Abstract
Loss of ATM kinase, a transducer of the DNA damage response and redox sensor, causes the neurodegenerative disorder ataxia-telangiectasia (A-T). While a great deal of progress has been made in elucidating the ATM-dependent DNA damage response (DDR) network, a key challenge remains in understanding the selective susceptibility of the nervous system to faulty DDR. Several factors appear implicated in the neurodegenerative phenotype in A-T, but which of them plays a crucial role remains unclear, especially since mouse models of A-T do not fully mirror the respective human syndrome. Therefore, a number of human neural stem cell (hNSC) systems have been developed to get an insight into the molecular mechanisms of neurodegeneration as consequence of ATM inactivation. Here we review the hNSC systems developed by us an others to model A-T.
Keywords: ATM, Neurodegeneration, Neural stem cells, iPS cells, DNA damage response, Hypoxia
1. Introduction
Ataxia-telangiectasia (A-T) is an inherited neurodegenerative disorder arising from inactivating mutations in ATM, a gene encoding a protein kinase regarded as a key regulator of the DNA damage response (DDR) to double-strand breaks (DSBs) [1], [2], [3]. Concordant with this function, cells from A-T patients are hypersensitive to ionizing radiation and defective in cell cycle checkpoint arrest [3], [4]. The most prominent neuropathological finding in A-T is cerebellar ataxia, which involves a significant loss of Purkinje and granule cells; to some extent, loss of neurons in striatum and substantia nigra is also found [5]. Although the neurodegenerative phenotype has been attributed to a defective response to DNA breaks in pre- and post-mitotic neurons [6], oxidative stress and reduced anti-oxidant defence may seemingly play a role [7]. For instance, A-T patients show persistent oxidative stress at cellular level, whereas brains and DNA from ATM knock-out mice exhibit increased signatures of reactive oxygen species (ROS) [8], [9], and furthermore dietary supplementation of NAC (N-acetyl-cysteine) ameliorates oxidative DNA damage [10] and protects Purkinje cells from redox-induced death [11]. At molecular level, the persistent oxidative stress in A-T cells was recently attributed to the capacity of ATM to undergo disulfide bond formation and activation in response to oxidants [12], a mechanism clearly distinct from that elicited by DNA breaks. Hence, ATM works in redox sensing and signalling, and the loss of redox balance in A-T may be central to the neuropathological phenotype. Moreover, unscheduled re-entry of terminally differentiated post-mitotic neurons into the cell cycle following exposure to stimuli such as chronic inflammation, hypoxia and oxidative stress initiates an apoptotic response [13], [14], and this cell cycle reactivation may be particularly noxious to Purkinje and granule cells in A-T. While mostly nuclear, in neurons a fraction of ATM is localized in the cytoplasm [15], [16], where it functionally interacts with the synaptic proteins VAMP2 and Synapsin-1. Together with the defective long term potentiation and synaptic vesicle recycling in ATM-deficient brains, these findings lead to hypothesize that the neurological symptoms of A-T may also arise from an impaired cytoplasmic activity of ATM separate from its DNA damage response [17]. Most recently, neurodegeneration in A-T has been linked to the loss of the cytoplasmic histone deacetylase HDAC4 and its nuclear accumulation, with consequent suppression of neuronal gene expression [18]. Interestingly, the evidence that the malfunction of ATM severely impairs glial cell functionality and vascular integrity, has led to the hypothesis that the cerebellar degeneration in A-T is a reflection of a dysfunctional neuro-glio-vascular unit [19]. In summary, several factors appear implicated in the neurodegenerative phenotype of A-T, but which of them are most crucial is still debated, primarily because of the unavailability of human model systems able to recapitulate the neurological disease in vitro.
A potent experimental tool to study neurodegeneration is offered by genetically stable neural stem cells (NSCs) derived from multiple locations within the mammalian brain, endowed with in vitro self renewal capacity and able to differentiatiate into neurons, astrocytes and oligodendrocytes, in proportions that mirror their physiological distribution [20], [21], [22]. Human NSCs (hNSCs), for instance, can generate neurons with a GABAergic, glutamatergic or dopaminergic phenotype and electrophysiological action potentials [22], [23], and upon transplantation can improve sensorimotor functions and generate synaptic junctions [21], [24], [25]. Ablation of ATM expression in these cells can be achieved by shRNA silencing, as we have recently shown [26]. The downregulation of ATM expression in human embryonic stem cells (hESCs), which can be directed to differentiate down specific neural cell lineages, also offers an in vitro model to study neurodegeneration [27], [28]. A prominent advance in stem cell biology is represented by the possibility of generating human induced pluripotent stem (iPS) cells by reprogramming fibroblasts and other somatic cells. Owing to their capacity to differentiate into pure populations of specific cell types, iPS cells provide a unique opportunity for disease modelling, which is of paramount importance for neuropathological disorders. In the case of A-T, the direct reprogramming of patients’ fibroblasts into iPS cells and conversion to functional neurons has been recently reported [29], [30].
