Abstract
Large number of epidemiological studies to know the effect of air pollution on the general mortality and morbidity, and the cardiopulmonary morbidity and mortality are concentrated in USA and Europe. Regional differences in air pollution necessitate regional level health effects studies. Present study is a cross sectional pilot study from India, an Asian country. A sample of population from an industrial town ‘Mandi Gobindgarh’ and a non-industrial town ‘Morinda’ were selected. A cross-sectional household survey was done in both the towns. One hundred subjects were selected from each of the towns. Ambient air quality data was collected for both towns over a period of 10-months to assess seasonal variations. In the present study the average PM10 (particulate matter with ≤ 10μm aerodynamic diameter) levels in Morinda were 99.54 μg/m3 and in Mandi Gobindgarh 161.20 μg/m3. As per NAAQS the permitted levels of PM10 is 50 μg/m3 taken as annual average (arithmetic mean). Elemental analysis of the aerosol samples found the concentration levels to be higher in Mandi- Gobindgarh than Morinda. The population in Gobindgarh shows a higher prevalence of symptoms of angina and cardiovascular disease considered in the study as compared to Morinda. When the same data is viewed in terms of male and female population, the female population is found to show these symptoms marginally higher than their counterparts. Considering the results of present study it can be stated that the increased levels of different pollutants and the higher prevalence of cardiovascular symptoms in Mandi-Gobindgarh (Industrial town) than the Morinda (Non-Industrial town) is because of the association of PM pollution with cardiovascular diseases. Keeping in view the current status of literature, further studies in this direction are needed in a country like India. Such data will also be globally relevant.
Keywords: Cardiovascular morbidity, air pollution, cross sectional study, India
Introduction
Air pollution including both the gaseous and suspended particulate matter (SPM) has become a matter of global concern, particularly in some of the world’s industrial cities. The ‘six criteria’ air pollutants currently identified by United States Environment Protection Agency (EPA) considered important from public health viewpoint are respirable particulate matter including both the PM10 (particulate matter with ≤ 10μm aerodynamic diameter) and PM2.5 (particulate matter with ≤ 2.5μm aerodynamic diameter), sulfur-dioxide (SO2), carbon monoxide (CO), nitrogen-dioxide (NO2), ozone (O3) and lead (Pb) [1].
Large numbers of epidemiological studies have been carried out throughout the world to know the effects of air pollution on the general mortality and morbidity, and the cardiopulmonary morbidity and mortality [2–9]. Recently a book entitled “Air Pollution and Health” edited by Holgate el al. has reviewed major aspects of air pollution and health [3]. During the 1990s, there has been rising use of time-series method to evaluate the effect of air pollution on daily mortality & morbidity measure, such as numbers of hospitalizations or emergency room visits [4]. These studies have been facilitated by new statistical methods for time series analysis, as well as availability of suitable hardware & software. The databases are typically assembled from routinely collected information on mortality & morbidity, monitoring data collected for regulatory purposes, & publicly available information on confounding factors, such as weather. An extensive series of analyses has been conducted by Pope III et al. [5], Dockery & Schwartz [6]. Time series studies in numerous cities, as well as recent multi-city studies, show associations between daily mortality and changes in PM10 and PM2.5[7, 8]. Prospective epidemiological studies reported significant associations between an increased risk of premature mortality and long-term exposure to PM2.5[2, 5]. These results were confirmed in extensive re-analyses and new analyses indicating premature mortality included not only cardiopulmonary causes, but cancer as well [9, 10]. Elevated levels of PM have also been linked to various indices of morbidity, ranging from hospitalization for respiratory or cardiovascular diseases to moderate exacerbation of respiratory diseases or decreases in lung function [7, 11]. A review of epidemiological studies related to Particulate Matter (PM) and human health was published by Englert [12]. Number of studies during last five years confirms the association of PM with cardiovascular morbidity and mortality and all age group population appears to be susceptible [11, 13–17].
Unlike most pollutants, ambient PM varies by chemical composition and size depending upon the particle formation processes. This leads to significant variability in PM characteristics across time and space, across source categories, and across individual sources within a single source category. Although a few air quality areas may have a PM character strongly influenced by a single source (e.g., a large power plant or smelter), PM for most air quality areas results from a mixture of locally generated emissions and those transported in from distant sources that are distinguished by high spatial and temporal variability [18].
A further complication exists in that any given individual particle can differ appreciably from another individual particle within a given size range. Particulate matter includes organic and inorganic matter, nitrogen compounds, sulphur compounds, polycyclic aromatic hydrocarbons, several metals and radionuclides. An estimate indicates that particulate contaminants contain nearly 22% metallic elements, the most abundant being Na, Ca, Al, Si, and Fe. Heavy metals include lead, chromium, arsenic, vanadium and selenium and many other elements. The concentration of these pollutants depends upon the source of their emission. Previous research confirms that PM2.5 composition varies by region [19]. Regional differences include the relative contribution of diesel and gasoline vehicles, coal and oil burning, the importance of secondary organic aerosol, and the proximity to large industrial sources of PM2.5[18]. Regional differences in composition and emission sources necessitate regional level health effects studies and emission control strategies. WHO’s estimates suggest that the health effect of outdoor air pollution in Asian cities is substantial. These estimates are based largely on the results of research conducted in Europe and North America that have been extrapolated to developing countries. Such extrapolation raises large uncertainties because Asia differs from Europe and North America in the nature of its air pollution, the conditions and magnitude of exposures to that pollution, and the health status of its populations. Within Asia also majority data is from Hong Kong, Taipei, China and South Korea. India despite having burgeoning mega cities, where rapid urban growth is accompanied by extremely high levels of air pollution compares poorly with China [20]. Thus conducting and evaluating epidemiologic studies in Asia is a priority.
The industrial towns deserve special attention in Asia as large population resides near the industrial area thus exposing themselves continuously to the industrial emissions. The occupational health problems in workers of the steel industry have drawn attention of the researchers [21–23]. References are also available regarding the epidemiological studies specific to the population residing in the vicinity of steel industries [24–25]. No studies have come to the notice of authors where the cardiovascular epidemiologies in the vicinity of steel industries have been addressed.
Recently Tata Energy Research Institute (TERI) has compiled a report where the status of PM2.5 and PM10 was assessed on the basis of previous work done in different urban cities of India and dearth of such data was highlighted [26]. No Epidemiological studies relating the air pollution with the health effects were reported. Only study after the TERI review is from an industrial town in Northern India where the association of outdoor air pollution with chronic respiratory morbidity was studied [27]. The urban population in India is particularly at risk due to a) expected high air pollution due to increasing vehicular traffic, growing industrialization and poor pollution control measures b) high population density and poor hygienic conditions in the crowded cities c) poor nutritional and health status of poor socioeconomic group of population. Keeping this in view, the present cross sectional study is designed to monitor the ambient air quality and correlate it with the cardiovascular health status of the general population in an industrial and a non-industrial town of Punjab. The particulate matter, both the PM10 and PM2.5 and the gaseous pollutants (CO, NOx, SOx) are estimated. To understand the composition of the PM the trace elements associated with the PM10 and PM2.5 are estimated using Proton Induced X-ray Emission (PIXE) techniques [28].
Methods
Study Area
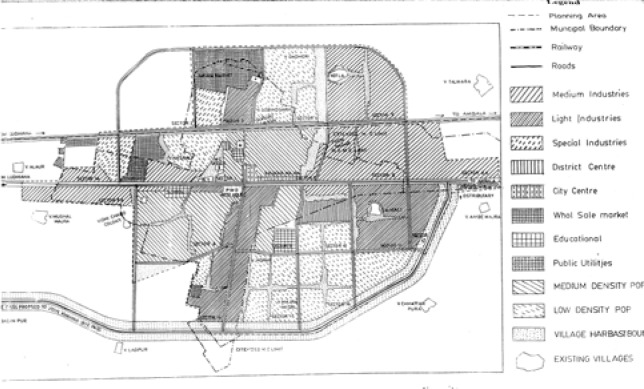
The study was carried out at Mandi-Gobindgarh, an industrial town and in Morinda, a non-industrial town of Punjab. These towns are located 35 km apart at latitude and longitude 30.66°N and 76.31°E, and 30.83°N and 76.58°E, respectively, in the Punjab State in northern India and have similar climatic and meteorological conditions. Mandi-Gobindgarh is one of the 17 critically polluted areas in the country identified by the Ministry of Environment and Forest, Govt. of India on the basis of available air and water monitoring data and location of hazardous industries. No epidemiological data on health status of population in these polluted areas are available. In Mandi-Gobindgarh, there are mainly steel rolling mills and foundries whereas in Morinda, there is only one Sugar Mill, which is located outside the city and works only in winter. Mandi-Gobindgarh is an example of mixed habitat where there is no distinction of industrial, commercial or residential areas as is demonstrated in the guideline map (Fig 1). Population of both the towns is approximately similar in occupation, and socio-economic factors. Gobindgarh and Morinda had 38,205 and 17,440 inhabitants as per 1991 census data.
