Summary
Sensing of an electric field (EF) by cells—galvanotaxis—is important in wound healing [1], development [2], cell division, nerve growth, and angiogenesis [3]. Different cell types migrate in opposite directions in EFs [4], and the same cell can switch the directionality depending on conditions [5]. A tug-of-war mechanism between multiple signaling pathways [6] can direct Dictyostelium cells to either cathode or anode. Mechanics of motility is simplest in fish keratocytes, so we turned to keratocytes to investigate their migration in EFs. Keratocytes sense electric fields and migrate to the cathode [7, 8]. Keratocyte fragments [9, 10] are the simplest motile units. Cell fragments from leukocytes are able to respond to chemotactic signals [11], but whether cell fragments are galvanotactic was unknown. We found that keratocyte fragments are the smallest motile electric field-sensing unit: they migrate to the anode, in the opposite direction of whole cells. Myosin II was essential for the direction sensing of fragments but not for parental cells, while PI3 kinase was essential for the direction sensing of whole cells but not for fragments. Thus, two signal transduction pathways, one depending on PI3K, another on myosin, compete to orient motile cells in the electric field. Galvanotaxis is not due to EF force and does not depend on cell or fragment size. We propose a “compass” model according to which protrusive and contractile actomyosin networks self-polarize to the front and rear of the motile cell, respectively, and the electric signal orients both networks toward cathode with different strengths.
Results and Discussion
Cells and Their Fragments Migrate in Opposite Directions in an EF
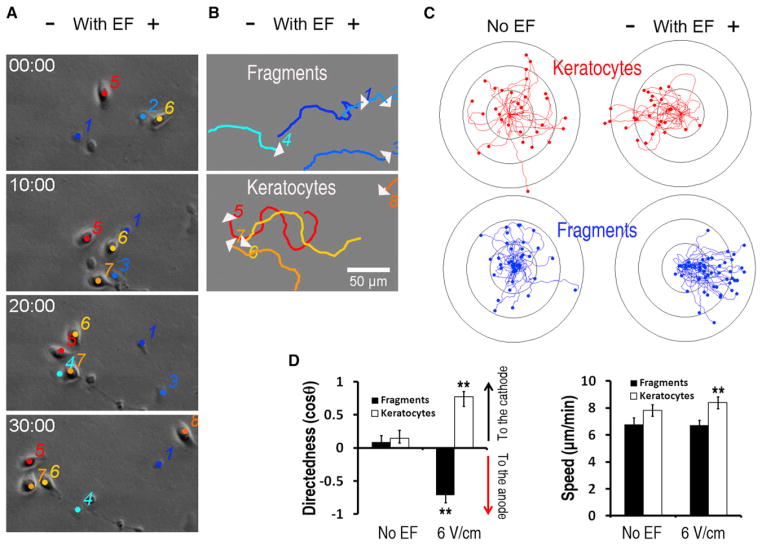
Keratocytes, as well as other motile cell types [12], can spontaneously detach cell fragments that move with shapes, speeds, and persistence similar to those of whole cells [9, 10]. Both fragments and parental cells cultured without application of an electric field (EF) showed migration in random directions, with a directedness value close to zero (Figures 1C and 1D). When exposed to EFs, both cells and fragments, which are devoid of nuclei and major organelles (Figure S1 available online), migrated directionally. Surprisingly, the cell fragments migrated to the anode, while the parental cells migrated to the cathode (Figure 1, Movie S1, and Movie S2).
Figure 1. EFs Direct Migration of Fragments to the Anode, in the Opposite Direction of that of the Parental Keratocytes.
(A) Fragments (1 to 4) migrate to the anode in EFs, in an opposite direction of their parental keratocytes (5 to 8). Time is in min:s. The scale bar represents 50 μm.
(B and C) Migration trajectories over 30 min of keratocytes (red in C) and fragments (blue in C) in the presence or absence of EFs with polarity as shown. White arrows indicate migration direction in (B).
(D) Directedness and trajectory speed of fragments and keratocytes. Data are shown as means ± SEM. See Figure S2 and Table S1 for related data. **p < 0.01 when compared to the control in the absence of an EF.
