Abstract
The amygdala-dependent molecular mechanisms driving the onset and persistence of posttraumatic stress disorder (PTSD) are poorly understood. Recent observational studies have suggested that opioid analgesia in the aftermath of trauma may decrease the development of PTSD. Using a mouse model of dysregulated fear, we found altered expression within the amygdala of the Oprl1 gene (opioid receptor–like 1), which encodes the amygdala nociceptin (NOP)/orphanin FQ receptor (NOP-R). Systemic and central amygdala infusion of SR-8993, a new highly selective NOP-R agonist, impaired fear memory consolidation. In humans, a single-nucleotide polymorphism (SNP) within OPRL1 is associated with a self-reported history of childhood trauma and PTSD symptoms (n = 1847) after a traumatic event. This SNP is also associated with physiological startle measures of fear discrimination and magnetic resonance imaging analysis of amygdala-insula functional connectivity. Together, these data suggest that Oprl1 is associated with amygdala function, fear processing, and PTSD symptoms. Further, our data suggest that activation of the Oprl1/NOP receptor may interfere with fear memory consolidation, with implications for prevention of PTSD after a traumatic event.
INTRODUCTION
Posttraumatic stress disorder (PTSD) is an anxiety disorder that is composed, in part, of altered fear learning that develops after exposure to a highly traumatic event. Current therapeutic approaches are used only after PTSD is already present and debilitating symptoms have appeared, but prevention of PTSD development is an important unmet medical need. Within the neural circuitry of fear formation, the amygdala is a critical site for development and storage of fear memory (1). At a molecular level within the amygdala, gene regulation and protein synthesis are needed for the consolidation of fear memory formation (2, 3). A new and more cost-effective therapeutic approach, although not yet a reality, would comprise early treatments that could prevent PTSD development by impairing fear memory consolidation (4). This early intervention would be especially appropriate for individuals at higher risk for PTSD after trauma, such as those with a history of previous trauma or those carrying genetic polymorphisms that have been associated with PTSD risk (4, 5).
There is little consensus as to what constitutes a PTSD-like rodent model (6). However, most models have in common the study of emotional memories according to a Pavlovian learning paradigm. This associative learning process consists of the pairing of a neutral conditioned stimulus with an aversive unconditioned stimulus eliciting a conditioned fear response. The conditioned stimulus can be a cue (for example, a tone) or a context (for example, a room). Examples of conditioned fear responses are increased freezing and heart rate. Fear extinction consists of new inhibitory learning after repeated or prolonged conditioned stimulus presentations, without the unconditioned stimulus, which causes a gradual decrease in the magnitude and frequency of the conditioned response (5). Here, we use a single severe stress exposure, immobilization to a wooden board (IMO), to model PTSD-like behavior in mice and to study resultant differential gene regulation in fear learning. We use this model for several reasons: A valid PTSD-like model requires a highly traumatic stress exposure, much more stressful than other mild stressors such as fear conditioning (6, 7). IMO is one of the most stressful among the emotional models used in the laboratory (7). Moreover, the severity of the stressor during trauma exposure is related to the likelihood of PTSD development (8). Previous IMO causes PTSD-like impaired fear extinction (9), whereas fear conditioning alone is not resistant to extinction (6). IMO is one of the few acute stressors that result in a wide range of long-term (more than 24 to 48 hours) PTSD-like symptoms after a single exposure in rodents (7). Among those symptoms, IMO elicits long-term impaired declarative memory and enhanced anxiety similar to what occurs in PTSD patients (4, 7–10).
RESULTS
Immobilization of mice causes long-term impaired declarative memory and enhanced anxiety
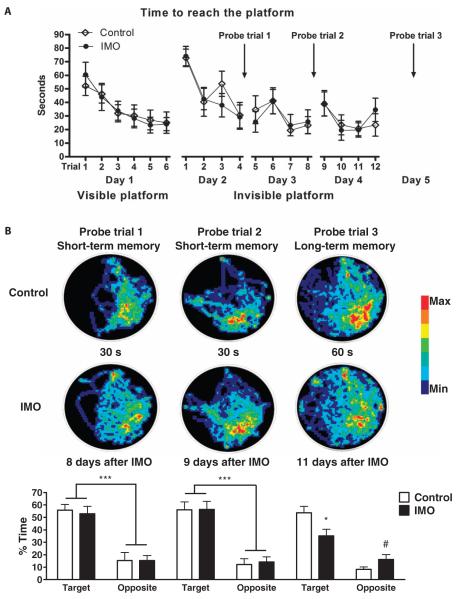
Mice were initially exposed for 2 hours using the IMO paradigm, followed 6 days later by a water maze task (Fig. 1A). There were differences in long-term, but not short-term, spatial memory on this spatial task after IMO (Fig. 1B). However, there was no difference in time to reach the platform between the groups during visible platform training, which indicated intact motivation and sensorimotor skills after IMO. Results from the long-term memory test showed that IMO animals spend less time than control animals in the target area (*P < 0.05). These data suggest that a single exposure to IMO elicits long-term declarative memory impairment. A separate group of mice was tested for anxiety-like measures 6 days after they were exposed for 2 hours to the IMO procedure. Data showed that IMO-treated mice exhibited enhanced anxiety in both the elevated plus maze (Fig. 2A) and the open-field maze (Fig. 2B). Overall, these findings suggest that mice, like rats, exhibit prolonged enhanced anxiety-like behavior and deficits in spatial learning after a single immobilization stress. We next examined whether previous IMO also altered fear extinction, another deficit observed in PTSD patients.
Fig. 1. Immobilization of mice causes long-term impaired declarative memory.
(A and B) Mice were exposed for 2 hours using the immobilization to a wooden board (IMO) procedure, 6 days before testing in the water maze test, which is used to evaluate spatial declarative memory. The water maze procedure lasted for 5 days. (A) Training started with six trials in 1 day of the visible platform test. Results showed no difference in the time to reach the platform between groups, which indicated intact motivation and sensorimotor skills. The day after, the invisible platform training started and consisted of four trials a day for three consecutive days, showing no differences in learning evaluated by time to reach the platform. (B) On days 2 (8 days after IMO) and 3 (9 days after IMO) after training, a probe trial was performed to assess short-term memory. Results showed no differences between IMO and control group in the short-term memory tests [***P < 0.001, Target versus Opposite, repeated-measures analysis of variance (ANOVA)]. On day 5 (11 days after IMO), only a probe trial was performed to assess long-term memory. Results showed that IMO animals spent less time than control animals in the target area (*P < 0.05 versus control in target area, repeated-measures ANOVA followed by Bonferroni post-test). IMO mice spent more time than the control mice in the opposite area of the maze (#P < 0.05 versus control in opposite area, repeated-measures ANOVA followed by Bonferroni post-test). These data replicate previous findings in rats (10).
