Abstract
Introduction:
In recent years there has been increase in the number of patients who need thoracic surgery – first of all different types of pulmonary resection because of primary bronchial cancer, and very often among patients whose lung function is impaired due to different degree of bronchial obstruction so it is necessary to assess functional status before and after lung surgery to avoid the development of respiratory insufficiency.
Objective:
To show the changes in the level of arterial blood gases after various ranges of lung resection.
Material and methods:
The study was done on 71 patients surgically treated at the Clinic for Thoracic Surgery KCU Sarajevo, who were previously treated at the Clinic for Pulmonary Diseases “Podhrastovi” in the period from 01. 06. 2009. to 01. 09. 2011. Different types of lung resection were made. Patients whose percentage of ppoFEV1 was (prognosed postoperative FEV1) was less than 30% of normal values of FEV1 for that patients were not given a permission for lung resection. We monitored the changes in levels-partial pressures of blood gases (PaO2, PaCO2 and SaO2) one and two months after resection and compared them to preoperative values. As there were no significant differences between the values obtained one and two months after surgery, in the results we showed arterial blood gas analysis obtained two months after surgical resection. Results were statistically analyzed by SPSS and Microsoft Office Excel. Statistical significance was determined at an interval of 95%.
Results:
In 59 patients (83%) there was an increase, and in 12 patients (17%) there was a decrease of PaO2, compared to preoperative values. In 58 patients (82%) there was a decrease, and in 13 patients (18%) there was an increase in PaCO2, compared to preoperative values. For all subjects (group as whole): The value of the PaO2 was significantly increased after lung surgery compared to preoperative values (p <0.05) so is the value of the SaO2%. The value of the PaCO2 was significantly decreased after lung surgery compared to preoperative values (p <0.05). Respiratory insufficiency was developed in none of patients.
Conclusion:
If the % ppoFEV1 (% prognosed postoperative FEV1) is bigger than 30% of normal values of FEV1 (according to sex, weight, height, age) in patient planned for lung resection surgery there is no development of respiratory insufficiency after resection.
Key words: lung resection, level of arterial blood gases.
1. INTRODUCTION
In recent years there has been increase in the number of patients who need thoracic surgery - first of all different types of pulmonary resection for primary bronchial cancer, particularly among older patients and patients whose lung function is impaired due to the different degree of bronchial obstruction. In all these patients it is necessary to assess functional status before and after lung surgery performed, although in patients with cancer we may ask - what is the risk of postoperative complication in relation to disease that certainly has fatal outcome if not treated surgically.
This problem is differently treated in the literature. Most authors (1-32) recommended to be done complete functional testing - complete spiroplethysmographic processing: forced expiratory volume in one second (FEV1), vital capacity (VC), forced vital capacity (FVC), flow - volume curve, pulmonary resistance (Rt), residual lung volume (RV), bronchodilator test, maximum voluntary minute ventilation (MMV) and total lung capacity (TLC), and in cases where diffuse interstitial changes of lung are radiologically presented, DLCO ( transfer factor for CO) should be also determined (2, 6, 7). There is also a need to predict the postoperative FEV1 (ppoFEV1-prognosed postoperative FEV1) that could be done by various methods (1, 4, 5-25). If the ppoFEV1 is lower than 30% of normal values of FEV1 for this patient, which is the great risk for postoperative complications and postoperative death, that patient is not allowed to be undergone to lung resection (7). All authors (1-32) require doing the analysis of gases in the blood before lung resection. If there is hypercapnia, i.e. high partial pressure of carbon dioxide in arterial blood (Pa CO2) >45 mm Hg (6 kPa) it does not give the approval for the surgery (1-32). If there is hypoxemia i.e. decreased partial pressure of oxygen in arterial blood (PaO2) <60 mmHg (8 kPa) or arterial oxygen saturation (SaO2) below 90% it does not give the approval for the surgery (1-32). We should try with oxygen therapy, along with other appropriate bronchodilator therapy, to achieve normalization of blood gases.
2. OBJECTIVE
Objective of the study is to show the changes in the level of arterial blood gases after various ranges of lung resection.
3. MATERIALS AND METHODS
The study was done on 71 patients surgically treated at the Clinic for Thoracic Surgery of Clinical Center of University of Sarajevo, who were previously treated at the Clinic for Pulmonary Diseases “Podhrastovi” in the period from 01. 06. 2009 to 01. 09. 2011. The following resection operative procedures were made in patients: pulmectomy (left, right), lobectomy (upper and lower: left, right and middle), bilobectomy (right: upper and lower), segmentectomies. The control group consisted of the same patients. Lung function tests were done when the patient was clinically stable and after taking the complete bronchodilator treatment, if it was needed.
Before surgery all patients were undergone to complete spiroplethysmographic processing as follows: FVC, FEVl, flow-volume curve, total pulmonary resistance (Rt) including bronchodilator test, RV, TLC. Arterial blood gas analysis was necessarily performed.
