Abstract
This is a descriptive case report of a 30-year-old man with massive epistaxis, echymosis on arms, abnormal CBC and increased plasma urea and creatinine level (i.e. above normal range). Probably, these are as side effects of interferon beta-1a injection. This is the first report according to our literature search (Pub Med, Google scholar, ISI web of knowledge, ProQuest, MD consult, Science Direct, and SCOPUS) about interferon beta-1a related abnormal kidney function tests hereafter. Abnormal kidney function tests (i.e. increased plasma urea and creatinie level) is not a known as side effect of interferon beta-1a. This case indicates, likely potential for development of these side effects with this medication.
Key words: Interferon beta, Side effect, Multiple sclerosis, Rebif.
1. INTRODUCTION
One of the small protein messengers is interferon that is called cytokine. It is generated by the immune system in responding to viral infection. There are three types of interferon: alpha, beta and gamma (1). Rebif is a type of protein that is called beta interferon (INF beta). It produces naturally in the body (2). Rebif (INF beta-1a) is produced as a purified 166 amino acid glycoprotein with a molecular weight of approximately 22,500 Daltons. It is generated by recombinant DNA technology using genetically engineered Chinese Hamster Ovary cells (3, 4).
It is used to treat the relapsing forms of multiple sclerosis (1). It may decrease the number of flare-ups of the disease and decelerate process of the occurrence of some of the physical disabilities that they are common in people with multiple sclerosis (MS) (2). MS is the autoimmune inflammatory chronic disease of the nervous system (CNS) that is demonstrated by demyelinization and loss of axons (5) and also it is demonstrated by clinical manifestations by episodes of focal and multifocal disorders of the brain, spinal cord and the optic nerve (6, 7). There are no exact evidences of that how the rebif works in MS (8).
In addition to the presented case, a literature search (Pub Med, Google scholar, MD consult, Science Direct, SCOPUS, ISI web of knowledge, ProQuest) for articles published from 2000 to present time has been performed, using the Medline subheading keywords “Interferon” and “side effects”, “Interferon” and “abnormal kidney function tests”, “Interferon beta” and “side effects”, “Interferon beta” and “Drug reactions”, “Interferon beta” and “abnormal kidney function tests”. We have found no case report or related article. The relevant references of side effects were retrieved, but nothing was found.
Here, we report a patient with massive epistaxis, abnormal kidney function tests and other uncommon side effects of INF beta-1a (Rebif) after using it for a period of time.
2. CASE REPORT
A 30-year-old male, resident of Yazd, Iran, was referred to our emergency ward on April 23, 2011, because of massive epistaxis and ecchymosis on arms. He was known as a case of MS and he was getting Rebif (Ares-Serono, Geneva, Switzerland), three days a week, and each time 44mcg subcutaneous, for three months.
He had no history of trauma, hematuria, hematochezia, melena, hematemesis, diabetic mellitus, hypertension, ischemic heart disease and hyper lipidemia. The findings in his admission were blood pressure of 102/72 mmHg, pulse rate of 90 beat/min, axillary temperature of 37.2°C and respiratory rate of 24/min. He was oriented to time, place and person. In addition to abnormal CBC and kidney function tests, RBC 2.77mil/cu mm, Hemoglobin (Hgb) 8g/dl, Hematocrite (HCT) 25%, MCV 75fl, platelet count (PLT) 48000, plasma urea 91 mg/dl and creatinine 3.3 mg/dl. PT, PTT and INR were normal. When we hospitalized the patient, routine tests in our unit were checked for him, so they are summarized in Table 1. We checked retic count that was 0.3%. He didn’t have any significant data In his urine analysis and echocardiography. After that, liver function tests [SGOT (AST)-SGPT (ALT)-ALP], total and direct bilirubin were checked, they were normal. We checked LDH that was 1050 U/L. Infectious diseases such as HIV, HBV, HCV, HSV and EBV serology and ASO were negative. Blood and urine cultures, PPD test, Wright, 2ME were negative, too. Also Direct Coombs and Indirect Coombs were checked. They were negative, too. All investigations for probable malignancies were negative.
Table 1.