Fig. 2. Immobilization causes long-term enhanced anxiety-like symptoms in mice.
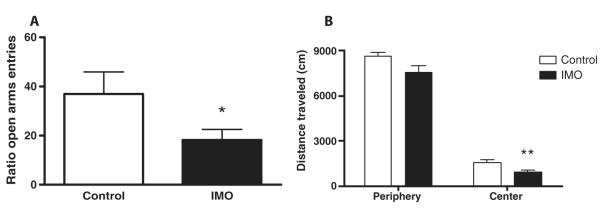
(A and B) Mice were exposed for 2 hours to immobilization to a wooden board (IMO) 6 days before testing in (A) an elevated plus maze or (B) an open-field test. (A) Five-minute exposure to elevated plus maze revealed that IMO mice exhibited long-term enhanced anxiety according to the ratio of the time spent in the open arms (*P < 0.05, two-tailed Student’s t test). (B) Concordantly, 30-min exposure to an open field resulted in mice showing enhanced anxiety when the time that control or IMO mice spent in the center of the apparatus was calculated (**P < 0.01, two-tailed Student’s t test). These data replicate previous findings in rats (49).
Amygdala Oprl1 gene regulation is altered in a PTSD-like mouse model during fear consolidation
We then studied cued-fear expression in mice with a single previous history of IMO versus compensatory handling (control) 6 days before fear conditioning. Here, we used auditory-fear conditioning where the conditioned stimulus was a tone (30 s, 6 kHz) that coterminated with an unconditioned stimulus, which was a mild electric footshock (0.5 s, 1-mA intensity). Mice that received fear conditioning showed significantly more freezing during this test, independently of having received IMO or not, than animals exposed to the fear conditioning box without receiving stimuli (box-only exposure) (main effect of fear conditioning: F1,36 = 42.5; P ≤ 0.0001, repeated-measures ANOVA followed by Bonferroni post-test; Fig. 3A). During the cued-fear expression test, the IMO-fear conditioning group presented more enhanced freezing than did the control-fear conditioning group (fear conditioning main effect: F1,36 = 508; P ≤ 0.0001; IMO main effect: F1,36 = 12; P ≤ 0.001; Fear conditioning × IMO interaction: F1,36 = 10.8; P ≤ 0.001, repeated-measures ANOVA followed by breakdown of the interaction; Fig. 3A). In the fear conditioning test, control-fear conditioned mice showed more freezing during the final conditioned stimulus than during the intertrial interval (Trial × Cue × Group interaction: F1,20 = 10.3; P ≤ 0.01, repeated-measures ANOVA; conditioned stimulus and intertrial interval 5 breakdown of the interaction: P ≤ 0.01, repeated-measures ANOVA), whereas the IMO-fear conditioning group had similar freezing during all of the conditioned stimulus and intertrial interval periods(Fig. 3B). This suggests that IMO mice have difficulties discriminating between nonthreat periods (intertrial interval) and danger signals (conditioned stimulus). This is concordant with a recent report that shows that PTSD patients present altered discrimination of safety and danger signals (11).
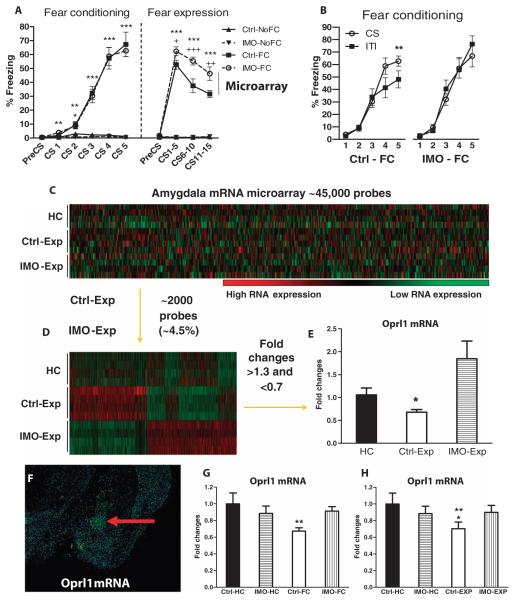
Fig. 3. Differential regulation of Oprl1 in the amygdala during cued-fear conditioning and cued-fear expression in a PTSD-like mouse model.
(A) Immobilization-fear conditioning (IMO-FC) and control-fear conditioning (Ctrl-FC) groups equally acquired cued-fear conditioning. IMO in the absence of fear conditioning did not elicit freezing (IMO-NoFC). During the cued-fear expression (Exp) test, the IMO-FC group presented enhanced freezing relative to the nonstressed fear conditioned group (***P ≤ 0.001, IMO-FC versus IMO-NoFC, repeated-measures ANOVA followed by Bonferroni post-test; +P < 0.05, ++P ≤ 0.01, +++P ≤ 0.001, IMO-FC versus Ctrl-FC, repeated-measures ANOVA followed by Bonferroni post-test) (n = 10 mice per group). IMO alone did not induce freezing in the fear expression test. The term “microarray” and the line (under fear expression) denote that at 2 hours after fear expression, microarray analysis was performed to identify differential gene expression in the IMO-FC and Ctrl-FC groups. (B) During fear conditioning, the control-fear conditioning group (Ctrl-FC) presents discrimination of the conditioned stimulus 5 (CS5) presentation versus the intertrial interval (ITI) (period between stimulus) (**P ≤ 0.01, repeated-measures ANOVA followed by Bonferroni post-test), whereas the IMO-fear conditioning group (IMO-FC) does not. (C) mRNA microarray analysis of amygdala tissue showing 45,281 transcripts of amygdala mRNA, obtained 2 hours after fear expression (n = 4 mice per group). (D) Selection of the 1963 probes (4.34%) that present statistically significant changes in control-fear expression (Ctrl-Exp) group versus the IMO-fear expression (IMO-Exp) group. (E) Oprl1 mRNA is down-regulated in the control-fear expression (Ctrl-Exp) group versus the IMO-fear expression (IMO-Exp) group (*P < 0.05, ANOVA followed by Bonferroni post-test). (F) Oprl1 mRNA is highly expressed in the mouse central amygdala (red arrow) (12). (G) In replication studies, fear conditioning induces down-regulation of Oprl1 mRNA in the amygdala of control-fear conditioned (Ctrl-FC) mice [*P < 0.05 versus home cage group (HC), ANOVA followed by Bonferroni post-test, n = 8 mice per group]. The mice in the home cage group were undisturbed in the vivarium and had compensatory handling the same days that the IMO mice were exposed to stress. (H) The cued-fear expression test also induced down-regulation of Oprl1 mRNA in the amygdala of the control-fear conditioning group (Crl-FC) (*P < 0.05 versus HC, **P ≤ 0.01 versus IMO-FC, ANOVA followed by Bonferroni post-test; n = 8 mice per group).
