Abstract
The unique vibrational properties inherent to the human vocal fold have a significant detrimental impact on wound healing and scar formation. Hydrogels have taken prominence as a tissue engineered strategy to restore normal vocal structure and function as cellularity is low. The frequent vibrational and shear forces applied to, and present in this connective tissue make mechanical properties of such hydrogels a priority in this active area of research. Hyaluronic acid has been chemically modified in a variety of ways to address cell function while maintaining desirable tissue mechanical properties. These various modifications have had mixed results when injected in vivo typically resulting in better biomechanical function but not necessarily with a concomitant decrease in tissue fibrosis. Recent work has focused on seeding mesenchymal progenitor cells within 3D architecture of crosslinked hydrogels. The data from these studies demonstrate that this approach has a positive effect on cells in both early and late wound healing, but little work has been done regarding the biomechanical effects of these treatments. This paper provides an overview of the various hyaluronic acid derivatives, their crosslinking agents, and their effect when implanted into the vocal folds of various animal models.
Keywords: vocal fold, tissue engineering, hyaluronic acid hydrogel, progenitor cell
Introduction
Vocal folds are two strips of tissue housed in the larynx, whose vibration results in voice. Voice disorders secondary to injury to these strips are the most common communication disorder seen across the lifespan.1 Further, conservative estimates suggest that 3 to 9% of the general population has some type of voice abnormality2,3at any given moment in time, and that 29% of the general population will have a voice disorder at least once in their life.3 Vocal fold scarring, a specific vocal fold injury is accompanied by a marked decrease in voice quality and control4 secondary to pathophysiologic changes of the vocal fold lamina propria extracellular matrix (ECM). These changes directly alter vocal quality and create debilitating dysphonias due to loss of normal vibratory function.3 Fibrosis induced vis-à-vis vocal fold scarring significantly increases stiffness and viscosity of the lamina propria, contributing to glottic incompetence.5-7 Treatment outcomes for patients with vocal fold ECM injury, loss, or scarring remain largely ineffective despite substantial remediative efforts that have been taken to date. For more information on these efforts, see refs 8 and 9.
The foremost reason for the inability to adequately treat vocal fold scarring is that current surgical options disrupt ECM biomechanical tissue properties and injectable gels or implants do not mimic the complex composition of the ECM. ECM composition and organization is a central issue due to its crucial contributions to vocal fold biomechanical properties and resultant voice quality. Collagen injections, fat injections, and microflaps have all been tried in an effort to remediate scarring with diminutive success.6,8 None of these interventions have been reported to yield appropriate biomechanical properties or long-term success. Human vocal fold lamina propria has an elastic shear modulus ranging from 10 Pa to 1 kPa over a frequency range of 0.01 to 10 Hz.10 Dynamic viscosity of the same tissue ranges from 1 to 0.1 kPa-s over the same frequency range.10 Ideally, hydrogels for injection should attempt to match these ranges, a goal which inhibits the usefulness of some current materials. For example, collagen has a dynamic viscosity that is an order of magnitude or greater than normal vocal folds.11 In addition, long-term collagen injection results have been compromised due to foreign body reaction and resorption. Most importantly, these materials have been unable to regenerate lost ECM when scarred.
In recent years, tissue engineering strategies for repair of vocal fold injury such as scarring have been introduced and center on the use of injectable hydrogels and their use as delivery vehicles for stem cells. Injectable biomaterials overcome a major limitation of most scaffold materials used for tissue engineering, the need for surgical implantation. For the vocal folds, injectable hydrogels are strongly preferred for three main reasons. First, an injectable material could be formed into any desired shape at the site of injury upon injection. Second, crosslinkable polymer mixtures would adhere to the tissue during gel formation and the resulting mechanical interlocking would strengthen the tissue-hydrogel interface. Third, introduction of a crosslinkable hydrogel could be accomplished by injection, thereby minimizing the invasiveness and potential trauma of the procedure. The lamina propria of the vocal folds is only 3 mm thick, so the possibility of creating vocal scar and therefore impairing the mucosal wave is present with every microlaryngeal procedure. An injectable treatment would not increase the incidence of additional scarring and have greater applicability. The use of hydrogels to promote cell growth, differentiation, and organization is a common strategy in tissue engineering. Hydrogels, defined as hydrated polymer materials,12 are pliable, hydrophilic networks composed of synthetic or natural materials.13 Due to their pliable nature, hydrogels are commonly employed as a synthetic ECM for soft tissues, such as skin or cartilage.14,15 These characteristics also make them ideal for the vocal fold. Several important physical and chemical properties must be considered when designing or selecting appropriate materials for the vocal folds. These properties include the biomechanical properties of the hydrogel, its interactions with cells and tissues, and its ability to be easily injected through a small gauge needle.
