Abstract
The expression of distinct keratin pairs during epidermal differentiation is assumed to fulfill specific and essential cytoskeletal functions. This is supported by a great variety of genodermatoses exhibiting tissue fragility because of keratin mutations. Here, we show that the loss of K10, the most prominent epidermal protein, allowed the formation of a normal epidermis in neonatal mice without signs of fragility or wound-healing response. However, there were profound changes in the composition of suprabasal keratin filaments. K5/14 persisted suprabasally at elevated protein levels, whereas their mRNAs remained restricted to the basal keratinocytes. This indicated a novel mechanism regulating keratin turnover. Moreover, the amount of K1 was reduced. In the absence of its natural partner we observed the formation of a minor amount of novel K1/14/15 filaments as revealed by immunogold electron microscopy. We suggest that these changes maintained epidermal integrity. Furthermore, suprabasal keratinocytes contained larger keratohyalin granules similar to our previous K10T mice. A comparison of profilaggrin processing in K10T and K10−/− mice revealed an accumulation of filaggrin precursors in the former but not in the latter, suggesting a requirement of intact keratin filaments for the processing. The mild phenotype of K10−/− mice suggests that there is a considerable redundancy in the keratin gene family.
INTRODUCTION
The epidermis has become a paradigm for the understanding of intermediate filament (IF) function. Its IF cytoskeleton is formed from several combinations of type I and II keratins. K5/14/15 are expressed in the basal layer, and they become sequentially replaced by K1/2e/10 in suprabasal keratinocytes during terminal differentiation (Moll et al., 1982; Fuchs and Weber, 1994). This change in keratin pattern is accompanied by an altered arrangement and a massive increase in the amount of suprabasal keratin; although basal cells present individual IF, K1/2e/10 are organized in bundles and become oriented parallel to the cell surface. This process, although not understood in molecular terms, is being taken as one indicator of a cell type–specific function of individual keratin pairs.
Keratins have a major function in providing stability to epithelial cells under conditions of mechanical stress. This is exemplified by cell fragility after keratin point mutations in human inherited keratin disorders, including epidermolytic hyperkeratosis (Cheng et al., 1992; Rothnagel et al., 1992; Yang et al., 1994), and in transgenic and knockout mice expressing dominant-negative keratin subunits (Fuchs et al., 1992; Bickenbach et al., 1996; Porter et al., 1996). Moreover, the massive cytolysis accompanying the knockout of K14 (Lloyd et al., 1995), K5 (Peters et al., 2001), K8/19 (Tamai et al., 2000), and K18/19 (Hesse et al., 2000) has demonstrated that an intact IF cytoskeleton is essential to maintain tissue integrity in the basal layer of epidermis.
Tissue integrity is normally maintained through an interaction of keratins with constituent proteins of hemidesmosomes and desmosomes (Guo et al., 1995). Biochemical and yeast 2-hybrid data have provided evidence that the latter associate predominantly with type II epidermal keratins (Kouklis et al., 1994; Meng et al., 1997), whereas the former interact with the type I keratin 14 (Geerts et al., 1999). In the upper epidermis, K1/10 become covalently cross-linked to cornified envelope proteins such as involucrin (Ming et al., 1994; Steinert and Marekov, 1995, 1997; Candi et al., 1998). Despite these well-known interactions, it is not yet known in mechanical terms how mutations in keratins or their loss lead to cytolysis. Most notably, the analysis of knockout mice for K8 and 18 demonstrated that their absence did not lead to increased tissue fragility in internal epithelia (Baribault et al., 1994; Magin et al., 1998). On the other hand, the expression of dominant-negative keratin 18 and the ablation of keratins 8/19 and 18/19 (Hesse et al., 2000; Tamai et al., 2000) were accompanied by tissue disease. Collectively, these data raise the issue of whether all keratins exert a purely structural function and how much keratin per cell is required to provide mechanical stability.
Previously, we have generated knockout mice expressing a deletion mutant of K10 (K10T), which represented a good model for epidermolytic hyperkeratosis (Porter et al., 1996; Reichelt et al., 1997). In those mice, the truncated K10 acted in a dominant-negative way and led to the accumulation of large keratin aggregates, followed by cytolysis of suprabasal keratinocytes, a strong induction of K6/16, and the perinatal death of homozygous mice. Now we have generated K10−/− mice to analyze the contribution of suprabasal keratins to the stability of the upper epidermis. We report that despite the absence of K10, which is the single most prominent protein in epidermis (Fuchs and Weber, 1994), K10−/− mice are viable and do not suffer from tissue fragility. Our data represent the first comparison of a dominant-negative keratin mutant with a complete knockout of the same keratin, generated by homologous recombination, revealing that both have completely different consequences for the stability of the concerned tissue.