Two hours after the cued-fear expression test, animals were sacrificed and amygdala tissue was collected to perform mRNA microarray analyses (Fig. 3, C to E, and fig. S1A). To focus on differential gene expression as a function of previous IMO history, and not fear conditioning per se, we then selected probes that were statistically different between control-fear expression versus IMO-fear expression groups. About 4.5% of the examined genes (45,281) were differentially expressed on the basis of previous IMO exposure (Fig. 3D). From those genes, we then selected those that presented >1.3- and <0.7-fold changes in both groups versus the home cage group (fig. S1B). Of those probes, the only one that represented a gene with highly specific expression in the amygdala compared to other areas of the brain was the opioid receptor–like 1 (Oprl1) gene (Fig. 3F).
Oprl1 is found in mice in the central amygdala, whereas its expression in other amygdala regions is relatively low (12). The Oprl1 gene encodes the nociceptin receptor (NOP-R), also known as the orphanin FQ receptor, which is activated by the nociceptin peptide (NOP) (or orphanin FQ) (13, 14). On the microarray, Oprl1 mRNA was significantly down-regulated in the control-fear expression group when compared to IMO-fear expression group (group effect: F2,11 = 30.7; P < 0.05, ANOVA followed by Bonferroni post-test; Fig. 3E). Additionally, quantitative polymerase chain reaction (qPCR) analysis confirmed the microarray finding: Oprl1 mRNA was down-regulated after fear conditioning and fear expression tests in control animals but not in animals with previous IMO exposure [Stress × Fear conditioning interaction: F1,29 = 5; P < 0.05 (Fig. 3G); Stress × Fear expression interaction: F1,29 = 5.5; P < 0.05, ANOVA followed by Bonferroni post-test (Fig. 3H)]. Moreover, Oprl1 mRNA down-regulation only occurred when the conditioned and unconditioned stimuli are paired but not when they are unpaired (fig. S1C). Oprl1 mRNA concentrations in the striatum do not change after fear conditioning or fear expression test in control or IMO groups (fig. S1, D and E). We hypothesize that amygdala-specific dysregulation of Oprl1 gene expression after fear conditioning and fear expression in IMO mice may partially explain the mechanisms of altered fear learning in this PTSD-like animal model.
SR-8993 is a new specific NOP-R agonist
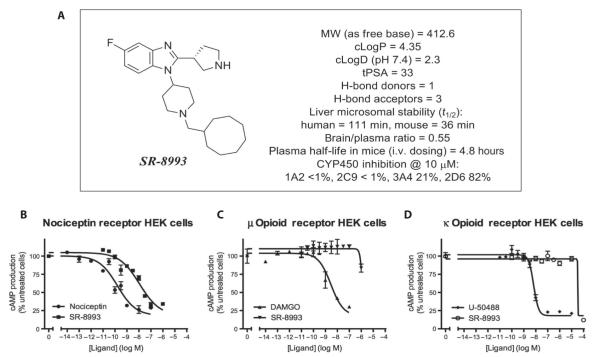
Using peptides and nonselective compounds, it has been suggested that NOP-R agonism impairs fear learning, whereas antagonism enhances fear (15, 16). We wished to examine a more selective NOP-R agonist, with >100-fold selectivity over the μ opioid receptor and essentially no κ or δ opioid receptor activity, in our dysregulated fear model. We thus used a newly developed and selective NOP agonist, SR-8993 (Fig. 4A and fig. S2), which potently activates NOP-R [median effective concentration (EC50) = 8.8 ± 1.38 nM; Fig. 4B]. SR-8993 is also characterized by unusually high selectivity for NOP-R over the closely related opioid receptors μ (EC50 = 4800 ± 3300 nM; Fig. 4C), κ (EC50 > 10,000 nM, estimated; Fig. 4D), and δ (no activity). SR-8993 passes common drug-likeness criteria and is not predicted to be associated with toxicity (17). Drug metabolism and pharmacokinetic studies in mice showed an in vivo half-life of 4.8 ± 0.6 hours and a brain/plasma ratio of 0.55, measured 2 hours after intravenous dosing. It is stable in human and mouse liver microsome preparations and does not show inhibition of most major CYP450 isoforms, with the exception of 2D6 (Fig. 4A).
Fig. 4. SR-8993 is a new specific and potent NOP-R agonist.
(A) SR-8993 is characterized by unusually high selectivity for the NOP-R over the closely related opioid receptors. Structure and physical and pharmacokinetic characteristics of SR-8993 are shown. (B to D) SR-8993 dose-response curves in human embryonic kidney (HEK) cells expressing the NOP/NOP-R (B), the μ opioid receptor (C), and the κ opioid receptor (D).
The drug metabolism, pharmacokinetic, and biochemical selectivity data were used in the design of experiments. An intraperitoneal injection of SR-8993 was used to obtain high brain concentrations of SR-8993 that activated NOP-R with minimal activation of μ or κ opioid receptors. A dose of 10 mg/kg achieved a 660 ± 51 nM brain concentration 120 min after intraperitoneal administration, which is 75-fold above the NOP-R EC50 and 7-fold below the μ EC50. We chose a somewhat lower injection dose (3 mg/kg) to allow targeting of NOP-R with a low probability of affecting other brain receptors.
