Abstract
This paper presents translational aspects of imaging and genetic studies of language and cognition in children with epilepsy of average intelligence. It also discusses current unanswered translational questions in each of these research areas. A brief review of multimodal imaging and language study findings shows that abnormal structure and function, as well as plasticity and reorganization in language-related cortical regions are found both in children with epilepsy with normal language skills and in those with linguistic deficits. The review on cognition highlights that multiple domains of impaired cognition and abnormalities in brain structure and/or connectivity are evident early on in childhood epilepsy and might be specific for epilepsy syndrome. The description of state of the art genetic analyses that can be used to explain the convergence of language impairment and Rolandic epilepsy includes a discussion of the methodological difficulties involved in these analyses. Two junior researchers describe how their current and planned studies address some of the unanswered translational questions regarding cognition and imaging and the genetic analysis of speech sound disorder, reading, and centrotemporal spikes in Rolandic epilepsy.
Keywords: language, cognition, imaging, genetics, pediatric epilepsy
INTRODUCTION
The integration of language and cognition matures by the end of adolescence and reflects age-related changes in cortical association areas and language-related brain regions and pathways (1). The wide range of linguistic (2), cognitive/academic (3), and related social and psychiatric comorbidities (See review in (4)) in children with epilepsy with average intelligence implies impairment in these higher-level linguistic/cognitive skills and their associated brain regions and neural pathways. Therefore, biomarkers of these deficits could promote early identification and treatment of children with epilepsy at risk for language impairment.
From a translational perspective there currently are no animal models of language and its impairments and no biological tests for risk of language impairment or other epilepsy comorbidities. In addition, models of cognitive abnormalities in animals with seizures cannot tap into the higher-level integration of language and cognition that enable children to function adequately across multiple domains. However, studies using multimodal imaging of the brain and genetics provide a window into the brain-behavior relationships involving language and cognition.
This paper briefly reviews current trends in the translational research of language, cognition, and the genetics of speech and language in pediatric epilepsy, and identifies unanswered translational questions. Two junior researchers describe how their current and planned studies address some of the unanswered translational questions regarding cognition and imaging (Dr. Lin) and genetic analysis of speech sound disorder, reading, and centrotemporal spikes in Rolandic epilepsy (Dr. Addis).
LANGUAGE AND IMAGING IN PEDIATRIC EPILEPSY
Rochelle Caplan, M.D.
State of the Art
Language, Development, and Pediatric Epilepsy
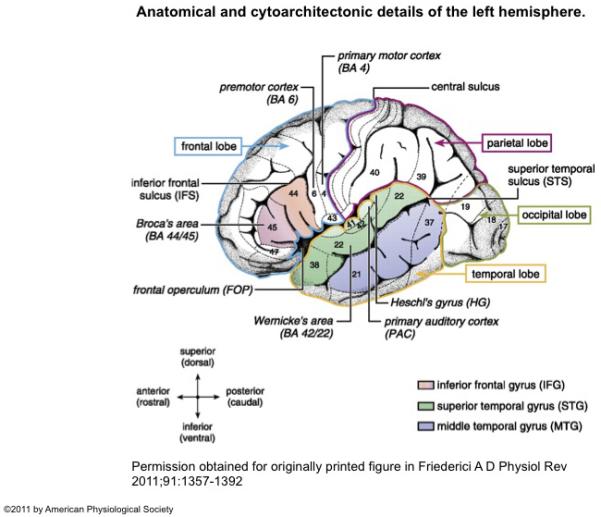
During the complex growth in thought, cognitive flexibility, and integration of knowledge with language in childhood and adolescence, there is an increase in syntactic complexity (5), advanced use of grammar and vocabulary, as well as abstraction (6-8). The parallel ongoing and protracted development of fronto-temporal language-related regions (e.g., superior temporal gyrus, Heschl’s gyrus, inferior frontal gyrus) and pathways (9) (Figure 1) increases their vulnerability to the effects of on-going seizures (10).
Figure 1.
Anatomical and cytoarchitectonic details of the left hemisphere. The different lobes (frontal, temporal, parietal, occipital) are marked by colored borders. Major language relevant gyri (IFG, STG, MTG) are color coded. Numbers indicate language-relevant Brodmann Areas (BA) which Brodmann (1909) defined on the basis of cytoarchitectonic characteristics. The coordinate labels superior/inferior indicate the position of the gyrus within a lobe (e.g., superior temporal gyrus) or within a BA (e.g., superior BA 44; the superior/inferior dimension is also labeled dorsal/ventral). The coordinate labels anterior/posterior indicate the position within a gyrus (e.g., anterior superior temporal gyrus; the anterior/posterior dimension is also labeled rostral/caudal). Broca’s area consists of the pars opercularis (BA 44) and the pars triangularis (BA 45). Located anterior to Broca’s area is the pars orbitalis (BA 47). The frontal operculum (FOP) is located ventrally and more medially to BA 44, BA 45. The premotor cortex is located in BA 6. Wernicke’s area is defined as BA 42 and BA 22. The primary auditory cortex (PAC) and Heschl’s gyrus (HG) are located in a lateral to medial orientation.
Permission obtained from originally printed figure in Friederici A D Physiol Rev 2011;91:1357-1392
Thus, particularly in older children with epilepsy, seizure variables are associated with impaired basic linguistic skills (e.g., syntax, semantics, and phonetics) (2, 11-15) and higher-level discourse skills (i.e., use of language to formulate and organize thoughts into coherent speech) (16). But evidence for linguistic deficits in children with new onset seizures (13, 17) and their continuation over time irrespective of seizure control in children with chronic epilepsy (18), together with impaired phonological awareness in benign Rolandic epilepsy (RE) (a disorder associated with few lifetime seizures) (14), also imply a role for the neuropathology underlying epilepsy. The findings of the few imaging studies conducted to date on language in children with epilepsy with average intelligence might reflect combined effects of the underlying neuropathology, ongoing seizures, or both factors on plasticity in these brain regions.
Structural Imaging Studies
Normal Brain Development
Gray matter volume and cortical thickness increases during childhood, peak at about puberty, and gradually decrease during adolescence (See reviews in (19) and (20)). These morphometric changes progress in an antero-posterior direction first involving the sensory-motor cortex and then secondary and multimodal cortical brain regions (See review in (20)). The frontal and temporal lobe, particularly the language-related superior temporal gyrus, are the last brain regions to undergo these maturational changes. Synaptic remodeling (pruning) with reduction in the number of synapses, neuropil, and number of glial cells and experience-dependent molding of the architecture of cortical columns along with dendritic spine and axonal remodeling underlie these developmental changes in gray matter volume and thickness (20-23). G proteins, such as the G protein-coupled receptor, the superconserved receptor expressed in brain (SREB2), play a prominent role in plasticity, neural integrity, and cortical thickness (24, 25).
Although white matter volume also increases markedly in the first few years of life, in contrast to gray matter, it expands linearly and simultaneously in the parietal, frontal, and temporal lobes from the onset of puberty, and continues into adulthood (See review in (26)). Myelination brings about the increase in white matter and the relative decrease in gray matter (23, 27).
In addition to age, twin studies have identified the role of heritability in the gray matter density of the (pre-) frontal and temporal areas (28) and in the cortical thickness of the left middle and inferior frontal gyri, lateral fronto-orbital and occipitotemporal gyri, pars opercularis, planum temporale, precentral and parahippocampal gyri as well as the medial region of the primary somatosensory cortex (29). In 9 and 12-year old twin cortical thinning is also highly heritable in both Broca’s and Wernicke’s areas (30). But heritability for later developing brain involved in language and executive function is higher in adolescence than in childhood (31). Although white matter density is highly heritable in fronto-occipital and superior longitudinal fascicles (28), this is not the case for the age-related growth in white matter (26).
Pediatric Epilepsy
Structural studies conducted to date on language in pediatric epilepsy include children of different ages and have been cross-sectional. Their findings, therefore, are averages of children who are at different stages of the previously described developmental changes in gray and white matter. Most notably, these studies have identified significantly different relationships of language measures with brain volumes and cortical thickness and depth in children with epilepsy compared to typically developing age and gender matched children. They demonstrate that both children with epilepsy with and without impaired language skill have abnormal development and reorganization of language-related brain regions including the inferior frontal gyrus, posterior superior temporal gyrus, temporal lobe, Heschl’s gyrus, orbital frontal gyrus, and dorsolateral prefrontal cortex (32, 33) (See brain regions in Figure 1).
Thus, whereas higher mean overall language scores were associated with increased gray and white matter total brain and dorsolateral prefrontal cortex volumes in healthy children, they were positively related to inferior frontal gyrus and temporal lobe gray matter volumes in 64% of children with epilepsy with average language scores (33). In contrast, the 36% of patients with linguistic deficits had significantly smaller anterior superior temporal gray matter volumes and language scores that were negatively associated with dorsolateral prefrontal gray matter volumes.
Measures of how a child coherently communicates thoughts and ideas so that the listener follows who and what the child is talking about were significantly associated with smaller orbital frontal gyrus and inferior frontal gyrus gray matter volumes, increased Heschl’s gyrus gray matter volumes, and smaller superior temporal gyrus white matter volumes in children with epilepsy. In healthy children, however, these measures were related to significantly larger orbital frontal gyrus, superior temporal gyrus, and temporal lobe gray matter volumes, as well as to decreased Heschl’s gyrus white matter volumes (32).
Similarly, higher verbal intelligence was related to greater sulcal depth in the left superior temporal gyrus, middle frontal, and superior frontal gyrus of healthy children but in the left inferior frontal and sulcal region between the paracentral lobule and superior frontal region in children with absence epilepsy (34). Whereas cortical thickness related to verbal and performance IQ scores had a similar brain region distribution in the control subjects, this was not the case in the epilepsy group. Verbal IQ was associated with right inferior temporal and left parieto-occipital cortical thickness, and performance IQ was related to left middle frontal and superior temporal cortical thickness.