Here, we review the human cell systems that have been developed in order to perform in vitro analysis regarding the outcome of ATM inactivation in relation to DNA damage response and repair, susceptibility to oxidative stress and neural differentiation, with the overall goal to get an insight into the molecular mechanisms of neurodegeneration in A-T.
2. Available hNSCs as in vitro models of A-T
NSCs are self-renewing, multipotent precursor cells that reside in specialized regions of the embryonic and adult central nervous system (CNS) and participate in its homeostasis.
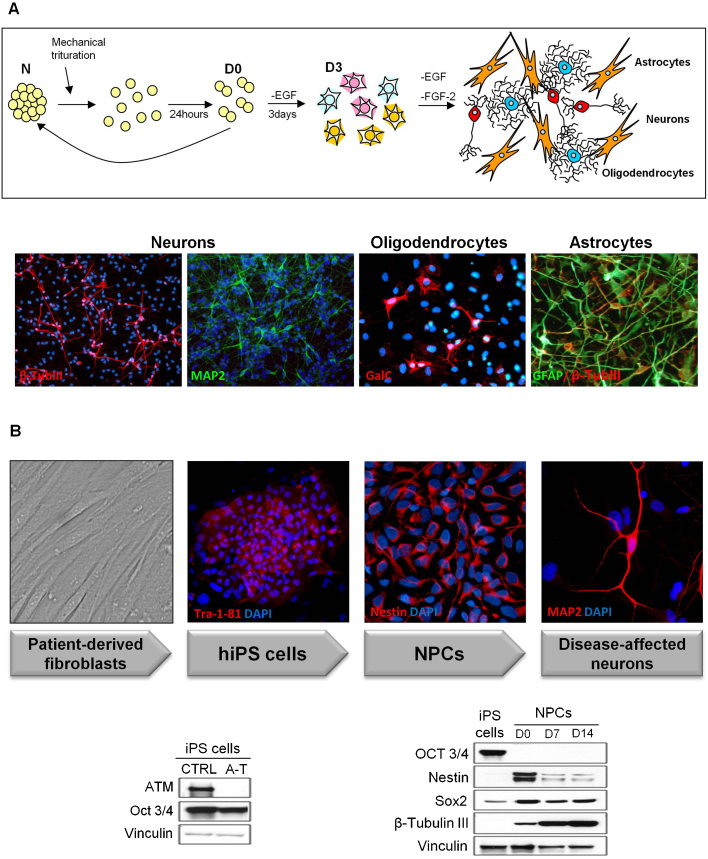
Primary cultures of human NSCs have been isolated from anatomically distinct fetal brain regions (e.g. midbrain and cortex) and grown in vitro as neurospheres (N) in the presence of EGF and FGF-2 [25], [31]. These cells can be maintained long-term in a proliferative undifferentiated state and can be induced to differentiate into the three major CNS cell types, i.e. neurons, astrocytes, and oligodendrocytes, in proportions that mirror their physiological distribution (Fig. 1A). To overcome some limitations (e.g. slow growth), primary NSCs have been immortalized with a retroviral vector encoding the v-myc oncogene (ihNSCs), and have shown to retain self-renewal and multipotential capacity [23], [31]. The impact of ATM on the biological properties of these cells has been generally studied in two ways, either by chemical inhibition of ATM with KU-55933 [32] or by lentiviral shRNA-mediated stable knockdown of ATM (shATM) [26], although it has been argued that inhibition of ATM activity and knockdown of the protein do not fully mirror the broad spectrum of A-T-causing mutations [33], [34], in line with recent findings showing that mice expressing a kinase-inactive ATM die during embryonic development, whereas ATM-null mice are viable [34].
Fig. 1.
ihNSC and iPS derived neurons. (A) The cartoon illustrates the maintenance and differentiation of ihNSCs. Cells are grown as neurospheres (N) in medium containing EGF and FGF-2, and mechanically disaggregated for expansion. After 24 h (D0; D: days of differentiation), cells appear as doublets which are plated on laminin-coated surfaces and grown without EGF for 3 days, in order to induce progenitor differentiation. FGF-2 is then removed to promote the generation of mature neurons, oligodendrocytes and astrocytes. The different cell types are detected by IF using lineage-specific phenotypic markers. (B) A-T patient-derived skin fibroblasts are reprogrammed into iPS cells. iPS cells express the pluripotency markers Tra-1-81 and upon neutralization give rise to a self-renewing population of NPCs expressing Nestin. NPCs can be further differentiated into mature neurons expressing MAP2. A-T iPS cells are ATM-deficient and express the pluripotency factor Oct3/4 (western blot, left). Upon neuralization, NPCs downregulate Oct3/4 and express the neural markers Nestin and Sox2, which decrease from D0 to D14 whereas β-Tubulin III increases (western blot, right). Protein loading per lane was verified with anti-vinculin antibodies.
Pluripotent human embryonic stem cells (hESCs), which can be differentiated into neural progenitors and mature neurons and astrocytes, provide another powerful model system to assess the effects of loss or inactivation of ATM. Also for these studies, ATM has either been stably knockdown by transducing hESCs with lentiviruses harbouring shRNA against ATM [27], [35], or chemically inhibited with KU-55933 [28].