Physical hydrogel properties are governed by repeating units of the main polymer backbone, crosslinking conditions, and processing environment.16 The most important physical properties are typically the mechanical properties of the material, including elastic and viscous moduli. Hydrogels can also be used as space filling scaffolds to fill defects and promote wound healing,17 with an emphasis on matching native tissue mechanics. Hydrogel mesh size, typically controlled by crosslinker concentration, can also play a critical role in cell fate processes. Different cell types may react to scaffold mesh size in different ways, which can affect proliferation rates, protein synthesis, ECM deposition and myofibroblast differentiation.18 Processing conditions, such as temperature and pH, also play an important role in the degree of crosslinking and gelation time. Chemical properties, governed by the interaction of cell surface receptors of resident cells with the chemical groups present on the hydrogel polymers, must also be considered for any implantable biomaterials. Downstream effects of the interaction between cell surface receptors and scaffold can influence inflammation, cell attachment, and proliferation. Scaffolds designed to avoid or even mitigate inflammation upon injection have met with some success in other parts of the body.19,20 Attachment motifs, such as the RGD peptide, are critical for adhesive cell survival and function. As such, scaffolds designed to interact with cells must have the necessary groups present.
A hydrogel scaffold designed for the human vocal fold lamina propria must take into account several unique considerations. The viscoelastic properties are especially crucial for the human vocal fold, due to the high frequency vibration required for voicing. When the viscoelastic properties of implantable biomaterials are used to treat vocal fold mucosa are greater than those of the vocal fold tissues being replaced then vocal fold oscillation and phonation becomes more difficult.11 This is particularly true when the vocal fold mucosa is directly involved in repair because the mucosa is the major vibratory portion of the vocal fold, especially in small-amplitude oscillations like phonation onset and offset. Viscoelastic shear property is one of the most important factors in the choice for optimal biomaterial for mucosal repair.11 Further, inflammation associated with injection and foreign substances in this tissue can have severe negative effects on wound healing and even life threatening airway edema. Finally, the resident cells within the lamina propria must be taken into account. The lamina propria consists primarily of vocal fold fibroblasts (VFF), which share many properties with mesenchymal stem cells (MSC),21 including differentiation potential. As such, any injectable hydrogel scaffold should be able to interact with VFF, and maintain them in their native, undifferentiated state.
Hyaluronic Acid
Hyaluronic acid (HA), a linear nonsulfated glycosaminoglycan, is a major component of the vocal fold ECM and is found in most tissues in the human body.22 Structurally, HA is a long chain composed of a disaccharide repeating unit containing D-glucuronic acid and N-acetyl-D-glucuronic acid (Fig. 1). The number of repeating units determines the molecular weight of the HA molecule, which can range from 1x105 Da to 2x106, as well as cellular interaction.23 Short chain HA fragments, typically in the 200-kDa range, elicit a response in inflammatory macrophages, causing expression in a number of inflammatory mediators.24 Long HA chains play an integral role in ECM organization and mechanical properties.25 The considerable chain length formed by the repeating structure allows HA to form an extensive hydrogen bond network with water across its entire length, resulting in a high viscosity solution.26 This not only restricts the diffusion of small molecules within the matrix, but also has a significant impact on the mechanical properties of tissue with high HA content. In the human vocal fold lamina propria, HA is the most prevalent glycosaminoglycan present, with roughly 6.4 μg of HA for each mg of total protein.27 This high concentration contributes significantly to the observed biological and mechanical properties and its subsequent effect on vibration.28 In particular, the large, loosely coiled molecular structure of HA allows it to function as a shock absorber, resisting tissue compression and cellular trauma. In this capacity, HA acts as a tissue damper that may protect the vocal fold edges from the oscillatory trauma experienced during phonation. Moreover, the osmotic, viscoelastic and space-filling properties of HA are important in voice because they directly affect the thickness and viscosity of the vocal fold.28,29 Maintenance of HA distribution, and ECM organization by local VFF is therefore important in voice production and wound healing. HA interacts with cells through various cell surface receptors, including CD44, which is present in both VFF and MSC.21,30 The major pathway for HA degradation in the vocal fold is through local metabolism by the hyaluronidase family of enzymes.22 As a result, HA injected into the vocal fold is typically rapidly degraded in as little as 3–5 d.31 The rapid degradation of HA injections makes its natural form unsuitable for tissue engineering, and necessitates chemical modifications. The most common modification is covalent bonding to the carboxylate or hydroxyl residues. The inherent properties of HA make it a promising candidate as a hydrogel platform for the delivery of progenitor cells, as well as providing a matrix for cell growth.
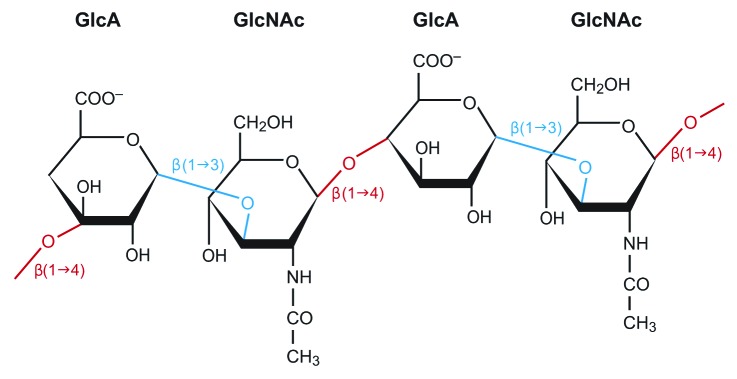
Figure 1. Hyaluronic acid chain showing repeating structure of D-glucuronic acid (GlcA) and N-acetyl-D-glucuronic acid (GlcNAc). The β linkages between residues are marked as well.