Summary
Due to the cross-sectional design and wide age range of children in these studies, 7-15 years, the study means do not represent the different stages of pruning and myelination in the individual children. Nevertheless, compared to typically developing children, the significantly different associations between language and gray and white matter volumes in both children with epilepsy with and without linguistic deficits imply reorganization and plasticity, respectively of brain regions involved in language in children with epilepsy.
Functional Imaging Studies
Functional study findings (35-38) also suggest that abnormalities in brain regions subserving language, their networks, and neuronal functioning play a role in the basic and higher-level linguistic skills/deficits of children with epilepsy. As in the structural studies, the functional study findings imply that plasticity and reorganization occur in the 60% of epilepsy youth with normal language skills.
A functional magnetic resonance imaging (fMRI) study of an auditory description decision task in 4.5 – 19-year old epilepsy youth with localization related epilepsy and control subjects, identified activation in the left inferior frontal gyrus and along the left superior temporal gyrus in 93% control subjects and 61% patients; left dominant pattern with greater activation in inferior frontal gyrus, mesial left frontal lobe, and right cerebellum in 5% controls and 26% of patients, most of whom had a right hemisphere epileptic focus; and activation in the right inferior frontal gyrus and superior temporal gyrus in 1 control and 14% patients with a left focus and symptomatic epilepsy (38). Similarly, a verb generation task revealed less left hemisphere lateralization, particularly in anterior regions (inferior frontal gyrus), in children with benign RE, aged 6.5–11.8 years, matched with healthy children (37).
In an fMRI study of higher-level discourse skills children listened to short question-answer dialogues and determined whether the answers made sense (half did) (39). Unknown to the subjects, features critical to making this decision varied so as to tap into either the reasoning (logical vs. illogical) or topic maintenance (topic maintained vs. unpredicted topic change) of the on-going conversation (39). Compared to healthy children, complex partial seizure subjects showed significantly decreased left inferior frontal gyrus activation during the reasoning condition and a lack of right temporal dominance in the topic maintenance condition (35) (Figure 2).
Figure 2.
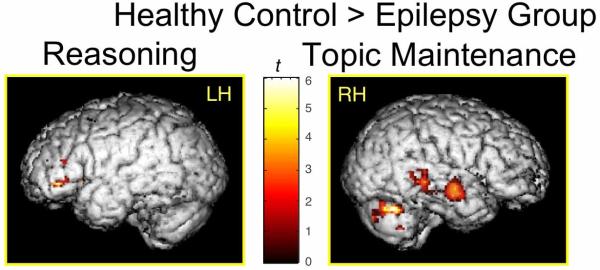
Between-group comparisons show significantly cortical greater activity in controls than children with epilepsy in the left inferior frontal gyrus for the reasoning condition and in the right temporal cortex for the topic maintenance condition.
A magnetic resonance spectroscopy study demonstrated that the reduced N-acetyl-aspartate (NAA) plus N-acetylaspartyl-glutamate (NAAG) (tNAA) in the right inferior frontal gyrus in children with complex partial seizures compared to control subjects was associated with illogical thinking and seizure frequency in the epilepsy group (35). Sensitivity of tNAA to this linguistic variable and its atypical lateralization suggest that effects of ongoing seizures on neuronal and glial function in inferior frontal gyrus might underlie illogical thinking in these children.
In summary, normal and abnormal lateralization patterns despite normal performance in different linguistic tasks in these fMRI studies suggest that localization related epilepsy is associated with reorganization and plasticity of neural circuits involved in basic and higher level language skill. The MRS findings imply that higher-level linguistic deficits reflect abnormal lateralization and chemical functioning of glial cells subserving language.
Unanswered Translation Questions and Future Directions
Evidence for brain imaging (structure, function), clinical epilepsy, and genetic/familial biomarkers of the linguistic phenotypes of pediatric epilepsy (i.e., children with and without linguistic deficits) will enable early identification and treatment of a comorbidity that impacts the educational, social, and behavioral functioning of children with epilepsy, as well as long-term vocational and independent living outcome (40). Furthermore, delineation of the cortical and subcortical neurodevelopmental mechanisms underlying plasticity and reorganization of the structure and function of language-related brain regions in children with normal language skills could lead to the development of innovative treatment techniques to augment plasticity and reorganization of the neural networks involved in language development. To achieve this goal, studies are needed on the role of early developing subcortical structures involved in language skill, such as the thalamus (41-43), basal ganglia (44), hippocampus (42, 45), and cerebellum (See review in (46)) in the plasticity and reorganization of language-related brain regions and pathways in children with epilepsy.
COGNITION AND IMAGING IN PEDIATRIC EPILEPSY
Bruce Hermann, Ph.D.
State of the Art
Moving Forward
Cognitive impairment is a common and widely appreciated complication of the childhood epilepsies. Much research has been devoted to identifying the nature and extent of these cognitive disorders and their related clinical seizure correlates. Reviews of studies of cognition in children with established epilepsy have shown that a number of epilepsy-related factors appear to contribute to these problems, such as seizure frequency, recurrent seizures, age at seizure onset, duration of illness, antiepileptic drugs (AEDs), type of epilepsy, and EEG findings (47). However, across studies, the effects of epilepsy-related variables are not consistently replicated and often other considerations such as family integrity, preexisting learning problems, and comorbid psychopathology are significant factors influencing academic underachievement and related cognitive and linguistic deficits (47). Among the questions that have arisen regarding the epilepsy-cognition link are when in the course of epilepsy do these abnormalities present themselves, how do they unfold over time, and what are the features of the underlying neurobiology that may be responsible for these cognitive impairments?
Cognition and Imaging in New Onset Epilepsy
One way to begin to untangle these issues is to pay greater attention to the natural history of the neurobehavioral complications of the childhood epilepsies. To that end, important issues are whether cognitive difficulties are apparent at epilepsy onset and how they develop over time in the context of recurrent seizures, treatment with AEDs, and the psychosocial complications that may accompany childhood epilepsy. Identification of cognitive abnormalities at the onset of epilepsy would also infer the potential presence of antecedent neurodevelopmental abnormalities and again the associated question of the neuroanatomic abnormalities that may underlie these effects is key.
So what then is the status of cognition in children with new onset epilepsy? A total of 11 papers have examined children with epilepsy at or near the diagnosis of epilepsy. Of these investigations, 7 assessed children prior to the initiation of AEDs (Table 1). As can be seen, there is near complete consensus that cognitive abnormalities are present early in the course of the disorder. While in agreement, these studies vary significantly in the nature of the administered test batteries, the epilepsy syndromes examined, the nature and degree of identified anomalies, the associated comparison groups, and many other characteristics of the investigations. But it is clear that cognitive disorders can be apparent very early in the course of childhood epilepsy.
Table 1. New onset epilepsy: Cognition.
| Authors | Year | AED Status | Controls | Abnormalities |
|---|---|---|---|---|
| Bourgeois et al. (95) | 1983 | Yes | Yes | |
| Stores et al. (96) | 1992 | Drug naïve | Yes | Yes |
| Mandelbaum & Burack (97) | 1997 | Drug naïve | No | --- |
| Williams et al. (98) | 1998 | Drug naïve | Yes | No |
| Kolk et al. (99) | 2001 | Drug naïve | Yes | Yes |
| Oostrom et al. (100) | 2003 | Drug naïve | Yes | Yes |
| Hermann et al. (101) | 2006 | Yes | Yes | |
| Fastenau et al. (102) | 2009 | Yes | Yes | |
| Bhise et al. (103) | 2010 | Drug naïve | No | --- |
| Jeong et al. (104) | 2011 | No | --- | |
| Vintan et al. (105) | 2012 | Drug naïve | Yes | Yes |
What is the status of brain structure in children with new onset epilepsy? There are now a total of 8 investigations that have undertaken neuroimaging in this population. These studies also vary in a number of ways. Studies differ in the imaging techniques used, the targeted regions of interest, the epilepsy syndromes under investigation, comparison groups, and other features. Here only one study has examined children prior to the initiation of AEDs. Again, there is near complete consensus that abnormalities in brain structure and/or connectivity are evident early on in childhood epilepsy.
Much remains to be learned about children with new onset epilepsy, but an interesting question is the specificity of the cognitive and neuroimaging impairments and their interrelationship, especially across epilepsy syndromes. To date, the pattern of cognitive profiles in new onset epilepsy seems to be affected in a generalized fashion, that is, compared to healthy controls, children with new onset epilepsy appear to exhibit a relatively broad range of abnormality affecting performance across several cognitive domains including intelligence, language, learning and memory, and executive function. In recent years, considerable interest has developed regarding the status of executive function (EF) in various childhood clinical populations including childhood epilepsy. From a neuropsychological perspective, EF is an umbrella term used to identify a broad and diverse set of self-regulatory processes encompassing behaviors such as initiation, planning, organization, purposive actions, self-monitoring, and self-regulation—all viewed as critical skills that are associated with successful day-to-day functioning.
The components of EF do not develop in a linear fashion or at the same rate. Development often occurs in a stage-like manner, consistent with known brain development (48, 49)). EF components also demonstrate different developmental trajectories, complicating study of the construct (48, 50). One of the difficulties in studying EF has been the availability of specific standardized assessment tools developed for this specific purpose, particularly with children. That said, the available literature suggests that EF is indeed a vulnerable domain of cognition among pediatric epilepsy patients regardless of epilepsy syndrome (51). Interestingly, impairments in EF have been documented using both formal neuropsychological tests (i.e., measures of response inhibition, novel problem solving, sustained attention) as well as parent-reported behavioral measures (e.g., BRIEF). That this is an important cognitive process that is adversely affected both from the perspective of the parent as well from traditional neuropsychological tests is now clear. The implications for ongoing development are less clear however. As the child with epilepsy develops and becomes more dependent on the child’s self-regulatory processes, what are the implications of disordered EF for independent living status, vocational status, and other critical components of independent living?
