Abstract
Purpose
Polysialic acid (polySA) is a polymer side chain bound to the neural cell adhesion molecule that is extensively expressed on the surface of small cell lung cancer (SCLC) cells. In our previous study, a robust antibody response was noted in patients with SCLC after vaccination with 30 μg of keyhole limpet hemocyanin (KLH)-conjugated N-propionylated (NP-) polySA, but peripheral neuropathy and ataxia were detected in several vaccinated patients. The objectives of the current trial were to establish the lowest optimal dose and to confirm the safety of the induction of antibodies against polySA with the NP-polySA vaccine.
Experimental design
Patients with SCLC who completed initial treatment and had no evidence of disease progression were injected with either 10 or 3 μg of NP-polySA conjugated to KLH and mixed with 100 μg of immunologic adjuvant (QS-21) at weeks 1, 2, 3, 4, 8, and 16.
Results
Nine patients were enrolled at each of the two dose levels. Prior to vaccination, one patient in each group had low-titer antibodies against polysialic acid. All patients at the 10 μg vaccine dose level responded to vaccination with IgM antibody titers against polysialic acid (median titer 1/1,280 by ELISA), and all but one patient made IgM and IgG antibodies against the artificial vaccine immunogen, NP-polysialic acid (median titer 1/10,240). The antibody responses at the 3 μg vaccine dose level were lower; six of nine patients developed antibodies against polysialic acid (median titer 1/160). Post-vaccination sera from 6/9 and 3/9 patients in the 10 and 3 μg groups reacted strongly with human SCLC cells by fluorescent-activated cell sorting (FACS). Sera from all patients in the 10 μg dose group also had bactericidal activity against group B meningococci with rabbit complement. Self-limited grade 3 ataxia of unclear etiology was seen in 1 of 18 patients.
Conclusions
Vaccination with NP-polySA–KLH resulted in consistent high-titer antibody responses, with the 10 μg dose significantly more immunogenic than the 3 μg dose. This study establishes the lowest optimally immunogenic dose of NP-polysialic acid in this NP-polysialic acid–KLH conjugate vaccine to be at least 10 μg, and it establishes the vaccine’s safety. We plan to incorporate NP-polySA into a polyvalent vaccine against SCLC with four glycolipid antigens also widely expressed in SCLC–GD2, GD3, fucosylated GM1, and globo H.
Keywords: Small cell lung cancer, Minimal residual disease, Polysialic acid
Introduction
Polysialic acid (PolySA) is a polymer of negatively charged sialic acid residues, which, in vertebrate embryonic neural tissue, is linked to the 5th immunoglobulin domain of the neural cell adhesion molecule (NCAM) [1, 2]. The large size and negative charge of this carbohydrate side-chain modulates cell–cell interactions at least in part by impeding NCAM binding [3]. As such, the varying levels of polySA expression play a role in controlling neural cell migration and differentiation in developing embryonal neural tissues and in adult brains in regions of neural plasticity [1, 4]. PolySA is also a component of the cell wall of group B meningococci [1], and several groups have identified high rates of polySA expression on nearly all human small cell lung cancers (SCLC) [5–7]. In these studies, monoclonal antibodies to the extended embryonic form of polySA were used for immunohistochemical staining. SCLC more frequently expresses polySA than other low-grade neuroendocrine tumors such as carcinoids, leading to the hypothesis that the inhibitory effect of polySA on cell adhesion contributes to the propensity for early metastases and the clinically aggressive nature of SCLC [5, 6]. Data from various animal tumor models support this idea. In a rat transplantable pituitary model, polySA-NCAM expression correlated with tumor invasiveness, metastases, and growth rate [8]. In nude mice injected intraperitoneally with human rhabdomyosarcoma TE671 cells, cleavage of polySA by endoneuraminidase delayed the formation of ascites and decreased the number of lung and liver metastases [9].
The restricted expression of polySA in normal human tissues, in contrast to the abundant expression in SCLC, suggests that polySA may be a rational, highly specific target for immunologically directed therapy [7]. The challenge with this approach is to overcome immunologic tolerance which is likely due to the transient expression of polySA in embryonal neural tissue and the limited expression in adult brain tissues. Chemical modification of polySA by N-propionylation augments the antibody response to meningococcal group B polysaccharide in mice [10]. In a prior clinical trial, we showed that this modification also boosts immunologic responses in humans [11]. Patients with SCLC who had completed first-line chemotherapy were vaccinated with either polySA or NP-polySA. Congruent with several other vaccine studies we have conducted [12, 13], both sialic acid preparations were conjugated to keyhole limpet hemocyanin (KLH) and administered with a saponin adjuvant, QS-21. All patients were treated at a dose of 30 μg. Native polySA conjugated to KLH did not induce any significant immune responses, but all six patients treated with 30 μg of NP-polySA–KLH developed high-titer antibodies. While complement-dependent cytotoxicity of polySA-positive tumor cells could not be demonstrated, the sera induced bactericidal activity against group B meningococcus in all but one case.
We now seek to expand our experience with the NP-polySA vaccine. Previously, toxicity was primarily limited to injection site reactions, though grade 2 or greater peripheral neuropathy was observed in 4 of 13 patients. We could not determine definitively whether these neurologic complaints were due to the vaccine-induced antibody responses against polysialic acid or to other treatment or disease-related causes. This current study was launched to better assess the immunogenicity of lower doses and to carefully monitor potential neurologic effects of the induced antibodies.
