Abstract
Cardiosphere-derived cells (CDCs) are under clinical development and are currently being tested in a clinical trial enrolling patients who have undergone a myocardial infarction. CDCs are presently administered via infusion into the infarct-related artery and have been shown in early clinical trials to be effective agents of myocardial regeneration. This review describes the administration of CDCs in a hyaluronan-gelatin hydrogel via myocardial injection and the subsequent improvements in therapeutic benefit seen in animal models. Development of a next generation therapy involving the combination of CDCs and hydrogel is discussed.
Keywords: cardiosphere-derived cells, myocardial infarction, hyaluronan-gelatin hydrogel, CADUCEUS trial, ALLSTAR trial
Introduction
Cardiosphere-derived cells (CDCs)1 have been under clinical development since 2009. The ongoing ALLSTAR trial (NCT01458405) is examining the safety and efficacy of allogeneic CDCs administered by intracoronary infusion in patients who have suffered a myocardial infarction (MI). Findings from the CADUCEUS trial,2 in which autologous CDCs were administered to post-MI patients, have already foreshadowed the potential clinical utility of CDCs in this patient population. Both cell therapies are believed to act via the same mechanisms, to stimulate endogenous regeneration and attenuate fibrosis, and do so without eliciting an immune response,3,4 in the case of allogeneic CDCs. The effects manifest preclinically as a decrease in cardiomyocyte apoptosis, recruitment of cardiac stem cells, stimulation of cardiomyocyte proliferation, increase in blood vessel density and decrease in collagen deposition;3,5 clinically as a reduction in infarct size, accumulation of viable myocardium and attenuation of left ventricular remodeling.2 Should ALLSTAR replicate the findings of CADUCEUS as expected based on preclinical studies,4 patients treated with allogeneic CDCs will experience a nearly 50% reduction in infarct size over the course of a year, commensurate with the addition of new myocardial mass.2
Despite the sizeable observed and expected benefits of CDC therapy in clinical studies, preclinical studies have shown that no more than 5% of cells survive longer than 24 h after intracoronary delivery in either saline or a cryopreservation solution containing DMSO (dimethyl sulfoxide).4,6 Presumably, poor cell retention and engraftment can be attributed to multiple factors, such as: the use of a minimally-invasive delivery approach, intracoronary infusion, which is not as effective as intramyocardial injection, the harsh ischemic microenvironment making transplanted cells susceptible to apoptosis, and the lack of space and anchorage sites available for transplanted cells making them susceptible to interstitial clearance by the lymphatic system. Furthermore, these retention and engraftment issues are common to most cell therapies, not specific to CDCs, although solutions may need to be tailored to cell type. While many possible solutions do exist, in the case of CDCs, intramyocardial injection in a hyaluronan-gelatin hydrogel has been shown to meaningfully improve retention, engraftment and efficacy in preclinical studies.7 A next generation therapy for MI patients may involve the combination of CDCs and hydrogel.
The current status of cell therapy for MI is summarized herein along with the preclinical data supporting the use of a CDC-hydrogel combination therapy. Plans to move that combination product toward the clinic are described as well.
Cell Therapy for Myocardial Infarction
This year 1.3M Americans will have a new or recurrent MI.8 Only 15% of MI sufferers will die as an immediate result,8 a mortality rate that has declined in recent years thanks to advances in the acute management of MI.9 However, 36% of MI survivors will develop heart failure (HF),10 and will consequently be at increased risk for death.11 Following an MI, ejection fraction (EF), end-systolic and end-diastolic volumes (ESV and EDV), and to a greater extent infarct size have been shown to predict subsequent HF development, adverse left ventricular (LV) remodeling, MACE (major adverse cardiac events), and all-cause mortality for patients.12-16 Infarct size alone is a rigorous, independent predictor of MACE-free survival that can be used to classify patients as at-risk (e.g., infarct size ≥ 18.5%) or not at-risk.14 Even with maximum medical care, however, once an infarct is established its size does not change.17 While long-term adverse neurohormonal responses can be countered with β blockers and ACE-inhibitors and the likelihood of recurrent ischemic events can be decreased with aggressive secondary prevention,18 no therapy currently available can reduce the size of an established infarct.