HA Hydrogels for Vocal Fold Augmentation
Several engineered HA hydrogels, ranging in complexity and purpose, have been investigated as potential scaffolds for progenitor cell delivery specifically for the vocal fold. One of the simplest hydrogels, the divinyl sulfone crosslinked HA derivative Hylan-B, has been shown to be non-antigenic, non-toxic, and non-inflammatory in animal models.32 Further modifications can be performed to add photo-polymerizable groups to Hylan-B, thereby creating a simple method to adjust the swelling ratio and degradation rate.33 To date, Hylan-B and its derivatives have only been used as a space filling hydrogel injection, not as delivery vehicles for progenitor cells to the vocal fold. Human use with Hylan B, or Hylaform (Allergan, Inc.), in the vocal fold lamina propria has not been reported in the literature and is no longer being marketed in the United State for clinical use. Recently, a new process utilizing a latent crosslinking agent has produced thiol-modified HA with considerable advantages, including tunability. Under appropriate conditions, HA can be reacted with thiol cross-linker 3,3′-dithiobis (DTP), to produce HA-DTPH (Fig. 2).34 The DTP crosslinker, a non-cytotoxic agent, allows for hydrogel formation through the formation of disulfide bonds at room temperature and reduced pH. Due to the disulfide bond nature of the cross-linked network, a simple reducing agent such as dithiothreitol can be used to dissolve the gels. The addition use of this crosslinking agent, as well as the mild conditions necessary for reaction to occur makes HA-DTPH capable of cell encapsulation. Indeed, murine fibroblasts seeded within the cross-linked gel not only showed 95% viability after 96 h of culture, but proliferated as well.34 The clinical use of HA-DTPH for cell encapsulation is limited however, with gels taking up to 120 min to form. This period of time is not ideal for clinicians or during a surgical operation, curbing its clinical usefulness. The use of disulfide bonds as crosslinkers readily lends itself to adding in functional groups capable of significantly altering hydrogel properties, such as gelation time. The ability to rapidly and easily alter hydrogel properties allows researchers, and eventually clinicians, to tailor HA hydrogels to specific applications as they are needed.
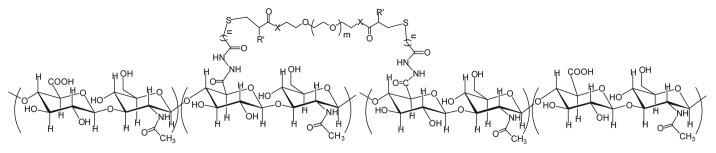
Figure 2. Hyaluronic acid backbone with attached DTP crosslinker. The 2-carboxylic acid group on D-glucuronic acid (GlcA) serves as the site for covalent attachment of the DTP crosslinker.
In order to promote faster gelation times, polyethylene glycol diacrylate (PEGDA) was incorporated into HA-DTPH hydrogels. By varying the ratio of PEGDA to HA-DTPH, gelation time can be decreased to as low as five minutes.35 As the ratio of HA-DTPH to PEGDA was increased, crosslinking density decreased and swelling ratio increased, with the 1:1 ratio having the highest crosslink density and the lowest swelling ratio.35 Thus, the mesh size, gelling time, and swelling ratio can all easily be altered simply by varying the ratio, offering a significant degree of control over the resultant hydrogel. Crosslinked gels can be naturally degraded by hyaluronidases in a manner consistent with non-crosslinked HA. This is a significant finding, as it allows cells to interact with and remodel the crosslinked HA using normal pathways and molecules. Like HA-DTPH, it is possible to encapsulate cells in the hydrogel by adding them to the solution before adding the crosslinker, in this case PEGDA. Using this method, human tracheal scar fibroblasts were shown to be viable for 28 d in vitro, increasing their number by nearly 10-fold. Similarly, cell-seeded hydrogels implanted subcutaneously in nude mice were shown to not induce necrosis or damage surrounding tissue, as well as maintain cellular phenotype.35 Recently, HA-DTPH has been slightly modified36 to include additional carboxylate groups on the HA backbone, effectively altering the viscosity and reducing the rate of degradation.37 This new HA derivative has been named CMHA-S or Carbylan-S, and will be referred to as CMHA-S from this point forward. The extra carboxylate groups on the HA backbone also allow further ease of chemical modification and crosslinking possibilities.38 For example, the crosslinking of CMHA-S to thiolated gelatin (Gtn-DTPH), resulting in a material designated Extracel® (a.k.a., HyStem-C®) allows for cell interaction through natural attachment motifs present on the denatured collagen present in the gelatin.39
Mechanical Effects of HA Gel Injection
Injection of HA gels, as well as their crosslinked derivatives, has shown to be a successful method for restoring normal vocal fold mechanical properties in vivo (Table 1). In vivo vocal fold studies with gel injection have been performed primarily in rabbits, due to their similarity of vocal fold tissue to human vocal fold and ease of access to the larynx intraorally.40 Early studies with Hylan-B show that injections into rabbit vocal fold lamina propria have mechanical results similar to normal vocal fold. Normal vocal folds from animals sacrificed 6 mo after injection have a dynamic viscosity lower than other injectable biomaterials such as collagen or Teflon32 and similar to non-injected vocal fold tissue, demonstrating efficacy.40
Table 1. HA hydrogel injections and their mechanical and fibrotic effects.