Understanding the neuroanatomy of EF is important. From an anatomic perspective, it has been shown that children with new onset epilepsies or specific epilepsy syndromes, exhibit abnormalities in neuroanatomic regions with connectivity known to be part of the distributed system supporting diverse executive functions. This system includes especially the fronto-striatal-thalamic structures. To give one example, a number of studies have provided evidence for executive dysfunction in Juvenile Myoclonic Epilepsy (JME). Specifically, impairments in concept formation, abstract reasoning, mental flexibility, cognitive speed, and planning have been reported (52-55). Converging evidence from neuroimaging and neuropsychological studies provide a basis to hypothesize that frontal lobe and thalamic dysfunction underlie executive impairments in JME (52, 56), and there are now a limited number of direct investigations demonstrating the structural brain correlates of executive dysfunction in children with JME.
Unanswered Translation Questions and Future Directions
Important unanswered questions for the future include the following. Focusing specifically on EF, the distribution of disruption in specific components of executive function and their neuroanatomical correlates remain to be clarified as does the variability that may be evident in these relationships across epilepsy syndromes. Further, the impact of recurrent seizures and medication treatment on the normal developmental trajectories of these important systems remains to be clarified and, among those children whose epilepsy remits, it is important to determine whether the cognitive functions and their underlying anatomic systems “recover” to age appropriate levels. Finally, the impact of disrupted executive functions on day-to-day functional status remains to be determined so that targeted interventions can be developed.
Beginning Answers: Cognition and Imaging
Jack J. Lin, M.D.
Temporal Lobe Epilepsy, Imaging, and Cognition
Whereas hippocampal sclerosis is the primary structural signature of mesial temporal lobe epilepsy (MTLE), accumulating evidence has shown that considerable anatomical abnormalities exist outside of this region. In concert with structural abnormalities, patients with MTLE exhibit a pattern of cognitive impairments affecting not only memory but also a broad array of cognitive domains including intelligence, executive function, language, and sensorimotor processing (57-59). Postulating specific links between distributed structure abnormalities and cognitive performance, my colleagues and I examined reduction in cortical thickness and complexity (60), aberrant white matter connections (61), as well as their selective cognitive correlates in TLE (62).
Comparing 15 left and 15 right TLE patients to 19 healthy controls (60), we found that both the left and right TLE groups had regions with up to 30% bilateral decrease in average cortical thickness with significant thinning of the bilateral frontal poles, frontal operculum, orbitofrontal, lateral temporal, and occipital regions, as well as the right angular gyrus and primary sensorimortor cortex surrounding the central sulcus (Figure 3a). Examining cortical complexity, the left TLE group showed significantly reduced complexity across all the left and right hemisphere lobes except the right frontal lobe. In contrast, the right TLE group exhibited significantly reduced cortical complexity across all lobes other than the right frontal and parietal lobes. These findings implied that the distributed nature of cognitive impairment (57-59) in TLE might involve a widespread and coordinated network of abnormalities.
Figure 3.
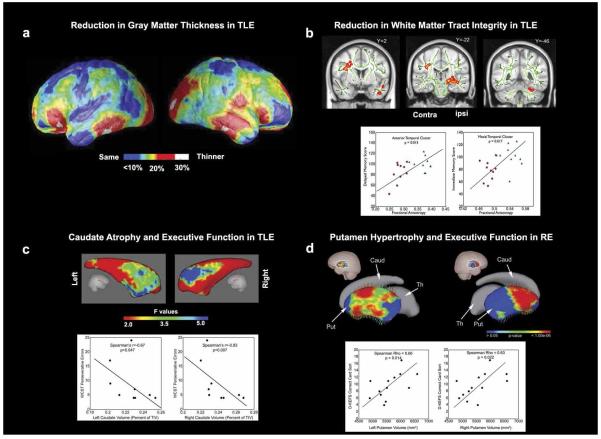
Brain structural abnormalities are linked to cognitive deficits in epilepsy. a) Reduced gray matter thickness in left MTLE. The color maps denote mean percent reduction in cortical thickness as a percentage of control average. Red areas in the bilateral frontal poles, frontal operculum, orbitalfrontal, lateral temporal and occipital regions, and the right angular gyrus and sensorimotor cortex denote up to 30% reduction in thickness. b) Whole brain diffusion tensor imaging reveals reduced fractional anisotropy (FA) in the anterior temporal lobe, mesial temporal lobe and cerebellum ipsilateral, as well as frontoparietal lobe contralateral to the side of seizure onset in TLE. FA is positively correlated with delayed memory scores in the anterior temporal lobe and with immediate memory scores in the mesial temporal lobe. c) Shape analysis shows that the caudate head is selectively atrophied bilaterally in left TLE. Caudate volumes are negatively correlated with Wisconsin Card Sorting Test perseverative errors. d) Bilateral ventral dorsal putamen hypertrophy is evident in children with new onset RE. Putamen volumes are positively correlated with Delis-Kaplan Executive Function System card sorting scores. Abbreviations: MTLE, mesial temporal lobe epilepsy; TLE, temporal lobe epilepsy; RE, Rolandic epilepsy.
We then investigated whether TLE disrupted cortical and subcortical connections that are germane to higher cognitive processing are related to cognitive deficits in multiple domains. In cortico to cortico pathways I found that white matter integrity was reduced in the mesial and anterior temporal lobe and in the connections between the frontal and temporal lobes (uncinate fasciculus and arcuate fasciculus) in TLE (61). Importantly, the degree of white matter abnormalities in the mesial temporal lobe correlated with immediate memory performance, and anterior temporal lobe abnormalities were linked to delayed memory scores (62) (Figure 3b). In the cortical to subcortical pathways, aberrant frontocerebellar and frontostriatal connections were associated with impaired executive function (62, 63) (Figure 3c). In summary, TLE is also associated with widespread white matter abnormalities and these changes have important cognitive correlates.
Brain Structure and Cognition in New Onset Pediatric Epilepsy
One of the fundamental limitations of any cross-sectional study in chronic adult TLE is that the timing of the structural abnormalities cannot be determined. Researchers often hypothesize that chronic epilepsy causes widespread structural abnormalities and cognitive comorbidities. However, the timing of epilepsy is also pivotal as the immature brain may be more vulnerable to the impact of early-life seizures. Our first insight into the neurodevelopmental impact of epilepsy came from the association between age of epilepsy onset and white matter brain connections in adult TLE patients. Earlier seizure onset was linked to lower integrity in the uncinate fasciculus (61), a key white matter tract connecting the mesial temporal lobe to the frontal lobe. Subsequent studies from my group (62) and others (64, 65) showed that the integrity of the splenium of the corpus callosum was also related to age of seizure onset.
Although these associations were intriguing, the nature and progression of cognitive abnormalities in epilepsy was unclear. The optimal method to examine the influence of early onset epilepsy on brain structure and cognition is a controlled prospective cohort investigation initiated at the time of epilepsy onset. To achieve this goal, I collaborated with Bruce Hermann, Ph.D. in his studies on brain and cognition in children with new onset epilepsy. At a syndrome-specific level, an important but unexpected finding was that children with new onset RE had hypertrophied caudate and putamen (66). Further, children with RE and larger striatum performed better on EF tasks compared to those with smaller putamen and healthy controls (Figure 3d).
Several lines of evidence suggest that these findings reflect neurodevelopmental and genetic factors rather than seizure-associated brain reorganization. First, whereas normal brain development occurs through anterior to posterior expansion, enlargement of the caudate and putamen in RE is coupled with shape alteration in the vertical direction. This finding suggests a neurodevelopmental derangement (67). Second, the EPL4 gene, critical for maturation of projection neurons and recently implicated in the centrotemporal EEG trait, is highly expressed in the putamen (Allen Brain Atlas, http://www.brain-map.org). Finally, better performance on the EF tasks by the children with RE and larger striatum compared to those with smaller putamen and the healthy controls implies that the hypertrophy might be an adoptive mechanism. Interestingly, hypertrophy in certain brain regions is associated with enhanced skills. For example, larger hippocampus is linked to better navigational skills (68) and increased amygdala is positively elated to social network size (69).
In summary, early onset epilepsy may have a deleterious impact on key white matter connections. Further, as described in the section on Cognition and Imaging in Pediatric Epilepsy, evidence for both aberrant brain structure and cognition are evident early in the course of epilepsy suggesting a neurodevelopmental contribution to the cognitive comorbidities of children with epilepsy.
Further directions
How can we use the evidence for brain structural abnormalities and their relationship to cognitive performances to improve cognition in adults and children with epilepsy? In order to develop epilepsy specific treatments, we must first understand baseline compensatory processes, mechanisms by which improvement occurs, and predictors of long-term gains. In this regard, animal models have provided considerable insights. For instance, successful working memory performance requires a symphony of brain oscillations between the prefrontal lobe, mesial temporal, and basal ganglia (70), regions that were abnormal in my studies (Figure 3). Further, successful performance on memory tasks after early-life seizures requiring increased reliance on the frontal lobe suggests possible compensation for a dysfunctional medial temporal lobe (71). Currently, we are using functional MRI and diffusion tensor imaging to investigate whether intensive working memory training in TLE enhances prefrontal and temporal lobe functional and structural connections. If confirmed, these compensatory mechanisms could be enhanced to accelerate memory and cognitive recovery.
GENETICS OF LANGUAGE IMPAIRMENT IN PEDIATRIC EPILEPSY
Deb K. Pal, M.D., Ph.D.