Laboratory findings in the first admission in our unit. FBS: fasting blood suger; WBC: white blood cell count; RBC: red blood cell count; Hgb: hemoglobin; HCT: hematocrite; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; PLT: platelet; ESR: erythrocyte sedimentation rate
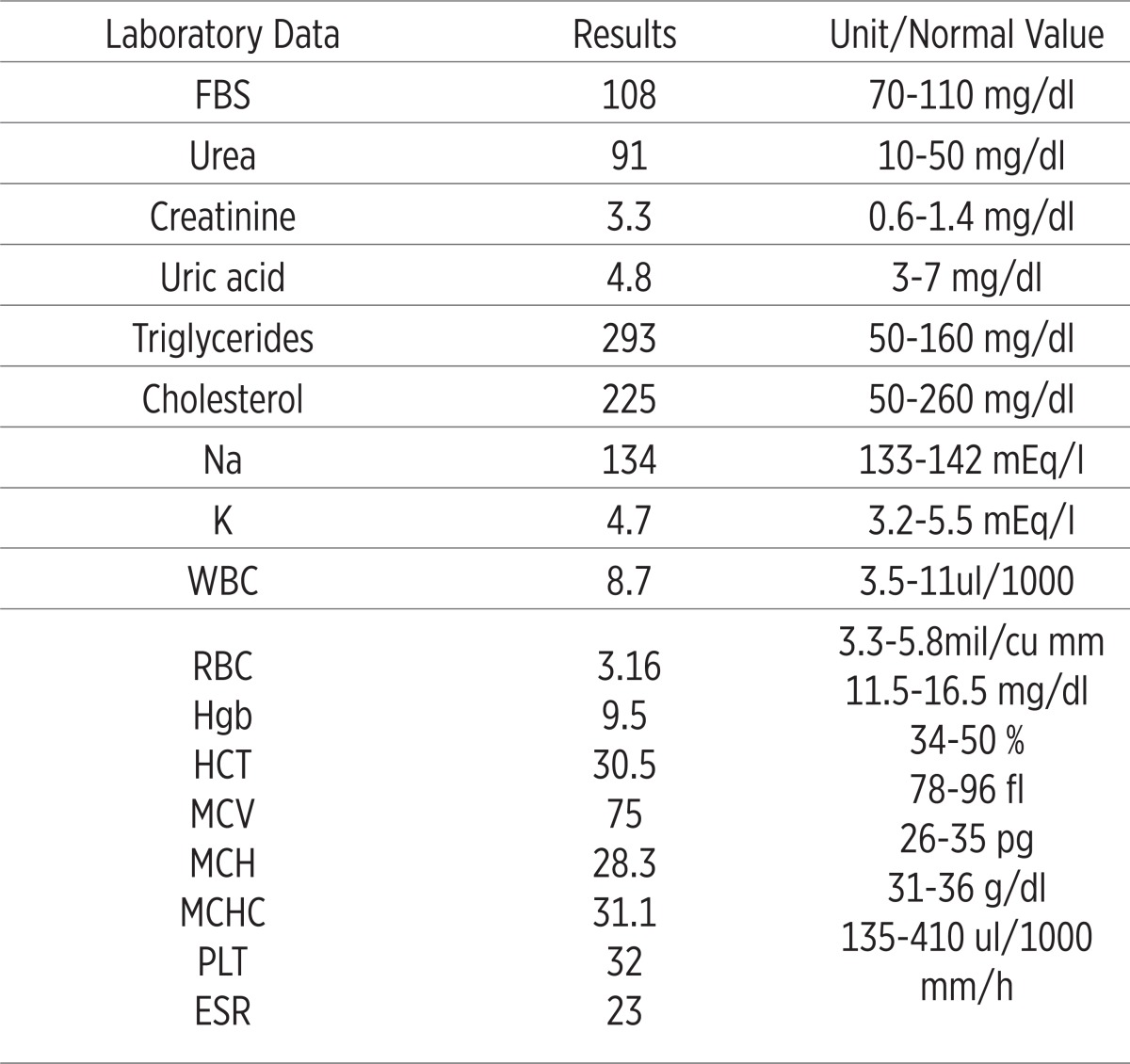 |
Thyroid function tests were normal. CBC, plasma urea and creatinine level were checked again two days later, yet his RBC, Hgb, HCT, MCV, PLT were low (i.e. under normal range) and his plasma urea and creatinine level were high (i.e. above normal range). 24 hours urine with a view to volume, creatinine, urea, uric acid and protein level was checked that was normal. Five days later, his CBC, plasma urea and creatinine level were checked again; RBC 2.88mil/cu mm, Hgb 8.4g/dl, HCT 26.2% and MCV 76fl, plasma urea 91 mg/dl and creatinine 3.2 mg/dl. He was treated with 50mg oral Prednisolone ½ Bid, 50mg Ranitidine IV Bid, one Folic acid tablet Bid, one Vitamin B12 tablet daily. He got 2 IU blood pack cell and Ringer serum 1000 cc/4hrs in the first admission. Peripheral blood smear had severe decrease in number of platelets. Erythrocytes were hypo-chromic severely with mild anisocytosis. White blood cell morphology and count were within normal limit and the most of them were nuetrophils. In bone marrow aspiration, cellularity appeared good (around 50%) and myeloid/erythroid (M/E) ratio was decreased. Blast count was less than 5% of nucleated cells. No dysplasia was detected. Megakaryocytes were increased. In bone marrow biopsy, megakaryocytes were increased. There was no evidence of malignancy. His chest radiograph, pelvic and abdominal sonography was normal. ANA, C3, C4, CH5, c and p ANCA and Anti-dsDNA and serum proteins