Patients and methods
Patient selection
We enrolled adults with SCLC, limited or extensive stage, who had completed their initial therapy with chemotherapy (and radiation if needed) at least four, but not more than 12 weeks previously. Patients needed to have a Karnofsky performance status of 70% or greater. Patients must not have had peripheral sensory neuropathy greater than grade 1. Required hematologic and biochemical parameters included a total white blood count ≥ 3,000/μl, a total lymphocyte count ≥ 500/μl, SGOT level ≤ 1.5 × upper limit of normal, serum bilirubin ≤ 1.5 mg/dl, and serum alkaline phosphatase level ≤ 1.5 × upper limit of normal. Patients with known immune deficiency or autoimmune disease, prior splenectomy or splenic radiation, or patients on oral corticosteroids were excluded. Pregnant or lactating women, patients with active infections, or patients with unstable medical illnesses were also excluded. This protocol was reviewed by the Memorial Sloan-Kettering Institutional Review Board. Written informed consent was obtained.
Within 2 weeks of starting treatment, all patients underwent a history and physical examination, complete blood count, biochemical profile, and amylase. A CT scan of the chest within 4 weeks of starting treatment was required to document ongoing partial or complete response to initial therapy. In order to more carefully monitor for potential neurologic toxicities, a baseline neurologic examination including assessment of sensation and coordination was performed by a neurologist (LA), and a contrast-enhanced CT or MRI of the brain was obtained before vaccination.
Vaccine preparation
Propionylation of polysialic acid
Polysialic acid (polySA) of Escherichia coli origin (colominic acid), keyhole limpet hemocyanin (KLH), and sodium cyanoborohydride were obtained from Sigma Chemical Co., (St. Louis, MO). PolySA was further purified over a size exclusion column to yield high molecular weight polySA of approximately 10,000 before propionylation and coupling with KLH. For the preparation of propionylated polySA, polySA was first deacetylated by treatment with 2 M NaOH at 107°C for 6 h. It was then mixed with sodium bicarbonate solution and propionic anhydride and incubated at room temperature for approximately 12 h, dialyzed, and lyophilized as previously described [10]. Replacement of CH3-CO-HN- acetyl groups with CH3-CH2-CO-NH- propionyl groups was confirmed by NMR (data not shown).
NP-polySA–KLH conjugation
NP-polySA–KLH conjugates were prepared as described by Jennings et al. [10]. The conjugation procedure cleaved the vicinyl hydroxyl group of the terminal sialic acid of the polySA by periodate. The periodate method involved reaction with meta-periodate of the vicinyl hydroxyl group in polySA resulting in the formation of an aldehyde at the end of each polySA chain. The aldehyde was then made to react with the free N-terminal amino group and to ε-amino groups on lysine of KLH by reductive amination in the presence of sodium cyanoborohydride. The linkage was stabilized by treatment with sodium cyanoborohydride, and the conjugate was washed extensively with normal saline and an Amicon Centriprep 30 unit concentration. Protein and sialic acid content were determined. The ratio of NP-polySA molecules conjugated to each KLH molecule was 80/1.
Immunization
Patients were vaccinated with either 10 or 3 μg of NP-polySA–KLH conjugate plus 100 μg of immunologic adjuvant QS-21 (Antigenics, New York, NY) [14] at weeks 1, 2, 3, 4, 8, and 16. Serum samples for immunologic studies were obtained at weeks 1, 2, 3, 4, 6, 8, 10, 16, and 18 and stored at −70°C until analysis.
Serologic assays
ELISA: IgM and IgG antibody titers were measured by ELISA as described previously [15]. Briefly, 96-well flat-bottomed plates (Nunc, Rochester, New York) were precoated with polySA-HSA or NP-polySA-HSA at 0.1 μg per well in carbonate buffer, or carbonate buffer alone. Serially diluted sera in 1% human serum albumin (HSA) were added along with sera from patients with known specific high-titer antibodies or no antibodies, which served as positive and negative controls, respectively. Goat anti-human IgG and goat anti-human IgM conjugated with alkaline phosphatase were purchased from KPL (Gaithersburg, MD) and were used to complete the assay. Plates were read at 10–15 min for IgG and IgM on an ELISA plate reader (Bio-Rad model 550 Microplate Reader) at 405 nm. Optical density (OD) for the carbonate buffer alone wells was subtracted at each serum dilution from the corresponding antigen wells to obtain the corrected OD. The titer was defined as the highest dilution yielding a corrected OD ≥0.1. For inhibition experiments, 0.1 ml of sera at the indicated dilutions (see Table 3) was preincubated for 1 h at 37°C with the indicated quantities of Sigma polySA, and the inhibited sera (plus inhibiting antigen) were then added to ELISA plates as described above. Percent inhibition was calculated as uninhibited OD minus inhibited OD divided by uninhibited OD X 100.
Table 3.
