Abstract
Focal stroke is a disabling disease with lifelong sensory, motor and cognitive impairments. Given the paucity of effective clinical treatments, basic scientists are developing novel options for protection of the affected brain and regeneration of lost tissue. Tissue bioengineering and stem/progenitor cell treatments have both been individually pursued for stroke neural repair therapies, with some benefit in tissue recovery. Emerging directions in stroke neural repair approaches combine these two therapies to use biopolymers with stem/progenitor transplants to promote greater cell survival in the transplant and directed delivery of bioactive molecules to the transplanted cells and the adjacent injured tissue. In this review the background literature on a combined use of neural stem/progenitor cells encapsulated in hyaluronan gels is discussed and the way this therapeutic approach can affect the important processes involved in brain tissue reconstruction, such as angiogenesis, axon regeneration, neural differentiation and inflammation is clarified. The glycosaminoglycan hyaluronan can optimize those processes and be employed in a successful neural tissue engineering approach.
Keywords: stroke, neural repair, regeneration, hyaluronan, brain
Introduction: Focal Ischemic Stroke
Acute ischemic stroke is one of the main causes of mortality and disability. It annually affects 93 new and recurrent cases per 100,000 population in the US, which imposes a direct economic cost of over $73 billion.1 There is only one approved medical therapy for stroke: tPA is a “clot busting” agent that acts to reperfuse damaged brain, and must be given within 4.5 h of the stroke onset. However, the affected patients are left with life-long motor, sensory and cognitive disabilities,2 which indicates that despite reperfusion associated with opening of an occluded blood vessel there exists non-salvaged and damaged brain tissues. Therefore neural repair strategies are required to improve neurological dysfunction.
The most common stroke subtype is non-hemorrhagic stroke, caused by a sudden arterial occlusion that disrupts blood flow to brain tissue3 and leads to tissue necrosis in the center of the territory of occluded artery, known as the infarct core.3 Brain areas that are adjacent to the stroke and connected to the region that will become the infarct core survive the stroke. Evidence from human and animal studies indicates that plasticity in the regions of the brain adjacent to and connected with the stroke exhibit the most recovery of function.4-6 Mechanisms of recovery in these areas include neurogenesis, in which newly born immature neurons migrate into the peri-infarct cortex in large numbers and may provide a direct trophic support or cell replacement in this tissue. Also, axons in peri-infarct cortex sprout new connections by activating a specific “sprouting transcriptome.” Recovery is also seen in the remapping of motor and sensory maps in peri-infarct cortex.4-6
However, this recovery is limited, making stroke the leading cause of adult disability. Neuronal circuits in peri-infarct cortex are stunned or dysfunctional, exhibiting increased inhibition7 and altered responses to excitatory signals.8 Experimental studies have shown that the self-repair processes of axonal sprouting and neurogenesis could be enhanced by specific treatment directed at tissue reorganization, as opposed to neuroprotective strategies which maximize the extent of spared neurological functions by protecting the brain tissue against active cell death mechanisms.9 Because neural repair processes occur over weeks and months after the stroke they may be amenable to more delayed treatments that could be given to a wider array of patients than acute neuroprotective therapies.10
Ischemia, among many changes, induces the proliferation and migration of endogenous neural stem and progenitor cells (NSPCs) in the brain.5,11 These play a neuroprotective role by modulating intrinsic inflammatory responses to injury.12,13 Transplanted stem/progenitor cells may enhance these endogenous processes after stroke such as axonal sprouting, neurogenesis and angiogenesis.13,14 Stem/progenitor cells can also directly integrate into damaged neural networks by differentiating into neurons and glial cells,15,16 although this does not appear to be a substantial element in the tissue repair after NSPC transplantation.17 Stem cell transplantation after stroke can both enhance endogenous repair processes and potentially create new neuronal circuits or brain architecture in partially damaged areas next to the stroke core. Therefore, stem cell transplantation is a therapeutic option for post-stroke neural damage.
However, there are pre-clinical and clinical challenges that limit the application of neural stem/progenitor cells in stroke. Peri-infarct tissue is the area where the most significant post-stroke regeneration occurs5 and therefore transplanted cells need to be delivered as close as possible to that area for a maximum effect on the recovering circuits after stroke. However, direct injection of stem/progenitor cells into the peri-infarct cortex carries the theoretical risk of damaging the very tissue that recovers after stroke.18 The infarct cavity is a compartmentalized area of dead, loose tissue that provides a potential space for cell transplantation and it is located adjacent to the peri-infarct zone.19 However, cells injected into this cavity encounter inflammation, lack of blood supply and pro-apoptotic factors and subsequently die.20 The necrotic core of infarcted tissue does not provide transplanted cells with a viable matrix and required growth factors to help them regenerate and reorganize the damaged tissue. As infarcted or damaged tissue is a target for neural repair in stroke and other CNS degenerative diseases the limited vascular and trophic support and increased inflammation in this target region can be an explanation as to why clinical stem cell therapy had limited success or variable outcome in patients suffering from disorders such as stroke and Parkinson.21
A potential solution to stem cell targeting in stroke is the encapsulation of neural stem/precursor cells in a bio-compatible matrix to enhance their survival and/or differentiation in stroke. Such a matrix might provide signals through the structure of the hydrogel backbone, the incorporation of growth factors or the inclusion of protein motifs that provide pro-survival, pro-growth or pro-differentiation effects. There have been several studies applying biopolymers as carrying scaffolds for neural stem/progenitor cells transplanted to brain focal ischemic lesions (Table 1). In this review, we will focus on the use of Hyaluronan (HA) and discuss options for a bio-compatible hydrogel matrix with a potential to enhance the outcomes of neural cell transplantation for brain ischemic lesions.