EF = 6 V/cm. Ring unit, 100 μm; duration, 30 min. See also Figure S2, Table S1, Movie S1, and Movie S2.
This directional migration was further confirmed with multiple reversal of the EF polarity, which induced rapid reversal of the migration direction every time in both fragments and cells (Figure S2). Starting from an EF strength of 0.5 V/cm, increase of EFs increased directedness for both cells and fragments (Table S1). The speed of cells, but not of fragments, increased moderately with the EF strength (Figure 1D).
The shapes of cells and fragments before EF application were not changed significantly by EFs (Figures S3A and S3B). For keratocyte cells [13] and fragments [10], it has been shown that quantitative changes in the principal modes of variations of cell shape are indicators of changes in the self-organization of the actin-myosin networks and consequent changes in biophysical motile machinery. Thus, the EF invariance of the shapes of fragments and cells indicates that an EF mainly orients fragments and cells without affecting organization of the actin-myosin networks. To assess the role of actin dynamics in EF sensing, we used the Arp2/3 inhibitor CK-666. Both perturbed cells and fragments slowed down significantly, yet remained strongly directional, to cathode and anode, respectively (Figure 2 and Table S1). Thus, actin machinery itself is unlikely to be a part of the signaling transduction pathway of galvanotaxis.
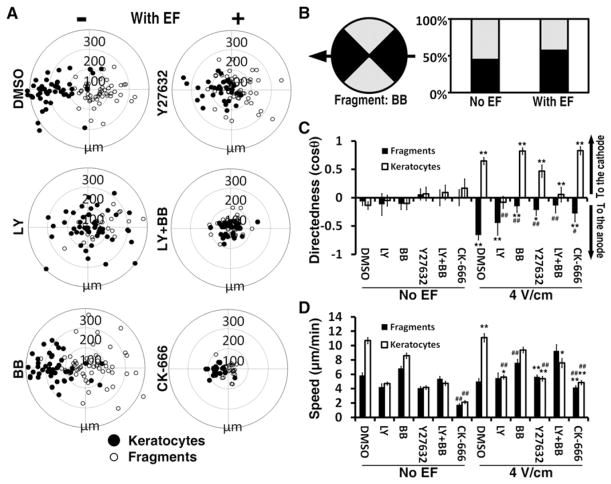
Figure 2. Distinctive Roles for Myosin and PI3K Signaling in Cells and Fragments in Determination of EF-Guided Migration Direction.
(A) Trajectories’ end points (after 30 min migration) of keratocytes (black circles) and fragments (white circles). PI3 kinase inhibition (LY) biases cells’ directionally in parallel to the EF vector, with the majority of cells going to the anode, although many cells still migrate to the cathode, and had no effects on directional migration of fragments. Inhibition of myosin (BB and Y27632) abolished directional migration of fragments but had no significant effects on keratocytes. Double inhibition of PI3K and myosin (LY+BB) rendered both cells and fragments nondirectional. Inhibition of Arp2/3 (CK-666) slowed down both cells and fragments but did not cancel their respective directionalities.
(B) EF biases fragments’ directionally in parallel to the EF vector (arrow) in the presence of myosin inhibitor. The percentage of the fragments in horizontal quadrants (dark) was increased upon EF stimulation. The differences between the no-EF and EF groups are significant (p < 0.01).
(C and D) Directedness (C) and trajectory speed (D) of fragments and keratocytes in (A). * (p < 0.05) and ** (p < 0.01) indicate that the difference between no EF and an EF is significant. ## (p < 0.01) indicates that the difference between drug treatment and DMSO vector control is significant. Data are shown as means ± SEM.
EF = 4 V/cm. Ring unit, 100 μm; duration, 30 min. See also Figure S4 and Table S1.
PI3K Inhibition Does Not Affect Fragments, but Reverses Cells to the Anode
What could be the mechanisms for fragments to migrate oppositely to their parental cells? In neutrophils, two antagonistic pathways—“frontness” and “backness”—were proposed to segregate the actin-protruding and actin-myosin contractile networks to the front and rear, respectively, and to orient the front up the chemotactic gradient [14]. When Rac was inhibited and the frontness pathway weakened, the cell rear oriented up the chemotactic gradient, and cell migration shifted down the gradient. Though galvanotaxis has a different sensor, it shares known signal transduction pathways and molecules with chemotaxis (PI3Ks, PIP2/PIP3, and PTEN in Dictyostelium [15] and microtubules and Rho GTPases in nerve growth cones [16]).