State-of-the-Art
Structural Genomic Variation: Basics
Genetic analyses are helping to explain the convergence of language impairments and epilepsy in children of average intelligence. Array comparative genomic hybridization (aCGH) is one recent method of genetic analysis that is now a routinely used clinical tool in the workup of neurodevelopmental disorders. aCGH identifies changes in gene dosage, either loss (deletions or null genotypes) or gain (insertions or duplications). Occasionally these copy number variants (CNVs) may be associated with disease (72). Depending on the size of the genomic material lost or gained, one or several genes may be implicated in the association. Where several genes are implicated, sometimes a minimal critical region may be defined by overlapping the genomic sequences of affected individuals who each may have differing length structural variations. Often microscopically visible CNVs are originally reported in association with severe phenotypes, and more subtle submicroscopic alterations are subsequently investigated (e.g. by DNA resequencing) as potential associations with milder disease forms. A common theme is the association of recurrent CNVs (CNVs that occur in multiple individuals) with several different neurodevelopmental phenotypes. aCGH has recently revealed aetiological connections between speech or language impairment and epilepsy (as well as with other disorders).
Structural Genomic Variants: Pleiotropic Effects
Pleiotropy is defined as multiple traits influenced by the same gene. Autism, developmental delay, schizophrenia-like psychosis, and AED-related weight gain are all found in children with epilepsy and some or all these traits may result from pleiotropy. An example is the chromosome 16p11.2 region, where deletions were reported initially associated with autism and developmental delay (73, 74) and subsequently obesity (75). Duplications of the same region were associated with seizures and speech and language delay (76), as well as with schizophrenia (77). Elsewhere on this chromosome, 16p13.2-13.13 deletion has been reported in association with absent or delayed speech, Rolandic seizures, intellectual disability and dysmorphic features (78). Overlapping deletions of this region in three patients implicate GRIN2A as a candidate gene in this syndrome (79).
The link between the rare epileptic encephalopathies, Landau-Kleffner syndrome (LKS) and Continuous Spikes in Slow-Wave Sleep (CSWSS), with autism has been consolidated through the discovery of CNVs in LKS/CSWSS that are common to those found in autism and speech and language disorders. Some of the implicated genes encode cell adhesion proteins (e.g. cadherins, contactins, catenins) that play a crucial role at the synapse (80).
Structural Genomic Variants: Experimental Design and Analysis Issues
There are however, many experimental challenges in the field and some of these are addressed below. First, associations with CNVs usually lack phenotypic specificity; studies that search for CNVs in one specific clinical population will likely miss association with a broader phenotype. Second, aside from very large CNVs that are likely causal, smaller or novel CNVs or CNVs that are not clearly pathogenic may have a modifying effect. In this case, it is helpful to study structural variation genomewide and to avoid reporting these variants of uncertain significance in isolation. Third, the calling of CNVs from aCGH and single nucleotide polymorphism (SNP) arrays is not uniformly reproducible and is somewhat dependent on the precise platform, quality control and analysis pipelines. Different algorithms result in different CNV frequencies and the use of at least two must be considered a gold standard. One must also bear in mind that clinical aCGH results may be filtered according to what is perceived to be pathogenic, and may not be comparable to an unbiased analysis of array data from a research laboratory. Fourth, the choice of suitable comparison data is a very important issue. Ancestry, cohort and possibly gender may influence the distribution of CNVs, yet many papers report the use of convenience samples with variable or unknown screening for the trait or related traits of interest and often called using different platforms and analysis pipelines. Clearly, unsuitable controls may bias disease associations in either direction. Fifth, as with any other epidemiological study, low sample sizes and selective ascertainment schemes may introduce bias and error into the interpretation of positive or negative CNV-disease associations. These in turn may limit the generalizability of findings and the chances of replication. Last, although in some instances CNVs are clearly strong risk factors for neurodevelopmental disorder, the attributable risk fraction for CNVs remains very low for the majority of individuals with neurodevelopmental disorders. Clearly the goal of elucidating the cause of common epilepsies in children of average intelligence must start with an explanatory genetic model that takes account of both genomic structural and sequence variants.
Unanswered Translation Questions and Future Directions
At the moment, the search for copy number variation has caught the popular imagination and the journals are filling with new CNV studies. But how will we judge the value of these studies in ten years’ time in the epilepsy field? I believe their value lies in how well they address the simple unanswered questions that we face today. For example, what is the role of CNVs in common complex epilepsies in children of average intelligence? Are children with rare recurrent CNVs genetically heterogeneous? Will the genes underlying CNVs yield hitherto undiscovered sequence susceptibility variants for common epilepsies? Or do the CNVs instead act as modifiers, interacting with other CNVs or with other genomic sequence variants? When the large proportion of potentially pathogenic or susceptibility-inducing CNVs have been discovered for epilepsy, will genetic pathway analysis of these CNVs in combination lead us to molecular maps of pathogenesis for epilepsy and its related comorbidities, including language and neurodevelopmental disorders? The frequency of recurrent and novel CNVs appears to be orders of magnitude higher in idiopathic focal compared to idiopathic generalized epilepsies - is this a real phenomenon, and, if it is, does it correlate with mild linguistic and cognitive impairments? If CNVs are to be useful for genetic counseling, what do we advise unaffected carriers of “pathogenic” or susceptibility CNVs and how do we calculate recurrence risk?
Major Genetic Effect Loci in Rolandic Epilepsy: Finding the Susceptibility Sequence Variants
We must not forget that aside from CNVs, considerable progress is being made on the major genetic influences that are known to act on common complex epilepsies like RE. Our approach to the complicated genetics of RE is to dissect the overall phenotype into its component clinical traits and endophenotypes (e.g. EEG). Linkage analysis of RE families has thus revealed major susceptibility loci for speech sound disorder (81) and reading disability (82), the two main neurodevelopmental comorbidities in RE. Speech-sound disorder (OMIM 608445) is defined as developmentally inappropriate errors in speech production that reduce intelligibility and which is distinct from stuttering and aphasia. Operationally we used ICD-10 definitions of speech articulation disorder (F80.0) (who.int/classifications/en). Intriguingly, the 11p13 locus containing candidate genes ELP4 and PAX6 are pleiotropic (one gene or variant influences multiple phenotypes) for both speech sound disorder and the centrotemporal spike EEG signature of RE (81). Acoustic analysis of speech distinguishes the physiologic mechanism of speech sound disorder in these individuals as dyspraxic, based on abnormalities of voice-onset time and vowel durations, suggesting the role of cortico-striatal networks (81). However, unlike in rare Mendelian epilepsies e.g. Roll et al 2006 (83) single causal coding mutations are not expected in a complex genetic model, and this is probably even more so in the case of subclinical traits (viz EEG) where we expect rare variants in several genes to be involved. Thus the primary challenge in finding sequence susceptibility variants, even where prior linkage and association evidence localize the critical genomic region, is to discover susceptibility variants in non-coding regions. Almost by definition, such regions will not be covered by exome (coding region) sequencing. Nevertheless, massively parallel sequencing is an absolutely necessary tool to interrogate intronic (within a gene, and between the coding exon blocks) and intergenic (between gene) regions where variants can act to control gene expression. At the moment, next-generation sequencing data is challenging in many respects ranging from the “capture” of delimited DNA segments, to the depth and extent of genomic coverage, and not least to the correct alignment of sequence reads to reference genomes and comparison with suitable control data – much of which is still emerging.
Tantalizing answers are expected from investigation of the 11p13 locus. Do all affected individuals have the same variants, or do different variants share the same molecular mechanism? Can the co-occurrence of speech sound disorder, or the differing severity of the EEG abnormality be explained by epistatic interaction at this locus? Does the 11p13 locus provide the common denominator between different epilepsies that exhibit focal sharp waves? And crucially, will the identification of risk alleles result in early detection of at-risk individuals and implementation of early intervention(s)?
Beginning Answers: Language and Genetics
RE, Speech, and Genetics
Laura Addis, BSc D.Phil
Copy Number Variation Analysis in RE
RE (84) has a variable course and progression in children with differences in seizure severity and frequency and the comorbidities experienced, such as speech and language impairments, dyslexia and attention problems (84-88). My pilot genome-wide investigation using a well-phenotyped cohort has provided evidence that both CNVs that occur more frequently in neurological disorders (e.g. at 15q11.2, 15q13.2, 16p11.2), as well as larger novel CNVs, play a role in the etiology of RE (89). In my current study I am testing the hypothesis that CNVs act as modifier loci in RE (i.e. different CNVs alter the disease severity and course, and influence the type of comorbidities a child will exhibit). CNVs can act as modifier loci in complex disorders because dosage differences in critical genes contained within the CNV amplify, change or suppress the phenotypic effect of other pathogenic variants in major effect genes (90, 91). The same CNV can also be involved in constellations of symptoms depending on the nature of other risk factors - called variable expressivity. As the comorbid traits associated with RE occur at a high frequency in parents and siblings of cases (85, 86), I have found them an ideal internal control group for studying CNV inheritance, variable expressivity, and sub-trait correlations.
Methodological Considerations for CNV Studies
When choosing an array for the CNV analysis, I was mindful of the control population to be used. It is not always financially viable to genotype a control set and then a public dataset can be chosen. To minimize bias when choosing a publicly available dataset it is best to use one that is assayed on the same platform as the cases. If the raw data is available this is also preferable because both datasets can be analyzed with the same ‘pipeline’ of software tools. But control datasets even two or three years old may have been assayed on now obsolete aCGH or single nucleotide polymorphism (SNP) arrays. Therefore, it is good practice to try and choose a current chip for cases that includes as many probes as possible from the control chip so that the analysis can be as similar as possible. However, the older the chip, the fewer probes are on it, and so small CNVs may be missed. Stringent criteria for analyzing CNVs should also be employed for robust calls (e.g. making sure >5 probes/SNPs cover the CNV), validated with an alternative method (e.g. aCGH vs. SNP array), especially for small CNVs which can be false positives, and breakpoints accurately mapped with the polymerase chain reaction (PCR).