Cell therapy aims to alter this fixed trajectory for MI survivors: to intervene in the process of adverse LV remodeling, to reduce infarct size and to actually regenerate viable myocardial tissue in its place. The field to date has focused primarily on when to administer cells and what cells to administer, while relying on minimally-invasive delivery approaches (i.e., intracoronary infusion) that could also be readily and widely adopted by clinicians. More novel delivery approaches (i.e., transendocardial injection) have begun to establish a decent clinical safety profile,19 but seem to offer marginal added efficacy benefits. The result of all attempts to date has been partial restoration of cardiac structure and function. On the whole (in a meta-analysis considering 50 studies enrolling 2625 patients) autologous bone marrow cells, by far the cell type most extensively studied clinically, have led to a 4.0% increase in EF, an 8.9 mL reduction in ESV, a 5.2 mL reduction in EDV, and a 4.0% reduction in infarct size compared with control.20 These primary efficacy data can be termed marginally positive at best. Although one of the first and most positive studies21 is now reporting unanticipated benefits on long-term clinical endpoints (e.g., death, recurrent MI, HF development, revascularization),22 room for improvement undeniably still exists.
Clinical Use of Cardiosphere-Derived Cells
Cardiosphere-derived cells have yet undergone limited clinical use, but may have come the closest to achieving the goals of cell therapy, including viable tissue regeneration. The CADUCEUS (CArdiosphere-Derived AUtologous Stem CElls to Reverse VentricUlar DySfunction) trial demonstrated the safety and efficacy of autologous CDC administration via intracoronary infusion in patients with LV dysfunction post-MI.2 In the randomized, controlled, dose-escalating Phase I trial, autologous CDCs manufactured from endomyocardial biopsy specimens were infused into the infarct-related artery in 17 patients. Eight patients were followed as standard-of-care controls. In > 12 months of follow-up, safety endpoints were equivalent. Contrast-enhanced magnetic resonance imaging (MRI) revealed reductions of infarct size (scar mass normalized to total LV mass) in CDC-treated patients (-7.7 ± 4.8%), but not in controls (+0.3 ± 5.4%) over a period of 6 mo. The treatment effect in CDC patients nearly doubled at 12 mo (-12.3 ± 5.0%), amounting to a 46% relative reduction of infarct size (from a baseline of 24%), but remained unchanged in controls (-2.2 ± 7.1%). In comparison to the overall effect reported for bone marrow cells on infarct size,20 CDCs elicited much larger reductions. Theoretically, tissue regeneration should be manifested not only by scar shrinkage but also by an increase in viable tissue (measured independently by MRI). Accordingly, while changes in scar mass mirrored changes in infarct size, viable tissue mass increased in CDC-treated patients (+13.0 ± 11.4 g at 6 mo), but not in controls (+0.9 ± 6.2 g at 6 mo), and the correlation between scar shrinkage and increased viability was highly significant (r = -0.59, p = 0.0007). This novel finding indicates that CDCs may in fact be truly regenerative.
Following the discovery that autologous and allogeneic CDCs act via the same mechanisms of action, and furthermore, that allogeneic CDCs could be safely administered in the setting of MI without eliciting an immune response,3,4 the ALLSTAR trial was initiated. ALLSTAR (ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration) is a Phase I/II randomized, double-blinded, placebo-controlled safety and efficacy study. The ongoing study is evaluating intracoronary infusion of allogeneic CDCs or placebo in 248 patients with LV dysfunction post-MI. Allogeneic CDCs are manufactured from a single donor for use in many recipients, and several donors will be utilized during the course of ALLSTAR, so as to demonstrate product comparability. The study will carefully monitor patients for the development of inflammation or an immune reaction in response to allogeneic CDC administration, while simultaneously assessing changes in infarct size, cardiac function, quality-of-life and cardiac biomarkers. ALLSTAR will establish much about the effectiveness of CDC therapy for post-MI patients. In the meantime, efforts to improve upon the therapy will continue.
Approaches to Enhance Efficacy of Cell Therapy
CDCs, much like other cell types, are retained in the heart more effectively when intramyocardial injection as opposed to intracoronary infusion is employed for cell administration. In a clinically-relevant preclinical model, use of a minimally-invasive catheter-based transendocardial injection system resulted in ~15% engraftment 24 h after delivery,23 as opposed to the ~5% achievable with intracoronary infusion.4,6 Prior preclinical studies have shown also that preventing mechanical loss and washout of CDCs from the contracting, perfused myocardium (by completely arresting the heart), can lead to a further 4-fold increase in 24 h engraftment.24 Ultimately, efficacy scales with engraftment, and novel approaches aimed at reducing or preventing mechanical loss while enhancing cell survival and subsequent engraftment could contribute greatly to the efficacy of CDC therapy.