Table 1. Application of biopolymers in neural stem/progenitor cell therapy of focal brain ischemic lesion.
| Article | Stroke model/ species |
Cells transplanted /density per animal |
Hydrogel | Outcomes |
|---|---|---|---|---|
| Bible et al. 2009 Biomaterials72 |
MCAO/rat |
NSC/ 3.15 × 105 cells in 30 µl |
Fibronectin-coated PLGA particles |
Descriptive analyses of cell survival, cell-scaffold-tissue integration, cell differentiation, angiogenesis and host inflammatory response |
| Jin et al. 2010 J Cereb Blood Flow Metab.73 |
MCAO/rat |
hES-NPC/ 6 × 106 cells in 50 µl |
Matrigel |
Reduction in lesion volume, improving cell survival, differentiation and behavioral indices |
| Yu et al., 2010 Anat Rec (Hoboken)74 |
MCAO/rat |
NSC/ 1.5 × 104 in 5 µl |
Collagen |
Cell survival, NSC synapse formation and neurological severity score improved |
| Zhong et al. 2010 Neurorehabil Neural Repair14 |
PT/mice |
ES-NPC / 105 cells in 7 µl |
Hyaluronan /Heparin /Collagen |
Improving transplant cell survival and host inflammatory response, angiogenesis and astrocytic reactivity |
| Matsuse et al. 2011 Tissue Eng Part A75 |
MCAO/rat |
MS-NSC/ 2 × 104 cells, 12 µl in striatum and 8 µl in cortex |
Collagen with bFGF in gelatin microspheres |
Infarct volume, cell survival and distribution, angiogenesis, number of host NSCs and motor behavior improved |
| Bible et al. 2012 Biomaterials76 | MCAO/rat | hNSC /2.1–2.5 × 106 cells in 25–40 µl |
Acellular ECM | Descriptive analyses of cell imaging by MRI, cell migration, differentiation and cell-host tissue interaction |
ES, embryonic stem; h, human; MCAO, middle cerebral artery occlusion; MS, mesenchymal stem; NPC, neural precursor cell; NSC, neural stem cell; PLGA, poly(lactic-co-glycolic acid; PT, photothrombotic.
Tissue engineering is a multi-faceted approach, as there are several pathologies in damaged tissues that should be corrected. The main deficit in stroke is due to the lost or damaged neuronal circuits. In order to repair injured circuits and possibly restore neurons glial support is needed, as well as a vasculature to supply oxygen and nutrients. Inflammation with both its destructive and constructive roles actively contributes to the brain tissue response to the implanted hydrogel. An efficacious hydrogel design, therefore, should take these into consideration and analyze interactions of the hydrogel elements with the injured brain tissue. We explore in this review the interactions of HA with the processes involved in inflammation, foreign body response, angiogenesis, NPC differentiation and axon growth.
Hyaluronan
HA is a glycosaminoglycan composed of repeated disacharid D-glucuronic acid and N-acetyl-D-glucosamine. It is produced by a membrane-bound group of enzymes named hyaluronan synthases, which organize D-glucuronic acid and N-acetyl-D-glucosamine disacharids into a non-branched linear polymer that can range between 1 MDa to 4 MDa in size; this is what generally known as high molecular weight (HMW) HA. HMW HA is very flexible and can readily form into coils, nets or fibers22 (see an electron micrograph in Fig. 1). HMW HA is strongly negatively charged and therefore absorbs up to 10–10,000 times its weight water.23 It is generally a bio-inert molecule that acts to maintain a hydrated and porous environment, absorbs mechanical shock and regulates osmotic balance. HMW HA can also sequester and gradually release growth factors and other bioactive molecules to communicate a local biological influence over cells. HMW HA is specifically interesting from a tissue engineering standpoint as it can diminish the interaction of encapsulated cells with other cells and growth factors and therefore “conceal” them from local harmful signals; this is mainly done by modulating inflammatory response of macrophages.24 HMW HA can be degraded by enzymes, hyaluronidases, into oligomers that, unlike high molecular HA, are highly bioactive (see below). HA degradation is facilitated in inflammation and injury by the production of reactive oxygen and nitrogen species.25
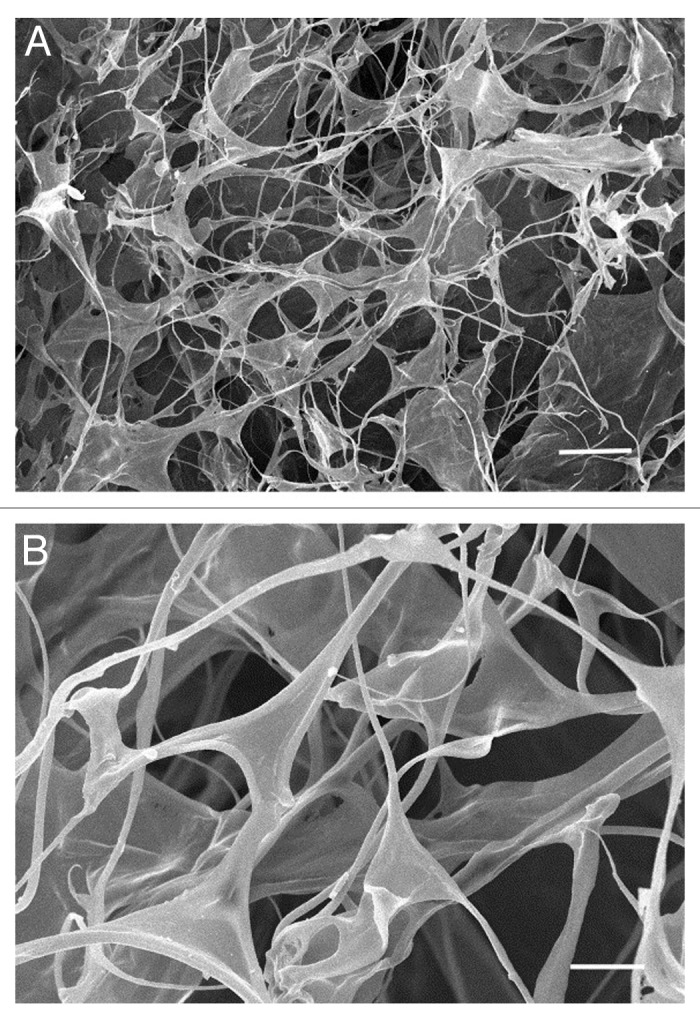
Figure 1. Scanning electron micrograph of HA hydrogel at (A) lower or (B) higher magnification. Reproduced with permission from ref. 77. Scale bar = (A) 50 µm and (B) 20 µm.