Patients were divided into 21 groups according to the range of lung resection surgery and a sex which is seen in Table 1.
Table 1.
The sample of treated patients according to the range of lung resection surgery
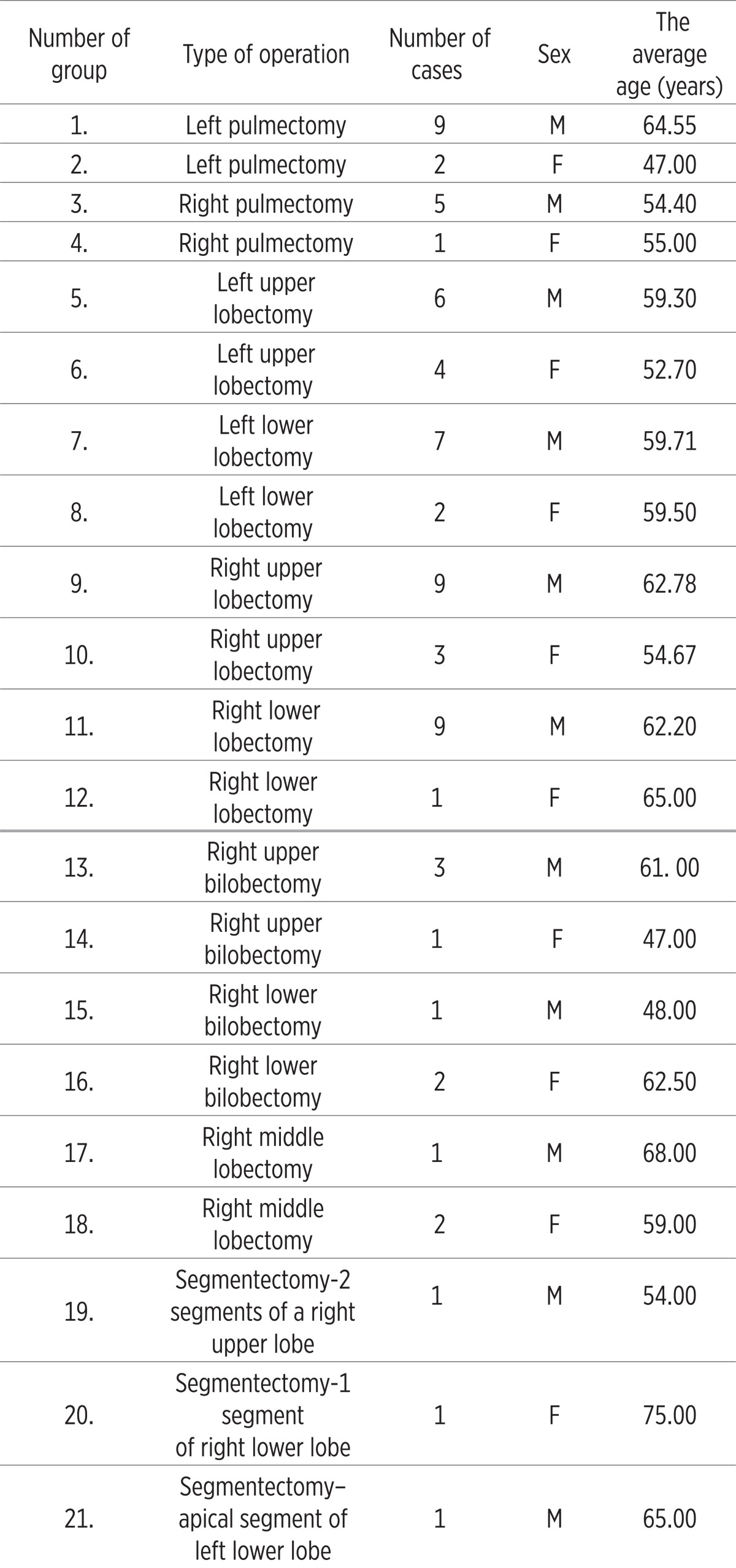 |
The study included 52 men, mean age 60, 96 years (45-73) and 19 women, mean age 56 years (47-75) with various type of lung resection.
* If there was a disturbance in blood gases: hypoxemia i.e. PaO2 below 60 mm Hg (8 kPa) or SaO2 below 90%, with or without hypercapnia i.e. PaCO2 above 45 mmHg (6 kPa) approval for operation was not given. We tried with bronchodilator therapy, because disorder of level of gases in blood indicate on deeper ventilatory disturbances, and with oxygen therapy, to achieve the normalization gases in blood. If the blood gases normalized, patients were involved in the study and given approval for the surgery.
* If there was bronchospasm we also did not give approval for the surgery because bronchospasm is accompanied with vasospasm, all of which leads to disturbances of ventilation - perfusion relationships in the lungs, and a significant increase in pulmonary vascular resistance and that is a major surgical risk. If bronchospasm is relieved by appropriate bronchodilator therapy patients were included into the study.
