Abstract
Background
Klebsiella pneumoniae is one of the major pathogens causing hospital-acquired multidrug-resistant infections. The capsular polysaccharide (CPS) is an important virulence factor of K. pneumoniae. With 78 capsular types discovered thus far, an association between capsular type and the pathogenicity of K. pneumoniae has been observed.
Methodology/Principal Findings
To investigate an initially non-typeable K. pneumoniae UTI isolate NTUH-K1790N, the cps gene region was sequenced. By NTUH-K1790N cps-PCR genotyping, serotyping and determination using a newly isolated capsular type-specific bacteriophage, we found that NTUH-K1790N and three other isolates Ca0507, Ca0421 and C1975 possessed a new capsular type, which we named KN2. Analysis of a KN2 CPS− mutant confirmed the role of capsule as the target recognized by the antiserum and the phage. A newly described lytic phage specific for KN2 K. pneumoniae, named 0507-KN2-1, was isolated and characterized using transmission electron microscopy. Whole-genome sequencing of 0507-KN2-1 revealed a 159 991 bp double-stranded DNA genome with a G+C content of 46.7% and at least 154 open reading frames. Based on its morphological and genomic characteristics, 0507-KN2-1 was classified as a member of the Myoviridae phage family. Further analysis of this phage revealed a 3738-bp gene encoding a putative polysaccharide depolymerase. A recombinant form of this protein was produced and assayed to confirm its enzymatic activity and specificity to KN2 capsular polysaccharides. KN2 K. pneumoniae strains exhibited greater sensitivity to this depolymerase than these did to the cognate phage, as determined by spot analysis.
Conclusions/Significance
Here we report that a group of clinical strains possess a novel Klebsiella capsular type. We identified a KN2-specific phage and its polysaccharide depolymerase, which could be used for efficient capsular typing. The lytic phage and depolymerase also have potential as alternative therapeutic agents to antibiotics for treating K. pneumoniae infections, especially against antibiotic-resistant strains.
Introduction
Klebsiella pneumoniae, a Gram-negative enteric bacterium, is a common pathogen that causes hospital-acquired urinary tract infections (UTIs), septicemia, and pneumonia [1]. In the recent decades, community-acquired pyogenic liver abscess (PLA) caused by K. pneumoniae complicated with metastatic meningitis and endophthalmitis has emerged globally, especially in Asia [2]–[16]. The capsule is an important virulence factor in K. pneumoniae. With at least 78 capsular serotypes defined, associations of certain serotypes with specific K. pneumoniae-induced diseases have been observed [17], [18]. The majority of K. pneumoniae isolates causing liver abscesses belong to serotypes K1 and K2 [10], [19], the most virulent of the known serotypes (Mizuta, K., 1983). Our studies showed that K. pneumoniae strains causing PLA belonged to capsular types K1 (∼80%), K2, K5, K20, K54, K57, and a new type [9], [20]. In a collection from a medical center in Taiwan, K1 was found most frequently among bacteremic isolates, and K2 was found mostly in respiratory tract specimens [10]. Among Klebsiella bacteremic isolates in Europe and North America, capsular serotypes K2, K21, and K55 were reported to be the most common [21]. The distribution of K. pneumoniae capsular serotypes appears to differ worldwide [22]–[25].
The capsule of KIebsiella spp. can be typed using a variety of techniques [26]. Serological diagnosis is commonly used to determine Klebsiella capsular serotypes, mainly via recognition of CPS variants by specific antibodies. However, serotyping is usually expensive, and serological cross-reactions between two or more capsular serotypes are common in clinical isolates.
Molecular techniques for capsular typing circumvent these problems and have thus become the current trend. Polymerase chain reaction (PCR) has been used to accurately and rapidly type Klebsiella based on DNA sequence variations in the capsular polysaccharide synthesis (cps) gene cluster [5]. The chromosomal cps region usually consists of 17∼25 genes encoding proteins involved in the translocation and assembly of surface polysaccharides. However, cps genotyping requires DNA sequences that can be distinguished between different capsular types. To date, only a limited number of specific cps sequences are known. Typing by use of bacteriophages(phages) that infect bacteria is an attractive method to diagnostic laboratories because it is simple and rapid, allowing analysis of large numbers of isolates [27]. Klebsiella phages have been used to differentiate serologically cross-reactive strains [28] and for epidemiological typing [29]. However, phage typing requires the isolation of phages that have high specificity for particular capsular serotypes. Because each of these typing methods has limitations or drawbacks, investigators usually employ 2 or more approaches to improve their typing accuracy.
Typing method limitations preclude serotype determination for some clinical isolates. For example, an Australian survey using antisera for typing reported that out of 293 K. pneumoniae isolates, 88 (30%) could not be typed and 54 (18%) had a positive reaction for more than one capsular type [22]. While typing K. pneumoniae clinical isolates in our laboratory using serotyping and cps-PCR, we found that UTI strain NTUH-K1790N could not be typed. In this study, we initially sequenced the cps locus of this strain. We tried to characterize this unidentified capsular type using several typing methods.
Methods
Bacterial Strains
We purchased 77 K. pneumoniae capsular serotype (K) reference strains from Statens Serum Institute (Copenhagen, Denmark). In addition, strain A1142, identified in our previous study as a new serotype (KN1) [20], was used. In total, 265 K. pneumoniae clinical isolates were analyzed using cps-PCR, including: 124 strains collected from the National Taiwan University Hospital (32 blood isolates from patients with no tissue-invasive disease, 22 non-blood isolates from non-septic patients, and 70 UTI strains); 13 strains from En Chu Kong Hospital, Taiwan; 34 strains from Far Eastern Memorial Hospital, Taiwan; 15 strains from Hong Kong [7]; and 79 isolates from blood or cerebrospinal fluid (CSF) of patients obtained from the Department of Medical Microbiology, University of Manitoba, Winnipeg, Manitoba, Canada [20].
Ethics Statement
The bacterial clinical strains used in this study were provided by collection of National Taiwan University Hospital, En Chu Kong Hospital, Far Eastern Memorial Hospital and University of Manitoba, described in previous publications [7], [20]. The Ethics Committee of National Taiwan University Hospital approved that no formal ethical approval was needed to use these bacterial strains, because the strains were remnant from patient samples, and the data were analyzed anonymously.