HA is a reasonable choice to encapsulate cells for transplantation into the brain because it is naturally and abundantly found in the brain.26 HA has a role in the brain more than just space-filling, hydration and matrix provision. HA influences cell adhesion, migration, axon path-finding, brain regional specificity and therefore, it is actively involved in normal development of the brain.26,27 HA is increasingly deposited in the aged brain and diminishes oligodendrocyte precursor (OPC) maturation.28 HA in the demyelinated plaques of multiple sclerosis prohibits OPC maturation and myelin repair.31 HA is a component of the perineuronal net that modulates mature cerebral neurons.29 HA is also involved in brain pathologies and diseases. HA promotes malignant glial cell adhesion, migration and metastasis in the brain.28 It also contributes to mossy fiber sprouting in the hippocampus that will eventually lead to temporal lobe epilepsy.27 HA is involved in immunomodulation, tissue injury and repair in the brain through the innate immune receptors toll-like receptors 2 and 4 (TLR2 and TLR4), signaling through the main inflammatory transcription factor NFκB, and tumor necrosis factor α secretion.26,30 These phenomena are mediated through HA receptors, mainly CD44, RHAMM and TLR4. Hence, HA affects a variety of physiologic and pathologic functions, which makes its application intriguing and challenging.
HA gives different biological signals depending on the molecular weight (see Table 2 for an overview). High molecular HA (> 500 kDa) that is normally found in the brain plays a structural role and silences inflammation, angiogenesis and neural differentiation.32-34 But in pathologies, such as brain ischemic stroke, HA is fragmented into 6- to 40-mers through the action of hyaluronidases and reactive oxygen or nitrogen species. Fragmented HA has a distinct role in activating innate and adaptive immune response, as well as inducing proliferation, motility, and tubule formation of endothelial cells and angiogenesis (refs. 35 and 36 and personal communication with M. Slevin). This effect is mediated by HA receptor CD44 on the endothelial cells.37 This allows tissue engineers to vary the molecular sizes of HA. By encapsulating cells in high molecular weight HA, cells can be transplanted and protected from inflammation upon transplantation in the acute phase of brain damage and through a controlled and gradual degradation, low molecular weight HA is released and can induce angiogenesis and differentiation of stem cell.
Table 2. A comparative summary of findings in high molecular weight HA vs. low molecular weight HA.
| High molecular weight hyaluronan | Low molecular weight hyaluronan |
|---|---|
| - provides hydration and porosity - sequesters and gradually releases bioactive molecules - conceals encapsulated cells from other cells and humoral factors - keeps stem/progenitor cells in a quiescence state - silences inflammation - prohibits angiogenesis - inhibits astrocyte activation and scar formation |
- activates immune response - induces angiogenesis - promotes proliferation, differentiation and migration of stem/progenitor cells |
Every material implanted in the brain is at risk of being isolated by the brain foreign body response.38 This starts with the brain’s attempt to heal the wound caused by implantation. Upon failure to recognize the implant as “self” or to degrade it, the brain mounts a chronic activation of inflammatory response and gliotic scar formation that will eventually seal-off the “stranger” from accessing normal brain tissue and will effectively cause implant malfunction. From the anti-inflammatory effects of HMW HA it might be predicted that HA will reduce scar formation in the brain and peripheral nerves. This is indeed true:39,40Figure 2 shows how applying HMW HA can attenuate astrocytic reactivity and scar formation in spinal cord injury. These data render HA gel a bio-compatible choice for neural tissue engineering.
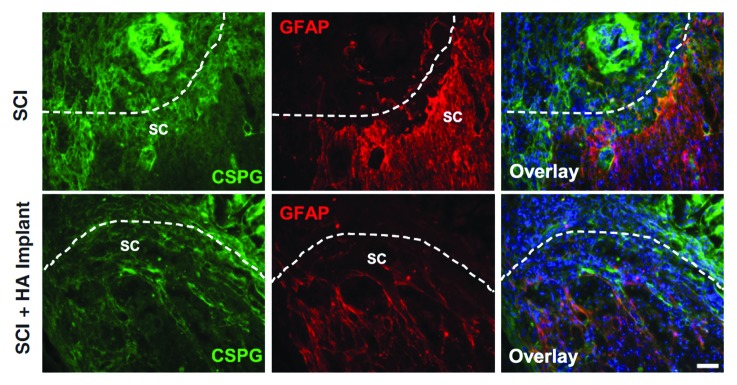
Figure 2. Attenuation of astrocyte activation and deposition of chondroitin sulfate proteoglycan (CSPG) by HMW HA gel injection in vivo. Longitudinal images of spinal cord from control (“SCI”) and HA-implanted animals are shown 10 d after spinal cord injury (SCI). Reproduced with permission fromref.39. Scale bar = 100 µm.
Hyaluronan and Angiogenesis
Nutrients and oxygen can functionally diffuse up to 150–250 µm from blood capillaries.25 The size of infarcted tissue in the brain is several fold larger than this critical range. Therefore any tissue engineering solution for the brain after stroke should consider reconstructing a normal microenvironment with a proper accessibility to oxygen and nutrients, and envisage a solution for the formation of new vessels in tissue constructs. Angiogenesis requires activation of endothelial cells with pro-angiogenic factors (such as vascular endothelial growth factor or VEGF, platelet-derived growth factor or PDGF, fibroblast growth factor or FGF, hepatic growth factor or HGF, angiopoietin-1 and transforming growth factor-β or TGF-β). These factors induce a process of endothelial branching from existing capillaries into adjacent tissue and penetration into an implanted matrix. Endothelial cells need to survive and proliferate inside such a transplanted matrix, and be capable of remodeling it to migrate through and establish preliminary cord-like capillary structures; these will advance into mature capillaries and further to small arteries and veins.