| HA derivative | Type of crosslinking | Animal model and vocal fold condition | Seeded cell type | Biomechanical properties, compared with saline injection | Fibrotic effects compared with saline controls |
|---|---|---|---|---|---|
|
HA-DTPH-PEGDA |
PEGDA |
Injured rabbit29 |
None |
-No change in G’ -Lower G” |
-Moderate fibrosis, no difference -No difference for Col -No difference for HA |
|
Carbylan-S® |
PEGDA |
Injured rabbit29 |
None |
-Lower G’ -Lower G” |
-Mild fibrosis, significant -difference -No difference for Col -No difference for HA |
|
Extracel® |
Thiolated gelatin (Gtn-DTPH) |
Scarred rabbit34 |
None |
-Lower G’ -Lower G” |
-Increased Col -Increased FN -Increased Procollagen |
|
Extracel® |
Thiolated gelatin (Gtn-DTPH) |
Scarred rabbit34 |
Autologous VFF |
-Lower G’ -Lower G” |
-Increased Col -Increased FN |
|
Extracel® |
Thiolated gelatin (Gtn-DTPH) |
Scarred rat35 |
Mouse bone marrow MSC |
-No data available |
-Increased Col-III -Increased FN -Increased TGF-β1 |
| Collagen-HA cogel | Physical entanglement | Injured rabbit38 | Rabbit adipose MSC | -No data available | -Increased Col for 3 mo, then normal -Increased HA for 3 mo, then normal |
G’, elastic modulus; G”, viscous modulus; Col, collagen; FN, fibronectin; MSC, mesenchymal stem cell; VFF, vocal fold fibroblasts.
In vitro rheology results on CMHA-S and HA-DTPH-PEGDA detailing the elastic shear viscous moduli of both materials indicated that the CMHA-S is the stiffer of the two materials, yet still within normal range to that of human vocal fold lamina propria. Excised tissue from injured rabbit vocal fold injected with CMHA-S had a lower elastic shear modulus than both the injured tissue treated with saline controls and injured tissue injected with HA-DTPH-PEGDA.37 A significant difference was also observed in the viscous modulus; both CMHA-S and HA-DTPH-PEGDA were significantly less viscous than the saline-treated samples. Overall, both gels were biomechanically compatible to human vocal fold mucosa, with the possibility of CMHA-S providing a better environment for subsequent biomechanical outcomes as determined by histological outcomes, as discussed below.37 Similar results were reported at six months in a rabbit model injected with Extracel®.31 A lower elastic shear modulus and lower viscous modulus compared with saline controls was reported, a feature indicative of a decrease in overall fibrosis in the injected tissue.31 This in vivo data indicates that these injected HA hydrogels have positive effect on the mechanical properties of the vocal fold following vocal fold injury. It has been well documented that HA hydrogels can be formulated to have viscoelastic properties that match the vocal fold lamina propria in vitro. It is also well documented that in vitro viscoelastic properties of HA gels correlates well observed in vivo viscoelastic properties upon injection in animal models.32
Fibrotic Effects of HA Gel on VFF and MSC
Injection of HA hydrogels without cells has been shown to have a positive effect on resident VFF response to wound healing. One of the earliest such methods employed CMHA-S and a HA-DTPH-PEGDA hydrogel, effectively comparing the effects of each on cell survival in an in vivo environment.37 Trichrome staining performed on sections of the excised larynges showed that the average fibrosis levels for animals injected with the HA-DTPH-PEGDA were moderate and not significantly different from saline-treated controls (Fig. 3). Average fibrosis levels for the CMHA-S treated group displayed only mild fibrosis, with a significant difference compared with controls, indicating less tissue fibrosis. It should also be noted that an ELISA assay determined that HA levels in the hydrogel treated vocal fold samples were the same as those in the saline controls, indicating that the injected gels had degraded. In an in vitro investigation to study the effects of various engineered HA hydrogels on progenitor cell fate, MSCs isolated from abdominal fat were encapsulated in several hydrogels, including Restylane® (crosslinked HA), and a cogel of Restylane® and fibrin.41 Cell morphology and proliferation in these gels showed increased elongation and DNA content in the fibrin-HA cogel, compared with the HA gel. An increase in elastin expression was also observed in the cogel samples, but decorin levels remained similar in both gel types. Finally, CD44 significantly decreased in the cogel sample, indicating a potential deregulation of support for the stem cell maintenance of the undifferentiated state. This is supported by lower expression levels of CD105, a cell surface marker highly expressed in undifferentiated MSCs. These results indicate that fibrin-HA cogels may be useful as an MSC delivery system while also increasing cell proliferation and elastogenesis. The lower expression levels for CD44 and CD105 may indicate that it is possible to affect MSC differentiation within the cogel as well.