We hypothesized that, similarly, two pathways—frontness and backness—compete to orient cells in EFs in opposite directions, and that the frontness is stronger than the backness in cells, with the opposite being true in fragments. This hypothesis predicts that if the frontness is weakened, the cells in an EF would reorient from the cathode to the anode by the previously overwhelmed backness. On the other hand, the fragments’ directionality should not be affected because in them the backness dominates, and weakening of the frontness would not change this balance.
PI3 kinase is a key part of direction-sensing frontness pathways relaying signals to actin [17]; therefore, we tested whether PI3K influences the migration direction of keratocytes and fragments. PI3K inhibition with LY294002, in agreement with our hypothesis, switched the directional migration of cells from the cathode to the anode (Figure 2). Although the overall number of motile cells and cell speed were decreased by this perturbation, a great fraction of keratocytes maintained persistent motility (Figure S4A). As can be seen clearly in stronger EFs (Figure S4B), the majority of cells migrate to the anode, but a few cells maintain the cathodal migration. As expected, LY294002 did not have significant effects on the anode-directed migration of fragments (Figure 2).
Myosin Inhibition Disrupts Directional Sensing of Fragments but Not Cells
According to our hypothesis, weakening of the backness should reorient the fragments from the anode to the cathode, but not the cells’ cathodal migration. We tested the effect of myosin inhibition, because myosin is associated with controlling the motile cell rear. Indeed, blebbistatin, a small-molecule inhibitor of myosin, did not affect the directional cell migration (Figure 2). However, rather than reorienting the fragments, blebbistatin treatment abolished the anode directed migration of fragments (Figure 2). Interestingly, fragments did not migrate in completely random direction: a statistically significant majority of the myosin-inhibited fragments in EFs migrated along the EF direction, to either the anode or the cathode (Figure 2B). We used another compound, Y27632, to inhibit the Rho-associated kinase (ROCK) and myosin downstream. ROCK inhibition abolished directional migration of fragments to the anode but had little effects on cathode directed migration of cells (Figure 2).
To investigate whether the signal transduction was limited to the PI3K- and myosin-mediated pathways, we applied LY294002 and blebbistatin simultaneously. When both pathways were inhibited, directionality of both cells and fragments in EFs was lost completely (Figure 2 and Table S1). Moreover, there was no longer a bias to the quadrants represented by either the cathode or anode as opposed to the up or down quadrants. Therefore, a parallel independent pathway transducing the EF signal is unlikely.
Depletion of Extracellular Calcium Disrupts Directional Sensing of Fragments but Not Cells
We then asked what could be the basis for different relative strengths of the frontness and backness pathways in cells and fragments and investigated possible regulators upstream of myosin. The fragments are devoid of endoplasmic reticulum (Figure S1), the major store of intracellular Ca2+, which could alter Ca2+ dynamics in fragments. Ca2+ channels are involved in keratinocytes’ galvanotaxis [18] and are proposed to regulate keratocytes’ response to EFs [7]. Varying extracellular Ca2+ affects the direction of granulocyte migration in EFs [5].
We used EGTA, a chelating agent, to deplete extracellular Ca2+. In addition to lowering the average speeds of cells and fragments, this perturbation rendered fragments nondirectional in EFs (Figures 3A and 3B). Because myosin and adhesion turnover are regulated through Ca2+ [19], one possibility is that due to different Ca2+ regulation in cells and fragments, myosin and adhesion strengths and distributions are affected differently, leading to different transduction of the galvanotactic signals along two pathways in cells and fragments.
