Abstract
Recent studies have demonstrated that cross talk between ceramide and redox signaling modulates various cell activities and functions and contributes to the development of cardiovascular diseases and renal dysfunctions. Ceramide triggers the generation of reactive oxygen species (ROS) and increases oxidative stress in many mammalian cells and animal models. On the other hand, inhibition of ROS-generating enzymes or treatment of antioxidants impairs sphingomyelinase activation and ceramide production. As a mechanism, ceramide-enriched signaling platforms, special cell membrane rafts (MR) (formerly lipid rafts), provide an important microenvironment to mediate the cross talk of ceramide and redox signaling to exert a corresponding regulatory role on cell and organ functions. In this regard, activation of acid sphingomyelinase and generation of ceramide mediate the formation of ceramide-enriched membrane platforms, where trans-membrane signals are transmitted or amplified through recruitment, clustering, assembling, or integration of various signaling molecules. A typical such signaling platform is MR redox signaling platform that is centered on ceramide production and aggregation leading to recruitment and assembling of NADPH oxidase to form an active complex in the cell plasma membrane. This redox signaling platform not only conducts redox signaling or regulation but also facilitates a feedforward amplification of both ceramide and redox signaling. In addition to this membrane MR redox signaling platform, the cross talk between ceramide and redox signaling may occur in other cell compartments. This book chapter focuses on the molecular mechanisms, spatial–temporal regulations, and implications of this cross talk between ceramide and redox signaling, which may provide novel insights into the understanding of both ceramide and redox signaling pathways.
Keywords: Sphingolipid, Membrane rafts, Free radicals, Signalosomes, Redoxosome, Caveolae, Acid sphingomyelinase, Lysosome, Methyl-β-cyclodextrin
1 Introduction
Redox signaling is a fundamental signaling mechanism in cell biology which importantly participates in a variety of cellular activities including cell proliferation (Burdon 1996; Cai 2006; Nicco et al. 2005), differentiation (Del Prete et al. 2008; Hansberg et al. 1993; Kusmartsev and Gabrilovich 2003; Sasaki et al. 2009; Sauer et al. 2001), and apoptosis (Hildeman 2004; Liu et al. 2009; Mates and Sanchez-Jimenez 2000; Perrone et al. 2008; Wolf 2005). Abnormal redox signaling is frequently involved in various pathophysiological processes such as senescence (Colavitti and Finkel 2005), inflammation (Azad et al. 2008; Muller-Peddinghaus 1989; Yamamoto et al. 2009), hypoxia (Bell and Chandel 2007; Guzy and Schumacker 2006; Kietzmann and Gorlach 2005; MacFarlane et al. 2008), and ischemia/reperfusion (Goswami et al. 2007; Szocs 2004; Toledo-Pereyra et al. 2004), which contribute to the progression of almost all diseases, from cardiovascular ones such as shock (Flowers and Zimmerman 1998; Gendzwill 2007a, b), hypertension (Delles et al. 2008; Hirooka 2008; Ong et al. 2008; Paravicini and Touyz 2008; Puddu et al. 2008; Zeng et al. 2009), and atherosclerosis (Kojda and Harrison 1999; Patel et al. 2000), to metabolic ones such as diabetes mellitus (Bagi et al. 2009; Ksiazek and Wisniewska 2001), to neurodegenerative ones such as Alzheimer's disease (Casadesus et al. 2004; Perry et al. 1998), infectious diseases (Jamaluddin et al. 2009; Mashimo et al. 2006; Ochsendorf 1998; Sun et al. 2008), and cancer (Azad et al. 2009; Oyagbemi et al. 2009; Weinberg and Chandel 2009). Currently, it is of high interest to explore how redox signaling is regulated under both physiological and pathological conditions.
Despite extensive studies, the precise mechanisms for rapid activation of redox enzymes by different stimuli are still poorly understood. Redox enzymes such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, unlike G-protein-coupled enzymes, are not coupled with any specific receptors. Therefore, a previous undefined mechanism must exist to bridge receptor activation and redox signaling. Recent studies in non-phagocytes revealed that membrane raft (MR, formerly lipid raft) signaling platforms might be responsible for activation of death receptors, in particular CD95 and tumor necrosis factor receptor 1 (TNFR1). These death receptors were found to be localized in MRs, and these receptors in MRs can interact to stabilize the MRs and allow raft aggregation (i.e., MRs clustering), by which many raftophilic molecules are invariably recruited into the complex with the MRs, producing massive signaling and effector responses. Furthermore, it has been well documented that ROS or oxidative stress is a downstream mechanism of receptor clustering in MR platforms. This MR-associated ROS generation, downstream of CD95 and TNFR1, may be of high importance in the early alterations of cell functions during activation of death receptors and in induction of apoptosis (Dumitru et al. 2007; Morgan et al. 2007). Indeed, recently collected evidence support the view that MR signaling platforms are an important mechanism for initiating and transmitting redox signaling in mammalian cells (Li and Gulbins 2007; Oakley et al. 2009; Yang and Rizzo 2007; Zhang and Li 2010). Given the central role of ceramide and related signaling in the formation or regulation of MR redox platforms through clustering of ceramide-enriched microdomains, the cross talk between ceramide and redox signaling is emerging as an important cellular signaling mechanism that mediates the regulation of cellular activities.
Ceramide is generated by several enzymatic pathways in mammalian cells. Two major pathways are the sphingomyelinase (SMase) pathway that generates ceramide from sphingomyelin (SM) by the activities of SMase and the de novo synthesis pathway that synthesizes ceramide from serine and palmitoyl-CoA by the activity of ceramide synthase. The biophysical properties of ceramide molecules predict a tight interaction of ceramide molecules with each other, resulting in the formation of stable and tightly packed ceramide-enriched membrane microdomains that spontaneously fuse to form large ceramide-enriched membrane macrodomains or platforms. Among SMases, acid SMase (ASMase) has been considered as the major enzyme responsible for the formation of ceramide-enriched membrane platforms. Recently, we and others have reported that various death factors bind to their receptors in or around individual MRs and stimulate ASMase to produce ceramide from SM, leading to the formation of ceramide-enriched membrane platforms. In such platforms, NADPH oxidase subunits such as gp91phox and p47phox and other redox molecules are aggregated, clustered, and/or recruited, leading to signal transduction by increase or scavenging of O2•– or ROS. Such redox signaling associated with ceramide-enriched membrane platforms has been found to contribute to the regulation of a variety of cellular activities and organ functions and lead to pathological changes such as endothelial dysfunction, cell apoptosis, and phagosomal action in neutrophils or macrophages (Jin et al. 2008b; Li et al. 2007; Zhang et al. 2006). Given the focus of this chapter on the cross talk between ceramide and redox signaling, we will discuss the role of ceramide in the regulation of MR or nonraft redox signaling and vice versa as well as summarize some evidence related to physiological and pathological relevances of this cross talk. Given that there are a lot of discussions about the basic knowledge of ceramide signaling in other chapters, here, we first provide some background information regarding current knowledge of redox signaling and regulation.
2 Redox Signaling and Oxidative Stress
2.1 Reactive Oxygen Species
Reactive oxygen species (ROS) is a collective term that often includes not only the oxygen radicals such as superoxide (O2•–), hydroxyl (OH–), peroxyl (RO2), alkoxyl (RO•), and hydroperoxyl (HO2•) but also non-radicals such as hydrogen peroxide (H2O2), hypochlorous acid (HOCl), ozone (O3), singlet oxygen (ΔgO2), and peroxynitrite (ONOO–). Since these oxygen derivatives, whether they are radicals or non-radicals, are very reactive, they can oxidize or reduce other molecules in living cells or tissues. Therefore, in general, redox signaling is often referred to as the signaling induced by ROS (Halliwell and Cross 1994; Stadtman 2004; Tang et al. 2002). The most important ROS is O2•–, which is unstable and short-lived because it has an unpaired electron, and it is highly reactive with a variety of cellular molecules, including proteins and DNA. O2•– is reduced to H2O2 by superoxide dismutase (SOD), and both O2•– and H2O2 can diffuse from their sites of generation to other cellular locations. H2O2 is further reduced to generate the highly reactive •OH through the Haber–Weiss or Fenton reaction under pathological conditions. In contrast to O2•– and H2O2, •OH is highly reactive and, therefore, causes primarily local damage. In addition, O2•– can also interact with NO to form another reactive oxygen free radical, ONOO•–. Under physiological conditions, O2– preferably produces H2O2 via the dismutation reaction. However, when excess O2•– is produced, a substantial amount of O2•– reacts with NO to produce ONOO•–. Taken together, these ROS constitute a redox regulatory network that controls cellular activity and function.
2.2 Redox Signaling and Injury
It has been reported that ROS can be produced as a basic signaling messenger to maintain cell or organ functions or increasingly generated or released in response to various stimuli. Meanwhile, these active molecules are constantly scavenged by the endogenous antioxidant systems, mainly composed of the enzyme-mediated pathways as SOD, catalase, glutathione peroxidase, glutathione-S-transferase, thioredoxin/thioredoxin reductase, and other peroxidases. In addition, direct reactions between ROS and different molecules may also result in antioxidant actions such as the interactions between ROS and NO, –SH, vitamin E, β-carotene, ceruloplasmin, ferritin, transferrin, hemoglobin, and ascorbates. Being tightly regulated under normal conditions, intracellular and extracellular ROS are maintained at very low levels (less than 1 % of produced ROS). If the generation of ROS exceeds its removal by scavengers, the intracellular and extracellular levels of ROS will increase, leading to oxidative stress and a progression of various pathophysiological processes and respective diseases. If the level of ROS increases to even higher levels, its damaging effects, to DNAs, proteins, lipids, and glycols, become inevitable. These damaging effects of ROS are often tightly correlated together and share a common redox system responsible for the generation and scavenging of ROS molecules.
2.3 ROS-Generating Systems
Among four common ROS including O2•–, H2O2, HO–, and ONOO–, O2•– has been considered as the progenitor of other common ROS. The production of O2•– and related regulation in biological systems have been intensively studied. In mammalian cells, many pathways are involved in the production of O2•–, including NADPH oxidase, xanthine oxidase, mitochondrial respiration chain, cytochrome P450, lipoxygenase, cyclooxygenase, peroxisomes, and NO synthase uncoupling. Some nonenzymatic derivatives of O2•– may be formed via photolysis, heme protein + Fe, and auto-oxidation reactions. Among these pathways, NADPH oxidase has been reported to be a major source of O2•– for the redox regulation in some cells such as vascular endothelial and smooth muscle cells (Griendling et al. 2000). It is estimated that this nonmitochondrial NADPH oxidase-derived O2•– constitutes more than 95 % of the production of O2•– in these cells, especially when they are stimulated (Mohazzab et al. 1994; Rajagopalan et al. 1996).