PCR-based Genotyping of cps
PCR primers used in this study are shown in Table S1. To obtain the full sequence of NTUH-K1790N cps, we used 3 PCR primer pairs for cps conserved sequences: (1) pre-galF-F, wzc-R1 (2) CPS-1, rCPS; and (3) gnd-1F, ugd. Purified PCR products of NTUH-K1790N cps were sequenced using a high-throughput sequencing service by Yang-Ming Genome Research Center: High-throughput Genome Analysis Core, using the Illumina/Solexa GAII sequencing platform. K. pneumoniae cps gene clusters usually consist of the conserved genes located at the 5′ and 3′ ends, and the variable genes in the middle. The variable cps genes are usually considered to be used to distinguish different capsular types. To determine the cps genotype, we designed 5 sets of PCR primer pairs for cps genes in the variable region: orf8(sharing amino-acid similarity with wzy), orf10, orf11, orf12, and wzx, according to DNA sequencing data of NTUH-K1790N (KN2) cps. These primers were all listed in Table S1 and designed to amplify PCR fragments <1.5 Kb in size. The specificity of these primers was tested by PCR using 77 K-serotype reference strains (Statens Serum Institute) and KN1 as templates [20]. In brief, 3 µL of an overnight bacterial culture was added to 10 µL of water and boiled for 15 min to release DNA, followed by addition of 2 µL of 10× reaction buffer, 2.5 U Taq polymerase (Bioman, Taipei, Taiwan), deoxynucleoside triphosphates at final concentrations of 0.1 mM each, and primers at final concentrations of 0.4 mM each to give a final volume of 20 µL. The PCR conditions were 96°C for 3 min, followed by 30 temperature cycles of 96°C for 30 s, 53°C for 15 s, and 72°C for 30 s.
Serotyping by Immunoblotting
Rabbit anti-KN2 antiserum was generated by a commercial company LTK BioLaboratories (Taipei, Taiwan). K. pneumoniae C1975 (KN2), pre-washed and resuspended in 5% formaldehyde, was used to immunize the rabbits by subcutaneous injections at Day 1, 21, 31, 41. The antiserum at Day 51 was tested for its sensitivity by Western Blot analysis. For capsular serotyping, capsules were extracted by a modified hot water-phenol extraction method [5]. In brief, 1 mL overnight-cultured bacteria was harvested and resuspended in 150 µL of water. An equal volume of hot phenol (pH 6.6) was added then vortexed vigorously. The mixture was incubated at 65°C for 20 min, followed by chloroform extraction and centrifugation. Ten µL of each capsular extract was vacuum spotted onto a nitrocellulose membrane by means of a slot blot device. The membrane was overlapped with a piece of filter and both were rinsed with Western transfer buffer containing 47.8 mM Tris, 38.6 mM glycine, 20% MeOH, and 0.037% sodium dodecyl sulfate. The membrane was dried and non-specific sites were blocked by soaking the membrane in 1× phosphate buffered saline with 0.5% Tween 20 (PBST) plus 5% milk for 1 h at room temperature. The membrane was then incubated with anti-KN2 antiserum (1∶5000 dilution) dissolved in PBST plus milk at 4°C overnight, washed 4 times with PBST for 10 min each, incubated with the secondary antibody conjugated with horseradish peroxidase (goat anti-rabbit IgG-HRP, 1∶10 000) for 1 h at room temperature, and washed 3 times with PBST for 10 min each. The ECL reagent was added for 3 min and the membrane was exposed to X-ray film in the dark.
Phage Isolation
To isolate a phage specific for KN2 K. pneumoniae from the natural environment, K. pneumoniae Ca0507 (KN2) was co-incubated with sewage collected at Taipei City in LB broth overnight. After centrifugation, the supernatant was filtered using a 0.45 µm filter and spotted onto LB plates overlaid with K. pneumoniae Ca0507 (KN2) to detect phage plaques. An agar overlay method was used for isolation of a pure phage preparation and to determine phage titers [28], [30]. Single plaque isolation, elution, and re-plating were performed repeatedly.
Determination of the Host Range of Phage/Polysaccharide Depolymerase
The spot test was performed to observe phage infection or polysaccharide depolymerase activity [31]. In brief, LB agar in a 6 or 24-well plate was overlaid with top agar that had been inoculated with 100 µL of a fresh bacterial culture. Phage or purified recombinant polysaccharide depolymerase (1 µL, 1 µg/µL) expressed by E. coli BL21(DE3) was spotted onto the plate after the top agar had solidified. After overnight incubation at 37°C, plates were observed for formation of lytic or semi-clear spots.
Electron Microscopy and Pulsed Field Gel Electrophoresis (PFGE) of Phage
The purified phage were adsorbed to carbon-coated copper grids, negatively stained with 3% uranyl acetate, and observed in a Hitachi-7100 Transmission Electron Microscope (TEM) at an accelerating voltage of 100 kV. Photographs were taken at a magnification of 70,000–150,000. For PFGE, phage suspensions were mixed 1∶1 with 2% low melt agarose and poured into molding blocks. A 4-mm block slice was put into the well of a 1% agarose gel (Bio-Rad, Hercules, CA, USA), and the well was sealed with 0.8% low melt agarose. The gel was run at 14°C, 6 V/cm, 15 h, switch time 1–12 s in a Biorad CHEF MAPPER XA using 0.5× TBE buffer. The gel was stained with ethidium bromide for 10 min and washed in distilled water before visualization under UV light.
Phage Genomic DNA Sequencing
Phage genomic DNA was extracted using a Qiagen Lamda kit (Qiagen, Valencia, CA). After phage were precipitated and lysed, the phage DNA was extracted using phenol/chloroform and then precipitated with ethanol. Genomic sequencing was performed using high-throughput sequencing by the Yang-Ming Genome Research Center: High-throughput Genome Analysis Core, using the Illumina/Solexa GAII sequencing platform. Putative open reading frames (ORFs) were predicted using the GeneMark network service (http://exon.gatech.edu/). The search for homologous genes was done using the BLAST network service (http://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome). The presentation of genome sequence features was done on the CGView server (http://stothard.afns.ualberta.ca/cgview_server/).
Protein Expression and Purification
PCR fragments of the phage gene orf96 encoding putative polysaccharide depolymerases were cloned into a pET-28c expression vector (Novagen, Madison, WI, USA) via the BamHI and SalI sites. The resulting orf96-pET28c plasmid was transformed into an E. coli BL21(DE3) strain. The recombinant His-tagged ORF96 protein was expressed under 0.05 mM IPTG induction at 16°C overnight, followed by purification from the soluble fraction using Ni-NTA (Qiagen, Valencia, CA) according to the manufacturer’s instructions and SDS-PAGE analysis.
DNA Sequencing Data
All new data on DNA sequencing in this study has been deposited in DDBJ/EMBL/GenBank databases. The cps sequences of NTUH-K1790N have been deposited under the accession number AB795939. The genome sequences of phage 0507-KN2-1 have been deposited under the accession number AB797215.
