Abstract
Background
NKX2-5 is a transcriptional factor, which plays an important role in heart formation and development. Two genetic variants in the coding region of NKX2-5, 63A>G (rs2277923) and 606G>C (rs3729753), have been investigated in the risk of congenital heart disease (CHD), although with inconsistent results. Thus, a meta-analysis was performed to clarify the associations between the two variants and CHD risk in the Chinese population.
Methods and Results
Relevant studies were identified by searching PubMed, ISI Web of Science and CNKI databases and by reviewing the reference lists of retrieved articles. Then, the data from eligible studies were combined in an allelic model. A total of 7 and 4 studies were ultimately included for 63A>G and 606G>C, respectively. The results of overall meta-analyses showed that significant association was detected for 63A>G (OR = 1.26, 95% CI = 1.02–1.56, P heterogeneity = 0.009, I 2 = 65.1%), but not for 606G>C (OR = 1.22, 95% CI = 0.75–1.96, P heterogeneity = 0.412, I 2 = 0.0%). Regarding 63A>G variant, positive results were also obtained in the subgroups of atrial septal defect and large-sample-size study. Besides, the sensitivity analysis indicated that significant association was still detected after deletion of the individual studies with positive result and striking heterogeneity.
Conclusion
Our results revealed that the 63A>G variant in NKX2-5, but not the 606G>C, may contribute to CHD risk for Chinese.
Introduction
Congenital heart disease (CHD) is defined as a gross structural anomaly of the heart or intrathoracic great vessels that is actually or potentially of functional significance [1]. As the most common developmental abnormality, CHD has a incidence of approximately 9.1 in 1000 new born babies worldwide and the situation is more severe in premature, death and abortion forms [2]. According to the report provided by China’s Ministry of Health in 2012, the incidence of CHD during perinatal period was estimated to be 40.95/10,000 [3]. CHD contains 15 clinical types at least and its morbidity and mortality are significantly greater than that of the general individuals, even after effective surgical correction [4], [5]. Despite its high prevalence and poor prognosis, the causes of CHD are largely unknown. Accumulating evidence has indicated that genetic factor plays a crucial role in CHD, with high degree of heritability [6]. In 2012, a systematic review conducted by Shieh JT et al. further demonstrated that CHD risk significantly increased in consanguineous unions of the studied populations, principally at first-cousin level [7].
Cardiac transcription factors, such as NKX2-5, GATA4, TBX5 and TBX20, strictly regulate the development of heart through a regulatory network [8]–[10]. Recently, a growing number of variants in these genes have been found to implicate in CHD cases [11]–[13], suggesting their potentially important roles in the occurrence of CHD. Among these cardiac transcription factors, the NKX2-5 gene, located at chromosome 5q34, was firstly identified to be involved in heart development of many organisms, such as zebrafish, frog, chicken, mouse and human being. A series of clinical manifestations for CHD including arrhythmia, cardiac contractility defects, cardiac structural defects and premature death were observed in NKX2-5 knocked out mice [14], [15]. Besides, family studies revealed that widely variable expressivity of NKX2-5 would also lead to CHD [16], [17]. Moreover, NKX2-5 not only individually acts in transcriptional regulation, but also interacts with other transcription factors in the early stage of cardiac development [18]–[24]. A synonymous variant 63A>G (rs2277923, Glu21Glu) located in the exon 1 was reported to be associated with risk of CHD. In 1999, Benson et al. firstly detected the variant in 52 normal controls [25]. Moreover, the variant was also identified by Reamon-Buettner et al. in the diseased heart tissues of 68 patients with CHD by direct sequencing, but the difference in allele frequency was insignificant between CHD cases and healthy controls [26]. However, Shi et al. observed significant association in 110 case-control pairs of Chinese [27], which was followed by a number of studies trying to replicate the attractive result in the Chinese population, but with inconsistent findings [28]–[33]. Another synonymous variant 606G>C (rs3729753, Leu202Leu) located in the exon 2 of NKX2-5, was also suspected to implicate in CHD risk in the Chinese population. Based on the controversial conclusions and limited statistical power of individual studies, as well as almost all relevant data obtained from the Chinese population, we performed a meta-analysis of published studies to provide more precise estimations for the associations between 63A>G and 606G>C variants and the risk of CHD in Chinese population.