* If there was hyperinflation of lung parenchyma of great degree that is if the RV was greater than 300% of standard values for that patient, which indicating a distinct bronchial obstruction, we did not give permission for operation. If the appropriate bronchodilator therapy decreased RV to values below 300% of standard values, patient was included into the study.
* The degree of damage to the lung function is primarily determined based on the FEV1 because it is the most objective parameter of lung function which indicates the obstructive and restrictive disorders of lung function and that indicates the type and degree of impairment of ventilatory lung function. At the same time it was examined and FVC, another very important parameter of lung function.
* If there was radiological evidence of diffuse interstitial lung disease, transfer factor – lung diffusion capacity for carbon monoxide (DLCO) was also determined.
In all patients on the basis of measured preoperative lung functional parameters we predicted postoperative lung function level in the scope of the planned lung resection and determined the maximum possible level of resection. We predicted postoperative FEV l (ppoFEVl) in absolute values (liter- L) and expressed it also as a percentage (%) of normal values of the patient’s FEV1. If the value of preoperative FEV 1 was below of 35% of the standard – normal values for that patient we did not give the permission for lobectomy, bilobectomy and pulmectomy. Th e maximum possible extent of resection is determined by% ppo FEV 1 (FVC) to be 30% of the normal value of FEV1 (FVC) for that patient regardless of how much it is in absolute values (L).
Complete spiroplethysmographic processing and measurement of diff usion capacity of the lungs or transfer factor (by single breath method) was done on the apparatus Master Lab-Jaeger, a determination of blood gases in the Radiometer ABL 505 apparatus in the Laboratory for clinical respiratory physiology - Department of Clinic for Pulmonary disease “Podhrastovi”.
We monitored the changes in levels of blood gases (PaO2, PaCO2, SaO2) one and two months aft er resection and compared them to preoperative values. As there were no signifi cant diff erences between the values obtained one and two months aft er surgery, in the results we showed arterial blood gas analysis obtained two months aft er surgical resection. Results were statistically analyzed by SPSS and Microsoft Offi ce Excel. Statistical signifi cance was determined at an interval of 95%.
We followed whether chronic pulmonary insufficiency possibly developed. Respiratory failure is defi ned as PaO2 <8 kPa (60 mmHg), PaCO2> 6 kPa (45 mmHg) and SaO2 <90%. If there is a decrease in PaO2 or SaO2, we talk about partial respiratory insufficiency, and if it is accompanied by increase in PaCO2 we are talking about a global respiratory insufficiency. If after 10 days of bronchodilator therapy with 24-hour oxygen therapy (for 10 days) there is no normalization of arterial blood gases we are talking about the chronic respiratory insufficiency (32).
4. RESULTS
Results of the study are presented in tables and graphs. A proper comment is given below each table and graph.
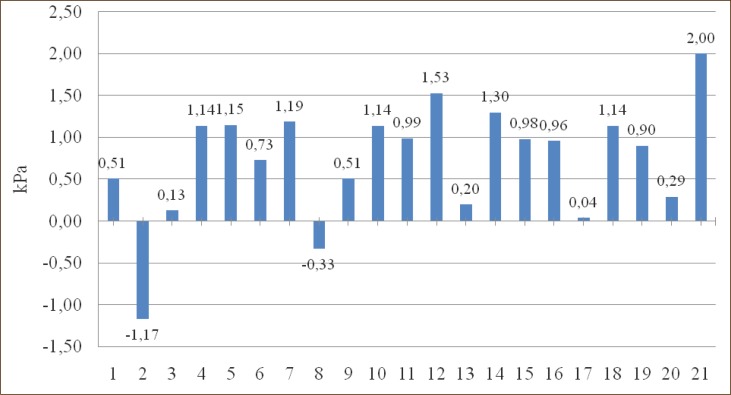
Among 71 patients operatively treated only one didn’t have cancer (Table 2). In group 2. (Left pulmectomy–women) and group 8. (Left lower lobectomy – women) there is a decrease in PaO2 for 1.17 kPa and 0.33 kPa, and in all other groups there is an increase of PaO2, compared to preoperative values.
Table 2.
Diagnoses of patients undergone to lung resection
 |
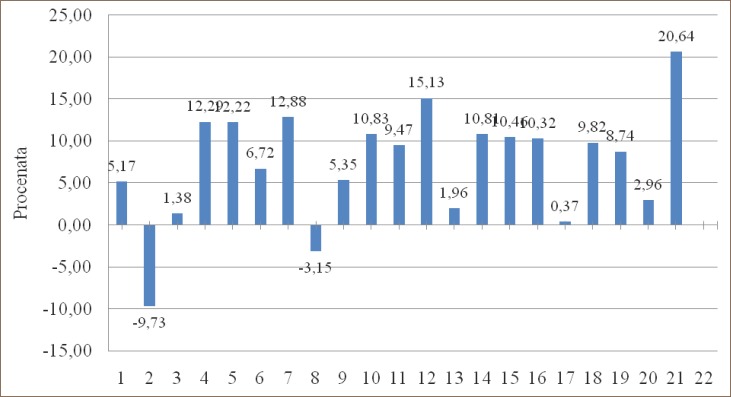
In group 2. (left pulmectomy - women) and group 8. (left lower lobectomy – women) there is a decrease of PaO2 of 9.73% and 3.15%, while in all other groups there is an increase of PaO2, compared to preoperative values.