Results
1. Identification of K. pneumoniae Clinical Isolates with a Novel cps Genotype
To characterize the undefined capsular type of K. pneumoniae NTUH-K1790N, we amplified and sequenced the cps region between the conserved genes galF and gnd. We found that this cps region of NTUH-K1790N was similar to that published for undefined strain NK29 (cpskpB, [32]) ( Figure 1A ). These 2 strains had the same cps gene annotation between galF and gnd, with a total DNA sequence similarity of 99%. In addition, the cps sequences of NTUH-K1790N and NK29 did not resemble any other publicly available cps sequences. To further characterize this unknown cps genotype, we designed PCR primers from 5 genes in the variable region of NTUH-K1790N cps ( Figure 1A ): orf8, orf10, orf11, orf12, and wzx. Orf8 encoded a putative protein with 11 predicted trans-membrane domains (by SMART program, http://smart.embl-heidelberg.de/), sharing an amino-acid sequence identity of 133/409 (33%) with Wzy polymerase of Escherichia coli (accession ADI43271). Wzx functions as a flippase to transfer repeat units of CPS across the plasma membrane, and Wzy polymerizes these repeating units in the periplasm [33]. Because wzx and wzy have low sequence similarity, they were assumed to be serotype-specific and could thus be used for typing [32], [34]–[36]. Orf10 and orf12 encoded putative glycosyltransferases, which transfer sugars to specific biomolecules and are diverse in nature. Orf11 encoded a hypothetical protein, sharing an amino-acid sequence identity of 92/342(27%) with beta-glucanase of Bacteroides sp. (accession ZP_07938801.1).
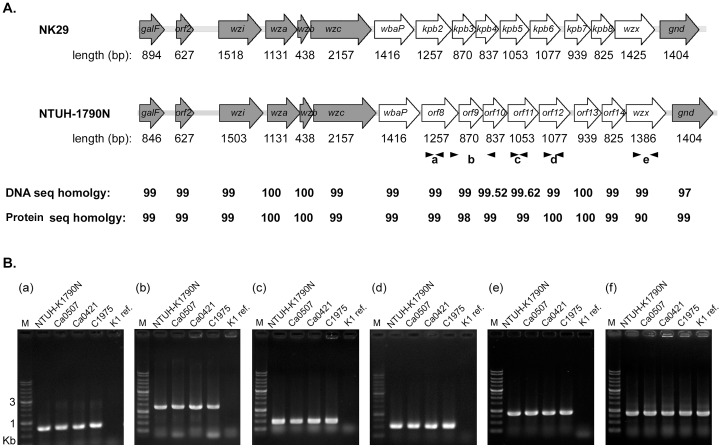
Figure 1. NTUH-K1790N cps-PCR genotyping in K. pneumoniae.
(A) Comparison of the capsular polysaccharide synthesis (cps) regions of NK29 (Shu et al., 2009) and NTUH-K1790N strains. ORFs are shown by arrows, with the length as indicated below. Gray arrows show the conserved ORFs, and white arrows show the variable ORFs in different serotypes. The homology score between NK29 and NTUH-K1790N is compared using the sequence alignment program ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Black arrowheads refer to 5 pairs of PCR primers (a-e) used for cps genotyping. (B) cps-PCR genotyping of K. pneumoniae strains using primers as indicated: a. KN2-orf8-F and KN2-orf8-R; b. KN2-orf8-F2 and KN2-orf10-R; c. KN2-orf11-F and KN2-orf11-R; d. KN2-orf12-F and KN2-orf12-R; e. KN2-wzx-F and KN2-wzx-R; f. 23S rRNA as control for K. pneumoniae. K1 ref. presents the reference strain for the K1 serotype.G.
PCR of NTUH-K1790N cps was performed using these different primer pairs in 77 Klebsiella reference strains of known capsular serotypes from the Statens Serum Institute and in a new serotype KN1 (strain A1517) [20]. All PCR results were negative in the 77 K serotype and KN1 reference strains (data not shown), suggesting that the NTUH-K1790N cps gene might differ from that of the test strains. We further applied NTUH-K1790N cps-PCR to other clinical isolates from Asia and Canada with unknown capsular types, including isolates from UTI, blood, non-blood, and CSF. Interestingly, of the 264 clinical isolates tested, we found that 3 strains, Ca0507, Ca0421, and C1975, were positive for the NTUH-K1790N cps gene in all PCR analyses ( Figure 1B ). Ca0507 and Ca0421 were isolated from blood of septic patients in Canada [20], and C1975 was isolated from sputum of a patient in NTUH, Taiwan [7]. Hence, NTUH-K1790N cps-PCR actually did recognize some K. pneumoniae strains. Our data indicated that NTUH-K1790N, Ca0507, Ca0421, C1975, and NK29 belonged to a group of Klebsiella isolates with the same cps genotype. This cps genotype differs from that of the 78 previously documented types and therefore could be novel.
2. Characterization of the Novel Capsular Serotype of K. pneumoniae by Immunoblotting
To further clarify whether NTUH-K1790N, Ca0507, Ca0421, C1975, and NK29 belonged to a new capsular serotype, we generated antiserum against C1975 for serotyping. Immunoblotting showed strong positive signals in the extracted capsular polysaccharides from all four strains, indicating that these four strains belonged to the same serotype ( Figure 2A ). In addition, we generated an isogenic Ca0507 CPS− mutant, in which orf8 (predicted to encode Wzy) of the cps locus was deleted by an unmarked deletion method using the temperature-sensitive plasmid pKO3-Km [37], [38]. Immunoblot serotyping showed that this mutant was no longer recognized by anti-C1975 antiserum ( Figure 2A ), confirming that orf8 was essential for capsular polysaccharide synthesis. We thus concluded that anti-C1975 antiserum was reactive to capsular polysaccharides.
Figure 2. Immunoblot serotyping of K. pneumoniae NTUH-K1790N, Ca0507, Ca0421, and C1975.
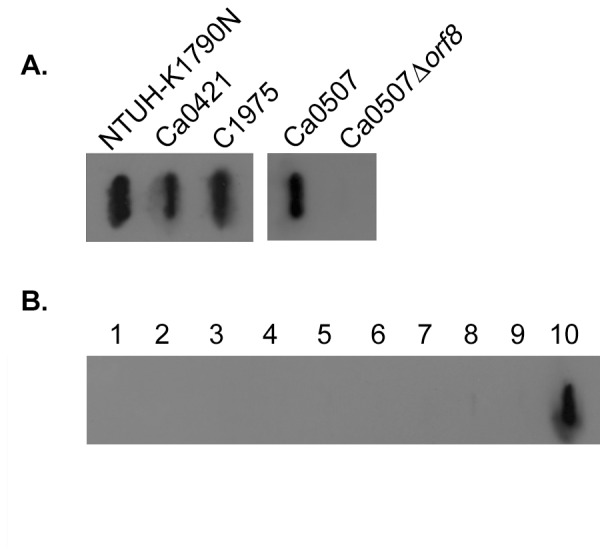
Rabbit anti-C1975 antiserum was used as the first antibody (1∶5000) and goat anti-rabbit IgG-HRP as the second antibody (1∶10 000). (A) The slot blot showing anti-C1975 antiserum reacts to the extracted capsular polysaccharides from strains NTUH-K1790N, C1975, Ca0421 and Ca0507. Ca0507Δorf8 presents the isogenic mutant with a deletion of gene orf8 in the cps. (B) The slot blot for the extracted capsular polysaccharides from 77 K serotype reference strains and a new type A1517. Sample1–9: K31 to K39; 10: C1975 as control. Negative reactions were observed in other reference strains and A1517 (data not shown).