MATERIALS AND METHODS
Generation of the Knockout
The 5′ flank was a 1.6-kb (–2015 to –414) fragment and the 3′ flank was a 4.3-kb EcoRI–BamHI (1595 to ∼5900) fragment of the mouse K10 gene. Both were derived from a 129 SVJ mouse genomic library (λ FIX II library; Stratagene, Heidelberg, Germany). The sequence of the K10 locus from –2578 to + 2524 was submitted to GenBank (accession no. AF245658). A lox P-flanked 2.7-kb phosphoglycerate kinase promoter-driven HPRT minigene (Porter et al., 1996) replaced 414 bp of the promoter region and exons 1 and 2 of the K10 gene (–414 to –1595). The construct was cloned in Bpt SKII+ (Stratagene) and electroporated into HM-1 cells (Magin et al., 1998). After PCR, positive clones were verified by Southern blotting, with the use of 5′ and 3′ probes as well as an HPRT probe. The targeting frequency was 8%. Correctly targeted embryonic stem cells were injected into blastocysts of BALB/c mice and returned to CBA recipients. Offspring of transgenic chimaeras were mated to BALB/c mice for further analysis. Additionally, some mice were back-crossed to yield a 129/Ola background.
Immunofluorescence and Electron Microscopy
For conventional electron microscopy (EM) of skin samples, see Bussow (1978) and Porter et al. (1996). For processing of cryosections, see Reichelt et al. (1999). Primary antibodies were anti-K6 (693-1), 1:1000; anti-K10 (LH2), undiluted; anti-K5 and anti-K1 (AF138 and AF109; Babco, Richmond, CA), 1:5000; anti-K15 serum, 1:200; and anti-K17 serum, 1:1000 (McGowan and Coulombe, 1998b). Secondary antibodies were Texas Red–coupled goat anti-mouse immunoglobulin G1 (Southern Biotechnology Associates, Birmingham, AL) and Alexa 594-coupled goat anti-rabbit (Molecular Probes, Eugene, OR). For immunogold EM, 4-μm sections on coverslips were fixed for 10 min with acetone at –20°C, permeabilized with 0.3% Triton-X 100, and after a short rinse with PBS, incubated for 2 h with antibodies against K1 (8.60; Sigma, Deisenhofen, Germany; 1:5000) and against K14 (guinea pig serum, 1:1000). After 3 washes with PBS, sections were incubated overnight with secondary antibodies coupled to 5- or 10-nm gold particles for double staining and with nanogold-coupled antibodies for single staining. Silver enhancement for the nanogold probes and fixation and embedding in Epon were carried out as described previously (Rose et al., 1995).
Northern Blotting and In Situ Hybridization
For Northern blot analysis, trunk skin was obtained from neonatal mice and immediately frozen in liquid nitrogen. RNA was isolated with TRIzol (Life Technologies, Karlsruhe, Germany). Fifteen micrograms of RNA were loaded per lane. For processing of gels and hybridization, see Reichelt et al. (1999). Probes for mouse K1, 5, 10, and 14 were derived from the 3′-noncoding regions (K5, laboratory of T.M.M.; K1, 10, and 14, kind gifts from H. Winter, German Cancer Research Centre, Heidelberg, Germany). Quantitative analysis was performed with Image Master VDS software (Amersham Pharmacia Biotech, Freiburg, Germany). The ribosomal RNA from ethidium bromide–stained gels was compared with that of the mRNA from the respective autoradiographs.