Methods
Identification and Eligibility of Studies
Relevant articles published up to February 2013 were identified by searching PubMed and ISI Web of Science databases using the key words “NKX/CSX” and “congenital heart disease/defects” without language restriction. To expand the coverage of our searches, we further performed search in China National Knowledge Infrastructure (CNKI) database applying the above key words. References of retrieved articles were also scanned. We conducted the meta-analysis and reported its results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (Checklist S1).
We used following criteria to select the eligible studies: (1) case-control studies in the Chinese population; (2) evaluation of the NKX2-5 63A>G and/or 606G>C variant(s) and CHD risk; (3) non-syndromic CHD confirmed histologically or pathologically; (4) presentation of data necessary for calculating odds ratios (ORs) with 95% confidence intervals (CIs). All clinical types, such as atrial septal defect (ASD), ventricular septal defect (VSD), patent ductus arteriosus and patent formen ovale were included in this meta-analysis. Review, case report, simply commentary, animal study, unpublished report and the study with CHD being considered as a component of well-known genetic syndromes or a multiple congenital anomaly syndrome were excluded.
Data Extraction
Data were extracted independently by two reviewers. The following information was extracted from the eligible studies: first author, year of publication, design type of study, types of CHD, source of DNA, whether Hardy-Weinberg equilibrium (HWE) accorded in control population and counts of alleles or genotypes in case and control groups.
Statistical Analysis
Goodness-of-fit x 2 test was applied to assess HWE in controls if there were no definitive statements about whether the genotype frequencies in control groups were compatible with HWE in the original. The associations between NKX2-5 63A>G, 606G>C variants and risk of CHD were evaluated by allelic ORs and their 95% CIs because of only allele frequencies reported in some studies. The Cochran’s Q statistic test was employed to estimate the between-study heterogeneity [34]. When the P value of Q statistic test was <0.1, the random-effects model was used due to significant heterogeneity [35]; otherwise, the fixed-effects model was applied [36]. Overall meta-analysis was initially conducted and then stratified analysis, if feasible, was carried out according to the types of CHD and sample size. Additionally, sensitivity analysis was conducted, in which the pooled ORs were calculated after omission of each study in turn. Publication bias was assessed by funnel plot and Egger’s test [37]. The above analyses were conducted in Stata10.0 and all P values less than 0.05 were considered statistically significant for all tests except for Q test. Besides, the power analysis was carried out by Power V3.0 software (http://dceg.cancer.gov/tools/design/POWER).
Results
Characteristic of Included Studies
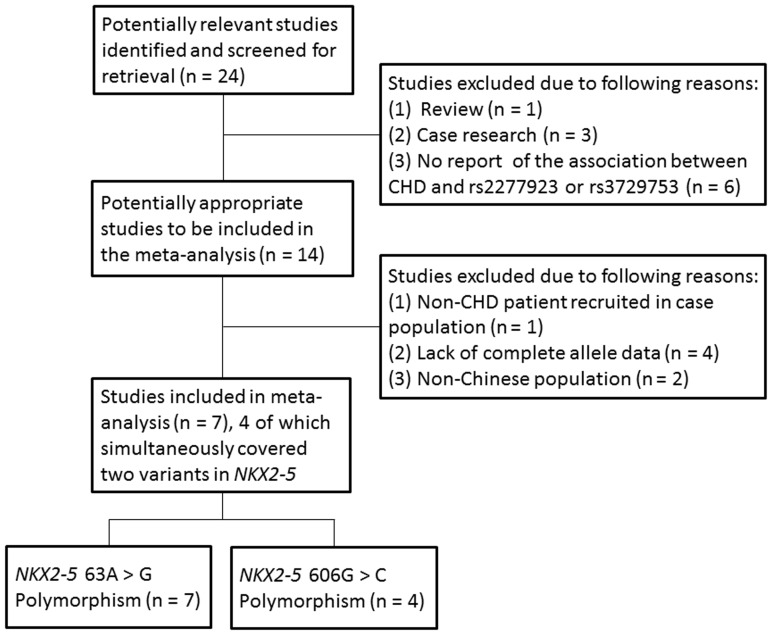
Figure 1 shows the literature search and study selection procedures. Twenty-four articles were initially identified through the above search strategy. After review of the titles and abstracts, 10 articles were excluded. Seven publications were further excluded after review of the full texts because of the following reasons: the cases in the study by Ouyang et al. suffered from coronary artery disease (CAD) and rheumatic heart disease (RHD) [38]; two studies carried out in non-Chinese populations, plus one of which extracted DNA from formalin fixed tissue samples [26], [39]; no complete allele data obtained in other four studies [18], [40], [41], [42]. Finally, 7 studies containing 1243 cases and 1139 controls were relevant to 63A>G and 4 studies containing 748 cases and 630 controls were relevant to 606G>C. Of these studies, 3 studies just explored a single type of CHD [28], [29], [33], whereas multiple types of CHD were involved in the other studies [27], [30], [31], [32]. A same set of control was applied across two studies by Liu et al [28], [29]. Besides, whether the control group in the study by Xiong et al. accordant with HWE was neither mentioned in the original, nor calculated by goodness-of-fit x 2 test due to lack of genotype frequencies [32]. The characteristics of the included studies are shown in Table 1 .