3 Interactions of Ceramide and Redox Signaling Pathways
There is accumulating evidence that ceramide induces the activation of ROS-generating enzymes, including NADPH oxidase, xanthine oxidase, NO synthase, and the mitochondrial respiratory chain (Corda et al. 2001; Lecour et al. 2006). In particular, ceramide has been shown to activate NADPH oxidase and to increase the production of O2•– in a variety of mammalian cells, including human aortic smooth muscle cells, endothelial cells (ECs), and macrophages (Bhunia et al. 1997; Zhang et al. 2007, 2008). Because many stimuli activate NADPH oxidase by translocation and aggregation of its subunits, it has been proposed that ceramide may mediate the fusion of small raft domains to ceramide-enriched membrane platforms, thereby clustering subunits of NADPH oxidase, assembling them into an active enzyme complex and producing of O2•–. In addition, ceramide has also been shown to interact with the mitochondrial electron transport chain leading to the generation of ROS (Corda et al. 2001). Given the crucial role of NADPH oxidase in the normal regulation of cell functions and as one of the most important redox signaling pathways (Dworakowski et al. 2006; Finkel and Holbrook 2000), we will focus on the cross talk between ceramide signaling pathway and NADPH oxidase-derived redox regulation.
3.1 Ceramide-Enriched Microdomains in MR Redox Signaling
Ceramide belongs to a highly hydrophobic lipid family, which consists of fatty acids with carbon chains in variable lengths (2–28 carbons) and sphingosine (Mathias and Kolesnick 1993). SMase is released to the cell surface or extracellular space in an autocrine or paracrine manner that hydrolyzes cell-surface SM, inducing cell–cell communications and exerting remote action through blood circulation. More importantly, SMase may act on SM incorporated in the MR area of cell membranes and thereby produce ceramide locally (Cremesti et al. 2002). Ceramide molecules spontaneously bind to each other to form microdomains that fuse to large ceramide-enriched membrane platforms (Brown and London 1998; Grassme et al. 2001). In such ceramide-enriched platforms, redox molecules such as NADPH oxidase subunits or cofactors can be aggregated to assemble into active NADPH oxidase complex, producing O2•– to conduct signaling (Jin et al. 2008b; Zhang et al. 2006).
This ceramide-enriched redox signaling platform has been found to be formed in response to different death receptor ligands such as CD95 ligand, TNF-α, or endostatin (Jin et al. 2008a, b; Zhang et al. 2006). Furthermore, ultraviolet irradiation also induces the formation of ceramide-enriched platforms that mediate ROS production (Chatterjee and Wu 2001). We recently demonstrated that a rapid movement and consequent fusion of lysosomes to supply ASMase into the MR area of cell membranes occur in response to various stimuli (Jin et al. 2007, 2008a). This lysosome fusion is critical for the formation of ceramide-enriched platforms and therefore determines MR redox signaling in different cells, in particular in ECs (Jin et al. 2008a, b).
Further studies have revealed that sortilin, a glycoprotein responsible for transferring ASMase from the Golgi apparatus to lysosomes, is also important in initiating the movement of lysosomes and promoting their fusion to the cell membrane in ECs (Bao et al. 2010a, b). Sortilin is a 95-kDa glycoprotein, which has been reported to play an important role in targeting or transferring proteins to lysosomes (Ni and Morales 2006). Its Vps10p domain in the luminal region may be the binding site for the saposin-like motif of ASMase, while its cytoplasmic tail containing an acidic cluster-dileucine motif binds the monomeric adaptor protein GGA and is structurally similar to the cytoplasmic domain of M6P. The coupled sortilin-1 and ASMase work together to promote the movement of lysosomes toward the cell membrane, which, in turn, leads to MRs clustering and NADPH oxidase activation in ECs. This ASMase-dependent clustering of receptors was also observed for other receptors such as CD20, CD40, TNFR, and epidermal growth factor receptors (EGFR) (Rodighiero et al. 2004). In addition, the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor)-centered exocytic machinery was found to be involved in MR clustering to form redox signaling platforms. SNAREs comprise a superfamily of small, mostly membrane-anchored proteins, which mediate membrane fusion between organelles or from organelles to cell plasma membranes (Gerst 1999). In particular, SNARE-mediated membrane fusion plays an essential role in the secretory pathway of various eukaryotic cells, which is named as the SNARE or SNARE-centered exocytic machinery (Blank et al. 2002). It seems that SNARE as a membrane fusion facilitator is also present in MR redox signaling platforms although its major function is to help lysosome fusion (Zhang and Li 2010).
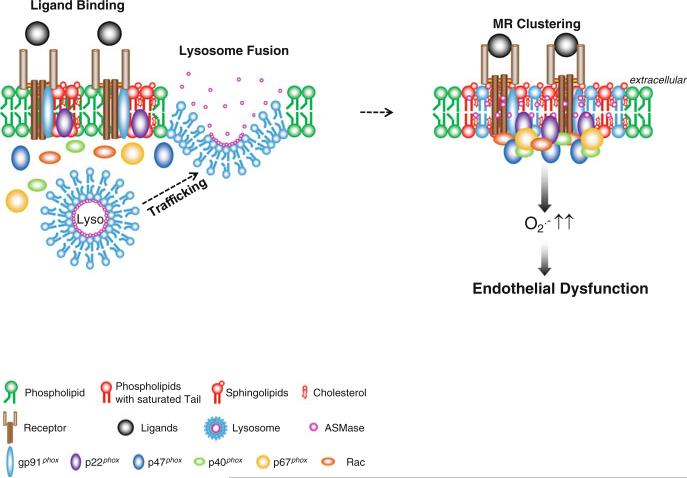
A comprehensive working model for the mediation of MR clustering and the formation of MR signaling platforms in arterial ECs is presented in Fig. 1; this model emphasizes the derivation of membrane ASMase as being from lysosomes, which target ASMase when it is synthesized from ER and transported through Golgi apparatus. Many mature lysosomes with ASMase are proximal to the cell membrane. When a receptor such as death receptor is activated by a ligand binding to it or by other stimulations, these lysosomes proximal to the cell membrane become mobilized to move and fuse with the cell membrane, activating ASMase and synthesizing ceramide, thereby resulting in MRs clustering and the formation of ceramide-enriched platforms. These MR platforms, in turn, recruit, translocate, and aggregate NOX and its subunits or cofactors and assemble them into an active enzyme complex, which produces O2•–, promoting transmembrane signaling.
Fig. 1.
Lysosome biogenesis and fusion to cell membrane to form ceramide-enriched redox signaling platforms. ASMase is synthesized from the ER and transported through Golgi apparatus to lysosomes. These lysosomes can be mobilized to traffic and fuse into cell membrane, where ASMase is activated and ceramide produced, resulting in MRs clustering and formation of ceramide-enriched platforms (adapted from Xia M, et al. Cardiovasc Res (2011) 89 (2): 401–409)
3.2 Redox Signaling Molecules Associated with Ceramide-Enriched Membrane Platforms
3.2.1 The NADPH Oxidase Family
NADPH oxidase, identified and characterized first in neutrophils, catalyzes the 1-electron reduction of oxygen producing O2•– using NADPH as the electron donor. This neutrophil oxidase consists of at least five subunits: two membrane-bound subunits gp91phox (also known as NOX2) and p22phox and three cytosolic subunits p47phox, p40phox, and p67phox. NOX2 and p22phox form an integral membrane complex termed cytochrome b558, and the other four subunits, p47phox, p67phox, p40phox, and the small GTPase Rac, localize in the cytosol in resting cells (Babior et al. 2002). In the classic model of phagocytic-type NADPH oxidase, activation involves translocation of the four cytosolic proteins to the cell membrane and interactions with the membrane spanning subunits p22phox and NOX2, resulting in the transfer of the NADPH electron to oxygen molecules and the generation of O2•– (Babior et al. 2002; Dang et al. 2002).
NOX protein family consists of six other homologues of NOX2 (gp91phox) catalytic subunits, namely, NOX1, NOX3, NOX4, NOX5, DUOX1 (dual oxidases1), and DUOX2, which determine ROS production in non-phagocytes (Cheng et al. 2001). NOX2 is also expressed in non-phagocytes, including neurons, cardiac cells, skeletal muscle cells, liver cells, ECs, B lymphocytes, epithelial cells, and hematopoietic cells (Piccoli et al. 2005). The structure and function of non-phagocytic NOX are very similar to NOX2. They can also catalyze a single-electron reduction of molecular oxygen, generating O2•– and other ROS. It is interesting to note that almost all NOXs were demonstrated to have some structural or functional link to or relationship with MRs (Ushio-Fukai et al. 2001; Zhang et al. 2006; Zuo et al. 2004, 2005). Given that NOX activation requires many cofactors to work together, MRs provide a wonderful platform for NOX and the other NADPH oxidase subunits and cofactors to assemble and then work as an active enzymatic complex. However, the driving force or actual physical platform for NADPH oxidase assembly as an active enzyme complex is still unknown. As noted above, the ceramide-enriched membrane macrodomains or platforms may represent an important mechanism mediating this assembly or activation process of NADPH oxidase.
3.2.2 Superoxide Dismutase
Recently, proteomic analysis demonstrated that membrane SOD (SOD1) is present in MR fractions (Zhai et al. 2009), a fact consistent with previous reports that SOD1 is detectable in MRs (Siafakas et al. 2006). Reported SOD1 levels, for example, in MR fractions were much higher than that in other areas of the plasma membrane. These results support the view that aggregation of MRs may play an important role for SOD1 actions. It is assumed that localization and subsequent aggregation of SOD1 in MRs could affect cellular functions as well as the interplay between different cell types, as MRs are rich in receptors and the signaling molecules necessary for cell–cell communications (Zhai et al. 2009). Indeed, a more recent study has reported that H2O2, generated extracellularly by extracellular SOD, anchored to ECs surface via the heparin-binding domain (HBD), enhances VEGF-induced VEGF receptor 2 (VEGFR2) autophosphorylation in caveolin-enriched MRs, but not in non-caveolar MRs. The HBD of endothelial SOD is required for its localization in plasma membrane MRs, suggesting that localization of endothelial SOD in caveolae/MRs via HBD can serve as an important mechanism by which SOD-derived extracellular H2O2 efficiently promotes VEGFR2 signaling in ECs and postnatal angiogenesis (Oshikawa et al. 2010).
3.2.3 Catalase
In neutrophils, recent proteomic analysis (Feuk-Lagerstedt et al. 2007) has also found catalase in MR fractions that play critical roles in redox signaling by cleavage of H2O2. Although some studies have demonstrated that MR-associated catalase may be related to peroxisome biogenesis, the function of this catalase association with MRs remains still largely unknown. It is possible that MRs in hepatic peroxisomal membrane cells are able to help catalase sorting and distribution to different compartments of these cells, assigning them an important role in hepatocyte proliferation and lipid metabolism. Given that hepatic caveolin-1 plays an important role in liver regeneration and lipid metabolism, caveolae with catalase may be critically involved in this liver regeneration and lipid metabolism. However, recent studies found that the absence of caveolin-1 did not affect the peroxisomal location of catalase in mouse liver. It seems caveolin-1 is not required for peroxisome biogenesis, whereas other types of peroxisomal MRs are required (Woudenberg et al. 2010). Obviously, more research and thinking need to be invested into the formation and function of MR-associated catalase complexes.