Interestingly, neuronal models of A-T based on the use of patients’ tissues are currently being developed. For example, olfactory mucosa-derived neurospheres (ONS) have recently been established from several A-T patients and have been shown to differentiate in vitro into neurons, astrocytes and oligodendrocytes [30].
Finally, patient-derived induced pluripotent stem (iPS) cells provide a powerful tool for disease modelling. To address the role of ATM in neurodegeneration, we and others [29], [30] have generated iPS cells by reprogramming fibroblasts from A-T patients and normal donors by the introduction of pluripotency factors (Oct4/Klf4/Sox2/cMyc). Moreover, we have also established conditions to generate from iPS cells stable proliferating neural precursor cell (NPC) lines expressing Nestin and Sox2, which can be induced to differentiate into β-Tubulin III and MAP2 positive neurons (Fig. 1B) expressing GABAergic and glutamatergic markers, and which acquire electrophysiological function upon maturation [29]. Although the differentiation in a complete range of neuronal subtypes has not yet been achieved, this model system is extremely promising as it allows to study patient-derived cells which retain the original disease-causing mutation and can be differentiated in virtually all cell types.
3. The DNA damage response and repair in pre- and post-mitotic neural cells
Upon terminal differentiation, rapidly proliferating neuroprogenitors exit the cell cycle and become postmitotic. As the repair of DNA DSBs occurs through either homologous recombination (HR) or non-homologous end joining (NHEJ), the former operating in dividing cells while the latter also in non-dividing cells [36], the cellular answer to genotoxic damage may differ according to the cell cycle phase. Moreover, it has been shown that in human neuron-like cells the levels of ATM and other DDR components, e.g. ATR and NBS1, are downregulated during neuronal differentiation, with consequent attenuation of the damage response in non-dividing versus dividing cells [37] (Fig. 2A). Clearly, the availability of hNSCs offers the opportunity to study these phenomena in relation to ATM deficiency.
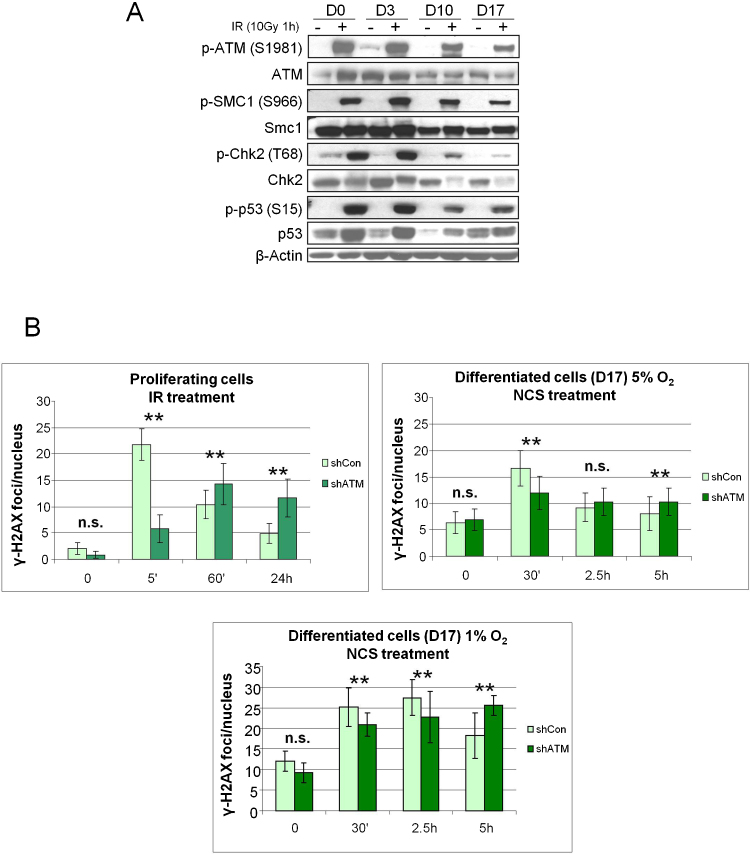
Fig. 2.
Response of ihNSC to IR-induced DNA damage. (A) At the indicated days of differentiation (D) cells were irradiated and analyzed 1 h later by western blot for the autophosphorylation of ATM-S1981 and phosphorylation of the ATM targets Smc1-S966, Chk2-T68 and p53-S15. Protein loading per lane was verified with anti-β-Actin antibodies (B) The formation and loss of damage-induced γ-H2AX nuclear foci was assessed on cells at D0 or D17 (cultured and differentiated in 5% or 1% O2, as indicated) which were treated with 1 Gy or 0.88 nM NCS respectively, collected at the indicated time points and IF-labeled for γ-H2AX. For each treatment, the number of foci was scored from over 100 cell nuclei per duplicate preparations and from three independent experiments (mean ± S.D.). For each time point, the difference between shCon and shATM was statistically significant (**P < 0.01) except for those indicated with n.s.