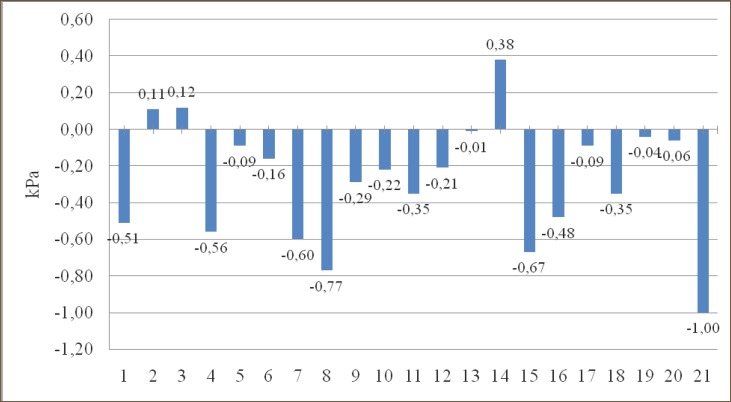
In group 2. (Left pulmectomy – women), group 3. (Right pulmectomy – men) and group 14. (Right upper bilobectomy – women) there is an increase in PaCO2 of 0.11 kPa, 0.12 kPa and 0.38 kPa, and in all other groups there is a fall in PaCO2, compared to preoperative values.
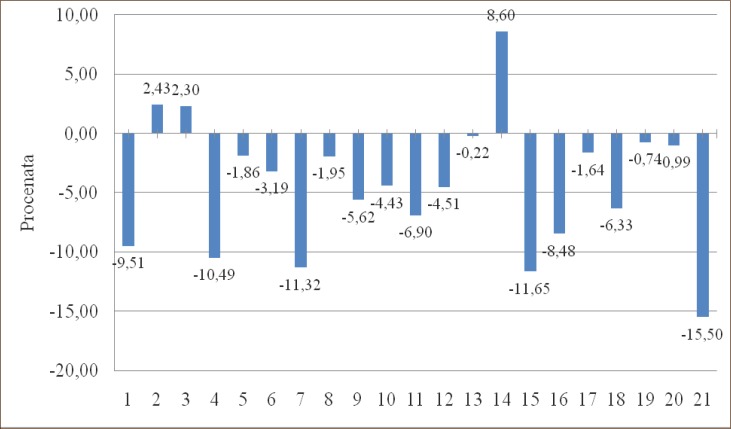
In group 2. (Left pulmectomy – women), group 3. (Right pulmectomy – men) and group 14. (Right upper bilobectomy – women) there is an increase in PaCO2 of 2.43%, 2.30% and 8.60% and in all other groups there is a decrease in PaCO2, compared to preoperative values. In group 2. (Left pulmectomy – women) there is a decrease of SaO2 for 1.10% compared to preoperative values and in all other groups there is a rise in SaO2.
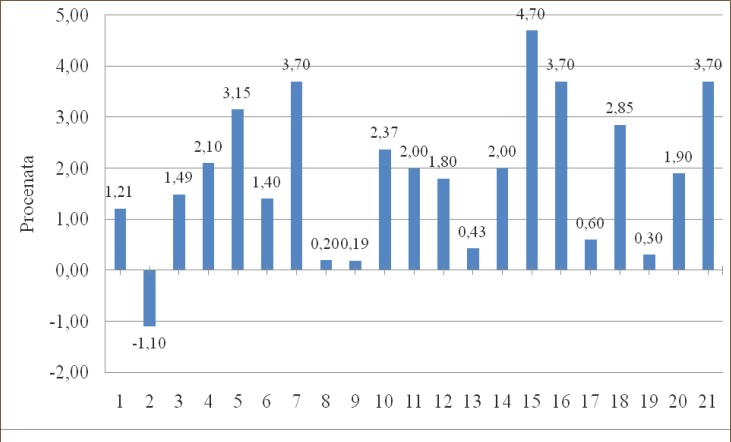
In 59 patients (83%) there was an increase, and in 12 patients (17%) there was a decrease of PaO2, compared to preoperative values, but in none of the patients postoperative PaO2 was lower than 8 kPa. (Graph 1, 2, 3, 4, 5, 6, 7).
Graph 1.