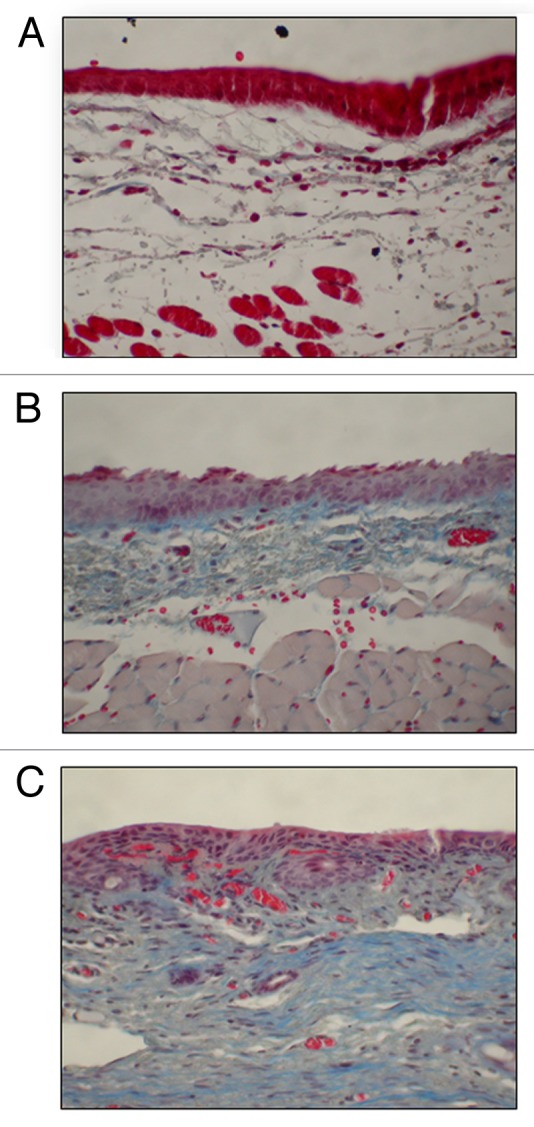
Figure 3. Representative 40x coronal sections of the vocal fold treated with a trichrome stain. Statistical significance established by blinded pathologist qualitatively categorizing the fibrosis level for each section. (A) CMHA-S treated vocal folds showing mild fibrosis. Visual inspection indicates a significant decrease in fibrosis between the CMHA-S treatment group and saline-treated controls (p = 0.0158). (B) HA-DTPH-PEGDA treated vocal folds showing moderate fibrosis. No statistical difference between fibrosis levels seen in saline-treated controls (p = 0.1645). (C) Saline treated controls showing moderate fibrosis.
Fibrotic Effects of Cell-Seeded HA Gel Injection
The first method to employ both a hyaluronic acid hydrogel and progenitor cells in vivo showed the potential of a cell-seeded scaffold to alter tissue viscoelastic properties to a comparable degree as hydrogel alone. Scarred rabbit vocal fold tissue was injected with one of four groups: saline, autologous VFF, Extracel®, or Extracel® with autologous VFF.42 Extracel® injected vocal fold tissue demonstrated increased collagen and fibronectin deposition compared with saline controls, but no increase in procollagen staining was observed. The tissue injected with seeded Extracel® had decreased elastic shear modulus and viscous modulus compared with untreated vocal folds, but lacked any significant difference when compared with any of the other treatment groups. Both viscous and elastic shear moduli for the autologous VFF group was significantly decreased compared with the moduli measured in saline controls and the other treatment groups. Decreased moduli are typically related to an improved mucosal wave and decrease in tissue fibrosis. Overall, the results indicate that injection of autologous VFF alone represents the best option for improved viscoelastic properties of vocal fold scar. The results demonstrating that VFF seeded Extracel® injections improve biomechanical properties are important, as wound healing strategies employing cell-matrix injections are viable without a loss of viscoelasticity.
The benefit of cell seeded Extracel® matrices, with respect to wound healing, has also been demonstrated in a rat model. In a fashion similar to the 2008 study, Johnson et al. seeded bone marrow derived mouse MSCs in an Extracel® matrix and injected it in a scarred rat animal model.43 This effect of the seeded Extracel® was compared with injections containing saline, stem cells alone, or Extracel® alone, with respect to gene expression and apoptosis. The cell-seeded matrix showed a significantly higher level of collagen III, fibronectin, and TGF-β1 over any of the other treatment groups. The increased expression of collagen and fibronectin, both fibrous proteins with extensive cell adhesion motifs, indicate the early establishment of a lattice for wound healing events. The concomitant upregulation of TGF-β1 further supports the establishment of an early lattice, as TGF-β1 plays a significant role as a promoter of ECM protein expression.44 Importantly, injection of the cell seeded scaffolds did not cause a rise in myofibroblasts within the lamina propria, as was evidenced by a lack of increase in smooth muscle actin expression and staining over saline controls. Increased myofibroblast levels and persistence of these levels can lead to the onset of hypertrophic scar formation.45 These investigations suggests that cell-seeded Extracel® promotes early wound healing by increasing the production ECM proteins necessary for early wound healing while simultaneously decreasing the likelihood of scar formation.