Determining if a CNV is potentially pathogenic is an area of concern for investigations of this type. I am using the following methods: a CNV is classed as novel if it is not seen in public and local control datasets e.g. the Database of Genomic Variants. CNVs will be further investigated if they or their constituent genes are known to be pathogenic in other disorders, if they are listed in the CNV morbidity map of developmental delay and neurological development (92), or if they unmask recessive alleles. CNVs that are known to be under negative genetic selection (i.e. they are rare but occur at increased frequency in disease populations and arise independently in multiple founders), may also be targets for further study (93). Lastly, CNVs that contain gene sets that are dosage balanced (i.e. very sensitive to the relative quantities of the gene), and thus rarely involved in copy number variation events, also become candidates. These genes are called ohnologs, are developmentally enriched, and have a strong association with disease (94).
For a particular trait, it is likely that dosage sensitive genes disrupted by CNVs will show significant enrichment in functional gene or pathway categories defined using network and pathway analysis software such as DAVID (http://david.abcc.ncifcrf.gov/) or Ingenuity Systems. These tools offer several options for clustering genes and their products into gene families, functional groups, physiological locations or biochemical pathways, and disease associations. Potentially functionally significant events (duplications or deletions) will be confirmed using comprehensive gene expression analysis (this will also identify non-coding CNVs with regulatory functions) and cellular models.
Next-Generation Sequencing of RE Genetic Locus
In the second aspect of my work on RE, using family-based linkage and association our group has mapped centrotemporal spikes, the autosomal dominant EEG hallmark of RE to the ELP4-PAX6 region on chromosome 11p13 (84). This region is also pleiotropic for speech-sound disorder in RE (81). We sought to uncover the causal variants for centrotemporal spikes and speech-sound disorder using Illumina next generation sequencing from long-range PCR of the 650Kb linkage region in 27 RE probands of European origin. This method was chosen because conventional Sanger sequencing of ELP4 coding regions had not uncovered any mutations, and the region contained a complex pattern of long and short-range enhancer elements for the developmental control gene and transcription factor PAX6. It was therefore unlikely that a conventional coding mutation would be found, but rather functional variant(s) that are in a highly conserved regulatory region would be causal. This method was fairly unique at the time, and I have overcome several challenges in data analysis to create a short-list of variants to take forward for genotyping and association analysis in a large number of cases, family members and controls.
Challenges in Next-Generation Sequencing Data Analysis
Our group has developed a pipeline to align, sort, merge and assemble the sequence reads of DNA to the reference genome, and then filter and call SNPs and insertions and deletions (InDels). I discovered the need for a tailored pipeline when we found that none of the 60 SNPs we had genotyped for the previous association analysis were called from a next-generation sequencing service pipeline in 12/27 of the same individuals! With the custom pipeline we developed, I also found it extremely important to align our sequence reads only to the captured region of 11p13. Some regions of DNA are identical repetitive sequences found at various locations throughout the genome. When aligning regional sequence reads back to the entire genome, DNA for these repetitive regions would mis-align to other chromosomes, leaving gaps in the regional data and consequent missing SNP information. As I knew our sequence reads were only from 11p13 because they were captured using a long-range PCR method when aligned back only to 11p13, the “holes” in the coverage were filled. Interestingly, the SNPs called in these repetitive regions are novel, and it is possible that these regions of public genomes are aligned incorrectly to the reference genome. Within our pipeline, removing PCR duplicates also facilitated quality SNP calling. I also had to decide to either call SNPs by pooling reads from all individuals in ‘batch mode,’ which gives more robustness to allele calls, or to call SNPs for each individual separately, which would facilitate more calls in regions where only some individuals had good quality coverage.
Another major challenge that we overcame during this study was the availability of suitable control data, and how to analyze it. Most publicly available control data from the National Center for Biotechnology Information (NCBI) or 1000Genomes is of low read depth (4-8×). Read depth describes how many strands of DNA sequence are created and aligned over a particular genomic region. Low read depth data introduces an information bias during comparison with our high depth (200×) data because high read-depth data is more robust especially for low-frequency variants. We have developed a novel statistical adjustment to overcome this, and validated the method with higher-depth controls not in the public domain. The list of overlapping variants between the datasets was prioritized for further genotyping in a larger case-control cohort to provide the most comprehensive list of possible causative variants for centrotemporal spikes and speech-sound disorder in RE. SNPs were prioritized if they were most highly associated with affection using both single-SNP and statistical binning (clustering) methods. Bins were defined both by gene and functional element, e.g. promoter or enhancer. SNPs were also prioritized if they were novel to our cases or predicted to be functional, e.g. splice-site, enhancer, coding. InDel calling is a future focus as they are difficult to reliably align to the genome and highly variable between individuals.
Future Directions
The goal of my work is to confirm the diagnosis of RE, predict the disease course and comorbidities, including speech and reading problems, and the risk that siblings will develop these traits. By identifying genetic markers of the disorder and of modifier loci, both with single SNPs and CNVs, I will contribute to the development of a comprehensive genetic test for RE and to the education of parents about the cause and course of the disorder. This testing panel will help parents as well as health and educational practitioners plan appropriate testing, monitoring, and treatment of children with RE and their siblings if/when needed.
CONCLUSION
As evident from this review, translational research of language and cognition in pediatric epilepsy is at beginning stages. Use of a translational approach to address the unanswered questions presented in this paper will help delineate the underlying mechanisms, identify possible biomarkers, and enable early identification and treatment of children at risk for the linguistic and cognitive comorbidities of pediatric epilepsy.
Supplementary Material
Table 2. New onset epilepsy: Neuroimaging.
| Authors | Primary Findings | Abnormalities? |
|---|---|---|
| Hermann et al. (13) | Lobar volumetrics in IGE & LRE | No |
| Hutchinson et al. (106) | DTI in IGE & LRE | Yes |
| Pulsipher et al. (107) | Thalamus volume in JME | Yes |
| Tosun et al. (108) | Cortical thickness in IGE & LRE | Yes |
| Jackson et al. (109) | Ventricular volume in IGE & LRE | Yes |
| Widjaja et al. (110) | Cortical thickness in IGE & LRE | Yes |
| Yang et al. (111) | DTI in Absence | Yes |
| Lin et al. (112) | Putamen volume in BECTS | Yes |
ACKNOWLEDGMENTS
Supported by grants K23 NS060993 (JJL), European Union Marie Curie International Reintegration Award of the Seventh Framework Programme (DKP); Epilepsy Research UK (DKP); Waterloo Foundation (DKP); Charles Sykes Epilepsy Research Trust (DKP); NIHR Specialist Biomedical Research Centre for Mental Health of South London and Maudsley NHS Foundation Trust (DKP), NIH 2RO1-44351 (BH), as well as NS32070 and MH 6718 (RC).
REFERENCES
- 1.Lenroot RK, Giedd J. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Caplan R, Siddarth P, Vona P, Stahl L, Bailey CE, Gurbani S, Sankar R, Donald WD. Language in pediatric epilepsy Epilepsia. 2009;50:2397–407. doi: 10.1111/j.1528-1167.2009.02199.x. [DOI] [PubMed] [Google Scholar]
- 3.Dunn DW, Johnson CS, Perkins SM, Fastenau PS, Byars AW, deGrauw TJ, Austin JK. Academic problems in children with seizures: Relationships with neuropsychological functioning and family variables during the 3 years after onset. Epilepsy Behav. 2010;19:455–461. doi: 10.1016/j.yebeh.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Hamiwka L, Jones JE, Salpekar J, Caplan R. Child psychiatry: Special edition on the future of clinical epilepsy research Epilepsy Behav. 2011;22:38–46. doi: 10.1016/j.yebeh.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Brauer J, Neumann J, Friederici AD. Temporal dynamics of perisylvian activation during language processing in children and adults. Neuroimage. 2008;41:1484–1492. doi: 10.1016/j.neuroimage.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman R, Nir-Sagiv B. Comparing narrative and expository text construction across adolescence: A developmental paradox. Discourse Processes. 2007;43:79–120. [Google Scholar]