Inhibition of ELISA and FACS reactivity by preincubation of sera with purified E. coli polysialic acid
| Pt. no. (10 μg dose) | Serum dilution | ELISA | FACS % inhibition | ||||
|---|---|---|---|---|---|---|---|
| % inhibition | Target cells | ||||||
| 0.08 mcg (%) | 5 mcg (%) | 25 mcg (%) | Sera dilution | N417 (%) | DMS79 (%) | ||
| 2 | 1/640 | 10 | 70 | 100 | 1/20 | 97 | 58 |
| 5 | 1/160 | 30 | 80 | 90 | 1/20 | 100 | 86 |
| 6 | 1/320 | 5 | 25 | 92 | 1/20 | 91 | 94 |
| 7 | 1/2,560 | 40 | 80 | 88 | 1/20 | 33 | 79 |
| 8 | 1/160 | 50 | 100 | 95 | 1/20 | 13 | 25 |
| 9 | 1/40 | 17 | 46 | 82 | 1/20 | –* | 73 |
* Post-immunization FACS reactivity unchanged from preimmunization reactivity in this experiment
Flow cytometric analysis: Human small cell lung cancer cell lines N417 and DMS-79 were purchased from ATCC. Goat anti-human IgM-fluorescein-isothiocyanate (FITC) and goat anti-human IgM-FITC were obtained from Southern Biotechnology Associates Inc., (Birmingham, AL). Fluorescent-activated cell sorting was performed as previously described [16] to demonstrate antibody binding to the cell surface of the cell lines. The polySA-positive SCLC cell line H345 served as the target. The cells were incubated with 20 μl of 1:20 diluted sera or anti-polySA mAb 5A5 for 30 min on ice. After washing, 20 μl of 1:25 goat anti-human IgM and IgG was added, mixed, and incubated for 30 min. After washing, the positive population and mean fluorescence intensity of the stained cells were analyzed by flow cytometry. (FACScan, Becton & Dickinson, San Jose, CA). Pre- and peak titer post-immunization sera were run together with the pretreatment percent positive cells set at about 10%. A positive response is defined as a tripling of the percent positive cells with at least a 50% increase in mean fluorescent intensity (MFI). For inhibition experiments, 0.1 ml of sera at a serum dilution of 1/20 (see Table 3) was preincubated for 1 h at 37°C with 50 mcg of Sigma polySA, and the inhibited sera were then added to cells as described above. Percent inhibition was calculated as 100 minus inhibited post-treatment MFI minus pretreatment MFI divided by uninhibited post-treatment MFI minus pretreatment MFI multiplied by 100.
Bactericidal assays
The determination of the bactericidal activity of serum samples against group B meningococcus using rabbit and human complement was performed as previously described [17]. Bacteria were incubated with diluted sera and complement in a 1-h assay and the number of colony-forming units was counted after overnight incubation. Percent killing was calculated relative to medium alone wells.
Statistical considerations
The primary goal of the study was to establish the lowest effective dose of N-propionylated polysialic acid capable of safely inducing maximal antibody production. Three dose levels were planned: 10, 3, and 1 μg. Using a dose de-escalation scheme, we attempted to maintain acceptable immunologic response, while reducing the risk of toxicity and expanding the ability to manufacture larger amounts of vaccine. The 1 μg dose was not pursued after the antibody titers in the 3 μg cohort were assessed and found to be less than at the other two doses.
A significant immune response was defined as (1) an antibody titer of ≥1:80 by ELISA against polysialic acid for patients with no detectable baseline titer; (2) an ELISA titer ≥eightfold increase over baseline for patients with a detectable baseline titer, or (3) by FACS a tripling of the percent positive cells with at least a 50% increase in mean fluorescent intensity (MFI). The frequency of positive responses was compared using the chi-square test [18].
Nine patients were planned for enrollment at each dose level [19]. Overall survival was estimated using Kaplan–Meier analysis.
Results
Twenty-two patients were enrolled. Three patients were ineligible due to disease progression, and one patient was ineligible due to grade 2–3 sensory neuropathy identified on the baseline examination by the neurologist. Eighteen patients were therefore treated with the vaccine, nine patients at each of the two doses. Their clinical characteristics are specified in Table 1.
Table 1.
Patient characteristics (n = 18)
| Men | 7 (39%) |
| Women | 11 (61%) |
| Median age (range) | 62 (48–72) |
| Karnofsky performance status | |
| 100% | 2 (11%) |
| 90% | 6 (33%) |
| 80% | 10 (56%) |
| Stage | |
| Limited | 11 (61%) |
| Extensive | 7 (39%) |
| Chemotherapy | |
| Etoposide, cisplatin | 11 (61%) |
| Etoposide, carboplatin | 4 (22%) |
| Irinotecan, carboplatin | 2 (11%) |
| Irinotecan, etoposide, carboplatin | 1 (6%) |
| Radiation | |
| Thoracic | 12 (67%) |
| Brain* | 11 (61%) |
| Bone metastasis (Shoulder) | 1 (6%) |
| None | 4 (22%) |
* Brain radiation was administered to treat brain metastases in one patient and for prophylaxis in 10 patients
Immunologic response
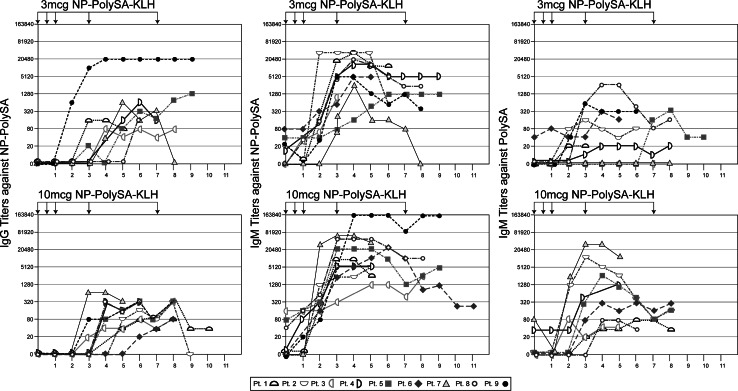
Table 2 and Fig. 1 display the antibody titers against the artificial immunogen NP-polySA and the natural SCLC antigen polySA observed for each patient. All patients at both dose levels rapidly mounted IgM antibodies to NP-polySA. High IgM antibody titers were observed by the third week and were then sustained at a relatively constant level for the duration of the study. IgG antibodies to NP-polySA at both dose levels rose more slowly and were of lower titer. IgM antibodies that cross-reacted with native polySA were observed in 5/9 and 9/9 patients at the 3 and 10 μg doses, respectively, and the median titers were lower at the 3 μg dose as well (1/160 vs. 1/1,280). As expected, these were at lower titers than those seen against the NP-polySA (median titers 1/10,240). Some decline in IgM antibody titers to native polySA was generally detected by week 8. No patients developed detectable IgG antibodies that cross-reacted with native polySA. The responses against polySA in patients vaccinated at the 10 μg dose were comparable to the responses demonstrated in our previous study with patients vaccinated at the 30 μg dose level (Table 2).