One such approach combines CDCs with an in situ polymerizable hydrogel (Hystem®-C™, BioTime Inc.) that can be delivered intramyocardially, either by direct surgical injection or by a transendocardial catheter. Multiple hydrogels alone have demonstrated a capacity for improving cardiac function in preclinical models25 and at least one26 is undergoing clinical testing in a post-MI patient population (NCT01226563). Cell-hydrogel combinations of various sorts have also been characterized preclinically as therapies for myocardial repair.27 A hyaluronan-gelatin hydrogel not dissimilar to that selected for use with CDCs has been shown to be particularly well-suited for withstanding the contractile forces of the heart.28 Hystem-C™ is a hyaluronan-based hydrogel crosslinked using thiol-reactive poly(ethylene glycol) diacrylate and covalently linked to thiolated collagen to aid cell attachment. The base product is chemically-defined and nonimmunogenic and the collagen is porcine derived. Hyaluronan is a glycosaminoglycan component of the extracellular matrix of all connective tissues, making it an attractive vehicle for cell delivery.29 Hyaluronan-based hydrogels can be formulated with varying gelation times depending on the concentrations of the individual monomers, making them suitable for catheter delivery and in situ polymerization. Collagen is a major component of the heart’s natural extracellular matrix. Furthermore, hyaluronan-gelatin hydrogels biodegrade in vivo over the course of four to eight weeks due to the action of hyaluronidases and collagenases produced naturally by cells.30 It has been demonstrated that Hystem-C™ promotes tissue repair in various organ systems,31 but our study represents its first use in the heart.7
The CDC-hydrogel combination therapy was intended to: (1) reduce cell loss due to leakage by virtue of hydrogel viscosity and by acting as a substrate to which CDCs can anchor; (2) bolster cell survival by reducing the level of apoptosis following transplantation by offering an environment in which CDCs are temporarily protected from the in vivo elements; (3) allow for the gradual migration of CDCs out of the hydrogel, concurrent with its degradation, and into the myocardium where they can form new cardiomyocytes and endothelial cells; and (4) improve cardiac function beyond the level seen with cells delivered in a saline vehicle due to improved cell engraftment and prolonged paracrine effects.
Hyaluronan-Gelatin Hydrogel Delivery of Cardiosphere-Derived Cells
CDCs, which express multiple collagen-binding integrins (α1, α2, α3; Fig. 1A–C) as well as the receptor for hyaluronic acid (CD44; Fig. 1D), were found to be highly compatible with Hystem-C™ when incorporated within the hydrogel and cultured for up to one week. Cells loaded with viable and dead cell fluorescent indicators, allowed for the visualization of CDC morphology and the quantitative assessment of viability over time. CDCs adopted a spread morphology, typical of that seen in culture, in Hystem-C™ (Fig. 2B), while CDCs in Hystem™ (the base product without collagen; Fig.2A) remained rounded. Furthermore, more than 80% of CDCs embedded in Hystem-C™ remained viable for one week, while more than 50% of CDCs embedded in Hystem™ were dead within the week (Fig. 2C). Additionally, in vitro migratory capacity of the CDCs was greatest when Hystem-C™ was the material from which they migrated, with Hystem™ acting no differently than culture media alone in terms of a migration platform. These data indicated that CDCs could survive embedded in Hystem-C™ short-term, for the amount of time it may take for the hydrogels to begin to biodegrade in vivo, and that Hystem-C™ as a delivery vehicle may in fact stimulate CDC migration into the surrounding myocardium in vivo at such a time when environmental cues are favorable.
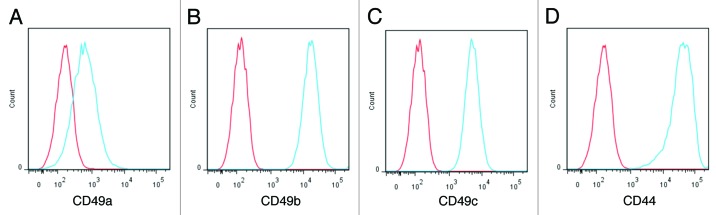
Figure 1. CDC surface markers compatible with hydrogel. Representative flow cytometry histograms showing expression of CD49a (A), CD49b (B), CD49c (C) and CD44 (D) in CDCs (in blue). Isotype controls are shown in red.