Figure 1:
A guideline map of Mandi-Gobindgarh indicating the sampling sites
Population Sample
A sample of population from an industrial town and a non-industrial town were selected. A cross-sectional household survey was done in both the towns to collect data on socio-demographic factors, occupational history, lifestyle (including smoking) and cardiovascular symptoms. Ambient air quality data was collected for both towns over a period of 10-months to assess seasonal variations.
For the cross-sectional household survey, one hundred subjects were selected from each of the towns. Individuals of either sex above 15-years were included. The final age breakup of the population sample for the respective sites is given in Table 1. The demographic trends as observed in 1991 census for India are also given in this table. The percentage representation of people in the sample is found to be decreasing with increase in age. Similar trend was observed in the study population also.
Table 1:
Age-wise breakup of the population sample for the study sites and demographic trends as per 1991 census
| Age groups (in years) | Morinda (Rest-House road) | Mandi-Gobindgarh (Premnagar) | Overall of present study sample | 1991 Census for India | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Males | Females | Males | Females | Total | % | Total (millions) | % | |
| 15–30 | 14 | 16 | 15 | 16 | 61 | 30.5 | 399.84 | 42.8 |
| 30–45 | 15 | 18 | 15 | 16 | 64 | 32.0 | 264.61 | 28.3 |
| 45–60 | 13 | 10 | 13 | 11 | 47 | 23.5 | 164.16 | 17.57 |
| 60 and above | 8 | 6 | 7 | 7 | 28 | 14.0 | 105.43 | 11.3 |
|
| ||||||||
| Total | 50 | 50 | 50 | 50 | 200 | 100.0 | 934.04 | 100.0 |
For the study, random samples were used from each population with equal distribution of males and females. A baseline survey including the data collection on risk factors for major cardiovascular and cardiopulmonary diseases was completed. This was done by interviewing the subjects using the individual schedule. The schedule consisted of questions on socio-economic factors, occupational history, cardiorespiratory symptoms, family and individual medical history.
An additional questionnaire developed at the London School of Hygiene and Tropical Medicine was administered to the subjects [29]. This questionnaire utilizes the characteristics of ischaemic pain, i.e., occurrence of pain usually on walking, in certain chest and leg sites, usually causing the individual to slow his/her pace or stop, and promptly relieved by rest. On the basis of the questionnaire the subjects’ symptoms could be diagnosed as Angina, Pain of Possible Infarction or Intermittent Claudication. The Epi Info-6 (version 6.04b) a word processing database and statistics program for public health was used for the analysis of above information [30].
Monitoring of Aerosols
Fifty samples were collected from August-1999 to May-2000 in both the study areas. This period include a monsoon and winter season, and moderate to heavy rains were received during this period in the region. The sampling time for all these samples was 12/36 hrs. The flow-rate of air was chosen as 3/8 lt./min. An overview of the sampling program is given in Table 2.
Table 2:
An overview of the sampling program, in the two study sites from August 1999 to May 2000
| Morinda | Mandi-Gobindgarh | |
|---|---|---|
| Number of samples | 25 | 35 |
| Collection time | 12/36 hrs. | 12/36 hrs. |
| Flow rate | 3/8 lt. per min. | 3/8 lt. per min. |
| Levels of PM2.5 | 24–90 μg/m3 | 32–100 μg/m3 |
| Levels of PMCF | 33–102 μg/m3 | 34–126 μg/m3 |
| Average PM2.5 | 42.18 μg/m3 | 70.00 μg/m3 |
| Average PM10 | 99.54 μg/m3 | 161.20 μg/m3 |
Aerosol sampling kit involved Millipore diaphragmatic vaccum pump (Catalog No. 5522050) and sequential filter unit (SFU) with coarse pored Nuclepore filters for collection of fractionated samples of particulate matter under ambient conditions. With this sampler outdoor aerosol can be separated in two fractions which roughly can be described as anthropogenic (PM2.5) and soil derived particles (PM10).
SFU consisted of 8 μm pore size nuclepore filter (110632 PC memb., 25 mm diameter) followed by a 0.4 μm pore size filter (110607 PC memb., 25 mm diameter) both held in a double 25 mm filter-holder (Nuclepore Corp., Pleasanton, CA, USA). The air through the filter mebranes was sucked at a required flow rate with the help of a diaphragmatic vacuum pump (Millipore Catalog No.-5522050). All the aerosol-sampling sites were located on the flat rooftops of building (20–40 feet high) to have an effective collection of the aerosols below 10 μm size.
Measurement of Loading of Particulate Matter
To perform weighing, the Mettler M3 microbalance, with an electrical weighing range of approximately 150 μg was used. The readability is 1μg. A 100 mg calibration weight is built in. The dried filter membranes were weighed (W1). The weighed filter membrane was placed in the filter-holder of the SFU air sampler. The flow rate was recorded with a rotameter. The sampled filter membranes were stored in clean petridishes so that they were moisture free, then they were weighed again (W2) (Bureau of Indian Standard: 5182, Part IV, 1973) [31].
Concentration of SPM was calculated as follows:
where PMx is particulate matter either:
-
a)
Smaller than or equal to 2.5 μm. (PM2.5)
-
b)
Smaller than or equal to 10 μm. (PM10)
-
c)
Fraction between PM10 and PM2.5 (PM10 − PM2.5 = PMCF)
PIXE Analysis
To study the composition of fractionated aerosols, about twenty samples were analyzed using Proton Induced X-ray Emission (PIXE) technique at Institute of Physics (IOP), Bhubaneshwar, Orissa, India. The PIXE is an analytical technique capable of trace element detection sensitivity upto a few parts per million. In PIXE technique, accelerated particle are incident on matter, they interact with the electrons in the atoms and thereby producing a vacancy. When interactions occur in an inner shell, the vacancy is filled by an electron from an outer shell, and an x-ray photon of characteristic energy is emitted. By measuring the energy, one can determine the atomic number and area under the x-ray peak indicates the amount of that element present [28].
Measurement of Gaseous Pollutants
Battery operated, compact pocket sized instruments (Endee Engg. Pvt. Ltd., Mumbai, India) were used for measuring levels of carbon monoxide (CO), oxides of nitrogen (NOx) and oxides of sulphur (SOx) in the ambient air of the two sampling sites.
ECG Recording and Analysis
The electrocardiogram (ECG), a graphic time based record of electrical events in the heart muscle was employed in cardiovascular epidemiology. It is useful in diagnosing manifestations of ischemic heart disease, i.e., myocardial infarction, ischemia and hypertrophy. The ECG machine used was Cardiart 108T (BPL, India) in both the study sites.
Electrocardiographic Coding
For the assessment of cardiovascular health in the sample population, ECG records were analyzed by using the ‘Minnesota’ code [32]. In the present study, the revised form of Minnesota Code was used to classify the ECGs in precisely defined classes and reducing the coding variability. Mutually Exclusive Subclasses: Codable ECG items could coexist in an individual record (e.g. commonly individuals with class 1-1 Q-waves have class 5-2 T-waves also). Tabulation was made of each major class, but if more than one item was present among subclasses only the most significant (lowest number) assignment was given, e.g., 1-1-2 took precedence over 1-2-4; 4-1 took precedence over 4-2 within separated lead groups.
After the coding of all the ECG samples, 33% ECG were cross-checked randomly. Comparison showed above 90% agreement in these coding. To minimise systematic bias in major codes between control and exposed samples, ECG coded in the first round were reread. In this latter sample, no major and only a few minor 4- or 9-codes were found upon re-coding.
Since Coronary Heart Disease (CHD) may take more than one form, various diagnostic categories mentioned below were used separately and in combination as response variable. The definition of CHD categories used in this study is based on patient history of chest pain, doctors’ diagnosis and ECG signs coded according to the Minnesota code.
Major ECG signs (Definite myocardial infarction): Minnesota code: 1.1 or 1.2 major Q-waves.
Positive ECG signs: (Possible myocardial infarction): Minnesota codes: 1.1–1.3 Q/QS waves; 4.1, 4.2 (ST-depression); 5.1, 5.2 (T-waves negativity); 6-1, 6-2 (AV-Block); 7-1 (LBBB), 7-2 (RBBB), 8-1 (Premature beats), 8.3 (Atrial fibrillation or flutter).
