Abstract
Objectives
We tested the hypothesis whether selective blunting of platelet-derived growth factor (PDGF)–dependent vascular smooth muscle cell (VSMC) proliferation and migration is sufficient to prevent neointima formation after vascular injury.
Background
To prevent neointima formation and stent thrombosis after coronary interventions, it is essential to inhibit VSMC proliferation and migration without harming endothelial cell function. The role of PDGF—a potent mitogen and chemoattractant for VSMC that does not affect endothelial cells—for neointima formation remains controversial.
Methods
To decipher the signaling pathways that control PDGF beta receptor (βPDGFR)–driven VSMC proliferation and migration, we characterized 2 panels of chimeric CSF1R/βPDGFR mutants in which the binding sites for βPDGFR-associated signaling molecules (Src, phosphatidylinositol 3-kinase [PI3K], GTPase activating protein of ras, SHP-2, phospholipase Cγ 1 [PLCγ]) were individually mutated. Based on in vitro results, the importance of PDGF-initiated signals for neointima formation was investigated in genetically modified mice.
Results
Our results indicate that the chemotactic response to PDGF requires the activation of Src, PI3K, and PLCγ, whereas PDGF-dependent cell cycle progression is exclusively mediated by PI3K and PLCγ. These 2 signaling molecules contribute to signal relay of the βPDGFR by differentially regulating cyclin D1 and p27kip1. Blunting of βPDGFR-induced PI3K and PLCγ signaling by a combination mutant (F3) completely abolished the mitogenic and chemotactic response to PDGF. Disruption of PDGF-dependent PI3K and PLCγ signaling in mice expressing the F3 receptor led to a profound reduction of neointima formation after balloon injury.
Conclusions
Signaling by the activated βPDGFR, particularly through PI3K and PLCγ, is crucial for neointima formation after vascular injury. Disruption of these specific signaling pathways is sufficient to attenuate pathogenic processes such as vascular remodeling in vivo.
Keywords: PI-3 kinase, PLCγ, platelet-derived growth factor, proliferation, restenosis
Despite novel treatment options to achieve vessel patency after percutaneous coronary interventions, vascular restenosis remains a significant clinical problem (1). Critical events for neointima formation at sites of vascular injury are the attraction of vascular smooth muscle cells (VSMCs) from the media, their neointimal proliferation, and extracellular matrix deposition (1–3). The inhibition of central cell cycle–regulating proteins such as mammalian target of rapamycin is efficient in reducing neointima formation after coronary stenting (4,5). However, this approach is hampered by the burden of in-stent thrombosis, because mammalian target of rapamycin inhibition also restrains the proliferation of endothelial cells and thus attenuates re-endothelialization (6,7). Sophisticated novel therapeutic approaches should selectively target VSMC proliferation and migration without affecting endothelial cell function.
Platelet-derived growth factor (PDGF) represents the most potent mitogen and chemoattractant for VSMCs (8,9), whereas it does not elicit significant effects on endothelial cells. Furthermore, PDGF and its beta receptor subtype (βPDGFR), which is abundantly expressed in VSMC (10,11), are significantly upregulated and activated at sites of vascular injury (12,13), making the βPDGFR a promising target for therapeutic interventions. However, recent studies on the role of the βPDGFR for neointima formation yielded conflicting results (14,15). Although inhibition of PDGFR signaling was effective in reducing restenosis formation in animal models, systemic administration of a PDGFR inhibitor failed to prevent the recurrence of in-stent restenosis in humans. Therefore, it appears crucial to more precisely define the role of PDGF for neointima formation and to identify the signaling pathways by which the βPDGFR mediates cellular responses that contribute to vascular disease. We used a genetic approach to address 3 important questions: 1) what are the signaling pathways that mediate PDGF-dependent migration and proliferation in VSMCs; 2) is βPDGFR signaling really critical for neointima formation in vivo; and 3) can the disruption of specific signaling pathways prevent neointima formation? Upon ligand binding, the βPDGFR recruits and activates several SH2 domain-containing signaling molecules that each stably associate with the receptor at phosphorylated tyrosine residues that serve as specific docking sites (16). These include Src family kinases (Src), phosphatidylinositol 3-kinase (PI3K), the GTPase-activating protein of Ras (RasGAP), SHP-2, and phospholipase Cγ1 (PLCγ). To systematically investigate the role of each individual signaling pathway, we used a series of chimeric colony-stimulating factor-1 receptor (CSF1R)/βPDGFR mutants in which the tyrosine residues required for binding of each signaling molecule were individually replaced with phenylalanine (17). Using this system, we were able to precisely characterize the signal relay mechanisms that govern PDGF-dependent chemotaxis and cell cycle progression in VSMCs. These studies particularly identified PI3K and PLCγ as critical signaling enzymes in vitro. Consequently, blunting of PDGF-dependent PI3K and PLCγ signaling in F3 mice, which lack the respective binding sites in the βPDGFR (18), dramatically reduced neointima formation after vascular injury in vivo.
Methods
Generation of chimeric CSF1R/βPDGFR mutants
The chimeric receptor (ChiR) cDNA was constructed from DNA encoding amino acids 1 to 513 of the human CSF1R and amino acids 528 to 1,106 of the human βPDGFR joined at an EcoRI site (19). Mutations in the βPDGFR sequence are as described (17).
Animals and diets
βPDGFR-F3 mice were generated by introducing point mutations into the βPDGFR locus by gene targeting as described (18). Male βPDGFRF3/F3 mice and littermate wild-type (WT) controls (C57BL/6J) were kept on a regular diet until experimentation. Mice were fed a chow diet (D12108 containing 1.25% cholesterol; Research Diets, New Brunswick, New Jersey) that was started 5 days before intervention and continued until harvesting. All animal experiments were performed in accordance with institutional guidelines and approved by the local animal committee.
Mouse carotid balloon injury
Balloon injury of the left proximal common carotid artery was performed in WT and βPDGFRF3/F3 mice (10 to 14 weeks of age) as described (20). Briefly, the mid-common carotid artery was dilated to approximately 1.3 times the unmanipulated diameter for 60 s, using a miniature angioplasty catheter (Schwager Medica, Winterthur, Switzerland). Mice were euthanized 21 days after balloon injury. Vessels were perfusion fixed with 4% paraformaldehyde. The injured left and the uninjured right common carotid artery were excised. After post-fixation with 4% paraformaldehyde overnight, the vessels were embedded in paraffin blocks and processed further.