We have previously demonstrated that the DDR of dividing ihNSCs is defective upon ablation of ATM by shRNA interference, as evident from the reduced phosphorylation of ATM substrates [26]. Moreover, the disappearance of γ-H2AX, an indicator of ongoing DSB repair, is markedly delayed in shATM cells, indicating that ATM deficiency, like in other dividing cell types, delays the DNA repair also in hNSCs. By contrast, in terminally differentiated shATM ihNSCs, the number of γ-H2AX foci 30 min following DNA damage is greater than in control cells, while the resolution of the foci is only modestly delayed (Fig. 2B), suggesting a minor dependence on ATM for DSB repair. It should be noted that, compared to ATM-proficient neurons, non-dividing neurons from ATM-deficient neural progenitors obtained from hESCs are virtually negative for γ-H2AX expression [27].
4. Oxidative stress
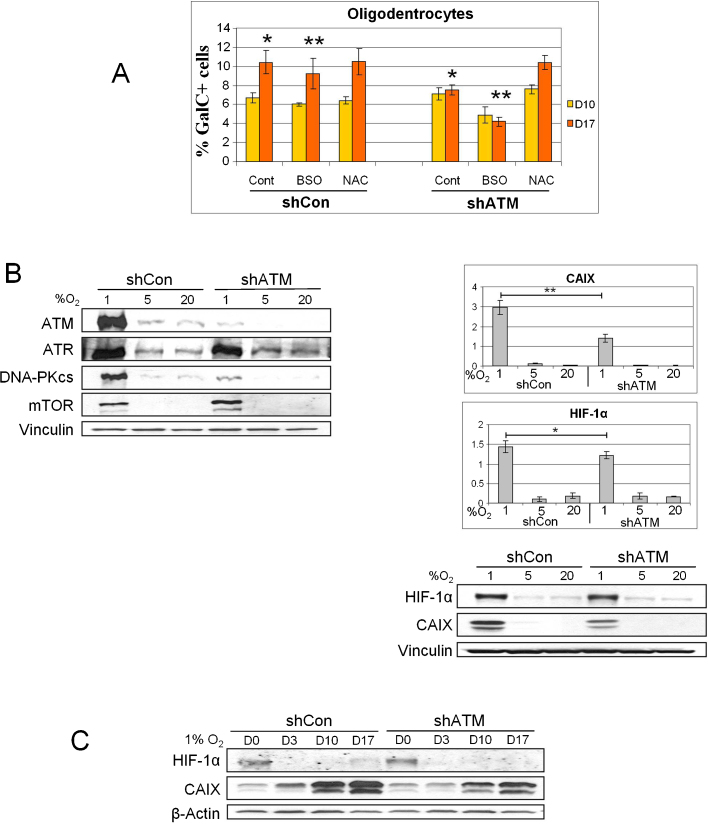
Oxygen regulates the homeostasis, apoptosis and differentiation of stem cells and committed progenitors in the central nervous system, where its levels range from about 0.55–8% [38], [39]. Low oxygen tension (hypoxia), a stress condition encountered in a variety of neuropathological states such as cerebral ischemia, is known to promote an undifferentiated state in several stem cells and progenitor cells [21] and to induce a paradoxical increase in the production of mitochondrial ROS, which further increase intracellular stress [40], [41]. In fact, hypoxia activates adaptive mechanisms involving the stabilization of the hypoxia-inducible factor 1α (HIF-1α) and the transcription of HIF-1α-regulated genes [3]. A link between hypoxia and ATM was recently established in studies demonstrating that ATM mediates the phosphorylation of HIF-1α on Ser696, and this modification causes the downregulation of the sensor of nutrient and energy sufficiency mTORC1, which acts as a restriction point under stress conditions [42]. Loss of hypoxia-dependent inhibition of mTORC1 sensitizes ATM-deficient cells to p53-dependent apoptosis [42]. Hypoxia-induced ROS are currently believed to be the key cytosolic signal that elicits the accumulation of HIF-1α by inhibiting prolyl-hydroxylase (PHD) activity through an unknown mechanism [43]. Deregulated ROS can be neuropathological and given that ROS can activate ATM [39] to downregulate mTORC1 [44], it is possible that ATM mediates certain cellular stress responses through the hypoxia-induced ROS. However, little is known about the role of hypoxia in neural cells in relation to ATM. We have recently investigated this aspect and one of the most striking findings in ATM-proficient ihNSCs was the dramatic upregulation by hypoxia (1% O2) of the levels of ATM and of its family members ATR, DNA-PKcs and mTOR (Fig. 3A, left), but not of the ATM substrates Smc1, NBS1, Mre11, Chk2 and Chk1 (not shown). This phenomenon appears to depend partly on enhanced transcription and partly on AMPK activity. We also found that ATM deficiency reduces the accumulation of HIF-1α and Carbonic Anhydrase IX (CAIX) in proliferating ihNSCs grown under hypoxia (Fig. 3A, right). This reduced accumulation of HIF-1α and especially CAIX is also observed in non-dividing terminally differentiated cells (Fig. 3B), indicating that the expression of these molecules is partially dependent on ATM.