HA has been widely studied and appreciated as a pro-angiogenic tool in tissue engineering because it can initiate and maintain angiogenesis. HMW HA promotes quiescence in endothelial cells, but oligomeric HA promotes their proliferation and migration; this effect is mediated directly through endothelial cells and also indirectly by inducing inflammatory cells to secrete pro-angiogenic factors. HA relays these messages through CD44, RHAMM and TLR-4 receptors.26,41,42 Long polymers of HA bind to CD44 and interfere with any biological signal transduction; this inhibits endothelial cell proliferation that leads to arrest of angiogenesis. Oligomeric HA, in contrast, attaches to CD44 and promote proliferation of endothelial cells. This molecule also enhances matrix metalloproteinase (MMP)-2 and -9 expression, promoting matrix degradation and progression of angiogenesis. MMPs also activate endogenous TGF-β that in turn contributes to angiogenesis. Interactions of short chain HA with RHAMM lead to cytoskeletal changes and subsequent migration of endothelial cells that further contribute to the formation of new vessels.26
Growing human umbilical vein endothelial cells (HUVECs) on HA gels improved their proliferation. This augmentation was proportional to the concentration of HA and by using 1% HA gel cells maximally increased their proliferation over 2-fold in a 48 h interval. HA also protects HUVECs against apoptosis induced by serum deprivation. An HA gel enhanced angiogenesis, arteriogensis and improved ischemia in an experimental model of mice limb ischemia when it encapsulated HUVECs.43 Implanting HA hydrogels in the brain has improved angiogenesis.44 HA is also important in maintaining vascular integrity.45 These data show HA has the potential to support the formation of a vasculature to allow generation and survival of engineered constructs in sizes required to replace the infarcted brain tissue. Some studies have bound oligomeric HA to culture surfaces and thereby induced endothelial cell proliferation and tube formation.46 An alternative approach would be to engineer HMW HA gels to persistently degrade and provide host tissue endothelial cells with a continuous source of pro-angiogenic oligomeric HA.47
Interaction of endothelial cells with a plain or unmodified HA gel is often not sufficient to make a patent vasculature. Chemical engineering can help to further enhance angiogenesis in HA gels by introducing components of extracellular matrix (ECM) molecules such as fibronectin48 or fibronectin-derived synthetic protein motifs.44 Other studies have shown HA gels that gradually release pro-angiogenic growth factors VEGF or bFGF have an improved angiogenesis.26
Hyaluronan and Neural Stem/Progenitor Cells
There are many indications that HA influences neural stem/progenitor cells in neural tissue development and therefore HA is a suitable candidate to encapsulate NSPCs in neural tissue engineering. HA content is high in developing brain and it declines to 25% two weeks after birth.49 In vitro studies show that the addition of HA contributes to increasing water absorption and porosity of bio-matrices.50 Water absorption and resultant changes in the three dimensional HA structure are responsible for some of its biological properties. For example, HA is associated with neural crest cell distribution along the neural tube perhaps by creating a porous milieu for cell migration through the tight cell-cell junction of neuroepithelial cells. Ninety percent of HA is associated with water, suggesting HA contributes to cell migration by providing a loose matrix. HA is diffusely found in the developing brain while NSPCs are migrating in radial or tangential directions. Although HA decreases in postnatal brain, it is still found in cerebellum and corpus callosum, where HA is re-organized into dense meshworks; interestingly, those areas are home to an extensive migration of progenitor cells.26
Besides being a permissive conduit for migration, HA actively interacts with cells to tune the cellular machinery for migration. Through RHAMM receptors HA induces calmodulin-mediated signaling that affects actin and microtubule.51 HA-rich fiber tracts may also function as physical cues guiding the migration of newly born cells in the CNS.26 It has been suggested that HA induces neural progenitor migration through RHAMM activation. CD44, another known receptor for the HA molecule, is found on NPCs and mediates their interaction with endothelial cells and their transmigration through vessels;52 these stem cell functions lead to establishing neurovascular stem cell niche that plays a crucial role in normal adult angiogenesis as well as integration of exogenous transplanted NSPCs.
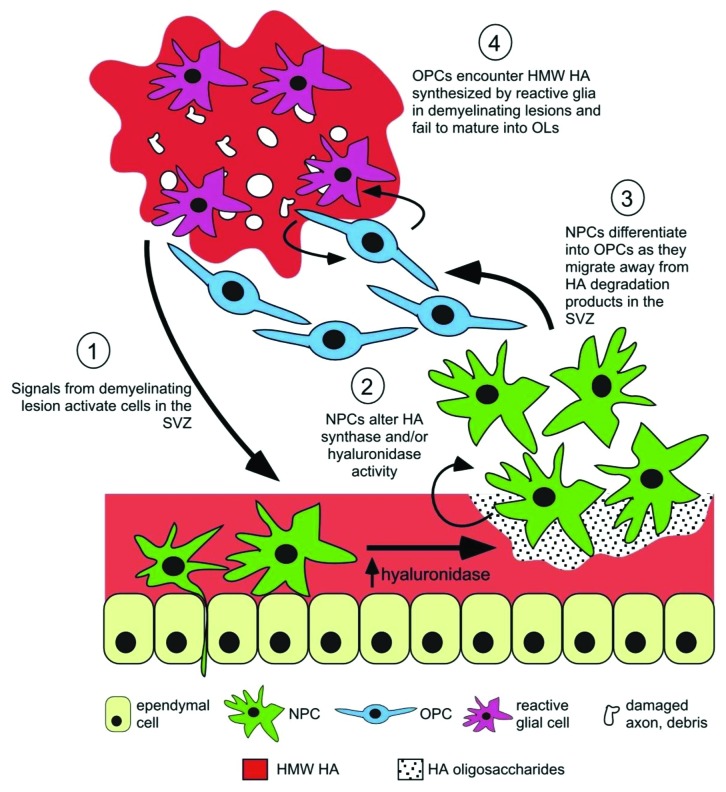
HA is found in the niche surrounding stem cells and it affects stem cell proliferation and differentiation. It is specifically found in the brain ventricular regions with ongoing stem cell proliferation.26 HA interaction with CD44 receptor on stem cells influences mitosis spindle formation, which in turn affects symmetric vs. asymmetric division and therefore controls self-renewal vs. differentiation.53 HA is generally believed to slow the proliferation of stem cells in the brain. Enzymatic HA removal induces proliferation of oligodendrocyte-type 2 astrocyte progenitors,54 now classified as oligodendrocyte progenitor cells. HA is partly the reason for failed remyelination of demyelinated axons in multiple sclerosis by oligodendrocyte progenitor cells (see Fig. 3 for an illustration): While HMW HA secreted by astrocytes in demyelinated lesions prohibits OPC differentiation to myelin-producing cells, removing HMW HA promotes oligodendrocyte maturation.31 On the other hand, it has been suggested that degradation of HA promotes proliferation of cells.55 Moreover, LMW HA (as the degradation product of HMW HA) can induce proliferation, differentiation and migration of NSPCs.26 Degradation of HA gels encapsulating neural progenitor cells causes differentiation and maturation of those cells; this fits well with the in vivo finding showing a decline in the brain HA content in the postnatal period.14 It is therefore possible to utilize the dual effect of HA on stem cells by encapsulation of NSPCs in high molecular size HA to prohibit proliferation and maturation, and by a timely degradation of HA and provision of cells with LMW HA turn on their proliferation and differentiation machinery.
Figure 3. An illustrative model on how changes in HA regulate NSPCs in normal state and in response to injury. (1) CNS insults activate NSPCs in the subventricular zone (SVZ); (2) HA in the niche is degraded due to increased hyaluronidase activity of NSPCs and other cell types and therefore (3) NSPCs proliferate, migrate toward the lesion and differentiate to OPCs. (4) However, their maturation is inhibited if they encounter HMW HA-rich chronic lesions. Reproduced with permission from ref. 26.