Most recently, adipose-derived MSCs (AdMSC) were injected into injured rabbit vocal folds with a collagen-hyaluronic acid composite hydrogel, with histological effects being investigated after 15 d, 40 d, 3 mo, 6 mo and 1 y after injection.46 This study differs from the other cell-seeded gel studies in several important ways, including gel crosslinking and time points. The collagen-hyaluronic acid gel utilized in this investigation was not chemically crosslinked in any way, but simply consists of collagen and hyaluronic acid gels mixed together. Further, gels seeded with AdMSC were cultured in vitro for one week with an air-liquid interface, indicative of promoting epithelial cell differentiation and stratification. Results demonstrated that collagen content in the vocal folds injected with cell seeded scaffolds increased until the 3 mo time point, where it peaked. At 12 mo, collagen content and distribution were close to that of normal controls. Hyaluronic acid content following injection followed a similar trend, with levels peaking at day 40, then declining and finally stabilizing at 12 mo. After injection of the cell-seeded HA-collagen composite, the fibronectin content was highest at 40 d, and then decreased until stabilizing around 12 mo. Hematoxylin and eosin staining also revealed physiologic differences between AdMSC-seeded HA-collagen, adipose-derived MSC implantation, and untreated (but still injured) vocal fold tissue. At 15 d, inflammatory cell migration and infiltration were present in all treatment groups. At 6 mo, untreated controls had large amounts of fibrosis and disorganized lamina propria ECM was observed. Tissue implanted with adispose-derived MSC showed a gradual decrease in fibrous tissue starting at 6 mo to nearly normal levels at 12 mo, with some irregular distribution. Finally, the cell-seeded HA-collagen composite gel showed the most improvement, with normal levels of fibrous tissue at 6 mo and normal organization at 12 mo. This study lends further proof of concept to the theory that progenitor-seeded HA supports in early wound healing, while not inducing fibrosis and scar formation. Despite the results demonstrated in this study, little was done to characterize the gel utilized itself. No data was reported on the hydrogel residence time in vivo nor the rheological properties of the gel in vitro or in vivo. Further an uncrosslinked gel is likely to be degraded quickly. Given the positive histological results reported, further investigation into this material is warranted.
Extracel®, Inflammation and Macrophage Phenotypes
Taking a step back to better understand why cell seeded Extracel is beneficial in vivo, a recent in vitro study has also shown this combination may have an impact on key players in inflammation. VFF cultured on the 2D surface of Extracel® hydrogels had a higher expression of IL-8 and TNF-α, two pro-inflammatory cytokines, compared with polystyrene controls. VFF seeded within 3D Extracel constructs have also been shown to increase IL-8 and TNF- α mRNA levels compared with polystyrene. It should be noted that increased expression of COX-2 and IL-6, two pro-inflammatory cytokines, was not observed in either condition.47 A similar study investigating the effect of macrophages grown on Extracel® or VFF-seeded Extracel® found differences between macrophage inflammatory phenotype. Macrophages grown on the cell-seeded Extracel® showed decreased CD116 and increased HLA-DR, indicative of an anti-inflammatory phenotype.48 Translation of these findings to in vivo work may have a significant effect on clinical vocal fold injections via modulation of the macrophage phenotype. Recent in vitro work found that CMHA-S could enhance the role TNF-α in remodeling the lamina propria layer via significantly downregulating TIMP3 and extracellular matrix-related mRNA transcript levels for collagen III and fibronectin and upregulation MMP1 and MMP2 expression, resulting in increased MMP/TIMP3 ratios.49 Taken together, the anti-inflammatory properties of cell-seeded Extracel® hydrogels show translational potential for clinical use.
Conclusions
Hyaluronic hydrogels has been investigated for vocal fold regeneration and wound healing since the early 2000s. They can be easily chemically modified to provide the necessary viscoelastic properties that match the vocal fold lamina propria which a paramount consideration for this tissue type. Early in vitro and in vivo animals studies have demonstrated that most HA hydrogels alone improve wound healing in injured and scarred models. More recently the delivery of mesenchymal stem cells to the vocal fold using a hyaluronic acid hydrogel augments and amplifies improved wound healing and minimizing scarring. Unique in vitro investigations have demonstrated benefits of these hydrogels in terms of inflammatory effects on both resident VFF and recruited macrophages. The in vitro and in vivo studies reported herein provide the necessary data to move forward with FDA approval for human clinical trials with hyaluronan hydrogels injections in isolation and with cell therapy.