In situ hybridization was performed with the use of RNA probes derived from 3′-noncoding sequences from K5 and 14. Probes were labeled with biotin-16-UTP (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer's instructions (RNA polymerases and ribonuclease inhibitor, Fermentas, St. Leon-Rot, Germany). Five-micrometer cryosections of neonatal back skin were placed on Superfrost slides (Menzel-Gläser, Braunschweig, Germany), air dried, and fixed with 4% paraformaldehyde (in PBS) for 20 min. Sections were washed 2 times for 5 min each with PBS and then blocked for 10 min with 0.1 M triethanolamine (Sigma; 2.7 ml triethanolamine, 200 ml double-distilled water, 0.33 ml HCl, and 533 μl acetic anhydride) followed by 2 washes with PBS for 5 min each. Prehybridization was performed with 50 μl of hybridization solution (0.3 M NaCl, 5 mM EDTA, 20 mM Na-phosphate, 20 mM Tris, pH 6.8, 50% deionized formamide [ultrapure, Merck, Darmstadt, Germany], 5% dextran sulfate, 1× Denhardt's, 10 mM DTT, 0.5 mg/ml yeast tRNA, and 100 μg/ml salmon sperm DNA) per section. After 1 h at 42°C, hybridization solution was replaced by 25 μl of fresh hybridization solution containing 250 ng biotin-labeled probe. A coverslip was placed on top, and the probes were heated for 5 min at 90°C before they were allowed to hybridize for 16 h at 42°C. The sections were then washed briefly with 2× SSC (prepared from a 20× stock: 3 M NaCl and 0.3 M Na citrate, pH 7.0) until the coverslips had come off, and then 30 min with 2× SSC, 50% formamide, and 20 mM DTT and another 30 min with 1× SSC, 50% formamide, and 20 mM DTT both at 50°C, followed by a 5-min wash with 1× SSC and 0.1% SDS at ambient temperature. Sections were incubated for 30 min at 37°C with 1× SSC containing 20 μg/ml RNase A (Roche Molecular Biochemicals) and afterward were washed for 30 min with 0.5× SSC, 50% formamide, and 20 mM DTT at 50°C. The slides were then washed 2 times with PBS for 5 min at ambient temperature and incubated for 30 min with streptavidine-alkaline phosphatase (Dako, Hamburg, Germany). After that, they were washed 2 times with PBS as before and incubated for 5 min in alkaline phosphatase buffer (100 mM Tris-HCl, pH 9.5, 100 mM NaCl, and 50 mM MgCl2). Finally, 5-bromo-4-chloro-3-indolyl phosphate–nitro blue tetrazolium substrate solution (Dako) was added. After 30 min, the reaction was terminated by washing with double-distilled water, and sections were embedded in Mowiol (Calbiochem, Schwalbach, Germany).
Two-dimensional Gel Electrophoresis and Western Blotting
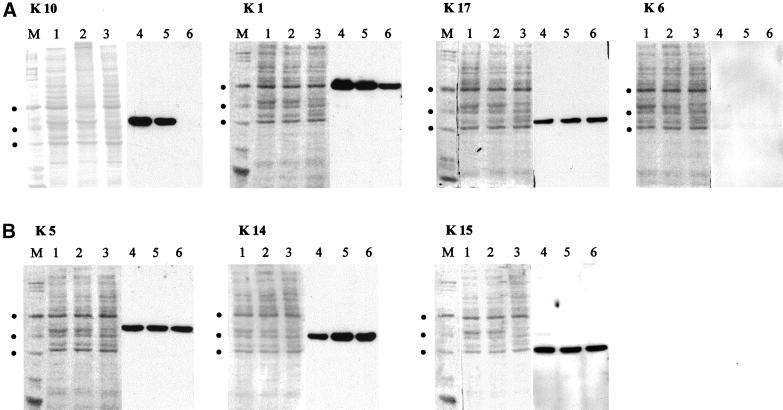
Trunk skin was prepared from neonatal mice and immediately frozen in liquid nitrogen. For SDS-PAGE, total protein extraction, separation and electrotransfer, see (Reichelt et al., 1999). For 2-dimensional gel electrophoresis of keratin complexes, protein extracts enriched in cytoskeletal proteins were prepared from neonatal trunk skin (Hatzfeld and Franke, 1985). The resulting pellet was resuspended in isoelectric focusing sample buffer as before (Magin et al., 1998), except that 5.5 M urea was used. This extract was dialyzed with the use of collodion bags (Sartorius, Göttingen, Germany) to urea concentrations up to 9.5 M. The samples were first separated by isoelectric focusing at the respective urea concentration and subsequently by SDS-PAGE. Western blotting was performed as described before (Reichelt et al., 1999). The composition of ampholines was 0.8% of pH 4–6 and pH 5–7 and 0.4% of pH 3–10 (Amersham Pharmacia Biotech). Primary antibodies were diluted as follows: anti-K10 (LH 2), 1:1000; anti-K1 (AF109), 1:400,000; anti-K5 (AF138), 1:100,000; anti-K6, 1:10,000; anti-K15, 1:5000; anti-K17, 1:1000; anti-K 14, 1:50,000; and anti-filaggrin (AF111), 1:10,000 (AF109, AF138, and AF111, Babco). Before application of antibodies, polyvinylidene difluoride membranes were stained with Coomassie blue and photographed.