These data have inspired researchers to employ HA hydrogel to recapitulate the normal stem cell niche: by providing NSPCs with a 3 dimensional matrix close in composition to the ECM, and by further supplementing HA hydrogel with collage-I, abundantly found in basal lamina of the subventricular zone.56 Cells growing in these gels have a lower proliferation rate compared with a 2-dimensional culture, and their neural differentiation improves by almost 5-fold. Other research groups have enriched HA gels with an optimized ratio of collagen57 or added carefully-tuned amounts of fibroin (produced by Bombyx mori silkworm50), heparin sulfate14 or IKVAV (a protein motif originally found in laminin) plus brain-derived neurotrophic factor58 to provide NSPCs with an optimally engineered microenvironment to improve their survival, proliferation and differentiation. By altering the hydrogel compliance instead of changing biochemical factors, differentiation of NSPCs to neurons was optimized in gels mimicking neonatal brain compliance vs. those gels similar to adult brain in elasticity.59 Neural progenitor cells (NPCs) encapsulated in a combined hydrogel made of HA, heparin sulfate and collagen transplanted to the stroke cavity were protected against the host inflammatory insult and their survival improved (Fig. 4).14 Applying this biopolymer matrix, however, did not influence NPCs differentiation.
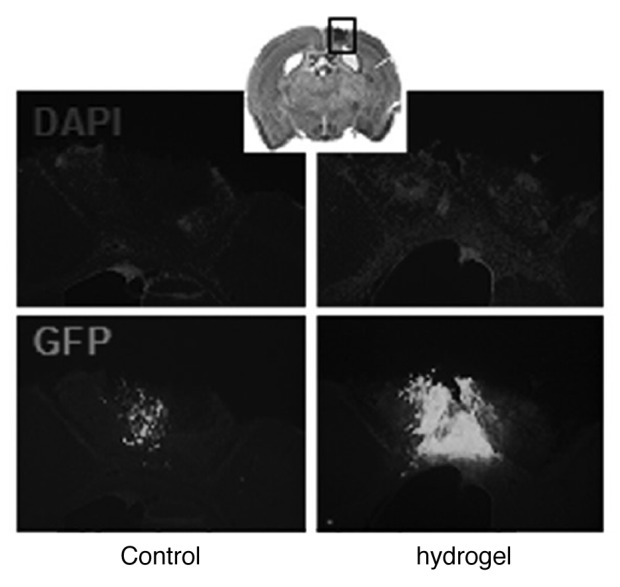
Figure 4. Hyaluronan–heparin–collagen hydrogel improves the survival of neural progenitor cells (NPCs) transplanted into the infarct cavity after stroke. NPCs derived from the embryonic cortex were transplanted into the infarct cavity 7 d after stroke, with or without hydrogel; 14 d after transplantation, transplanted cells were identified by the immunostaining of green fluorescent protein (GFP); the infarct cavity was identified by counterstaining with DAPI (4',6-diamidino- 2-phenylindole). Reproduced with permission from ref. 14.
Hyaluronan and Axon Growth
Attempts to study the role of HA in axonal local sprouting and long-distance regeneration started with simple histological observations in late 1980s.60 These found HA as a fine mesh around brain and spinal cord myelinated axons,61,62 as well as in endoneurial tubes in the peripheral nerves.63 HA was also found in the perineuronal nets, the specialized ECM structures around cell bodies in the CNS that among many roles also regulate synaptic connectivity.61,62
These phenomenological studies had a clear conclusion: HA is likely to affect a neuron’s physiological functions in the developing and adult nervous system, and also could determine the regenerative response to nervous system injuries. These were the foci for further investigations. HA was found to fine-tune axon pathfinding and contribute to architecturally specific areas in the brain: layer-specific termination of entorhinal fibers to the dentate gyrus, an area responsible for formation of new memories, was abolished by degrading HA molecules.64 Another region that HA serves as a guidance molecule is the optic chiasm, where those axons of the optic nerve coming from the nasal halves of retina will cross and give rise to the very specific optic pathway. HA localizes in the medial part of the optic chiasm, the point where axon crossing takes place.65 Data suggests the HA receptor CD44 is involved in mediating the HA role in axon crossing. Further experiments revealed HA removal deters axon crossing in the optic chiasm.66
HA is also involved in the nervous system response to injuries and pathological conditions. The presence of HA was correlated with the axon growth in regenerating limbs of larval ambystoma.60 This finding was echoed in a murine model of peripheral nerve injury: HA was associated with successful axon regeneration and it was co-localized in Schwann cells proliferation areas and the bands of Büngner, two heralds of endogenous peripheral nerve repair.63 Mossy fiber sprouting in hippocampus is responsible for the formation of a recurrent aberrant excitatory network and the consequent temporal lobe epilepsy. Increases in HA expression in the hippocampus are associated with temporal lobe epilepsy and removing HA in the dentate gyrus protects the brain from epileptogenic chemicals (e.g., kainic acid) that induce mossy fiber sprouting.27
These findings prompted researchers to apply HA to promote regeneration in the peripheral and the central nervous systems. Their initial concept considered HA as a hydrated open lattice to allow axon growth as well as free diffusion of nutrients and growth factors. Early attempts applied an injectable form of HA to amplify axon growth, myelination and conduction velocity in regenerating peripheral nerve.67,68 HA also diminished the perineural scar formation, a known impediment to the regeneration, in re-opposed stamps of peripheral nerves.40
HA promoted regeneration of dorsal root ganglia axons in vitro, but it failed in vivo to induce spinal cord regeneration.69 These findings revisit the established concept of compromised axon regeneration in the CNS. CNS axons are facing multiple barriers in their internal machinery as well as their surrounding environment in order to mount an effective regenerative response and therefore additional molecules are required to persuade severed axons to re-grow. Hence plain HA gels were supplemented with elements of the ECM or membrane receptor ligands to promote axon growth into the implanted HA matrix in the lesioned brain70 or spinal cord.71
Perspectives and Caveats