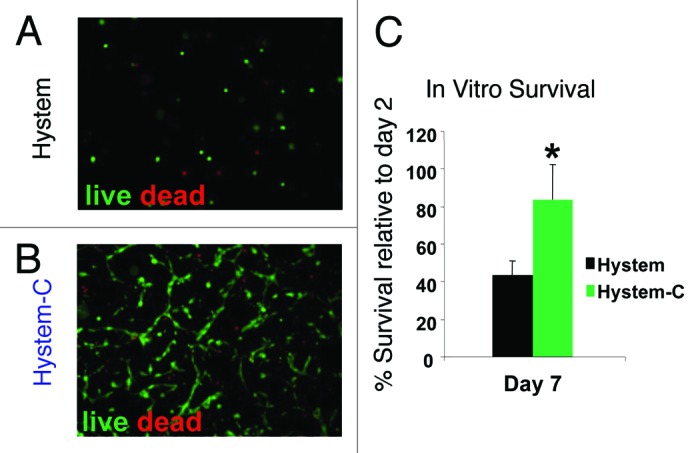
Figure 2. CDC survival in the hydrogel. (A and B) Representative fluorescent micrographs showing live (Calcien-AM: green) and dead (EthD: red) staining of CDCs cultured in Hystem™ and Hystem-CTM for 7 d. (C) CCK-8 assay quantifying cell survival rates in HystemTM (black bars) or Hystem-CTM (green bars) (n = 3). * indicates p < 0.05 when compared with HystemTM.
A mouse model of MI was next employed to investigate the combination product in vivo. CDCs were incorporated within Hystem-C™, Hystem™ or PBS (phosphate-buffered saline) and delivered as an aqueous solution (such that gelation occurred in situ), using a needle and syringe, intramyocardially in mice. Cell retention 24 h after delivery was dramatically increased in the Hystem-C™ condition (Fig. 3B), by more than 7-fold compared with both the Hystem™ and PBS conditions (Fig. 3A), resulting in an average retention of ~35% of the total cells delivered. Long-term cell engraftment (3 weeks after delivery) was significantly increased for the Hystem-C™ group compared with the PBS group (Fig. 3C), though expectedly reduced compared with 24 h. The results in terms of cardiac function and structure revealed improvements in left ventricular ejection fraction (LVEF; Fig. 4A) and additions of viable myocardial mass (Fig. 4B) for the Hystem-C™ group that exceeded the effects seen in all other groups (p < 0.05 vs all other groups). The two other treatment groups included for comparison, Hystem-C™ alone (no cells) and CDCs in PBS, showed a preservation of LVEF over the study period, as opposed to the clear improvement seen in the CDCs in Hystem-C™ group, while the PBS only control group deteriorated. Severe adverse remodeling (chamber dilatation and infarct wall thinning) and a limited amount of viable mass were observed in the control group. The treatment groups showed significantly reduced degrees of adverse remodeling and significantly greater amounts of viable mass, with benefits increasing across the Hystem-C™ alone, CDCs in PBS and CDCs in Hystem-C™ groups. An analysis of CDC differentiation capacity revealed that delivery in Hystem-C™ did not impair their ability to form new cardiomyocytes or endothelial cells, that more differentiation occurred as a consequence of greater engraftment. An analysis of the angiogenic effect, one of several manifestations of the paracrine effects of CDC treatment, demonstrated that neovascularization was improved when Hystem-C™ was used for CDC delivery (Fig. 5). These data illustrated that Hystem-C™ as a delivery vehicle could in fact improve both short-term retention and long-term engraftment of CDCs in the setting of MI, and could also lead to improvements in treatment efficacy as assessed by cardiac function and cell activity in vivo. These data in total serve as compelling proof-of-concept for the CDC-hydrogel combination therapy.