Table 2.
Immune response: peak reciprocal IgM and IgG ELISA titers for antibodies binding to polySA according to dose
| NP-polySA–KLH + QS-21 Vaccine | Patient | Peak IgM ELISA | Peak IgG ELISA | FACs reactivity against SCLC cell lines DMS 79 | |
|---|---|---|---|---|---|
| % Positive cells (MFI) | |||||
| Pre | Post | ||||
| 30 μg* | 1 | 1,280 | 10 | 11% (63) | 85% (263)** |
| 2 | 160 | 0 | 10% (95) | 57% (201) | |
| 3 | 1,280 | 0 | 12% (77) | 41% (125) | |
| 4 | 40 | 0 | 11% (78) | 62% (168) | |
| 5 | 640 | 20 | 10% (165) | 27% (228) | |
| 6 | 1,280 | 0 | 10% (190) | 45% (352) | |
| 10 μg | 1 | 1,280 | 0 | 10% (72) | 21% (88) |
| 2 | 5,120 | 0 | 10% (58) | 37% (113) | |
| 3 | 80 | 0 | 10% (170) | 21% (225) | |
| 4 | 1,280 | 0 | 10% (93) | 9% (70) | |
| 5 | 2,560 | 20 | 10% (95) | 55% (179) | |
| 6 | 320 | 0 | 10% (70) | 36% (106) | |
| 7 | 2,560 | 0 | 10% (42) | 63% (148) | |
| 8 | 80 | 0 | 10% (47) | 44% (85) | |
| 9 | 80 | 0 | 10% (50) | 82% (164) | |
| 3 μg | 1 | 0 | 0 | 10% (197) | 17% (205) |
| 2 | 160 | 0 | 10% (6) | 89% (17) | |
| 3 | 0 | 0 | 10% (27) | 30% (38) | |
| 4 | 40 | 0 | 10% (31) | 29% (47) | |
| 5 | 320 | 0 | 10% (52) | 24% (81) | |
| 6 | 320 | 0 | 10% (101) | 19% (132) | |
| 7 | 0 | 0 | 10% (61) | 10% (56) | |
| 8 | 2,560 | 0 | 10% (41) | 72% (173) | |
| 9 | 2,560 | 0 | 10% (38) | 60% (101) | |
Percentage positive cells and mean fluorescent intensity are indicated for pre- and post-immunization peak ELISA titer sera
* Patients from prior study [11]
** Bold values are significant immune responses
Fig. 1.
IgM and IgG antibody responses against the synthetic vaccine component NP-polySA and IgM antibody responses in against the natural tumor antigen polySA (nine patients) in ELISAs after vaccination with NP-polySA–KLH at the 3 or 10 μg dose levels. Arrows indicate the time of vaccinations. The 10 μg dose is significantly more immunogenic than the 3 μg dose
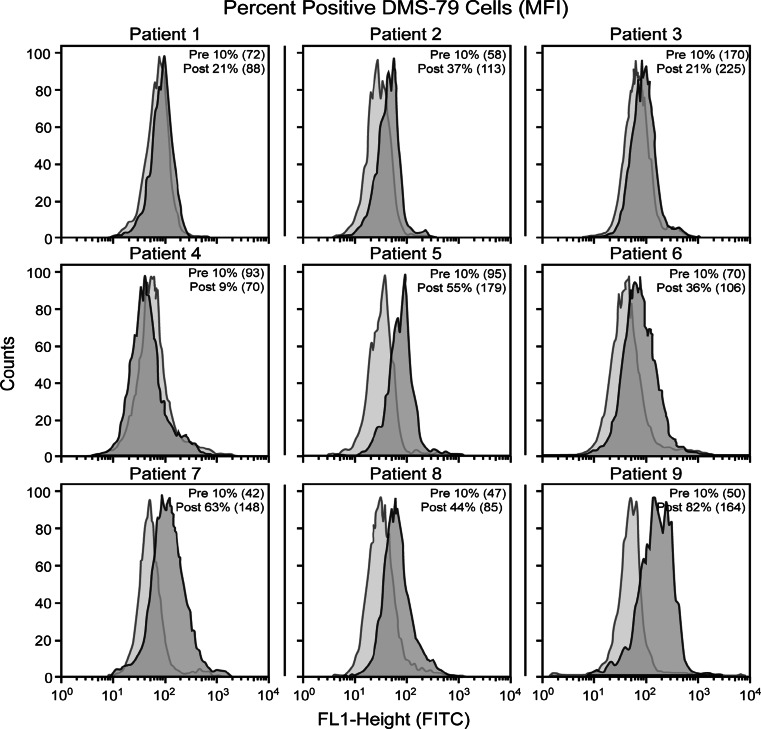
Using FACS to measure binding to DMS-79 cells, immune responses capable of reacting with polySA expressed on these cell lines were confirmed in 6/9 patients vaccinated at the 10 mcg dose level (see Fig. 2) and in 3/9 patients at the 3 μg dose level. Culture of these target cells in medium containing human serum instead of FCS had no significant impact on the cell surface reactivity (data not shown). Results for the patients vaccinated here with the 3 and 10 mcg doses were compared to the results in our previous trial with the 30 mcg dose where sera from 5 of 6 patients reacted with SCLC cells (see Table 2). Cell surface reactivity was demonstrated in 3 of 9, 6 of 9, and 5 of 6 patients at the 3, 10, and 30 μg doses, respectively. Again, responses in patients vaccinated at the 10 μg dose were comparable to the responses demonstrated in our previous study with patients vaccinated at the 30 μg dose level, and sera from the 30 μg dose patients demonstrating significantly more cell surface reactivity than sera from the 3 μg dose patients (P = 0.02).