Tissue engineering will fail if it does not address the many pathologies present in the damaged brain tissue. In early stages of neural repair a focus was on neuronal replacement. However, controlling inflammation, securely delivering stem/progenitor cells, promoting angiogenesis, inducing axon regeneration and evading the foreign body reaction are examples of demands from the local environment in a more biologically active tissue repair approach. Further, these are not distinct processes but are inter-related. As discussed in this review, HA is present in the developing and adult brain with a functional consequence for interaction with angiogenesis, inflammation, axonal and cell migration guidance and with effects on cell proliferation and differentiation. This implies applying HA in tissue constructs will modulate inflammation, angiogenesis, stem cell proliferation/differentiation and axon regeneration and therefore improve brain ischemic lesions. In addition to those, tissue engineering capabilities of HA for protein motif incorporation indicate a future in which HA can serve to influence not just the individual elements of tissue repair, but multiple aspects of tissue repair at the same time. However there exists a dual functional role of HA by size (see Table 2) and therefore a successful tissue engineering approach requires an intelligent construction, maintenance and degradation of the HA hydrogel.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/23863
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Dobkin BH. Training and exercise to drive poststroke recovery. Nat Clin Pract Neurol. 2008;4:76–85. doi: 10.1038/ncpneuro0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wechsler LR. Intravenous thrombolytic therapy for acute ischemic stroke. N Engl J Med. 2011;364:2138–46. doi: 10.1056/NEJMct1007370. [DOI] [PubMed] [Google Scholar]
- 4.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–72. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 5.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–42. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 6.Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci. 2009;29:1719–34. doi: 10.1523/JNEUROSCI.4249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–9. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarkson AN, Overman JJ, Zhong S, Mueller R, Lynch G, Carmichael ST. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci. 2011;31:3766–75. doi: 10.1523/JNEUROSCI.5780-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 10.Carmichael ST. Targets for neural repair therapies after stroke. Stroke. 2010;41(Suppl):S124–6. doi: 10.1161/STROKEAHA.110.597146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, et al. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–74. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiol Dis. 2010;37:275–83. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Locatelli F, Bersano A, Ballabio E, Lanfranconi S, Papadimitriou D, Strazzer S, et al. Stem cell therapy in stroke. Cell Mol Life Sci. 2009;66:757–72. doi: 10.1007/s00018-008-8346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong J, Chan A, Morad L, Kornblum HI, Fan G, Carmichael ST. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil Neural Repair. 2010;24:636–44. doi: 10.1177/1545968310361958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns TC, Verfaillie CM, Low WC. Stem cells for ischemic brain injury: a critical review. J Comp Neurol. 2009;515:125–44. doi: 10.1002/cne.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Y. Cell-based therapy for stroke. J Neural Transm. 2011;118:61–74. doi: 10.1007/s00702-010-0478-4. [DOI] [PubMed] [Google Scholar]
- 17.Bliss T, Guzman R, Daadi M, Steinberg GK. Cell transplantation therapy for stroke. Stroke. 2007;38(Suppl):817–26. doi: 10.1161/01.STR.0000247888.25985.62. [DOI] [PubMed] [Google Scholar]
- 18.Mountz JM. Nuclear medicine in the rehabilitative treatment evaluation in stroke recovery. Role of diaschisis resolution and cerebral reorganization. Eura Medicophys. 2007;43:221–39. [PubMed] [Google Scholar]
- 19.Lin TN, Sun SW, Cheung WM, Li F, Chang C. Dynamic changes in cerebral blood flow and angiogenesis after transient focal cerebral ischemia in rats. Evaluation with serial magnetic resonance imaging. Stroke. 2002;33:2985–91. doi: 10.1161/01.STR.0000037675.97888.9D. [DOI] [PubMed] [Google Scholar]
- 20.Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101:11839–44. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aurand ER, Lampe KJ, Bjugstad KB. Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci Res. 2012;72:199–213. doi: 10.1016/j.neures.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spicer AP, Tien JY. Hyaluronan and morphogenesis. Birth Defects Res C Embryo Today. 2004;72:89–108. doi: 10.1002/bdrc.20006. [DOI] [PubMed] [Google Scholar]
- 23.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 24.Austin JW, Gilchrist C, Fehlings MG. High molecular weight hyaluronan reduces lipopolysaccharide mediated microglial activation. J Neurochem. 2012 doi: 10.1111/j.1471-4159.2012.07789.x. In press. [DOI] [PubMed] [Google Scholar]
- 25.Pardue EL, Ibrahim S, Ramamurthi A. Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering. Organogenesis. 2008;4:203–14. doi: 10.4161/org.4.4.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preston M, Sherman LS. Neural stem cell niches: roles for the hyaluronan-based extracellular matrix. Front Biosci (Schol Ed) 2011;3:1165–79. doi: 10.2741/218. [Schol Ed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bausch SB. Potential roles for hyaluronan and CD44 in kainic acid-induced mossy fiber sprouting in organotypic hippocampal slice cultures. Neuroscience. 2006;143:339–50. doi: 10.1016/j.neuroscience.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 28.Cargill R, Kohama SG, Struve J, Su W, Banine F, Witkowski E, et al. Astrocytes in aged nonhuman primate brain gray matter synthesize excess hyaluronan. Neurobiol Aging. 2012;33:830 e13–24. doi: 10.1016/j.neurobiolaging.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carulli D, Rhodes KE, Brown DJ, Bonnert TP, Pollack SJ, Oliver K, et al. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol. 2006;494:559–77. doi: 10.1002/cne.20822. [DOI] [PubMed] [Google Scholar]