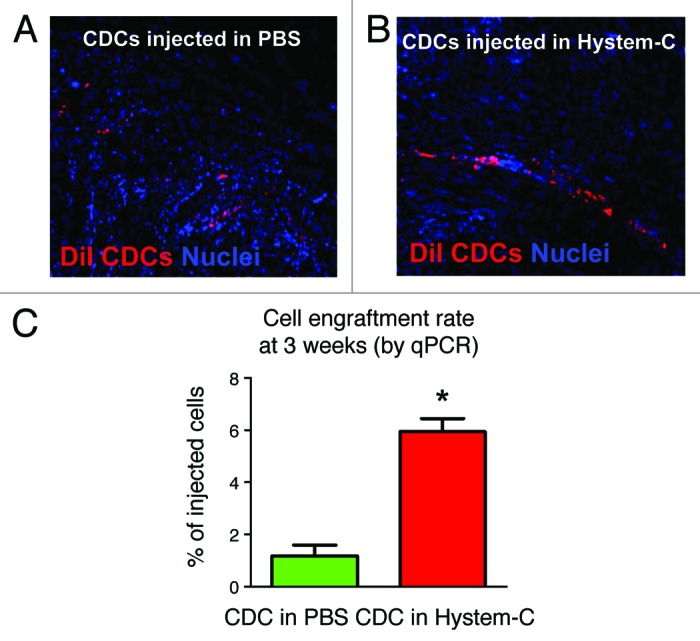
Figure 3. Enhanced cell engraftment by delivering CDCs in Hystem-CTM. (A and B) Representative confocal images showing engraftment of DiI-labeled human CDCs (red) 24 h after injection into post-MI mouse hearts. (C) Quantitative PCR analysis of cell engraftment rates in the mouse hearts 3 weeks post injection (n = 3). * indicates p < 0.05 when compared with “CDC in PBS.”
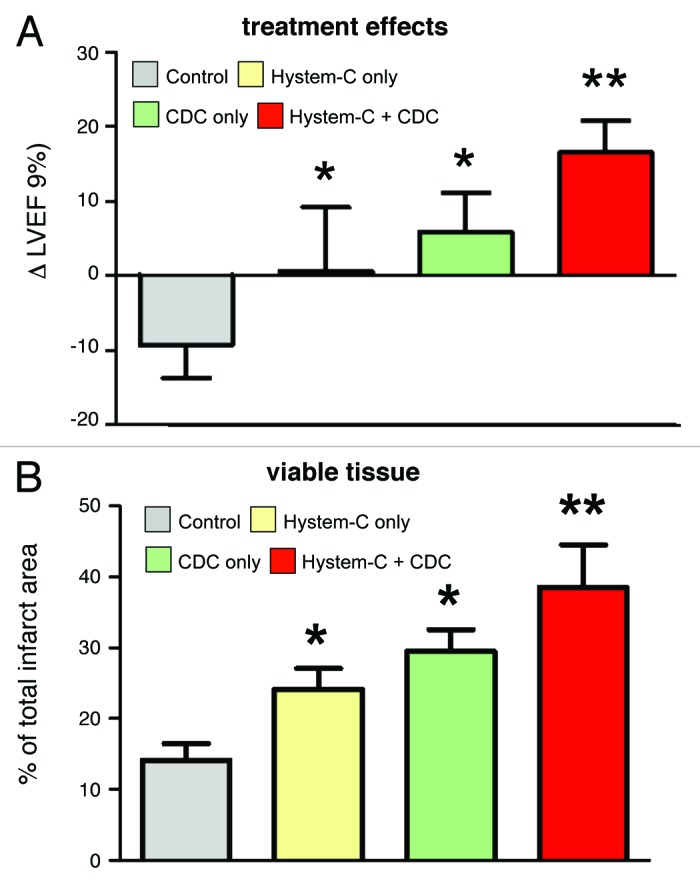
Figure 4. Cardiac function and heart morphometry. (A) Changes of left ventricular ejection fraction (LVEF) measured by echocardiography from baseline to 3 weeks in each group. (B) Quantitative analysis and LV morphometric parameters of Masson’s trichrome images (n = 3–5 mice per group). * indicates p < 0.05 when compared with Control. ** indicates p < 0.05 when compared with any other group.