We performed immunoblot serotyping of the 77 Klebsiella K reference strains and A1517. The reaction to anti-C1975 antiserum was negative in all 78 strains ( Figure 2B ). These results indicate that anti-C1975 antiserum is specific and does not react to the other 78 documented capsular serotypes. This group of K. pneumoniae was thus determined to belong to a novel serotype. We named this new capsular serotype ‘KN2.’
3. Isolation of a Phage Specific for KN2 K. pneumoniae
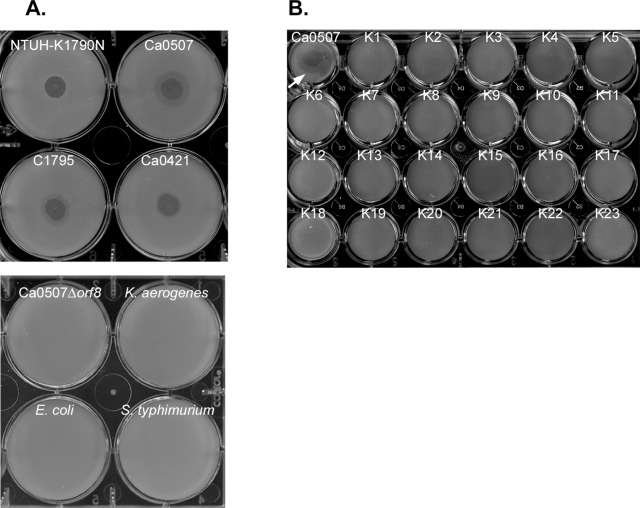
In nature, encapsulated bacteria can be infected by phages, which often carry capsule-degrading enzymes. The use of phages for Klebsiella capsular typing has been previously described [27], [28], [39]. Such lytic phages can also be used as therapeutic agents [40]–[43]. In this study, we sought to isolate a lytic phage of the KN2 K. pneumoniae strain from the natural environment. A phage was isolated from sewage collected at Taipei city by co-incubation with K. pneumoniae Ca0507. In spot tests, this phage caused a lytic spot on K. pneumoniae Ca0507 (KN2) and also on three other KN2 strains ( Figure 3A ). Lysis did not occur in the other Enterobacteriaceae tested, including Klebsiella aerogenes, Escherichia coli, Salmonella typhimurium, and interestingly, the KN2 CPS− mutant. This result suggests that infection of the phage might occur via targeting and recognition of the capsular polysaccharides in K. pneumoniae.
Figure 3. Bacterial host range of phage 0507-KN2-1.
(A) Upper panel showing spot tests of 0507-KN2-1 (109) on K. pneumoniae KN2 strains as indicated. Lower panel showing spot tests on Ca0507 isogenic mutant with a deletion of gene orf8 in cps (Ca0507Δorf8), K. aerogenes, E. coli 29522 and S. typhimurium ATCC14028. (B) Spot tests of 0507-KN2-1 (109) on K. pneumoniae 77 K serotype reference strains and a new type A1517. Ca0507 was used as a positive control (indicated by a white arrow). Negative reactions were observed in all reference strains and A1517 (data on K24-K77 and A1517 not shown).
When testing K. pneumoniae with different capsular serotypes, this bacteriophage did not cause lytic infections in any of the 77 K reference strains or in KN1 ( Figure 3B ). Therefore, this phage (named 0507-KN2-1) is specific to KN2 K. pneumoniae and can be used for KN2 typing. Consistent with the results of cps-PCR genotyping and immunoblot serotyping, phage typing indicated again that these KN2 K. pneumoniae strains have a novel capsular structure that differs from the 78 documented capsule types.
4. Morphological and Molecular Characterization of Phage 0507-KN2-1
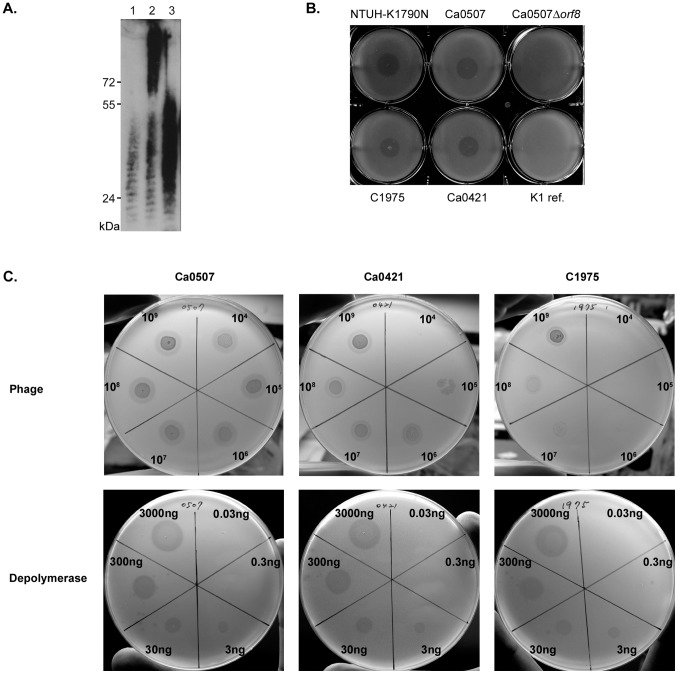
Phage 0507-KN2-1 produced plaques with small clear centers surrounded by hazy rings (halo) ( Figure 4A ), suggesting the production of soluble phage enzymes like exopolysaccharide depolymerases [44]. The morphology of purified 0507-KN2-1 phage particles were further studied using TEM ( Figure 4B ). The average length of the phage tails was approximately 107 nm, and the average diameter of the phage heads was approximately 66 nm. The morphological characteristics of 0507-KN2-1 resembled Myoviridae family members, which have an icosahedral head and a contractile tail [45].
Figure 4. Characterization of phage 0507-KN2-1.