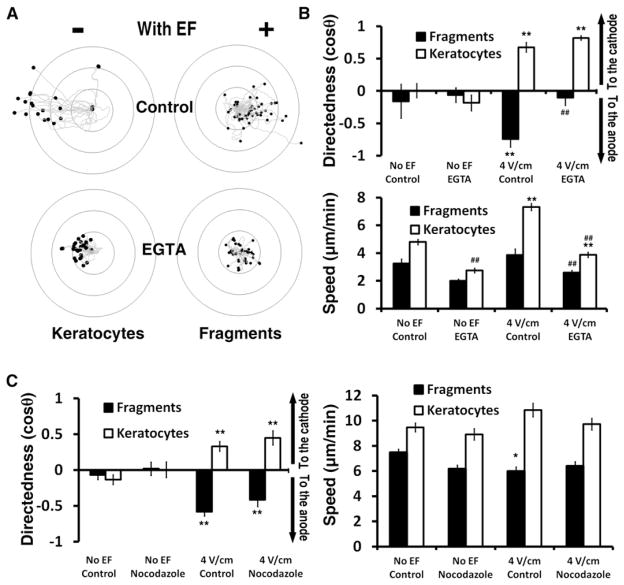
Figure 3. Calcium Signaling Is Required for Galvanotaxis of Fragments but Not Cells, Whereas Microtubules Are Dispensable for Both.
(A) EGTA (5 mM) abolished the directional migration of fragments, but not that of keratocytes. Migration speed decreased significantly in both the fragments and keratocytes.
(B) Quantification of directionality and speed of keratocytes and fragments with or without Ca2+ depletion. EGTA impairs fragment directionality and slows down both cell and fragment migration. **p < 0.01 when compared to no EF control; ##p < 0.01 when compared to that in EF without treatment of EGTA.
(C) Directionality and speed of keratocytes and fragments in the presence and absence of Nocodazole. * (p < 0.05) and ** (p < 0.01) indicate that the difference between no EF and EF is significant. ## (p < 0.01) indicates that the difference between Nocodazole and DMSO vector control is significant.
EF = 4 V/cm. Ring unit, 100 μm; duration, 30 min. In (B) and (C), data are shown as means ± SEM. See also Figure S1 and Table S1.
Perturbation of Microtubules Has No Effect on Cell and Fragment Galvanotaxis
Another difference between the cells and fragments is the presence of microtubules in the former and absence in the latter [20]. Microtubules do not affect keratocyte cell motility [13], and disruption of microtubules does not affect keratocytes’ galvanotaxis [10]. Disruption of microtubules with nocodazole did not affect the directedness of either cells or fragments in EFs (Figure 3C). Thus, different microtubule-mediated signaling is not the cause of the different balance of the anode- and cathode-directing pathways of galvanotaxis.
Directional Sensing Does Not Depend on Cell or Fragment Sizes
Yet another difference between the cells and fragments is their size, with cells having four times greater surface area than fragments (Figure S3C). This could potentially lead to differences in both initial EF-sensing mechanisms (the effect of the membrane potential gradient is proportional to both the cell size and EF strength [21]) and signal transduction (i.e., reaction-diffusion-based sensing depends on proper scaling of chemical concentrations with size [22]). However, neither cell nor fragment directedness depends on their sizes, while the directedness of both depends on the EF strength (Figure S3C). In fact, the smaller fragments are more sensitive to weaker EFs (~0.5 V/cm) than the larger cells (~1 V/cm) (Table S1).
The Galvanotactic Effect Is Not the Result of Direct Electric Force Sensing
In principle, if the cell and fragment bear opposite surface charges, the electrostatic and osmotic forces on their surfaces in EF would be opposite [23]. However, in suspension, both cells and fragments are dragged to the cathode with similar speeds in EFs (Figure S3D), so their surfaces have charges of the same sign and similar density, and the electric forces would drag them in the same direction.
Another serendipitous observation argues against the direct force-sensing: often, fragments stay connected to mother cells by membrane tethers. (The diffusion of signaling molecules through the tether is much slower than the characteristic migration time scale [24], so the mother cell and fragment do not share signaling pathways.) In this state, cell or fragment pairs often moved in opposite directions (cathode or anode, respectively) for minutes, until the tether was stretched up to tens of microns (Figure 4 and Movie S3), at which point both cell and fragment were stalled. When PI3K was inhibited, both cell and fragment connected by the tether migrated to anode, but the cell was usually faster and would outrun the fragment, after which the cell and fragment in its wake traveled together without stretching the tether further (Figure 4). When myosin was inhibited, the cathode-directed cell rapidly left the fragment behind, stretching the tether sometime by more than 100 μm (Figures 4A and 4B). This indicates that cells and fragments are unlikely to respond to mechanical force exerted by EFs on charged cells, because such force of at most 10 pN [23] would be overwhelmed by the opposite tether force of tens of piconewtons. The EF signal orients the tethered cell and fragment effectively enough for the cell and fragment to persist despite the opposing mechanical forces.
