Abstract
During metamorphosis in holometabolous insects, the nervous system undergoes dramatic remodeling as it transitions from its larval to its adult form. Many neurons are generated through post-embryonic neurogenesis to have adult-specific roles, but perhaps more striking is the dramatic remodeling that occurs to transition neurons from functioning in the larval to the adult nervous system. These neurons exhibit a remarkable degree of plasticity during this transition; many subsets undergo programmed cell death, others remodel their axonal and dendritic arbors extensively, whereas others undergo trans-differentiation to alter their terminal differentiation gene expression profiles. Yet other neurons appear to be developmentally frozen in an immature state throughout larval life, to be awakened at metamorphosis by a process we term temporally-tuned differentiation. These multiple forms of remodeling arise from subtype-specific responses to a single metamorphic trigger, ecdysone. Here, we discuss recent progress in Drosophila melanogaster that is shedding light on how subtype-specific programs of neuronal remodeling are generated during metamorphosis.
Keywords: neuronal identity, plasticity, neuropeptide, differentiation, network remodeling
The mechanisms underlying cellular stability and plasticity have recently received increasing attention in large part spurred by discoveries showing that adult “stable” cells can be trans-differentiated to other specific cellular subtypes1-6 or even driven toward an induced pluripotent stem cell state by the addition of a few choice transcription factors.7,8 These studies demonstrate that cells can display spectacular plasticity in the presence of the right combination of modulatory factors, findings that have profound implications for therapeutic approaches to human disorders. An important goal in neuroscience has been to induce neuronal plasticity in mature dysfunctional neurons in order to restore function. In the majority of the nervous system, dysfunctional neurons cannot be replaced by newborn neurons, and therefore individual neurons must last a lifetime. Mature neurons typically only possess a limited degree of plasticity that is largely restricted to modulation of synaptic contacts. Thus, a longstanding challenge has been how to reactivate plasticity in otherwise stable neurons. Studying mechanisms that underlie such dramatic neuronal plasticity as observed during insect metamorphosis is a rational approach to identifying potential therapeutic approaches.
Metamorphosis in holometabolous insects and amphibians represents a profound alteration of the body plan and of behavior, which both require dramatic nervous system remodeling. In insects, a surge of steroid hormone 20-hydroxyecdysone (ecdysone) at the end of the 3rd larval stage initiates a diversity of metamorphic responses in neuroblasts and postmitotic neurons.9 First, ecdysone triggers post-embryonic neurogenesis that accounts for a tremendous increase in the number of neurons within the nervous system.10 Second, many postmitotic neurons remodel to generate adult circuits (Fig. 1). Dependent upon the neuronal subtype, this may involve programmed cell death,11–13 radical alteration in axonal and dendritic arbors,14 trans-differentiation from one stable cell identity into another15 or the terminal differentiation of developmentally frozen neurons.16
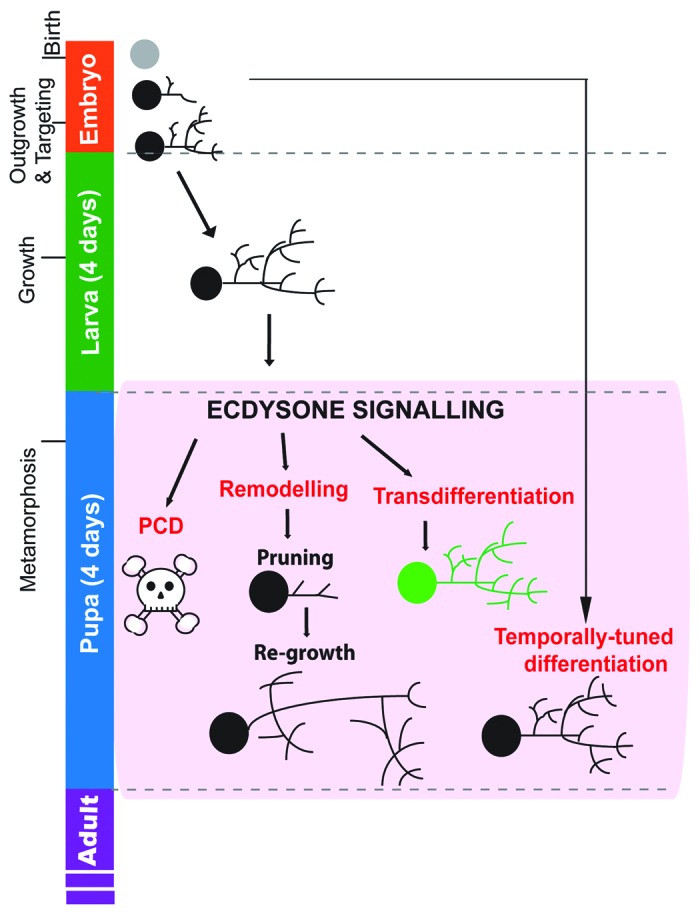
Figure 1. Major forms of neuronal remodeling during ecdysone-driven metamorphosis in Drosophila. Developmental stages of Drosophila melanogaster are shown (at 25°C). During embryonic development (24 h), the embryonic neurons are born and their axons and dendrites subsequently grow to their targets and connect into appropriate circuits for function during larval stages. Most differentiate at this time into mature functional neurons. During larval stages, most neurons undergo expansional growth to accommodate the increasing animal size. Throughout larval stages, subsets of post-embryonic neuroblast lineages (not shown in figure) undergo division to produce new postmitotic neurons. This peaks during the final 48 h of larval life. Ecdysone signaling increases prior to pupariation and prompts metamorphosis (in combination with a drop in juvenile hormone titer and the attainment of critical weight). Ecdysone titers fluctuate throughout metamorphosis (not shown here). Ecdysone signaling prompts 4 major forms of neuronal remodeling that occurs during metamorphosis in the pupa; Programmed cell death (PCD), structural remodeling, transdifferentiation or temporally-tuned differentiation. The exact time of remodeling differs between neuronal subsets. Programmed cell death kills specific neuronal subsets with high segmental and subtype specificity. Structural remodeling is neuronal subtype-specific and remodels larval arbors into adult-specific circuits. Certain neurons remodel only axons, or only dendrites, or both. The first step is pruning of branches back to proximal projections, followed by re-targeting, often to distinct targets. Transdifferentiation is rare and involves the changing of effector gene expression so that the neurons adopt a novel function. Temporally-tuned differentiation has been observed for numerous neuronal populations, involving the developmental freezing of neurons at earlier stages of development prior to mature effector gene expression and axo-dendritic arbor outgrowth.