- 30.Wang MJ, Kuo JS, Lee WW, Huang HY, Chen WF, Lin SZ. Translational event mediates differential production of tumor necrosis factor-alpha in hyaluronan-stimulated microglia and macrophages. J Neurochem. 2006;97:857–71. doi: 10.1111/j.1471-4159.2006.03776.x. [DOI] [PubMed] [Google Scholar]
- 31.Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–72. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 32.Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–9. doi: 10.1016/S0945-053X(01)00184-6. [DOI] [PubMed] [Google Scholar]
- 33.Slevin M, Kumar S, Gaffney J. Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing responses. J Biol Chem. 2002;277:41046–59. doi: 10.1074/jbc.M109443200. [DOI] [PubMed] [Google Scholar]
- 34.West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–6. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- 35.Al Qteishat A, Gaffney JJ, Krupinski J, Slevin M. Hyaluronan expression following middle cerebral artery occlusion in the rat. Neuroreport. 2006;17:1111–4. doi: 10.1097/01.wnr.0000227986.69680.20. [DOI] [PubMed] [Google Scholar]
- 36.Kilia V, Skandalis SS, Theocharis AD, Theocharis DA, Karamanos NK, Papageorgakopoulou N. Glycosaminoglycan in cerebrum, cerebellum and brainstem of young sheep brain with particular reference to compositional and structural variations of chondroitin-dermatan sulfate and hyaluronan. Biomed Chromatogr. 2008;22:931–8. doi: 10.1002/bmc.1010. [DOI] [PubMed] [Google Scholar]
- 37.Wang YZ, Cao ML, Liu YW, He YQ, Yang CX, Gao F. CD44 mediates oligosaccharides of hyaluronan-induced proliferation, tube formation and signal transduction in endothelial cells. Exp Biol Med (Maywood) 2011;236:84–90. doi: 10.1258/ebm.2010.010206. [DOI] [PubMed] [Google Scholar]
- 38.Zhong Y, Bellamkonda RV. Biomaterials for the central nervous system. J R Soc Interface. 2008;5:957–75. doi: 10.1098/rsif.2008.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khaing ZZ, Milman BD, Vanscoy JE, Seidlits SK, Grill RJ, Schmidt CE. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J Neural Eng. 2011;8:046033. doi: 10.1088/1741-2560/8/4/046033. [DOI] [PubMed] [Google Scholar]
- 40.Ozgenel GY. Effects of hyaluronic acid on peripheral nerve scarring and regeneration in rats. Microsurgery. 2003;23:575–81. doi: 10.1002/micr.10209. [DOI] [PubMed] [Google Scholar]
- 41.Matou-Nasri S, Gaffney J, Kumar S, Slevin M. Oligosaccharides of hyaluronan induce angiogenesis through distinct CD44 and RHAMM-mediated signalling pathways involving Cdc2 and gamma-adducin. Int J Oncol. 2009;35:761–73. doi: 10.3892/ijo_00000389. [DOI] [PubMed] [Google Scholar]
- 42.Gao F, Yang CX, Mo W, Liu YW, He YQ. Hyaluronan oligosaccharides are potential stimulators to angiogenesis via RHAMM mediated signal pathway in wound healing. Clin Invest Med. 2008;31:E106–16. doi: 10.25011/cim.v31i3.3467. [DOI] [PubMed] [Google Scholar]
- 43.Tang ZC, Liao WY, Tang AC, Tsai SJ, Hsieh PC. The enhancement of endothelial cell therapy for angiogenesis in hindlimb ischemia using hyaluronan. Biomaterials. 2011;32:75–86. doi: 10.1016/j.biomaterials.2010.08.085. [DOI] [PubMed] [Google Scholar]
- 44.Cui FZ, Tian WM, Hou SP, Xu QY, Lee IS. Hyaluronic acid hydrogel immobilized with RGD peptides for brain tissue engineering. J Mater Sci Mater Med. 2006;17:1393–401. doi: 10.1007/s10856-006-0615-7. [DOI] [PubMed] [Google Scholar]
- 45.Lennon FE, Singleton PA. Hyaluronan regulation of vascular integrity. Am J Cardiovasc Dis. 2011;1:200–13. [PMC free article] [PubMed] [Google Scholar]
- 46.Ibrahim S, Joddar B, Craps M, Ramamurthi A. A surface-tethered model to assess size-specific effects of hyaluronan (HA) on endothelial cells. Biomaterials. 2007;28:825–35. doi: 10.1016/j.biomaterials.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 47.Collier JH, Camp JP, Hudson TW, Schmidt CE. Synthesis and characterization of polypyrrole-hyaluronic acid composite biomaterials for tissue engineering applications. J Biomed Mater Res. 2000;50:574–84. doi: 10.1002/(SICI)1097-4636(20000615)50:4<574::AID-JBM13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 48.Seidlits SK, Drinnan CT, Petersen RR, Shear JB, Suggs LJ, Schmidt CE. Fibronectin-hyaluronic acid composite hydrogels for three-dimensional endothelial cell culture. Acta Biomater. 2011;7:2401–9. doi: 10.1016/j.actbio.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 49.Margolis RU, Margolis RK, Chang LB, Preti C. Glycosaminoglycans of brain during development. Biochemistry. 1975;14:85–8. doi: 10.1021/bi00672a014. [DOI] [PubMed] [Google Scholar]
- 50.Ren YJ, Zhou ZY, Liu BF, Xu QY, Cui FZ. Preparation and characterization of fibroin/hyaluronic acid composite scaffold. Int J Biol Macromol. 2009;44:372–8. doi: 10.1016/j.ijbiomac.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Lynn BD, Turley EA, Nagy JI. Subcellular distribution, calmodulin interaction, and mitochondrial association of the hyaluronan-binding protein RHAMM in rat brain. J Neurosci Res. 2001;65:6–16. doi: 10.1002/jnr.1122. [DOI] [PubMed] [Google Scholar]
- 52.Rampon C, Weiss N, Deboux C, Chaverot N, Miller F, Buchet D, et al. Molecular mechanism of systemic delivery of neural precursor cells to the brain: assembly of brain endothelial apical cups and control of transmigration by CD44. Stem Cells. 2008;26:1673–82. doi: 10.1634/stemcells.2008-0122. [DOI] [PubMed] [Google Scholar]
- 53.Fujiwara T, Kawakatsu T, Tayama S, Kobayashi Y, Sugiura N, Kimata K, et al. Hyaluronan-CD44 pathway regulates orientation of mitotic spindle in normal epithelial cells. Genes Cells. 2008;13:759–70. doi: 10.1111/j.1365-2443.2008.01203.x. [DOI] [PubMed] [Google Scholar]
- 54.Marret S, Delpech B, Delpech A, Asou H, Girard N, Courel MN, et al. Expression and effects of hyaluronan and of the hyaluronan-binding protein hyaluronectin in newborn rat brain glial cell cultures. J Neurochem. 1994;62:1285–95. doi: 10.1046/j.1471-4159.1994.62041285.x. [DOI] [PubMed] [Google Scholar]