Figure 4. Galvanotaxis of Fragments Tethered to Mother Cells.
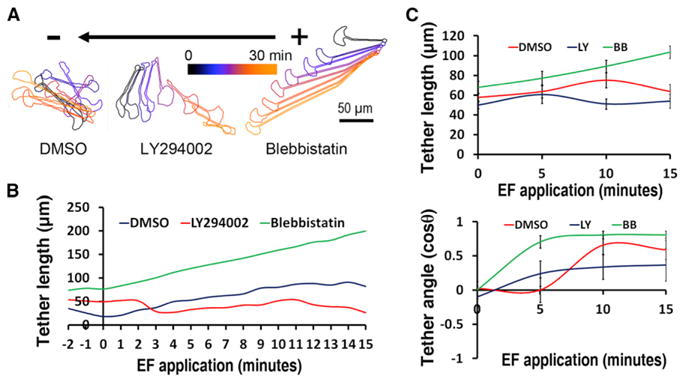
(A) Migration of tethered fragments and cells in the presence of DMSO (left), LY294002 (middle) or Blebbistatin (right), in an EF of 4 V/cm in the indicated orientation. The overlaid contours of different time point after EF application are color coded. The scale bar represents 50 μm.
(B) Typical scatter plots of tether lengths in the presence of blebbistatin (green line, increasing length) and LY294002 (red line, decreasing length) when compared to DMSO (blue line, increasing length).
(C) Quantification of average tether lengths and tether angles after EF exposure in the presence of blebbistatin (green line) and LY294002 (red line) when compared to DMSO (blue line). Note that the average direction of the tether in the control case does not change for a while because initially the cell and fragment pass each other side by side in the opposite directions, and the tether simply rotates. After that, the cell is closer to the cathode and the fragment is closer to the anode, and their further divergence orients the tether along the EF. In the case of the perturbations, the tether is oriented by the leading cell much faster. Data are shown as means ± SEM.
Finally, we tested how the cell and fragment directedness depended on external pH and found that, in agreement with [23], the cells maintained the cathodal-oriented motility in the wide range of pH only losing the directedness at pH < 6 (Figure S4C). Interestingly, the fragments sensed EFs in the range of pH similar to that of the EF-sensing cells (Figure S4C), pointing indirectly to the same physical EF sensor in the cells and fragments.
“Compass” Model of Galvanotaxis
We conclude that the cell fragment—the simplest and smallest cell migration unit—is also the smallest EF-sensing unit, containing both the EF sensor and the relevant signal transduction pathways. These PI3K- and myosin-dependent pathways perform differently in cells and fragments. We propose that two competing pathways direct cells in an EF (see the graphical abstract available online): a PI3K-dependent pathway signaling to the protruding actin network at the cell front, orienting the front toward cathode, and a myosin-dependent pathway signaling to the contractile actin-myosin network at the cell rear, orienting the rear also toward the cathode. The PI3K-dependent pathway dominates in cells, while the myosin-dependent pathway dominates in fragments. In the whole cell, the signal orienting the protruding actin network toward the cathode is stronger, so the cell front is facing the cathode. In the fragment, the signal orienting the contractile actin-myosin network at the rear toward the cathode is stronger, so the fragment rear is facing the cathode and the front is facing the anode.