Angina Pectoris: Positive Angina Questionnaire.
Clinical diagnosis: History of doctors’ diagnosis of myocardial infarction or Angina. Probable CHD: Presence of major ECG signs or Angina Pectoris or clinical diagnosis.
Results
Environmental Studies
Fractionated Aerosol Sampling
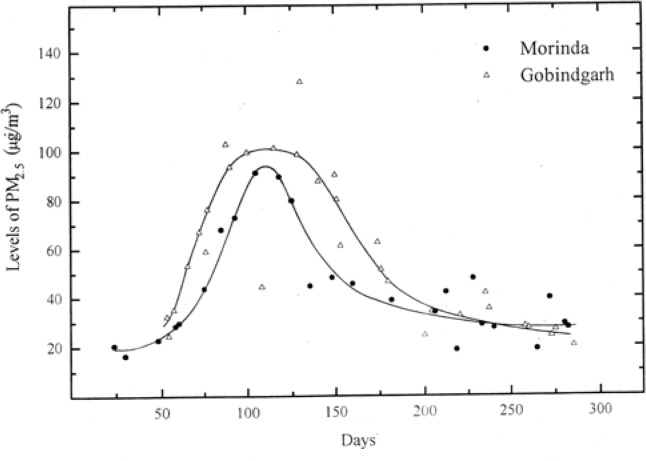
Figure 2 gives a comparative view of PM2.5 levels in ambient air of the two mentioned study sites. Similarly, Figure 3 compares the levels of PM10. It was found that the particulate matter levels increased with the onset of winter and reached a maximum in the months of Nov.–Dec. (Figures 2 and 3). The levels again started declining with the onset of summer. The possible reason for the increase in levels of particulate matter in winter could be the fact that in this region, the weather conditions in winter season are generally stable. These conditions result in thermal inversions concentrating the pollutants. Parr et al. has reported similar observations for the apportionment of air pollution for a city in New South Wales, Australia [33].
Figure 2:
Ambient air levels of PM2.5 in the two study sites
Figure 3:
Ambient air levels of PM10 in the two study sites
The levels of PM2.5 vary from 24 μg/m3 to 90 μg/m3 in Morinda and 32 μg/m3 to 100 μg/m3 in Gobindgarh. The levels of PMCF vary from 33 μg/m3 to 102 μg/m3 in Morinda and 34 μg/m3 to 126 μg/m3 in Gobindgarh. The PM10 is sum total of PM2.5 and PMCF. As per NAAQS the permitted levels of PM10 is 50 μg/m3 taken as annual average (Arithmetic mean). In the present study the PM10 levels both at Morinda and Gobindgarh are higher than the permitted levels. As reported by Punjab State Council for Science and Technology (PSCST), levels of total suspended particulate (TSP) from Gobindgarh are 400–750 μg/m3[34]. No reports are available on PM2.5 or PM10. It may be mentioned that marked reduction in TSP concentrations can be achieved without significantly altering the ambient concentrations of inhalable particles that penetrate the thoracic airways. Therefore, available TSP data is not of much significance from health point of view. Another significant observation is that PM2.5 levels are almost comparable to PMCF levels. It is well established that from cardiorespiratory health aspect the PM2.5 plays much more significant role than the PM10. Also the sources of PM2.5 are anthropogenic whereas the source of PMCF is normally natural. The higher levels of PM2.5 at Morinda are difficult to understand. The only possible reason is the transport of PM2.5 from industrial cities to Morinda.
Thirteen elements namely S, Cl, K, Ca, Ti, Cr, Mn, Fe, Ni, Zn, As, Br and Pb were detected. The error in concentrations can be up to 30%. This includes the error of air volume sucked and charge collection and other physical parameters used by GUPIX software for quantitative estimation [35]. The arithmetic mean and standard deviation of the concentration levels of different elements obtained in samples from Mandi-Gobindgarh and Morinda are given in Table 3a and 3b respectively.
Table 3a:
Arithmetic mean concentrations (ng/m3) and standard deviation (σ) of various elements detected in aerosol samples from Mandi-Gobindgarh. MDL stands for minimum detection limit of the PIXE set up at Bhubaneshwar for the aerosol samples in (ng/m3). To calculate the arithmetic mean the concentrations of the elements in the samples where these were below detection limits were taken to be zero.
| Elements | MDL | PM2.5 | PMcf | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean | σ | Min | Max | Mean | σ | Min | Max | ||
| Silicon (Si) | 80090 | 11288 | 28856 | 0 | 94125 | 0 | 0 | 0 | 0 |
| Sulphur (S) | 769 | 6315 | 6031 | 0 | 19136 | 901 | 1620 | 0 | 5009 |
| Chlorine (Cl) | 229 | 1897 | 2125 | 0 | 18598 | 5096 | 4228 | 0 | 13910 |
| Potasium (K) | 86 | 2833 | 3243 | 878 | 22339 | 3769 | 3911 | 499 | 15506 |
| Calcium (Ca) | 78 | 713 | 571 | 0 | 3609 | 6054 | 5276 | 1031 | 19428 |
| Titanium (Ti) | 54 | 74 | 83 | 0 | 329 | 612 | 522 | 98 | 1945 |
| Chromium (Cr) | 45 | 92 | 87 | 0 | 258 | 199 | 238 | 0 | 828 |
| Manganese (Mn) | 44 | 140 | 168 | 0 | 3012 | 351 | 313 | 66 | 1221 |
| Iron (Fe) | 15 | 4189 | 4325 | 488 | 14021 | 20931 | 22587 | 3534 | 82239 |
| Nickel (Ni) | 54 | 24 | 35 | 0 | 908 | 105 | 167 | 0 | 522 |
| Zinc (Zn) | 37 | 14707 | 16109 | 1307 | 51808 | 11607 | 13740 | 88 | 44637 |
| Arsenic (As) | 167 | 96 | 175 | 0 | 601 | 384 | 782 | 0 | 2381 |
| Bromine(Br) | 153 | 2960 | 2062 | 44 | 7311 | 227 | 681 | 0 | 2417 |
| Lead (Pb) | 301 | 3783 | 4811 | 0 | 14550 | 1283 | 1952 | 0 | 7304 |
Table 3b:
Arithmetic mean concentrations (ng/m3) and standard deviation (σ) of various elements detected in aerosol samples from Morinda. MDL stands for minimum detection limit of the PIXE set up at Bhubaneshwar for the aerosol samples in (ng/m3). To calculate the arithmetic mean the concentrations of the elements in the samples where these were below detection limits were taken to be zero.
| Elements | MDL | PM2.5 | PMcf | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean | σ | Min | Max | Mean | σ | Min | Max | ||
| Silicon (Si) | 80090 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sulphur (S) | 769 | 3602 | 533 | 3264 | 4217 | 298 | 516 | 0 | 894 |
| Chlorine (Cl) | 229 | 1132 | 644 | 667 | 1868 | 3002 | 1991 | 1332 | 5207 |
| Potasium (K) | 86 | 4720 | 3670 | 1595 | 8762 | 3711 | 3160 | 1152 | 7243 |
| Calcium (Ca) | 78 | 643 | 734 | 0 | 1461 | 7816 | 5377 | 2841 | 13522 |
| Titanium (Ti) | 54 | 61 | 90 | 0 | 165 | 746 | 500 | 349 | 1307 |
| Chromium (Cr) | 45 | 187 | 60 | 120 | 236 | 195 | 96 | 99 | 292 |
| Manganese (Mn) | 44 | 127 | 120 | 47 | 265 | 254 | 199 | 60 | 458 |
| Iron (Fe) | 15 | 1611 | 1330 | 726 | 3141 | 10348 | 7926 | 3773 | 19149 |
| Nickel (Ni) | 54 | 41 | 60 | 0 | 111 | 0 | 0 | 0 | 0 |
| Zinc (Zn) | 37 | 5957 | 7560 | 592 | 14605 | 849 | 385 | 411 | 1139 |
| Arsenic (As) | 167 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bromine(Br) | 153 | 4745 | 203 | 4542 | 4948 | 42 | 37 | 0 | 70 |
| Lead (Pb) | 301 | 0 | 0 | 0 | 0 | 247 | 428 | 0 | 742 |
The minimum and maximum concentration values and also the minimum detection limits (MDL) for each element by PIXE set up at IOP Bhubaneshwar for aerosol samples is given. The concentration levels are found to be, in general, higher in Mandi-Gobindgarh as compared to Morinda. The large deviation from the mean concentration is indicative of highly varying day to day industrial activity and weather conditions.