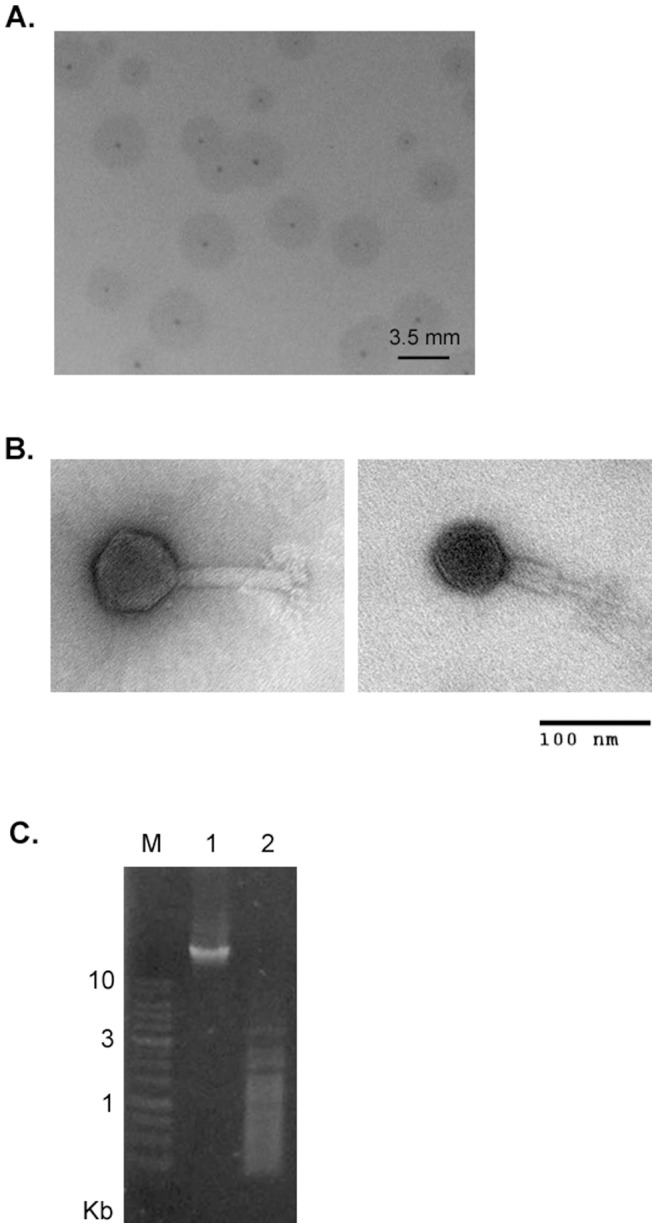
(A) Plaque morphology of 0507-KN2-1 on K. pneumoniae Ca0507. Scale bar, 3.5 mm. (B) TEM images of 0507-KN2-1 taken at 150 000 × magnification. Scale bars for both pictures, 100 nm. (C) Restriction digestion of 0507-KN2-1 genome. M: DNA marker; lane1: uncut genomic DNA, 2 µg; lane2: genomic DNA 2 µg incubated with HinPI1 at 37°C for 5 h.
We further characterized the genome of 0507-KN2-1. Analysis by PFGE showed that the phage genome has an approximate size of 145.5–194 kb (not shown). The extracted phage DNA was sensitive to digestion with the restriction enzyme HinP1I ( Figure 4C ), indicating a double-stranded DNA genome. Whole-genome sequencing of phage 0507-KN2-1 was carried out using high-throughput sequencing. We obtained a circular map of the 159 991 bp genome, which has a G+C content of 46.7% and 154 predicted ORFs encoding hypothetical proteins with at least 100 amino acid residues (Table S2). BlastN analysis revealed that the most related genome sequences in the database were Salmonella phage PhiSH19 (accession JN126049.1, covering 50% of the 0507-KN2-1 genome with 72–99% identity), Salmonella phage Vi01 (accession FQ312032.1, covering 48% of the 0507-KN2-1 genome with 72–100% identity), Salmonella phage SFP10 (accession HQ259103, covering 47% of the 0507-KN2-1 genome with 72–96% identity), and Escherichia phage vB_EcoM_CBA120 (accession JN593240.1, covering 46% of the 0507-KN2-1 genome with 72–100% identity). All of these phages are Myoviridae members and have genome sizes of 157–158 kb. By BlastP search, 0507-KN2-1 potential gene products showed significant sequence similarity to proteins from different phages infecting Salmonella, Shigella, Escherichia, and Dickeya (Table S2). The combined results of phage morphology, genome size, and sequence similarity suggest that 0507-KN2-1 is a Myoviridae virus.
5. Expression of a Capsular Polysaccharide Depolymerase Specific for KN2
We observed that the plaques of phage 0507-KN2-1 were surrounded by halos, indicative of bacterial cell decapsulation. This observation suggested the phage produced a depolymerase enzyme that could diffuse through the agar layer. Phage depolymerases, often a part of the tail spike or tail fiber, can degrade bacterial capsular polysaccharides into their component oligosaccharide units during infection. Capsular polysaccharide depolymerases have multiple applications, including use as therapeutic agents against bacterial pathogens [46], [47], for the prevention or eradication of biofilms [44], [48], and for the production of oligosaccharides from polysaccharides [49]–[51]. We therefore sought to isolate and purify the depolymerase of this phage.
Analysis of the genome sequence of phage 0507-KN2-1 revealed that orf96 (3738 bp) encoded a putative protein (1245 amino acids) sharing amino-acid sequence similarity to other known phage tail fiber or spike proteins in a 170-amino-acid region of the N-terminus. For example, this coding region shares identity of 125/167 amino acids (75%) with the tail fiber protein of Escherichia phage PhaxI (total 927 amino acids, accession number YP_007002808.1). Interestingly, the C-terminal ∼200 amino-acid region shares a sequence similarity to the polysaccharide lyase of Enterobacteria phage K5 (total 632 amino acids, accession number: CAA71133.2) with an identity of 61/222 amino acids (27%).
To determine whether the product of this gene had depolymerase activity, we cloned orf96 into the pET28c expression vector. His-tagged ORF96 protein with a predicted size of ∼138 kDa was expressed and purified. Immunoblotting showed that the purified protein caused depolymerization of isolated KN2 capsular polysaccharides ( Figure 5A ). In spot tests, purified ORF96 protein caused decapsulation in the KN2 K. pneumoniae strains ( Figure 5B ) but in none of the 77 K reference strains or KN1 (data not shown). In addition, the purified ORF96 protein did not cause digestion-like spots on the KN2 CPS− mutant (Ca0507Δorf8) bacterial lawns. Therefore, this depolymerase was specific for KN2 capsular polysaccharides. In contrast to phage infection, the purified depolymerase exhibited a consistent sensitivity between different KN2 strains ( Figure 5C ), suggesting that the depolymerase was more ideal for typing than phage did. After validation of the sensitivity and specificity in more strains, this KN2 polysaccharide depolymerase could be considered its application to large-scale capsular typing for K. pneumoniae clinical strains.
Figure 5. Bacteriophage-derived depolymerase specific for KN2 capsular polysaccharides.
(A) Immunoblot showing depolymerzation of KN2 extracted capsular polysaccharides by purified ORF96 proteins. The extracted capsular polysaccharides alone or incubated with ORF96 proteins (10 µg) at 37°C for 6 h were separated by SDS-PAGE, transferred onto a nitrocellulose membrane and detected using rabbit anti-C1975 antiserum (1∶5000) and goat anti-rabbit IgG-HRP (1∶10 000). Lane1: Ca0507Δorf8 alone; lane2: wild-type Ca0507 alone; lane3: wild-type Ca0507 plus ORF96 proteins. (B) A spot test showing ORF96 proteins with the depolymerase activity to KN2. Purified ORF96 proteins (1 µg) was spotted on the bacterial lawn and incubated at 37°C overnight. Decapsulation of KN2 K. pneumoniae NTUH-K1790N, Ca0507, Ca0421 and C1975 was observed. K1 ref. presents the reference strain of the K1 serotype. (C) Comparison of sensitivity of phage 0507-KN2-1 and capsular polysaccharide depolymerase. Spot tests with serial titration (counter-clockwise) of phage 0507-KN2-1 (upper panels) or purified ORF96 proteins (lower panels) on KN2 strains as indicated.