SR-8993 prevents fear memory consolidation when injected into the central amygdala or systemically
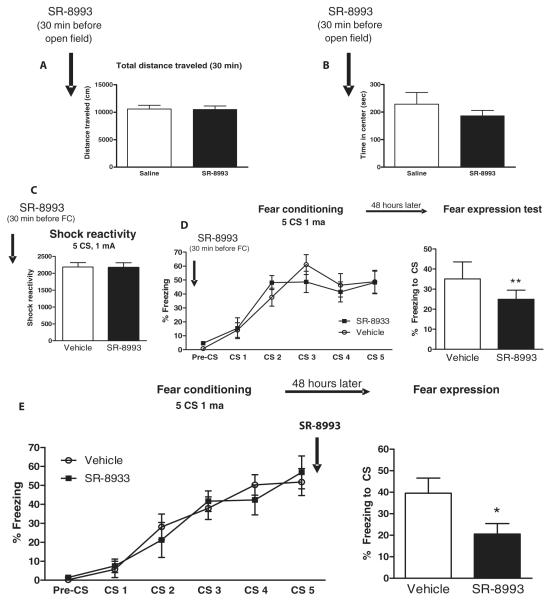
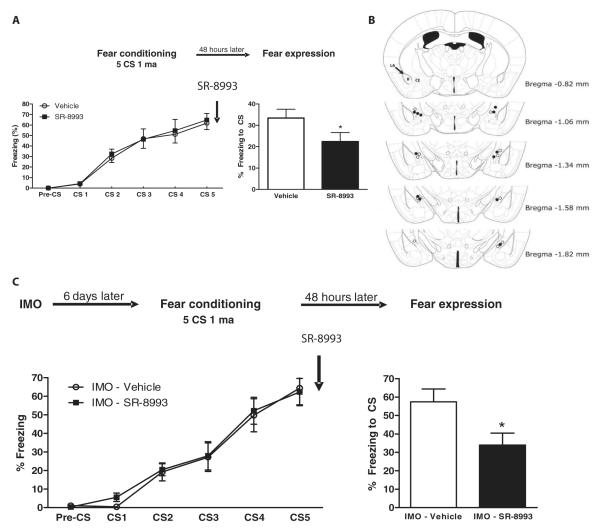
SR-8993, given intraperitoneally, does not induce differences in locomotor activity, anxiety-like behavior, footshock reactivity, or cued-fear acquisition (Fig. 5, A to D). In contrast, it has clear on-target NOP-R activation effects. SR-8993 impaired cued-fear memory consolidation when injected 30 min before fear conditioning. Concordantly, SR-8993 dosing immediately after fear conditioning caused impaired cued-fear memory consolidation (t11 = 2.3; P < 0.05, two-tailed Student’s t test; Fig. 5E). Similarly, SR-8993 injected into the central amygdala immediately after fear conditioning also impaired cued-fear memory consolidation (t10 = 2.4; P < 0.05, two-tailed Student’s t test; Fig. 6, A and B). Moreover, SR-8993 impaired cued-fear memory consolidation when mice had a previous IMO exposure before fear conditioning (t14 = 2.5; P < 0.05, two-tailed Student’s t test; Fig. 6C). SR-8993, which targets NOP-R, is a potent, selective, and effective agent to block cued-fear memory consolidation in control animals and a PTSD-like model. This suggests that activation of Oprl1 expression in the amygdala with an NOP-R agonist is sufficient to impair fear consolidation even in a robust model of fear dysregulation.
Fig. 5. The NOP-R agonist SR-8993 impairs cued-fear memory consolidation in mice.
The NOP-R agonist SR-8993 (3 mg/kg), when systemically injected, impairs cued-fear memory consolidation but has no effects on anxiety, shock reactivity, or fear acquisition. (A) SR-8993 does not elicit locomotor changes evaluated with the open field (n = 8 mice per group). (B) SR-8993 has no effect on anxiety evaluated by the time spent in the center of the apparatus in the open field (n = 8 mice per group). (C) SR-8993 does not induce changes in pain sensitivity to mild electric footshock evaluated in the startle chamber (n = 8 mice per group). (D) SR-8993 does not alter freezing during cued-fear conditioning but impairs fear memory consolidation when evaluated 48 hours later in the fear expression test (**P < 0.01, two-tailed Student’s t test; n = 8 mice per group). (E) Systemic injection of SR-8993 given immediately after cued-fear conditioning. Upon cue fear expression testing 48 hours later, the immediate post-training SR-8993 impaired fear memory consolidation, as shown by reduced freezing in the fear expression test (*P < 0.05, two-tailed Student’s t test; n = 6 to 7 mice per group).
Fig. 6. SR-8993 impairs cued-fear memory when infused in the central amygdala and in a PTSD-like mouse model.
(A) SR-8993 bilaterally injected into the central amygdala immediately after fear conditioning causes impaired fear memory consolidation as shown by the degree of freezing in the cued-fear expression test (*P < 0.05, two-tailed Student’s t test; n = 6 mice per group). (B) Histological verification of SR-8993 infusion sites. The dots indicate the lowest point of the injector tip. Bregma is the anatomical point on the skull at which the coronal suture is intersected perpendicularly by the sagittal suture used as a reference point. (C) Systemic SR-8993 given immediately after fear conditioning in mice with a previous IMO exposure impaired fear memory consolidation as determined by reduced freezing in the fear expression test (*P < 0.05, two-tailed Student’s t test; n = 8 mice per group).