The distribution of elements in the PM2.5 and PMcf fractions give an idea about the impact of the possible pollution sources at the measurement site [36]. It can be seen elements such as S and Pb are higher in the PM2.5 in Mandi-Gobindgarh samples which are related to coal and oil burning in furnaces. The elements related to natural dust such as K, Ca, Ti, Mn, and Fe are mainly distributed in PMcf fraction from the samples from both the towns [35].
Gas Pollutants
Table 4 gives the concentration of gaseous pollutants (CO, NOx, SOx) at the respective sites. In Morinda, the levels of NOx and SOx were zero ppm for both residential area and Grand Trunk (GT) road, while CO level ranged from 0 to 1 ppm for residential area and 2 to 3 ppm for GT road. In Gobindgarh, the levels of NOx and SOx for the colony (residential and industrial) were found to range from 0 to 2 and 0 to 1 ppm, respectively. For Gobindgarh, SOx was found to range from 0 to 2 ppm and NOx ranged from 1 to 4 ppm for GT-road. CO levels were found to range from 0 to 3 ppm for residential area and ranged from 1 to 3 ppm for GT-road. With the present equipment used for gaseous monitoring, it is not possible to monitor upto the sensitivity of 0.03 ppm for SO2 and 0.053 ppm for NO2. However, even with the present measurements it can be safely interpreted that the levels of these gases do exceed the permissible levels from time to time. The only possible explanation for this is the burning of coal in cupola furnaces.
Table 4:
Levels of gaseous pollutants in the two sites.
| Gas pollutant | Morinda | Mandi-Gobindgarh | ||
|---|---|---|---|---|
|
| ||||
| Colon* | GT Road | Colon# | GT Road | |
| CO (ppm) | 0–1 | 2–3 | 0–3 | 1–3 |
| SOx (ppm) | 0 | 0 | 0–1 | 0–2 |
| NOx (ppm) | 0 | 0 | 0–2 | 1–4 |
purely residential
mixed habitat; no separation of residential or industrial zones
Taking into consideration the higher levels of PM2.5 and PM10 in Gobindgarh (as well as in Morinda) compared to the NAAQS values (Table 5) and the presence of NOx and SOx beyond permissible level, the prevalence of COPD is expected to rise in the population residing in Gobindgarh. The population is particularly vulnerable as the residential and industrial zones are not planned separately and thus, the population is continuously exposed to the pollutants emitted from industry.
Table 5:
National Ambient Air Quality Standards Ref. 1.
| Pollutant | Primary standards* | Type of average |
|---|---|---|
| Ozone (O3) | 0.12 ppm (235 μg/m3) | Maximum daily 1h average# |
|
| ||
| PM10 | 50 μg/m3 | Annual arithmetic mean** |
| 150 μg/m3 | 24 h** | |
|
| ||
| Sulphur dioxide (SO2) | 0.03 ppm (80 μg/m3) | Annual arithmetic mean |
| 0.14 ppm (365 μg/m3) | 24 h## | |
|
| ||
| Nitrogen dioxide (NO2) | 0.053 ppm (100 μg/m3) | Annual arithmetic mean |
|
| ||
| Carbon monoxide (CO) | 9 ppm (10 mg/m3) | 8 h## |
| 35 ppm (40 mg/m3) | 1 h## | |
|
| ||
| Lead (Pb) | 1.5 μg/m3 | Maximum quarterly average |
The primary standard is to protect against adverse health effects.
The O3 standard is attained when the expected number of days per calendar year with maximum hourly average concentrations > 0.12 ppm is ≤ 1 (averaged over the previous three years).
Particulate standards use PM10 as the indicator pollutant. The annual standard is attained when the expected annual arithmetic mean concentration is ≤ 50 μg/m3; the 24 h standard is attained when the expected number of days per calendar year >150 μg/m3 is ≤ 1.
Not to be exceeded more than once a year.
Population Survey Studies
Susceptibility Factors
The study of susceptibility factors in the male and female populations in both study sites is represented age wise in Tables 6a and 6b respectively. After adding the results of male and female populations the total hypertension cases were 11 in Morinda and 10 in Gobindgarh. Number of diagnosed diabetes cases was one for both the sites. One case in Morinda and 3 in Gobindgarh were found to be on asthma treatment. While there was no case of heart attack or stroke at both the sites, there were 2 cases of tuberculosis, one for each site. Family history of various ailments was found in 21 cases in Morinda and 13 for Gobindgarh.
Table 6a:
Prevalence of different susceptibility factors in the male population of different age groups in the two study sites.
| Age groups | 15–30 | 30–45 | 45–60 | 60 and above | Totals | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Morinda | Gobindgarh | Morinda | Gobindgarh | Morinda | Gobindgarh | Morinda | Gobindgarh | Morinda | Gobindgarh | |
| Diagnosed hypertension | 0 | 1 | 3 | 0 | 1 | 1 | 0 | 1 | 4 | 3 |
|
| ||||||||||
| Diagnosed diabetes | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
|
| ||||||||||
| Suffered or treated | ||||||||||
| Asthma | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 2 |
| Stroke | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tuberculosis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
|
| ||||||||||
| Family history | ||||||||||
| Hypertension | 4 | 1 | 2 | 0 | 1 | 2 | 0 | 0 | 7 | 3 |
| Diabetes | 1 | 3 | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 4 |
| Heart problems | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| Stroke | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| Cancer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Any others | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 3 | 0 |
Table 6b:
Prevalence of different susceptibility factors in female population of different age groups in the two study sites.
| Age groups | 15–30 | 30–45 | 45–60 | 60 and above | Totals | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Morinda | Gobindgarh | Morinda | Gobindgarh | Morinda | Gobindgarh | Morinda | Gobindgarh | Morinda | Gobindgarh | |
| Diagnosed hypertension | 1 | 2 | 3 | 4 | 3 | 0 | 0 | 1 | 7 | 7 |
|
| ||||||||||
| Diagnosed diabetes | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
|
| ||||||||||
| Suffered or treated | ||||||||||
| Asthma | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Stroke | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tuberculosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||||
| Family history | ||||||||||
| Hypertension | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3 | 0 |
| Diabetes | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3 | 0 |
| Heart problem | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stroke | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 |
| Cancer | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Any others | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
With respect to the male and female population data, it was found that greater number of females had been diagnosed with hypertension as compared to males. For the diagnosis of diabetes, figures were same for male and female population. Subjects with past history of treatment of asthma, tuberculosis or stroke were marginally higher for males as compared to the female counterparts. Number of cases for family history for different ailments was significantly higher for males as compared to females.
Angina Questionnaire
Prevalence of different angina related symptoms in the male and female population for Morinda and Gobindgarh is presented in Table 7. The results have been grouped in different age groups as well.
Table 7:
Frequency of chest pain and intermittent claudication in the population of different age groups in the two study sites.
| Age groups | 15–30 | 30–45 | 45–60 | 60 and above | Totals | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Morinda | Gobindgarh | Morinda | Gobindgarh | Morinda | Gobindgarh | Morinda | Gobindgarh | Morinda | Gobindgarh | |
| Chest Pain | 4 | 5 | 4 | 8 | 0 | 8 | 3 | 0 | 11 | 21 |
| Angina | 0 | 4 | 0 | 4 | 0 | 6 | 0 | 0 | 0 | 14* |
| Possible infraction | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 |
| Intermittent Claudication | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 2 | 4 |
| Past medical history (specify) | 0 | 0 | 0 | 1(Septal defect) | 0 | 0 | 0 | 0 | 0 | 1 |
(P,0.05)
For Morinda, in total 11 chest pain complaints were recorded while the number was 21 for Gobindgarh. For angina no record was found in Morinda, whereas 14 persons in total were classified having angina in Gobindgarh. Only 1 case for possible infarction was found in Morinda and 2 persons in Gobindgarh had this condition. Two persons were classified as case of intermittent claudication in Morinda while in Gobindgarh, there were 4 such cases. In the whole study of 200 persons, only 1 case of past medical history was reported from Gobindgarh and was diagnosed, as congenital disorder.
Thus it can be said that considering the two study sites, the population in Gobindgarh shows a higher prevalence of symptoms of angina and cardiovascular disease considered in the study as compared to Morinda. When the same data is viewed in terms of male and female population, the female population is found to show these symptoms marginally higher than their counterparts.