Discussion
The capsule, one of the most important K. pneumoniae virulence factors, protects the bacterium from the lethal serum factors and phagocytosis that are part of the host immune response. Based on the structural variability of the capsular polysaccharides, Klebsiella sp. has been traditionally classified into 77 K serotypes for several decades [52]. No further capsular serotypes were reported until recently, when a new serotype (KN1) was found in our lab during studies of PLA-inducing K. pneumoniae [20]. Through serological and molecular typing, the pathogenicity and epidemiological relevance of the different capsular serotypes of K. pneumoniae in various infectious diseases have been identified [53]–[56]. In this study, we found a UTI strain with a capsular type that was initially not recognized using immunoserotyping or cps-PCR. Our findings showed that this strain had a novel capsular type, which we called KN2. Our experience suggests that the inability to type some clinical strains may be due to the presence of new capsular types that have not yet been identified and that more Klebsiella capsule types may exist than are presently known.
Our data indicated that K. pneumoniae KN2 strains had the same cps genotype and shared capsule structural characteristics, including reactivity to antiserum, susceptibility to phage infection, and decapsulation by a capsular polysaccharide depolymerase. These features distinguish the KN2 strains from the documented K reference strains. No cross-reactions were observed between KN2 strains and the other 79 documented capsular types in any our assays, indicating the novelty of their capsule structures and a high degree of specificity in typing. The prevalence of this new capsular type in 265 clinical isolates of K. pneumoniae from Asia and Canada is ∼1.5%, including 1 isolate from UTI, 2 bacteremic isolates, and 1 non-blood isolate.
Serological diagnosis is often used to identify Klebsiella capsular serotypes, but this method has drawbacks. The antisera are usually expensive, and their limited sensitivity and specificity result in inconsistent results in serotype prevalence studies [21], [22], [57], [58]. Molecular typing is more accurate but requires type-specific sequence data for the cps regions, which are not yet available for all types up to now. Phage-based capsular typing is rapid, simple, and low-cost, but requires phage with a narrow host range and no cross-reactivity to other types. Here, we further isolated and purified a phage enzyme that can be used for efficient typing. The stability and sensitivity of purified KN2 depolymerase for inducing decapsulation spots is better than phage infection, and the amount of enzyme used can be easily quantified. Therefore, enzyme-based typing is more suitable for large-scale epidemiological studies of capsular type prevalence. Using this more efficient typing method, the prevalence of KN2 capsular type in different K. pneumoniae-mediated diseases can be further investigated.
Phage 0507-KN2-1 is a double-stranded DNA virus that belongs to Myoviridae. This virus shares genome characteristics with other phages that infect Enterobacteriaceae, such as Salmonella phage Vi01-related viruses. To date, all K. pneumoniae phages with published whole genome sequences in the database belonged to 3 families in the tailed dsDNA Caudovirales order: Podoviridae, Siphoviridae, and Myoviridae. Phage 0507-KN2-1 shares less genome similarity with previously reported K. pneumoniae phages KP34 (Podoviridae, 43.81 kb, accession GQ413938), phiKO2 (Siphoviridae, 51.6 kb, accession AY374448), KP15 (Myoviridae, 174.44 kb, accession GU295964), K11 (Podoviridae, 41.18 kb, accession EU734173), KP32 (Podoviridae, 41.12 kb, accession GQ413937), KP36 (Siphoviridae, 49.82 kb, accession JQ267364), and JD001 (Myoviridae, 48.81 kb, accession JX866719). Although several K. pneumoniae phages have been reported, associations between the molecular characteristics of the phage or polysaccharide depolymerase and the specificity to host capsular serotypes have not been clearly described.
In this study, we provide molecular data for the phage and polysaccharide depolymerase together with well-defined characteristics and host specificity. The host range of phage 0507-KN2-1 is narrow and specific, only to K. pneumoniae that have the KN2 capsular type. In the first step of infection, phages need to attach and absorb to specific targets on the bacterial surface. Our results indicate that capsular polysaccharides may be the specific receptors for 0507-KN2-1, since neither the phage nor the depolymerase causes lytic or semi-turbid spots on Ca0507Δorf8 mutant. The specificity of 0507-KN2-1 could be due to the phage-carried polysaccharide depolymerase, which can recognize the KN2 capsular structure distinct from other capsular types. The well-characterized host specificity and full sequences of phage and polysaccharide depolymerase revealed by this study will be helpful for elucidating the mechanistic details that determine the host range of this virus. In addition, KN2 depolymerase, for which we have provided the sequence data and demonstrated in vitro activity, can be used to investigate its functional regions, the structure of KN2 capsule polysaccharides, and the interactions between this enzyme and its polysaccharide substrates.
In many hospitals, K. pneumoniae is the second most frequent etiological agent of Gram-negative bacteraemia and UTIs. Drug resistant isolates remain a problem, rendering current antibiotic therapy relatively ineffective. K. pneumoniae reportedly produces extended-spectrum β-lactamases, resulting in multidrug resistance [59]–[62]. In addition, development of antibiotic resistance can be enhanced by formation of protective biofilms that can impede the penetration of drugs. Thus, alternative therapeutic methods are needed. Lytic phages have been considered possible alternatives to antibiotics for treating Klebsiella infections [40], [41], [63]. The narrow host range displayed by phages can be advantageous for use as therapeutic agents since it has limited adverse effects on the natural bacterial population compared to chemical antibiotics. Administration of capsular depolymerase has been shown to reduce bacteraemia and deaths in animals [46] and to exhibit protective effects by promoting in vivo killing of bacteria by neutrophils [47]. We found KN2 possessed by clinical K. pneumoniae causing bacteraemia and UTIs. The potential therapeutic effects of phage 0507-KN2-1 and recombinant KN2 depolymerase can be further investigated for use in so-called phage therapy and glycosidase therapy. Because the mechanism of bacterial killing by lytic phage and glycosidases differ from that of antibiotics, these agents have the potential to treat antibiotic-resistant K. pneumoniae strains. Moreover, their ability to generate oligosaccharides from polysaccharides could be applied to polysaccharide vaccine production.
In summary, we have identified and characterized a new capsular type of K. pneumoniae, its specific bacteriophage, and the polysaccharide depolymerase of this phage. These results may be applicable to capsular typing in further epidemiological investigations and to the development of new treatments for antibiotic-resistant K. pneumoniae infections.
Supporting Information
PCR Primers used in this study.
(DOC)
Annotation and features of predicted ORFs in bacteriophage Ca0507-KN2 genome.