The OPRL1 gene is associated with altered fear learning and amygdala-insula functional connectivity in PTSD patients
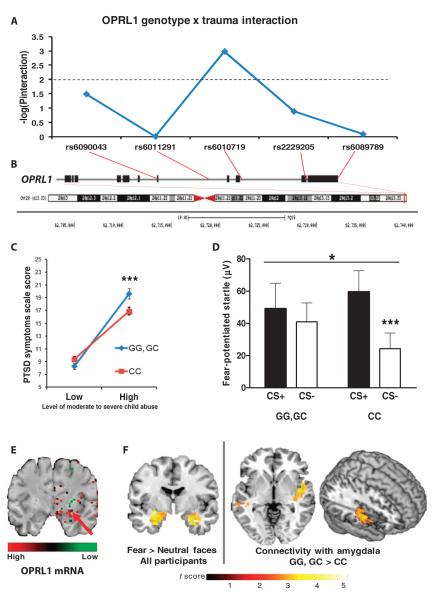
We next examined if OPRL1 is involved in PTSD and fear learning in a traumatized human population. For that purpose, we performed a tag single-nucleotide polymorphism (SNP) analysis (18, 19), spanning 20 kb of the OPRL1 locus with a total of five SNPs obtained from an Illumina OmniQuad 1M array. We examined whether each SNP was associated with PTSD diagnosis in this cohort of highly traumatized urban civilian subjects (n = 1847). SNP rs6010719 was associated with increased PTSD symptoms in individuals exposed to moderate to severe child abuse [SNP × Child trauma interaction: F2,1847 = 6.4; P < 0.005, univariate ANOVA (UNIANOVA)]. Because OPRL1 has also previously been associated with substance abuse (20) and PTSD is often comorbid with substance abuse (21, 22), and because PTSD is sometimes differentially associated with sex and age, we then reran the interaction, controlling for age, sex, and substance abuse. After these covariates were added, rs6010719 remained significant in the interaction (SNP × Child trauma interaction: F2,1793 = 6.9; P ≤ 0.001, ANOVA; Fig. 7, A and B). Additionally, we found no interaction with this SNP, and sex, age, or substance abuse was able to predict PTSD symptoms (all P values >0.1). Compared to CC carriers, the genetic association of G allele carriers for PTSD risk increased with the degree of trauma exposure (Fig. 7C).
Fig. 7. In humans, OPRL1 is associated with PTSD and altered fear processing in PTSD.
(A) The significance level of each of the five OPRL1 SNPs examined is shown as “log(Pinteraction)” for the interaction of each genotype and level of self-reported child abuse [using the Childhood Trauma Questionnaire (CTQ)]. For a Bonferroni-corrected P value of 0.01 (for five SNPs), the log(P) would be 2. We find that the rs6010719 SNP survives correction for the interaction test at P ≤ 0.005. (B) Location of SNPs within the OPRL1 gene (average 3.5-kb inter-SNP interval) and location of the gene on chromosome 20. (C) Specific interactions of the G allele carriers (GG, GC) versus CC allele carriers of the rs6010719 SNP in OPRL1, demonstrating that G allele carriers who have experienced greater trauma are at higher risk for PTSD symptoms (F1,1847 = 10.5; P < 0.001, UNIANOVA). Additionally, we found no interactions between this SNP and variables such as sex, age, or substance abuse that predicted PTSD symptoms (all P values >0.1). (D) G allele carriers of the rs6010719 SNP (n = 49) showed no discrimination between CS+ (danger signal) and CS− (safety signal) measured by the FPS response. In contrast, individuals of the CC genotype (n = 70) showed normal discrimination (interaction: *P < 0.05, ***P ≤ 0.001, ANOVA). (E) OPRL1 mRNA is highly expressed in the human central amygdala [picture modified from Allen Brain Atlas (12, 50)]. The arrow indicates the central amygdala. (F) (Left) Within-group random-effects analysis showed enhanced bilateral amygdala activation in response to fearful versus neutral face stimuli in all participants, irrespective of genotype (local maximum for left amygdala: Z = 4.32; x, y, z = −32, −8, −16; Pcorr < 0.05; local maximum for right amygdala: Z = 3.12; x, y, z = 28, −4, −28; Pcorr < 0.05). (Right) For fearful versus neutral face stimuli, G carriers (n = 10) have increased functional connectivity between amygdala (seed region) and right posterior insula, relative to CC allele carriers (n = 19) (Pcorr < 0.05). Results are overlaid on a representative structural anatomical image in standard Montreal Neurological Institute space.
To examine the effect of this OPRL1 variant on human fear processing, we examined fear-potentiated startle (FPS) by measuring the acoustic startle reflex in a subset of this highly traumatized cohort. Given the above finding, we collapsed the GG and GC genotypes into G allele carriers versus the CC genotype during differential fear conditioning, in which one conditioned stimulus was paired with an aversive airblast (+), whereas a second conditioned stimulus was not (−) (11, 23). A significant interaction of genotype and type of conditioned stimulus (F1,118 = 4.4; P < 0.05, ANOVA) indicated that G allele carriers did not discriminate on the FPS response between danger signals (+) and safety signals (−) during late fear conditioning. In contrast, those with the CC genotype showed an increase in the FPS response to the (+) conditioned stimulus versus the (−) conditioned stimulus (F1,69 = 12.3; P ≤ 0.001, ANOVA; Fig. 7D). Examining the difference score between the (+) and (−) conditioned stimuli, G allele carriers presented a significantly decreased discrimination rate even after taking into account covariance for sex, age, and degree of childhood trauma and PTSD symptoms (SNP effect: F1,113 = 5.7; P < 0.05, ANOVA).
Notably, the OPRL1 gene is highly expressed in the human amygdala in concordance with the mouse gene expression pattern [Figs. 3A and 7E and (12)]. To examine OPRL1 association with fear processing within the brain, traumatized women underwent functional magnetic resonance imaging (fMRI) as they viewed fearful and neutral face stimuli. We predicted that in G carriers, the amygdala would show increased reactivity and increased functional coupling with other fear-related regions such as the insula and anterior cingulate (24, 25). Random-effects analysis of the main effect of fearful relative to neutral face stimuli showed robust bilateral amygdala activation within our sample with no differences between G and CC carriers. However, for fearful relative to neutral faces, a two-group t test comparing task-based functional connectivity for G versus CC carriers showed greater functional connectivity between the amygdala and posterior insula in the GG/GC group (Fig. 7F; Pcorr < 0.05).
DISCUSSION
This study demonstrates translational convergence in a gene associated with PTSD in human patients and a PTSD-like mouse model. Moreover, the SNP rs6010719 of the OPRL1 gene is associated with PTSD symptoms, and this may be explained by its effect on altered fear learning and fear discrimination mechanisms. Additionally, this SNP is associated with differential amygdala-insula functional connectivity, and the insula has been reported to be involved with PTSD (26). Given the potential role of the nociceptin peptide in pain perception, the amygdala-insula functional connectivity is intriguing given this circuit’s role in central pain processing (27).