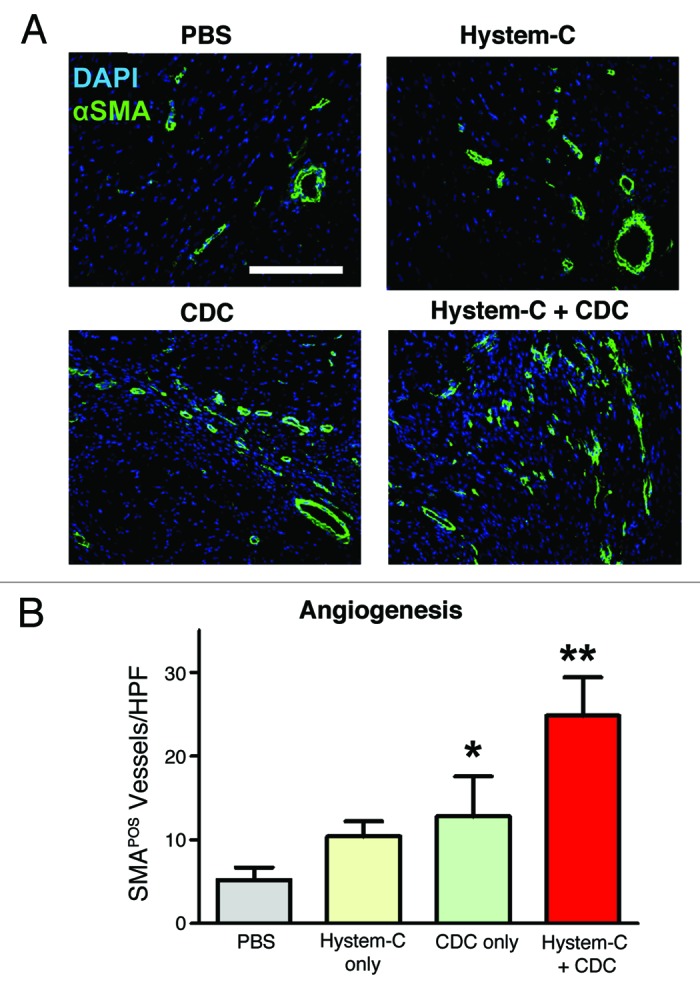
Figure 5. Promotion of angiogenesis by CDC/hydrogel transplantation. (A) Representative confocal images showing α smooth muscle actin-positive vasculature in the hearts receiving various treatment products. (B) Quantitation of α smooth muscle actin-positive vasculature in various groups (n = 5 mice per group). * indicates p < 0.05 when compared with Control. ** indicates p < 0.05 when compared with any other group. Bar = 200 µm.
Advancing a Cardiosphere-Derived Cell and Hydrogel Combination Therapy to the Clinic
Next steps for the CDC-hydrogel combination therapy will include compatibility testing with one of several catheter-based transendocardial injection systems and large animal studies to evaluate safety and efficacy in a clinically-relevant model. An appropriate patient population, perhaps one in which intracoronary infusion in a previously infarcted artery poses a safety risk, can then be targeted for a first clinical study. In the arena of cell therapy for MI, a new product that can overcome the widespread issues affecting cell engraftment should ultimately result in greater clinical benefits for patients. Cardiosphere-derived cells paired with Hystem-C™ have shown great promise thus far in preclinical testing. Such a product may also reduce the manufacturing time and cost needed to generate an adequate therapeutic dosage, making the therapy more accessible to patients. The general techniques developed and knowledge gained from this study may be applicable to other cell types as well,32 and delivery with Hystem-C™ may in fact benefit the field of cell therapy for MI as a whole.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/24490
References
- 1.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 2.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–12. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith RR, Kreke M, Malliaras K, Kanazawa H, Huang CH, Valle I, et al. Allogeneic cardiosphere-derived cells are safe and effective in a pig model of myocardial infarction. American Heart Association Annual Scientific Sessions 2012. [Google Scholar]
- 5.Tseliou E, Pollan S, Malliaras K, Terrovitis J, Sun B, Galang G, et al. Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. J Am Coll Cardiol. 2013;61:1108–19. doi: 10.1016/j.jacc.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 6.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–83, 7, 1083. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng K, Blusztajn A, Shen D, Li TS, Sun B, Galang G, et al. Functional performance of human cardiosphere-derived cells delivered in an in situ polymerizable hyaluronan-gelatin hydrogel. Biomaterials. 2012;33:5317–24. doi: 10.1016/j.biomaterials.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–65. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 10.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–46. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly DJ, Gershlick T, Witzenbichler B, Guagliumi G, Fahy M, Dangas G, et al. Incidence and predictors of heart failure following percutaneous coronary intervention in ST-segment elevation myocardial infarction: the HORIZONS-AMI trial. Am Heart J. 2011;162:663–70. doi: 10.1016/j.ahj.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Burns RJ, Gibbons RJ, Yi Q, Roberts RS, Miller TD, Schaer GL, et al. CORE Study Investigators The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol. 2002;39:30–6. doi: 10.1016/S0735-1097(01)01711-9. [DOI] [PubMed] [Google Scholar]
- 13.Roes SD, Kelle S, Kaandorp TA, Kokocinski T, Poldermans D, Lamb HJ, et al. Comparison of myocardial infarct size assessed with contrast-enhanced magnetic resonance imaging and left ventricular function and volumes to predict mortality in patients with healed myocardial infarction. Am J Cardiol. 2007;100:930–6. doi: 10.1016/j.amjcard.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Wu E, Ortiz JT, Tejedor P, Lee DC, Bucciarelli-Ducci C, Kansal P, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart. 2008;94:730–6. doi: 10.1136/hrt.2007.122622. [DOI] [PubMed] [Google Scholar]
