: can we learn something from our elders? Dev Med Child Neurol. 2009;51(suppl 4):59–63 [DOI] [PubMed] [Google Scholar]
- 19. Howcroft J, Klejman S, Fehlings D, et al. Active video game play in children with cerebral palsy: potential for physical activity promotion and rehabilitation therapies. Arch Phys Med Rehabil. 2012;93:1448–1456 [DOI] [PubMed] [Google Scholar]
- 20. Worley JR, Rogers SN, Kraemer RR. Metabolic responses to Wii Fit video games at different game levels. J Strength Cond Res. 2011;25:689–693 [DOI] [PubMed] [Google Scholar]
- 21. Lanningham-Foster L, Foster RC, McCrady SK, et al. Activity-promoting video games and increased energy expenditure. J Pediatr. 2009;154:819–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ballaz L, Plamondon S, Lemay M. Ankle range of motion is key to gait efficiency in adolescents with cerebral palsy. Clin Biomech (Bristol, Avon). 2010;25:944–948 [DOI] [PubMed] [Google Scholar]
- 23. Retarekar R, Fragala-Pinkham MA, Townsend EL. Effects of aquatic aerobic exercise for a child with cerebral palsy: single-subject design. Pediatr Phys Ther. 2009;21:336–344 [DOI] [PubMed] [Google Scholar]
- 24. Mahon AD, Marjerrison AD, Lee JD, et al. Evaluating the prediction of maximal heart rate in children and adolescents. Res Q Exerc Sport. 2010;81:466–471 [DOI] [PubMed] [Google Scholar]
- 25. Machado FA, Denadai BS. Validity of maximum heart rate prediction equations for children and adolescents. Arq Bras Cardiol. 2011;97:136–140 [DOI] [PubMed] [Google Scholar]
- 26. Verschuren O, Maltais DB, Takken T. The 220−age equation does not predict maximum heart rate in children and adolescents. Dev Med Child Neurol. 2011;53:861–864 [DOI] [PubMed] [Google Scholar]
- 27. van den Berg-Emons RJ, Saris WH, Westerterp KR, van Baak MA. Heart rate monitoring to assess energy expenditure in children with reduced physical activity. Med Sci Sports Exerc. 1996;28:496–501 [DOI] [PubMed] [Google Scholar]
- 28. Achten J, Jeukendrup AE. Heart rate monitoring: applications and limitations. Sports Med. 2003;33:517–538 [DOI] [PubMed] [Google Scholar]
- 29. Koopman AD, Eken MM, van Bezeij T, et al. Does clinical rehabilitation impose sufficient cardiorespiratory strain to improve aerobic fitness? J Rehabil Med. 2013;45:92–98 [DOI] [PubMed] [Google Scholar]
- 30. Strong WB, Malina RM, Blimkie CJ, et al. Evidence based physical activity for school-age youth. J Pediatr. 2005;146:732–737 [DOI] [PubMed] [Google Scholar]
- 31. Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207 [DOI] [PubMed] [Google Scholar]
- 32. Mutlu A, Livanelioglu A, Gunel MK. Reliability of Ashworth and Modified Ashworth scales in children with spastic cerebral palsy. BMC Musculoskelet Disord. 2008;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Balaban B, Tok F, Tan AK, Matthews DJ. Botulinum toxin a treatment in children with cerebral palsy: its effects on walking and energy expenditure. Am J Phys Med Rehabil. 2012;91:53–64 [DOI] [PubMed] [Google Scholar]
- 34. Waters RL, Mulroy S. The energy expenditure of normal and pathologic gait. Gait Posture. 1999;9:207–231 [DOI] [PubMed] [Google Scholar]
- 35. Gajdosik RL, Bohannon RW. Clinical measurement of range of motion: review of goniometry emphasizing reliability and validity. Phys Ther. 1987;67:1867–1872 [DOI] [PubMed] [Google Scholar]
- 36. Goh HT, Thompson M, Huang WB, Schafer S. Relationships among measures of knee musculoskeletal impairments, gross motor function, and walking efficiency in children with cerebral palsy. Pediatr Phys Ther. 2006;18:253–261 [DOI] [PubMed] [Google Scholar]
- 37. Taylor NF, Dodd KJ, Graham HK. Test-retest reliability of hand-held dynamometric strength testing in young people with cerebral palsy. Arch Phys Med Rehabil. 2004;85:77–80 [DOI] [PubMed] [Google Scholar]
- 38. Damiano DL, Abel MF. Functional outcomes of strength training in spastic cerebral palsy. Arch Phys Med Rehabil. 1998;79:119–125 [DOI] [PubMed] [Google Scholar]
- 39. Berry T, Howcroft J, Klejman S, et al. Variations in movement patterns during active video game play in children with cerebral palsy. J Bioeng Biomed Sci. 2011;Sci S1:001 [Google Scholar]
- 40. Leung ML, Chung PK, Leung RW. An assessment of the validity and reliability of two perceived exertion rating scales among Hong Kong children. Percept Mot Skills. 2002;95:1047–1062 [DOI] [PubMed] [Google Scholar]
- 41. McNevin NH, Coraci L, Schafer J. Gait in adolescent cerebral palsy: the effect of partial unweighting. Arch Phys Med Rehabil. 2000;81:525–528 [DOI] [PubMed] [Google Scholar]
- 42. Maltais D, Wilk B, Unnithan V, Bar-Or O. Responses of children with cerebral palsy to treadmill walking exercise in the heat. Med Sci Sports Exerc. 2004;36:1674–1681 [DOI] [PubMed] [Google Scholar]
- 43. Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 2nd ed NJ: Upper Saddle River: Prentice Hall; 2000 [Google Scholar]
- 44. Foley L, Maddison R. Use of active video games to increase physical activity in children: a (virtual) reality? Pediatr Exerc Sci. 2010;22:7–20 [DOI] [PubMed] [Google Scholar]
- 45. Mockford M, Caulton JM. Systematic review of progressive strength training in children and adolescents with cerebral palsy who are ambulatory. Pediatr Phys Ther. 2008;20:318–333 [DOI] [PubMed] [Google Scholar]
- 46. Desloovere K, Molenaers G, Feys H, et al. Do dynamic and static clinical measurements correlate with gait analysis parameters in children with cerebral palsy? Gait Posture. 2006;24:302–313 [DOI] [PubMed] [Google Scholar]
- 47. Baranowski T, Abdelsamad D, Baranowski J, et al. Impact of an active video game on healthy children's physical activity. Pediatrics. 2012;129:e636–e642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Snider L, Majnemer A, Darsaklis V. Virtual reality as a therapeutic modality for children with cerebral palsy. Dev Neurorehabil. 2010;13:120–128 [DOI] [PubMed] [Google Scholar]
- 49. Roemmich JN, Barkley JE, Lobarinas CL, et al. Association of liking and reinforcing value with children's physical activity. Physiol Behav. 2008;93:1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. DiLorenzo TM, Stucky-Ropp RC, Vander Wal JS, Gotham HJ. Determinants of exercise among children, II: a longitudinal analysis. Prev Med. 1998;27:470–477 [DOI] [PubMed] [Google Scholar]
- 51. Abel MF, Damiano DL. Strategies for increasing walking speed in diplegic cerebral palsy. J Pediatr Orthop. 1996;16:753–758 [DOI] [PubMed] [Google Scholar]