Fig. 2.
Fluorescent cell-sorting results for the nine patients immunized with the NP-polySA–KLH at the 10 μg dose plus 100 μg QS-21. Percentage positive cells and mean fluorescent intensity (MFI) are indicated for pre- and post-immunization peak ELISA titer sera. Significant reactivity was detected with sera from 6 to 9 patients (all but patients 1, 3, and 4)
The specificity for polysialic acid of sera from the six patients vaccinated at the 10 μg dose level demonstrating strong cell surface reactivity was confirmed by inhibition studies. As summarized in Table 3, ELISA reactivity against polysialic acid was almost completely inhibited by preincubation with E. coli colominic. FACS reactivity for 5 of the 6 patients against N417 and/or DMS79 cells was decreased by >70% by preincubating sera with colominic acid. Also, post-vaccination sera, but not prevaccination sera, from 9/9 patients vaccinated with 10 μg NP-polySA were bactericidal against group B meningococcus in the presence of rabbit complement (Table 4).
Table 4.
Bactericidal activity of sera from patients vaccinated with 10 μg NP-polySA against group B meningococcus in the presence of rabbit complement
| Patient | Bactericidal activity antisera dilution able to give | |
|---|---|---|
| 50% killing | 80% killing | |
| 1 | 512 | 256 |
| 2 | 128 | 4 |
| 3 | 128 | 4 |
| 4 | 1,024 | 1,024 |
| 5 | 2,048 | 2,048 |
| 6 | 512 | 64 |
| 7 | 8,192 | 2,048 |
| 8 | 1,024 | 1,024 |
| 9 | 2,048 | 512 |
Toxicity
Toxicities are exhibited in Table 5. The most common side effect was grade 1 injection site reaction. One patient (patient 4 at the 10 μg dose level) treated with etoposide, cisplatin, and concurrent thoracic radiation followed by prophylactic cranial irradiation experienced grade 2 sensory neuropathy and grade 3 ataxia after vaccination. She was extensively evaluated by a neurologist. Gadolinium-enhanced MRI of the brain was unremarkable. Lumbar puncture showed no evidence of infection, and cytology was negative. Because of these symptoms, she was discontinued from the study after the fourth vaccination. Her symptoms ultimately resolved spontaneously, and she remains asymptomatic and free of SCLC or neurologic symptoms 4 years later. One patient with limited stage SCLC developed radiation pneumonitis after two vaccinations and was taken off study because of the need to treat her with corticosteroids.
Table 5.
Toxicities felt possibly or definitely related to vaccination and occurring in more than one patient
| NP-polySA–KLH + QS-21 (n = 18) | Grade | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Ataxia* | – | – | 1 | – |
| Constipation | 2 | – | – | – |
| Diarrhea | 3 | – | – | – |
| Fatigue | 1 | 2 | – | – |
| Fever | 2 | – | – | – |
| Headache | 2 | – | – | – |
| Injection site reaction | 10 | – | – | – |
| Nausea | 3 | 2 | – | – |
| Neuropathy (sensory) | 1 | 1 | – | – |
| Vomiting | 3 | 1 | – | |
* Although this toxicity only occurred in one patient, it was included due to the severity
Survival
Survival was not a primary endpoint; however, we reviewed these data to look for a signal of efficacy. Table 6 shows the time to progression, the site of progression, and the survival time from the first vaccination. Immune titers are shown alongside these data. Four patients are still alive, all of whom had limited stage disease at the time of diagnosis.
Table 6.