Change in PaO2 (kPa) compared to preoperative values in groups.
Graph 2.
Change in PaO2 in percentages compared to preoperative values in groups.
Graph 3.
Change in PaCO2 (kPa) compared to preoperative values in groups.
Graph 4.
Change in PaCO2 (kPa) in percentages compared to preoperative values in groups.
Graph 5.
Change in SaO2 % compared to preoperative values in groups.
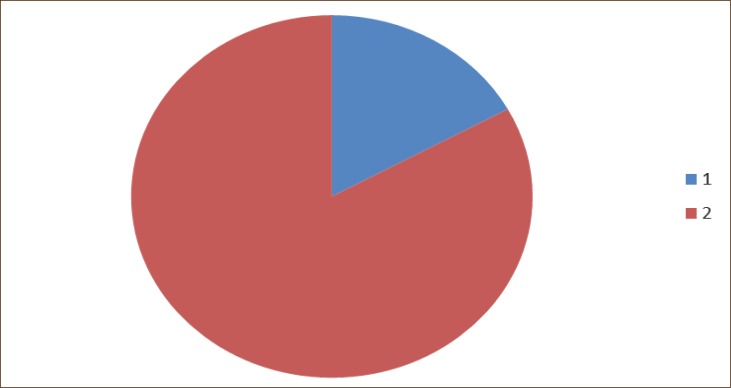
Graph 6.
The change of PaO2 (kPa) compared with preoperative values. Legend 1. Patents who achieved decreased values of PaO2 after operation. 2. Patients who achieved increased values of PaO2 after operation.
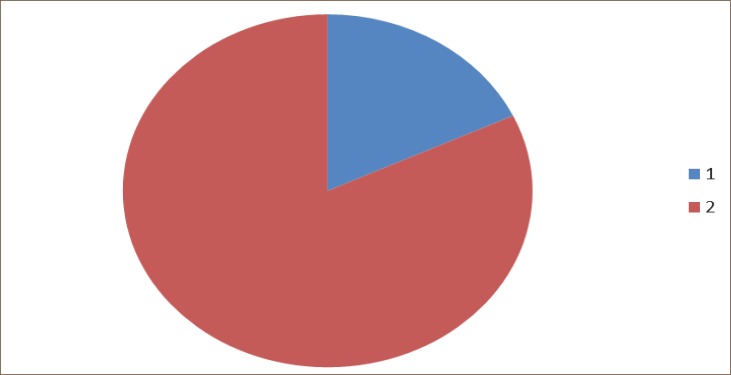
Graph 7.
The change of PaCO2 (kPa) compared with preoperative values. Legend 1. Patients who achieved increased values of PaCO2 after operation. 2. Patients who achieved decreased values of PaCO2 after operation.
In 58 patients (82%) there was a decrease, and in 13 patients (18%) there was an increase in PaCO2, compared to preoperative values, but in none of the patients postoperative PaCO2 was higher than 6kPa.
For all patients included in study:
The value of the PaO2 was significantly increased after surgery compared to preoperative values (p <0.05).
The value of the PaCO2 was significantly decreased after surgery compared to preoperative values (p <0.05).
The value of the SaO2 was significantly increased after surgery compared to preoperative values (p <0.05).
5. DISCUSSION
Arterial blood gas analysis is indicated in all patients who are scheduled for pulmonary resection (1-35). Many authors have attempted to defi ne the postoperative complications according to the values of gases in the blood preoperatively. Preoperative arterial hypoxemia (i.e. PaO2 <60 mm Hg) is considered as a contraindication for lung resection (1-32). However, in some patients, especially those with lung cancer, pulmonary zones to be surgically removed may be an area that has no ventilation (V’) (due to bronchial obstruction), but has a perfusion (Q’); this zone can be a zone of right–left shunt (V ’/ Q’ = 0) and a potential cause of arterial hypoxemia (3). Resection of such area could cause the PaO2 to return to normal values after resection (3). So it would be need to determine the causes of preoperative arterial hypoxemia (1, 3, 7, 8). In most patients in our study there was a statistically significant increase of oxygen and arterial oxygen saturation (PaO2 and SaO2) (59 patients or 83.1%) and statistically significant decreases of carbon dioxide (PaCO2) (58 patients or 81.7%) after resection surgery.
Hypercapnia (i.e. PaCO2> 45 mm Hg) is a contraindication for surgery because it marks a significant loss of lung function, advanced pulmonary disease and minimal pulmonary reserve, but in some patients it may be due to reversible airway obstruction or treatable infection. Historically hypercapnia is the criterion that excludes resection (3, 7,8,14) because it is associated with poor ventilatory function. According to the BTS guideline (7) hypercapnia by itself is not a prediction of complications after lung resection, but in such patients there is usually % ppoFEV1 <40% of normal values (7). In two series (2, 33) of patients with cancer with preoperative hypercapnia, who were undergone surgery, peri - and postoperative complications were not more frequent than usual, indicating that preoperative hypercapnia is not an independent risk factor but requires further testing of lung function (34). In two other studies preoperative hypoxemia (PaO2<60 mmHg) and arterial oxygen saturation (SaO2) <90% was associated with increased levels of postoperative complications (25, 30). In our study, patients with hypoxemia with or without hypercapnia were not given permission for resection surgery.