Morphometric analysis
Three serial cross-sections (5-μm thickness, 1,000 μm apart) were taken from the midportion of the dilated segment for histomorphological analysis. Corresponding sections were obtained from the uninjured right common carotid artery. Morphometric analyses were performed using Image J analysis software (National Institutes of Health, Bethesda, Maryland).
Immunoblotting
Quiescent VSMCs were left resting or stimulated with 50 ng/ml of PDGF-BB or macrophage colony-stimulating factor (M-CSF) for times indicated in the presence or absence of inhibitors. Cells were washed and lysed as described (21,22), and lysates were subjected to immunoblot analysis.
Chemotaxis assay
PDGF-dependent chemotaxis was assayed utilizing a 48-well modified Boyden chemotaxis chamber (NeuroProbe Inc., Baltimore, Maryland) and collagen-coated polyvinylpyrrolidone-free polycarbonate filters (8-μm pore size) (Poretics Corp., Livermore, California) as described (21,22).
DNA synthesis assay
DNA synthesis was measured by a 5-bromodeoxyuridine (BrdU)-incorporation assay, carried out according to the manufacturer’s specifications (Roche, Mannheim, Germany) with an incubation time of 5 h as previously described (22).
Statistical analysis
All data are expressed as mean ± SEM. Statistical significance of differences was calculated using analysis of variance with post-hoc Tukey test or Student unpaired t test. p < 0.05 was considered statistically significant.
Results
The main goal of our study was to investigate whether selective blunting of PDGF-dependent VSMC proliferation and migration is sufficient to prevent neointima formation after vascular injury in vivo. To identify the crucial targets for neointima prevention, we performed in vitro studies utilizing chimeric CSF1R/βPDGFR mutants (ChiR) which contain the ligand-binding domain of the CSF1R (c-fms) and the cytoplasmic domain of the WT or mutated βPDGFR (17). The subtraction panel contains mutants in which the binding site for either one of the βPDGFR -associated signaling enzymes is mutated (Fig. 1A). The add-back panel is based on the F5 receptor and contains mutants that have the binding sites for one of the associated proteins restored (Fig. 1B). A kinase inactive mutant (R634) served as a negative control. The chimeric approach enabled us to selectively stimulate PDGF-dependent signaling cascades via ChiR mutants by M-CSF and to bypass endogenous βPDGFR in VSMCs. The ChiR mutant-expressing cell lines have been characterized previously (17).
Figure 1. Schematic Diagram Illustrating the Cytoplasmic Domain of Chimeric Colony-Stimulating Factor 1 Receptor/Platelet-Derived Growth Factor Beta Receptor (ChiR) Mutants (17).
Tyrosine phosphorylation sites are represented as P, and Tyr-to-Phe substitutions are indicated as black squares. The signaling enzymes that associate with the receptor are shown at the top of the schemes. The nomenclature of the substraction panel (A) and add-back panel (B) of chimeric receptor (ChiR) mutants is indicated to the right of each receptor representation. In the subtraction panel, the names indicate which of the Tyr residues have been replaced with Phe (“F-mutants”), and in the add-back panel, the name of each mutant denotes which of the mutations in the F5 construct has been repaired (“Y-mutants”). JM = juxtamembrane; KI = kinase insert; PI3K = phosphatidylinositol 3-kinase; PLCγ = phospholipase Cγ 1; RasGAP = GTPase activating protein of ras.
Functionality of ChiR mutants in VSMCs
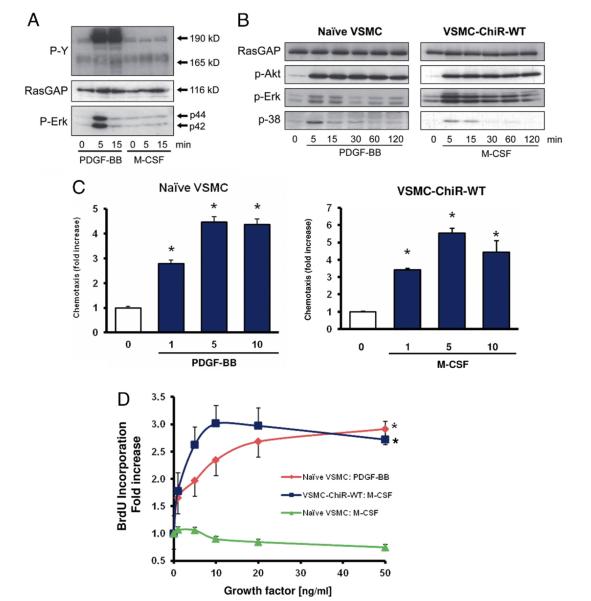
Because our approach required that VSMCs do not express functional CSF1R, we first compared their responsiveness to PDGF-BB and M-CSF. As expected, stimulation of non-transfected VSMCs with PDGF-BB led to tyrosine phosphorylation of the βPDGFR and to activation of downstream signaling molecules such as Erk1/2. In contrast, M-CSF did not mediate the tyrosine phosphorylation of the CSF1R and did not induce Erk phosphorylation in VSMCs (Fig. 2A), whereas it triggered these responses in human monocytes (not shown). M-CSF triggered the phosphorylation of downstream signaling molecules such as Akt, Erk, and p38, and VSMC migration and proliferation in ChiR-WT–expressing cells to a similar extent as PDGF-BB in non-transfected cells (Figs. 2B, 2C, and 2D). These data demonstrate that the system of ChiR mutants allows the systematic evaluation of βPDGFR signaling in VSMCs without the need for pharmacological inhibitors.
Figure 2. The M-CSF–Stimulated ChiR-WT Mimics PDGF-Induced Responses in VSMCs.
(A) Responsiveness of naive vascular smooth muscle cells (VSMCs) to platelet-derived growth factor (PDGF)-BB (50 ng/ml) and macrophage colony-stimulating factor (M-CSF) (50 ng/ml). Shown are immunoblots probed with antibodies recognizing phospho-tyrosine (P-Y), GTPase activating protein of ras (RasGAP) (lysate control), and phospho-Erk. (B) Activation of downstream signaling molecules in PDGF-BB–stimulated naive VSMCs and M-CSF–stimulated chimeric receptor (ChiR) wild-type (WT)–expressing cells. (C) Comparison of the chemotactic response of naive VSMCs to PDGF-BB (10 ng/ml) and ChiR-WT–expressing cells to M-CSF (10 ng/ml). (D) Comparison of growth factor–dependent cell cycle progression in PDGF-BB– or M-CSF–stimulated naive or ChiR-WT–expressing VSMCs as indicated. Data represent means ± standard error of the mean from at least 3 independent experiments (*p < 0.05 vs. buffer).