3.2.4 Thioredoxin
Although it is not yet extensively studied, thioredoxin (TRX) has also been reported as a MR-associated protein. In some reports, MRs have been shown to mediate the effects of TRX. There is convincing evidence that MRs may mediate the actions of TRX on leukocyte–EC interaction related to redox regulation during inflammation. TRX is a ubiquitous protein with a redox-active disulfide that functions in concert with NADPH and TRX reductase to control the redox state of cysteine residues of different oxidant-targeted proteins. Given the antioxidant role of TRX, the MR-mediated role of TRX in the interaction between leukocytes and ECs may importantly regulate inflammatory responses through counteracting oxidative stress and ROS. In addition, TRX can be internalized into the cells through MR-mediated endocytosis. In particular, a TRX mutant, TRX-C35S (with replacement of cysteine 35 by serine), was found to bind rapidly to the cell surface and be internalized into the cells through MRs in the plasma membrane. This indicates that the cysteine at the active site of TRX is important for the internalization and signal transduction of extracellular TRX through MRs (Hara et al. 2007; Kondo et al. 2007).
3.2.5 Transient Receptor Protein C3 and C4 (TRPC3 and TRPC4)-Redox Sensors
MRs have also been reported to promote molecules aggregation, gating, or activation producing their downstream impact on redox sensing or enhancement of effector responses to redox signaling. Among these molecules, a currently identified redox-sensitive protein-transient receptor protein (TRP) is particularly noteworthy. TRPs are a family of voltage-independent nonspecific cation-permeable channels. Evidence exists that TRPC3 and TRPC4 localize or relocalize in MRs and can form a TRPC3–TRPC4 complex with different properties from their respective homomeric channels, which are redox sensitive (Poteser et al. 2006). Perhaps these TRP channels are directly gated or influenced by the formation of MR platforms, and therefore, their redox-sensing function is altered. Indeed, the TRPC3 channel activity is increased by cholesterol loading of the cell membrane when TRPC3 is overexpressed. This increased channel activity may lead to enhanced redox sensitivity of the channels, exerting an important redox regulation or resulting in pathologic consequences in different cells (Poteser et al. 2006).
3.3 Redox Regulation of Ceramide-Enriched Membrane Platform Formation
There is increasing evidence that the formation of MR-derived signaling platforms can also be regulated by redox molecules. For example, the formation of ceramide-enriched membrane platforms in the membrane of coronary arterial ECs can be reduced by SOD but increased by O2•– donor or generating systems (Zhang et al. 2007). H2O2 was also found to activate pro-survival signaling pathways, including activation of PI3 kinase/Akt and extracellular signal-regulated kinases (ERK)1/2 by changes in MRs behaviors (Yang et al. 2006a). Exogenous administration of xanthine/xanthine oxidase, a O2•– generating system, has demonstrated a dramatic increase in MRs clustering and ceramide-enriched membrane platform formation in the membrane of ECs (Qiu et al. 2003; Zhang et al. 2007). Furthermore, ROS in T lymphocytes were also shown to enhance MR signaling, and blockade of ROS production by the SOD-mimic MnTBAP reduced the localization of several signaling molecules such as LAT, phospho-LAT, and PLC-gamma in MRs fractions. Treatment of T cells with the ROS synthesizer, tert-butyl hydrogen peroxide (TBHP), greatly enhanced MR formation and the distribution of phospho-LAT into MRs. Moreover, lipid peroxides were found to promote the formation of larger rafts or platforms on the membrane, and photooxidation, at the lipid double bonds, caused raft enlargement (Ayuyan and Cohen 2006). These observations corroborate and reinforce the conclusion that ROS are able to enhance MR clustering or formation of macrodomains and must contribute to the formation of MR platforms (Lu et al. 2007). In addition to the direct regulation of SMase/ceramide pathway, various ROS were found to influence MR signaling or function through their actions on many other MR constituents such as caveolin-1, cholesterol, and related raft proteins (Dumitru et al. 2007; Morgan et al. 2007).
3.3.1 ROS Interact with Caveolin-1
Biochemical and morphological experiments have shown that at least two subtypes of lipid microdomains are present in mammalian cells: caveolar and noncaveolar MRs. The size of noncaveolar MRs is 50–100 nm or even smaller, and each of them contains 10–30 protein molecules. Caveolae, cave-like plasma membrane subdomains, are considered as another subtype of lipid domains. Caveolin-1 is the major protein component of caveolae, and its polymerization forms a rigid scaffold that maintains the characteristic cave-like morphology. In addition to its structural function, caveolin-1 has several important regulatory activities through direct interaction with other functional proteins and signaling molecules. Caveolin-1 is subject to two types of posttranslational modification that might be critical for regulating its intracellular activity and localization, namely, phosphorylation and palmitoylation. Recent studies have indicated that both phosphorylation and palmitoylation of caveolin-1 can be regulated by ROS and ultimately affect caveolar functions. In vascular ECs, H2O2 causes increased tyrosine phosphorylation of caveolins (Brown and London 1998). In addition, Parat and colleagues showed that exogenous H2O2 did not alter the intracellular localization of caveolin-1 in ECs, but it inhibited the trafficking of newly synthesized caveolin-1 to MRs (Brown et al. 1998). They further demonstrated that H2O2 did not alter the rate of caveolin-1 depalmitoylation but rather decreased the “on-rate” of palmitoylation (Brown et al. 1998). Functional studies substantiated that caveolin-1 is a sensitive target of oxidative stress and that the oxidation of caveolar membrane cholesterol causes the translocation of caveolin-1 from the plasma membrane to the Golgi apparatus (Grassme et al. 2001). In a separate study, treatment of ECs with ROS caused a release of caveolin-1 from membranes and also a decrease in the number of caveolae detected by electron microscopy (van den Elzen et al. 2005). In summary, these results suggest that oxidative stress modulates caveolin-1 function and cellular levels, which may ultimately affect caveolar function and plasma membrane composition, namely, alteration in the ratio of caveolar vs. noncaveolar MRs or membrane signaling platforms.
3.3.2 ROS Interact with Cholesterol
It has been known that the formation of MRs is driven by tight packing between cholesterol and sphingomyelin and other sphingolipids. Oxysterols are derivatives of cholesterol that contain a second oxygen atom as a carbonyl, hydroxyl, or epoxide group (Morita et al. 2004). Cytotoxic oxysterols formed by nonspecific oxidative mechanisms can affect many cellular processes that contribute to the pathogenesis of disease. According to their biophysical properties, which can be distinct from those of cholesterol, oxysterols can promote or inhibit the formation of membrane microdomains or MRs (Byfield et al. 2006; Scheel-Toellner et al. 2004). For instance, the activities of receptor tyrosine kinases such as the epidermal growth factor (EGF) receptor and insulin receptors, which are found in MR/caveolae, can be modulated by changes in cellular cholesterol content. EGF stimulation-induced phosphatidylinositol 4,5-bisphosphate turnover is inhibited by depletion of cholesterol, but the effects of repletion with different oxysterols varied according to their structure. Turnover was not restored by 25-hydroxycholesterol, while 7-ketocholesterol and 5α, 6α-epoxycholesterol restored turnover (Morita et al. 2004). Likewise, desmosterol, another oxysterol, impaired raft-dependent signaling via the insulin receptor, while nonraft-dependent protein secretion was not affected (Morita et al. 2004). Therefore, these studies suggest that ROS may oxidize cholesterol to various oxysterols, which affect the stability of MRs and formation of MR-derived signaling platforms.
3.4 Redox Regulation of SMase Activity
The role of SMase in the formation of ceramide-enriched membrane platforms has been extensively studied. Recently, several studies have indicated that the generation of ROS may be involved in the activation of the enzyme in response to various stimuli (Charruyer et al. 2005; Dumitru and Gulbins 2006; Malaplate-Armand et al. 2006; Scheel-Toellner et al. 2004). Scheel-Toellner and colleagues demonstrated that ASMase activation, ceramide generation, and CD95 clustering play a crucial role in the spontaneous apoptosis of neutrophils; apoptosis was substantially delayed in Asm-deficient mice (Scheel-Toellner et al. 2004). Based on the observations that the intracellular redox balance changes in aging neutrophils, the authors investigated the possibility that ROS may be involved in ASMase activation. They demonstrated that pretreating neutrophils with the antioxidants N-acetylcysteine (NAC) and desferrioxamine substantially inhibited the events downstream of ASMase, such as ceramide generation and CD95 clustering, indicating that ROS release is required for ASM activation (Scheel-Toellner et al. 2004). Similarly, pretreatment with the antioxidant, pyrrolidine dithiocarbamate (PDTC), abolished ASMase activation by ultraviolet (UV)-C light in U937 cells (Charruyer et al. 2005).
In other studies, neuronal stimulation with soluble oligomers of amyloid-beta peptide was found to result in the release of ROS and the subsequent activation of SMases (Malaplate-Armand et al. 2006). Treatment of these neurons with antioxidant molecules and a cPLA2-specific inhibitor or antisense oligonucleotide led to inhibition of SMase activation and subsequent apoptosis, suggesting that amyloid-beta oligomers induce neuronal death by activating both NSMase and ASMase via a redox-sensitive cPLA2-arachidonic acid pathway (Malaplate-Armand et al. 2006). In addition, Dumitru and colleagues have also demonstrated the involvement of ROS in TRAIL-induced activation of ASMase and apoptosis (Dumitru and Gulbins 2006). Stimulation with TRAIL/DR5 led to the activation of ASMase and the subsequent formation of ceramide-enriched membrane platforms, DR5 clustering, and consequent apoptosis. Pretreatment with antioxidants NAC and Tiron substantially inhibited TRAIL-induced ASMase activation, ceramide/DR5 clustering, and apoptosis, demonstrating that ROS play a crucial role in TRAIL-associated signaling pathway (Dumitru and Gulbins 2006). Finally, studies investigating the cellular effects of Cu2+ showed that Cu2+ also promotes the ROS-dependent activation of ASMase and leads to the death of hepatocytes (Dumitru and Gulbins 2006). It was shown that the accumulation of Cu2+, as occurred in Wilson disease, activates ASMase in hepatocytes and triggers the release of ceramide in these cells. This process results in Cu2+-induced hepatocyte death, which can be prevented by a deficiency in ASMase (Lang et al. 2007).