Acknowledgments
The authors would like to acknowledge funding from NIH NIDCD R01 4336 and T32 DC009401.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/23799
References
- 1.Verdolini K, Ramig LO. Review: occupational risks for voice problems. Logoped Phoniatr Vocol. 2001;26:37–46. doi: 10.1080/140154301300109125. [DOI] [PubMed] [Google Scholar]
- 2.Roy N, Merrill RM, Thibeault S, Parsa RA, Gray SD, Smith EM. Prevalence of voice disorders in teachers and the general population. J Speech Lang Hear Res. 2004;47:281–93. doi: 10.1044/1092-4388(2004/023). [DOI] [PubMed] [Google Scholar]
- 3.Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002;16:96–104. doi: 10.1016/S0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 4.Smith E, Verdolini K, Gray SD, Nichols S, Lemke J. Effect of voice disorders on quality of life. J Med Speech-Lang Pathol. 1996;4:223–44. [Google Scholar]
- 5.Ford CN. Advances and refinements in phonosurgery. Laryngoscope. 1999;109:1891–900. doi: 10.1097/00005537-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Ford C. Collagen injection in the scarred vocal fold. J Voice. 1987;1:116–8. doi: 10.1016/S0892-1997(87)80034-6. [DOI] [Google Scholar]
- 7.Ford CN, Bless DM. Phonosurgery: Assessment and surgical management of voice disorders New York: Raven Press; 1991. [Google Scholar]
- 8.Benninger MS, Alessi D, Archer S, Bastian R, Ford C, Koufman J, et al. Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg. 1996;115:474–82. doi: 10.1016/S0194-5998(96)70087-6. [DOI] [PubMed] [Google Scholar]
- 9.Hansen JK, Thibeault SL. Current understanding and review of the literature: vocal fold scarring. J Voice. 2006;20:110–20. doi: 10.1016/j.jvoice.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Chan RW, Titze IR. Viscoelastic shear properties of human vocal fold mucosa: measurement methodology and empirical results. J Acoust Soc Am. 1999;106:2008–21. doi: 10.1121/1.427947. [DOI] [PubMed] [Google Scholar]
- 11.Rosen CA, Thibeault SL, Klemuk S, Smith ME. In reference to Viscoelasticity of hyaluronan and nonhyaluronan based vocal fold injectables: implications for mucosal versus muscle use. Laryngoscope. 2007;117:1506–8, author reply 1506-8. doi: 10.1097/MLG.0b013e31806842ec. [DOI] [PubMed] [Google Scholar]
- 12.Ratner BD. Biomaterials science: an introduction to materials in medicine. Amsterdam; Boston: Elsevier Academic Press, 2004. [Google Scholar]
- 13.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–79. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 14.Balakrishnan B, Mohanty M, Umashankar PR, Jayakrishnan A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials. 2005;26:6335–42. doi: 10.1016/j.biomaterials.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Suh JKF, Matthew HWT. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589–98. doi: 10.1016/S0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 16.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–51. doi: 10.1016/S0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 17.Hoemann CD, Sun J, Légaré A, McKee MD, Buschmann MD. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthritis Cartilage. 2005;13:318–29. doi: 10.1016/j.joca.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Liao HM, Munoz-Pinto D, Qu X, Hou Y, Grunlan MA, Hahn MS. Influence of hydrogel mechanical properties and mesh size on vocal fold fibroblast extracellular matrix production and phenotype. Acta Biomater. 2008;4:1161–71. doi: 10.1016/j.actbio.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27:2370–9. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Fraser IP, Yeo Y, Highley CB, Bellas E, Kohane DS. Anti-inflammatory function of an in situ cross-linkable conjugate hydrogel of hyaluronic acid and dexamethasone. Biomaterials. 2007;28:1778–86. doi: 10.1016/j.biomaterials.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Hanson SE, Kim J, Johnson BHQ, Bradley B, Breunig MJ, Hematti P, et al. Characterization of mesenchymal stem cells from human vocal fold fibroblasts. Laryngoscope. 2010;120:546–51. doi: 10.1002/lary.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraser JRE, Laurent TC, Laurent UBG. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 23.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–61. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 24.Hodge-Dufour J, Noble PW, Horton MR, Bao C, Wysoka M, Burdick MD, et al. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J Immunol. 1997;159:2492–500. [PubMed] [Google Scholar]
- 25.Chen WYJ, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79–89. doi: 10.1046/j.1524-475X.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 26.Lapcík L, De Smedt S, Demeester J, Chabrecek P, Lapcík L Jr and Hyaluronan: Preparation, structure, properties, and applications. Chem Rev. 1998;98:2663–84. doi: 10.1021/cr941199z. [DOI] [PubMed] [Google Scholar]
- 27.Hahn MS, Jao CY, Faquin W, Grande-Allen KJ. Glycosaminoglycan composition of the vocal fold lamina propria in relation to function. Ann Otol Rhinol Laryngol. 2008;117:371–81. doi: 10.1177/000348940811700508. [DOI] [PubMed] [Google Scholar]