- 15.Yokota H, Heidary S, Katikireddy CK, Nguyen P, Pauly JM, McConnell MV, et al. Quantitative characterization of myocardial infarction by cardiovascular magnetic resonance predicts future cardiovascular events in patients with ischemic cardiomyopathy. J Cardiovasc Magn Reson. 2008;10:17. doi: 10.1186/1532-429X-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klem I, Shah DJ, White RD, Pennell DJ, van Rossum AC, Regenfus M, et al. Prognostic value of routine cardiac magnetic resonance assessment of left ventricular ejection fraction and myocardial damage: an international, multicenter study. Circ Cardiovasc Imaging. 2011;4:610–9. doi: 10.1161/CIRCIMAGING.111.964965. [DOI] [PubMed] [Google Scholar]
- 17.Ganame J, Messalli G, Masci PG, Dymarkowski S, Abbasi K, Van de Werf F, et al. Time course of infarct healing and left ventricular remodelling in patients with reperfused ST segment elevation myocardial infarction using comprehensive magnetic resonance imaging. Eur Radiol. 2011;21:693–701. doi: 10.1007/s00330-010-1963-8. [DOI] [PubMed] [Google Scholar]
- 18.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction) J Am Coll Cardiol. 2004;44:E1–211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, et al. Cardiovascular Cell Therapy Research Network (CCTRN) Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307:1717–26. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–68. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, et al. REPAIR-AMI Investigators Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 22.Assmus B, Rolf A, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, et al. REPAIR-AMI Investigators Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3:89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- 23.Yee K, Malliaras K, Kanazawa H, Tseliou E, Marban L, Makkar R, et al. Dose-dependent regenerative efficacy and functional improvement in pigs with ischemic cardiomyopathy injected transendocardially with allogeneic cardiospheres. American Heart Association Annual Scientific Sessions 2011. [Google Scholar]
- 24.Terrovitis J, Lautamäki R, Bonios M, Fox J, Engles JM, Yu J, et al. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol. 2009;54:1619–26. doi: 10.1016/j.jacc.2009.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson TD, Christman KL. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin Drug Deliv. 2013;10:59–72. doi: 10.1517/17425247.2013.739156. [DOI] [PubMed] [Google Scholar]
- 26.Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol. 2009;54:1014–23. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Ye Z, Zhou Y, Cai H, Tan W. Myocardial regeneration: Roles of stem cells and hydrogels. Adv Drug Deliv Rev. 2011;63:688–97. doi: 10.1016/j.addr.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Dahlmann J, Krause A, Möller L, Kensah G, Möwes M, Diekmann A, et al. Fully defined in situ cross-linkable alginate and hyaluronic acid hydrogels for myocardial tissue engineering. Biomaterials. 2013;34:940–51. doi: 10.1016/j.biomaterials.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Hyaluronan AA. Cell Mol Life Sci. 2007;64:1591–6. doi: 10.1007/s00018-007-7032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shu XZ, Ahmad S, Liu Y, Prestwich GD. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J Biomed Mater Res A. 2006;79:902–12. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 31.Shu XZ, Ahmad S, Liu Y, Prestwich GD. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J Biomed Mater Res A. 2006;79:902–12. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 32.Prestwich GD, Erickson IE, Zarembinski TI, West M, Tew WP. The translational imperative: making cell therapy simple and effective. Acta Biomater. 2012;8:4200–7. doi: 10.1016/j.actbio.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]