One of the mechanisms by which ASMase activity is regulated by ROS has been described by Qiu and coworkers (Qiu et al. 2003). It has been proposed that C-terminal cysteine (Cys629) plays a crucial role in the enzymatic activity of recombinant human ASMase (rhASM). The loss of the free sulfhydryl group on this amino acid results in activation of the enzyme, and this loss of free sulfhydryl group may be due to copper-promoted dimerization of rhASM by C-terminal cysteine, thiol-specific chemical modification of this cysteine to form a mixed disulfide bond or a sulfur-carbon linkage, deletion of this cysteine by carboxypeptidase or recombinant DNA technology, and site-specific mutation to change the cysteine to a serine residue. Because zinc is required for ASMase activity, the effect of C-terminal cysteine modification on the activation of ASMase may be associated with zinc coordination. It is known that zinc coordinates with a water molecule to produce an optimal structure for catalysis of ASMase. This zinc coordination model is essentially identical to the “cysteine switch” activation mechanism described previously for the matrix metalloproteinase family (Van Wart and Birkedal-Hansen 1990).
Another redox regulatory mechanism of various SMases has been described in a number of studies (Bezombes et al. 2002; Hernandez et al. 2000; Mansat-de Mas et al. 1999), which is related to the effect of glutathione (GSH) (Liu and Hannun 1997; Martin et al. 2007). It has been demonstrated that the GSH/GSSG ratio is critical to such redox regulation of SMase activity. In this regard, the influence of glutathione, its analogs, and individual fragments on the activity of various SMase isoforms have been studied (Liu and Hannun 1997; Liu et al. 1998). In particular, the inhibitory effect of GSH on the neutral Mg2+-dependent SMase was shown to be associated with the g-glutamyl-group of GSH. Since the effect of GSH is accompanied by decrease in diene conjugate and diene ketone levels, the ability of GSH to inhibit oxidative processes in the cell due to its antioxidative properties may be mainly responsible for its effect to inhibit SMase activity. In other words, ROS-mediated oxidation of NSMase or AMSase may enhance their activity (Tsyupko et al. 2001). In addition, the intracellular GSH concentration is thought to be involved in the regulation of SMase activity by increase in its expression (Yoshimura et al. 1999).
3.5 Feedforward Amplifying Mechanism
MRs signaling platforms usually contain different proteins including different signaling molecules and cross-linkers or enzymes (Simons and Ikonen 1997; Simons and Toomre 2000). The formation of MR platforms activates, facilitates, and/or amplifies signal transductions. As mentioned above, if MR clustering forms a ceramide-enriched membrane platform, the ceramide production or enrichment is mainly from SMase-catalyzed cleavage of SM cholines in individual MRs (Gulbins and Kolesnick 2003; Hoekstra et al. 2003). Redox regulation of SMase, MRs clustering, and ceramide-enriched platform formation suggest that MRs and ROS may constitute an amplification system of redox signals and ceramide signaling cell membranes, which insures the efficiency of signal transduction. The formation of such feedforward amplifying loop for MR redox and ceramide signaling may also be responsible for the tempospatial regulation of a complex signalosome that precisely and efficiently control cell function. If the activity of this regulatory loop is excessively enhanced, excessive production of both ROS and ceramide may result in the progress and development of different diseases or pathological processes.
It should be noted that ceramide-enriched membrane platforms might also be formed without the presence of classically defined MRs simply through a fusion of several ceramide molecules. These ceramide molecules can come from MRs or other membrane fractions. The clustering of receptor molecules within ceramide-enriched membrane platforms might well have several important functions such as the aggregation in close proximity of many receptor molecules (Gulbins and Grassme 2002), the facilitation of the transactivation of signaling molecules associating or interacting with a receptor, and the amplification of the specific signals generated by activated receptors. However, in some studies the formation of ceramide or ceramide platforms may not play roles in signaling, but contribute to the scrambling of the cell membrane as shown at the erythrocyte surface. It is shown that the eryptosis may be linked to apoptotic pathways via ceramide, which may be causally cross talked to local oxidative stress. This may represent another type of MR redox signaling in erythrocytes (Lang et al. 2006, 2010).
4 Functional Relevance of the Cross Talk
It has been known that the biological responses to cellular or tissue ROS levels are very different and vary from physiological to pathological reactions. Therefore, the cross talk between ceramide and redox signaling may be implicated in various cell and organ functions, depending upon the amount of ROS and ceramide. With respect to ROS, when small amounts of ROS are produced, they may mediate physiological redox signaling, but when large amounts of ROS are produced, which refer to increased oxidative stress, cell/tissue damage may occur, resulting in cellular apoptosis and necrosis and ultimately causing various systemic or organ-based diseases. In regard to ceramide, largely increased ceramide production through such feedwarding mechanism may also contribute to the development of different diseases. This section will focus on the functional relevance of ceramide–redox signaling to the regulation of endothelial function and renal glomerular and tubular functions.
4.1 Regulation of Endothelial Function
Ceramide was demonstrated to increase endothelial O2•– in the endothelium of isolated small coronary arteries, which was blocked by different NADPH oxidase inhibitors such as N-vanillylnonanamide, apocynin, and diphenyleneiodonium. By analysis of the enzyme activity, ceramide was found to significantly stimulate the activity of NADPH oxidase in ECs, which was prevented by NADPH oxidase inhibitors, but not by inhibitors of NOS, xanthine oxidase, and mitochondrial electron transport chain enzymes. In addition, inhibition of NADPH oxidase by different NADPH oxidase inhibitors largely prevented ceramide-induced and O2•–-mediated impairment of endothelium-dependent relaxation to agonists in small bovine coronary arteries (Zhang et al. 2001). These studies very clearly indicate that NADPH oxidases mediate dysfunction of ECs induced by ceramide. In additional studies, ceramide-induced activation of NADPH oxidase was associated with a rapid translocation of p47phox to the cytoplasmic membrane. As discussed above, p47phox translocation is a crucial step leading to activation of NADPH oxidase in phagocytes, these data suggest that p47phox translocation may initiate ceramide-induced activation of NADPH oxidase in coronary ECs. However, the signaling mechanisms that initiate p47phox translocation are unclear. It has been suggested that TNF-α activates PKC-ζ, which in turn phosphorylates p47phox, thereby inducing the translocation of this subunit to the membrane where it associates with gp91phox to form the active enzyme complex (Frey et al. 2002). It is possible that ceramide employs this kinase to regulate NADPH oxidase in ECs. On the other hand, it might be also possible that ceramide-enriched membrane platforms recruit the subunits of NADPH oxidase to assemble and activate the oxidase at the cell membrane after treatment with TNF-α.
Although it is very attractive to speculate that MRs and ceramide-enriched membrane platforms are involved in the homeostasis of ECs and the response of these cells to cytokines, little is known about the role of these domains for the regulation of vascular endothelial functions. Recently, work in our laboratory has tested whether MR clustering and trafficking on the cell membrane of ECs are associated with ceramide production and action (Jin et al. 2007, 2008a; Zhang et al. 2006, 2007). It was found that ASMase and ceramide are of importance in CD95 ligand-induced formation of MR clusters on the EC membrane. We also demonstrated that ceramide-mediated clustering of MRs is involved in the regulation of O2•– production in coronary ECs via NADPH oxidase. This effect was associated with the recruitment and aggregation of the NADPH oxidase subunits gp91phox and p47phox in MRs. It was shown that silencing the ASMase gene by siRNA reduced CD95 ligand-induced gp91phox aggregation in MR clusters and p47phox translocation and completely inhibited CD95 ligand-induced O2•– production in these cells. In isolated small bovine coronary arteries, transfection of ASMase siRNA markedly attenuated CD95 ligand-induced inhibition of endothelium-dependent vasorelaxation (a response to bradykinin) by 60 % (Zhang et al. 2007). The results suggest that ASMase, the release of ceramide, and MR-derived ceramide-enriched membrane platforms are involved in the activation of NADPH oxidase in response to cytokines in coronary ECs, consequently leading to endothelial dysfunction (Jin et al. 2007; Zhang et al. 2006, 2007).
Recently, the MR redox signaling platform associated with NADPH oxidase has been demonstrated to be responsible for endothelial dysfunction induced by various stimuli such as death receptor activation, homocysteine, cytokines, or adipokines (Jin et al. 2008b; Xia et al. 2011; Zhang et al. 2006). As a commonly used functional study, endothelium-dependent vasodilation (EDVD) response in isolated perfused arteries was intensively tested. It was found that various stimulations which led to the formation of MR redox signaling platforms such as CD95 ligand, endostatin, homocysteine, and visfatin all led to impairment of EDVD. This impairment was homeostatically recovered by NADPH oxidase inhibition using apocynin, M-β-CD, filipin, or ASMase siRNA, suggesting that MR redox signaling platforms with NADPH oxidase participate in the impairment of endothelial function (Jin et al. 2007, 2008b; Zhang et al. 2006, 2007). This MR redox enhancement in endothelial injury and dysfunction may be intimately involved in the pathophysiology of diverse cardiovascular diseases such as atherosclerosis, hypertension, shock, and ischemia/reperfusion injuries.
In addition, the formation or enhancement of MR redox signaling platforms may contribute to macrophage reprogramming, foam cell formation, and cell deformability. Induction of lipid oxidation through ROS was found to amplify foam cell formation through oxidized low-density lipoprotein (Ox-LDL) uptake and a subsequent clustering of ceramide-enriched lipid domains (Morita et al. 2004). In addition, Ox-LDL was found to affect cell-surface turnover of ceramide-backbone sphingolipids and apoE-mediated uptake, by low-density lipoprotein receptor-related protein (MRP) family members, leading, in turn, to cell-surface expansion of ceramide-enriched domains and activation of apoE-/MRP1-/CD1-mediated antigen presentation (van den Elzen et al. 2005). On the other hand, high-density lipoprotein (HDL)-mediated lipid efflux can disrupt MRs and prevent foam cell formation. It has been suggested that MR redox signaling or regulation plays an important role in the formation of foam cells and thus in the progression of atherosclerosis (Schmitz and Grandl 2007).
In addition to the role in alterations of macrophage behavior, MR redox signaling may also play an important role in cell deformability, thereby initiating or promoting atherogenesis (Levitan and Gooch 2007). Studies have demonstrated that disruption of MRs by oxidants such as Ox-LDL altered the cytoskeletal structure, including the extent of polymerization, stabilization, cross-linking, and membrane association (Byfield et al. 2006). These molecular alterations may increase the force generated by the cytoskeleton, resulting in a stiffening of the cytoskeleton and hence stiffening of the cell and plasma membrane. Increased force in the cytoskeleton and its downstream increased stiffness may also elevate membrane tension and thereby influence the activity of various mechanosensitive ion channels. Direct evidence suggests that Ox-LDL can disrupt MRs, resulting in a series of pathological changes in the biomechanical properties of vascular ECs and ultimately induce endothelial dysfunction and atherogenesis (Blair et al. 1999; Byfield et al. 2006).