Figure 1. Flow diagram of the study selection procedure.
Table 1. Characteristics of included studies.
| First author | Publication year | Country | Study type | Variants | Types of CHD | Case/control | DNA source | HWE |
| Shi | 2005 | China | Case-control | 63A>G | Multiple | 110/110 | Blood | Y |
| Liub | 2009 | China | Case-control | 63A>G | ASD | 180/200 | Blood | Y |
| Liub | 2009 | China | Case-control | 63A>G 606G>C | VSD | 160/200 | Blood | Y |
| Zhang | 2009 | China | Case-control | 63A>G 606G>C | Multiple | 230/200 | Blood | Y |
| Peng | 2010 | China | Case-control | 63A>G 606G>C | Multiple | 63A>G: 126/114606G>C: 134/109 | Blood | Y |
| Xiong | 2012 | China | Case-control | 63A>G606G>C | Multiple | 224/121 | Blood | –a |
| Pang | 2012 | China | Case-control | 63A>G | VSD | 213/194 | Blood | Y |
Abbreviations: HWE, Hardy-Weinberg equilibrium; CHD, congenital heart disease; ASD, atrial septal defect; VSD, ventricular septal defect.
Not mentioned nor could be figured out.
A same set of control applied across two case-control analyses.
Overall Meta-analysis
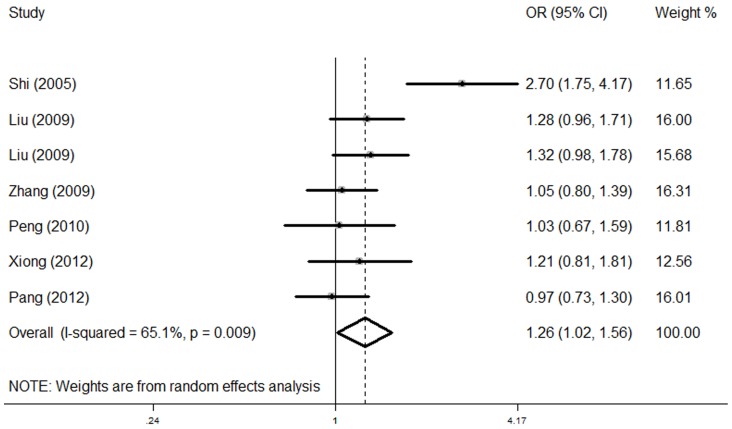
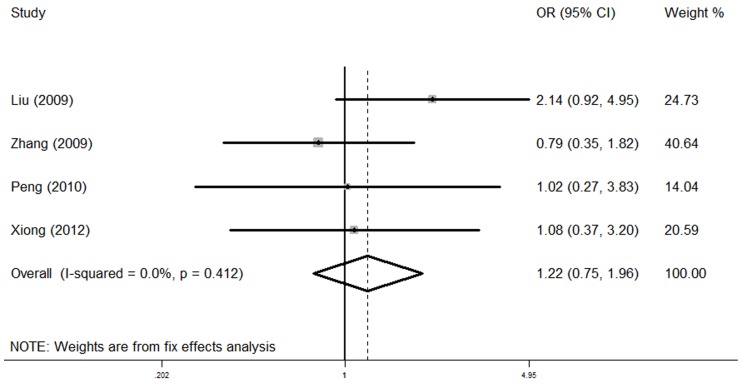
Figure 2 shows the combined result for 63A>G in associated with CHD. A random-effects model was used because of significant heterogeneity (P heterogeneity = 0.009, I 2 = 65.1%). Individuals with the NKX2-5 63G allele showed a significantly increased CHD risk compared with those with the A allele (OR = 1.26, 95% CI = 1.02–1.56, P = 0.034). For 606G>C, no substantial heterogeneity was detected (P heterogeneity = 0.412). In the fixed-effects model, no significant association between this variant and CHD was found (OR = 1.22, 95% CI = 0.75–1.96, P = 0.422, P heterogeneity = 0.412, I 2 = 0.0%, Figure 3 ). Besides, for NKX2-5 A63G with minor allele frequency of 0.433, we calculated that the power for our sample size to detect an OR of 1.26 was 0.801; for NKX2-5 G606C with minor allele frequency of 0.022, the power for our sample size to detect an OR of 1.22 was 0.079.