Arterial blood gas analysis in the study of Pierce RJ et al. (18) showed that there was a small drop of PaO2 (12%) aft er pulmectomy, and no signifi cant decreases aft er minor resections. In our study, in a whole group, there was a statistically significant increase of PaO2 two months after surgery compared to preoperative values (p <0.05). In 59 patients (83.1%) PaO2 was higher, and in 12 (16.9%) lower than preoperatively. Th e largest decline was observed in two patients (female 47 and male 61 years old) who were undergone left pulmectomy and where the PaO2 decreased by 1.65 kPa (12.38 mmHg), respective 1.59 kPa (11.93 mmHg) as compared to the preoperative value i.e. by 14.35%, or 19.23%, but it did not develop respiratory failure that is falling PaO2 below 60 mm Hg (8 kPa). In the fi rst case ppo FEV1 and FEV1 obtained were identical, while the in the second achieved FEV1 was lower than the ppoFEV1 by 16.98% compared to the prediction in L, and realized FEV1 expressed as a percentage of the normal value for this patient was for 8% lower than the % ppo FEV1.Increase of oxemia or increase PaO2 aft er resection can be explained by repairing ventilation - perfusion relationship aft er resection of the lung in which there was a right-left shunt (3). In Pierce’s study (18) PaCO2 was not signifi cantly changed for any type of resection. With us there was a statistically significant decreases in PaCO2 for the group as a whole (p <0.05). In 58 patients (81.7%) aft er resection surgery there was decrease, and in 13 (18.3%) patients there was an increase in PaCO2. The largest increase was recorded in one patient (male of 53 years) with right pulmectomy where PaCO2 increased by 1,03 kPa (7,73 mm Hg), or 22.78% compared to preoperative values, but even with him, as well as in other patients hypercapnia i.e. the level of PaCO2> 45 mm Hg (6kPa) did not develop.
Fee JH et al. (26) have studied the level of preoperative blood gases, spirometric tests, pulmonary vascular resistance (PVR) in 30 patients. Eighteen patients were preoperatively assessed low-risk (PaO2> 50 mmHg, FEV1> 50% of normal, FVC> 50% of normal, and PVR <190 dynes cm ‘sec’) and all survived. Five patients who were considered high risk based on PVR died aft er surgery from respiratory failure (4 of them before surgery were considered low risk based on spirometry). If these figures are correct, then patients with high PVR cannot tolerate resection, regardless of its size and this fi nding supports the opinion of the author that preoperative reduction in lung capillaries may be a crucial factor in the development of postoperative respiratory failure and cor pulmonale.
We need to know that immediately aft er the operation drop in PaO2 comes from 10% to 30% due to disorders of ventilation – perfusion relationship with poor ventilation of the region that continues to have normal perfusion (27). Th is type of atelectasis is not easily seen on X-rays and is called “micro atelectasis”. If previously there was no disorder in the exchange of gases, there is no increase in PaCO2. In patients with preoperative normal or minimally impaired lung function, these events remain silent (low risk), but in patients with impaired preoperative pulmonary function, signifi cant pulmonary complications may occur (high risk) (35). Increased work of breathing to maintain an adequate PaO2 and PaCO2 further stress the respiratory muscles and may lead to the development of their weaknesses with further disruption of blood gases (35).
Diff erent, oft en unexpected, changes in blood gases level aft er resection of lung parenchyma can be explained by the fact that diff erent types of relations between ventilation and perfusion (V ‘/ Q’) may exist in lungs. According to GM Tiss (3) there are following types of relationships between ventilation and perfusion (V’/ Q’): a) pulmonary zone to be removed has the same distribution of ventilation and perfusion as well as the rest of the lung. It does not mean that V ‘/ Q’ is normal, but is the same in all parts of the lungs. In this case, loss of lung function aft er resection would be proportional to the degree of resection; b) Th e area to be removed of the lung has an invaluable pulmonary function (no function–neither ventilation nor perfusion). In this case, resection will have no impact on postoperative pulmonary function because the patient has a functional either autolobectomy or autopulmectomy before surgery; c) Pulmonary zone to be resected carries the main part of the functions – ventilation and perfusion (main part of the V`/ Q`). In this case, the postoperative pulmonary function would be severely damaged with a disorder of blood gases; d) Pulmonary zone to be removed is the seat of an abnormal relationship between ventilation and perfusion (V ‘/Q’) because of the lack of ventilation due to the bronchial obstruction, and well-preserved perfusion. Th is is the site of right-left shunt and could lead to arterial hypoxemia. In this case, the resection of these areas could lead to correcting the cause of hypoxemia and normalization of blood gases (3).