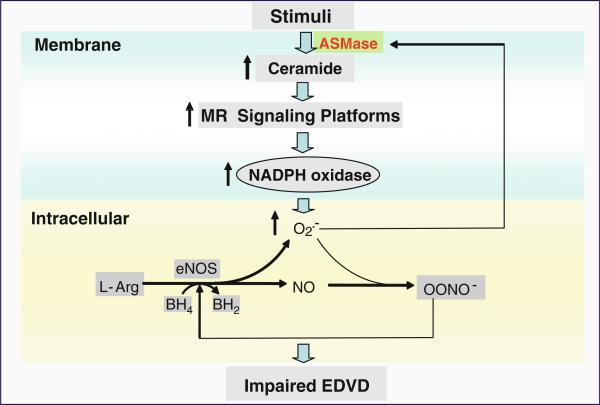
As summarized in Fig. 2, ASMase-mediated ceramide signaling and ROS-based redox signaling interact through a MR signaling platforms. As a molecular cross talk, this interaction of ceramide and ROS may play an important role in the regulation of endothelial function. When various death factors or other stimuli act on ECs, ASMase located in situ or translocated from lysosomes or lysosome-like vesicles is activated to produce ceramide from SM, resulting in the formation of a number of ceramide-enriched membrane signaling platforms. In these platforms, ASMase, NADPH oxidase subunits such as gp91phox and p47phox, and other proteins are aggregated and activated, producing O2•–. O2•– reacts with NO to decrease NO bioavailability and to produce peroxynitrite (ONOO–). Increased ONOO– uncouples NOS to produce more O2•– but less NO. O2•– or ROS may feedforward enhance MRs clustering by enhancement of ASMase and alteration of MR clustering process, forming positive amplifications. All these together constitute a redox signaling network resulting in endothelial dysfunction and impairment of endothelium-dependent vasodilation, which may be the basis for difference cardiovascular diseases such as atherosclerosis, coronary artery disease, hypertension, and peripheral arterial disease.
Fig. 2.
Ceramide-enriched redox signaling platforms associated with NADPH oxidase in endothelial dysfunction. Upon ASMase stimulation, ceramide is released to promote MR clustering and form ceramide-enriched MR platforms, with aggregation and assembling of NADPH oxidase subunits and other proteins such as Rac GTPase. Then, NADPH oxidase is activated to produce O2•–, which reacts with NO to produce ONOO– resulting in endothelial dysfunction in coronary arteries. Further, NADPH oxidase-derived O2•– regulates ASMase activation and ceramide production in a feedforward manner
4.2 Regulation of Renal Function
Recent studies have indicated that ceramide may be implicated in the regulation of kidney function and seems to be involved in renal glomerular and tubular pathology (Kaushal et al. 1998; Ueda et al. 2000; Yi et al. 2004; Yin et al. 1997). More recently, our group demonstrated that ceramide importantly contributes to the development of chronic glomerular injury associated with hyperhomocysteinemia, and thereby ceramide may serve as an important mechanism of end-stage renal disease (Yi et al. 2004, 2007, 2009b). Several studies that employed TLC and HPLC analysis reported the detection of ceramide in the kidney, leading to the hypothesis that ceramide might be involved already in the regulation of normal renal function (Kaushal et al. 1998; Ueda et al. 2000; Yi et al. 2004; Yin et al. 1997). To determine whether ceramide also participates in the development of chronic renal failure, we employed a model of hyperhomocysteinemia-induced renal injury. These studies revealed that hyperhomocysteinemia significantly increased ceramide levels in the renal cortex from rats. Likewise, treatment of cultured mesangial cells with l-homocysteine resulted in a concentration-dependent increase in ceramide. Evidence for a de novo synthesis of ceramide by l-homocysteine was provided in studies that employed fumonisin B1 and myriocin, inhibitors of the de novo synthesis pathway of ceramide. These inhibitors prevented l-homocysteine-induced ceramide formation in mesangial cells as well as in vivo in the kidney and attenuated glomerular injury and proteinuria (Yi et al. 2004, 2009b). These data provide direct evidence that the ceramide pathway is critically involved in L-homocysteine-induced glomerular injury and glomerular sclerosis.
Further mechanistic studies have demonstrated that l-homocysteine stimulated ceramide production in different glomerular cells such as glomerular capillary ECs, podocytes, and mesangial cells and that ceramides appear to be an important regulator of the function of glomerular filtration membrane, which is consistent with previous results that ceramide may be involved in the regulation of normal renal function (Kaushal et al. 1998; Ueda et al. 2000). It was also found that blockade of ceramide production in hyperhomocysteinimic rats substantially inhibited the enhancement of NADPH oxidase activity and production of O2•– in the kidney (Yi et al. 2004). Although translocation of p47phox, seen in ECs, was not shown to occur in l-homocysteine- or ceramide-induced activation of NADPH oxidase in rat mesangial cells (Yi et al. 2004), in podocytes and glomerular capillary ECs, homocysteine was shown to induce the formation of MR redox signaling platforms associated with NADPH oxidase (Yi et al. 2009a; Zhang et al. 2010). Perhaps, the transformation of small MRs to ceramide-enriched membrane platforms results in a clustering of NADPH oxidase molecules, producing redox signaling or injury in these glomerular cells, resulting in local oxidative stress and ultimate glomerular injury. This oxidative stress mediated by NADPH oxidase has been indicated to play an important role in progressive glomerular injuries or glomerulosclerosis associated with hyperhomocysteinemia and other diseases such as diabetes and hypertension (Eid et al. 2009; Fujimoto et al. 2008; Yi et al. 2004). It is now known that the formation of MR redox platforms and ROS production is a major mechanism responsible for hyperhomocysteinemia-induced enhancement of glomerular permeability, thereby producing glomerular injuries and consequent sclerosis, which is associated with the regulation of microtubule stability. It seems that the early injurious effects of hyperhomocysteinemia and other pathogenic factors acting on NADPH oxidase are associated with the formation of redox signaling platforms via MR clustering and consequent increases in glomerular permeability due to disruption of microtubule networks in the glomerular filtration membrane (Yi et al. 2007; Zhang et al. 2010).
In addition to their role in the regulation of renal glomerular function, MRs-associated NADPH oxidase may maintain an inactive state of this enzyme in human renal proximal tubule (RPT) cells. Disruption of such inactive MRs may result in their activation (Han et al. 2008). Different cells use MRs to conduct redox signaling in different ways. As Li et al. have reported, NADPH oxidase-dependent ROS production is differentially regulated in MRs and non-MR compartments of RPT epithelial cells (Yi et al. 2009b). This differential regulation or MR-associated inactive NADPH oxidase is mainly attributed to the action of the neurotransmitter dopamine. Dopamine is an essential neurotransmitter involved, mainly via its peripheral receptors, in the control of blood pressure, sodium balance, and various renal and adrenal functions (Jose et al. 2002). As G-protein-coupled receptors, dopamine receptors are associated with both caveolar and noncaveolar MRs (Allen et al. 2007; Lingwood et al. 2009; Simons and Ikonen 1997; Yu et al. 2004). It has been shown that D1-like receptors can exert an inhibitory action on ROS production in VSM and RPT cells (White and Sidhu 1998; Yang et al. 2006b; Yasunari et al. 2000). However, the molecular mechanisms involved still remain unknown. By sucrose density gradient ultracentrifugation and analysis of NADPH oxidase isoforms and subunits in MRs, it was found that the majority of membrane proteins was in non-MR fractions; only a small portion of proteins were in MR fractions. The D1-like receptor agonist, fenoldopam, decreases NOX2 and Rac1 proteins in MRs, albeit to a greater extent in hypertensive than normotensive rats. Fenoldopam decreased the amount of NOX2 that co-immunoprecipitated with p67phox in cells from normotensive rats. These observations suggest that fenoldopam causes a redistribution of NOX2, NOX4, and Rac1 from MRs and to non-MR fractions. Further studies have shown that disruption of MRs results in the reactivation of NADPH oxidase that was destroyed by antioxidants and the silencing of NOX2 or NOX4. Perhaps this explains why in human RPT cells, MRs maintain NADPH oxidase in an inactive state (Han et al. 2008).
5 Concluding Remarks
In summary, there is no doubt that redox signaling through NADPH oxidase and other ROS producing or scavenging systems is correlated with the unique membrane structures known as MRs, where MRs serve as platforms to aggregate the membrane spanning or cytosolic components of the enzymes subunits or cofactors. In particular, MRs clustering may be a major mechanism for the assembly of NADPH oxidase subunits and cofactors into an active enzyme complex. Such MR redox platforms produce O2•– and thereby conduct redox signaling with compartmentalization and amplifications in response to different receptor bindings or other stimuli. It is well known that the formation of this MR redox signaling platform associated with NADPH oxidase is associated with activation of ASMase and production of ceramide. On the other hand, ROS production may enhance ASMase activity or alter MR components to promote MRs clustering. It is clear that ROS-based redox signaling and ceramide producing system and consequent signaling interact under control condition or upon different stimuli, constituting a temperospatial cross talk between two signaling pathways. Such cross talk may be significantly implicated in the regulation of organ functions. As an example, the regulation of endothelial function and renal glomerular or tubular functions is closely associated with ceramide–redox cross talk, and their interplay, if excessively enhanced, may result in endothelial dysfunction and renal glomerular or tubular dysfunction, leading to various cardiovascular and renal diseases. In perspective, it is imperative to develop new in vivo research strategies that are able to address the contribution of ceramide–redox interaction to organ functions and related regulatory mechanisms. More studies may also be needed to translate experimental results related to MR redox signaling platforms and ceramide–redox cross talk to clinical use.
Acknowledgment
The works cited in the authors’ laboratory were supported by the National Institutes of Health grants (HL075316 and HL057244).