Figure 2. The forest plots of ln (OR) with 95% CIs for the NKX2-5 63A>G for CHD.
Random-effects pooled OR = 1.26, 95% CI = 1.02–1.56, P = 0.034; P heterogeneity = 0.009.
Figure 3. The forest plots of ln (OR) with 95% CIs for the NKX2-5 606G>C for CHD.
Fixed-effects pooled OR = 1.22, 95% CI = 0.75–1.96, P = 0.422; P heterogeneity = 0.412.
Stratified Analysis
The data were firstly stratified by the types of CHD into two subgroups, the VSD and ASD subgroups. Significant association was observed between 63A>G and ASD (OR = 1.31, 95% CI = 1.00–1.71, P = 0.048, P heterogeneity = 0.719, I 2 = 0.0%). However, no statistical evidence for the association between 63A>G and VSD was detected (OR = 1.67, 95% CI = 0.87–3.21, P = 0.124, P heterogeneity = 0.000, I 2 = 89.5%, Table 2 ).
Table 2. Associations between NKX2-5 63A>G and CHD stratified by types of CHD and sample size.
| Variables | No.a | Case/control | OR (95% CI) | I-square (%) | P for heterogeneityb |
| Type | |||||
| VSD | 3 | 433/504 | 1.67 (0.87–3.21) | 89.5% | P = 0.000 |
| ASD | 2 | 202/310 | 1.31 (1.00–1.71) | 0.0% | P = 0.719 |
| Sample sizec | |||||
| Small | 2 | 236/224 | 1.67 (0.65–4.27) | 89.4% | P = 0.002 |
| Large | 5 | 1007/665 | 1.15 (1.01–1.32) | 0.0% | P = 0.538 |
Abbreviations: OR, Odds ratio; CI, confidence interval.
The number of articles.
When P value of the heterogeneity test was >0.1, the fixed-effects model was used; otherwise, the random-effects model was applied.
The study was regarded as large-sample-size study, if the number of case was greater than 150; otherwise, the study was defined as small-sample-size study.
The data were then stratified by sample size. The study was regarded as large-sample-size if the number of case is greater than 150; otherwise, the study was defined as small-sample-size [43], [44]. In the large-sample-size subgroup, significant association between 63A>G and CHD was detected (OR = 1.15, 95% CI = 1.01–1.32, P = 0.040, P heterogeneity = 0.538, I 2 = 0.0%). While no significant risk of CHD associated with 63A>G was observed in the small-sample-size subgroup (OR = 1.67, 95% CI = 0.65–4.27, P = 0.285, P heterogeneity = 0.002, I 2 = 89.4%, Table 2 ).
The association regarding 606G>C and CHD could not be assessed due to insufficient data.
Sensitivity Analysis
Given the significant between-study heterogeneity, sensitivity analysis was carried out to assess the effect of each study on the overall estimate. The association between 63A>G and CHD turned to be marginal statistical significance when several studies were omitted. Besides, the heterogeneity was drastically reduced after deletion of the study by Shi et al. (P heterogeneity = 0.648, I 2 = 0.0%)( Table 3 ). In terms of 606G>C, the sensitivity analysis demonstrated relatively robust results with no reverse outcome ( Table 3 ).