Fig. 3.
ATM depletion and hypoxia markers accumulation. (A, left) Upregulation by hypoxia (1% O2) of ATM and other family members in proliferating ihNSCs as detected by western blot. (A, right) HIF-1α and CAIX protein levels in proliferating ihNSCs in relation to hypoxia and ATM deficiency. The graphs refers to the densitometry quantification of the western blot bands (**P < 0.01; *P < 0.05). (B) Hypoxic markers accumulation during differentiation. Cells grown in 1% O2 were collected at various days of differentiation and the expression of HIF-1α and CAIX was examined by western blot. Protein loading per lane was verified with anti-vinculin or anti-β-Actin antibodies.
5. Differentiation/maturation
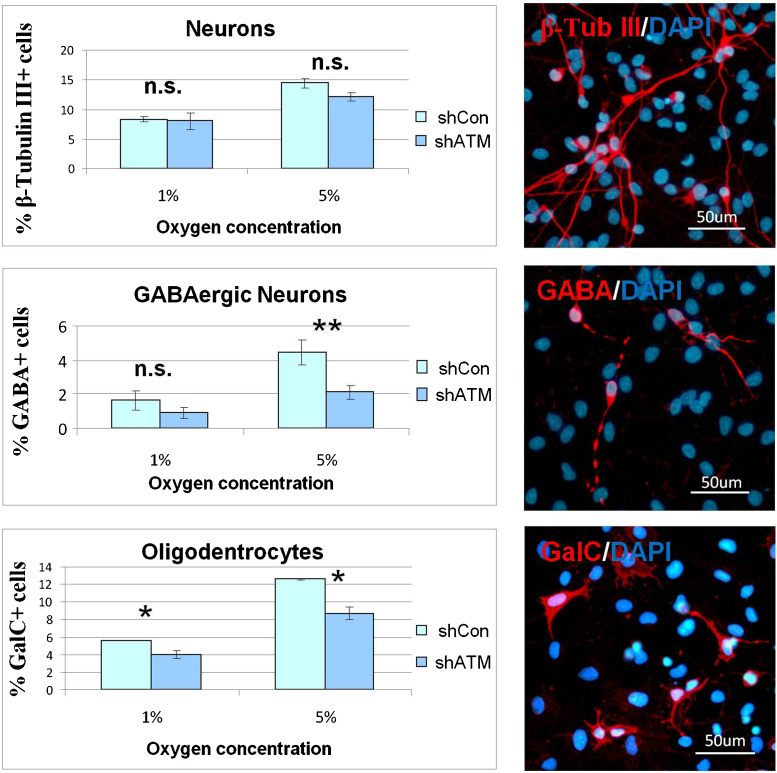
ATM deficiency appears to impact on hNSC differentiation. Indeed, while the yield of neurons expressing MAP2 and β-Tubulin III markers is unaffected by ATM deficiency, that of GABAergic neurons falls by 50% (Fig. 4A). Notably, GABA signaling plays an important role in adult neurogenesis, through a mechanism in which GABA induces the phosphorylation of H2AX by ATM/ATR, and this epigenetic change within the subventricular zone niche restricts the proliferation of neural stem cells and neuronal output [45], [46]. Whether the reduced yield of GABAergic neurons in ATM-deficient ihNSCs arises from a dysfunctional GABA signaling owing to ATM deficiency and somehow limiting a specific neuronal output, remains unknown. Nevertheless, this in vitro result agrees with pathological and clinical findings showing a GABA deficiency in the cerebellum of an A-T patient [47], and amelioration of the ataxia manifestation by treatment with a GABA-analog [48].
Fig. 4.
ATM depletion, hypoxia and effects on differentiation. ihNSCs at D17 of differentiation in the indicated O2 concentrations were IF-labelled with antibodies specific for neurons (β-Tubulin III), GABAergic neurons (GABA) and oligodendrocytes (GalC). Nuclei were counterstained with DAPI and cells scored by fluorescence microscopy. Results in each graph were obtained from the analysis of three independent experiments, and significant differences between shCon and shATM are indicated (**P < 0.01; *P < 0.05; n.s.: not significant). The representative IF photos refer to shCon at D17 of differentiation in 5% O2. (B) ihNSCs were induced to differentiate on glass coverslips in medium containing 1 mM NAC or 1 mM BSO, a glutathione-depleting and oxidative stress-inducing agent, and then IF-labeled with antibodies specific for oligodendrocytes (GalC) (**P < 0.01; *P < 0.05).
Interestingly, oxygen levels also affect the differentiation of neural progenitor cells [49]. Concordant with this, we have observed that the amount of neurons expressing β-Tubulin III is markedly reduced in ihNSCs differentiating under hypoxia, although to the same extent in ATM-deficient and–proficient cells. Hypoxia also affects the yield of GABAergic neurons, but to a greater extent in ATM-deficient than in control ihNSCs, and the potassium channel-interacting protein-1 (KCHIP-1), predominantly expressed by GABAergic neurons [43], also appears to be markedly dependent on oxygen tension, since the fraction of positive cells falls substantially under hypoxia, and to a higher degree in ATM-deficient ihNSCs (Fig. 4A).