References
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- Ayuyan AG, Cohen FS. Lipid peroxides promote large rafts: effects of excitation of probes in fluorescence microscopy and electrochemical reactions during vesicle formation. Biophys J. 2006;91:2172–2183. doi: 10.1529/biophysj.106.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health. 2008;11:1–15. doi: 10.1080/10937400701436460. [DOI] [PubMed] [Google Scholar]
- Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777–790. doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Feher A, Beleznai T. Preserved coronary arteriolar dilatation in patients with type 2 diabetes mellitus: implications for reactive oxygen species. Pharmacol Rep. 2009;61:99–104. doi: 10.1016/s1734-1140(09)70011-8. [DOI] [PubMed] [Google Scholar]
- Bao JX, Jin S, Zhang F, Wang ZC, Li N, Li PL. Activation of membrane NADPH oxidase associated with lysosome-targeted acid sphingomyelinase in coronary endothelial cells. Antioxid Redox Signal. 2010a;12:703–712. doi: 10.1089/ars.2009.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao JX, Xia M, Poklis JL, Han WQ, Brimson C, Li PL. Triggering role of acid sphingomyelinase in endothelial lysosome-membrane fusion and dysfunction in coronary arteries. Am J Physiol Heart Circ Physiol. 2010b;298:H992–H1002. doi: 10.1152/ajpheart.00958.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EL, Chandel NS. Mitochondrial oxygen sensing: regulation of hypoxia-inducible factor by mitochondrial generated reactive oxygen species. Essays Biochem. 2007;43:17–27. doi: 10.1042/BSE0430017. [DOI] [PubMed] [Google Scholar]
- Bezombes C, de Thonel A, Apostolou A, Louat T, Jaffrezou JP, Laurent G, et al. Overexpression of protein kinase Czeta confers protection against antileukemic drugs by inhibiting the redox-dependent sphingomyelinase activation. Mol Pharmacol. 2002;62:1446–1455. doi: 10.1124/mol.62.6.1446. [DOI] [PubMed] [Google Scholar]
- Bhunia AK, Han H, Snowden A, Chatterjee S. Redox-regulated signaling by lactosyl-ceramide in the proliferation of human aortic smooth muscle cells. J Biol Chem. 1997;272:15642–15649. doi: 10.1074/jbc.272.25.15642. [DOI] [PubMed] [Google Scholar]
- Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem. 1999;274:32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- Blank U, Cyprien B, Martin-Verdeaux S, Paumet F, Pombo I, Rivera J, et al. SNAREs and associated regulators in the control of exocytosis in the RBL-2H3 mast cell line. Mol Immunol. 2002;38:1341–1345. doi: 10.1016/s0161-5890(02)00085-8. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol. 1998;164:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- Brown DL, Doubilet PM, Miller FH, Frates MC, Laing FC, DiSalvo DN, et al. Benign and malignant ovarian masses: selection of the most discriminating gray-scale and Doppler sono-graphic features. Radiology. 1998;208:103–110. doi: 10.1148/radiology.208.1.9646799. [DOI] [PubMed] [Google Scholar]
- Burdon RH. Control of cell proliferation by reactive oxygen species. Biochem Soc Trans. 1996;24:1028–1032. doi: 10.1042/bst0241028. [DOI] [PubMed] [Google Scholar]
- Byfield FJ, Tikku S, Rothblat GH, Gooch KJ, Levitan I. OxLDL increases endothelial stiffness, force generation, and network formation. J Lipid Res. 2006;47:715–723. doi: 10.1194/jlr.M500439-JLR200. [DOI] [PubMed] [Google Scholar]
- Cai H. A new mechanism for flow-mediated vasoprotection? Focus on “lung endothelial cell proliferation with decreased shear stress is mediated by reactive oxygen species”. Am J Physiol. 2006;290:C35–C36. doi: 10.1152/ajpcell.00414.2005. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Smith MA, Zhu X, Aliev G, Cash AD, Honda K, et al. Alzheimer disease: evidence for a central pathogenic role of iron-mediated reactive oxygen species. J Alzheimers Dis. 2004;6:165–169. doi: 10.3233/jad-2004-6208. [DOI] [PubMed] [Google Scholar]
- Charruyer A, Grazide S, Bezombes C, Muller S, Laurent G, Jaffrezou JP. UV-C light induces raft-associated acid sphingomyelinase and JNK activation and translocation independently on a nuclear signal. J Biol Chem. 2005;280:19196–19204. doi: 10.1074/jbc.M412867200. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Wu S. Cell line dependent involvement of ceramide in ultraviolet light-induced apoptosis. Mol Cell Biochem. 2001;219:21–27. doi: 10.1023/a:1011083818452. [DOI] [PubMed] [Google Scholar]
- Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Colavitti R, Finkel T. Reactive oxygen species as mediators of cellular senescence. IUBMB Life. 2005;57:277–281. doi: 10.1080/15216540500091890. [DOI] [PubMed] [Google Scholar]
- Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol. 2001;24:762–768. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- Cremesti AE, Goni FM, Kolesnick R. Role of sphingomyelinase and ceramide in modulating rafts: do biophysical properties determine biologic outcome? FEBS Lett. 2002;531:47–53. doi: 10.1016/s0014-5793(02)03489-0. [DOI] [PubMed] [Google Scholar]
- Dang PM, Cross AR, Quinn MT, Babior BM. Assembly of the neutrophil respiratory burst oxidase: a direct interaction between p67PHOX and cytochrome b558 II. Proc Natl Acad Sci USA. 2002;99:4262–4265. doi: 10.1073/pnas.072345299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete A, Zaccagnino P, Di Paola M, Saltarella M, Oliveros Celis C, Nico B, et al. Role of mitochondria and reactive oxygen species in dendritic cell differentiation and functions. Free Radic Biol Med. 2008;44:1443–1451. doi: 10.1016/j.freeradbiomed.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Delles C, Miller WH, Dominiczak AF. Targeting reactive oxygen species in hypertension. Antioxid Redox Signal. 2008;10:1061–1077. doi: 10.1089/ars.2007.2008. [DOI] [PubMed] [Google Scholar]
- Dumitru CA, Gulbins E. TRAIL activates acid sphingomyelinase via a redox mechanism and releases ceramide to trigger apoptosis. Oncogene. 2006;25:5612–5625. doi: 10.1038/sj.onc.1209568. [DOI] [PubMed] [Google Scholar]
- Dumitru CA, Zhang Y, Li X, Gulbins E. Ceramide: a novel player in reactive oxygen species-induced signaling? Antioxid Redox Signal. 2007;9:1535–1540. doi: 10.1089/ars.2007.1692. [DOI] [PubMed] [Google Scholar]
- Dworakowski R, Anilkumar N, Zhang M, Shah AM. Redox signalling involving NADPH oxidase-derived reactive oxygen species. Biochem Soc Trans. 2006;34:960–964. doi: 10.1042/BST0340960. [DOI] [PubMed] [Google Scholar]
- Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, et al. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58:1201–1211. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuk-Lagerstedt E, Movitz C, Pellme S, Dahlgren C, Karlsson A. Lipid raft proteome of the human neutrophil azurophil granule. Proteomics. 2007;7:194–205. doi: 10.1002/pmic.200600482. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Flowers F, Zimmerman JJ. Reactive oxygen species in the cellular pathophysiology of shock. New Horizons. 1998;6:169–180. [PubMed] [Google Scholar]
- Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB. PKCzeta regulates TNF-alpha-induced activation of NADPH oxidase in endothelial cells. Circ Res. 2002;90:1012–1019. doi: 10.1161/01.res.0000017631.28815.8e. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Satoh M, Horike H, Hatta H, Haruna Y, Kobayashi S, et al. Olmesartan ameliorates progressive glomerular injury in subtotal nephrectomized rats through suppression of superoxide production. Hypertens Res. 2008;31:305–313. doi: 10.1291/hypres.31.305. [DOI] [PubMed] [Google Scholar]
- Gendzwill A. Reactive oxygen species and vascular hyporeactivity in septic shock. Part II–Scavengers and vascular hyporeactivity in septic shock. Pol Merkur Lekarski. 2007a;23:284–287. [PubMed] [Google Scholar]
- Gendzwill A. Reactive oxygen species and vascular hyporeactivity in septic shock. Part I–Reactive oxygen species and vascular hyporeactivity. Pol Merkur Lekarski. 2007b;23:280–283. [PubMed] [Google Scholar]
- Gerst JE. SNAREs and SNARE regulators in membrane fusion and exocytosis. Cell Mol Life Sci. 1999;55:707–734. doi: 10.1007/s000180050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami SK, Maulik N, Das DK. Ischemia-reperfusion and cardioprotection: a delicate balance between reactive oxygen species generation and redox homeostasis. Ann Med. 2007;39:275–289. doi: 10.1080/07853890701374677. [DOI] [PubMed] [Google Scholar]
- Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, et al. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Grassme H. Ceramide and cell death receptor clustering. Biochim Biophys Acta. 2002;1585:139–145. doi: 10.1016/s1388-1981(02)00334-7. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Cross CE. Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect. 1994;102(Suppl 10):5–12. doi: 10.1289/ehp.94102s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Li H, Villar VA, Pascua AM, Dajani MI, Wang X, et al. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension. 2008;51:481–487. doi: 10.1161/HYPERTENSIONAHA.107.103275. [DOI] [PubMed] [Google Scholar]
- Hansberg W, de Groot H, Sies H. Reactive oxygen species associated with cell differentiation in Neurospora crassa. Free Radic Biol Med. 1993;14:287–293. doi: 10.1016/0891-5849(93)90025-p. [DOI] [PubMed] [Google Scholar]
- Hara T, Kondo N, Nakamura H, Okuyama H, Mitsui A, Hoshino Y, et al. Cell-surface thioredoxin-1: possible involvement in thiol-mediated leukocyte-endothelial cell interaction through lipid rafts. Antioxid Redox Signal. 2007;9:1427–1437. doi: 10.1089/ars.2007.1661. [DOI] [PubMed] [Google Scholar]
- Hernandez OM, Discher DJ, Bishopric NH, Webster KA. Rapid activation of neutral sphingomyelinase by hypoxia-reoxygenation of cardiac myocytes. Circ Res. 2000;86:198–204. doi: 10.1161/01.res.86.2.198. [DOI] [PubMed] [Google Scholar]
- Hildeman DA. Regulation of T-cell apoptosis by reactive oxygen species. Free Radic Biol Med. 2004;36:1496–1504. doi: 10.1016/j.freeradbiomed.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Hirooka Y. Role of reactive oxygen species in brainstem in neural mechanisms of hypertension. Auton Neurosci. 2008;142:20–24. doi: 10.1016/j.autneu.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Hoekstra D, Maier O, van der Wouden JM, Slimane TA, van ISC. Membrane dynamics and cell polarity: the role of sphingolipids. J Lipid Res. 2003;44:869–877. doi: 10.1194/jlr.R300003-JLR200. [DOI] [PubMed] [Google Scholar]