Concordantly, the amygdala Oprl1 gene is dysregulated in a PTSD-like mouse model after fear learning. A previous study found that a single exposure to stress elicited Oprl1 mRNA expression changes in amygdala (28). Thus, individual episodes of significant traumatic stress may alter amygdala Oprl1 function, which affects later fear conditioning and cued-fear expression in our PTSD-like model. Additionally, using Oprl1 knockout mice, Oprl1 has been shown to be involved in contextual fear conditioning (29). That study did not find an effect on cued-fear learning, which might be explained by developmental compensation in a knockout mouse model. A recent report in rats suggests that nociceptive sensitivity and NOP concentrations are enhanced in a different PTSD-like model (single prolonged stress) than the one we have used in the present study (IMO) (30). Notably, the NOP agonist SR-8993 does not alter reactivity to footshock (Fig. 5C). This suggests that NOP-R regulation of nociception may be strongly influenced by the nature of the test, as previously reported (31).
This study does not support a model in which Oprl1 within the amygdala is solely responsible for fear consolidation, but rather that it is required for normal fear memory formation to occur. Together, our data suggest that early intervention (shortly after fear learning) with a selective, centrally acting NOP agonist reduces fear memory consolidation. In humans, this may translate to the ability to prevent the likelihood of developing PTSD in the aftermath of trauma. Notably, the opiate agonist morphine, a primarily μ opioid receptor agonist given within 48 hours after trauma, reduces the risk of subsequent development of PTSD in observational studies (32, 33). The μ opioid receptor and NOP-R have a high level of homology but are involved in different biological functions (34). For example, the NOP agonist Ro 64-6198 has been shown to block the rewarding effects of morphine in mice (35) and does not induce rewarding effects per se in monkeys (36). Thus, the selective agonism of NOP-R in humans may mimic morphine’s beneficial effect in reducing fear memory consolidation without the well-known undesired effects of opioids (for example, dependence, addiction, tolerance, gastrointestinal symptoms, and respiratory impairment). If this hypothesis is clinically validated and our studies are replicated, NOP-R agonists may be important candidates for the prevention of PTSD, particularly in an early intervention setting, shortly after exposure to traumatic experiences.
MATERIALS AND METHODS
Animals
All experiments were performed on adult (2 to 3 months old) wild-type strain C57BL/6J from Jackson Labs, male mice that were group-housed in a temperature-controlled vivarium, with ad libitum access to food and water. They were maintained on a 12-hour/12-hour light/dark cycle, with all behavioral procedures being performed during the light cycle. All procedures used were approved by the Institutional Animal Care and Use Committee of Emory University and in compliance with National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Immobilization to wooden board (IMO)
Mice were exposed once for 2 hours to IMO, which was performed as previously described (9).
Water maze
The apparatus was a 122-cm-diameter pool (San Diego Instruments). Water was at room temperature between 19° and 20°C and made opaque by adding nontoxic latex. Swim paths were monitored and stored with the video-tracking system SMART 2.5.19 (Panlab, Harvard Apparatus) for later analysis. Atlantis platform was used as previously described (10).
mRNA extraction and microarray hybridization
Total mRNA was isolated and purified from the tissue with the RNeasy Mini Kit (catalog 74106, Qiagen). Illumina Mouse WG-6 v2 Expression BeadChip microarray (Illumina Inc.) was assayed for 45,281 transcripts. We initially selected probes that were statistically different between control-fear expression (Ctrl-Exp) and IMO-fear expression (Exp) groups. From those genes, we initially selected for genes with >1.5- and <0.5-fold changes in both groups versus home cage (HC) group. However, no genes survived this combined stringent criteria. We then relaxed the criteria to select those that presented >1.3- and <0.7-fold change in both groups versus the home cage group, and those genes were prioritized on the basis of amygdala expression for further replication.
Complementary DNA synthesis and qPCR
Total mRNA was reverse-transcribed with the RT2 First Strand Kit (catalog 330401, Qiagen). The primer used for the qPCR was the TaqMan Oprl1 Mm00440563_m1 from Applied Biosystems.
SR-8993 synthesis and pharmacokinetics
Steps 1 and 2: Synthesis of 1-(cyclooctylmethyl)piperidin-4-amine. Steps 3 and 4: Synthesis of N1-(1-(cyclooctylmethyl)piperidin-4-yl)-4-fluorobenzene-1,2-diamine. Steps 5 to 7: Synthesis of (R)-1-(1-(cyclooctylmethyl)piperidin-4-yl)-5-fluoro-2-(pyrrolidin-3-yl)-1H-benzo[d]imidazole (SR-8993). The cAMP (adenosine 3′,5′-monophosphate) biosensor assay, ACTOne Membrane Potential assay kit, was purchased from Codex BioSolutions. Hepatic microsomal stability assays and brain penetration assays were performed as previously described (37).
SR-8993 administration
SR-8993 was dissolved in physiological saline. Systemic intraperitoneal volume of injection was 8 μl/g, and the dose was 3 mg/kg for behavioral experiments and 10 mg/kg for brain penetration assays. Intramygdalar SR-8993 volume of injection was 0.5 μl with 100 ng of dose per side. Vehicle was physiological saline at the same volume of injection.
Stereotactic surgery and infusion of SR-8993
Stereotactic surgery was performed as previously described (38). Administration of a volume of 0.5 μl per side of SR-8993 was delivered over a period of 60 s.
Cued-fear conditioning and fear expression test
Cued-fear conditioning and fear expression tests were performed as previously described (9).
Human subjects
Detailed trauma interviews were performed using the PTSD Symptom Scale (PSS) on about 2000 highly traumatized males and females and the Clinician-Administered PTSD Scale (CAPS) on a smaller subset after informed consent were collected. As noted in some of our previous works (18, 19), these subjects are adult (average age, ~40 years), primarily female (60%), highly traumatized, impoverished, primarily African American, and with very large rates of current and lifetime PTSD. Other phenotype measurements included in the data collection were the CTQ as our primary child abuse measure, and current substance abuse.