PI3-kinase and phospholipase Cγ mediate the βPDGFR’s mitogenic signal
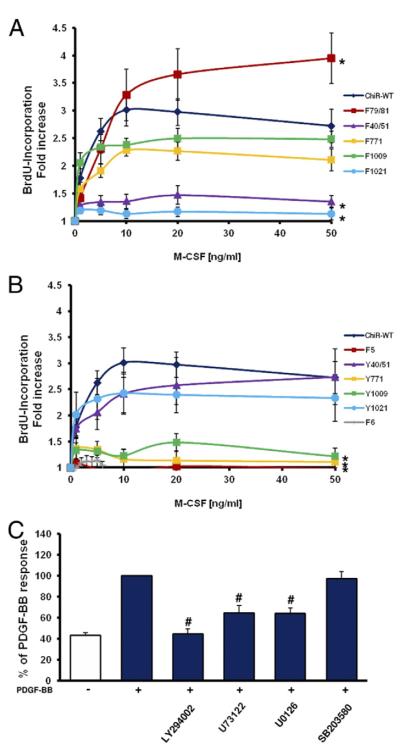
Stimulation of ChiR-WT expressing cells resulted in a concentration-dependent increase in BrdU uptake (Fig. 3A). Deletion of the binding sites for PI3K (F40/51) or PLCγ (F1021) almost completely suppressed DNA synthesis even at saturating ligand concentrations, whereas deletion of the Src binding site (F79/81) led to an enhanced mitogenic response. The latter is consistent with previous reports, identifying Src as a negative regulator of PDGFR signaling (21). ChiR mutants that only bind either PI3K (Y40/51) or PLCγ (Y1021) were able to partially mediate the ChiR-WT response (Fig. 3B). A potential caveat of utilizing receptor mutants is the possibility that in addition to the known binding partners of phosphotyrosines, other unknown signaling molecules may also interact with these sites. Therefore, all measurements were repeated in a second approach in PDGF-stimulated VSMCs in the presence of pharmacological inhibitors. Consistent with the findings obtained by the ChiR system, pharmacological inhibition of PI3K or PLCγ suppressed PDGF-BB mediated BrdU incorporation (Fig. 3C). Furthermore, inhibition of MEK1/2 also led to a decrease of VSMC proliferation, whereas suppression of p38 activity had no effect.
Figure 3. Role of Signal Relay Enzymes in PDGF Beta Receptor–Mediated DNA Synthesis.
Vascular smooth muscle cells (VSMCs) expressing either the chimeric receptor (ChiR) wild-type (WT), the subtraction panel of ChiR mutants (A), or the add-back panel of ChiR mutants (B) were arrested by serum deprivation and exposed to buffer or various concentrations of macrophage colony-stimulating factor (M-CSF). DNA synthesis was measured by 5-bromodeoxyuridine (BrdU) incorporation. Data are expressed as fold increase over buffer. (C) Effect of pharmacological inhibitors against phosphatidylinositol 3-kinase (LY294002), phospholipase Cγ 1 (U73122), MEK (U0126), and p38 (SB203580) on platelet-derived growth factor (PDGF)-BB–dependent cell cycle progression in non-transfected VSMCs. Data are expressed as the percentage of PDGF-BB–stimulated cells. All data represent mean ± standard error of the mean from at least 3 independent experiments (*p < 0.05 vs. WT at 50 ng/ml M-CSF, #p < 0.05 vs. PDGF alone).
Multiple signaling enzymes are required for PDGF-dependent chemotaxis
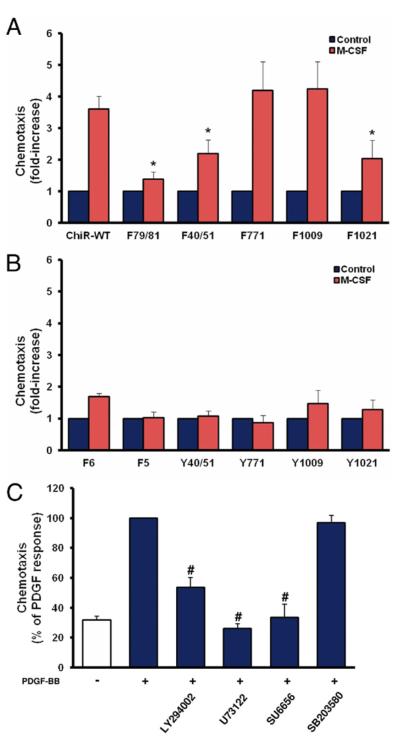
Stimulation of ChiR-WT expressing VSMCs led to an increase in cell migration to approximately 4-fold the basal level (Fig. 4A). Mutation of the Src binding site (F79/81) almost completely abolished M-CSF–induced chemotaxis, and mutation of the binding sites for PI3K (F40/51) or PLCγ (F1021) significantly reduced M-CSF–dependent chemotaxis by 55% and 60%, respectively. Activation of either 1 signaling molecule alone (add-back panel) was not sufficient to rescue the chemotactic response (Fig. 4B). Consistent with the use of ChiR mutants, pharmacological inhibition of Src abolished PDGF-dependent chemotaxis in naive VSMCs, whereas inhibition of either PI3K or PLCγ led to a partial inhibition of this response (Fig. 4C). These data indicate that PI3K, PLCγ, and Src are required for PDGF-dependent chemotaxis in VSMCs.
Figure 4. Role of Signal Relay Enzymes in PDGF Beta Receptor–Mediated Chemotaxis.
(A) Subtraction panel of chimeric receptor (ChiR) mutants. (B) Add-back panel of ChiR mutants. (C) Naive vascular smooth muscle cells that have been stimulated with platelet-derived growth factor (PDGF)-BB in the presence of pharmacological inhibitors against phosphatidylinositol 3-kinase (LY294002), phospholipase Cγ 1 (U73122), Src (SU6656), and p38 (SB203580). All data represent mean ± standard error of the mean from at least 3 independent experiments (*p < 0.05 vs. wild type; #p < 0.05 vs. PDGF alone).