Electrocardiographic Studies
For the assessment of cardiovascular health in the sample populations, ECG records were analyzed by using the ‘Minnesota code’. This scheme of classification of ECG findings in adult population provided a framework for reporting ECG items in uniform and clearly defined terms. The ECG findings were classified by the lead groups as given below:
-
a)
Anterolateral site is represented by I, aVL, V6
-
b)
Posterior (inferior) site is represented by II, III, aVF
-
c)
Anterior site is represented by V1 – V5
| Code Item | Aspect Analysed |
|---|---|
| 1 | Q & QS waves |
| 2 | QRS axis deviation |
| 3 | High amplitude R waves |
| 4 | ST junction (J) & segment depression |
| 5 | T wave items |
| 6 | AV conduction defect |
| 7 | Ventricular conduction defect |
| 8 | Arrhythmias |
| 9 | ST segment elevation |
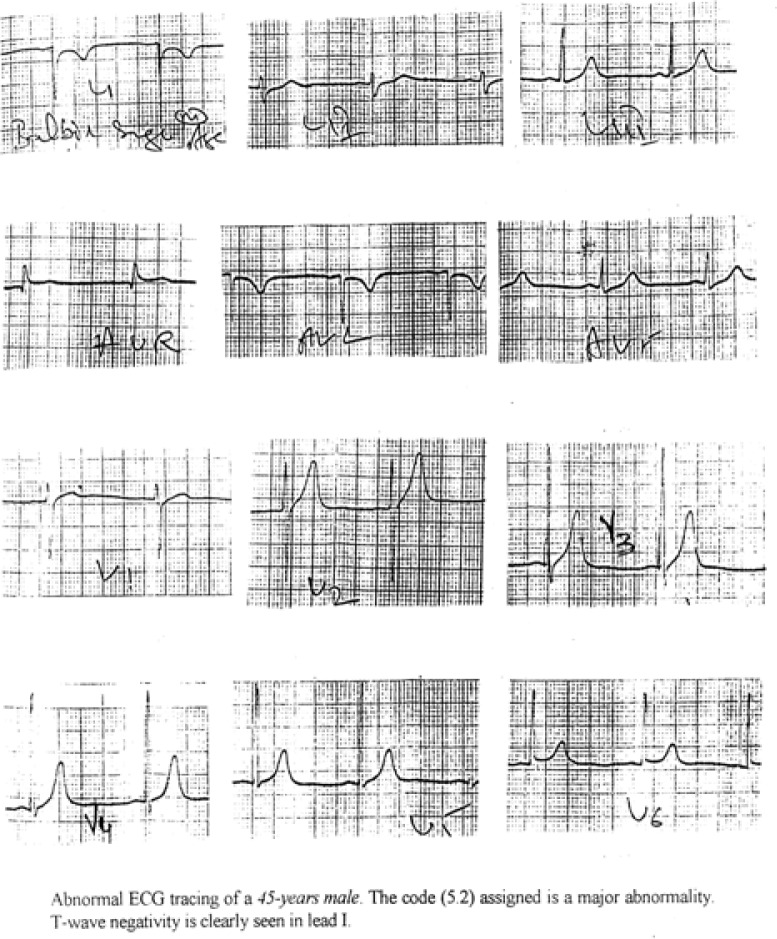
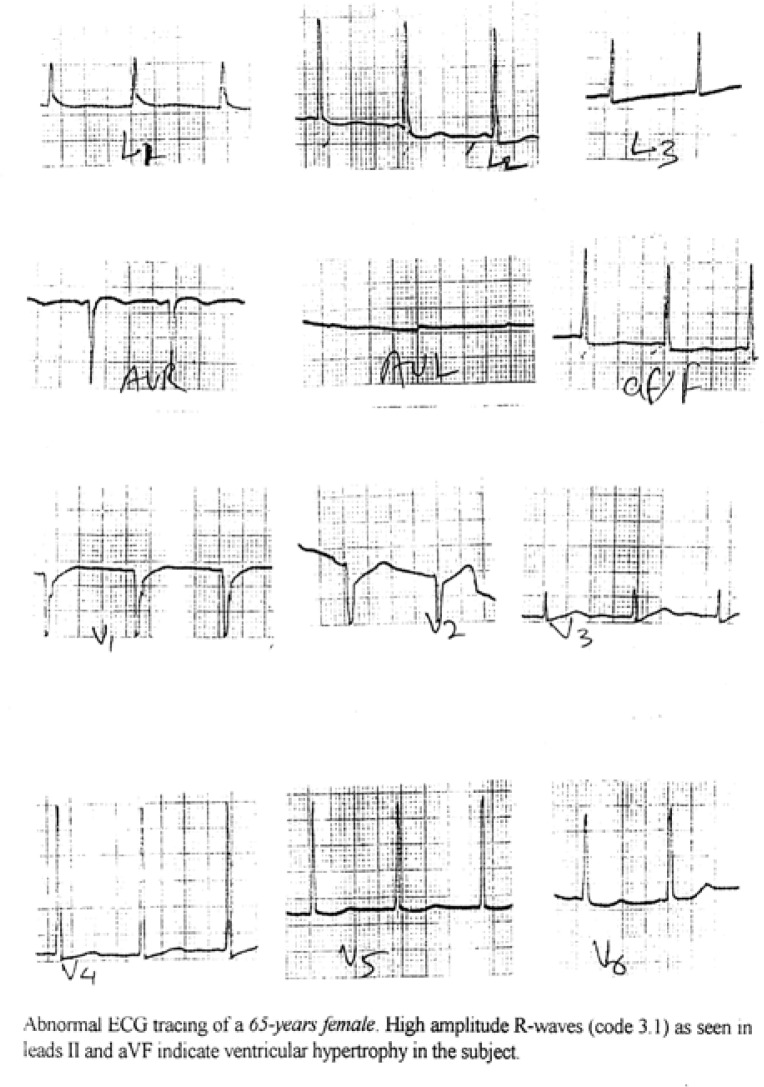
The different codes represented different classes of ECG and these major classes were further split into subclasses to include all possible normal and abnormal variants of ECG. Out of these only those subclasses which signify abnormal pattern were taken for the present study. The same are shown in Tables 8(a) and 8(b). It was observed that there was significant (P<0.05) difference between ST-elevation of males of Morinda and Mandi Gobindgarh. At the same time for the females there were significant (P<0.05) differences for ST-segment depression and T-wave negativity for the Morinda and Mandi Gobindgarh population. These abnormal subclasses of ECG codes obtained from an individual were analysed together to obtain the final information about the prevalence of a particular cardiovascular symptoms. Figures 4(a) and 4(b) represent an example of an abnormal ECG tracings where code 3-1 plus code (4-1, 4-2, 4-3) or code (5-1,5-2 or 5-3) classify left ventricular hypertrophy. In the Table 9(a) abnormalities have been defined as major and minor abnormalities while the Table 9(b) gives the prevalence of different Coronary Artery Diseases on the basis of these significant codes and the information obtained through questioning of the subjects. Table 9(c) gives the frequency of Left Ventricular Hypertrophy in the two population samples. There were significant (P<0.05) differences between minor abnormality and probable CHD in the population of Morinda and Mandi Gobindgarh.
Table 8(a):
Frequency of various Minnesota codes in the male population of different age groups in the two study sites.
| ECG findings | Minnesota Code | 15–30 | 30–45 | 45–60 | 60 and above | All Age Groups | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Site 1 | Site 2 | Site 1 | Site 2 | Site 1 | Site 2 | Site 1 | Site 2 | Site1 | Site 2 | ||
| Q-Wave Amplitude | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Axis Deviation QRS-Complex | 2 | 1 | 2 | 0 | 2 | 1 | 0 | 2 | 2 | 4 | 6 |
| High Amp. R-waves | 3 | 0 | 4 | 3 | 1 | 1 | 1 | 2 | 0 | 6 | 6 |
| ST-segment depression | 4 | 2 | 10 | 5 | 4 | 3 | 2 | 2 | 3 | 12 | 19 |
| T-wave negativity | 5 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 3 |
| AV Conduction Defect | 6 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Ventricular block | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arrhythmias | 8 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 2 |
| Low QRS amplitude | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ST-elevation | 9 | 3 | 8 | 1 | 9 | 1 | 3 | 2 | 0 | 7 | 20* |
(P<0.05): Site 1: Morinda: Site 2: Gobindgarh
Table 8(b):
Frequency of various Minnesota codes in the female population of different age groups in the two study sites.