- 7.Nippold M, Hesketh LJ, Duthie JK, Mansfield TC. Conversational versus expository discourse: a study of syntactic development in children, adolescents, and adults. J Speech Lang Hear Res. 2005;48:1048–1064. doi: 10.1044/1092-4388(2005/073). [DOI] [PubMed] [Google Scholar]
- 8.Ravid D. Semantic development in textual contexts during the school years: Noun Scale analyses. J Child Lang. 2006;33:791–821. doi: 10.1017/s0305000906007586. [DOI] [PubMed] [Google Scholar]
- 9.Friederici AD. The brain basis of language processing: From structure to function. Physiological Reviews. 2011;91:1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006;5:1055–1063. doi: 10.1016/S1474-4422(06)70626-3. [DOI] [PubMed] [Google Scholar]
- 11.Fastenau P, Shen J, Dunn DW, Perkins SM, Hermann BP, Austin JK. Neuropsychological predictors of academic underachievement in pediatric epilepsy: moderating roles of demographic, seizure, and psychosocial variables. Epilepsia. 2004;45:1261–72. doi: 10.1111/j.0013-9580.2004.15204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henkin Y, Kishon-Rabin L, Pratt H, Kivity S, Sadeh M, Gadoth N. Linguistic processing in idiopathic generalized epilepsy: an auditory event-related potential study. Epilepsia. 2003;44:1207–1217. doi: 10.1046/j.1528-1157.2003.65402.x. [DOI] [PubMed] [Google Scholar]
- 13.Hermann B, Jones J, Sheth R, Dow C, Koehn M. Seidenberg M. Children with new-onset epilepsy: Neuropsychological status and brain structure. Brain. 2006;129:2609–2619. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- 14.Northcott E, Connolly AM, Berroya A, Sabaz M, McIntyre J, Christie J, Taylor A, Batchelor J, Bleasel AF, Lawson JA, Bye AM. The neuropsychological and language profile of children with benign rolandic epilepsy. Epilepsia. 2005;46:924–930. doi: 10.1111/j.1528-1167.2005.62304.x. [DOI] [PubMed] [Google Scholar]
- 15.Schoenfeld J, Seidenberg M, Woodard A, Hecox K, Inglese C, Mack K, Hermann B. Neuropsychological and behavioral status of children with complex partial seizures. Dev Med Child Neurol. 1999;41:724–31. doi: 10.1017/s0012162299001486. [DOI] [PubMed] [Google Scholar]
- 16.Caplan R, Siddarth P, Gurbani S, Lanphier E, Koh S, Sankar R. Thought disorder: A developmental disability in pediatric epilepsy. Epilepsy Behav. 2006;8:726–735. doi: 10.1016/j.yebeh.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Fastenau PS, Johnson CS, Perkins SM, Byars AW, deGrauw TJ, Austin JK, Dunn DW. Neuropsychological status at seizure onset in children: Risk factors for early cognitive deficits. Neurology. 2009;73:526–534. doi: 10.1212/WNL.0b013e3181b23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones JE, Siddarth P, Gurbani S, Shields WD, Caplan R. Cognition, academic achievement, language, and psychopathology in pediatric chronic epilepsy: Short-term outcomes. Epilepsy Behav. 2010;18:211–217. doi: 10.1016/j.yebeh.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giedd J, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:465–70. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw P, Kaban NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–31. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chavarria-Siles I, Rijpkema M, Lips E, Arias-Vasquez A, Verhage M, Franke B, Fernandez G, Posthuma D. Genes encoding heterotrimeric G-proteins are associated with gray matter volume variations in the medial frontal cortex. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto M, Straub RE, Marenco S, Nicodemus KK, Matsumoto S-i, Fujikawa A, Miyoshi S, Shobo M, Takahashi S, Yarimizu J, Yuri M, Hiramoto M, Morita S, Yokota H, Sasayama T, Terai K, Yoshino M, Miyake A, Callicott JH, Egan MF, Meyer-Lindenberg A, Kempf L, Honea R, Vakkalanka RK, Takasaki J, Kamohara M, Soga T, Hiyama H, Ishii H, Matsuo A, Nishimura S, Matsuoka N, Kobori M, Matsushime H, Katoh M, Furuichi K, Weinberger DR. The evolutionarily conserved G protein-coupled receptor SREB2/GPR85 influences brain size, behavior, and vulnerability to schizophrenia. Proc Natl Acad Sci U S A. 2008;105:6133–6138. doi: 10.1073/pnas.0710717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brouwer RM, Mandl RCW, Schnack HG, van Soelen ILC, van Baal GC, Peper JS, Kahn RS, Boomsma DI, Pol HEH. White matter development in early puberty: A longitudinal volumetric and diffusion tensor imaging twin study. PLoS ONE. 2012;7:e32316. doi: 10.1371/journal.pone.0032316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yakovlev PI, Lecours A. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Vol. 3. Oxford Blackwell; Oxford: 1967. p. 70. [Google Scholar]
- 28.Peper JS, Schnack HG, Brouwer RM, Van Baal GCM, Pjetri E, Székely E, van Leeuwen M, van den Berg SM, Collins DL, Evans AC, Boomsma DI, Kahn RS, Hulshoff Pol HE. Heritability of regional and global brain structure at the onset of puberty: A magnetic resonance imaging study in 9-year-old twin pairs. Hum Brain Mapp. 2009;30:2184–2196. doi: 10.1002/hbm.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon U, Fahim C, Perusse D, Evans AC. Lateralized genetic and environmental influences on human brain morphology of 8-year-old twins. NeuroImage. 2010;53:1117–1125. doi: 10.1016/j.neuroimage.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Soelen ILC, Brouwer RM, van Baal GCM, Schnack HG, Peper JS, Collins DL, Evans AC, Kahn RS, Boomsma DI, Hulshoff Pol HE. Genetic influences on thinning of the cerebral cortex during development. NeuroImage. 2012;59:3871–3880. doi: 10.1016/j.neuroimage.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 31.Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Kendler KS, Evans AC, Giedd JN. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp. 2009;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caplan R, Levitt J, Siddarth P, Taylor J, Daley M, Wu KN, Gurbani S, Shields WD, Sankar R. Thought disorder and fronto-temporal volumes in pediatric epilepsy. Epilepsy Behav. 2008;13:593–599. doi: 10.1016/j.yebeh.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caplan R, Levitt JG, Siddarth P, Wu KW, Gurbani S, Donald WD, Sankar R. Language and fronto-temporal volumes in pediatric epilepsy. Epilepsy Behav. 2009;50:2466–72. [Google Scholar]
- 34.Tosun D, Siddarth P, Seidenberg M, Toga A, Hermann B, Caplan R. Effects of childhood absence epilepsy on associations between regional cortical morphometry and aging and cognitive abilities. Hum Brain Mapp. 2010;32:580–91. doi: 10.1002/hbm.21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dapretto M, Gurbani S, McNealy K, Malin E, Caplan R. Making sense of conversation in pediatric epilepsy: An fMRI study. America Epilepsy Society Proceedings. 2006 [Google Scholar]
- 36.O’Neill J, Seese R, Hudkins M, Siddarth P, Levitt J, Tseng PB, Wu KN, Gurbani S, Shields WD, Caplan R. 1H MRSI and social communication deficits in pediatric complex partial seizures. Epilepsia. 2011;52:1705–1714. doi: 10.1111/j.1528-1167.2011.03114.x. [DOI] [PubMed] [Google Scholar]
- 37.Lillywhite LM, Saling MM, Simon Harvey A, Abbott DF, Archer JS, Vears DF, Scheffer IE, Jackson GD. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia. 2009;50:2276–2284. doi: 10.1111/j.1528-1167.2009.02065.x. [DOI] [PubMed] [Google Scholar]
- 38.You X, Adjouadi M, Guillen MR, Ayala M, Barreto A, Rishe N, Sullivan J, Dlugos D, VanMeter J, Morris D, Donner E, Bjornson B, Smith ML, Bernal B, Berl M, Gaillard WD. Sub-patterns of language network reorganization in pediatric localization related epilepsy: A multisite study. Hum Brain Mapp. 2011;32:784–799. doi: 10.1002/hbm.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dapretto M, Lee SS, Caplan R. A functional magnetic resonance study of discourse coherence in typically developing children. NeuroReport. 2005;16:1661–1665. doi: 10.1097/01.wnr.0000183332.28865.11. [DOI] [PubMed] [Google Scholar]
- 40.Dodrill C, Clemmons D. Use of neuropsychological tests to identify high school students with epilepsy who later demonstrate inadequate performances in life. J Consult Clin Psychol. 1984;52:520–7. doi: 10.1037//0022-006x.52.4.520. [DOI] [PubMed] [Google Scholar]
- 41.Johnson MD, Ojemann GA. The role of the human thalamus in language and memory: Evidence from electrophysiological studies. Brain Cogn. 2000;42:218–230. doi: 10.1006/brcg.1999.1101. [DOI] [PubMed] [Google Scholar]
- 42.Preston JL, Frost SJ, Mencl WE, Fulbright RK, Landi N, Grigorenko E, Jacobsen L, Pugh KR. Early and late talkers: school-age language, literacy and neurolinguistic differences. Brain. 2012;133:2185–2195. doi: 10.1093/brain/awq163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, Holland SK. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan S-H, Ryan L, Bever TG. Role of the striatum in language: Syntactic and conceptual sequencing. Brain Lang. 2012 doi: 10.1016/j.bandl.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Duff MC, Brown-Schmidt S. The hippocampus and the flexible use and processing of language. Front Hum Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Halloran CJ, Kinsella GJ, Storey E. The cerebellum and neuropsychological functioning: A critical review. J Clin iExper Neuropsychol. 2012;34:35–56. doi: 10.1080/13803395.2011.614599. [DOI] [PubMed] [Google Scholar]
- 47.Jones JE, Siddarth P, Gurbani S, Shields WD, Caplan R. Cognition, academic achievement, language, and psychopathology in pediatric chronic epilepsy: Short-term outcomes. Epilepsy Behav. 2010;18:211–7. doi: 10.1016/j.yebeh.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol. 2002;8:71–82. doi: 10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- 49.Anderson V. Assessing executive functions in children: biological, psychological, and developmental considerationst. Pediatr Rehabil. 2001;4:119–36. doi: 10.1080/13638490110091347. [DOI] [PubMed] [Google Scholar]