PI3K and PLCγ differentially regulate the expression of cyclin D1 and p27kip1
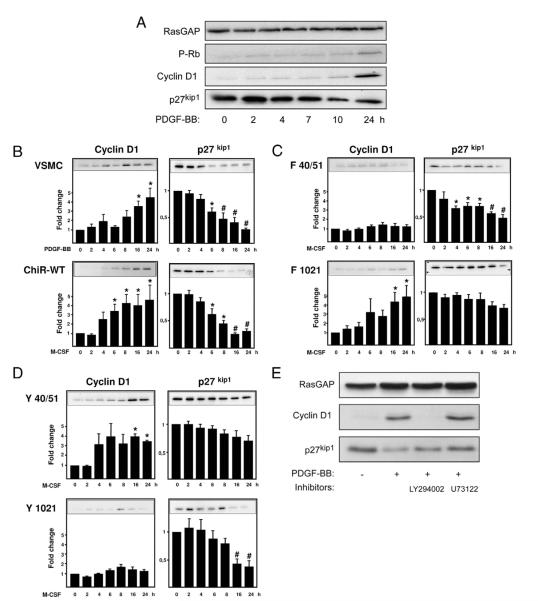
Growth factors regulate the cell cycle from the exit from G0 until late G1. Phosphorylation of the retinoblastoma protein (Rb) depends on G1 cyclin-dependent kinases (cdks), which are activated by G1 cyclins and inhibited by cdk inhibitors. The main growth factor–induced events in early G1 are the up-regulation of cyclin D1 and the down-regulation of the cdk inhibitor p27Kip1 (23). Stimulation of the endogenous βPDGFR with PDGF-BB and ChiR-WT with M-CSF led to a time-dependent increase of cyclin D1 expression and degradation of p27kip1, resulting in efficient Rb phosphorylation at 24 h (Figs. 5A and 5B). As expected, the F5 receptor did not influence p27kip1 and cyclin D1 levels or mediate Rb phosphorylation (not shown). When the various ChiR mutants were compared for their ability to regulate cell cycle controlling molecules, disruption of PI3K binding to the ChiR (F40/51) abolished the induction of cyclin D1, whereas binding of PI3K alone (Y40/51) was sufficient to induce cyclin D1 expression (Figs. 5C and 5D). Conversely, disruption of all other signaling pathways including PLCγ (F1021) did not affect M-CSF–dependent cyclin D1 induction (Fig. 5C, and not shown). Interestingly, the F40/51 mutation diminished the receptor’s capability to down-regulate p27kip1 to a much lesser extent than the F1021 mutation (Fig. 5C). Additionally, the Y1021 mutant but not the Y40/51 mutant was able to efficiently down-regulate p27kip1 (Fig. 5D). Pharmacological inhibition of PI3K in naive VSMC completely suppressed PDGF-dependent cyclin D1 induction, whereas inhibition of PLCγ had no influence (Fig. 5E). Conversely, inhibition of PLCγ abolished the down-regulation of p27kip1, whereas inhibition of PI3K had little effect. Hence induction of cyclin D1 is primarily mediated by PI3K, whereas PLCγ primarily promotes the degradation of p27kip1.
Figure 5. Regulation of Cell Cycle–Controlling Proteins Through PI3K and PLCγ.
(A) Platelet-derived growth factor (PDGF)-BB causes induction of cyclin D1 and down-regulation of p27 kip1 protein at 24 h, resulting in retinoblastoma protein phosphorylation (P-Rb). (B) The activated chimeric receptor wild type (ChiR-WT) mimics the PDGF-BB–induced response in naive vascular smooth muscle cells (VSMCs). (C) Regulation of cyclin D1 and p27 kip1 by the ligand-induced F40/51 and F1021 mutants. (D) Regulation of cyclin D1 and p27 kip1 by the ligand-induced Y40/51 and Y1021 mutants. (E) Naive VSMCs were treated with 50 ng/ml of PDGF-BB for 24 h in the presence of pharmacological inhibitors of phosphatidylinositol 3-kinase (PI3K; LY294002) or phospholipase Cγ 1 (PLCγ; U73122). (B to D) Cyclin D1 and p27kip1 signals were scanned and quantified by densitometry, and the values were normalized for GTPase activating protein of ras (RasGAP) levels (lysate control). Data represent mean ± standard error of the mean from 3 independent experiments (*p < 0.05, #p < 0.01 vs. buffer).
Disruption of PDGF-dependent PI3K and PLCγ activity by a combination mutant
Based on the preceding findings, we generated a combination mutant that lacks the binding sites for PI3K and PLCγ (F3 receptor; Fig. 6A) in an attempt to completely abolish PDGF-dependent proliferation and migration. The chimeric F3 mutant was stably expressed in VSMCs to a similar level than the ChiR-WT and did not associate with either PI3K or PLCγ upon stimulation with M-CSF (Figs. 6B and 6C). VSMCs transfected with the F3 mutant were unable to mediate a chemotactic or mitogenic signal upon M-CSF stimulation (Figs. 6D and 6E) and failed to efficiently induce cyclin D1 or down-regulate p27kip1 in response to M-CSF (Fig. 6F). Hence our in vitro studies identified PI3K and PLCγ as main mediators of PDGF-dependent proliferation and migration of VSMCs, and the F3 mutant appeared as the ideal tool to verify their importance in vivo.
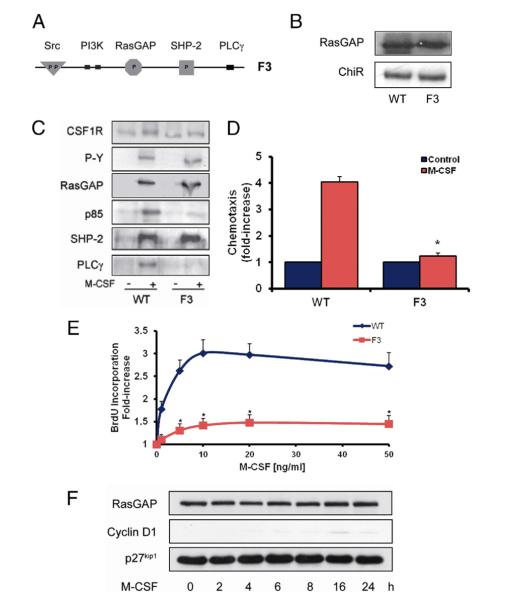
Figure 6. Blunting of Mitogenic and Chemotactic Signaling by a Combination Mutant That Lacks the PI3K and PLCγ Binding Sites (F3 Receptor).