(DOC)
Funding Statement
This study was supported by grants from the National Science Council, National Taiwan University, and National Taiwan University Hospital in Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Podschun R, Ullmann U (1998) Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11: 589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang SC, Fang CT, Hsueh PR, Chen YC, Luh KT (2000) Klebsiella pneumoniae isolates causing liver abscess in Taiwan. Diagn Microbiol Infect Dis 37: 279–284. [DOI] [PubMed] [Google Scholar]
- 3. Cheng DL, Liu YC, Yen MY, Liu CY, Wang RS (1991) Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med 151: 1557–1559. [PubMed] [Google Scholar]
- 4. Chiu CT, Lin DY, Liaw YF (1988) Metastatic septic endophthalmitis in pyogenic liver abscess. J Clin Gastroenterol 10: 524–527. [DOI] [PubMed] [Google Scholar]
- 5. Chuang YP, Fang CT, Lai SY, Chang SC, Wang JT (2006) Genetic determinants of capsular serotype K1 of Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis 193: 645–654. [DOI] [PubMed] [Google Scholar]
- 6. Chung DR, Lee SS, Lee HR, Kim HB, Choi HJ, et al. (2007) Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea. J Infect 54: 578–583. [DOI] [PubMed] [Google Scholar]
- 7. Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT (2004) A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med 199: 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fang FC, Sandler N, Libby SJ (2005) Liver abscess caused by magA+ Klebsiella pneumoniae in North America. J Clin Microbiol 43: 991–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, et al. (2007) Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clinical Infectious Diseases 45: 284–293. [DOI] [PubMed] [Google Scholar]
- 10. Fung CP, Chang FY, Lee SC, Hu BS, Kuo BI, et al. (2002) A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut 50: 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keynan Y, Karlowsky JA, Walus T, Rubinstein E (2007) Pyogenic liver abscess caused by hypermucoviscous Klebsiella pneumoniae. Scand J Infect Dis 39: 828–830. [DOI] [PubMed] [Google Scholar]
- 12. Ko WC, Paterson DL, Sagnimeni AJ, Hansen DS, Von Gottberg A, et al. (2002) Community-acquired Klebsiella pneumoniae bacteremia: Global differences in clinical patterns. Emerging Infectious Diseases 8: 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu YC, Cheng DL, Lin CL (1986) Klebsiella-Pneumoniae Liver-Abscess Associated with Septic Endophthalmitis. Archives of Internal Medicine 146: 1913–1916. [PubMed] [Google Scholar]
- 14. Nadasy KA, Domiati-Saad R, Tribble MA (2007) Invasive Klebsiella pneumoniae syndrome in North America. Clinical Infectious Diseases 45: e25–28. [DOI] [PubMed] [Google Scholar]
- 15. Wang JH, Liu YC, Lee SSJ, Yen MY, Chen YS, et al. (1998) Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clinical Infectious Diseases 26: 1434–1438. [DOI] [PubMed] [Google Scholar]
- 16. Yang CC, Yen CH, Ho MW, Wang JH (2004) Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect 37: 176–184. [PubMed] [Google Scholar]
- 17. Cortes G, Borrell N, de Astorza B, Gomez C, Sauleda J, et al. (2002) Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect Immun 70: 2583–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mizuta K, Ohta M, Mori M, Hasegawa T, Nakashima I, et al. (1983) Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect Immun 40: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Struve C, Bojer M, Nielsen EM, Hansen DS, Krogfelt KA (2005) Investigation of the putative virulence gene magA in a worldwide collection of 495 Klebsiella isolates: magA is restricted to the gene cluster of Klebsiella pneumoniae capsule serotype K1. J Med Microbiol 54: 1111–1113. [DOI] [PubMed] [Google Scholar]
- 20. Pan YJ, Fang HC, Yang HC, Lin TL, Hsieh PF, et al. (2008) Capsular polysaccharide synthesis regions in Klebsiella pneumoniae serotype K57 and a new capsular serotype. J Clin Microbiol 46: 2231–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cryz SJ Jr, Mortimer PM, Mansfield V, Germanier R (1986) Seroepidemiology of Klebsiella bacteremic isolates and implications for vaccine development. J Clin Microbiol 23: 687–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jenney AW, Clements A, Farn JL, Wijburg OL, McGlinchey A, et al. (2006) Seroepidemiology of Klebsiella pneumoniae in an Australian Tertiary Hospital and its implications for vaccine development. J Clin Microbiol 44: 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Legakis NJ, Tzouvelekis LS, Hatzoudis G, Tzelepi E, Gourkou A, et al. (1995) Klebsiella pneumoniae infections in Greek hospitals. Dissemination of plasmids encoding an SHV-5 type beta-lactamase. J Hosp Infect 31: 177–187. [DOI] [PubMed] [Google Scholar]
- 24. Smith SM, Digori JT, Eng RH (1982) Epidemiology of Klebsiella antibiotic resistance and serotypes. J Clin Microbiol 16: 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blanchette EA, Rubin SJ (1980) Seroepidemiology of clinical isolates of Klebsiella in Connecticut. J Clin Microbiol 11: 474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ayling-Smith B, Pitt TL (1990) State of the art in typing: Klebsiella spp. J Hosp Infect 16: 287–295. [DOI] [PubMed] [Google Scholar]
- 27. Gaston MA, Ayling-Smith BA, Pitt TL (1987) New bacteriophage typing scheme for subdivision of the frequent capsular serotypes of Klebsiella spp. J Clin Microbiol 25: 1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pieroni P, Rennie RP, Ziola B, Deneer HG (1994) The use of bacteriophages to differentiate serologically cross-reactive isolates of Klebsiella pneumoniae. J Med Microbiol 41: 423–429. [DOI] [PubMed] [Google Scholar]
- 29. Sechter I, Mestre F, Hansen DS (2000) Twenty-three years of Klebsiella phage typing: a review of phage typing of 12 clusters of nosocomial infections, and a comparison of phage typing with K serotyping. Clin Microbiol Infect 6: 233–238. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J, Russell D (2001) Molecular Cloning: A Laboratoty Manual, Edn.: CSHL, New York.