electrophoresis were in normal range. His kidney biopsy had no significant thing. We consulted with neurologist and she proposed to disconnect interferon beta-1a (Rebif). After all of these investigations, we disconnected Rebif and after two days his CBC, plasma urea and creatinine level were checked again and their results were very fantastic. His RBC, Hgb, HCT, MCV and PLT had started to rise toward normal range, plasma urea and creatinine level had begun to fall. After seven days, they became normal.
They are summarized in Table 2.
Table 2.
Laboratory findings after seven days from disconnecting Rebif. RBC: red blood cell count; Hgb: hemoglobin; HCT: hematocrite; MCV: mean corpuscular volume; PLT: platelet
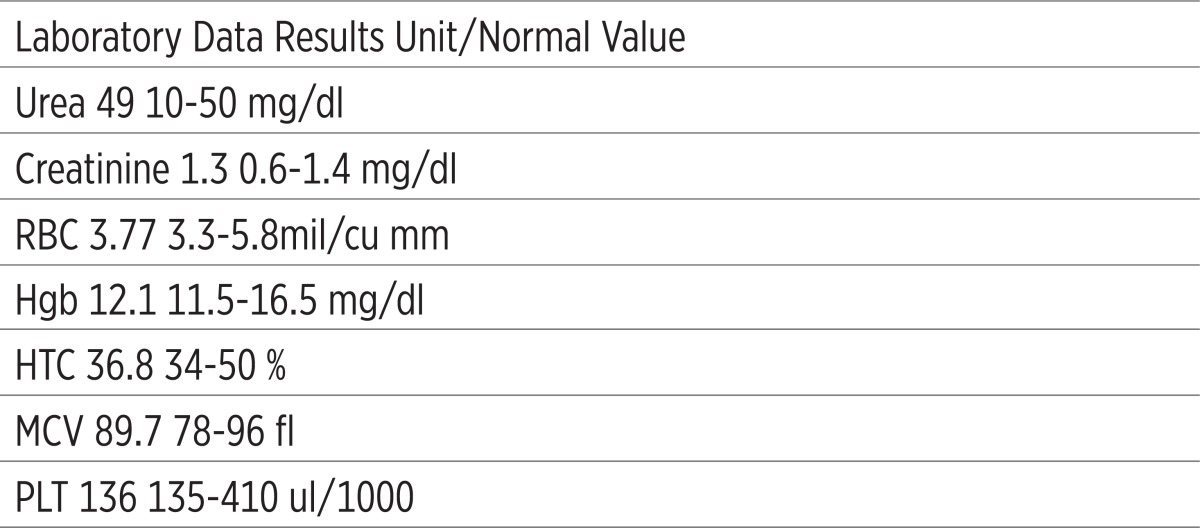 |
3. DISCUSSION
One of the small protein messengers is interferon that is called cytokine. It is generated by the immune system in responding to viral infection. There are three types of interferon: alpha, beta and gamma (1, 8). They are a group of naturally-occurring proteins that are made and secreted by cells of the immune system (for example, white blood cells, natural killer cells, fibroblasts, and epithelial cells) (9). Commercially available interferons are human interferons manufactured, using recombinant DNA technology. The mechanism of action of interferon is complex and is not well understood. Interferons calm the response of the immune system to viruses, bacteria, cancer, and other foreign substances that invade the body. Interferons do not directly kill viral or cancerous cells; they increase response of the immune system and reduce the growth of cancer cells by regulating the action of several genes that control the secretion of numerous cellular proteins that affect growth (9).