Table 3. Sensitivity analysis of pooled studies for CHD on NKX2-5 63A>G and 606G>C.
| Variant | Study omitted | OR (95% CI) | I-square (%) | P for heterogeneitya |
| 63A>G | Shi | 1.14 (1.00–1.30) | 0.0 | 0.648 |
| Liu | 1.26 (0.97–1.64) | 70.7 | 0.004 | |
| Liu | 1.25 (0.97–1.62) | 70.3 | 0.005 | |
| Zhang | 1.31 (1.02–1.68) | 68.3 | 0.007 | |
| Peng | 1.30 (1.02–1.65) | 69.8 | 0.005 | |
| Xiong | 1.27 (1.00–1.63) | 70.9 | 0.004 | |
| Pang | 1.32 (1.05–1.68) | 64.9 | 0.014 | |
| 606G>C | Liu | 0.91 (0.51–1.64) | 0.0 | 0.890 |
| Zhang | 1.51 (0.83–2.73) | 0.0 | 0.507 | |
| Peng | 1.25 (0.75–2.08) | 28.4 | 0.247 | |
| Xiong | 1.25 (0.74–2.13) | 29.1 | 0.244 |
When P value of the heterogeneity test was >0.1, the fixed-effects model was used. Otherwise, the random-effects model was used.
Publication Bias
As reflected by funnel plots (Figure S1 and Figure S2) and Egger’s test, no publication bias was detected for 63A>G (P = 0.249) and 606G>C in NKX2-5 (P = 0.797).
Discussion
The current meta-analysis suggested that the 63A>G variant in NKX2-5 was significantly associated with the risk of CHD in the Chinese population, whereas the 606 G>C did not appear to have an effect on CHD susceptibility. Besides, positive results with regard to 63A>G were found in the subgroups of ASD and large-sample-size study with no heterogeneity.
NKX2-5 acts as a prominent candidate CHD-associated gene given its crucial role in heart morphogenesis and function. Embryonic lethality, growth retardation and abnormal heart morphogenesis were observed in mice with targeted NKX2-5 disruption due to impaired cardiac looping [45]. NKX2-5, as the fifth gene identified in the NK-2 homeobox gene family, consists of two exons which encode a 324-amino-acid protein [46], [47]. The protein encoded by NKX2-5 is a transcription factor comprising homeodomain (HD), TN and NK2-specific (NK2-SD) domains. It is estimated that there are more than 33 variants detected in NKX2-5 as yet, in which, the 63A>G and 606G>C polymorphsims studied most extensively were included in our meta-analysis [31], [48]. The 63A>G variant was found to be significantly associated with CHD risk in this meta-analysis. Synonymous variants are gradually acknowledged to alter protein expression, conformation and function through mechanisms of affecting splicing accuracy, translation fidelity, mRNA structure and protein folding [49]. It has reported that the 63A>G variant weakened the transactivation activity of NKX2.5 by approximately 20% [38]. Furthermore, the RESCUE ESE, a program for validation of exonic splicing enhancers, demonstrated that 63A>G could repeal an exonic splice site starting 4 base pairs upstream of the variant [50], exerting an influence on translational kinetics. Although there are some evidence for the role of 63A>G variant in CHD, the underlying mechanism is required further investigation. Alternatively, we cannot rule out the possibility of its involvement to linkage disequilibrium (LD) with other disease causing variants. For another variant 606G>C, no promising association was found in this meta-analysis, which was consistent with multiple studies [29], [30], [31], [32]. For instance, Zhang et al. indicated that there was no significant difference for this variant in the allele and genotype frequencies between CHD and controls [30]. Besides, no biological and functional analyses have been reported about this variant yet. Thus, the 606G>C variant may not contribute significantly to CHD risk, but the result should be treated with caution because of the low power obtained from our sample size.
To explore the sources of heterogeneity, we further conducted stratified and sensitivity analyses for 63A>G variant. Stratified analyses by the types of CHD and sample size suggested that heterogeneity only presented in the VSD and small-sample-size subgroups, while heterogeneity was effectively removed after deletion of the study by Shi et al. in the sensitivity analysis. Interestingly, the study by Shi et al. was included in the VSD and small-sample-size subgroups, implying that the heterogeneity found in the two subgroups might result from this study. Under review of this report, Shi et al. proposed that 63A>G variant was not significantly associated with ASD, but with VSD. Individuals carrying G allele had a 4.32 - fold increased risk of VSD than those with A allele, which did not conform to other studies. But positive result was still observed after removal of the study conducted by Shi et al., indicating the relatively stability of current meta-analysis. Besides, marginal statistical significance was presented in the sensitivity analysis when several studies were omitted, which was probably caused by limited data and modest effect of this variant.