The identity of the factors that govern these two pathways differently in cells and fragments remains unknown. One possibility could stem from different PIP3/PTEN or other activator/inhibitor ratios in cells and fragments. Another is that fragments’ ability to regulate intracellular Ca2+ levels is diminished, so EF could create a Ca2+ gradient in a fragment but not in a whole cell, rendering myosin-related signaling at the fragment rear relatively stronger. No long-lasting Ca2+ gradient within the keratocyte cell lamellipodium has been observed [8, 23], so it is likely that in whole cells Ca2+ is buffered and does not develop spatial gradients. Yet another possibility is that, mechanically, myosin could have a weaker steering effect on the whole cells than on fragments. Related to that, fragments are unlikely to inherit much myosin from mother cells; the myosin that they inherit is weakened and does not affect the motility mechanics [10, 24], so the fact that myosin-mediated steering in EFs is crucial is puzzling. Perhaps a signaling, not mechanical, function of myosin is essential for the EF sensing. Our data argue against involvement of the actin assembly dynamics in the galvanotaxis, but whether all actin networks are only downstream readouts of signaling pathways, unlike in neutrophils [25], requires further investigation.
Many studies have gradually converged on a number of hypotheses about how cells can sense EFs. First, an EF induces a gradient in the membrane electric potential [21], which can affect asymmetrically ion fluxes that could signal to the motile machinery. Second, cells are oriented by fluid flow [8], and the EF creates an electro-osmotic flow near the charged plasma membrane. Third, charged signaling proteins in the plasma membrane can be dragged by an electric force aggregating at one cell edge [26] and spatially biasing the signaling. Fourth, the cell can feel the force of an EF on the charged proteins in the plasma membrane. Following [23], our data suggest that electro-osmosis is unlikely to be the factor triggering galvanotaxis: since cells and fragments have similar surface charges and very different shapes, the electro-osmotic flow should pull both cell and fragment in the same direction, and the effect of fluid flow on the great mound of the cell body would be different from that on the flat and small fragment. The observation that a mechanical opposing force could not reorient cells and fragments suggests that direct electric force sensing is unlikely to be the galvanotactic mechanism. Our data agree with the hypothesis that electrophoresis of charged membrane proteins could be the initial signal of galvanotaxis in the keratocytes [23], as cells and fragments maintain their directedness in EF in similar ranges of pH (loss of the signal in acidic medium likely indicates that protonation of some charged proteins on the cell surface abolishes their drift in EFs). This also hints at the same upstream EF sensor in cells and fragments.
Supplementary Material
Acknowledgments
We are grateful to Greg Allen, Orion Weiner, and Roy Wollman for fruitful discussions, useful suggestions, and critical reading of this manuscript. We thank Noa Ofer and Kinneret Keren for sharing endoplasmic reticulum images. This work was supported by National Institutes of Health grants GM068952 to A.M. and 1R01EY019101 to M.Z., as well as by California Institute of Regenerative Medicine Research grant RB1-01417 and National Science Foundation Grant MCB-0951199 to M.Z and P. Devreotes, whose support we gratefully acknowledge.
Footnotes
Supplemental Information includes four figures, one table, Supplemental Experimental Procedures, and three movies and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2013.02.026.
References
- 1.Huttenlocher A, Horwitz AR. Wound healing with electric potential. N Engl J Med. 2007;356:303–304. doi: 10.1056/NEJMcibr066496. [DOI] [PubMed] [Google Scholar]
- 2.Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- 3.Zhao M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin Cell Dev Biol. 2009;20:674–682. doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Guo A, Song B, Reid B, Gu Y, Forrester JV, Jahoda CA, Zhao M. Effects of physiological electric fields on migration of human dermal fibroblasts. J Invest Dermatol. 2010;130:2320–2327. doi: 10.1038/jid.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franke K, Gruler H. Galvanotaxis of human granulocytes: electric field jump studies. Eur Biophys J. 1990;18:335–346. doi: 10.1007/BF00196924. [DOI] [PubMed] [Google Scholar]