- 55.Struve J, Maher PC, Li YQ, Kinney S, Fehlings MG, Kuntz C, 4th, et al. Disruption of the hyaluronan-based extracellular matrix in spinal cord promotes astrocyte proliferation. Glia. 2005;52:16–24. doi: 10.1002/glia.20215. [DOI] [PubMed] [Google Scholar]
- 56.Brännvall K, Bergman K, Wallenquist U, Svahn S, Bowden T, Hilborn J, et al. Enhanced neuronal differentiation in a three-dimensional collagen-hyaluronan matrix. J Neurosci Res. 2007;85:2138–46. doi: 10.1002/jnr.21358. [DOI] [PubMed] [Google Scholar]
- 57.Wang TW, Spector M. Development of hyaluronic acid-based scaffolds for brain tissue engineering. Acta Biomater. 2009;5:2371–84. doi: 10.1016/j.actbio.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 58.Park J, Lim E, Back S, Na H, Park Y, Sun K. Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive, hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor. J Biomed Mater Res A. 2010;93:1091–9. doi: 10.1002/jbm.a.32519. [DOI] [PubMed] [Google Scholar]
- 59.Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, et al. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010;31:3930–40. doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 60.Mescher AL, Cox CA. Hyaluronate accumulation and nerve-dependent growth during regeneration of larval Ambystoma limbs. Differentiation. 1988;38:161–8. doi: 10.1111/j.1432-0436.1988.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 61.Bignami A, Asher R. Some observations on the localization of hyaluronic acid in adult, newborn and embryonal rat brain. Int J Dev Neurosci. 1992;10:45–57. doi: 10.1016/0736-5748(92)90006-L. [DOI] [PubMed] [Google Scholar]
- 62.Bignami A, Asher R, Perides G. The extracellular matrix of rat spinal cord: a comparative study on the localization of hyaluronic acid, glial hyaluronate-binding protein, and chondroitin sulfate proteoglycan. Exp Neurol. 1992;117:90–3. doi: 10.1016/0014-4886(92)90115-7. [DOI] [PubMed] [Google Scholar]
- 63.Tona A, Perides G, Rahemtulla F, Dahl D. Extracellular matrix in regenerating rat sciatic nerve: a comparative study on the localization of laminin, hyaluronic acid, and chondroitin sulfate proteoglycans, including versican. J Histochem Cytochem. 1993;41:593–9. doi: 10.1177/41.4.8450198. [DOI] [PubMed] [Google Scholar]
- 64.Förster E, Zhao S, Frotscher M. Hyaluronan-associated adhesive cues control fiber segregation in the hippocampus. Development. 2001;128:3029–39. doi: 10.1242/dev.128.15.3029. [DOI] [PubMed] [Google Scholar]
- 65.Lin L, Wang J, Chan CK, Chan SO. Localization of hyaluronan in the optic pathway of mouse embryos. Neuroreport. 2007;18:355–8. doi: 10.1097/WNR.0b013e32802b70e2. [DOI] [PubMed] [Google Scholar]
- 66.Chan CK, Wang J, Lin L, Hao Y, Chan SO. Enzymatic removal of hyaluronan affects routing of axons in the mouse optic chiasm. Neuroreport. 2007;18:1533–8. doi: 10.1097/WNR.0b013e3282efa065. [DOI] [PubMed] [Google Scholar]
- 67.Seckel BR, Jones D, Hekimian KJ, Wang KK, Chakalis DP, Costas PD. Hyaluronic acid through a new injectable nerve guide delivery system enhances peripheral nerve regeneration in the rat. J Neurosci Res. 1995;40:318–24. doi: 10.1002/jnr.490400305. [DOI] [PubMed] [Google Scholar]
- 68.Wang KK, Nemeth IR, Seckel BR, Chakalis-Haley DP, Swann DA, Kuo JW, et al. Hyaluronic acid enhances peripheral nerve regeneration in vivo. Microsurgery. 1998;18:270–5. doi: 10.1002/(SICI)1098-2752(1998)18:4<270::AID-MICR11>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 69.Horn EM, Beaumont M, Shu XZ, Harvey A, Prestwich GD, Horn KM, et al. Influence of cross-linked hyaluronic acid hydrogels on neurite outgrowth and recovery from spinal cord injury. J Neurosurg Spine. 2007;6:133–40. doi: 10.3171/spi.2007.6.2.133. [DOI] [PubMed] [Google Scholar]
- 70.Wei YT, Tian WM, Yu X, Cui FZ, Hou SP, Xu QY, et al. Hyaluronic acid hydrogels with IKVAV peptides for tissue repair and axonal regeneration in an injured rat brain. Biomed Mater. 2007;2:S142–6. doi: 10.1088/1748-6041/2/3/S11. [DOI] [PubMed] [Google Scholar]
- 71.Wei YT, He Y, Xu CL, Wang Y, Liu BF, Wang XM, et al. Hyaluronic acid hydrogel modified with nogo-66 receptor antibody and poly-L-lysine to promote axon regrowth after spinal cord injury. J Biomed Mater Res B Appl Biomater. 2010;95:110–7. doi: 10.1002/jbm.b.31689. [DOI] [PubMed] [Google Scholar]
- 72.Bible E, Chau DY, Alexander MR, Price J, Shakesheff KM, Modo M. The support of neural stem cells transplanted into stroke-induced brain cavities by PLGA particles. Biomaterials. 2009;30:2985–94. doi: 10.1016/j.biomaterials.2009.02.012. [DOI] [Google Scholar]
- 73.Jin K, Mao X, Xie L, Galvan V, Lai B, Wang Y, et al. Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. J Cereb Blood Flow Metab. 2010;30:534–44. doi: 10.1038/jcbfm.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu H, Cao B, Feng M, Zhou Q, Sun X, Wu S, et al. Combinated transplantation of neural stem cells and collagen type I promote functional recovery after cerebral ischemia in rats. Anat Rec (Hoboken) 2010;293:911–7. doi: 10.1002/ar.20941. [DOI] [PubMed] [Google Scholar]
- 75.Matsuse D, Kitada M, Ogura F, Wakao S, Kohama M, Kira J, et al. Combined transplantation of bone marrow stromal cell-derived neural progenitor cells with a collagen sponge and basic fibroblast growth factor releasing microspheres enhances recovery after cerebral ischemia in rats. Tissue Eng Part A. 2011;17:1993–2004. doi: 10.1089/ten.tea.2010.0585. [DOI] [PubMed] [Google Scholar]
- 76.Bible E, Dell’Acqua F, Solanky B, Balducci A, Crapo PM, Badylak SF, et al. Non-invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke-damaged rat brain by (19)F- and diffusion-MRI. Biomaterials. 2012;33:2858–71. doi: 10.1016/j.biomaterials.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hou S, Tian W, Xu Q, Cui F, Zhang J, Lu Q, et al. The enhancement of cell adherence and inducement of neurite outgrowth of dorsal root ganglia co-cultured with hyaluronic acid hydrogels modified with Nogo-66 receptor antagonist in vitro. Neuroscience. 2006;137:519–29. doi: 10.1016/j.neuroscience.2005.09.029. [DOI] [PubMed] [Google Scholar]