Time to progression and survival after the initial vaccination
| Initial stage | Peak IgM titer to polySA | TTP (mo) | Site of progression | Survival (mo) | |
|---|---|---|---|---|---|
| 10 μg dose level | |||||
| Patient 1 | Extensive | 1,280 | 1 | Lung, LN | 3 |
| Patient 2 | Extensive | 5,120 | 8 | LN, adrenal, liver | 29 |
| Patient 3 | Limited | 80 | 4 | Brain, lung, LN, bone | 19 |
| Patient 4 | Limited | 1,280 | N/A | N/A | 45+ |
| Patient 5 | Limited | 2,560 | N/A | N/A | 72+ |
| Patient 6 | Limited | 320 | N/A | N/A | 70+ |
| Patient 7 | Extensive | 2,560 | 2 | Unknown | 6 |
| Patient 8 | Extensive | 80 | 6 | Lung | 11 |
| Patient 9 | Limited | 80 | N/A | N/A | 58 |
| 3 μg dose level | |||||
| Patient 1 | Extensive | 0 | 5 | Brain | 11 |
| Patient 2 | Limited | 160 | 3 | Unknown | 7 |
| Patient 3 | Limited | 0 | 8 | Lung, adrenal | 37 |
| Patient 4 | Limited | 40 | N/A | N/A | 56+ |
| Patient 5 | Limited | 320 | 10 | Liver | 33 |
| Patient 6 | Extensive | 320 | 2 | Brain, liver | 6 |
| Patient 7 | Extensive | 0 | 4 | Lung, LN | 22 |
| Patient 8 | Limited | 2,560 | 4 | LN | 13 |
| Patient 9 | Limited | 2,560 | 3 | Lung, liver | 24 |
+ indicates the patient remains alive at the time of this analysis
N/A not applicable
The survival data from this study (IRB Protocol # 02-097) were combined with those from the prior study (IRB Protocol # 98-065) in which patients were treated with the same vaccine but at a dose of 30 μg. One- and two-year survival rates and median survival times were calculated as measured from the start of NP-polySA immunization (Table 7). The inclusion of both limited and extensive stage patients in these trials confounds the results. The median survival of 22.9 months is encouraging though this was a small sample of selected patients.
Table 7.
Survival rates in prior clinical trials with polysialic acid vaccines
| Trial 02-097 (n = 18) | Trial 98-065 (n = 6) | Trial 02-097 and trial 98-65 combined (n = 24) | |
|---|---|---|---|
| 1-year survival rate (±standard deviation) | 66.7 (±11.1) | 50.0 (±20.4) | 62.5 (±9.9) |
| 2-year survival rate (±standard deviation) | 44.4 (±11.7) | 33.3 (±19.2) | 45.8 (±10.2) |
| Median survival, months (95% confidence interval) | 22.9 (11.3–NR) | 17.6 (7.97–NR) | 22.9 (11.3–37.2) |
NR not reached
Discussion
Although SCLC is initially responsive to chemotherapy, even to the point of complete radiologic regression in some cases, proliferation of residual chemoresistant tumor cells often results in rapid disease recurrence. Thus far, all agents administered with the hopes of controlling lethal residual disease have failed. This includes the anti-idiotype vaccine, BEC-2 (administered with BCG adjuvant) targeting GD3 ganglioside, which was tested in a randomized trial as maintenance therapy in patients with limited stage SCLC, but yielded limited antibody responses against GD3 and no improvement in survival or progression-free survival [20]. Despite the lack of benefit seen in that phase III trial, the interest in developing immunologic therapies in SCLC remains. We have pursued immunologic strategies against other carbohydrate antigens known to have restricted expression in SCLC such as polysialic acid. In this study, we further elucidate the safety and immunogenicity of the NP-polySA vaccine with a goal of selecting the lowest vaccine dose that safely induces the highest-titer antibody response against polysialic acid.
Our first goal was to determine whether a polysialic acid vaccine could be administered safely. This concern is based on the known expression of polysialic acid on neurons and neuronal precursor cells in the central nervous system and other locations such that induction of antibodies against polysialic acid might result in evidence of neurologic or other autoimmunity. We have previously described a study in which groups of six patients were immunized with either polySA–KLH or NP-polySA–KLH at an antigen dose of 30 μg in each case [11]. Three out of seven patients in the polySA–KLH group experienced grade 2 sensory peripheral neuropathy. All patients had previously received cisplatin, and two had received whole brain radiation. The fact that only one of these seven patients receiving the polySA–KLH vaccine developed any detectable antibody response against polySA, and this patient remained asymptomatic, suggested that these symptoms were not a consequence of antibody-induced autoimmunity. One out of six patients in the NP-polySA–KLH group developed numbness and weakness in her lower extremities that left her wheelchair bound. It was ultimately determined that she developed radiation myelitis resulting from thoracic radiation at the same site as thymic radiation she had received as a child. It was, however, impossible to determine definitively whether the vaccine contributed to any of these symptoms. Of the 18 patients vaccinated with the NP-polySA–KLH vaccine in the study described here, 14 produced antibodies against polySA and only one patient developed evidence of neurologic toxicity, grade 2 sensory neuropathy, and grade 3 ataxia after vaccination (patient 4 at the 10 μg dose level). Once again it was difficult to exclude the possibility that this was vaccine induced; however, her extensive previous treatment with etoposide, cisplatin, concurrent thoracic radiation, and prophylactic cranial radiation suggests that this could have been a consequence of previous therapy. In any case, her signs and symptoms resolved spontaneously, and the patient remains well and asymptomatic now 4 years later.
The second goal of this study was to confirm the immunogenicity of the NP-polySA–KLH vaccine and to select a dose for future trials. All patients produced a high-titer IgM antibody response against the artificial immunogen, NP-polySA, and most patients at both dose levels produced IgG antibodies against NP-polySA as well. IgM antibodies from 9 of 9 patients at the 10 μg dose level and 5 of 9 patients at the 3 μg dose level cross-reacted with the actual SCLC antigen polySA, but none of the IgG antibodies reacted with polySA. The IgM antibody response against both NP-polySA and polySA were significantly higher in the 10 μg dose level group than the 3 μg dose level group. In most cases, these antibody responses against polySA detected by ELISA also reacted with polySA as it is naturally expressed on the cell surface of SCLC cells. Cell surface reactivity was detected in 5 of 6 patients in our previous trial at the 30 μg dose level and in the trial described here in 6 of 9 patients at the 10 μg dose level and 3 of 9 patients at the 3 μg dose level. Based on these findings we have selected a dose level of 30 μg of NP-polySA for inclusion in our polyvalent KLH conjugate vaccine against SCLC.