It is believed that the remaining lung tissue adapts to the loss of surface area for gas exchange by creating a new functional area of ventilation and perfusion to increase the RV / TLC and that this adaptation occurs within the first few months after surgery (29, 30).
6. CONCLUSION
If the % ppoFEV1 (% prognosed postoperative FEV1) is bigger than 30% of normal values of FEV1 (according to sex, weight, height, age) in patient planned for lung resection surgery there is no development of respiratory insufficiency after resection. Th at shows that the lower threshold of lung function can be safely applied in surgical resection of lung tissue; we should consider the current guidelines for the assessment of lung resectability in patients with various diseases. Using a lower threshold for preoperative and prognosed postoperative values of pulmonary function will increase the number of operations, the level of healing and reduce mortality from various diseases that require thoracic surgical treatment.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Win T, Jackson A, Shraples L, Groves A, Wells FC, Ritchie AJ, Laroche CM. Relationship between pulmonary function and lung cancer surgical outcome. Eur Respir J. 2005;25:594–599. doi: 10.1183/09031936.05.00077504. [DOI] [PubMed] [Google Scholar]
- 2.Kearney DJ, Lee T, Reilly J, Decamp M, Surgabaker DJ. Assessment of operative risk in patients undergoing lung resection. Importance of predicted pulmonary function. Chest. 1994;105:753–759. doi: 10.1378/chest.105.3.753. [DOI] [PubMed] [Google Scholar]
- 3.Tissi GM. Preoperative evaluation of pulmonary function. Am Rev Respir Dis. 1979;119:293–310. doi: 10.1164/arrd.1979.119.2.293. [DOI] [PubMed] [Google Scholar]
- 4.Gerson G. Preoperative respiratory function tests and postoperative mortality. A study of patients undergoing surgery for carcinoma of the bronchus. Br J Anesth. 1969;41:967–971. doi: 10.1093/bja/41.11.967. [DOI] [PubMed] [Google Scholar]
- 5.Gass GD, Olsen GN. Preoperative pulmonary function testing to predict postoperative morbidity and mortality. Chest. 1986;89:127–135. doi: 10.1378/chest.89.1.127. [DOI] [PubMed] [Google Scholar]
- 6.Berchard D. Pulmonary function testing in diagnostic procedures for thoracic diseases. III. Philadelphia: WB Saunders, LoCicero J; 1992. [Google Scholar]
- 7.British Thoracic Society, Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001;56:89–108. doi: 10.1136/thorax.56.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckles MA, Spiro SG, Colice GL, Rudd RM American College of Chest Physicians. The physiologic evaluation of patients with lung cancer being considered for resection surgery. Chest. 2003;(Suppl.1):105S–114S. doi: 10.1378/chest.123.1_suppl.105s. [DOI] [PubMed] [Google Scholar]
- 9.Stein M, Koota G, Simon M, Frank H. Pulmonary evaluation of surgical patients. JAMA. 1962;181:765–768. doi: 10.1001/jama.1962.03050350027006. [DOI] [PubMed] [Google Scholar]
- 10.Markos J, Mullan BP, Hillman DR. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis. 1989;139:902–910. doi: 10.1164/ajrccm/139.4.902. [DOI] [PubMed] [Google Scholar]
- 11.Nomura A, Stemmermann G, Chyon P, Marcus GB, Buist AS. Prospective study of pulmonary function and lung cancer. Am Rev Respir Dis. 1991;144:307–311. doi: 10.1164/ajrccm/144.2.307. [DOI] [PubMed] [Google Scholar]
- 12.Skillrud DM, Offord KP, Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease. Ann Intern Med. 1986;105:503–507. doi: 10.7326/0003-4819-105-4-503. [DOI] [PubMed] [Google Scholar]
- 13.Pecora DV. Evaluation of cardiopulmonary reserve in candidates for chest surgery. J Thorac Cardiovasc Surg. 1962;44:60–66. [PubMed] [Google Scholar]
- 14.Miller JI, Grossman GD, Hatcher CR. Pulmonary functiontest criteria for operability and pulmonary resection. Surg Gynecol Obstet. 1981;153:893–895. [PubMed] [Google Scholar]
- 15.Miller JI. Physiologic evaluation of pulmonary function in the candidate for lung resection. J Thorac Cardiovasc Surg. 1993;105:347–351. [PubMed] [Google Scholar]