Dye Penetration Assay
The assay was used before by Hardman et al. (1998) on mouse embryos. Neonatal mice were killed by ether inhalation and then taken up and down a methanol series (25, 50, 75, and 100% methanol, 1 min each), equilibrated for 1 min in PBS, and subsequently stained with 0.2% toluidine blue (in water) for 5 min. Mice were rinsed 3 times for 1 min in 90% ethanol. After a brief wash in water, they were embedded in 0.4% agarose and immediately photographed.
RESULTS
Compensation by Basal Keratins 5, 14, and 15 Prevents Cytolysis and Permits Normal Epidermal Differentiation in K10–/– Mice
In accordance with their well-documented cytoskeletal function, keratins 10 and 1 are the most abundant proteins in epidermis, representing ∼60% of total protein (Fuchs and Green, 1980). In accordance with previous K10 transgenic and knockout mice (Fuchs et al., 1992; Bickenbach et al., 1996), we expected neonates to display a fragile epidermis. Surprisingly, all K10−/− mice analyzed so far were born at the expected Mendelian ratio, were fully viable, and showed no obvious skin defect resulting from birth stress. Histological analysis of neonatal skin revealed normal stratification and an unaltered number of epidermal cell layers, although we noticed a slight increase in granular cell size and the size of keratohyalin granules and a loose appearance of the stratum corneum (Figure 1B). Most importantly, we detected no cytolysis in K10−/− epidermis, suggesting that filaments consisting of K10 and 1 are not essential for maintaining the integrity of suprabasal epidermis in these mice.
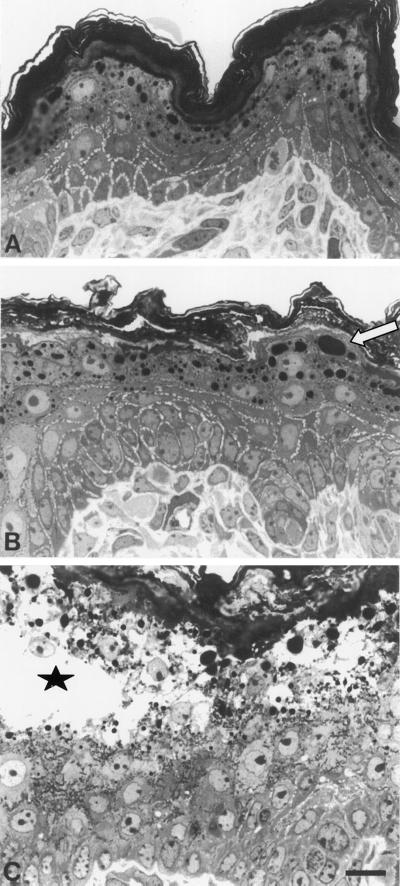
Figure 1.
Semithin sections of mouse back skin. Comparison of K10−/− (B) with wild-type (A) mice revealed a normal differentiation in the K10−/− animal. In the granular layer of K10−/− mice, cell size was increased, but the number of cell layers was identical in both genotypes. We noted an increase in the size of keratohyalin granules (B, arrow) in K10−/− mice. The stratum corneum appeared irregular compared with that of the wild-type mice. In contrast to homozygous neonatal K10T mice (C, asterisk), which carry the dominant-negatively acting K10T, the K10−/− epidermis did not show any sign of cytolysis (B). In K10T skin the massive suprabasal accumulation of keratin aggregates was clearly visible (C, dark matter), whereas K10−/− keratinocytes exhibited a clear cytoplasm (B). Bar, 10 μm.