Genome-wide association studies
All DNA for genotyping was quantified by gel electrophoresis with Quantity One (Bio-Rad) and then normalized to 400 ng. Using the Illumina Human Omni1-Quad BeadChip (Illumina Inc.), we performed SNP genotyping according to instructions by the manufacturer. Data were analyzed with PLINK.
Human FPS
Data were collected and analyzed as previously described (11, 23).
Neuroimaging
Eight fearful and eight neutral (four male and four female) faces were selected from the stimulus set of Ekman and Friesen (39). Faces were presented in a random order for 500 ms with a 500-ms presentation of a fixation cross separating each face stimulus. Brain imaging data were acquired on a Siemens 3.0-T Magnetom Trio TIM whole-body MR scanner (Siemens) with a standard head coil. Functional images were acquired with the Z-SAGA pulse sequence (40). Structural images were acquired with a gradient-echo, T1-weighted pulse sequence (repetition time, 2600 ms; echo time, 3.02 ms; voxel size, 1 mm × 1 mm × 1 mm).
Statistics
Statistics were performed with IBM SPSS Statistics 19.0. Detection of outliers was performed and, when necessary, removed from analyses. Repeated-measures ANOVA, one- or two-way ANOVA, or Student’s t test (two-tailed) for independent samples was tested. Bonferroni was the post-hoc analysis. The results are presented as means ± or + SEM, and statistical significance was set at P < 0.05.
Supplementary Material
Acknowledgments
We appreciate the technical assistance of G. Doho (Emory Cancer Genomics Shared Resource) with the microarray analyses. We also thank M. Dierssen and A. Regi for sharing the software jTracks, which was used to analyze the water maze.
Funding: This work was supported by NIH grants MH071537 (K.J.R.), MH096764 (K.J.R.), MH092576 (T.J.), and MH098212 (T.J.) as well as Brain and Behavior Research Foundation, the Burroughs Wellcome Fund, and Emory Medical Care Foundation (T.J.). Support was received from the NIH/National Center for Research Resources base grant P51RR000165 to Yerkes National Primate Research Center. The chemistry/new compound work was supported by the National Institute on Alcohol Abuse and Alcoholism (AA017943 to C.W., AA018665 to M.C.), the National Institute on Drug Abuse (DA035055 to C.W. and S.P.B., DA035056 to T.D.B.), and the National Center for Advancing Translational Sciences (UL1TR000454).
Footnotes
SUPPLEMENTARY MATERIALS www.sciencetranslationalmedicine.org/cgi/content/full/5/188/188ra73/DC1 Methods
Author contributions: R.A. and K.J.R. conceived and designed the study. R.A. performed the behavioral experiments in mice, systemically injected SR-8993, and analyzed the data. R.A. performed the micropunches and the mRNA isolation. R.A. analyzed the microarray. R.A. analyzed and performed the qPCR experiments. R.A. performed stereotactic surgeries, intra-amygdala infusion of SR-8993, and histological experiments. Y.T.C. and T.D.B. synthesized SR-8993. M.C. performed and analyzed the pharmacokinetic experiments. S.P.B. and H.S.-U. performed mouse behavior studies. J.S.S. and T.J. performed and analyzed the fMRI experiments. L.A. obtained the PSS data, and L.A. and K.J.R. analyzed the PSS data. L.A. performed the genome-wide association studies, and L.A. and K.J.R. analyzed the data. T.J. performed the FPS and analyzed the data. K.J.R., S.P.B., T.J., T.D.B., B.B., E.B.B., and C.W. obtained the funding. R.A. and K.J.R. wrote the manuscript.
Competing interests: S.P.B., Y.T.C., H.S.-U., T.D.B., and C.W. have filed a patent related to this work: Provisional Patent Application Serial No. 61804316 “Substituted benzimidazoles as nociceptin receptor modulators” docket # 1361.184PV2. The other authors declare that they have no competing interests.
Data and materials availability: The microarray data are publicly available in the Gene Expression Omnibus database under accession number GSE44944.
REFERENCES AND NOTES
- 1.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 2.Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J. Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J. Neurosci. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearns MC, Ressler KJ, Zatzick D, Rothbaum BO. Early interventions for PTSD: A review. Depress. Anxiety. 2012;29:833–842. doi: 10.1002/da.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andero R, Ressler KJ. Fear extinction and BDNF: Translating animal models of PTSD to the clinic. Genes Brain Behav. 2012;11:503–512. doi: 10.1111/j.1601-183X.2012.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: An animal model of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Armario A, Escorihuela RM, Nadal R. Long-term neuroendocrine and behavioural effects of a single exposure to stress in adult animals. Neurosci. Biobehav. Rev. 2008;32:1121–1135. doi: 10.1016/j.neubiorev.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Hinton DE, Lewis-Fernández R. The cross-cultural validity of posttraumatic stress disorder: Implications for DSM-5. Depress. Anxiety. 2011;28:783–801. doi: 10.1002/da.20753. [DOI] [PubMed] [Google Scholar]
- 9.Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am. J. Psychiatry. 2011;168:163–172. doi: 10.1176/appi.ajp.2010.10030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andero R, Daviu N, Escorihuela RM, Nadal R, Armario A. 7,8-Dihydroxyflavone, a TrkB receptor agonist, blocks long-term spatial memory impairment caused by immobilization stress in rats. Hippocampus. 2012;22:399–408. doi: 10.1002/hipo.20906. [DOI] [PubMed] [Google Scholar]
- 11.Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, Ressler KJ, Jovanovic T. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depress. Anxiety. 2011;28:1058–1066. doi: 10.1002/da.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen Brain Atlas Resources. http://www.brain-map.org.