(A) Schematic diagram illustrating the cytoplasmic domain of the chimeric receptor (ChiR)-F3 mutant harboring Tyr-to-Phe substitutions at tyrosine residues 740/51 and 1021. (B) Expression levels of ChiR-F3 in comparison with ChiR-wild type (WT)–expressing cells. (C) Binding characteristics of platelet-derived growth factor beta receptor–associated signaling molecules in quiescent and macrophage colony-stimulating factor (M-CSF)–stimulated ChiR-WT and ChiR-F3 expressing cells. (D, E) M-CSF–dependent chemotaxis and cell cycle progression in WT and ChiR-F3 expressing vascular smooth muscle cells (VSMCs). (F) Effect of M-CSF on cyclin D1 and p27kip1 levels in ChiR-F3 expressing VSMCs. Data in D and E represent mean ± SEM from 3 independent experiments (*p < 0.05 vs. WT). BrdU = 5-bromodeoxyuridine; CSF1R = colony-stimulating factor-1 receptor; other abbreviations as in Figure 1.
Disruption of PDGF-dependent PI3K and PLCγ signaling abolishes neointima formation after balloon injury in vivo
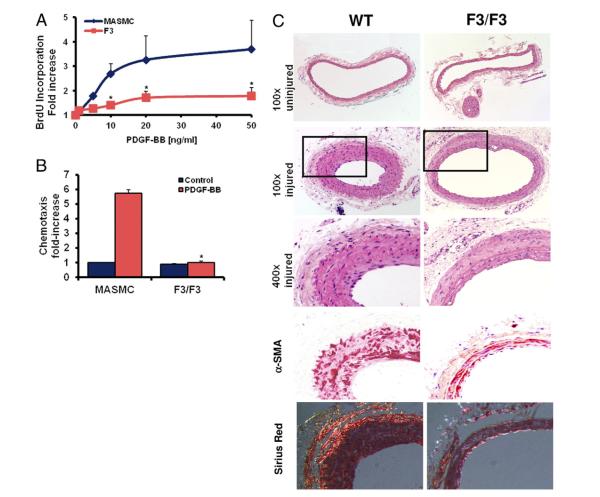
To evaluate the importance of PDGF-dependent PI3K and PLCγ signaling for neointima formation in vivo, we performed balloon injury in carotid arteries of mice that are homozygous for the murine βPDGFR F3 mutant. VSMCs derived from βPDGFRF3/F3 mice neither elicited a mitogenic signal, nor were they able to migrate toward PDGF (Figs. 7A and 7B). Balloon injury was performed in the common carotid arteries of male βPDGFRF3/F3 mice and littermate WT controls. Three weeks after balloon injury, WT mice displayed a robust neointima formation at the site of vascular injury, and this response was dramatically reduced in mice harboring the F3 mutation (Fig. 7C). Consequently, the intimal area and the intima-media ratio were significantly decreased in βPDGFRF3/F3 mice, whereas the patent lumen area was increased (Table 1). Outward remodeling in the βPDGFRF3/F3 mice was excluded, and there were no detectable differences in SMC differentiation markers and extracellular matrix (ECM) components (Fig. 7C, Online Fig. 1A). Consistently, activation of βPDGFR signaling in ChiR–expressing VSMCs did not affect the expression of differentiation markers (smoothelin, smooth muscle myosin heavy chain, alpha actin) or induce ECM components such as collagens I and Va (Online Figs. 1B and 2). The decreased intimal area in F3/F3 mice was paralleled by a decreased number of neointimal nuclei and proliferating VSMCs, whereas the area per nucleus (ECM index) was equal in WT and βPDGFRF3/F3 mice (Table 1), indicating that decreased VSMC accumulation rather than diminished matrix deposition accounts for the reduced neointima formation.
Figure 7. Disruption of PDGF-Dependent PI3K and PLCγ Signaling Attenuates Neointima Formation In Vivo.
(A, B) Platelet-derived growth factor (PDGF)–dependent cell cycle progression and chemotaxis in vascular smooth muscle cells isolated from wild type (WT) or PDGF beta receptor (βPDGFR)-F3/F3 mice (*p < 0.05 vs. WT-mouse aortic smooth muscle cells [MASMC]). (C) Representative histological cross-sections (hematoxylin eosin staining; 100×; inset: 400×; alpha-smooth muscle actin and Sirius red staining) of uninjured and injured common carotid arteries from male βPDGFRF3/F3 mice and littermate WT controls 3 weeks after balloon injury.
Table 1.
Morphometric Analyses of Carotid Arteries From WT or Platelet-Derived Growth Factor Beta Receptor F3/F3 Mice 21 Days After Balloon Injury
| Intimal Area (μm2) | Medial Area (μm2) | Lumen Area (μm2) | Intima-Media Ratio | |
|---|---|---|---|---|
| WT (n = 10) | 19,276 ± 5,409 | 30,071 ± 2,158 | 44,982 ± 9,130 | 0.64 ± 0.19 |
| F3/F3 (n = 11) | 6,309 ± 978* | 28,887 ± 3,527 | 79,780 ± 8,689* | 0.23 ± 0.03* |
|
| ||||
| Intimal Nuclei | ECM Index (μm2/cell) | PCNA Positive Cells (%) | ||
|
| ||||
| WT (n = 8) | 88.58 ± 29.66 | 359.8 ± 78.4 | 16.79 ± 2.44 | |
| F3/F3 (n = 7) | 28.19 ± 10.43 | 427.6 ± 68.2 | 5.48 ± 0.83* | |
Data are represented as mean ± SEM.
p < 0.05 versus WT.
ECM = extracellular matrix; PCNA = proliferating cell nuclear antigen; WT = wild-type.
Discussion
In this study, we systematically analyzed the contribution of the activated βPDGFR and its associated signaling molecules to cellular responses of VSMCs in vitro and neointima formation in vivo. The data presented herein are consistent with the schematic diagram outlined in Figure 8 and allow 2 important conclusions: 1) signaling by the activated βPDGFR (particularly through PI3K and PLCγ) is crucial for neointima formation after vascular injury; and 2) disruption of specific signaling pathways is sufficient to attenuate pathogenic processes such as neointima formation and constrictive vascular remodeling in vivo.