Use of interferons in multiple sclerosis has been studied for more than 20 years. Interferons are used in multiple sclerosis; their antiviral action, and their pleiotropic effects on the immune system and blood brain barrier could be advantage in patients with multiple sclerosis (10). Recombinant interferons were approved by many national regulatory agencies for the treatment of relapsing remitting multiple sclerosis, after clinical trials established that interferon beta preparations reduced disease activity (11, 12, 13, 14, 15). Interferons have been used for the treatment of multiple sclerosis for almost a decade, and are available for this use free of charge from many national health services (16). MS is a disease of the nerves, in which inflammation destroys the protective sheath around the nerves. This is called ‘demyelination’. The active substance in Rebif, interferon beta-1a, belongs to the group ‘interferons’. Interferons are natural substances that they are produced by the body to help it to war against attacks such as infections caused by viruses. There are no exact evidences of that how the rebif works in MS, but interferon beta seems to calm the immune system down and prevent relapses of MS (8).
Interferon beta-1a is produced by a method known as ‘recombinant DNA technology’: it is made by a cell that has received a gene (DNA), which makes it able to produce interferon beta-1a (8). The most common side effects of interferons are flu-like symptoms such as headaches, muscle aches, joint aches, fevers/chills and feeling sick, vomiting, loss of appetite, feeling tired, and diarrhea, depression mood, swings, poor concentration, vagueness. The flu-like symptoms are likely to occur. They are most common at the start of therapy and may decrease with continuing use. Over-the-counter fever reducers such as acetaminophen (Tylenol, others), ibuprofen (Motrin, Advil, others), and naproxen (Aleve), plenty of fluids, and taking the medication at bedtime may help to reduce these symptoms (1, 17). Less common effects may include; metallic taste, dry skin, dry mouth, loss or thinning of hair (temporary), pins and needles in the hands and toes and difficulty sleeping (1, 17).
While on interferon treatment, temporary reduction in the white blood cells and platelets (clotting blood cells), and thyroid problems may occur. This makes the patients with MS more vulnerable to infection, bleeding or bruising. When the treatment is stopped, the bone marrow returns to its normal state (1). Rebif should not be used in people who have a history of hypersensitivity (allergy) to natural or recombinant interferon beta, or any of the other ingredients. It must not be used during pregnancy. Also It must not be started in patients who are suffering from severe depression or having thoughts about committing suicide (8).
As a summary, the side effects of Rebif are Flu-like symptoms; most patients have flu-like symptoms (fever, chills, sweating, muscle aches and tiredness) (2); skin reactions, soreness, redness, pain, bruising or swelling may occur at the place of injection (2); depression and anxiety (2); Liver problems (2); blood problems, the patients may have a drop in the levels of infection-fighting blood cells, red blood cells or cells that help to form blood clots. If the drop in levels is severe, it can lessen patients’ ability to attack infections, make them feel tired or sluggish or cause them to bruise or bleed easily (2); thyroid problems, the thyroid function may change in the patients (2); allergic reactions, some patients have had hives, rash, skin bumps or itching while they were taking Rebif. Other patients have had more serious allergic reactions such as difficulty breathing, or feeling light-headed (2). anaphylaxis has been reported as a rare complication of using of Rebif (2); eye disorders, retinal vascular disorders (e.g. retinopathy, cotton wool spots or obstruction of retinal artery or vein) (3, 4); hepatobiliary disorders, rare cases of severe liver dysfunction, including hepatic failure requiring liver transplantation are other complications (3, 4).