- 13.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 14.Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr., Civelli O. Orphanin FQ: A neuropeptide that activates an opioidlike G protein–coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 15.Roozendaal B, Lengvilas R, McGaugh JL, Civelli O, Reinscheid RK. Orphanin FQ/nociceptin interacts with the basolateral amygdala noradrenergic system in memory consolidation. Learn Mem. 2007;14:29–35. doi: 10.1101/lm.403607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goeldner C, Reiss D, Wichmann J, Kieffer BL, Ouagazzal AM. Activation of nociceptin opioid peptide (NOP) receptor impairs contextual fear learning in mice through glutamatergic mechanisms. Neurobiol. Learn. Mem. 2009;91:393–401. doi: 10.1016/j.nlm.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walters WP. Going further than Lipinski’s rule in drug design. Expert Opin. Drug Discov. 2012;7:99–107. doi: 10.1517/17460441.2012.648612. [DOI] [PubMed] [Google Scholar]
- 18.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J, Young B, Pletcher MT, Heilig M, Wahlestedt C. Association between the nociceptin receptor gene (OPRL1) single nucleotide polymorphisms and alcohol dependence. Addict. Biol. 2008;13:88–94. doi: 10.1111/j.1369-1600.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- 21.Khoury L, Tang YL, Bradley B, Cubells JF, Ressler KJ. Substance use, childhood traumatic experience, and posttraumatic stress disorder in an urban civilian population. Depress. Anxiety. 2010;27:1077–1086. doi: 10.1002/da.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Najavits LM, Weiss RD, Shaw SR. The link between substance abuse and posttraumatic stress disorder in women. A research review. Am. J. Addict. 1997;6:273–283. [PubMed] [Google Scholar]
- 23.Jovanovic T, Phifer JE, Sicking K, Weiss T, Norrholm SD, Bradley B, Ressler KJ. Cortisol suppression by dexamethasone reduces exaggerated fear responses in posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36:1540–1552. doi: 10.1016/j.psyneuen.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Büchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 25.Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans: The influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33:653–663. doi: 10.1016/s0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- 26.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am. J. Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 27.Bornhövd K, Quante M, Glauche V, Bromm B, Weiller C, Büchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: A single-trial fMRI study. Brain. 2002;125:1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- 28.Green MK, Devine DP. Nociceptin/orphanin FQ and NOP receptor gene regulation after acute or repeated social defeat stress. Neuropeptides. 2009;43:507–514. doi: 10.1016/j.npep.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamiya T, Yamada K, Miyamoto Y, König N, Watanabe Y, Noda Y, Nabeshima T. Neuronal mechanism of nociceptin-induced modulation of learning and memory: Involvement of N-methyl-D-aspartate receptors. Mol. Psychiatry. 2003;8:752–765. doi: 10.1038/sj.mp.4001313. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Gandhi PR, Standifer KM. Increased nociceptive sensitivity and nociceptin/orphanin FQ levels in a rat model of PTSD. Mol. Pain. 2012;8:76. doi: 10.1186/1744-8069-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiss D, Wichmann J, Tekeshima H, Kieffer BL, Ouagazzal AM. Effects of nociceptin/orphanin FQ receptor (NOP) agonist, Ro64-6198, on reactivity to acute pain in mice: Comparison to morphine. Eur. J. Pharmacol. 2008;579:141–148. doi: 10.1016/j.ejphar.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N. Engl. J. Med. 2010;362:110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- 33.Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. A study of the protective function of acute morphine administration on subsequent posttraumatic stress disorder. Biol. Psychiatry. 2009;65:438–440. doi: 10.1016/j.biopsych.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 34.Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115:1363–1381. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoblock JR, Wichmann J, Maidment NT. The effect of a systemically active ORL-1 agonist, Ro 64-6198, on the acquisition, expression, extinction, and reinstatement of morphine conditioned place preference. Neuropharmacology. 2005;49:439–446. doi: 10.1016/j.neuropharm.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Ko MC, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J, Prinssen EP. Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology. 2009;34:2088–2096. doi: 10.1038/npp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brothers SP, Saldanha SA, Spicer TP, Cameron M, Mercer BA, Chase P, McDonald P, Wahlestedt C, Hodder PS. Selective and brain penetrant neuropeptide Y Y2 receptor antagonists discovered by whole-cell high-throughput screening. Mol. Pharmacol. 2010;77:46–57. doi: 10.1124/mol.109.058677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maguschak KA, Ressler KJ. Wnt signaling in amygdala-dependent learning and memory. J. Neurosci. 2011;31:13057–13067. doi: 10.1523/JNEUROSCI.3248-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ekman P, Friesen WV. Pictures of Facial Affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- 40.Heberlein KA, Hu X. Simultaneous acquisition of gradient-echo and asymmetric spin-echo for single-shot z-shim: Z-SAGA. Magn. Reson. Med. 2004;51:212–216. doi: 10.1002/mrm.10680. [DOI] [PubMed] [Google Scholar]
- 41.Sturn A, Quackenbush J, Trajanoski Z. Genesis: Cluster analysis of microarray data. Bioinformatics. 2002;18:207–208. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. ed. 2 Academic Press; San Diego, CA: 2004. [Google Scholar]
- 44.Arqué G, Fotaki V, Fernández D, Lagrán M. Martínez de, Arbonés ML, Dierssen M. Impaired spatial learning strategies and novel object recognition in mice haploinsufficient for the dual specificity tyrosine-regulated kinase-1A (Dyrk1A) PLoS One. 2008;3:e2575. doi: 10.1371/journal.pone.0002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holmes AP, Friston KJ. Generalisability, random effects and population inference. Neuroimage. 1998;7:S754. [Google Scholar]
- 47.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 48.Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belda X, Fuentes S, Nadal R, Armario A. A single exposure to immobilization causes long-lasting pituitary-adrenal and behavioral sensitization to mild stressors. Horm. Behav. 2008;54:654–661. doi: 10.1016/j.yhbeh.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, Abajian C, Beckmann CF, Bernard A, Bertagnolli D, Boe AF, Cartagena PM, Chakravarty MM, Chapin M, Chong J, Dalley RA, Daly BD, Dang C, Datta S, Dee N, Dolbeare TA, Faber V, Feng D, Fowler DR, Goldy J, Gregor BW, Haradon Z, Haynor DR, Hohmann JG, Horvath S, Howard RE, Jeromin A, Jochim JM, Kinnunen M, Lau C, Lazarz ET, Lee C, Lemon TA, Li L, Li Y, Morris JA, Overly CC, Parker PD, Parry SE, Reding M, Royall JJ, Schulkin J, Sequeira PA, Slaughterbeck CR, Smith SC, Sodt AJ, Sunkin SM, Swanson BE, Vawter MP, Williams D, Wohnoutka P, Zielke HR, Geschwind DH, Hof PR, Smith SM, Koch C, Grant SG, Jones AR. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.