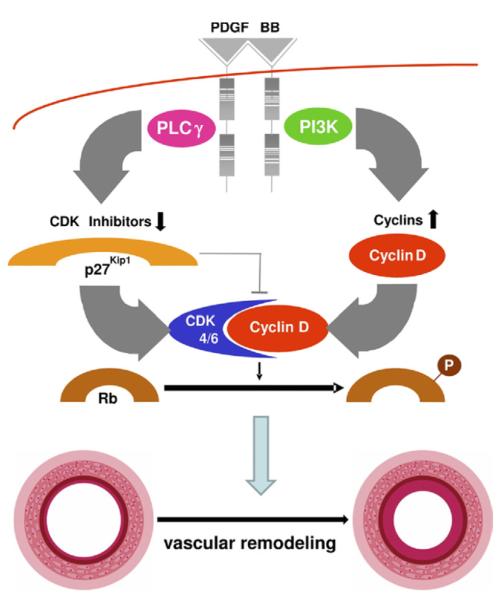
Figure 8. Schematic Diagram Illustrating the Differential Roles of PDGF-Dependent PI3K and PLCγ Activity for Cellular Responses and Neointima Formation In Vivo.
Upon ligand binding, the platelet-derived growth factor (PDRF) beta receptor recruits multiple signaling molecules, including phosphatidylinositol 3-kinase (PI3K) and phospholipase Cγ 1 (PLCγ). Although PI3K is required for efficient up-regulation of cyclin D1, PLCγ is mainly responsible for down-regulation of p27kip1. Both events are required for retinoblastoma protein (Rb) phosphorylation, which is crucial for cellular events such as cell cycle progression and chemotaxis. At least in mice, these events appear to be crucial for neointima formation after balloon injury. CDK = cyclin-dependent kinase.
Although previous studies that applied pharmacological approaches or neutralizing antibodies have indicated that PDGF is an important contributor to neointima formation (12,14), some studies argued against a significant role of PDGF. For instance, the systemic administration of the tyrosine kinase inhibitor imatinib failed to reduce the recurrence of coronary in-stent restenosis after coronary interventions in humans (15). To directly prove the importance of βPDGFR-initiated signals for neointima formation and to identify the crucial signaling pathways, we took advantage of a targeted genetic modification. In mice that are homozygous for the F3 mutation, the βPDGFR is normally expressed but is unable to recruit and activate PI3K and PLCγ upon ligand binding (18). Based on the previous observations that deletion of either the βPDGFR or the PDGF-B gene resulted in embryonic lethality that was in part due to vascular defects, the fact that F3/F3 mice are viable and have no obvious phenotype appears rather surprising (18). However, chimeric analysis revealed that when F3-receptor– bearing cells competed with WT βPDGFR-bearing cells in the same animals, there was a strong selection against βPDGFRF3/F3 cells in the layer of smooth muscle cells surrounding the vasculature. This finding, which indicates that the F3 mutant receptor does not transmit signals as efficiently as the WT receptor in vivo, prompted us to hypothesize that the F3 mutation may become relevant under pathological conditions. Indeed, when we performed balloon injury in the carotid arteries of βPDGFRF3/F3 mice, the remodeling process at the site of vascular injury was profoundly reduced when compared with that of WT mice.
Our results are in line with findings of other investigators showing that inhibition of βPDGFR signaling by the use of tyrosine kinase inhibitors or neutralizing antibodies prevented restenosis formation (12,14), as well as other consequences of vascular remodeling, such as atherogenesis and pulmonary arterial hypertension (24,25). The failure of oral imatinib to prevent the recurrence of in-stent restenosis in humans as shown by Zohlnhöfer et al. (15) does not discard the role of PDGF in this context, but may be explained by several reasons, including insufficient dosage, nonspecific inhibition of several tyrosine kinases instead of specific PDGF receptor inhibition, systemic instead of local application, and subtherapeutic tissue concentrations. Indeed, it has been suggested that imatinib administered orally may not reach sufficient serum concentrations (>10 μmol/l) to function as a potent inhibitor of PDGFR signaling in tissues (26). Our genetic approach circumvents the problem that a pharmacological agent may not reach sufficient tissue concentrations or may inhibit other unknown kinases and clearly showed that blunting of PDGF-dependent PI3K and PLCγ activity in vivo dramatically reduces neointima formation and constrictive arterial narrowing after vascular injury.
PDGF is known to exert mitogenic and migratory responses in VSMCs, both of which are thought to contribute to neointima formation. Previous studies have shown that neointimal VSMC accumulation in the first days after balloon injury occurs as a result of PDGF-dependent VSMC migration from the media to the intima (27). Although we did not particularly determine VSMC migration in our in vivo model, we show that the βPDGFR F3 mutation significantly reduced the number of proliferating VSMCs in the intima at 21 days after injury and that mutation of the PI3K and PLCγ binding sites abolished both PDGF-dependent VSMC proliferation and chemotaxis in vitro. Taken together, these data indicate that PDGF-dependent migration and proliferation are both important for neointima formation and that disruption of PDGF mediated PI3K and PLCγ signaling attenuates this process after balloon injury by affecting both responses.
The approach to investigate vascular remodeling in βPDGFRF3/F3 mice was based on in vitro experiments utilizing chimeric CSF1R/βPDGFR mutants that allowed the systematic characterization of βPDGFR signaling in VSMCs. We found that multiple signaling enzymes including PI3K, PLCγ, and Src contribute to PDGF-dependent chemotaxis. Because the directed migration of cells requires a number of cellular events, it is likely that each of the signaling molecules contributes to PDGF-mediated chemotaxis in a different way. Previous studies have indicated that PI3K- and PLCγ-dependent pathways are involved in actin rearrangement, cytoskeletal changes, and lamellipodia formation (28–31), whereas Src activity may be crucial for the direction of cellular movement by mediating receptor tyrosine kinase internalization and cap formation in 1 region of the cell (32). In contrast to chemotaxis, βPDGFR-mediated cell cycle progression depends on 2 of the receptor-associated signaling molecules, PI3K and PLCγ. These results are consistent with a previous study in HepG2 cells (33). Although deletion of the binding sites for PI3K or PLCγ almost completely abrogated DNA synthesis, the restoration of either binding site in the F5 receptor partially rescued βPDGFR-mediated proliferation. This may be explained by the fact that in contrast to the add-back mutants, the subtraction mutants (F40/51 and F1021) both bind and activate negative regulators of PDGFR signaling such as RasGAP and SHP-2 (16). Rat VSMCs expressing the F3 mutant and murine VSMCs isolated from βPDGFRF3/F3 mice were not able to mediate a mitogenic or chemotactic signal, indicating that the inability of the βPDGFR to associate with PI3K and PLCγ fully abrogated these responses. Our genetic approach bears one potential caveat: We cannot completely rule out that proteins other than the known binding partners of the mutated tyrosine residues also associate with these sites and thereby contribute to βPDGFR-mediated proliferation or migration. However, this seems very unlikely because pharmacological inhibition of PI3K or PLCγ also abrogated these cellular responses. In addition to mouse and rat VSMCs, we found that pharmacological inhibition of PI3K and PLCγ also attenuated PDGF-induced chemotaxis and proliferation of human coronary VSMCs (not shown), suggesting species independency.