- 28.Chan RW, Gray SD, Titze IR. The importance of hyaluronic acid in vocal fold biomechanics. Otolaryngol Head Neck Surg. 2001;124:607–14. doi: 10.1067/mhn.2001.115906. [DOI] [PubMed] [Google Scholar]
- 29.Butler JE, Hammond TH, Gray SD. Gender-related differences of hyaluronic acid distribution in the human vocal fold. Laryngoscope. 2001;111:907–11. doi: 10.1097/00005537-200105000-00029. [DOI] [PubMed] [Google Scholar]
- 30.Entwistle J, Hall CL, Turley EA. HA receptors: regulators of signalling to the cytoskeleton. J Cell Biochem. 1996;61:569–77. doi: 10.1002/(SICI)1097-4644(19960616)61:4<569::AID-JCB10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 31.Thibeault SL, Klemuk SA, Chen X, Quinchia Johnson BH. In Vivo engineering of the vocal fold ECM with injectable HA hydrogels-late effects on tissue repair and biomechanics in a rabbit model. J Voice. 2011;25:249–53. doi: 10.1016/j.jvoice.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahlqvist A, Gärskog O, Laurent C, Hertegård S, Ambrosio L, Borzacchiello A. Viscoelasticity of rabbit vocal folds after injection augmentation. Laryngoscope. 2004;114:138–42. doi: 10.1097/00005537-200401000-00025. [DOI] [PubMed] [Google Scholar]
- 33.Jia XQ, Burdick JA, Kobler J, Clifton RJ, Rosowski JJ, Zeitels SM, et al. Synthesis and characterization of in situ cross-linkable hyaluronic acid-based hydrogels with potential application for vocal fold regeneration. Macromolecules. 2004;37:3239–48. doi: 10.1021/ma035970w. [DOI] [Google Scholar]
- 34.Shu XZ, Liu YC, Luo Y, Roberts MC, Prestwich GD. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3:1304–11. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Shu X, Liu YC, Palumbo FS, Luo Y, Prestwich GD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25:1339–48. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Shu XZ, Liu Y, Prestwich GD. Modified macromolecules and methods of making and using thereof. 2011 [Google Scholar]
- 37.Hansen JK, Thibeault SL, Walsh JF, Shu XZ, Prestwich GD. In vivo engineering of the vocal fold extracellular matrix with injectable hyaluronic acid hydrogels: early effects on tissue repair and biomechanics in a rabbit model. Ann Otol Rhinol Laryngol. 2005;114:662–70. doi: 10.1177/000348940511400902. [DOI] [PubMed] [Google Scholar]
- 38.Serban MA, Prestwich GD. Modular extracellular matrices: solutions for the puzzle. Methods. 2008;45:93–8. doi: 10.1016/j.ymeth.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duflo S, Thibeault SL, Li WH, Shu XZ, Prestwich GD. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006;12:2171–80. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 40.Borzacchiello A, Mayol L, Gärskog O, Dahlqvist A, Ambrosio L. Evaluation of injection augmentation treatment of hyaluronic acid based materials on rabbit vocal folds viscoelasticity. J Mater Sci Mater Med. 2005;16:553–7. doi: 10.1007/s10856-005-0531-2. [DOI] [PubMed] [Google Scholar]
- 41.Park H, Karajanagi S, Wolak K, Aanestad J, Daheron L, Kobler JB, et al. Three-dimensional hydrogel model using adipose-derived stem cells for vocal fold augmentation. Tissue Eng Part A. 2010;16:535–43. doi: 10.1089/ten.tea.2009.0029. [DOI] [PubMed] [Google Scholar]
- 42.Thibeault SL, Klemuk SA, Smith ME, Leugers C, Prestwich G. In vivo comparison of biomimetic approaches for tissue regeneration of the scarred vocal fold. Tissue Eng Part A. 2009;15:1481–7. doi: 10.1089/ten.tea.2008.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson BQ, Fox R, Chen X, Thibeault S. Tissue regeneration of the vocal fold using bone marrow mesenchymal stem cells and synthetic extracellular matrix injections in rats. Laryngoscope. 2010;120:537–45. doi: 10.1002/lary.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duflo S, Thibeault SL, Li WH, Shu XZ, Prestwich G. Effect of a synthetic extracellular matrix on vocal fold lamina propria gene expression in early wound healing. Tissue Eng. 2006;12:3201–7. doi: 10.1089/ten.2006.12.3201. [DOI] [PubMed] [Google Scholar]
- 45.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 46.Xu W, Hu R, Fan EZ, Han DM. Adipose-derived mesenchymal stem cells in collagen-hyaluronic acid gel composite scaffolds for vocal fold regeneration. Ann Otol Rhinol Laryngol. 2011;120:123–30. doi: 10.1177/000348941112000209. [DOI] [PubMed] [Google Scholar]
- 47.Chen X, Thibeault SL. Biocompatibility of a synthetic extracellular matrix on immortalized vocal fold fibroblasts in 3-D culture. Acta Biomater. 2010;6:2940–8. doi: 10.1016/j.actbio.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanson SE, King SN, Kim J, Chen X, Thibeault SL, Hematti P. The effect of mesenchymal stromal cell-hyaluronic acid hydrogel constructs on immunophenotype of macrophages. Tissue Eng Part A. 2011;17:2463–71. doi: 10.1089/ten.tea.2010.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, Thibeault SL. Role of tumor necrosis factor-alpha in wound repair in human vocal fold fibroblasts. Laryngoscope. 2010;120:1819–25. doi: 10.1002/lary.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]