| ECG findings | Minnesota Code | 15–30 | 30–45 | 45–60 | 60 and above | Totals | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Site 1 | Site 2 | Site 1 | Site 2 | Site 1 | Site 2 | Site 1 | Site 2 | Site1 | Site 2 | ||
| Q & QS Waves | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 3 | 2 | 3 |
| Axis Deviation | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 2 |
| High Amp. R-waves | 3 | 0 | 2 | 2 | 3 | 0 | 3 | 1 | 0 | 3 | 8 |
| ST-segment depression | 4 | 5 | 15 | 10 | 12 | 6 | 7 | 4 | 4 | 25 | 38* |
| T-wave negativity | 5 | 3 | 4 | 0 | 7 | 2 | 1 | 1 | 2 | 6 | 14* |
| AV Conduction Defect | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Ventricular block | 7 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 |
| Arrhythmias | 8 | 2 | 0 | 1 | 1 | 0 | 2 | 1 | 1 | 4 | 4 |
| Low QRS amplitude | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ST-elevation | 9 | 1 | 5 | 0 | 1 | 3 | 1 | 0 | 2 | 4 | 9 |
(P<0.05): Site 1: Morinda: Site 2: Gobindgarh
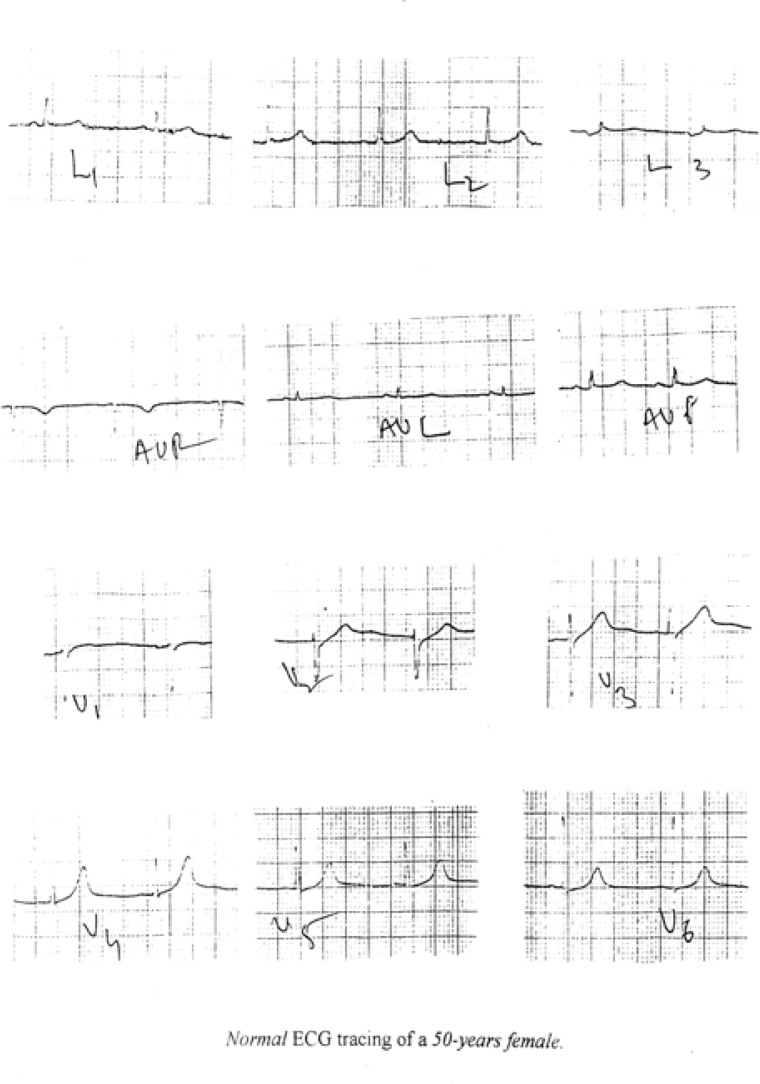
Figure 4a:
A normal ECG tracing from the study sample
Figure 4b:
An abnormal ECG tracing from the study sample
Table 9(a):
Prevalence of major and minor abnormalitya in the population of the two study sites.
| Gender (No) | Major Abnormalityb | Minor Abnormalityc | ||
|---|---|---|---|---|
|
| ||||
| Morinda | Gobingarh | Morinda | Gobindgarh | |
| Male (100) | 5 | 6 | 6 | 11 |
| Female (100) | 12 | 18 | 6 | 19 |
| Total (200) | 17 | 24 | 12 | 30* |
Abnormality is defined as presence of any major or minor abnormality.
Major abnormality is defined as presence of codes 1-1, 1-2; 4-1, 4-2; 5-1, 5-2; 6-1, 6-2, 7-1, 7-2, 7-4, 8-1, 8-3.
Minor abnormality is defined as presence of codes 1-3, 2-1, 2-2, 3-1, 3-2, 4-3, 5-3, 6-3, 9-1.
Table 9(b):
Prevalence of Coronary Heart Disease (CHD) spectrum in the populations of the two study sites.
| Gender (No) | Definite MIa | Possible MIb | Probable CHDc | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Morinda | Gobindgarh | Morinda | Gobindgarh | Morinda | Gobindgarh | |
| Male (100) | - | - | 5 | 8 | - | 6 |
| Female (100) | 1 | - | 12 | 18 | 1 | 8 |
| Total (200) | 1 | - | 17 | 26 | 1 | 14* |
Definite Myocardial infarction is defined as presence of code 1-1 or 1-2 major Q-waves.
Possible Myocardial infarction is defined as presence of codes 1-1, 1–2, 1–3 (Q/QS waves), 4.1 4.2 (ST-depression), 5.1, 5.2 (T-waves negativity), 6-1, 6-2 (AV-block), 7-1 (LBBB), 7-2 (RBBB), 8-1 (Premature beats), 8-3 (Atrial fibrillation or flutter).
Probable CHD is defined as presence of major Q-waves or Angina Pectoris or clinical diagnosis.
Table 9(c):
Prevalence of Left Ventricular Hypertrophy (LVH) in the populations of the two study sites.
| Gender (No) | LVHa | |
|---|---|---|
|
| ||
| Morinda | Gobingarh | |
| Male (100) | 1 | 1 |
| Female (100) | 1 | 2 |
| Total (200) | 2 | 3 |
LVH is defined as the presence of codes 3-1 plus (code 4-1, 4-2, 4-3) or (code 5-1, 5-2 or 5-3).
Coronary Heart Disease and Left Ventricular Hypertrophy
Prevalence of definite Myocardial Infarction (MI) was quite rare in the whole sample population, as only 1 female ECG from Morinda was coded (code 1-1). Possible MI was more prevalent among Gobindgarh sample (26 cases) than that of Morinda (17 cases). Probable MI defined as the combination of codes and patient’s past history was much higher for the Gobindgarh population. In total, Morinda reported a single case and Gobindgarh had 14 such cases (Table 9(b) and Figure 4(b)). Left Ventricular Hypertrophy was found in 5 cases in all. The prevalence per 100 subjects was 2 for Morinda and 3 for Gobindgarh (Table 9(c)).
On considering the results of electrocardiographic studies in terms of gender, it was found that the prevalence of both major and minor abnormalities was higher for females as compared to male population of same sample size. The same held true for coronary heart diseases, where the number of females with CHD spectrum was significantly higher than their counterparts. For LVH, the results were marginally higher for females.
Discussion
Present study attempts to address the problem of air pollution and its association with cardiovascular diseases from India using cross sectional model. The PM2.5 and PMcf were measured over a period of ten months Aug-1999 to May-2000. Both the PM2.5 and PMcf values are higher for Mandi-Gobindgarh than Morinda. The SOX and NOX levels also exceeded the NAAQS levels during the random survey in Mandi-Gobindgarh. The distribution of elements in the PM2.5 and PMcf fractions give an idea about the impact of the possible pollution sources at the measurement site [36]. Elements such as S, Cl, Fe, Zn and Pb are high by factor of 2–5 in both the PM2.5 and PMcf fractions in the Mandi-Gobindgarh than Morinda. Elements such as S, Cl and Zn are known to originate from coal and oil burning [36]. Furnaces generates heavy metals, including cadmium, chromium, lead, manganese, and nickel. Therefore in general higher concentration of elements in Mandi-Gobingarh shows the contribution from the integrated iron and steel industry activities in mandi-Gobindgarh. Normally the PM2.5 fraction is known to be from the anthropogenic sources and PMcf from the natural sources. The higher concentration of these elements in both fractions indicates the nucleation of pollutants even to PMcf fraction.