Our further investigations focused on cyclin D1 and p27kip1, which are critical for both cell cycle progression and chemotaxis (23,34,35). Although the role of PI3K for the regulation of cell cycle–controlling proteins has been addressed in multiple studies, the importance of PLCγ in this context was less clear. Our data indicate that PI3K and PLCγ regulate cell cycle progression at least in part in a non-redundant manner. PI3K was identified as the main regulator of βPDGFR-dependent cyclin D1 expression, whereas PLCγ appeared to be of minor importance for this event. This is consistent with other studies demonstrating that cyclin D1 is mainly regulated by PI3K in numerous systems (36–38). Surprisingly, our comparative analysis revealed that PLCγ is the main mediator of p27kip1 down-regulation. A recent report demonstrated that PDGF-BB–dependent degradation of p27Kip1 in VSMC is mediated by shortening the half-life of p27Kip1 mRNA via an Erk1/2-dependent pathway (39). Conventional protein kinase C isoforms that act downstream of PLCγ and contribute to p27Kip1 degradation are known to activate Erk1/2. Consistently, we found that inhibition of either Erk1/2 or protein kinase C almost completely abrogated PDGF-dependent down-regulation of p27Kip1 (not shown), suggesting a prominent role for these PLCγ effectors for the regulation of p27Kip1. Nevertheless, p27kip1 seems to be differentially regulated in different cell types and through different growth factors, as PDGF-dependent degradation of p27kip1 in fibroblasts occurs independently from PI3K and is controlled by c-myc, whereas interleukin-2–induced repression of p27kip1 requires PI3K and Akt (40). Hence, growth factor–dependent degradation of p27kip1 may be targeted in a cell-type–specific manner. Although both pathways were found to be redundant to some extent, our comparative approach identified PI3K as the main regulator of PDGF-dependent induction of cyclin D1 in VSMCs, whereas PLCγ mainly controls the expression of p27kip1.
Conclusions
In summary, our data indicate that PI3K and PLCγ mediate βPDGFR-dependent proliferation and migration of VSMCs and provide direct proof that these signaling pathways are essential for neointima formation after vascular injury in vivo. The identification of these signal relay mechanisms provides the basis for the development of novel therapeutic and preventive strategies that selectively target VSMCs without affecting endothelial cell function.
Supplementary Material
Acknowledgments
The authors thank Andrius Kazlauskas and Alex Toker (Harvard Medical School) for generously supplying antibodies against the βPDGFR (97A), RasGAP (69.3), and p85; and Jessica Schnitker and Deborah Vieth for excellent technical assistance. This manuscript contains parts of a doctoral thesis by F.G.
Acknowledgments
This work was supported in part by the Deutsche Forschungs-gemeinschaft (Grants No. Ro 1306/2-1 and 2-2), the Center for Molecular Medicine Cologne (project A6), and the Marga und Walter Boll-Stiftung (Kerpen, Germany).
Abbreviations and Acronyms
- BrdU
5-bromodeoxyuridine
- cdk
cyclin-dependent kinase
- ChiR
chimeric receptor
- CSF1R
colony-stimulating factor-1 receptor
- ECM
extracellular matrix
- M-CSF
macrophage colony-stimulating factor
- PDGF
platelet-derived growth factor
- PI3K
phosphatidylinositol 3-kinase
- PLCγ
phospholipase Cγ 1
- RasGAP
GTPase activating protein of ras
- VSMC
vascular smooth muscle cell
- WT
wild type
APPENDIX
For accompanying data and supplemental figures, please see the online version of this article.
Footnotes
The authors have reported that they have no relationships to disclose.
REFERENCES
- 1.Weintraub WS. The pathophysiology and burden of restenosis. Am J Cardiol. 2007;100:3K–9K. doi: 10.1016/j.amjcard.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Calvert PA, Bennett MR. Restenosis revisited. Circ Res. 2009;104:823–25. doi: 10.1161/CIRCRESAHA.109.196345. [DOI] [PubMed] [Google Scholar]
- 3.Bennett MR, O’Sullivan M. Mechanisms of angioplasty and stent restenosis: implications for design of rational therapy. Pharmacol Ther. 2001;91:149–66. doi: 10.1016/s0163-7258(01)00153-x. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–31. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 5.Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 6.Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Nakazawa G, Vorpahl M, Finn AV, Narula J, Virmani R. One step forward and two steps back with drug-eluting-stents: from preventing restenosis to causing late thrombosis and nouveau atherosclerosis. J Am Coll Cardiol Img. 2009;2:625–28. doi: 10.1016/j.jcmg.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz SM. Perspectives series: cell adhesion in vascular biology. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997;99:2814–16. doi: 10.1172/JCI119472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellgren AM, Smith CL, Olsen GS, et al. Platelet-derived growth factor receptor beta signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations. Circ Res. 2008;103:1393–1401. doi: 10.1161/CIRCRESAHA.108.176768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klinghoffer RA, Mueting-Nelsen PF, Faerman A, Shani M, Soriano P. The two PDGF receptors maintain conserved signaling in vivo despite divergent embryological functions. Mol Cell. 2001;7:343–54. doi: 10.1016/s1097-2765(01)00182-4. [DOI] [PubMed] [Google Scholar]
- 12.Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–54. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Abe J, Deguchi J, Takuwa Y, et al. Tyrosine phosphorylation of platelet derived growth factor beta receptors in coronary artery lesions: implications for vascular remodelling after directional coronary atherectomy and unstable angina pectoris. Heart. 1998;79:400–6. doi: 10.1136/hrt.79.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levitzki A. PDGF receptor kinase inhibitors for the treatment of restenosis. Cardiovasc Res. 2005;65:581–86. doi: 10.1016/j.cardiores.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Zohlnhofer D, Hausleiter J, Kastrati A, et al. A randomized, double-blind, placebo-controlled trial on restenosis prevention by the receptor tyrosine kinase inhibitor imatinib. J Am Coll Cardiol. 2005;46:1999–2003. doi: 10.1016/j.jacc.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 16.Rosenkranz S, Kazlauskas A. Evidence for distinct signaling properties and biological responses induced by the PDGF receptor alpha and beta subtypes. Growth Factors. 1999;16:201–16. doi: 10.3109/08977199909002130. [DOI] [PubMed] [Google Scholar]