ATM-deficiency also attenuates the yield of GalC- and CNPase-expressing oligodendrocytes [26] and furthermore, when ihNSCs are induced to differentiate under oxidative stress conditions achieved by the glutathione-depleting agent BSO (Buthionine Sulfoximine) or by hypoxia, a remarkable vulnerability of oligodendrocytes to ATM deficiency is observed (Fig. 4A and B).
Quite significantly, these results indicate that ATM deficiency does not appear to impair overall neurogenesis, though it partially affects oligodendrogenesis.
6. Concluding remarks
As animal models of A-T do not faithfully reproduce the human syndrome, attempts have been made to generate and use human neural stem cell lines derived from embryos, fetal brain or adult brain tissues, to study the mechanisms that underlie the neurodegenerative disease. hNSCs are a renewable source of undifferentiated cells, can be induced to differentiate into functional neurons and glia, and are amenable to experimental genetic manipulation. Studies with such cells are certainly providing important clues on the role of ATM in DDR and neuronal differentiation progression and maturation, as well as on the vulnerability to genotoxic and oxidative agents. However, since the characteristics of hNSCs either derived from ES cells or isolated from different regions of the brain can differ, possibly reflecting normal developmental patterns, it must be recognized that despite many advantages, the use of these stem cells for disease modelling has limitations [50]. Human iPS cells generated from reprogrammed A-T fibroblasts and capable of generating a variety of functional neurons offer an additional tool to study neurodegeneration also as function of the disease-associated genetic mutation. A current challenge is to direct the neural differentiation of iPS cells towards specific neuronal subtypes, e.g. Purkinje cerebellar neurons, which are specifically sensitive to ATM deficiency. This would allow the investigation of the effects of A-T-causing mutations using the cell population which is directly involved in the neurodegenerative phenotype of A-T.
Acknowledgments
This work was financially supported by the Italian Telethon Foundation grant GGP10066 (to D.D.) and by the Italian Association for Cancer Research (AIRC) grant IG10248.
References
- 1.Chun H.H., Gatti R.A. Ataxia-telangiectasia, an evolving phenotype. DNA Repair. 2004;3:1187–1196. doi: 10.1016/j.dnarep.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 2.McKinnon. P.J. ATM and the molecular pathogenesis of ataxia telangiectasia. Annu. Rev. Pathol. 2012;7:303–321. doi: 10.1146/annurev-pathol-011811-132509. [DOI] [PubMed] [Google Scholar]
- 3.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 4.Lavin M.F., Kozlov S. ATM activation and DNA damage response. Cell Cycle. 2007;6:931–942. doi: 10.4161/cc.6.8.4180. [DOI] [PubMed] [Google Scholar]
- 5.Lavin M.F. Ataxia-telangiectasia. From a rare disorder to a paradigm for cell signalling and cancer. Nat. Rev. Mol. Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y., McKinnon. P.J. Responding to DNA double strand breaks in the nervous system. Neuroscience. 2007;145:1365–1374. doi: 10.1016/j.neuroscience.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Barzilai A., Biton S., Shiloh Y. The role of the DNA damage response in neuronal development, organization and maintenance. DNA Repair. 2008;7:1010–1027. doi: 10.1016/j.dnarep.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Barzilai A., Rotman G., Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair. 2002;1:3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 9.Liu N., Stoica G., Yan M., Scofield V.L., Qiang W., Lynn W.S., Wong P.K. ATM deficiency induces oxidative stress and endoplasmic reticulum stress in astrocytes. Lab. Invest. 2005;85:1471–1480. doi: 10.1038/labinvest.3700354. [DOI] [PubMed] [Google Scholar]
- 10.Reliene R., Fischer E., Schiestl R.H. Effect of N-acetyl cysteine on oxidative DNA damage and the frequency of DNA deletions in ATM-deficient mice. Cancer Res. 2004;64:5148–5153. doi: 10.1158/0008-5472.CAN-04-0442. [DOI] [PubMed] [Google Scholar]
- 11.Arakawa M., Ushimaru N., Osada N., Oda T., Ishige K., Ito Y. N-acetylcysteine selectively protects cerebellar granule cells from 4-hydroxynonenal-induced cell death. Neurosci. Res. 2006;55:255–263. doi: 10.1016/j.neures.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Guo Z., Kozlov S., Lavin M.F., Person M.D., Paull T.T. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 13.Kruman I.I., Wersto R.P., Cardozo-Pelaez F., Smilenov L., Chan S.L., Chrest F.J., Emokpae R., Jr., Gorospe M., Mattson M.P. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004;41:549–561. doi: 10.1016/s0896-6273(04)00017-0. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y., Herrup K. Loss of neuronal cell cycle control in ataxia-telangiectasia: a unified disease mechanism. J. Neurosci. 2005;25:2522–2529. doi: 10.1523/JNEUROSCI.4946-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boehrs J.K., He J., Halaby M., Yang D. Constitutive expression and cytoplasmic compartmentalization of ATM protein in differentiated human neuron-like SH-SY5Y cells. J. Neurochem. 2007;100:337–345. doi: 10.1111/j.1471-4159.2006.04254.x. [DOI] [PubMed] [Google Scholar]