Both the angina related symptoms and ECG recordings show the higher prevalence of cardiovascular diseases (Possible infarction, Intermittent Claudication, Left ventricular hypertrophy) in Mandi-Gobindgarh than Morinda. Though the population size is small the significant (P<0.05) differences in the angina related symptoms, T-wave negativity, ST-segment depression or elevation clearly shows the association of PM with the increased cardiovascular diseases. Kumar et al. has already reported the association of outdoor air pollution with chronic respiratory morbidity from the town of present study [27]. Another study having with small population size observed that the healthy subjects taking no medications had decreases in heart rate associated with indoor and outdoor PM2.5 and PM [37]. Healthy subjects on medication had small increases in systolic blood pressure associated with indoor PM2.5 and outdoor PM10. Heterogeneity analysis found differences among the health groups for associations with particulate air pollution in heart rate but not in blood pressure.
Majority of data available till late 1990 is based on time-series analyses. Health Effect Institute reanalyzed the data from the large studies like NMMAPS (National Morbidity, Mortality, and Air Pollution study) in the US and APHEA 2 (Air Pollution and Health, a European Approach) in Europe [38,39]. The steel industry specific studies addressing the problems of effect of industrial emissions on the health of general population are scarce. Therefore present results are discussed keeping in view the occupational health of steel workers, morbidity in the population living near steel industries and the effect of air pollution on cardiovascular morbidity in general.
In the study carried by Duan et al. to evaluate the major causes of death and risk factors among male steelworkers in Beijing indicates that malignant neoplasms, cerebrovascular diseases and heart diseases were major causes of death [21]. In another study hypertension and hearing impairment were found to be prevalent in the workers continuously exposed to high levels of occupational noise in steel industry workers [22]. No significant excesses of lung cancer were observed among workers employed in the manufacture of ferroalloys and in the melting and casting of SS, whereas a significant excess appeared among SS foundry workers in a study carried by Moulin et al. [23]. This excess was higher and remained significant among workers with more than 30 years since first employment in the foundry area. Compared to Italian national data, regional age-standardized estimates were higher in Sardinia region (consisting of many industrial towns) for infectious diseases (23% in males and 12% in females), respiratory diseases (22% and 14%: pneumoconiosis was more than 6 times more frequent in Sardinia than in Italy), diseases of the digestive system (26% and 9%: for liver cirrhosis, the excess was 33% in males and 9% in females; corresponding figures for liver cancer were 13% and 16%), breast cancer in females (5%). Environmental (non occupational) pollution was considered the possible causal effect of the observed excesses of disease in the investigated industrial areas of Sardinia, particularly in women, less likely to be exposed to hazards in the work environment [25]. Lung cancer mortality in two areas of the Province of La Spezia (Northern Italy) exposed to environmental pollution emitted by a coal-fired power station and other industrial sources, including a waste incinerator was studied by Parodi et al. [24]. The risk observed among females was consistent with pollution measurements, whereas a strong confounding from occupational exposures and smoking habit was considered to be responsible for the lack of an excess risk in males.
The relative risk (RR) estimate for total mortality for a 10 μg/m3 increase in PM10 concentration was reported to be 1.0041 for the NMMAPS based on 90 US cities [38]. Based on the APHEA 2 study the RR per 10 μg/m3 increase in PM10 for cardiac problems was found to be 1.005 [38,39]. In the review Englert tried to address several questions relevant to health effects of PM [12]. Based on the available body of epidemiological data he concluded that there is a need to consider all PM fractions and health end point. He further said that not all particles are equal with respect to health effects. The constituents of PM vary from region to region, but in all, PM seems to be a health relevant kind of air pollution. A threshold below which no more effects may be expected cannot be described.
Zanobetti and Schwartz conducted a multicity case-crossover study of the acute effects of PM10 on the increased risk of being admitted to the hospital for myocardial infarction (MI) among the elderly (Age ≥ 65 years) in 21 US cities covering a population of 300,167 [16]. They found that for each 10 μg/m3 increase in the concentration of PM10, there was a 0.65% increase in the risk of hospitalization for an MI among the study population. In subjects with a previous admissions for chronic obstructive pulmonary disease (COPD) or with a secondary diagnosis of pneumonia risk was doubled. They also showed that the effects of PM10 on hospital for all other cardiovascular causes are not greatly different from the effects of MI admissions. They also observed a linear dose-response relationship between MI emergency hospital admissions and PM10 exposure, with slope changes at 20 μg/m3 and 50 μg/m3. Sullivan et al. observed only a relative risk of 1.07 per 10 μg/m3 of PM2.5 on MI onset in a study 5793 subjects [17]. On the whole there is a substantial body of epidemiologic literature showing a clear and consistent association between concentrations of ambient PM and negative health effects. Less clear is the biologic mechanism by which PM could be causing this morbidity and mortality.
Compositional analyses of ambient air in Quebec found that particulate matter with high sulphate fractions were more strongly associated with increased hospitalizations for cardiac disease [40]. The transition metal and sulphate content of particles may determine the level of oxidative stress induced by PM2.5 [41]. Physiological and histologic studies in susceptible rodents show cardiac injury after episodic, long-term inhalation of ambient-like combustion PM2.5 with high concentrations of transition metals [42]. Tracheal instillation studies of single metal components document that vanadium exposure induces cardiac arrhythmias in pulmonary hypertensive rats and nickel induces a late onset bradycardia [43]. In the present study also quite high concentrations of different metals have been found have been found (Table 3). As per the WHO air quality guidelines the potential pollutants which are of concern for public health and exceeds the recommended values are Cr, Mn, Ni, As and Pb [44].
Conclusion
The present study is a cross sectional study in which the air pollution measurements as well as the epidemiological data for cardiovascular diseases in subjects greater than fifteen years of age from a Industrial town is compared with a non-industrial town. To the authors knowledge except for this no other studies exists in literature where cross sectional model has been used to see the association of air pollution, in particular PM, with cardiovascular diseases. However Kumar et al. from the town of present study found a association of the outdoor air pollution with the chronic respiratory morbidity [27]. Considering the results of present despite the small population size the significant (P<0.05) differences in the angina related symptoms, T-wave negativity, ST-segment depression or elevation in the population of Mandi-Gobindgarh than Morinda clearly shows the association of PM with the increased cardiovascular diseases. Further studies in this direction are warranted in a country like India. Such data will also be globally relevant.