As we have previously described [11], complement lysis of tumor cells was not induced by these antibodies in the presence of human complement. This is attributed to the great distance from the cell surface that complement is activated when antibodies bind to polySA. It is not a consequence of the two target cell lines being resistant to CDC because the same target cell lines are readily lysed by antibodies against GM2, fucosylated GM1, and globo H, other SCLC target antigens that are glycolipids and intimately associated with the cell membrane [21]. Binding of IgM antibodies to target antigens inevitably results in the activation of the complement cascade resulting in opsonization, activation of leukocytes and macrophages, and increased vascular permeability. These actions are thought to be at least as important in protection from tumor challenge in in vivo models [21] as complement mediated lysis so this does not necessarily reflect poorly on polySA as a target for cancer therapy. These antibodies were however bactericidal for group B meningococci where polySA is more intimately associated with the cell membrane.
We have incorporated NP-PolySA into a pentavalent vaccine that includes NP-PolySA and four glycolipids that are over-expressed in SCLC–fucosyl GM1, GD2, GD3, and globo H. These five antigens are among the most highly expressed antigens on SCLC biopsy specimens. A mixture of monoclonal antibodies against these antigens was highly reactive with all of the 10 SCLC cell lines tested [22]. The safety and immunogenicity of each of these five antigen–KLH conjugates has now been separately tested in humans, and each has demonstrated robust immunogenicity [16, 23–26]. A clinical trial administering a pentavalent vaccine containing these five conjugates in patients with SCLC in complete or partial remission after chemotherapy is currently ongoing at MSKCC.
Acknowledgments
The authors wish to thank the research nurses Barbara Pizzo and Leslie Tyson and the data manager Valerie Baez for their assistance in carrying out this study. Tony Riley produced Fig. 1. We would also like to thank Professor Seppo Meri (Helsinki, Fi) for insightful comments about the complement system and MSKCC’S Clinical Grade Production (CGP) facility for vaccine preparation.
Conflict of interest
Dr. Livingston and Dr. Ragupathi are paid consultants and share holders of MabVax Therapeutics Inc. MabVax has licensed all of the KLH conjugate vaccines from MSKCC.
Footnotes
Supported by an American Society of Clinical Oncology Career Development Award (LMK), R41 CA128363 from the NIH, the Lawrence and Selma Ruben Foundations and the Flight Attendants Medical Research Institute.
References
- 1.Rutishauser U. Polysialic acid at the cell surface: biophysics in service of cell interactions and tissue plasticity. J Cell Biochem. 1998;70:304–312. doi: 10.1002/(SICI)1097-4644(19980901)70:3<304::AID-JCB3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 2.Finne J, Finne U, Deagostini-Bazin H, Goridis C. Occurrence of alpha 2, 8 linked polysialosyl units in a neural cell adhesion molecule. Biochem Biophys Res Commun. 1983;112:482–488. doi: 10.1016/0006-291X(83)91490-0. [DOI] [PubMed] [Google Scholar]
- 3.Rutishauser U, Acheson A, Hall AK, Mann DM, Sunshine J. The neural cell adhesion molecule (NCAM) as a regulator of cell–cell interactions. Science. 1988;240:53–57. doi: 10.1126/science.3281256. [DOI] [PubMed] [Google Scholar]
- 4.Rutishauser U, Landmesser L. Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell–cell interactions. Trends Neurosci. 1996;19:422–427. doi: 10.1016/0166-2236(96)10041-2. [DOI] [PubMed] [Google Scholar]
- 5.Komminoth P, Roth J, Lackie PM, Bitter-Suermann D, Heintz PU. Polysialic acid of the neural cell adhesion molecule distinguishes small cell lung carcinoma from carcinoids. Am J Pathol. 1991;139:297–304. [PMC free article] [PubMed] [Google Scholar]
- 6.Lantuejoul S, Moro D, Michalides RJ, Brambilla C, Brambilla E. Neural cell adhesion molecules (NCAM) and NCAM-PSA expression in neuroendocrine lung tumors. Am J Surg Pathol. 1998;22:1267–1276. doi: 10.1097/00000478-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Cordon-Cardo C, Zhang H, Reuter V, Adluri S, Hamilton W, Lloyd K, Livingston P. Selection of tumor antigens as targets for immune attack using immunuhistochemistry: I. Focus on gangliosides. Int J Cancer. 1997;73:42–49. doi: 10.1002/(SICI)1097-0215(19970926)73:1<42::AID-IJC8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Daniel L, Trouillas J, Renaud W, Chevallier P, Gouvernet J, Rougon G, Figarella-Branger D. Polysialylated-neural cell adhesion molecule expression in rat pituitary transplantable tumors (spontaneous mammotropic transplantable tumor in Wistar-Furth rats) is related to growth rate and malignancy. Cancer Res. 2000;60:80–85. [PubMed] [Google Scholar]