- 31. Verma V, Harjai K, Chhibber S (2009) Characterization of a T7-like lytic bacteriophage of Klebsiella pneumoniae B5055: a potential therapeutic agent. Curr Microbiol 59: 274–281. [DOI] [PubMed] [Google Scholar]
- 32. Shu HY, Fung CP, Liu YM, Wu KM, Chen YT, et al. (2009) Genetic diversity of capsular polysaccharide biosynthesis in Klebsiella pneumoniae clinical isolates. Microbiology 155: 4170–4183. [DOI] [PubMed] [Google Scholar]
- 33. Whitfield C, Roberts IS (1999) Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol Microbiol 31: 1307–1319. [DOI] [PubMed] [Google Scholar]
- 34. Jiang SM, Wang L, Reeves PR (2001) Molecular characterization of Streptococcus pneumoniae type 4, 6B, 8, and 18C capsular polysaccharide gene clusters. Infect Immun 69: 1244–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DebRoy C, Fratamico PM, Roberts E, Davis MA, Liu Y (2005) Development of PCR assays targeting genes in O-antigen gene clusters for detection and identification of Escherichia coli O45 and O55 serogroups. Appl Environ Microbiol 71: 4919–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kong F, Wang W, Tao J, Wang L, Wang Q, et al. (2005) A molecular-capsular-type prediction system for 90 Streptococcus pneumoniae serotypes using partial cpsA-cpsB sequencing and wzy- or wzx-specific PCR. J Med Microbiol 54: 351–356. [DOI] [PubMed] [Google Scholar]
- 37. Hsieh PF, Lin TL, Lee CZ, Tsai SF, Wang JT (2008) Serum-induced iron-acquisition systems and TonB contribute to virulence in Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis 197: 1717–1727. [DOI] [PubMed] [Google Scholar]
- 38. Link AJ, Phillips D, Church GM (1997) Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol 179: 6228–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rieger-Hug D, Stirm S (1981) Comparative study of host capsule depolymerases associated with Klebsiella bacteriophages. Virology 113: 363–378. [DOI] [PubMed] [Google Scholar]
- 40. Karamoddini MK, Fazli-Bazzaz BS, Emamipour F, Ghannad MS, Jahanshahi AR, et al. (2011) Antibacterial efficacy of lytic bacteriophages against antibiotic-resistant Klebsiella species. ScientificWorldJournal 11: 1332–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vinodkumar CS, Neelagund YF, Kalsurmath S (2005) Bacteriophage in the treatment of experimental septicemic mice from a clinical isolate of multidrug resistant Klebsiella pneumoniae. J Commun Dis 37: 18–29. [PubMed] [Google Scholar]
- 42. Slopek S, Durlakowa I, Weber-Dabrowska B, Dabrowski M, Kucharewicz-Krukowska A (1984) Results of bacteriophage treatment of suppurative bacterial infections. III. Detailed evaluation of the results obtained in further 150 cases. Arch Immunol Ther Exp (Warsz) 32: 317–335. [PubMed] [Google Scholar]
- 43. Sakandelidze VM, Meipariani AN (1974) [Use of combined phages in suppurative-inflammatory diseases]. Zh Mikrobiol Epidemiol Immunobiol 51: 135–136. [PubMed] [Google Scholar]
- 44. Hughes KA, Sutherland IW, Jones MV (1998) Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology 144 (Pt 11): 3039–3047. [DOI] [PubMed] [Google Scholar]
- 45.Ackermann HW (1987) Bacteriophage taxonomy. In Viruses of prokaryotes. Florida, Boca Raton: CRC Press, inc. 16 p.
- 46. Mushtaq N, Redpath MB, Luzio JP, Taylor PW (2004) Prevention and cure of systemic Escherichia coli K1 infection by modification of the bacterial phenotype. Antimicrob Agents Chemother 48: 1503–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scorpio A, Tobery SA, Ribot WJ, Friedlander AM (2008) Treatment of experimental anthrax with recombinant capsule depolymerase. Antimicrob Agents Chemother 52: 1014–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verma V, Harjai K, Chhibber S (2010) Structural changes induced by a lytic bacteriophage make ciprofloxacin effective against older biofilm of Klebsiella pneumoniae. Biofouling 26: 729–737. [DOI] [PubMed] [Google Scholar]
- 49. Niemann H, Frank N, Stirm S (1977) Klebsiella serotype-13 capsular polysaccharide: primary structure and depolymerization by a bacteriophage-borne glycanase. Carbohydr Res 59: 165–177. [DOI] [PubMed] [Google Scholar]
- 50. Niemann H, Kwiatkowski B, Westphal U, Stirm S (1977) Klebsiella serotype 25 capsular polysaccharide: primary structure and depolymerization by a bacteriophage-borne glycanase. J Bacteriol 130: 366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Altmann F, Christian R, Czerny T, Nimmich W, Marz L (1990) Bacteriophage-associated glycan hydrolases specific for Escherichia coli capsular serotype K12. Eur J Biochem 189: 307–312. [DOI] [PubMed] [Google Scholar]
- 52. Ørskov I, Fife-Asbury MA (1979) New Kiebsiella capsular antigen, K82, and the deletion of five of those previously assigned. Int J Syst Bacteriol 27: 2. [Google Scholar]
- 53. Podschun R (1990) Phenotypic properties of Klebsiella pneumoniae and K. oxytoca isolated from different sources. Zentralbl Hyg Umweltmed 189: 527–535. [PubMed] [Google Scholar]
- 54. Podschun R, Heineken P, Ullmann U, Sonntag HG (1986) Comparative investigations of Klebsiella species of clinical origin: plasmid patterns, biochemical reactions, antibiotic resistances and serotypes. Zentralbl Bakteriol Mikrobiol Hyg A 262: 335–345. [DOI] [PubMed] [Google Scholar]
- 55. Riser E, Noone P (1981) Klebsiella capsular type versus site of isolation. J Clin Pathol 34: 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Casewell MW, Phillips I (1978) Epidemiological patterns of Klebsiella colonization and infection in an intensive care ward. J Hyg (Lond) 80: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fung CP, Hu BS, Chang FY, Lee SC, Kuo BI, et al. (2000) A 5-year study of the seroepidemiology of Klebsiella pneumoniae: high prevalence of capsular serotype K1 in Taiwan and implication for vaccine efficacy. J Infect Dis 181: 2075–2079. [DOI] [PubMed] [Google Scholar]
- 58. Tsay RW, Siu LK, Fung CP, Chang FY (2002) Characteristics of bacteremia between community-acquired and nosocomial Klebsiella pneumoniae infection: risk factor for mortality and the impact of capsular serotypes as a herald for community-acquired infection. Arch Intern Med 162: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 59. Gniadkowski M (2001) Evolution and epidemiology of extended-spectrum beta-lactamases (ESBLs) and ESBL-producing microorganisms. Clin Microbiol Infect 7: 597–608. [DOI] [PubMed] [Google Scholar]
- 60. Nijssen S, Florijn A, Bonten MJ, Schmitz FJ, Verhoef J, et al. (2004) Beta-lactam susceptibilities and prevalence of ESBL-producing isolates among more than 5000 European Enterobacteriaceae isolates. Int J Antimicrob Agents 24: 585–591. [DOI] [PubMed] [Google Scholar]
- 61.Coque TM, Baquero F, Canton R (2008) Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill 13. [PubMed]
- 62. Miriagou V, Papagiannitsis CC, Kotsakis SD, Loli A, Tzelepi E, et al. (2010) Sequence of pNL194, a 79.3-kilobase IncN plasmid carrying the blaVIM-1 metallo-beta-lactamase gene in Klebsiella pneumoniae. Antimicrob Agents Chemother 54: 4497–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chhibber S, Kaur S, Kumari S (2008) Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice. J Med Microbiol 57: 1508–1513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR Primers used in this study.
(DOC)
Annotation and features of predicted ORFs in bacteriophage Ca0507-KN2 genome.
(DOC)