Figure 4c:
An abnormal ECG tracing for left ventricular hypertrophy
References
- 1.EPA (U.S. Environmental Protection Agency) Air quality criteria for particulate matter. Research Triangle Park, NC: National Center for Environmental Assessment-RTP Office; 1996. http://www.epa.gov/air/criteria.html Report Nos. EPA/600/P-95/001aF-cF. [Google Scholar]
- 2.Dockery DW, Pope CA, 3 rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Jr, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24):1753–9. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 3.Holgate ST, Samet JM, Koren HS, Maynard RL. Air Pollution and Health. Academic Press; USA: 1999. [Google Scholar]
- 4.American Thoracic Society. Committee for Environmental and Occupational Health Assembly. Bescom R, Bromberg PA, Costa DA, et al. Health effects of outdoor air pollution. Part 2. Am J Respir Crit Med. 1996;153:477–498. doi: 10.1164/ajrccm.153.2.8564086. [DOI] [PubMed] [Google Scholar]
- 5.Pope CA, III, Dockery DW, Sachwartz J. Review of epidemiological evidence of health effects of particulate air pollution. Inhal Toxicol. 1995;7:1–18. [Google Scholar]
- 6.Schwartz J, Morris R. Air pollution and hospital admissions for cardiovascular disease in Detroit, Michigan. American Journal of Epidemiology. 1995;142(1):23–5. doi: 10.1093/oxfordjournals.aje.a117541. [DOI] [PubMed] [Google Scholar]
- 7.Samet JM, Zeger SL, Dominici F, Curriero F, Coursac I, Dockery DW, Schwartz J, Zanobetti A. National morbidity, mortality, and air pollution study. Part II: morbidity and mortality from air pollution in the United States. Cambridge, MA. Health Effects Institute; Research Report No. 94. 2000 [PubMed] [Google Scholar]
- 8.Dominici F, McDermott A, Zeger SL, Samet JM. On the use of generalized additive models in time series of air pollution and health. Am J Epidemiol. 2002;156(3):193–203. doi: 10.1093/aje/kwf062. [DOI] [PubMed] [Google Scholar]
- 9.Krewski D, Burnett RT, Goldberg MS, Hoover K, Siemiatycki J, Jerret M, Abrahamowicz M, While H. Health Effects Institute. A Special Report of the Institute’s Particle Epidemiology Reanalysis Project; Cambridge, MA: 2000. Reanalysis of the Harvard Six Cities Study and the American Cancer Society Study of Particulate Pollution and Mortality. [Google Scholar]
- 10.Pope CA, I, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality and long-term exposure to fine particulate air pollution. J Am Med Assoc. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103(23):2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 12.Englert N. Fine particles and human health—a review of epidemiological studies. Toxicology Letters. 2004;149:235–242. doi: 10.1016/j.toxlet.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 13.Peters A, Liu E, Verrier RL, Schwartz J, Gold DR, Mittleman M, Baliff J, Oh JA, Allen G, Monahan K, Dockery DW. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11(1):11–17. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 14.D’Ippoliti D, Forastiere F, Ancona C, Agabiti N, Fusco D, Michelozzi P, Perucci CA. Air pollution and myocardial infarction in Rome: a case-crossover analysis. Epidemiology. 2003;14(5):528–35. doi: 10.1097/01.ede.0000082046.22919.72. [DOI] [PubMed] [Google Scholar]
- 15.Pope CA, III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution, epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109(1):71–7. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 16.Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ Health Perspect. 2005;113(8):978–82. doi: 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan J, Sheppard L, Schreuder A, Ishikawa N, Siscovick D, Kaufman J. Relation between short-term fine-particulate matter exposure and onset of myocardial infarction. Epidemiology. 2005;16(1):41–8. doi: 10.1097/01.ede.0000147116.34813.56. [DOI] [PubMed] [Google Scholar]
- 18.NARSTO Particulate Matter Science for Policy Makers. A NARSTO Assessment. 2003. see http://www.cgenv.com/Narsto/
- 19.Tolocka MP, Solomon PA, Mitchell W, Norris GA, Gemmill DB, Wiener RW, Vanderpool RW, Homolya JB, Rice J. A East versus West in the US: Chemical Characteristics of PM2.5 during the Winter of 1999. Aerosol Sci Technol, Special Issue for PM2000. 2001;34(1):88–96. [Google Scholar]
- 20.HEI, Special Report 15, Health Effects of Outdoor Air Pollution in Developing Countries of Asia: A Literature Review. Health Effects Institute; Cambridge, MA: 2004. [Google Scholar]
- 21.Duan XF, Wu XG, Yu XH, Zheng RP, Li JX, Wang JH, Chen JC, Gu DF. Major causes of death among male steelworkers in Beijing: A prospective study. Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34(7):651–654. [PubMed] [Google Scholar]
- 22.Narlawar UW, Surjuse BG, Thakre SS. Hypertension and hearing impairment in workers of iron and steel industry. Indian J Physiol Pharmacol. 2006;50(1):60–6. [PubMed] [Google Scholar]
- 23.Moulin JJ, Wild P, Mantout B, Fournier-Betz M, Mur JM, Smagghe G. Mortality from Lung Cancer and Cardiovascular Diseases among Stainless-Steel Producing Workers. Cancer Causes & Control. 1993;4(2):75–81. doi: 10.1007/BF00053147. [DOI] [PubMed] [Google Scholar]
- 24.Parodi S, Baldi R, Benco C, Franchini M, Garrone E, Vercelli M, Pensa F, Puntoni R, Fontana V. Lung cancer mortality in a district of La Spezia (Italy) exposed to air pollution from industrial plants. Tumori. 2004;90(2):181–5. doi: 10.1177/030089160409000204. [DOI] [PubMed] [Google Scholar]
- 25.Biggeri A, Lagazio C, Catelan D, Pirastu R, Casson F, Terracini B. Report on health status of residents in areas with industrial, mining or military sites in Sardinia, Italy. Epidemiol Prev. 2006;30(1 Suppl 1):5–95. [PubMed] [Google Scholar]
- 26.TERI Report for World Bank No. 2001EE41, Review of past and on-going work on Urban air quality in India. Tata Energy Research Institute; New Delhi, India: 2001. [Google Scholar]
- 27.Kumar R, Sharma M, Srivastva A, Thakur JS, Jindal SK, Parwana HK. Association of Outdoor Air Pollution with Chronic Respiratory Morbidity in an Industrial Town in Northern India. Archives of Environmental Health. 2004;59(9):471–477. doi: 10.1080/00039890409603428. [DOI] [PubMed] [Google Scholar]
- 28.Johansson SAE, Campbell JL, Malmquist KG. Particle- Induced X-Ray Emission Spectrometry (PIXE) New York: Wiley; 1995. [Google Scholar]
- 29.Fletcher C, Peto R, Tinker C, Speizer FE. An eight-year study of early chronic obstructive lung disease in working man in London. The natural history of chronic bronchitis and emphysema. Oxford University Press; New York: 1965. pp. 11–189. [Google Scholar]
- 30.United States Department of Health and Human Services. Centers for Disease Control and Prevention (CDC), Epidemiology Program Office, Division of Public Health Surveillance and Informatics “Epi Info” is a trademark of the Centers for Disease Control and Prevention (CDC) 2005. http://www.cdc.gov/epiinfo/Epi6/ei6.htm.
- 31.1973. Bureau of Indian Standard: 5182, part IV, Suspended Particulate Matter.
- 32.Rose GA, Blackburn H, Gillium RF, Prineas RJ. Cardiovascular survey methods. Geneva: World Health organization; 1982. [Google Scholar]
- 33.Parr RM, Stone SF, Zeisler R. Environmental Protection: Nuclear Analytical Techniques in Air Pollution Monitoring and Research. IAEA Bulletin. 1996;38:16. [Google Scholar]
- 34.1995. Punjab State Council for Science and Technology, Chandigarh, UT, India. Punjab Environment, Status Report.
- 35.Kumar A, Sidhu P, Nautiyal J, Rautray TR, Sudarshan M, Kumar R, Singh N, Garg ML, Dhawan DK. Elemental Analysis of Aerosol Samples Collected from an Industrial and a Non-industrial Town of Punjab (India) using PIXE Technique. Journal of Environmental Science & Engineering. 2007;49:41–47. [PubMed] [Google Scholar]
- 36.Hopke PK, editor. Chemical Analysis, Receptor modeling in environment chemistry. Wiley; New York: 1985. [Google Scholar]
- 37.Mar TF, Koenig JQ, Jansen K, Sullivan J, Kaufman J, Trenga CA, Seyed H, Siahpush SH, Liu L-JS, Neas L. Fine Particulate Air Pollution and Cardiorespiratory Effects in the Elderly. Epidemiology. 2005;16:681–687. doi: 10.1097/01.ede.0000173037.83211.d6. [DOI] [PubMed] [Google Scholar]
- 38.HEI Particle Epidemiology Reanalysis Project. Revised Analyses of Time-Series Studies of Air Pollution and Health. Health Effects Institute; Cambridge, MA: 2003. [Google Scholar]
- 39.Health Aspects of Air Pollution with Particulate Matter, Ozone and Nitrogen Dioxide, Report on a WHO Working Group, Bonn, Germany. WHO, Regional Office for Europe; Copenhagen: 2003. [Google Scholar]
- 40.Goldberg MS, Burnett RT, Bailar JC, III, et al. The association between daily mortality and ambient air particle pollution in Montreal, Quebec. 2. Cause-specific mortality. Environ Res. 2001;86:26–36. doi: 10.1006/enrs.2001.4243. [DOI] [PubMed] [Google Scholar]
- 41.Molinelli AR, Madden MC, McGee JK, et al. Effect of metal removal on the toxicity of airborne particulate matter from the Utah Valley. Inhal. Toxicol. 2002;14:1069–1086. doi: 10.1080/08958370290084737. [DOI] [PubMed] [Google Scholar]
- 42.Kodavanti UP, Schladweiler MC, Ledbetter AD, et al. The spontaneously hypertensive rat as a model of human cardiovascular disease: evidence of exacerbated cardiopulmonary injury and oxidative stressfrom inhaled emission particulate matter. Toxicol Appl Pharmacol. 2000;164:250–263. doi: 10.1006/taap.2000.8899. [DOI] [PubMed] [Google Scholar]
- 43.Campen MJ, Nolan JP, Schladweiler MC, et al. Cardiovascular and thermoregulatory effects of inhaled PM-associated transition metals: a potential interaction between nickel and vanadium sulfate. Toxicol Sci. 2001;64:243–252. doi: 10.1093/toxsci/64.2.243. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organisation. Air Quality Guidelines for Europe. 2nd Edition. WHO Regional Publication; Copenhagen, Denmark: 2000. (European Series). No. 91. [PubMed] [Google Scholar]