- 16.Putnam JB, Colon R, McMutray MJ, Ali MK, Roth JA. Predicted pulmonary function and survival after pneumectomy for primary lung carcinoma. Ann Thorac Surg. 1990;49:909–914. doi: 10.1016/0003-4975(90)90864-3. [DOI] [PubMed] [Google Scholar]
- 17.Bolliger CT, Guckel S, Engel H, et al. Prediction of functional reserves after lung resection: comparison between quantitative computered tomography, scintigraphy and anatomy. Respiration. 2002;69:482–489. doi: 10.1159/000066474. [DOI] [PubMed] [Google Scholar]
- 18.Pierce RJ, Copland KS, Barter CE. Preoperative risk evaluation for lung cancer. Predicted postoperative product as a predictor of surgical mortality. Am J Respir Crit Care Med. 1994;150:947–955. doi: 10.1164/ajrccm.150.4.7921468. [DOI] [PubMed] [Google Scholar]
- 19.Nakahara K, Ohno K, Hashimoto J, et al. Prediction of postoperative respiratory failure in patients undergoing lung resection for lung cancer. Ann Thorac Surg. 1988;46:549–552. doi: 10.1016/s0003-4975(10)64694-2. [DOI] [PubMed] [Google Scholar]
- 20.Segal JJ, Buterworthh BA. Ventilatory capacity in chronic bronchitis in relation to carbon dioxide retention. Scand J Resp Dis. 1966;47:215–220. [PubMed] [Google Scholar]
- 21.Zether BG, Gross TJ, Kern JA, et al. Predicting postoperative pulmonary function in patients undergoing lung resection. Chest. 1995;108:69–72. doi: 10.1378/chest.108.1.68. [DOI] [PubMed] [Google Scholar]
- 22.Andjelić B, Milicević B, Major Z. Preoperativno ispitivanje plućne funkcije. Najnovija shvatanja. Pneumon. 2000;38(1-20):95–99. [Google Scholar]
- 23.Le Roy Ladurie M, Ranson- Bitker B. Uncertainties in the expected value for forced expiratory volume in one second after surgery. Chest. 1986;90:223–228. doi: 10.1378/chest.90.2.222. [DOI] [PubMed] [Google Scholar]
- 24.Boysen PG. Pulmonary resection and postoperative pulmonary function. Chest. 1980;77:718–719. doi: 10.1378/chest.77.6.718. [DOI] [PubMed] [Google Scholar]
- 25.Fergusson MK, Reader LB, Mick R. Optimising selection of patients for major lung resection. J Thorac Cardiovasc Surg. 1995;109:275–283. doi: 10.1016/S0022-5223(95)70389-6. [DOI] [PubMed] [Google Scholar]
- 26.Fee JH, Holmes EC, Swenson EV, et al. Role of pulmonary vascular resistance measurements in preoperative evaluation of candidates for pulmonary resection. J Thorac Cardiovasc Surg. 1975;75:519–524. [PubMed] [Google Scholar]
- 27.Ali J, Weisel RD, Layung B, et al. Consequences of postoperative alterations in respiratory mechanisms. Am J Surg. 1974;128:376–382. doi: 10.1016/0002-9610(74)90176-7. [DOI] [PubMed] [Google Scholar]
- 28.Laws AK. Effects of induction of anaesthesia and muscle paralysis on functional residual capacity of the lungs. Can Anaesth Soc. 1968;15:325–331. doi: 10.1007/BF03006957. [DOI] [PubMed] [Google Scholar]
- 29.Rendher K, Hatch DJ, Sessler AD, et al. Effects of general anesthesia, muscle paralysis and mechanical ventilation on pulmonary nitrogen clearance. Anesthesiology. 1971;35:591–601. doi: 10.1097/00000542-197112000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Gournand A, Reily BL, Himmelstein A, Austrian A. Pulmonary circulation and alveolar-perfusion relationship after pneumonectomy. J Thorac Surg. 1950;19:80–116. [PubMed] [Google Scholar]
- 31.Eugene J, Brown SE, Ligh RV, et al. Maximum oxygen consumption: a physiologic guide to pulmonary resection. Surg Forum. 1982;33:260–262. [Google Scholar]
- 32.Hamzagić H. U: Savremene mogućnosti klinicke fiziologije disanja. Univerzitetska knjiga Sarajevo; 1999. Ocjena operabilnosti bolesnika; pp. 327–333. [Google Scholar]
- 33.Harpole DH, Liptay MJ, DeCamp MM. Prospective analysis of pneumonectomy risk factors for major morbidity and cardiac disrrhythmias. Ann Thorac Surg. 1966;61:977–982. doi: 10.1016/0003-4975(95)01174-9. [DOI] [PubMed] [Google Scholar]
- 34.Damhuis RA, Schutte PR. Resection rates and postoperative mortality in 7899 patients with lung cancer. Eur Respir J. 1996;9:7–10. doi: 10.1183/09031936.96.09010007. [DOI] [PubMed] [Google Scholar]
- 35.Wahi R, McMurty MJ, DeCaro LF, et al. Determinations of preoperative morbidity and mortality after pneumonectomy. Ann Thorac Surg. 1989;48:33–37. doi: 10.1016/0003-4975(89)90172-0. [DOI] [PubMed] [Google Scholar]