Although metamorphosis is ultimately triggered by a hormonal surge of ecdysone, the great diversity of neuronal responses points to subtype-specific mechanisms that differentially interpret this signal. In this review, we discuss gene-regulatory cascades that operate within postmitotic neurons to direct their specific programs of metamorphic remodeling, with a focus on processes and mechanisms that provide evidence for subtype-specific gene regulatory control.
Hormonal and Transcriptional Cascades of Insect Metamorphosis
Ecdysone signaling and the resulting nuclear receptor transcriptional cascades direct and coordinate the phases of Drosophila metamorphosis.17-19 Critical players in the initial hormonal signals that drive commitment to metamorphosis are juvenile hormone (JH), insulin signaling and ecdysone. Juvenile hormone is a generic term for a class of sesquiterpenoids secreted by the corpora allata (CA) gland that acts to maintain a larval character through developmental transitions known as ecdyses.20,21 In numerous insect species, experimental manipulation of JH shows that it prevents larval-pupal transformation and its removal can lead to precocious pupariation.22 Work in numerous insects has indicated that the onset of metamorphosis, rather that re-entry into another larval stage, occurs due to a decline in JH titer coupled to the attainment of a critical weight (signaled by insulin signaling23) that ensures sufficient nutritional support for the entire process of metamorphosis.21 Both mechanisms contribute to elevated synthesis and secretion of ecdysteroids by the prothoracic gland.23,24 JH has been shown to interact with multiple pathways including the insulin pathway; however, neither its exact mechanism of action nor any instructional role in neuronal remodeling during metamorphosis are well understood.21
Ecdysteroids are secreted by the prothoracic gland and thereafter converted by peripheral tissues into its bioactive form, 20-hydroxyecdysone (20E) or ecdysone, which drives the onset of metamorphosis.25 Ecdysone binds its nuclear receptor, ecdysone receptor (EcR), which mediates transcriptional responses as a heterodimeric receptor complex with Ultraspiracle (Usp).9,19,26 EcR has three isoforms EcR-A, -B1 and -B2 that differ in their N-termini but share the same ecdysone and DNA binding domains. These isoforms mediate different temporal and spatial outcomes from ecdysone signaling by virtue of differences in their expression and transcriptional activity.27,28
Ecdysone signaling mediates many of its effects through a cascade of nuclear receptors.17-19,27 First identified as primary response genes within ecdysone-induced polytene chromosomal puffs in the salivary gland,29 these include the nuclear receptors, Drosophila hormone receptor DHR3, DHR4 and DHR39, Ecdysone-induced protein E74, E75, E78 and E93), Ftz transcription factor 1 (Ftz-f1) and the zinc-finger transcription factor isoforms that comprise the Broad Complex (BR-C). These are each expressed in complex temporal and spatial patterns that are shaped by ecdysone signaling and also their own elaborate cross-regulatory relationships. In turn, the combinatorial spatiotemporal expression patterns of these nuclear receptors guide each step of metamorphosis at both the global and cell-subtype-specific level, as will be discussed in detail.17-19,27
Programmed Cell Death
Numerous larval neuronal subtypes are not utilized in adults and undergo ecdysone-triggered programmed cell death (PCD) during metamorphosis.13,30 Well-characterized models for metamorphic PCD in Drosophila are the ventral corazonin-expressing peptidergic neurons (vCrz),31 RP2 motoneurons,11 and a population of ~300 neurons known as type II neurons.32 In all three cases, the timed activation of PCD is mediated by caspases and the pro-apoptotic gene reaper (and in the case of type II neurons also grim) in a highly subtype-specific manner.11,12,31,32 Work in vCrz neurons, which die shortly after pupariation, demonstrates that ecdyone signaling induces subtype-specific expression of reaper, the initiator caspases dronc and strica as well as the effector caspases dcp-1 and ice.31
How is metamorphic neuronal PCD triggered in only specific neuronal subsets? The regulatory steps between ecdysone signaling and cell death gene expression in neurons are poorly defined. It is curious that EcR-B1 and B2 mediate PCD of vCrz and RP2 motoneurons in early pupa,11,12 but that the PCD of type II neurons soon after eclosion is correlated with high EcR-A expression (in the absence of EcR-B1).32 Nevertheless, differential expression of specific EcR isoforms is not on its own sufficient to drive a neuron into PCD. Insights derived from studies of ecdysone-induced PCD of other Drosophila tissues offer potential clues for how PCD may be activated.13 Direct binding of EcR-B1 to EcR-response elements (EcR-RE) in the cis-regulatory regions of reaper and dronc is required for their expression and subsequent PCD of salivary glands.33,34 As only cells programmed to die express reaper or dronc, key regulatory steps must occur upstream of cell death gene trans-activation. This makes subtype transcription factor codes, which act with EcR-B1 to induce reaper and dronc expression, a candidate mechanism. BR-C and the nuclear receptors E74 and E93 are required with EcR for reaper and dronc expression, and early ftz-f1 induction can also promote precocious salivary gland death.35 Direct binding of BR-C proteins to cis-regulatory sequences of dronc has been confirmed;33,35 however, cross-regulatory relationships between BR-C and these nuclear receptors complicate an interpretation of direct vs. indirect regulation of cell death gene expression. Chromatin regulation by the arginine-histone methyltransferase Art4 (also CARM1 or CARMER) is also required for dronc expression in a Drosophila cell line, l(2)mbn cells.36 However, as this is recruited to the EcR-RE of dronc upon EcR-B1 induction, to methylate Histone3R17 (H3R17) to promote transcription,37 it would appear that this is permissive rather than instructive for subtype-specific expression.
Is this combination of factors sufficient for the subtype-specificity of PCD? After all, these factors are co-expressed in cells undergoing many different forms of metamorphic change. Cells have different combinations of transcription factors, non-coding RNA’s and genome accessibility, all of which may contribute to cell-specific gene expression. Genomic approaches in salivary glands that have identified genes that change during metamorphosis have listed many transcriptional regulators, some of which may direct a PCD fate for the salivary gland.38,39 These candidates now provide a directed approach to identify such regulators. With respect to vCrz and RP2 neurons, both have served as models for transcriptional assignment of developmental neuronal subtype identity.40,41 Although less is known about their subtype transcription profile during PCD in early pupae, their well-defined developmental transcriptional codes provides a list of candidates that may act in concert with ecdysone-triggered nuclear receptor cascades to drive their subtype-specific PCD. A putative role for such “cell fate” transcription factors is supported by studies of embryonic PCD in specific neuronal lineages of C.elegans and Drosophila. In such cases, newly born postmitotic neurons utilize transcription factors mostly associated with subtype fate determination to regulate hard-wired PCD.42 These include transcription factors that direct lineage- and/or segment-specific cell fates.43,44 As the metamorphic PCD of specific vCrz and RP2 neurons is highly subtype- or segment-specific11,12 we would predict that integration of subtype-transcription factor codes with ecdysone-triggered cascades of nuclear receptors determines which neuronal subtypes undergo metamorphic PCD.