- Jamaluddin M, Tian B, Boldogh I, Garofalo RP, Brasier AR. Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. J Virol. 2009;83:10605–10615. doi: 10.1128/JVI.01090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Yi F, Li PL. Contribution of lysosomal vesicles to the formation of lipid raft redox signaling platforms in endothelial cells. Antioxid Redox Signal. 2007;9:1417–1426. doi: 10.1089/ars.2007.1660. [DOI] [PubMed] [Google Scholar]
- Jin S, Yi F, Zhang F, Poklis JL, Li PL. Lysosomal targeting and trafficking of acid sphingomyelinase to lipid raft platforms in coronary endothelial cells. Arterioscler Thromb Vasc Biol. 2008a;28:2056–2062. doi: 10.1161/ATVBAHA.108.172478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Zhang Y, Yi F, Li PL. Critical role of lipid raft redox signaling platforms in endostatin-induced coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2008b;28:485–490. doi: 10.1161/ATVBAHA.107.159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose PA, Eisner GM, Felder RA. Role of dopamine receptors in the kidney in the regulation of blood pressure. Curr Opin Nephrol Hypertens. 2002;11:87–92. doi: 10.1097/00041552-200201000-00013. [DOI] [PubMed] [Google Scholar]
- Kaushal GP, Singh AB, Shah SV. Identification of gene family of caspases in rat kidney and altered expression in ischemia-reperfusion injury. Am J Physiol. 1998;274:F587–F595. doi: 10.1152/ajprenal.1998.274.3.F587. [DOI] [PubMed] [Google Scholar]
- Kietzmann T, Gorlach A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin Cell Dev Biol. 2005;16:474–486. doi: 10.1016/j.semcdb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysio-logical importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Kondo N, Ishii Y, Kwon YW, Tanito M, Sakakura-Nishiyama J, Mochizuki M, et al. Lipid raft-mediated uptake of cysteine-modified thioredoxin-1: apoptosis enhancement by inhibiting the endogenous thioredoxin-1. Antioxid Redox Signal. 2007;9:1439–1448. doi: 10.1089/ars.2007.1665. [DOI] [PubMed] [Google Scholar]
- Ksiazek K, Wisniewska J. The role of glucose and reactive oxygen species in the development of vascular complications of diabetes mellitus. Przegl Lek. 2001;58:915–918. [PubMed] [Google Scholar]
- Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol. 2003;74:186–196. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- Lang F, Lang KS, Lang PA, Huber SM, Wieder T. Mechanisms and significance of eryptosis. Antioxid Redox Signal. 2006;8:1183–1192. doi: 10.1089/ars.2006.8.1183. [DOI] [PubMed] [Google Scholar]
- Lang PA, Schenck M, Nicolay JP, Becker JU, Kempe DS, Lupescu A, et al. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat Med. 2007;13:164–170. doi: 10.1038/nm1539. [DOI] [PubMed] [Google Scholar]
- Lang F, Gulbins E, Lang PA, Zappulla D, Foller M. Ceramide in suicidal death of erythrocytes. Cell Physiol Biochem. 2010;26:21–28. doi: 10.1159/000315102. [DOI] [PubMed] [Google Scholar]
- Lecour S, Van der Merwe E, Opie LH, Sack MN. Ceramide attenuates hypoxic cell death via reactive oxygen species signaling. J Cardiovasc Pharmacol. 2006;47:158–163. doi: 10.1097/01.fjc.0000198520.28674.41. [DOI] [PubMed] [Google Scholar]
- Levitan I, Gooch KJ. Lipid rafts in membrane-cytoskeleton interactions and control of cellular biomechanics: actions of oxLDL. Antioxid Redox Signal. 2007;9:1519–1534. doi: 10.1089/ars.2007.1686. [DOI] [PubMed] [Google Scholar]
- Li PL, Gulbins E. Lipid rafts and redox signaling. Antioxid Redox Signal. 2007;9:1411–1415. doi: 10.1089/ars.2007.1736. [DOI] [PubMed] [Google Scholar]
- Li PL, Zhang Y, Yi F. Lipid raft redox signaling platforms in endothelial dysfunction. Antioxid Redox Signal. 2007;9:1457–1470. doi: 10.1089/ars.2007.1667. [DOI] [PubMed] [Google Scholar]
- Lingwood D, Kaiser HJ, Levental I, Simons K. Lipid rafts as functional heterogeneity in cell membranes. Biochem Soc Trans. 2009;37:955–960. doi: 10.1042/BST0370955. [DOI] [PubMed] [Google Scholar]
- Liu B, Hannun YA. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. J Biol Chem. 1997;272:16281–16287. doi: 10.1074/jbc.272.26.16281. [DOI] [PubMed] [Google Scholar]
- Liu B, Andrieu-Abadie N, Levade T, Zhang P, Obeid LM, Hannun YA. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-alpha-induced cell death. J Biol Chem. 1998;273:11313–11320. doi: 10.1074/jbc.273.18.11313. [DOI] [PubMed] [Google Scholar]
- Liu CY, Lee CF, Wei YH. Role of reactive oxygen species-elicited apoptosis in the pathophysiology of mitochondrial and neurodegenerative diseases associated with mitochondrial DNA mutations. J Formosan Med Assoc. 2009;108:599–611. doi: 10.1016/s0929-6646(09)60380-6. [DOI] [PubMed] [Google Scholar]
- Lu SP, Lin Feng MH, Huang HL, Huang YC, Tsou WI, Lai MZ. Reactive oxygen species promote raft formation in T lymphocytes. Free Radic Biol Med. 2007;42:936–944. doi: 10.1016/j.freeradbiomed.2006.11.027. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Wilkerson JE, Lovett-Barr MR, Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol. 2008;164:263–271. doi: 10.1016/j.resp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaplate-Armand C, Florent-Bechard S, Youssef I, Koziel V, Sponne I, Kriem B, et al. Soluble oligomers of amyloid-beta peptide induce neuronal apoptosis by activating a cPLA2-dependent sphingomyelinase-ceramide pathway. Neurobiol Dis. 2006;23:178–189. doi: 10.1016/j.nbd.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Mansat-de Mas V, Bezombes C, Quillet-Mary A, Bettaieb A, D'Orgeix AD, Laurent G, et al. Implication of radical oxygen species in ceramide generation, c-Jun N-terminal kinase activation and apoptosis induced by daunorubicin. Mol Pharmacol. 1999;56:867–874. doi: 10.1124/mol.56.5.867. [DOI] [PubMed] [Google Scholar]
- Martin SF, Sawai H, Villalba JM, Hannun YA. Redox regulation of neutral sphingomyelinase-1 activity in HEK293 cells through a GSH-dependent mechanism. Arch Biochem Biophys. 2007;459:295–300. doi: 10.1016/j.abb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Mashimo M, Nishikawa M, Higuchi K, Hirose M, Wei Q, Haque A, et al. Production of reactive oxygen species in peripheral blood is increased in individuals with Helicobacter pylori infection and decreased after its eradication. Helicobacter. 2006;11:266–271. doi: 10.1111/j.1523-5378.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- Mates JM, Sanchez-Jimenez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol. 2000;32:157–170. doi: 10.1016/s1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- Mathias S, Kolesnick R. Ceramide: a novel second messenger. Adv Lipid Res. 1993;25:65–90. [PubMed] [Google Scholar]
- Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol. 1994;266:H2568–H2572. doi: 10.1152/ajpheart.1994.266.6.H2568. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Kim YS, Liu Z. Lipid rafts and oxidative stress-induced cell death. Antioxid Redox Signal. 2007;9:1471–1483. doi: 10.1089/ars.2007.1658. [DOI] [PubMed] [Google Scholar]
- Morita SY, Kawabe M, Sakurai A, Okuhira K, Vertut-Doi A, Nakano M, et al. Ceramide in lipid particles enhances heparan sulfate proteoglycan and low density lipoprotein receptor-related protein-mediated uptake by macrophages. J Biol Chem. 2004;279:24355–24361. doi: 10.1074/jbc.M402035200. [DOI] [PubMed] [Google Scholar]
- Muller-Peddinghaus R. Reactive oxygen species and inflammation. Dtsch Tierarzti Wochenschr. 1989;96:210–212. [PubMed] [Google Scholar]
- Ni X, Morales CR. The lysosomal trafficking of acid sphingomyelinase is mediated by sortilin and mannose 6-phosphate receptor. Traffic. 2006;7:889–902. doi: 10.1111/j.1600-0854.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- Nicco C, Laurent A, Chereau C, Weill B, Batteux F. Differential modulation of normal and tumor cell proliferation by reactive oxygen species. Biomed Pharmacother. 2005;59:169–174. doi: 10.1016/j.biopha.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Oakley FD, Abbott D, Li Q, Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal. 2009;11:1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsendorf FR. Infection and reactive oxygen species. Andrologia. 1998;30(Suppl 1):81–86. doi: 10.1111/j.1439-0272.1998.tb02830.x. [DOI] [PubMed] [Google Scholar]
- Ong SL, Zhang Y, Whitworth JA. Reactive oxygen species and glucocorticoid-induced hypertension. Clin Exp Pharmacol Physiol. 2008;35:477–482. doi: 10.1111/j.1440-1681.2008.04900.x. [DOI] [PubMed] [Google Scholar]
- Oshikawa J, Urao N, Kim HW, Kaplan N, Razvi M, McKinney R, et al. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS One. 2010;5:e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyagbemi AA, Azeez OI, Saba AB. Interactions between reactive oxygen species and cancer: the roles of natural dietary antioxidants and their molecular mechanisms of action. Asian Pac J Cancer Prev. 2009;10:535–544. [PubMed] [Google Scholar]
- Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31(Suppl 2):S170–S180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- Patel RP, Moellering D, Murphy-Ullrich J, Jo H, Beckman JS, Darley-Usmar VM. Cell signaling by reactive nitrogen and oxygen species in atherosclerosis. Free Radic Biol Med. 2000;28:1780–1794. doi: 10.1016/s0891-5849(00)00235-5. [DOI] [PubMed] [Google Scholar]
- Perrone GG, Tan SX, Dawes IW. Reactive oxygen species and yeast apoptosis. Biochim Biophys Acta. 2008;1783:1354–1368. doi: 10.1016/j.bbamcr.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Perry G, Castellani RJ, Hirai K, Smith MA. Reactive oxygen species mediate cellular damage in Alzheimer disease. J Alzheimers Dis. 1998;1:45–55. doi: 10.3233/jad-1998-1103. [DOI] [PubMed] [Google Scholar]
- Piccoli C, Ria R, Scrima R, Cela O, D'Aprile A, Boffoli D, et al. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J Biol Chem. 2005;280:26467–26476. doi: 10.1074/jbc.M500047200. [DOI] [PubMed] [Google Scholar]
- Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, et al. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem. 2006;281:13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- Puddu P, Puddu GM, Cravero E, Rosati M, Muscari A. The molecular sources of reactive oxygen species in hypertension. Blood Press. 2008;17:70–77. doi: 10.1080/08037050802029954. [DOI] [PubMed] [Google Scholar]