Some limitations of this meta-analysis should be acknowledged. First, there is evidence that the roles of variants in NKX2-5 gene may differ across racial backgrounds, while the current meta-analysis was specifically based on the Chinese population, baffling the generalization of our conclusion to other ethnic populations. Second, the sample size of our meta-analysis was relatively small, especially for NKX2-5 606G>C. Besides, all included studies were retrospectively designed. Thus, additional large-scale and well designed studies will be required to further confirm our results. Third, we only considered the allelic model in current study because of limited data. Other genetic models should be taken into account to validate our results. Fourth, some heterogeneous natures of studies, including different phenotypes of CHD, female/male ratios and match conditions of control group probably affected our results. However, we were unable to perform further analyses due to lack of detail data.
In summary, our meta-analysis helped for clarifying the discrepancies of genetic studies into associations of NKX2-5 63A>G and 606G>C variants with CHD and revealed that the 63A>G, but not the 606G>C was significantly associated with the risk of CHD in the Chinese population. Although the summary risk for developing CHD with the variants of NKX2-5 gene may be small, CHD occurs in high incidence in China and even a small increase in risk will translate to a large number of potential CHD cases.
Supporting Information
The funnel plot for NKX2-5 63A>G.
(TIF)
The funnel plot for NKX2-5 606G>C.
(TIF)
The PRISMA 2009 Checklist.
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Mitchell SC, Korones SB, Berendes HW (1971) Congenital Heart Disease in 56,109 Births Incidence and Natural History. Circulation 43: 323–332. [DOI] [PubMed] [Google Scholar]
- 2. van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, et al. (2011) Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 58: 2241–2247. [DOI] [PubMed] [Google Scholar]
- 3.National Health and Family Planning Commission Website. Available: http://www.moh.gov.cn/mohfybjysqwss/s7901/201209/55840.shtml. Accessed 2013 May 1.
- 4. van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, et al. (2011) The changing epidemiology of congenital heart disease. Nat Rev Cardiol 8: 50–60. [DOI] [PubMed] [Google Scholar]
- 5. Verheugt CL, Uiterwaal CS, van der Velde ET, Meijboom FJ, Pieper PG, et al. (2010) Mortality in adult congenital heart disease. Eur Heart J 31: 1220–1229. [DOI] [PubMed] [Google Scholar]
- 6. Pierpont ME, Basson CT, Benson DW Jr, Gelb BD, Giglia TM, et al. (2007) Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 115: 3015–3038. [DOI] [PubMed] [Google Scholar]
- 7. Shieh JTC, Bittles AH, Hudgins L (2012) Consanguinity and the risk of congenital heart disease. American Journal of Medical Genetics Part A 158A: 1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunwoodie SL (2007) Combinatorial signaling in the heart orchestrates cardiac induction, lineage specification and chamber formation. Seminars in Cell & Developmental Biology 18: 54–66. [DOI] [PubMed] [Google Scholar]
- 9. Rochais F, Mesbah K, Kelly RG (2009) Signaling Pathways Controlling Second Heart Field Development. Circulation Research 104: 933–942. [DOI] [PubMed] [Google Scholar]
- 10. Scholl AM, Kirby ML (2009) Signals controlling neural crest contributions to the heart. Wiley Interdisciplinary Reviews: Systems Biology and Medicine 1: 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Granados-Riveron JT, Pope M, Bu'Lock FA, Thornborough C, Eason J, et al. (2012) Combined Mutation Screening of NKX2–5, GATA4, and TBX5 in Congenital Heart Disease: Multiple Heterozygosity and Novel Mutations. Congenital Heart Disease 7: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu G, Shan J, Pang S, Wei X, Zhang H, et al. (2012) Genetic analysis of the promoter region of the GATA4 gene in patients with ventricular septal defects. Transl Res 159: 376–382. [DOI] [PubMed] [Google Scholar]