Specific examples of fate-determining transcription factors in regulating subtype-specific PCD in embryonic neurons have been uncovered (reviewed in ref. 42). The sensory-neuron determinant transcription factor senseless has been implicated in the protection of a subset of Bolwig’s organ photoreceptor neurons from PCD at pupariation.15 Also, dMP2 neurons undergo segment-specific PCD in late embryos, with anterior dMP2 neurons dying and posterior abdominal segment A6-A8 dMP2 neurons surviving.45 The A5/A6 transition is marked by Abdominal-B (Abd-B) expression in only the surviving A6-A8 dMP2 neurons. Remarkably, overexpression of Abd-B in all early anterior and posterior dMP2 neurons using the selective odd-GAL4 prevented PCD of anterior dMP2 neurons.45 In contrast, another study demonstrated a lack of anterior dMP2 neurons PCD in either Hb9 or Fkh mutants. Restoration of the pertinent transcription factor in either mutant in early dMP2 neurons (using odd-GAL4) rescued PCD.43 Thus, the cell-fate-determinants HB9, Fkh and Abd-B combinatorially determine segment-specific PCD of dMP2 neurons in embryos. These examples indicate a role for subtype transcription factors in subtype PCD (reviewed in ref. 42), but we have yet to determine how such factors regulate PCD and how they functionally intersect with ecdysone signaling to direct metamorphic PCD. Toward this end, noteworthy are studies that have performed global and tissue-specific microarrays for genes that are altered around pupariation in specific tissues,46,47 or are globally affected by EcR knockdown.47 Moreover, the modENCODE project is expanding the number of transcription factors for which genome-wide binding sites are being determined.48 In this way, one could identify genes that are regulated by EcR and whose cis-regulatory regions are chromatin immunoprecipitated by other transcription factors. Such comparison of available data sets would likely provide novel genes and hypotheses to explore the mechanisms that direct subtype-specific programs of metamorphosis.
Morphological Remodeling: Pruning
Many larval neurons that persist in the adult nervous system undergo extensive re-structuring and re-wiring of their axonal and dendritic arbors at metamorphosis. This spectacular form of neuronal plasticity has been the focus of intense analysis for several decades.14,49 In most neurons, larval arbors first prune back through retraction and/or degeneration, and this is followed by arbor regrowth and re-targeting. Particularly instructive models include mushroom body (MB) γ-neurons,49,50 thoracic ventral Tv4 neurons,28,51,52 peripheral dendritic arborizing (Da) sensory neurons53 and motoneurons.54,55 A number of common principles have emerged from these studies that define core transcriptional and cellular mechanisms of morphological plasticity. Best defined are the elaborate mechanisms for subtype-specific induction of EcR-B1 expression in these neuronal subtypes, which is required to initiate pruning. However, how EcR activation in these neurons promotes arbor remodeling as opposed to PCD or trans-differentiation is not well established.
EcR-B1 function is required for axonal or dendritic pruning of Tv4 neurons,51 MB γ-neurons,56 dendritic arborizing (Da) sensory neurons53,57 and motoneurons55 during early pupariation. While it is evident that EcR-B1 is expressed in many neurons during metamorphosis, its expression appears to be under strict subtype-specific control. For example, EcR-B1 expression in the mushroom body is selectively upregulated only in the subset of neurons destined to remodel, the γ-neurons.56 Recent studies have pointed to two parallel pathways that act non-redundantly to induce subtype-specific EcR-B1 expression in remodeling neurons: Extrinsic TGFβ-signaling and an intrinsic interplay between the nuclear receptors Ftz-f1 and Hr39.49,55
ftz-f1 is essential for EcR-B1 activation and axon pruning in MB γ-neurons.49ftz-f1 null mutant MB neuron clones fail to express EcR-B1 and retain a larval phenotype into adulthood that is rescued by restoration of EcR-B1.58 Prompted by evidence for functional antagonism between ftz-f1 and Hr39, in part mediated through competition at the same DNA motif,59,60 the authors examined a potential relationship between ftz-f1 and Hr39. Intriguingly, Hr39 mutants did not affect MB γ-neuron remodeling but its overexpression reduced EcR-B1 expression and inhibited pruning. This, too, was rescued by co-overexpression of EcR-B1.58 Detailed epistatic analysis clarified the relationship between ftz-f1 and Hr39. First, the expression of Hr39 was increased in ftz-f1 mutant clones. Second, the loss of pruning in ftz-f1 mutants was partially restored in Hr39/ftz-f1 double mutant clones, commensurate with a rescue of EcR-B1 expression. Thus, a key role for ftz-f1 appears to be the downregulation of Hr39. Competition between the two at the level of EcR-B1 regulation was further uncovered by showing that pruning and EcR-B1 expression, observed upon Hr39 overexpression, was rescued by co-overexpression of UAS-ftz-f1. As Ftz-f1 and Hr39 compete for binding to the same DNA motif in cis-regulatory regions for numerous genes,59,60 these data suggest that Ftz-f1 likely plays a triple-assurance role to increase EcR-B1 expression: It represses Hr39 expression, activates EcR-B1 by out-competing the repressive-acting Hr39 and acts as a trans-activator itself.