- Qiu H, Edmunds T, Baker-Malcolm J, Karey KP, Estes S, Schwarz C, et al. Activation of human acid sphingomyelinase through modification or deletion of C-terminal cysteine. J Biol Chem. 2003;278:32744–32752. doi: 10.1074/jbc.M303022200. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodighiero S, De Simoni A, Formenti A. The voltage-dependent nonselective cation current in human red blood cells studied by means of whole-cell and nystatin-perforated patch-clamp techniques. Biochim Biophys Acta. 2004;1660:164–170. doi: 10.1016/j.bbamem.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Yamamoto H, Tominaga K, Masuda K, Kawai T, Teshima-Kondo S, et al. NADPH oxidase-derived reactive oxygen species are essential for differentiation of a mouse macrophage cell line (RAW264.7) into osteoclasts. J Med Invest. 2009;56:33–41. doi: 10.2152/jmi.56.33. [DOI] [PubMed] [Google Scholar]
- Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- Scheel-Toellner D, Wang K, Assi LK, Webb PR, Craddock RM, Salmon M, et al. Clustering of death receptors in lipid rafts initiates neutrophil spontaneous apoptosis. Biochem Soc Trans. 2004;32:679–681. doi: 10.1042/BST0320679. [DOI] [PubMed] [Google Scholar]
- Schmitz G, Grandl M. Role of redox regulation and lipid rafts in macrophages during Ox-LDL-mediated foam cell formation. Antioxid Redox Signal. 2007;9:1499–1518. doi: 10.1089/ars.2007.1663. [DOI] [PubMed] [Google Scholar]
- Siafakas AR, Wright LC, Sorrell TC, Djordjevic JT. Lipid rafts in Cryptococcus neoformans concentrate the virulence determinants phospholipase B1 and Cu/Zn superoxide dismutase. Eukaryot Cell. 2006;5:488–498. doi: 10.1128/EC.5.3.488-498.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Role of oxidant species in aging. Curr Med Chem. 2004;11:1105–1112. doi: 10.2174/0929867043365341. [DOI] [PubMed] [Google Scholar]
- Sun G, Xu X, Wang Y, Shen X, Chen Z, Yang J. Mycoplasma pneumoniae infection induces reactive oxygen species and DNA damage in A549 human lung carcinoma cells. Infect Immun. 2008;76:4405–4413. doi: 10.1128/IAI.00575-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szocs K. Endothelial dysfunction and reactive oxygen species production in ischemia/reperfusion and nitrate tolerance. Gen Physiol Biophys. 2004;23:265–295. [PubMed] [Google Scholar]
- Tang XL, Takano H, Rizvi A, Turrens JF, Qiu Y, Wu WJ, et al. Oxidant species trigger late preconditioning against myocardial stunning in conscious rabbits. Am J Physiol Heart Circ Physiol. 2002;282:H281–H291. doi: 10.1152/ajpheart.2002.282.1.H281. [DOI] [PubMed] [Google Scholar]
- Toledo-Pereyra LH, Lopez-Neblina F, Toledo AH. Reactive oxygen species and molecular biology of ischemia/reperfusion. Ann Transplant. 2004;9:81–83. [PubMed] [Google Scholar]
- Tsyupko AN, Dudnik LB, Evstigneeva RP, Alessenko AV. Effects of reduced and oxidized glutathione on sphingomyelinase activity and contents of sphingomyelin and lipid peroxidation products in murine liver. Biochemistry. 2001;66:1028–1034. doi: 10.1023/a:1012381928535. [DOI] [PubMed] [Google Scholar]
- Ueda N, Kaushal GP, Shah SV. Apoptotic mechanisms in acute renal failure. Am J Med. 2000;108:403–415. doi: 10.1016/s0002-9343(00)00311-9. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Hilenski L, Santanam N, Becker PL, Ma Y, Griendling KK, et al. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem. 2001;276:48269–48275. doi: 10.1074/jbc.M105901200. [DOI] [PubMed] [Google Scholar]
- van den Elzen P, Garg S, Leon L, Brigl M, Leadbetter EA, Gumperz JE, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437:906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg F, Chandel NS. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci. 2009;66:3663–3673. doi: 10.1007/s00018-009-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BH, Sidhu A. Increased oxidative stress in renal proximal tubules of the spontaneously hypertensive rat: a mechanism for defective dopamine D1A receptor/G-protein coupling. J Hypertens. 1998;16:1659–1665. doi: 10.1097/00004872-199816110-00013. [DOI] [PubMed] [Google Scholar]
- Wolf G. Role of reactive oxygen species in angiotensin II-mediated renal growth, differentiation, and apoptosis. Antioxid Redox Signal. 2005;7:1337–1345. doi: 10.1089/ars.2005.7.1337. [DOI] [PubMed] [Google Scholar]
- Woudenberg J, Rembacz KP, van den Heuvel FA, Woudenberg-Vrenken TE, Buist-Homan M, Geuken M, et al. Caveolin-1 is enriched in the peroxisomal membrane of rat hepatocytes. Hepatology. 2010;51:1744–1753. doi: 10.1002/hep.23460. [DOI] [PubMed] [Google Scholar]
- Xia M, Zhang C, Boini KM, Thacker AM, Li PL. Membrane raft-lysosome redox signalling platforms in coronary endothelial dysfunction induced by adipokine visfatin. Cardiovasc Res. 2011;89:401–409. doi: 10.1093/cvr/cvq286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Shimizu S, Mori Y. Involvement of TRPM2 channel in amplification of reactive oxygen species-induced signaling and chronic inflammation. Nippon Yakurigaku Zasshi. 2009;134:122–126. doi: 10.1254/fpj.134.122. [DOI] [PubMed] [Google Scholar]
- Yang B, Rizzo V. TNF-alpha potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H954–H962. doi: 10.1152/ajpheart.00758.2006. [DOI] [PubMed] [Google Scholar]
- Yang B, Oo TN, Rizzo V. Lipid rafts mediate H2O2 prosurvival effects in cultured endothelial cells. FASEB J. 2006a;20:1501–1503. doi: 10.1096/fj.05-5359fje. [DOI] [PubMed] [Google Scholar]
- Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, et al. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006b;290:R96–R104. doi: 10.1152/ajpregu.00434.2005. [DOI] [PubMed] [Google Scholar]
- Yasunari K, Kohno M, Kano H, Minami M, Yoshikawa J. Dopamine as a novel antioxidative agent for rat vascular smooth muscle cells through dopamine D(1)-like receptors. Circulation. 2000;101:2302–2308. doi: 10.1161/01.cir.101.19.2302. [DOI] [PubMed] [Google Scholar]
- Yi F, Zhang AY, Janscha JL, Li PL, Zou AP. Homocysteine activates NADH/NADPH oxidase through ceramide-stimulated Rac GTPase activity in rat mesangial cells. Kidney Int. 2004;66:1977–1987. doi: 10.1111/j.1523-1755.2004.00968.x. [DOI] [PubMed] [Google Scholar]
- Yi F, Chen QZ, Jin S, Li PL. Mechanism of homocysteine-induced Rac1/NADPH oxidase activation in mesangial cells: role of guanine nucleotide exchange factor Vav2. Cell Physiol Biochem. 2007;20:909–918. doi: 10.1159/000110451. [DOI] [PubMed] [Google Scholar]
- Yi F, Jin S, Zhang F, Xia M, Bao JX, Hu J, et al. Formation of lipid raft redox signalling platforms in glomerular endothelial cells: an early event of homocysteine-induced glomerular injury. J Cell Mol Med. 2009a;13:3303–3314. doi: 10.1111/j.1582-4934.2009.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Xia M, Li N, Zhang C, Tang L, Li PL. Contribution of guanine nucleotide exchange factor Vav2 to hyperhomocysteinemic glomerulosclerosis in rats. Hypertension. 2009b;53:90–96. doi: 10.1161/HYPERTENSIONAHA.108.115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T, Sandhu G, Wolfgang CD, Burrier A, Webb RL, Rigel DF, et al. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. J Biol Chem. 1997;272:19943–19950. doi: 10.1074/jbc.272.32.19943. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Banno Y, Nakashima S, Hayashi K, Yamakawa H, Sawada M, et al. Inhibition of neutral sphingomyelinase activation and ceramide formation by glutathione in hypoxic PC12 cell death. J Neurochem. 1999;73:675–683. doi: 10.1046/j.1471-4159.1999.0730675.x. [DOI] [PubMed] [Google Scholar]
- Yu P, Yang Z, Jones JE, Wang Z, Owens SA, Mueller SC, et al. D1 dopamine receptor signaling involves caveolin-2 in HEK-293 cells. Kidney Int. 2004;66:2167–2180. doi: 10.1111/j.1523-1755.2004.66007.x. [DOI] [PubMed] [Google Scholar]
- Zeng C, Villar VA, Yu P, Zhou L, Jose PA. Reactive oxygen species and dopamine receptor function in essential hypertension. Clin Exp Hypertens. 2009;31:156–178. doi: 10.1080/10641960802621283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J, Strom AL, Kilty R, Venkatakrishnan P, White J, Everson WV, et al. Proteomic characterization of lipid raft proteins in amyotrophic lateral sclerosis mouse spinal cord. FEBS J. 2009;276:3308–3323. doi: 10.1111/j.1742-4658.2009.07057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li PL. Membrane raft redox signalosomes in endothelial cells. Free Radic Res. 2010;44(8):831–842. doi: 10.3109/10715762.2010.485994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Zou AP, Li PL. Ceramide reduces endothelium-dependent vasodilation by increasing superoxide production in small bovine coronary arteries. Circ Res. 2001;88:824–831. doi: 10.1161/hh0801.089604. [DOI] [PubMed] [Google Scholar]
- Zhang AY, Yi F, Zhang G, Gulbins E, Li PL. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension. 2006;47:74–80. doi: 10.1161/10.1161/01.HYP.0000196727.53300.62. [DOI] [PubMed] [Google Scholar]
- Zhang AY, Yi F, Jin S, Xia M, Chen QZ, Gulbins E, et al. Acid sphingomyelinase and its redox amplification in formation of lipid raft redox signaling platforms in endothelial cells. Antioxid Redox Signal. 2007;9:817–828. doi: 10.1089/ars.2007.1509. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li X, Carpinteiro A, Gulbins E. Acid sphingomyelinase amplifies redox signaling in Pseudomonas aeruginosa-induced macrophage apoptosis. J Immunol. 2008;181:4247–4254. doi: 10.4049/jimmunol.181.6.4247. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hu JJ, Xia M, Boini KM, Brimson C, Li PL. Redox signaling via lipid raft clustering in homocysteine-induced injury of podocytes. Biochim Biophys Acta. 2010;1803:482–491. doi: 10.1016/j.bbamcr.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Ushio-Fukai M, Hilenski LL, Alexander RW. Microtubules regulate angiotensin II type 1 receptor and Rac1 localization in caveolae/lipid rafts: role in redox signaling. Arterioscler Thromb Vasc Biol. 2004;24:1223–1228. doi: 10.1161/01.ATV.0000132400.25045.2a. [DOI] [PubMed] [Google Scholar]
- Zuo L, Ushio-Fukai M, Ikeda S, Hilenski L, Patrushev N, Alexander RW. Caveolin-1 is essential for activation of Rac1 and NAD(P)H oxidase after angiotensin II type 1 receptor stimulation in vascular smooth muscle cells: role in redox signaling and vascular hypertrophy. Arterioscler Thromb Vasc Biol. 2005;25:1824–1830. doi: 10.1161/01.ATV.0000175295.09607.18. [DOI] [PubMed] [Google Scholar]