- 9.Daniel L, Durbec P, Gautherot E, Rouvier E, Rougon G, Figarella-Branger D. A nude mice model of human rhabdomyosarcoma lung metastases for evaluating the role of polysialic acids in the metastatic process. Oncogene. 2001;20:997–1004. doi: 10.1038/sj.onc.1204176. [DOI] [PubMed] [Google Scholar]
- 10.Jennings HJ, Roy R, Gamian A. Induction of meningococcal group B polysaccharide-specific IgG antibodies in mice by using an N-propionylated B polysaccharide-tetanus toxoid conjugate vaccine. J Immunol. 1986;137:1708–1713. [PubMed] [Google Scholar]
- 11.Krug LM, Ragupathi G, Ng KK, Hood C, Jennings HJ, Guo Z, Kris MG, Miller V, Pizzo B, Tyson L, Baez V, Livingston PO. Vaccination of small cell lung cancer patients with polysialic acid or N-propionylated polysialic acid conjugated to keyhole limpet hemocyanin. Clin Cancer Res. 2004;10:916–923. doi: 10.1158/1078-0432.CCR-03-0101. [DOI] [PubMed] [Google Scholar]
- 12.Helling F, Zhang S, Shang A, Adluri S, Calves M, Koganty R, Longenecker BM, Yao TJ, Oettgen HF, Livingston PO. GM2-KLH conjugate vaccine: increased immunogenicity in melanoma patients after administration with immunological adjuvant QS-21. Cancer Res. 1995;55:2783–2788. [PubMed] [Google Scholar]
- 13.Livingston PO, Adluri S, Helling F, Yao TJ, Kensil CR, Newman MJ, Marciani D. Phase 1 trial of immunological adjuvant QS-21 with a GM2 ganglioside-keyhole limpet haemocyanin conjugate vaccine in patients with malignant melanoma. Vaccine. 1994;12:1275–1280. doi: 10.1016/S0264-410X(94)80052-2. [DOI] [PubMed] [Google Scholar]
- 14.Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991;146:431–437. [PubMed] [Google Scholar]
- 15.Ragupathi G, Meyers M, Adluri S, Ritter G, Livingston PO. Phase I trial with GD3-lactone-KLH conjugate and immunological adjuvant QS-21 vaccine with malignant melanoma. Proc Am Assoc Cancer Res. 1997;38:398. [Google Scholar]
- 16.Dickler MN, Ragupathi G, Liu NX, Musselli C, Martino DJ, Miller VA, Kris MG, Brezicka F, Livingston PO, Grant SC. Immunogenicity of a Fucosyl-GM1-keyhole limpet hemocyanin conjugate vaccine in patients with small cell lung cancer. Clin Cancer Res. 1999;5:2773–2779. [PubMed] [Google Scholar]
- 17.Pon RA, Lussier M, Yang QL, Jennings HJ. N-Propionylated group B meningococcal polysaccharide mimics a unique bactericidal capsular epitope in group B Neisseria meningitidis. J Exp Med. 1997;185:1929–1938. doi: 10.1084/jem.185.11.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher RA, Yates F (1963) Statistical tables for biological agricultural and medical research, 6th ed., Table IV ed., Oliver & Boyd Ltd., Edinburgh
- 19.Yao TJ, Begg CB, Livingston PO. Optimal sample size for a series of pilot trials of new agents. Biometrics. 1996;52:992–1001. doi: 10.2307/2533060. [DOI] [PubMed] [Google Scholar]
- 20.Giaccone G, Debruyne C, Felip E, Chapman PB, Grant SC, Millward M, Thiberville L, D’Addario G, Coens C, Rome LS, Zatloukal P, Masso O, Legrand C. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971–08971B; Silva Study) J Clin Oncol. 2005;23:6854–6864. doi: 10.1200/JCO.2005.17.186. [DOI] [PubMed] [Google Scholar]
- 21.Imai M, Landen C, Ohta R, Cheung NK, Tomlinson S. Complement-mediated mechanisms in anti-GD2 monoclonal antibody therapy of murine metastatic cancer. Cancer Res. 2005;65:10562–10568. doi: 10.1158/0008-5472.CAN-05-1894. [DOI] [PubMed] [Google Scholar]
- 22.Livingston PO, Hood C, Krug LM, Warren N, Kris MG, Brezicka T, Ragupathi G. Selection of GM2, fucosyl GM1, globo H and polysialic acid as targets on small cell lung cancers for antibody mediated immunotherapy. Cancer Immunol Immunother. 2005;54:1018–1025. doi: 10.1007/s00262-005-0663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krug LM, Ragupathi G, Hood C, Kris MG, Miller VA, Allen JR, Keding SJ, Danishefsky SJ, Gomez J, Tyson L, Pizzo B, Baez V, Livingston PO. Vaccination of patients with small-cell lung cancer with synthetic fucosyl GM-1 conjugated to keyhole limpet hemocyanin. Clin Cancer Res. 2004;10:6094–6100. doi: 10.1158/1078-0432.CCR-04-0482. [DOI] [PubMed] [Google Scholar]
- 24.Kirkwood JM, Ibrahim JG, Sosman JA, Sondak VK, Agarwala SS, Ernstoff MS, Rao U. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 25.Slovin SF, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, Spassova M, Bornmann WG, Fazzari M, Dantis L, Olkiewicz K, Lloyd KO, Livingston PO, Danishefsky SJ, Scher HI. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc Natl Acad Sci USA. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilewski T, Ragupathi G, Bhuta S, Williams LJ, Musselli C, Zhang X, Bencsath KP, Panageas KS, Chin J, Hudis CA, Norton L, Houghton A, Livingston PO, Danishefsky SJ. Immunization of metastatic breast cancer patients with a fully synthetic globo H conjugate: a phase I trial. Proc Natl Acad Sci USA. 2001;98:3270–3275. doi: 10.1073/pnas.051626298. [DOI] [PMC free article] [PubMed] [Google Scholar]