- 13.Beffagna G, Cecchetto A, Dal Bianco L, Lorenzon A, Angelini A, et al.. (2012) R25C mutation in the NKX2.5 gene in Italian patients affected with non-syndromic and syndromic congenital heart disease. J Cardiovasc Med (Hagerstown). [DOI] [PubMed]
- 14. Akazawa H, Komuro I (2005) Cardiac transcription factor Csx/Nkx2–5: Its role in cardiac development and diseases. Pharmacology & Therapeutics 107: 252–268. [DOI] [PubMed] [Google Scholar]
- 15. Terada R, Warren S, Lu JT, Chien KR, Wessels A, et al. (2011) Ablation of Nkx2–5 at mid-embryonic stage results in premature lethality and cardiac malformation. Cardiovascular Research 91: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kasahara H, Ueyama T, Wakimoto H, Liu Margaret K, Maguire Colin T, et al. (2003) Nkx2.5 homeoprotein regulates expression of gap junction protein connexin 43 and sarcomere organization in postnatal cardiomyocytes. Journal of Molecular and Cellular Cardiology 35: 243–256. [DOI] [PubMed] [Google Scholar]
- 17. Benson D (2010) Genetic Origins of Pediatric Heart Disease. Pediatric Cardiology 31: 422–429. [DOI] [PubMed] [Google Scholar]
- 18. Hamanoue H, Rahayuningsih SE, Hirahara Y, Itoh J, Yokoyama U, et al. (2009) Genetic screening of 104 patients with congenitally malformed hearts revealed a fresh mutation of GATA4 in those with atrial septal defects. Cardiol Young 19: 482–485. [DOI] [PubMed] [Google Scholar]
- 19. Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S (1999) The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development 126: 1269–1280. [DOI] [PubMed] [Google Scholar]
- 20. Zou Y, Evans S, Chen J, Kuo HC, Harvey RP, et al. (1997) CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2–5 homeobox gene pathway. Development 124: 793–804. [DOI] [PubMed] [Google Scholar]
- 21. Kiewitz R, Lyons GE, Schäfer BW, Heizmann CW (2000) Transcriptional regulation of S100A1 and expression during mouse heart development. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1498: 207–219. [DOI] [PubMed] [Google Scholar]
- 22. Torrado M, Nespereira B, López E, Centeno A, Castro-Beiras A, et al. (2005) ANKRD1 specifically binds CASQ2 in heart extracts and both proteins are co-enriched in piglet cardiac Purkinje cells. Journal of Molecular and Cellular Cardiology 38: 353–365. [DOI] [PubMed] [Google Scholar]
- 23. Willingham-Rocky LA, Golding MC, Wright JM, Kraemer DC, Westhusin ME, et al. (2007) Cloning of GJA1 (connexin43) and its expression in canine ovarian follicles throughout the estrous cycle. Gene Expression Patterns 7: 66–71. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe Y, Zaffran S, Kuroiwa A, Higuchi H, Ogura T, et al.. (2012) Fibroblast growth factor 10 gene regulation in the second heart field by Tbx1, Nkx2–5, and Islet1 reveals a genetic switch for down-regulation in the myocardium. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed]
- 25. Benson DW, Silberbach GM, Kavanaugh-McHugh A, Cottrill C, Zhang Y, et al. (1999) Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. The Journal of Clinical Investigation 104: 1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reamon-Buettner SM, Borlak J (2004) Somatic NKX2–5 mutations as a novel mechanism of disease in complex congenital heart disease. J Med Genet 41: 684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi L, Shen AD, Li XF, Bai S, Guan XL, Li ZZ (2005) Mutation Screening of Nkx2.5 Gene and Associated Study in Exon1 in Chinese with Congenital Heart Disease. Journal of Capital University Medical Sciences 26.