The importance of extrinsic context in selecting neurons for remodeling has been illuminated by studies showing that TGFβ-signaling is required for EcR-B1 expression in MB γ-neurons. Glia that infiltrate the MB, prior to pruning, secrete the TGFβ-ligand myoglianin, which triggers TGFβ-signal transduction within the MB γ-neurons. Glial-specific RNAi-mediated knockdown of Myoglianin blocked EcR-B1 expression and axon pruning of MB γ-neurons.61 Likewise, MB γ-neuronal clones mutant for TGFβ receptors or their signal transducer, dSmad2, failed to express EcR-B1 and failed to prune, and this was rescued by restoration of EcR-B1.50,62
A recent study has pointed to parallel-acting TGFβ and ftz-f1/Hr39 pathways in the pruning of other neurons.55 Within the first 12 h of puparium formation, motoneurons prune back from their larval muscle targets. Motoneuron retraction fails in ftz-f1 mutants, is inhibited by overexpression of Hr39, but is reestablished by Hr39 and EcR-B1 co-expression. Motoneuron pruning also requires extrinsic signaling from the muscles they target, much as MB γ-neurons require glial signaling. In muscle, ftz-f1 is required to shut off Hr39 and this is believed to be upstream of a muscle-derived TGFβ signal that the motoneurons require for their expression of EcR-B1, and ultimately pruning.55
Events downstream of EcR-B1 expression that result in an axon pruning response are poorly defined. It should first be mentioned that not all arbors prune in the same way; it can occur by retraction (motoneuron axons55), fragmentation and degeneration (MB γ-neuronal axons63) or a combination of the two (da sensory neuronal dendrites53). Intriguingly, the few detailed studies performed have found that degenerative mechanisms correlate with microtubule dismantling,63 while retractive mechanisms correlate primarily with the initial removal of actin-organizing proteins.55 Numerous studies have started to identify genes and pathways that are induced by EcR and contribute to pruning, most notably a genomic analysis of EcR-dependent gene expression in remodeling MB γ-neurons.64 The ubiquitin-proteasome protein pathway (UPS), which mediates protein degradation, is required for pruning in MB γ-neurons63 and peripheral sensory neurons,65 and an analysis of EcR-B1-dependent gene expression in MB γ-neurons demonstrated that most genes within the UPS are upregulated by EcR-B1.64 Other genes that play functional roles in pruning are implicated in cytoskeletal-remodeling including Ik2 kinase, Katanin p60-like 1 (Kat-60L1)66 and Mical.57
What regulatory mechanisms exist downstream of or in parallel to EcR-B1 expression to coordinate the induction of these effector genes? Surprisingly, certain effector pathways that would a priori appear to be logical players, such as the BR-C genes, E74, E75 and Kr-H1, all classically-defined EcR targets and ecdysone signaling effectors,9 are not essential for MB γ-neuron pruning, even though they are induced by EcR-B1 in these neurons.56,64,67 An important clue came from an RNAi screen for genes previously shown to be EcR-dependent in numerous tissues.57 The authors found that the transcription factor Sox14 is upregulated by EcR-B1 in peripheral sensory neurons and is necessary for pruning. Importantly, forced early expression of sox14 induced precocious pruning, showing it to be a sufficient step in pruning. It is interesting to note that sox14 is upregulated in an EcR-B1-dependent manner in remodeling MB γ-neurons.64 The authors also provide a robust link from EcR-B1 through Sox14 to an effector of cytoskeletal organization. They show that Sox14 upregulates Mical, an actin-filament interacting protein, that is also required for pruning. Moreover, the loss of pruning, due to disruption of Sox14 or EcR-B1 function, can be rescued by restoration of Mical expression.57
These data fill important gaps in our understanding of intrinsic neuronal cascades that direct subtype-specific responses yet much has yet to be learnt. For example, Sox14 is also upregulated by EcR-B1 at metamorphosis in another subset of sensory neurons and is required for them to undergo PCD, but Mical is not required for PCD of those neurons.
Morphological Remodeling: Regrowth
Following pruning, neuronal arbors are re-grown and re-targeted to form functional adult specific neuronal networks. Compared with pruning, relatively little is understood regarding the mechanisms of regrowth, however studies in FMRF-expressing Tv4 neurons,28,51 MB γ-neurons68 and olfactory projections neurons69 have proven illuminating. Pruning of Tv4 neurons is lost in EcR mutants, in which the B isoforms are not expressed, and this is rescued by a Tv4-specific GAL4 driving expression of either B1 or B2, but not EcR-A isoforms.28,51 Interestingly, while Tv4 pruning was inhibited by overexpression of two dominant negative forms of EcR (F645A and W650A) that block transcriptional activation, Tv4 regrowth was inhibited only by the W650A form, which, unlike F645A, is thought to lack ligand-dependent transcriptional de-repression. These results led the authors to propose that Tv4 axon pruning is directed by ligand-mediated transcriptional activation and regrowth is mediated by transcriptional de-repression. Results such as these demonstrate the complexity of EcR activity and the care that must be taken in interpreting the results of EcR manipulation.
MB γ-neurons undergo regrowth following their pruning (detailed above). A MARCM screen for piggyBac insertional mutants that disrupted MB γ-neuron remodeling identified the nuclear receptor UNF (also Hr51) as specifically blocking regrowth.68 Further analysis showed that UNF selectively regulated regrowth but neither developmental growth nor pruning, even though UNF is expressed throughout all these stages. Taking a lead from mouse studies that identified genes regulated by the mouse UNF ortholog, Nr2e3, the authors explored a potential role for TOR signaling in UNF-dependent regrowth. In confirmation of their hypothesis, MARCM clones for TOR mutant alleles specifically blocked regrowth, and expression of UAS-TOR in UNF mutant clones rescued axon regrowth. Further detailed analysis provided evidence for the role of the Tsc1/Rheb/TOR/S6K pathway in stimulating regrowth downstream of UNF, yet remarkably, not the appropriate guidance of regrowing γ-neurons. These results demonstrate that UNF coordinates growth and guidance through distinct molecular mechanisms. Many subtype-specific transcription factors have been identified to direct guided growth in development, but not to affect growth itself.70 We would anticipate that such subtype-specific transcription factors act within MB γ-neurons to direct guidance of Tor-stimulated regrowing axons. However, it will be particularly interesting to determine why these transcription factors would act in an UNF-dependent manner. Are they regulated transcriptionally by UNF, or is UNF required to act cooperatively with these transcription factors?
Olfactory circuits provide a powerful model to decipher subtype-specific mechanism of neuronal remodeling. Olfactory projection neurons (PNs) are second-order olfactory neurons that receive input from olfactory receptor neurons (ORNs) and relay this to the MB and lateral horn. PN dendrites synapse with ORN axons within one or a few of the 50 antennal lobe glomeruli. Subsets of embryonic-born PNs have been identified that connect to larval ORNs in glomeruli of the larval olfactory lobe. During metamorphosis, the larval ORNs undergo PCD and the larval antennal lobe degenerates. However, the PNs persist; they retract their dendrites and then regrow them into specific glomeruli of the newly forming adult antennal lobe to receive axonal contact from incoming newly generated adult ORNs.71 This pruning is under the direction of ecdysone and TGFß signaling, as are MB γ-neurons and motoneurons (see above).69 Important to furthering our understanding of the subtype-specific mechanisms of neuronal remodeling, the lineage, birth order and an elaborate combinatorial code of transcription factors that differentiate PN subtype identity and their specific connectivity has been characterized.69,72,73 Primarily by mutant analysis in MARCM clones, a combination of transcription factors, acj6, drifter,74 lola,73 islet, lim1, squeeze, cut,75 Chinmo76 and empty spiracles,77 have been shown to control precise dendritic targeting of PNs. However, details of the downstream genes targeted by such combinatorial transcriptional codes awaits discovery. With such detailed information regarding the trigger for remodeling (TGFß signaling and EcR-B1), as well as the subtype-specific transcriptional codes that determine selective axodendritic targeting, we anticipate this model to provide tremendous advances in our understanding of how these intersect to coordinate remodeling, and perhaps also provide insight into why these neurons undergo arbor remodeling, as opposed to any other form of metamorphic change.