- 17.Vantler M, Caglayan E, Zimmermann WH, Baumer AT, Rosenkranz S. Systematic evaluation of anti-apoptotic growth factor signaling in vascular smooth muscle cells. Only phosphatidylinositol 3′-kinase is important. J Biol Chem. 2005;280:14168–76. doi: 10.1074/jbc.M413310200. [DOI] [PubMed] [Google Scholar]
- 18.Tallquist MD, Klinghoffer RA, Heuchel R, et al. Retention of PDGFR-beta function in mice in the absence of phosphatidylinositol 3′-kinase and phospholipase Cgamma signaling pathways. Genes Dev. 2000;14:3179–90. doi: 10.1101/gad.844700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Symes K, Mercola M. Embryonic mesoderm cells spread in response to platelet-derived growth factor and signaling by phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 1996;93:9641–4. doi: 10.1073/pnas.93.18.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matter CM, Ma L, von Lukowicz T, et al. Increased balloon-induced inflammation, proliferation, and neointima formation in apolipoprotein E (ApoE) knockout mice. Stroke. 2006;37:2625–32. doi: 10.1161/01.STR.0000241068.50156.82. [DOI] [PubMed] [Google Scholar]
- 21.Rosenkranz S, Ikuno Y, Leong FL, Miyake S, Band H, Kazlauskas A. Src family kinases negatively regulate platelet-derived growth factor α receptor-dependent signaling and disease progression. J Biol Chem. 2000;275:9620–7. doi: 10.1074/jbc.275.13.9620. [DOI] [PubMed] [Google Scholar]
- 22.Rosenkranz S, Knirel D, Dietrich H, Flesch M, Erdmann E, Bohm M. Inhibition of the PDGF receptor by red wine flavonoids provides a molecular explanation for the “French paradox”. FASEB J. 2002;16:1958–60. doi: 10.1096/fj.02-0207fje. [DOI] [PubMed] [Google Scholar]
- 23.Kazlauskas A. The priming/completion paradigm to explain growth factor-dependent cell cycle progression. Growth Factors. 2005;23:203–10. doi: 10.1080/08977190500096020. [DOI] [PubMed] [Google Scholar]
- 24.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–32. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 25.Schermuly RT, Dony E, Ghofrani HA, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–21. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng B, Hayes M, Resta D, et al. Pharmacokinetics and pharmaco-dynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol. 2004;22:935–42. doi: 10.1200/JCO.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 27.Jackson CL, Raines EW, Ross R, Reidy MA. Role of endogenous platelet-derived growth factor in arterial smooth muscle cell migration after balloon catheter injury. Arterioscler Thromb. 1993;13:1218–26. doi: 10.1161/01.atv.13.8.1218. [DOI] [PubMed] [Google Scholar]
- 28.Choi JH, Yang YR, Lee SK, et al. Phospholipase C-gamma1 potentiates integrin-dependent cell spreading and migration through Pyk2/paxillin activation. Cell Signal. 2007;19:1784–96. doi: 10.1016/j.cellsig.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Kim EK, Tucker DF, Yun SJ, et al. Linker region of Akt1/protein kinase B-alpha mediates platelet-derived growth factor-induced translocation and cell migration. Cell Signal. 2008;20:2030–7. doi: 10.1016/j.cellsig.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Monypenny J, Zicha D, Higashida C, Oceguera-Yanez F, Narumiya S, Watanabe N. Cdc42 and Rac family GTPases regulate mode and speed but not direction of primary fibroblast migration during platelet-derived growth factor-dependent chemotaxis. Mol Cell Biol. 2009;29:2730–47. doi: 10.1128/MCB.01285-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sossey-Alaoui K, Li X, Ranalli TA, Cowell JK. WAVE3-mediated cell migration and lamellipodia formation are regulated downstream of phosphatidylinositol 3-kinase. J Biol Chem. 2005;280:21748–55. doi: 10.1074/jbc.M500503200. [DOI] [PubMed] [Google Scholar]
- 32.Broudy VC, Lin NL, Liles WC, et al. Signaling via Src family kinases is required for normal internalization of the receptor c-Kit. Blood. 1999;94:1979–86. [PubMed] [Google Scholar]
- 33.Valius M, Kazlauskas A. Phospholipase C-gamma 1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor’s mitogenic signal. Cell. 1993;73:321–34. doi: 10.1016/0092-8674(93)90232-f. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Jiao X, Wang C, et al. Cyclin D1 induction of cellular migration requires p27(KIP1) Cancer Res. 2006;66:9986–94. doi: 10.1158/0008-5472.CAN-06-1596. [DOI] [PubMed] [Google Scholar]
- 35.McClellan KA, Ruzhynsky VA, Douda DN, et al. Unique requirement for Rb/E2F3 in neuronal migration: evidence for cell cycle-independent functions. Mol Cell Biol. 2007;27:4825–43. doi: 10.1128/MCB.02100-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alt JR, Cleveland JL, Hannink M, Diehl JA. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000;14:3102–14. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takuwa N, Fukui Y, Takuwa Y. Cyclin D1 expression mediated by phosphatidylinositol 3-kinase through mTOR-p70(S6K)-independent signaling in growth factor-stimulated NIH 3T3 fibroblasts. Mol Cell Biol. 1999;19:1346–58. doi: 10.1128/mcb.19.2.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakakibara K, Kubota K, Worku B, et al. PDGF-BB regulates p27 expression through ERK-dependent RNA turn-over in vascular smooth muscle cells. J Biol Chem. 2005;280:25470–7. doi: 10.1074/jbc.M502320200. [DOI] [PubMed] [Google Scholar]
- 40.Bagui TK, Cui D, Roy S, et al. Inhibition of p27Kip1 gene transcription by mitogens. Cell Cycle. 2009;8:115–24. doi: 10.4161/cc.8.1.7527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.