- 50.Stuss DT. Biological and psychological development of executive functions. Brain Cogn. 1992;20:8–23. doi: 10.1016/0278-2626(92)90059-u. [DOI] [PubMed] [Google Scholar]
- 51.Parrish J, Geary E, Jones J, Seth R, Hermann B, Seidenberg M. Executive functioning in childhood epilepsy: parent-report and cognitive assessment. Dev Med Child Neurol. 2007;49:412–6. doi: 10.1111/j.1469-8749.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 52.Devinsky O, Gershengorn J, Brown E, Perrine K, Vazquez B, Luciano D. Frontal functions in juvenile myoclonic epilepsy. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:243–6. [PubMed] [Google Scholar]
- 53.Pascalicchio TF, de Araujo Filho GM, da Silva Noffs MH, Lin K, Caboclo LO, Vidal-Dourado M, Ferreira Guilhoto LM, Yacubian EM. Neuropsychological profile of patients with juvenile myoclonic epilepsy: a controlled study of 50 patients. Epilepsy Behav. 2007;10:263–7. doi: 10.1016/j.yebeh.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Piazzini A, Turner K, Vignoli A, Canger R, Canevini MP. Frontal cognitive dysfunction in juvenile myoclonic epilepsy. Epilepsia. 2008;49:657–62. doi: 10.1111/j.1528-1167.2007.01482.x. [DOI] [PubMed] [Google Scholar]
- 55.Sonmez F, Atakli D, Sari H, Atay T, Arpaci B. Cognitive function in juvenile myoclonic epilepsy. Epilepsy Behav. 2004;5:329–36. doi: 10.1016/j.yebeh.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain. 1999;122(Pt 11):2101–8. doi: 10.1093/brain/122.11.2101. [DOI] [PubMed] [Google Scholar]
- 57.Oyegbile T, Hansen R, Magnotta V, O’Leary D, Bell B, Seidenberg M, Hermann BP. Quantitative measurement of cortical surface features in localization-related temporal lobe epilepsy. Neuropsychology. 2004;18:729–37. doi: 10.1037/0894-4105.18.4.729. [DOI] [PubMed] [Google Scholar]
- 58.Bell B, Lin JJ, Seidenberg M, Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol. 2011;7:154–64. doi: 10.1038/nrneurol.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hermann BP, Lin JJ, Jones JE, Seidenberg M. The emerging architecture of neuropsychological impairment in epilepsy. Neurol Clin. 2009;27:881–907. doi: 10.1016/j.ncl.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin JJ, Salamon N, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Luders E, Toga AW, Engel J, Jr., Thompson PM. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex. 2007;17:2007–18. doi: 10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- 61.Lin JJ, Riley JD, Juranek J, Cramer SC. Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy Res. 2008;82:162–70. doi: 10.1016/j.eplepsyres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 62.Riley JD, Franklin DL, Choi V, Kim RC, Binder DK, Cramer SC, Lin JJ. Altered white matter integrity in temporal lobe epilepsy: association with cognitive and clinical profiles. Epilepsia. 2010;51:536–45. doi: 10.1111/j.1528-1167.2009.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riley JD, Moore S, Cramer SC, Lin JJ. Caudate atrophy and impaired frontostriatal connections are linked to executive dysfunction in temporal lobe epilepsy. Epilepsy Behav. 2011;21:80–7. doi: 10.1016/j.yebeh.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hermann B, Hansen R, Seidenberg M, Magnotta V, O’Leary D. Neurodevelopmental vulnerability of the corpus callosum to childhood onset localization-related epilepsy. Neuroimage. 2003;18:284–92. doi: 10.1016/s1053-8119(02)00044-7. [DOI] [PubMed] [Google Scholar]
- 65.Weber B, Luders E, Faber J, Richter S, Quesada CM, Urbach H, Thompson PM, Toga AW, Elger CE, Helmstaedter C. Distinct regional atrophy in the corpus callosum of patients with temporal lobe epilepsy. Brain. 2007;130:3149–54. doi: 10.1093/brain/awm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Cohen DJ, Gore JC. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome [see comments] Arch Gen Psychiatry. 2000;57:331–40. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 67.Vannucci RC, Barron TF, Lerro D, Anton SC, Vannucci SJ. Craniometric measures during development using MRI. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 68.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nat Neurosci. 2011;14:163–4. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fujisawa S, Buzsaki G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 2011;72:153–65. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kleen JK, Wu EX, Holmes GL, Scott RC, Lenck-Santini PP. Enhanced oscillatory activity in the hippocampal-prefrontal network is related to short-term memory function after early-life seizures. J Neurosci. 2011;31:15397–406. doi: 10.1523/JNEUROSCI.2196-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee C, Scherer SW. The clinical context of copy number variation in the human genome. Expert Rev Mol Med. 2010;12:e8. doi: 10.1017/S1462399410001390. [DOI] [PubMed] [Google Scholar]
- 73.Kumar RA, KaraMohamed S, Sudi J, Conrad DF, Brune C, Badner JA, Gilliam TC, Nowak NJ, Cook EH, Jr., Dobyns WB, Christian SL. Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet. 2008;17:628–38. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- 74.Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, Shago M, Moessner R, Pinto D, Ren Y, Thiruvahindrapduram B, Fiebig A, Schreiber S, Friedman J, Ketelaars CE, Vos YJ, Ficicioglu C, Kirkpatrick S, Nicolson R, Sloman L, Summers A, Gibbons CA, Teebi A, Chitayat D, Weksberg R, Thompson A, Vardy C, Crosbie V, Luscombe S, Baatjes R, Zwaigenbaum L, Roberts W, Fernandez B, Szatmari P, Scherer SW. Structural variation of chromosomes in autism spectrum disorder. American journal of human genetics. 2008;82:477–88. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walters RG, Jacquemont S, Valsesia A, de Smith AJ, Martinet D, Andersson J, Falchi M, Chen F, Andrieux J, Lobbens S, Delobel B, Stutzmann F, El-Sayed Moustafa JS, Chevre JC, Lecoeur C, Vatin V, Bouquillon S, Buxton JL, Boute O, Espinasse M, Cuisset JM, Lemaitre MP, Ambresin AE, Brioschi A, Gaillard M, Giusti V, Fellmann F, Ferrarini A, Hadjikhani N, Campion D, Guilmatre A, Goldenberg A, Calmels N, Mandel JL, Le Caignec C, David A, Isidor B, Cordier MP, Dupuis-Girod S, Labalme A, Sanlaville D, Beri-Dexheimer M, Jonveaux P, Leheup B, Ounap K, Bochukova EG, Henning E, Keogh J, Ellis RJ, MacDermot KD, van Haelst MM, Vincent-Delorme C, Plessis G, Touraine R, Philippe A, Malan V, Mathieu-Dramard M, Chiesa J, Blaumeiser B, Kooy RF, Caiazzo R, Pigeyre M, Balkau B, Sladek R, Bergmann S, Mooser V, Waterworth D, Reymond A, Vollenweider P, Waeber G, Kurg A, Palta P, Esko T, Metspalu A, Nelis M, Elliott P, Hartikainen AL, McCarthy MI, Peltonen L, Carlsson L, Jacobson P, Sjostrom L, Huang N, Hurles ME, O’Rahilly S, Farooqi IS, Mannik K, Jarvelin MR, Pattou F, Meyre D, Walley AJ, Coin LJM, Blakemore AIF, Froguel P, Beckmann JS. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature. 2010;463:671–675. doi: 10.1038/nature08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenfeld JA, Coppinger J, Bejjani BA, Girirajan S, Eichler EE, Shaffer LG, Ballif BC. Speech delays and behavioral problems are the predominant features in individuals with developmental delays and 16p11.2 microdeletions and microduplications. J Neurodev Disord. 2008;2:26–38. doi: 10.1007/s11689-009-9037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCarthy SE, Makarov V, Kirov G, Addington AM, McClellan J, Yoon S, Perkins DO, Dickel DE, Kusenda M, Krastoshevsky O, Krause V, Kumar RA, Grozeva D, Malhotra D, Walsh T, Zackai EH, Kaplan P, Ganesh J, Krantz ID, Spinner NB, Roccanova P, Bhandari A, Pavon K, Lakshmi B, Leotta A, Kendall J, Lee Y-h, Vacic V, Gary S, Iakoucheva LM, Crow TJ, Christian SL, Lieberman JA, Stroup TS, Lehtimaki T, Puura K, Haldeman-Englert C, Pearl J, Goodell M, Willour VL, DeRosse P, Steele J, Kassem L, Wolff J, Chitkara N, McMahon FJ, Malhotra AK, Potash JB, Schulze TG, Nothen MM, Cichon S, Rietschel M, Leibenluft E, Kustanovich V, Lajonchere CM, Sutcliffe JS, Skuse D, Gill M, Gallagher L, Mendell NR, Craddock N, Owen MJ, O’Donovan MC, Shaikh TH, Susser E, DeLisi LE, Sullivan PF, Deutsch CK, Rapoport J, Levy DL, King M-C, Sebat J. Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet. 2009;41:1223–1227. doi: 10.1038/ng.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reutlinger C, Helbig I, Gawelczyk B, Subero JI, Tonnies H, Muhle H, Finsterwalder K, Vermeer S, Pfundt R, Sperner J, Stefanova I, Gillessen-Kaesbach G, von Spiczak S, van Baalen A, Boor R, Siebert R, Stephani U, Caliebe A. Deletions in 16p13 including GRIN2A in patients with intellectual disability, various dysmorphic features, and seizure disorders of the rolandic region. Epilepsia. 2010;51:1870–3. doi: 10.1111/j.1528-1167.2010.02555.x. [DOI] [PubMed] [Google Scholar]
- 79.Endele S, Rosenberger G, Geider K, Popp B, Tamer C, Stefanova I, Milh M, Kortum F, Fritsch A, Pientka FK, Hellenbroich Y, Kalscheuer VM, Kohlhase J, Moog U, Rappold G, Rauch A, Ropers HH, von Spiczak S, Tonnies H, Villeneuve N, Villard L, Zabel B, Zenker M, Laube B, Reis A, Wieczorek D, Van Maldergem L, Kutsche K. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat Genet. 2010;42:1021–6. doi: 10.1038/ng.677. [DOI] [PubMed] [Google Scholar]
- 80.Lesca G, Rudolf G, Labalme A, Hirsch E, Arzimanoglou A, Genton P, Motte J, de Saint Martin A, Valenti MP, Boulay C, de Bellescize J, Keo-Kosal P, Boutry-Ktyza N, Edery P, Sanlaville D, Szepetowski P. Epileptic encephalopathies of the Landau-Kleffner and continuous spike and waves during slow-wave sleep types: genomic dissection makes the link with autism. Epilepsia. 2012 doi: 10.1111/j.1528-1167.2012.03559.x. in press. [DOI] [PubMed] [Google Scholar]