Trans-Differentiation
Developmental trans-differentiation in postmitotic neurons has been reported in vertebrates; zebrafish dorsal root ganglia (DRG) sensory neurons trans-differentiate into sympathetic ganglia neurons upon migration from the DRG to a new environment.78 Also, opsin gene expression in rainbow trout and Pacific pink salmon photoreceptors changed from UV- to blue-sensing in response to maturation and lifestyle change.79,80 However, it is in the realm of cellular reprogramming that the mechanisms of trans-differentiation might be most gainfully understood. For example, adult cells can be forcibly trans-differentiated in a directed manner by adding a limited cocktail of key transcription factors,5,6,81 presenting novel avenues to cellular replacement as a therapy.2 Thus, analysis of metamorphic trans-differentiation will likely reveal basic mechanisms that may prove useful to understanding how to manipulate trans-differentiation in a therapeutic setting.
To our knowledge, only one clear case of neuronal trans-differentiation in a functioning postmitotic neuron has been described in Drosophila. In larvae, each Bolwig’s organ (a larval light-sensing organ) comprises 12 photoreceptors; 8 green-sensitive (expressing Rhodopsin Rh6) and 4 blue sensitive (expressing Rh5). During metamorphosis, the Rh6-photoreceptor neurons undergo PCD, but Rh5-photoreceptors trans-differentiate into Rh6-expressing photoreceptors. Functionally, this switches these neurons from blue to green light sensitivity. Both PCD and trans-differentiation require cell-autonomous EcR function, as Rh5-neuron trans-differentiation and Rh6 neuron death can both be prevented by cell-autonomous blockade of EcR. However, whereas there is evidence indicating that senseless may act to spare Rh6-photpreceptors from PCD, the mechanisms that underlie the Rh5 to Rh6 switch are unknown.
Aside from the simple possibility that it may not be a widespread phenomenon, the rarity of reported cases of trans-differentiation may reflect the technical challenges associated with identifying neurons that undergo trans-differentiation. Such studies require establishing the expression of a perdurant or permanent marker from a subtype-specific promoter that can then be tracked at later stages. In Drosophila, this can be achieved using a subtype-specific promoter to drive tagged histone proteins that perdure through the trans-differentiation period15 or the activation of Flp recombinase in specific cells prior to trans-differentiation that excises a FRT-flanked stop cassette to bring a reporter under the control of a ubiquitous promoter.16,82 Other approaches such as MARCM (mosaic analysis with a repressible cell marker56) can be used to study trans-differentiation assuming that specific neurons can be reproducibility labeled, as has been performed for embryonic-born olfactory projection neurons that remodel their arbors at metamorphosis.69
Temporally-Tuned Differentiation
Numerous neuronal subtypes become postmitotic in early development, either in the embryo or early larval stages, but remain in a developmentally-frozen state until their eventual terminal differentiation around the time of pupariation.16 This process that we term “temporally-tuned differentiation”16 has been observed in mesothoracic motoneuron 5 (MN5),83 FMRFa-expressing Tv3 (Tva) neurons,84,85 the giant fiber neurons86 and a subset of CCAP-expressing efferent neurons (CCAP-EN).16 In each case, neurons appear to be morphologically and/or molecularly undifferentiated until pupariation, at which time they functionally mature and are incorporated into adult networks.
Recent analysis of the CCAP-neuronal network by our group has provided insight into the underlying mechanisms. CCAP-neurons and their peptide hormone battery of CCAP, Bursicon-α and Bursicon-β are required for the execution of pupal ecdysis, wherein the head and appendages are everted early in metamorphosis to establish the adult’s body plan. We found that a late differentiating subset of 12 CCAP-ENs are sufficient (after the ablation of 48 early-differentiating CCAP-neurons) for the proper execution of pupal ecdysis. These neurons become postmitotic and start to express a CCAP-EN subtype-specific combination of markers in the embryo, but remain immature throughout larval development. Beginning in late L3 larva and proceeding through the first 12 h of pupariation, they terminally differentiate; they initiate expression of CCAP, Bursicon-α and Bursicon-β and project their axon out of the CNS via peripheral segmental nerves. By expression of dominant negative (W650A) EcR receptors, EcR-A or -B1 were found to be non-redundantly required for terminal differentiation and that forced early expression of Ftz-f1 in mid-stage larvae preciously induced expression of all peptide hormones.
Studies described in detail above show that Ftz-f1 induces EcR-B1 expression in MB γ-neurons and motoneurons in part through repression of Hr39 expression.55,58 It will be intriguing to examine whether this relationship exists to differentiate late CCAP-ENs and, moreover, how Ftz-f1 acts in a sufficient manner. Further work to identify the subtype transcription factor code acting in CCAP-neurons would also allow a dissection of the intersection of nuclear receptor function with subtype transcription networks to generate such precise temporally-tuned terminal differentiation programs.
Another regulator of temporally-induced differentiation is TGFβ signaling. A subset of ~40 atonal-positive neurons known as the Dorsal Cluster (Dc) neurons is born in early larval stages and retains an immature morphology until metamorphosis.62 TGFβ signaling is required within these neurons for differentiation of a mature arbor, where it appears to act permissively, as precocious activation of TGFβ signaling failed to induce early remodeling
Concluding Remarks
Tremendous progress has been made in identifying mechanisms that direct metamorphic remodeling of Drosophila neurons. As would be expected, nuclear receptors play a critical triggering and organizing role. However, much work remains in order to understand how the type of metamorphic remodeling (PCD, remodeling dendritic and/or axonal arbors, trans-differentiation or temporally-tuned differentiation) is coordinated with the regulation of subtype-specific metamorphic changes (activation/changing of specific subtype-defining or guidance molecule genes). Currently, it is unclear if these are distinct pathways that act in parallel, or if the coordination of both is mediated by shared regulators with overlapping function. It would certainly be informative to identify signatures of distinct metamorphic events by genome-wide gene-expression analysis of multiple remodeling neuronal subsets, as has been performed in MB γ-neurons.64 Also, in neuronal subsets where elaborate subtype-specific transcriptional codes have been defined, it would be informative to examine whether the same transcription factors shape the “type” of metamorphic remodeling as well as regulate the subtype-specific gene expression profiles that are induced to effect the changes that are observed. As noted in the introduction, mature cells appear to retain the capacity for plasticity if provided an appropriate cocktail of transcriptional regulators. This offers us the opportunity to therapeutically target the mature and aging nervous system to restore function by rescuing gene expression and promoting regrowth. In this light, we anticipate that exploration of how stable larval neurons in the insect nervous system are triggered to undergo elaborate forms of plastic change during metamorphosis will continue to provide key insight into how we might approach such therapies.