- 28. Liu XY, Yang YQ, Yang Y, Lin XP, Chen YH (2009) Mutation of NKX2–5 gene in patients with atrial septal defect. Zhonghua Er Ke Za Zhi 47: 696–700. [PubMed] [Google Scholar]
- 29. Liu XY, Yang YQ, Yang Y, Lin XP, Chen YH (2009) Novel NKX2–5 mutations identified in patients with congenital ventricular septal defects. Zhonghua Yi Xue Za Zhi 89: 2395–2399. [PubMed] [Google Scholar]
- 30. Zhang W, Li X, Shen A, Jiao W, Guan X, et al. (2009) Screening NKX2.5 mutation in a sample of 230 Han Chinese children with congenital heart diseases. Genet Test Mol Biomarkers 13: 159–162. [DOI] [PubMed] [Google Scholar]
- 31. Peng T, Wang L, Zhou SF, Li X (2010) Mutations of the GATA4 and NKX2.5 genes in Chinese pediatric patients with non-familial congenital heart disease. Genetica 138: 1231–1240. [DOI] [PubMed] [Google Scholar]
- 32.Xiong F, Li Q, Zhang C, Chen Y, Li P, et al.. (2012) Analyses of GATA4, NKX2.5, and TFAP2B genes in subjects from southern China with sporadic congenital heart disease. Cardiovasc Pathol. [DOI] [PubMed]
- 33. Pang S, Shan J, Qiao Y, Ma L, Qin X, et al. (2012) Genetic and Functional Analysis of the NKX2–5 Gene Promoter in Patients With Ventricular Septal Defects. Pediatr Cardiol 33: 1355–1361. [DOI] [PubMed] [Google Scholar]
- 34. Zintzaras E, Ioannidis JP (2005) HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics 21: 3672–3673. [DOI] [PubMed] [Google Scholar]
- 35. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 36. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 37. Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ouyang P, Saarel E, Bai Y, Luo C, Lv Q, et al. (2011) A de novo mutation in NKX2.5 associated with atrial septal defects, ventricular noncompaction, syncope and sudden death. Clinica Chimica Acta 412: 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dinesh SM, Kusuma L, Ramachandra NB (2010) The Most Frequent c.239A>G SNP of NKX2.5 is not Involved in Congenital Heart Disease. New York Science Journal 3(8): 43–47. [Google Scholar]
- 40. Ding JD, Li KR, Zhang XL, Yao YY, Reng LQ, et al. (2009) [Preliminary exploration of transcription factor Nkx2.5 mutations and congenital heart diseases]. Zhonghua Yi Xue Za Zhi 89: 1114–1116. [PubMed] [Google Scholar]
- 41. Draus JM Jr, Hauck MA, Goetsch M, Austin EH 3rd, Tomita-Mitchell A, et al (2009) Investigation of somatic NKX2–5 mutations in congenital heart disease. J Med Genet 46: 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khetyar M, Tinworth L, Syrris P, Abushaban L, Abdulazzaq Y, et al. (2008) NKX2.5/NKX2.6 mutations are not a common cause of isolated type 1 truncus arteriosus in a small cohort of multiethnic cases. Genet Test 12: 467–469. [DOI] [PubMed] [Google Scholar]
- 43. Zou L, Chen W, Shao S, Sun Z, Zhong R, et al. (2012) Genetic variant in KIAA0319, but not in DYX1C1, is associated with risk of dyslexia: An integrated meta-analysis. Am J Med Genet B Neuropsychiatr Genet 159B: 970–976. [DOI] [PubMed] [Google Scholar]
- 44. Song RR, Zou L, Zhong R, Zheng XW, Zhu BB, et al. (2011) An integrated meta-analysis of two variants in HOXA1/HOXB1 and their effect on the risk of autism spectrum disorders. PLoS One 6: e25603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, et al. (1995) Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2–5. Genes Dev 9: 1654–1666. [DOI] [PubMed] [Google Scholar]
- 46. Turbay D, Wechsler SB, Blanchard KM, Izumo S (1996) Molecular cloning, chromosomal mapping, and characterization of the human cardiac-specific homeobox gene hCsx. Mol Med 2: 86–96. [PMC free article] [PubMed] [Google Scholar]
- 47. Shiojima I, Komuro I, Mizuno T, Aikawa R, Akazawa H, et al. (1996) Molecular Cloning and Characterization of Human Cardiac Homeobox Gene CSX1. Circulation Research 79: 920–929. [DOI] [PubMed] [Google Scholar]
- 48. Stallmeyer B, Fenge H, Nowak-Gottl U, Schulze-Bahr E (2010) Mutational spectrum in the cardiac transcription factor gene NKX2.5 (CSX) associated with congenital heart disease. Clin Genet 78: 533–540. [DOI] [PubMed] [Google Scholar]
- 49. Sauna ZE, Kimchi-Sarfaty C (2011) Understanding the contribution of synonymous mutations to human disease. Nat Rev Genet 12: 683–691. [DOI] [PubMed] [Google Scholar]
- 50. Fairbrother WG, Holste D, Burge CB, Sharp PA (2004) Single nucleotide polymorphism-based validation of exonic splicing enhancers. PLoS Biol 2: E268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The funnel plot for NKX2-5 63A>G.
(TIF)
The funnel plot for NKX2-5 606G>C.
(TIF)
The PRISMA 2009 Checklist.
(DOC)