There is difficult to distinguish idiopathic from drug induced these side effects (i.e. abnormal CBC and kidney function tests). However, it is extremely important to identify the offending drug, because the discontinuation of the drug is often followed by improvement of these side effects. All the investigation that we did makes it reasonable to attribute the symptoms to interferon beta-1a. In this case, symptoms didn’t relieve simply with conservative and drug treatment; we followed the patients for six months. We checked his CBC and kidney function tests every month during these six months. They were in normal range. Also we had no previous side effects and also no new complications in this patient. In our opinion, for this patient, it is important to be aware about using this medication again.
According to this case, it is likely appropriate to consider interferon beta-1a as a potential cause of these side effects in our further practice. The side effects that presented in this patient can be because of interferon beta-1a (Rebif) when we disconnected it, all of the side effects were relieved and his CBC, plasma urea and creatinine level became normal. We propose further researches and studies about interferon beta and in general all the interferons.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Bertrand Toulouse. Interferon therapy, National AIDS Treatment Advocacy Project (NATAP) New York [Online]. Available from: http://www.hepcadvocacy.org/factsheets/Interferon.pdf . 2010.
- 2.EMD Serono, Medication Guide, approved by the U.S. Food and Drug Administration; Rev (7), [Online] ; 2009-09-20480. Available from: http://www.emdserono.com/cmg.emdserono_us/en/images/rebif_med_guide_tcm115_19766.pdf .
- 3.Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon ß-1a treatment regimens in MS. The EVIDENCE Trial. Neurology. 2002;59:1496–1506. doi: 10.1212/01.wnl.0000034080.43681.da. [DOI] [PubMed] [Google Scholar]
- 4.PRISMS Study Group. Randomized double-blind placebo-controlled study of interferon ß-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352:1498–1504. [PubMed] [Google Scholar]
- 5.Ropper AH, Brown RH. Multiple sclerosis and allied demyelinative disease. In: Victors M, Ropper AH, editors. Adam’s and Victor’s Principles of Neurology. 7th. NewYork: Mc Graw-Hill; 2005. pp. 771–796. [Google Scholar]
- 6.Olek MJ, Dawson DM. Multiple sclerosis and other inflammatory demyelinating diseases of the central nervous system. In: Bradley GW, Daroff RB, Fenichel GM, Jankovic J, editors. Neurology in Clinical Practice. 4th. Philadelphia: Butterworth & Heinemann; 2004. pp. 1631–1664. [Google Scholar]
- 7.Weinstock-Guttman B, Bakshi R. Combination therapy for multiple sclerosis: the treatment strategy of the future? CNS Drugs. 2004;18(12):777–792. doi: 10.2165/00023210-200418120-00003. [DOI] [PubMed] [Google Scholar]
- 8.European Medicine Agency. [Online]. Available from: www.ema.europa.eu/docs/en_GB/.../WC500048682.pdf . 2010.
- 9.Omudhome O, Jay W. Marks FDA Prescribing Information. [Online]. Available from: http://www.medicinenet.com/interferon/article.htm . 2010.
- 10.Hohlfeld R. Biotechnological agents for the immunotherapy of multiple sclerosis: principles, problems and perspectives. Brain. 1997;120:865–916. doi: 10.1093/brain/120.5.865. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol. 1996;39:285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- 12.The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology. 1995;45:1277–1285. [PubMed] [Google Scholar]
- 13.Jacobs LD, Cookfair DL, Rudick RA, et al. A phase III trial of intramuscular recombinant interferon beta as treatment for exacerbating-remitting multiple sclerosis: design and conduct of study and baseline characteristics of patients. Mult Scler. 1995;1:118–135. doi: 10.1177/135245859500100210. [DOI] [PubMed] [Google Scholar]
- 14.The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis: I Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–661. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- 15.Paty DW, Li DKB the UBC MS/MRI Study Group, the IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis: II MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:662–667. doi: 10.1212/wnl.43.4.662. [DOI] [PubMed] [Google Scholar]
- 16.Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple Sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 17.A service of the U.S. National Library of Medicine National Institutes of Health. Interferon beta-1a Subcutaneous Injection, [Online] Available from: http://www.nlm.nih.gov/medlineplus/druginfo/meds/a604005.html . 2008.