Acknowledgments
The authors are very grateful to the Dr. Michael Gordon (UBC, Canada) for valuable critique of the manuscript prior to submission.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/23969
References
- 1.Graf T, Enver T. Forcing cells to change lineages. Nature. 2009;462:587–94. doi: 10.1038/nature08533. [DOI] [PubMed] [Google Scholar]
- 2.Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A. 2011;108:10343–8. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–86. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–11. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704–12. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gáliková M, Klepsatel P, Senti G, Flatt T. Steroid hormone regulation of C. elegans and Drosophila aging and life history. Exp Gerontol. 2011;46:141–7. doi: 10.1016/j.exger.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Truman JW, Bate M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol. 1988;125:145–57. doi: 10.1016/0012-1606(88)90067-X. [DOI] [PubMed] [Google Scholar]
- 11.Winbush A, Weeks JC. Steroid-triggered, cell-autonomous death of a Drosophila motoneuron during metamorphosis. Neural Dev. 2011;6:15. doi: 10.1186/1749-8104-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi YJ, Lee G, Park JH. Programmed cell death mechanisms of identifiable peptidergic neurons in Drosophila melanogaster. Development. 2006;133:2223–32. doi: 10.1242/dev.02376. [DOI] [PubMed] [Google Scholar]
- 13.Yin VP, Thummel CS. Mechanisms of steroid-triggered programmed cell death in Drosophila. Semin Cell Dev Biol. 2005;16:237–43. doi: 10.1016/j.semcdb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Williams DW, Truman JW. Remodeling dendrites during insect metamorphosis. J Neurobiol. 2005;64:24–33. doi: 10.1002/neu.20151. [DOI] [PubMed] [Google Scholar]
- 15.Sprecher SG, Desplan C. Switch of rhodopsin expression in terminally differentiated Drosophila sensory neurons. Nature. 2008;454:533–7. doi: 10.1038/nature07062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veverytsa L, Allan DW. Temporally tuned neuronal differentiation supports the functional remodeling of a neuronal network in Drosophila. Proc Natl Acad Sci U S A. 2012;109:E748–56. doi: 10.1073/pnas.1114710109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan AA, Thummel CS. Temporal profiles of nuclear receptor gene expression reveal coordinate transcriptional responses during Drosophila development. Mol Endocrinol. 2003;17:2125–37. doi: 10.1210/me.2002-0430. [DOI] [PubMed] [Google Scholar]
- 18.Thummel CS. Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev Cell. 2001;1:453–65. doi: 10.1016/S1534-5807(01)00060-0. [DOI] [PubMed] [Google Scholar]
- 19.King-Jones K, Thummel CS. Nuclear receptors--a perspective from Drosophila. Nat Rev Genet. 2005;6:311–23. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- 20.Bendena WG, Zhang J, Burtenshaw SM, Tobe SS. Evidence for differential biosynthesis of juvenile hormone (and related) sesquiterpenoids in Drosophila melanogaster. Gen Comp Endocrinol. 2011;172:56–61. doi: 10.1016/j.ygcen.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 22.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2012;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 23.Mirth CK, Riddiford LM. Size assessment and growth control: how adult size is determined in insects. Bioessays. 2007;29:344–55. doi: 10.1002/bies.20552. [DOI] [PubMed] [Google Scholar]
- 24.Marchal E, Vandersmissen HP, Badisco L, Van de Velde S, Verlinden H, Iga M, et al. Control of ecdysteroidogenesis in prothoracic glands of insects: a review. Peptides. 2010;31:506–19. doi: 10.1016/j.peptides.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Rees HH. Ecdysteroid biosynthesis and inactivation in relation to function. Eur J Entomol. 1995;92:9–39. [Google Scholar]
- 26.Thummel CS. From embryogenesis to metamorphosis: the regulation and function of Drosophila nuclear receptor superfamily members. Cell. 1995;83:871–7. doi: 10.1016/0092-8674(95)90203-1. [DOI] [PubMed] [Google Scholar]
- 27.Cherbas L, Hu X, Zhimulev I, Belyaeva E, Cherbas P. EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development. 2003;130:271–84. doi: 10.1242/dev.00205. [DOI] [PubMed] [Google Scholar]
- 28.Schubiger M, Tomita S, Sung C, Robinow S, Truman JW. Isoform specific control of gene activity in vivo by the Drosophila ecdysone receptor. Mech Dev. 2003;120:909–18. doi: 10.1016/S0925-4773(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 29.Baehrecke EH. Ecdysone signaling cascade and regulation of Drosophila metamorphosis. Arch Insect Biochem Physiol. 1996;33:231–44. doi: 10.1002/(SICI)1520-6327(1996)33:3/4<231::AID-ARCH5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 30.Tissot M, Stocker RF. Metamorphosis in drosophila and other insects: the fate of neurons throughout the stages. Prog Neurobiol. 2000;62:89–111. doi: 10.1016/S0301-0082(99)00069-6. [DOI] [PubMed] [Google Scholar]
- 31.Lee G, Wang Z, Sehgal R, Chen CH, Kikuno K, Hay B, et al. Drosophila caspases involved in developmentally regulated programmed cell death of peptidergic neurons during early metamorphosis. J Comp Neurol. 2011;519:34–48. doi: 10.1002/cne.22498. [DOI] [PubMed] [Google Scholar]
- 32.Robinow S, Draizen TA, Truman JW. Genes that induce apoptosis: transcriptional regulation in identified, doomed neurons of the Drosophila CNS. Dev Biol. 1997;190:206–13. doi: 10.1006/dbio.1997.8696. [DOI] [PubMed] [Google Scholar]
- 33.Jiang C, Lamblin AF, Steller H, Thummel CS. A steroid-triggered transcriptional hierarchy controls salivary gland cell death during Drosophila metamorphosis. Mol Cell. 2000;5:445–55. doi: 10.1016/S1097-2765(00)80439-6. [DOI] [PubMed] [Google Scholar]
- 34.Cakouros D, Daish TJ, Kumar S. Ecdysone receptor directly binds the promoter of the Drosophila caspase dronc, regulating its expression in specific tissues. J Cell Biol. 2004;165:631–40. doi: 10.1083/jcb.200311057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CY, Simon CR, Woodard CT, Baehrecke EH. Genetic mechanism for the stage- and tissue-specific regulation of steroid triggered programmed cell death in Drosophila. Dev Biol. 2002;252:138–48. doi: 10.1006/dbio.2002.0838. [DOI] [PubMed] [Google Scholar]