- 16.Gorodetsky E., Calkins S., Ahn J., Brooks P.J. ATM, the Mre11/Rad50/Nbs1 complex, and topoisomerase I are concentrated in the nucleus of purkinje neurons in the juvenile human brain. DNA Repair. 2007;6:1698–1707. doi: 10.1016/j.dnarep.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J., Han Y.R., Plummer M.R., Herrup K. Cytoplasmic ATM in neurons modulates synaptic function. Curr. Biol. 2009;19:2091–2096. doi: 10.1016/j.cub.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J., Chen J., Ricupero C.L., Hart R.P., Schwartz M.S., Kusnecov A., Herrup K. Nuclear accumulation of HDAC4 in ATM deficiency promotes neurodegeneration in ataxia telangiectasia. Nat. Med. 2012;18:783–790. doi: 10.1038/nm.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barzilai A. The neuro-glial-vascular interrelations in genomic instability symptoms. Mech. Ageing Dev. 2011;132:395–404. doi: 10.1016/j.mad.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Villa A., Navarro-Galve B., Bueno C., Franco S., Blasco M.A., Martinez-Serrano. A. Long-term molecular and cellular stability of human neural stem cell lines. Exp. Cell Res. 2004;294:559–570. doi: 10.1016/j.yexcr.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Pollock K., Stroemer P., Patel S., Stevanato L., Hope A., Miljan E., Dong Z., Hodges H., Price J., Sinden J.D. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp. Neurol. 2006;199:143–155. doi: 10.1016/j.expneurol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Donato R., Miljan E.A., Hines S.J., Aouabdi S., Pollock K., Patel S., Edwards F.A., Sinden J.D. Differential development of neuronal physiological responsiveness in two human neural stem cell lines. BMC Neurosci. 2007;8:36. doi: 10.1186/1471-2202-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Filippis L., Lamorte G., Snyder E.Y., Malgaroli A., Vescovi A.L. A novel, immortal, and multipotent human neural stem cell line generating functional neurons and oligodendrocytes. Stem Cells. 2007;25:2312–2321. doi: 10.1634/stemcells.2007-0040. [DOI] [PubMed] [Google Scholar]
- 24.Rota Nodari L., Ferrari D., Giani F., Bossi M., Rodriguez-Menendez V., Tredici G., Delia D., Vescovi A.L., De Filippis L. Long-term survival of human neural stem cells in the ischemic rat brain upon transient immunosuppression. PLoS ONE. 2010;5:e14035. doi: 10.1371/journal.pone.0014035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari D., Zalfa C., Rota Nodari L., Gelati M., Carlessi L., Delia D., Vescovi A., De Filippis L. Differential pathotropism of non-immortalized and immortalized human neural stem cell lines in a focal demyelination model. Cell. Mol. Life Sci. 2012;68:1193–1210. doi: 10.1007/s00018-011-0873-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlessi L., De Filippis L., Lecis D., Vescovi A., Delia D. DNA-damage response, survival and differentiation in vitro of a human neural stem cell line in relation to ATM expression. Cell Death Differ. 2009;16:795–806. doi: 10.1038/cdd.2009.10. [DOI] [PubMed] [Google Scholar]
- 27.Biton S., Gropp M., Itsykson P., Pereg Y., Mittelman L., Johe K., Reubinoff B., Shiloh Y. ATM-mediated response to DNA double strand breaks in human neurons derived from stem cells. DNA Repair. 2007;6:128–134. doi: 10.1016/j.dnarep.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Adams B.R., Golding S.E., Rao R.R., Valerie K. Dynamic dependence on ATR and ATM for double-strand break repair in human embryonic stem cells and neural descendants. PLoS ONE. 2010;5:e10001. doi: 10.1371/journal.pone.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delia D., Carlessi L., Fusar Poli E. Establishment of induced pluripotent stem (IPS) cells from A-T patient and characterization of the neural-specified progeny. ATW 2012, 14th International Workshop on Ataxia-Telangiectasia; New Delhi, India; 2012. [Google Scholar]
- 30.Nayler S., Gatei M., Kozlov S., Gatti R., Mar J.C., Wells C.A., Lavin M., Wolvetang E. Induced pluripotent stem cells from ataxia-telangiectasia recapitulate the cellular phenotype. Stem Cells Trans. Med. 2012;1:523–535. doi: 10.5966/sctm.2012-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S.U., Nagai A., Nakagawa E., Choi H.B., Bang J.H., Lee H.J., Lee M.A., Lee Y.B., Park I.H. Production and characterization of immortal human neural stem cell line with multipotent differentiation property. Methods Mol. Biol. 2008;428:103–121. doi: 10.1007/978-1-59745-133-8_10. [DOI] [PubMed] [Google Scholar]