- 6.Sato MJ, Kuwayama H, van Egmond WN, Takayama AL, Takagi H, van Haastert PJ, Yanagida T, Ueda M. Switching direction in electric-signal-induced cell migration by cyclic guanosine monophosphate and phosphatidylinositol signaling. Proc Natl Acad Sci USA. 2009;106:6667–6672. doi: 10.1073/pnas.0809974106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper MS, Schliwa M. Motility of cultured fish epidermal cells in the presence and absence of direct current electric fields. J Cell Biol. 1986;102:1384–1399. doi: 10.1083/jcb.102.4.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang L, Cormie P, Messerli MA, Robinson KR. The involvement of Ca2+ and integrins in directional responses of zebrafish keratocytes to electric fields. J Cell Physiol. 2009;219:162–172. doi: 10.1002/jcp.21660. [DOI] [PubMed] [Google Scholar]
- 9.Verkhovsky AB, Svitkina TM, Borisy GG. Self-polarization and directional motility of cytoplasm. Curr Biol. 1999;9:11–20. doi: 10.1016/s0960-9822(99)80042-6. [DOI] [PubMed] [Google Scholar]
- 10.Ofer N, Mogilner A, Keren K. Actin disassembly clock determines shape and speed of lamellipodial fragments. Proc Natl Acad Sci USA. 2011;108:20394–20399. doi: 10.1073/pnas.1105333108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malawista SE, de Boisfleury-Chevance A. Cryopreserved cytoplasts from human polymorphonuclear leukocytes (cytokineplasts) are chemotactic at speeds comparable to those of fresh intact cells. J Leukoc Biol. 1991;50:313–315. doi: 10.1002/jlb.50.3.313. [DOI] [PubMed] [Google Scholar]
- 12.Keller HU, Bessis M. Migration and chemotaxis of anucleate cytoplasmic leukocyte fragments. Nature. 1975;258:723–724. doi: 10.1038/258723a0. [DOI] [PubMed] [Google Scholar]
- 13.Keren K, Pincus Z, Allen GM, Barnhart EL, Marriott G, Mogilner A, Theriot JA. Mechanism of shape determination in motile cells. Nature. 2008;453:475–480. doi: 10.1038/nature06952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 16.Rajnicek AM, Foubister LE, McCaig CD. Growth cone steering by a physiological electric field requires dynamic microtubules, microfilaments and Rac-mediated filopodial asymmetry. J Cell Sci. 2006;119:1736–1745. doi: 10.1242/jcs.02897. [DOI] [PubMed] [Google Scholar]
- 17.Kölsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trollinger DR, Isseroff RR, Nuccitelli R. Calcium channel blockers inhibit galvanotaxis in human keratinocytes. J Cell Physiol. 2002;193:1–9. doi: 10.1002/jcp.10144. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Ishihara A, Oxford G, Johnson B, Jacobson K. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature. 1999;400:382–386. doi: 10.1038/22578. [DOI] [PubMed] [Google Scholar]
- 20.Euteneuer U, Schliwa M. The function of microtubules in directional cell movement. Ann N Y Acad Sci. 1986;466:867–886. doi: 10.1111/j.1749-6632.1986.tb38473.x. [DOI] [PubMed] [Google Scholar]
- 21.Minc N, Chang F. Electrical control of cell polarization in the fission yeast Schizosaccharomyces pombe. Curr Biol. 2010;20:710–716. doi: 10.1016/j.cub.2010.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iglesias PA, Devreotes PN. Navigating through models of chemotaxis. Curr Opin Cell Biol. 2008;20:35–40. doi: 10.1016/j.ceb.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Allen GM, Mogilner A, Theriot JA. Electrophoresis of cellular membrane components creates the directional cue guiding keratocyte galvanotaxis. Curr Biol. 2013 doi: 10.1016/j.cub.2013.02.047. Published online March 28, 2013. http://dx.doi.org/10.1016/j.cub.2013.02.047. [DOI] [PMC free article] [PubMed]
- 24.Houk AR, Jilkine A, Mejean CO, Boltyanskiy R, Dufresne ER, Angenent SB, Altschuler SJ, Wu LF, Weiner OD. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 2012;148:175–188. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong K, Pertz O, Hahn K, Bourne H. Neutrophil polarization: spatiotemporal dynamics of RhoA activity support a self-organizing mechanism. Proc Natl Acad Sci USA. 2006;103:3639–3644. doi: 10.1073/pnas.0600092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin S, Poo MM. The role of electro-osmosis in the electric-field-induced movement of charged macromolecules on the surfaces of cells. Biophys J. 1981;34:85–93. doi: 10.1016/S0006-3495(81)84838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.