- 36.Cakouros D, Daish TJ, Mills K, Kumar S. An arginine-histone methyltransferase, CARMER, coordinates ecdysone-mediated apoptosis in Drosophila cells. J Biol Chem. 2004;279:18467–71. doi: 10.1074/jbc.M400972200. [DOI] [PubMed] [Google Scholar]
- 37.Cakouros D, Mills K, Denton D, Paterson A, Daish T, Kumar S. dLKR/SDH regulates hormone-mediated histone arginine methylation and transcription of cell death genes. J Cell Biol. 2008;182:481–95. doi: 10.1083/jcb.200712169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CY, Clough EA, Yellon P, Teslovich TM, Stephan DA, Baehrecke EH. Genome-wide analyses of steroid- and radiation-triggered programmed cell death in Drosophila. Curr Biol. 2003;13:350–7. doi: 10.1016/S0960-9822(03)00085-X. [DOI] [PubMed] [Google Scholar]
- 39.Gorski SM, Chittaranjan S, Pleasance ED, Freeman JD, Anderson CL, Varhol RJ, et al. A SAGE approach to discovery of genes involved in autophagic cell death. Curr Biol. 2003;13:358–63. doi: 10.1016/S0960-9822(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 40.McDonald JA, Fujioka M, Odden JP, Jaynes JB, Doe CQ. Specification of motoneuron fate in Drosophila: integration of positive and negative transcription factor inputs by a minimal eve enhancer. J Neurobiol. 2003;57:193–203. doi: 10.1002/neu.10264. [DOI] [PubMed] [Google Scholar]
- 41.Karcavich R, Doe CQ. Drosophila neuroblast 7-3 cell lineage: a model system for studying programmed cell death, Notch/Numb signaling, and sequential specification of ganglion mother cell identity. J Comp Neurol. 2005;481:240–51. doi: 10.1002/cne.20371. [DOI] [PubMed] [Google Scholar]
- 42.Miguel-Aliaga I, Thor S. Programmed cell death in the nervous system--a programmed cell fate? Curr Opin Neurobiol. 2009;19:127–33. doi: 10.1016/j.conb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Miguel-Aliaga I, Thor S, Gould AP. Postmitotic specification of Drosophila insulinergic neurons from pioneer neurons. PLoS Biol. 2008;6:e58. doi: 10.1371/journal.pbio.0060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suska A, Miguel-Aliaga I, Thor S. Segment-specific generation of Drosophila Capability neuropeptide neurons by multi-faceted Hox cues. Dev Biol. 2011;353:72–80. doi: 10.1016/j.ydbio.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miguel-Aliaga I, Thor S. Segment-specific prevention of pioneer neuron apoptosis by cell-autonomous, postmitotic Hox gene activity. Development. 2004;131:6093–105. doi: 10.1242/dev.01521. [DOI] [PubMed] [Google Scholar]
- 46.Li TR, White KP. Tissue-specific gene expression and ecdysone-regulated genomic networks in Drosophila. Dev Cell. 2003;5:59–72. doi: 10.1016/S1534-5807(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 47.Beckstead RB, Lam G, Thummel CS. The genomic response to 20-hydroxyecdysone at the onset of Drosophila metamorphosis. Genome Biol. 2005;6:R99. doi: 10.1186/gb-2005-6-12-r99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nègre N, Brown CD, Ma L, Bristow CA, Miller SW, Wagner U, et al. A cis-regulatory map of the Drosophila genome. Nature. 2011;471:527–31. doi: 10.1038/nature09990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Awasaki T, Lee T. Orphan nuclear receptors control neuronal remodeling during fly metamorphosis. Nat Neurosci. 2011;14:6–7. doi: 10.1038/nn0111-6. [DOI] [PubMed] [Google Scholar]
- 50.Zheng X, Wang J, Haerry TE, Wu AY, Martin J, O’Connor MB, et al. TGF-beta signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell. 2003;112:303–15. doi: 10.1016/S0092-8674(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 51.Schubiger M, Wade AA, Carney GE, Truman JW, Bender M. Drosophila EcR-B ecdysone receptor isoforms are required for larval molting and for neuron remodeling during metamorphosis. Development. 1998;125:2053–62. doi: 10.1242/dev.125.11.2053. [DOI] [PubMed] [Google Scholar]
- 52.Brown HL, Cherbas L, Cherbas P, Truman JW. Use of time-lapse imaging and dominant negative receptors to dissect the steroid receptor control of neuronal remodeling in Drosophila. Development. 2006;133:275–85. doi: 10.1242/dev.02191. [DOI] [PubMed] [Google Scholar]
- 53.Williams DW, Truman JW. Cellular mechanisms of dendrite pruning in Drosophila: insights from in vivo time-lapse of remodeling dendritic arborizing sensory neurons. Development. 2005;132:3631–42. doi: 10.1242/dev.01928. [DOI] [PubMed] [Google Scholar]
- 54.Liu Z, Chen Y, Wang D, Wang S, Zhang YQ. Distinct presynaptic and postsynaptic dismantling processes of Drosophila neuromuscular junctions during metamorphosis. J Neurosci. 2010;30:11624–34. doi: 10.1523/JNEUROSCI.0410-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boulanger A, Farge M, Ramanoudjame C, Wharton K, Dura JM. Drosophila motor neuron retraction during metamorphosis is mediated by inputs from TGF-β/BMP signaling and orphan nuclear receptors. PLoS One. 2012;7:e40255. doi: 10.1371/journal.pone.0040255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee T, Marticke S, Sung C, Robinow S, Luo L. Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron. 2000;28:807–18. doi: 10.1016/S0896-6273(00)00155-0. [DOI] [PubMed] [Google Scholar]
- 57.Kirilly D, Gu Y, Huang Y, Wu Z, Bashirullah A, Low BC, et al. A genetic pathway composed of Sox14 and Mical governs severing of dendrites during pruning. Nat Neurosci. 2009;12:1497–505. doi: 10.1038/nn.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boulanger A, Clouet-Redt C, Farge M, Flandre A, Guignard T, Fernando C, et al. ftz-f1 and Hr39 opposing roles on EcR expression during Drosophila mushroom body neuron remodeling. Nat Neurosci. 2011;14:37–44. doi: 10.1038/nn.2700. [DOI] [PubMed] [Google Scholar]
- 59.Crispi S, Giordano E, D’Avino PP, Furia M. Cross-talking among Drosophila nuclear receptors at the promiscuous response element of the ng-1 and ng-2 intermolt genes. J Mol Biol. 1998;275:561–74. doi: 10.1006/jmbi.1997.1473. [DOI] [PubMed] [Google Scholar]