- 32.Hitomi M., Stacey D.W. The checkpoint kinase ATM protects against stress-induced elevation of cyclin D1 and potential cell death in neurons. Cytometry Part A. 2010;77A:524–533. doi: 10.1002/cyto.a.20885. [DOI] [PubMed] [Google Scholar]
- 33.Gilad S., Chessa L., Khosravi R., Russell P., Galanty Y., Piane M., Gatti R.A., Jorgensen T.J., Shiloh Y., Bar-Shira A. Genotype-phenotype relationships in ataxia-telangiectasia and variants. Am. J. Hum. Genet. 1998;62:551–561. doi: 10.1086/301755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniel J.A., Pellegrini M., Lee B.S., Guo Z., Filsuf D., Belkina N.V., You Z., Paull T.T., Sleckman B.P., Feigenbaum L., Nussenzweig A. Loss of ATM kinase activity leads to embryonic lethality in mice. J. Cell Biol. 2012;198:295–304. doi: 10.1083/jcb.201204035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coufal N.G., Garcia-Perez J., Peng G.E., Marchetto M.C.N., Muotri A.R., Mu Y., Carson C.T., Macia A., Moran J.V., Gage F.H. Ataxia telangiectasia mutated (ATM) modulates long interspersed element-1 (L1) retrotransposition in human neural stem cells. Proc. Natl. Acad. Sci. U.S.A. 2011;108:20382–20387. doi: 10.1073/pnas.1100273108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKinnon. P.J. DNA repair deficiency and neurological disease. Nat. Rev. Neurosci. 2009;10:100–112. doi: 10.1038/nrn2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biton S., Dar I., Mittelman L., Pereg Y., Barzilai A., Shiloh Y. Nuclear ataxia-telangiectasia mutated (ATM) mediates the cellular response to DNA double strand breaks in human neuron-like cells. J. Biol. Chem. 2006;281:17482–17491. doi: 10.1074/jbc.M601895200. [DOI] [PubMed] [Google Scholar]
- 38.Panchision D.M. The role of oxygen in regulating neural stem cells in development and disease. J. Cell. Physiol. 2009;220:562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 39.De Filippis L., Delia D. Hypoxia in the regulation of neural stem cells. Cell. Mol. Life Sci. 2011;68:2831–2844. doi: 10.1007/s00018-011-0723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamanaka R.B., Chandel N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamanaka R.B., Chandel N.S. Mitochondrial reactive oxygen species regulate hypoxic signaling. Curr. Opin. Cell Biol. 2009;21:894–899. doi: 10.1016/j.ceb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takai H., Wang R.C., Takai K.K., Yang H., de Lange T. Tel2 regulates the stability of PI3K-related protein kinases. Cell. 2007;131:1248–1259. doi: 10.1016/j.cell.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 43.Xiong H., Xia K., Li B., Zhao G., Zhang K.ChI.P1. Z. A potential modulator to GABAergic system. Acta Biochim. Biophys. Sin. (Shanghai) 2009;41:295–300. doi: 10.1093/abbs/gmp013. [DOI] [PubMed] [Google Scholar]
- 44.Zhang C.P., Zhu L.L., Zhao T., Zhao H., Huang X., Ma X., Wang H., Fan M. Characteristics of neural stem cells expanded in lowered oxygen and the potential role of hypoxia-inducible factor-1Alpha. Neurosignals. 2006;15:259–265. doi: 10.1159/000103385. [DOI] [PubMed] [Google Scholar]
- 45.Fernando R.N., Eleuteri B., Abdelhady S., Nussenzweig A., Andang M., Ernfors P. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc. Natl. Acad. Sci. U.S.A. 2011;108:5837–5842. doi: 10.1073/pnas.1014993108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andang M., Hjerling-Leffler J., Moliner A., Lundgren T.K., Castelo-Branco G., Nanou E., Pozas E., Bryja V., Halliez S., Nishimaru H., Wilbertz J., Arenas E., Koltzenburg M., Charnay P., El Manira A., Ibanez C.F., Ernfors P. Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature. 2008;451:460–464. doi: 10.1038/nature06488. [DOI] [PubMed] [Google Scholar]
- 47.Perry T.L., Kish S.J., Hinton D., Hansen S., Becker L.E., Gelfand E.W. Neurochemical abnormalities in a patient with ataxia-telangiectasia. Neurology. 1984;34:187–191. doi: 10.1212/wnl.34.2.187. [DOI] [PubMed] [Google Scholar]
- 48.Gazulla J., Benavente I., Sarasa Barrio M. Adult-onset ataxia-telangiectasia. A clinical and therapeutic observation. Neurologia. 2006;21:447–451. [PubMed] [Google Scholar]
- 49.Santilli G., Lamorte G., Carlessi L., Ferrari D., Nodari L.R., Binda E., Delia D., Vescovi A.L., De Filippis L. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS ONE. 2010;5:e8575. doi: 10.1371/journal.pone.0008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jakel R.J., Schneider B.L., Svendsen C.N. Using human neural stem cells to model neurological disease. Nat. Rev. Genet. 2004;5:136–144. doi: 10.1038/nrg1268. [DOI] [PubMed] [Google Scholar]