- 81.Pal DK, Li W, Clarke T, Lieberman P, Strug LJ. Pleiotropic effects of the 11p13 locus on developmental verbal dyspraxia and EEG centrotemporal sharp waves. Genes Brain Behav. 2010;9:1004–12. doi: 10.1111/j.1601-183X.2010.00648.x. [DOI] [PubMed] [Google Scholar]
- 82.Strug LJ, Addis L, Clarke T, Baskurt Z, Li W, Chiang T, Hardison H, Kugler SL, Mandelbaum DE, Novotny EJ, Wolf SM, Pal DK. The Genetics of Reading Disability in an Often Excluded Sample: Novel Loci Suggested for Reading Disability in Rolandic Epilepsy. PLoS One. 2012 doi: 10.1371/journal.pone.0040696. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roll P, Rudolf G, Pereira S, Royer B, Scheffer IE, Massacrier A, Valenti MP, Roeckel-Trevisiol N, Jamali S, Beclin C, Seegmuller C, Metz-Lutz MN, Lemainque A, Delepine M, Caloustian C, de Saint Martin A, Bruneau N, Depétris D, Mattéi MG, Flori E, Robaglia-Schlupp A, Lévy N, Neubauer BA, Ravid R, Marescaux C, Berkovic SF, Hirsch E, Lathrop M, Cau P, Szepetowski P. SRPX2 mutations in disorders of language cortex and cognition. Hum Mol Genet. 2006;15:1195–207. doi: 10.1093/hmg/ddl035. [DOI] [PubMed] [Google Scholar]
- 84.Strug LJ, Clarke T, Chiang T, Chien M, Baskurt Z, Li W, Dorfman R, Bali B, Wirrell E, Kugler SL, Mandelbaum DE, Wolf SM, McGoldrick P, Hardison H, Novotny EJ, Ju J, Greenberg DA, Russo JJ, Pal DK. Centrotemporal sharp wave EEG trait in rolandic epilepsy maps to Elongator Protein Complex 4 (ELP4) Eur J Hum Genet. 2009;17:1171–1181. doi: 10.1038/ejhg.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clarke T, Strug LJ, Murphy PL, Bali B, Carvalho J, Foster S, Tremont G, Gagnon BR, Dorta N, Pal DK. High risk of reading disability and speech sound disorder in rolandic epilepsy families: case-control study. Epilepsia. 2007;48:2258–65. doi: 10.1111/j.1528-1167.2007.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clarke T, Baskurt Z, Strug LJ, Pal DK. Evidence of shared genetic risk factors for migraine and rolandic epilepsy. Epilepsia. 2009;50:2428–33. doi: 10.1111/j.1528-1167.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- 87.Smith AB, Kavros PM, Clarke T, Dorta NJ, Tremont G, Pal DK. A neurocognitive endophenotype associated with rolandic epilepsy. Epilepsia. 53:705–11. doi: 10.1111/j.1528-1167.2011.03371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tovia E, Goldberg-Stern H, Ben Zeev B, Heyman E, Watemberg N, Fattal-Valevski A, Kramer U. The prevalence of atypical presentations and comorbidities of benign childhood epilepsy with centrotemporal spikes. Epilepsia. 2011;52:1483–8. doi: 10.1111/j.1528-1167.2011.03136.x. [DOI] [PubMed] [Google Scholar]
- 89.Addis L, Breen G, Strug LJ, Pal DK. A pilot study of copy number variation in rolandic epilepsy. Epilepsia. 2011;52:90. [Google Scholar]
- 90.Mefford HC, Mulley JC. Genetically complex epilepsies, copy number variants and syndrome constellations. Genome Med. 2010;2:71. doi: 10.1186/gm192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Girirajan S, Eichler EE. Phenotypic variability and genetic susceptibility to genomic disorders. Hum Mol Genet. 2010;19:R176–87. doi: 10.1093/hmg/ddq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cooper GM, Coe BP, Girirajan S, Rosenfeld JA, Vu TH, Baker C, Williams C, Stalker H, Hamid R, Hannig V, Abdel-Hamid H, Bader P, McCracken E, Niyazov D, Leppig K, Thiese H, Hummel M, Alexander N, Gorski J, Kussmann J, Shashi V, Johnson K, Rehder C, Ballif BC, Shaffer LG, Eichler EE. A copy number variation morbidity map of developmental delay. Nat Genet. 2011;43:838–46. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, Buizer-Voskamp JE, Hansen T, Jakobsen KD, Muglia P, Francks C, Matthews PM, Gylfason A, Halldorsson BV, Gudbjartsson D, Thorgeirsson TE, Sigurdsson A, Jonasdottir A, Bjornsson A, Mattiasdottir S, Blondal T, Haraldsson M, Magnusdottir BB, Giegling I, Moller HJ, Hartmann A, Shianna KV, Ge D, Need AC, Crombie C, Fraser G, Walker N, Lonnqvist J, Suvisaari J, Tuulio-Henriksson A, Paunio T, Toulopoulou T, Bramon E, Di Forti M, Murray R, Ruggeri M, Vassos E, Tosato S, Walshe M, Li T, Vasilescu C, Muhleisen TW, Wang AG, Ullum H, Djurovic S, Melle I, Olesen J, Kiemeney LA, Franke B, Sabatti C, Freimer NB, Gulcher JR, Thorsteinsdottir U, Kong A, Andreassen OA, Ophoff RA, Georgi A, Rietschel M, Werge T, Petursson H, Goldstein DB, Nothen MM, Peltonen L, Collier DA, Clair D, Stefansson K. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–6. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Makino T, McLysaght A. Ohnologs in the human genome are dosage balanced and frequently associated with disease. Proc Natl Acad Sci U S A. 107:9270–4. doi: 10.1073/pnas.0914697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bourgeois BF, Prensky AL, Palkes HS, Talent BK, Busch SG. Intelligence in epilepsy: a prospective study in children. Ann Neurol. 1983;14:438–44. doi: 10.1002/ana.410140407. [DOI] [PubMed] [Google Scholar]
- 96.Stores G, Williams PL, Styles E, Zaiwalla Z. Psychological effects of sodium valproate and carbamazepine in epilepsy. Arch Dis Child. 1992;67:1330–7. doi: 10.1136/adc.67.11.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mandelbaum DE, Burack GD. The effect of seizure type and medication on cognitive and behavioral functioning in children with idiopathic epilepsy. Dev Med Child Neurol. 1997;39:731–5. doi: 10.1111/j.1469-8749.1997.tb07374.x. [DOI] [PubMed] [Google Scholar]
- 98.Williams J, Bates S, Griebel ML, Lange B, Mancias P, Pihoker CM, Dykman R. Does short-term antiepileptic drug treatment in children result in cognitive or behavioral changes? Epilepsia. 1998;39:1064–9. doi: 10.1111/j.1528-1157.1998.tb01291.x. [DOI] [PubMed] [Google Scholar]
- 99.Kolk A, Beilmann A, Tomberg T, Napa A, Talvik T. Neurocognitive development of children with congenital unilateral brain lesion and epilepsy. Brain Dev. 2001;23:88–96. doi: 10.1016/s0387-7604(01)00180-2. [DOI] [PubMed] [Google Scholar]
- 100.Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, Peters AC, Jennekens-Schinkel A. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”--a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003;112:1338–44. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- 101.Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006;129:2609–19. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- 102.Fastenau PS, Johnson CS, Perkins SM, Byars AW, deGrauw TJ, Austin JK, Dunn DW. Neuropsychological status at seizure onset in children: risk factors for early cognitive deficits. Neurology. 2009;73:526–34. doi: 10.1212/WNL.0b013e3181b23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhise VV, Burack GD, Mandelbaum DE. Baseline cognition, behavior, and motor skills in children with new-onset, idiopathic epilepsy. Dev Med Child Neurol. 2010;52:22–6. doi: 10.1111/j.1469-8749.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 104.Jeong MH, Yum MS, Ko TS, You SJ, Lee EH, Yoo HK. Neuropsychological status of children with newly diagnosed idiopathic childhood epilepsy. Brain Dev. 2011;33:666–71. doi: 10.1016/j.braindev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 105.Vintan M, Palade S, Cristea A, Benga I, Muresanu D. A neuropsychological assessment, using computerized battery tests (CANTAB), in children with benign rolandic epilepsy before AED therapy. J Med Life. 2012;5:114–9. [PMC free article] [PubMed] [Google Scholar]
- 106.Hutchinson E, Pulsipher D, Dabbs K, Myers y, Gutierrez A, Sheth R, Jones J, Seidenberg M, Meyerand E, Hermann B. Children with new-onset epilepsy exhibit diffusion abnormalities in cerebral white matter in the absence of volumetric differences. Epilepsy Res. 2010;88:208–14. doi: 10.1016/j.eplepsyres.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pulsipher DT, Seidenberg M, Guidotti L, Tuchscherer VN, Morton J, Sheth RD, Hermann B. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia. 2009;50:1210–9. doi: 10.1111/j.1528-1167.2008.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tosun D, Dabbs K, Caplan R, Siddarth P, Toga A, Seidenberg M, Hermann B. Deformation-based morphometry of prospective neurodevelopmental changes in new onset paediatric epilepsy. Brain. 2011;134:1003–14. doi: 10.1093/brain/awr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jackson DC, Irwin W, Dabbs K, Lin JJ, Jones JE, Hsu DA, Stafstrom CE, Seidenberg M, Hermann BP. Ventricular enlargement in new-onset pediatric epilepsies. Epilepsia. 2011;52:2225–32. doi: 10.1111/j.1528-1167.2011.03323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Widjaja E, Zarei Mahmoodabadi S, Go C, Raybaud C, Chuang S, Snead OC, Smith ML. Reduced cortical thickness in children with new-onset seizures. AJNR Am J Neuroradiol. 2012;33:673–7. doi: 10.3174/ajnr.A2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang T, Guo Z, Luo C, Li Q, Yan B, Liu L, Gong Q, Yao D, Zhou D. White matter impairment in the basal ganglia-thalamocortical circuit of drug-naive childhood absence epilepsy. Epilepsy Res. 2012;99:267–73. doi: 10.1016/j.eplepsyres.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 112.Lin JJ, Riley JD, Hsu DA, Stafstrom CE, Dabbs K, Becker T, Seidenberg M, Hermann BP. Striatal hypertrophy and its cognitive effects in new-onset benign epilepsy with centrotemporal spikes. Epilepsia. 2012;53:677–85. doi: 10.1111/j.1528-1167.2012.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.