- 60.Ayer S, Walker N, Mosammaparast M, Nelson JP, Shilo BZ, Benyajati C. Activation and repression of Drosophila alcohol dehydrogenase distal transcription by two steroid hormone receptor superfamily members binding to a common response element. Nucleic Acids Res. 1993;21:1619–27. doi: 10.1093/nar/21.7.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Awasaki T, Huang Y, O’Connor MB, Lee T. Glia instruct developmental neuronal remodeling through TGF-β signaling. Nat Neurosci. 2011;14:821–3. doi: 10.1038/nn.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng X, Zugates CT, Lu Z, Shi L, Bai JM, Lee T. Baboon/dSmad2 TGF-beta signaling is required during late larval stage for development of adult-specific neurons. EMBO J. 2006;25:615–27. doi: 10.1038/sj.emboj.7600962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watts RJ, Hoopfer ED, Luo L. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;38:871–85. doi: 10.1016/S0896-6273(03)00295-2. [DOI] [PubMed] [Google Scholar]
- 64.Hoopfer ED, Penton A, Watts RJ, Luo L. Genomic analysis of Drosophila neuronal remodeling: a role for the RNA-binding protein Boule as a negative regulator of axon pruning. J Neurosci. 2008;28:6092–103. doi: 10.1523/JNEUROSCI.0677-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuo CT, Jan LY, Jan YN. Dendrite-specific remodeling of Drosophila sensory neurons requires matrix metalloproteases, ubiquitin-proteasome, and ecdysone signaling. Proc Natl Acad Sci U S A. 2005;102:15230–5. doi: 10.1073/pnas.0507393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee HH, Jan LY, Jan YN. Drosophila IKK-related kinase Ik2 and Katanin p60-like 1 regulate dendrite pruning of sensory neuron during metamorphosis. Proc Natl Acad Sci U S A. 2009;106:6363–8. doi: 10.1073/pnas.0902051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi L, Lin S, Grinberg Y, Beck Y, Grozinger CM, Robinson GE, et al. Roles of Drosophila Kruppel-homolog 1 in neuronal morphogenesis. Dev Neurobiol. 2007;67:1614–26. doi: 10.1002/dneu.20537. [DOI] [PubMed] [Google Scholar]
- 68.Yaniv SP, Issman-Zecharya N, Oren-Suissa M, Podbilewicz B, Schuldiner O. Axon regrowth during development and regeneration following injury share molecular mechanisms. Curr Biol. 2012;22:1774–82. doi: 10.1016/j.cub.2012.07.044. [DOI] [PubMed] [Google Scholar]
- 69.Marin EC, Watts RJ, Tanaka NK, Ito K, Luo L. Developmentally programmed remodeling of the Drosophila olfactory circuit. Development. 2005;132:725–37. doi: 10.1242/dev.01614. [DOI] [PubMed] [Google Scholar]
- 70.Landgraf M, Thor S. Development and structure of motoneurons. Int Rev Neurobiol. 2006;75:33–53. doi: 10.1016/S0074-7742(06)75002-4. [DOI] [PubMed] [Google Scholar]
- 71.Jefferis GS, Vyas RM, Berdnik D, Ramaekers A, Stocker RF, Tanaka NK, et al. Developmental origin of wiring specificity in the olfactory system of Drosophila. Development. 2004;131:117–30. doi: 10.1242/dev.00896. [DOI] [PubMed] [Google Scholar]
- 72.Jefferis GS, Marin EC, Stocker RF, Luo L. Target neuron prespecification in the olfactory map of Drosophila. Nature. 2001;414:204–8. doi: 10.1038/35102574. [DOI] [PubMed] [Google Scholar]
- 73.Spletter ML, Liu J, Liu J, Su H, Giniger E, Komiyama T, et al. Lola regulates Drosophila olfactory projection neuron identity and targeting specificity. Neural Dev. 2007;2:14. doi: 10.1186/1749-8104-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Komiyama T, Johnson WA, Luo L, Jefferis GS. From lineage to wiring specificity. POU domain transcription factors control precise connections of Drosophila olfactory projection neurons. Cell. 2003;112:157–67. doi: 10.1016/S0092-8674(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 75.Komiyama T, Luo L. Intrinsic control of precise dendritic targeting by an ensemble of transcription factors. Curr Biol. 2007;17:278–85. doi: 10.1016/j.cub.2006.11.067. [DOI] [PubMed] [Google Scholar]
- 76.Zhu S, Lin S, Kao CF, Awasaki T, Chiang AS, Lee T. Gradients of the Drosophila Chinmo BTB-zinc finger protein govern neuronal temporal identity. Cell. 2006;127:409–22. doi: 10.1016/j.cell.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 77.Lichtneckert R, Nobs L, Reichert H. Empty spiracles is required for the development of olfactory projection neuron circuitry in Drosophila. Development. 2008;135:2415–24. doi: 10.1242/dev.022210. [DOI] [PubMed] [Google Scholar]
- 78.Wright MA, Mo W, Nicolson T, Ribera AB. In vivo evidence for transdifferentiation of peripheral neurons. Development. 2010;137:3047–56. doi: 10.1242/dev.052696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng CL, Flamarique IN. Chromatic organization of cone photoreceptors in the retina of rainbow trout: single cones irreversibly switch from UV (SWS1) to blue (SWS2) light sensitive opsin during natural development. J Exp Biol. 2007;210:4123–35. doi: 10.1242/jeb.009217. [DOI] [PubMed] [Google Scholar]
- 80.Cheng CL, Novales Flamarique I. Opsin expression: new mechanism for modulating colour vision. Nature. 2004;428:279. doi: 10.1038/428279a. [DOI] [PubMed] [Google Scholar]
- 81.Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, et al. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9:374–82. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–40. doi: 10.1016/0092-8674(93)90072-X. [DOI] [PubMed] [Google Scholar]
- 83.Consoulas C, Restifo LL, Levine RB. Dendritic remodeling and growth of motoneurons during metamorphosis of Drosophila melanogaster. J Neurosci. 2002;22:4906–17. doi: 10.1523/JNEUROSCI.22-12-04906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schneider LE, Sun ET, Garland DJ, Taghert PH. An immunocytochemical study of the FMRFamide neuropeptide gene products in Drosophila. J Comp Neurol. 1993;337:446–60. doi: 10.1002/cne.903370308. [DOI] [PubMed] [Google Scholar]
- 85.Schneider LE, Roberts MS, Taghert PH. Cell type-specific transcriptional regulation of the Drosophila FMRFamide neuropeptide gene. Neuron. 1993;10:279–91. doi: 10.1016/0896-6273(93)90318-L. [DOI] [PubMed] [Google Scholar]
- 86.Allen MJ, Drummond JA, Moffat KG. Development of the giant fiber neuron of Drosophila melanogaster. J Comp Neurol. 1998;397:519–31. doi: 10.1002/(SICI)1096-9861(19980810)397:4<519::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]