Next, we performed immunofluorescence (Figures 2 and 3), Northern blotting (Figure 4), and Western blotting (Figure 5), all confirming the absence of K10 in all epidermal strata of the knockout mice. The distribution of K1 was unaltered, although immunofluorescence (Figure 2) and Western blotting demonstrated a noticeable reduction in homozygotes (Figure 5A) and a milder reduction in heterozygotes (Figure 5A), in agreement with immunofluorescence data (see Figure 2). This prompted us to investigate whether K1 remained as a single keratin or formed complexes with another partner in vivo. To investigate whether other keratins or IF proteins were expressed in K10−/− mice as a means to stabilize the epidermis, we examined the expression patterns of potential candidate skin keratins (K2e, 5, 6, 9, 14, 15, 16, and 17), of keratins specific for internal epithelia (K8, 18, and 19) and of vimentin by immunofluorescence staining. Most of these IF proteins were either not expressed or not up-regulated (our unpublished data). Surprisingly, the distribution of K5, 14, and 15 was altered in the epidermis of K10−/− mice. In comparison with normal epidermis, K10−/− mice showed an increased staining throughout the suprabasal epidermis up to the uppermost granular layer (Figure 3). As judged by immunofluorescence, all 3 keratins were expressed at similar levels. Western blotting, however, revealed an increase in K14 but not in K5 or 15 (Figure 5B). This result suggested that the synthesis of various epidermal keratins is regulated individually. To get an insight into at which level the expression of keratins was regulated, we performed Northern blotting. This demonstrated that the mRNAs of K1 and 5 remained unaltered, whereas that of K14 was increased (Figure 4A). Quantitative analysis of the mRNA levels showed that the increase in K14 was ∼36%, whereas the amount of K1 and 5 remained unaltered. Remarkably, neither K14 nor K5 mRNA was expressed suprabasally but remained exclusively restricted to the basal epidermal layer, as indicated by in situ hybridization (Figure 4B). At present, our data do not allow us to discriminate whether the increase in K14 mRNA results from increased transcription or increased stability. The persistent suprabasal expression of the K14 protein suggests that the half-life of K14 is increased in K10−/− mice.
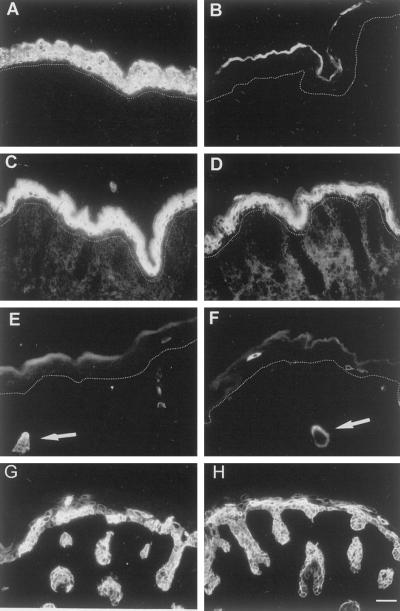
Figure 2.
Immunofluorescence analysis of suprabasal keratins. The loss of K10 in the knockout mice (B) was confirmed (A, wild-type) and the suprabasal K1 expression was clearly reduced in in the K10−/− mice (D) compared with the wild-type mice (C). K6 expression was not induced in K10−/− (F) but showed the normal expression pattern of wild-type skin in hair follicles (E and F, arrow) as well as in rare single interfollicular cells. K17 expression was patchy in both wild-type (G) and K10−/− (H) epidermis. The dotted line in A–F marks the basal membrane, ignoring hair follicles. Bar, 64 μm.
Figure 3.
Immunofluorescence analysis of basal keratins. We noted a suprabasal increase in the basal cell keratins K5 (A and B), K14 (C and D), and K15 (E and F). In the wild-type mice, these keratins are predominantly found in the basal layer (A, C, and E), whereas the knockout had a significant suprabasal persistence of these keratins (B, D, and F). Bar, 64 μm.
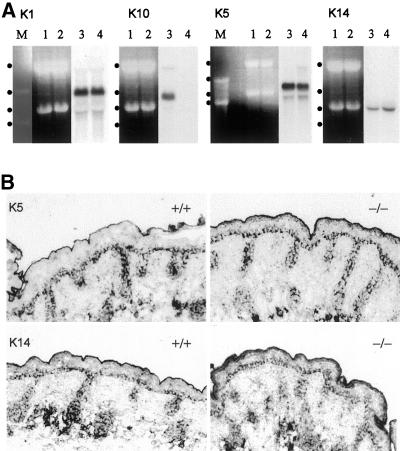
Figure 4.
K14 mRNA is increased, but it remains restricted to basal cells. (A) Northern blot analysis revealed that K1 and 5 expression were unaltered in K10 knockout mice (−/−), whereas K14 mRNA was increased by 36%. In addition, the complete loss of K10 in the knockout mice was confirmed. Quantitation was performed by densitometric measurement. Lanes M, marker; lanes 1 and 2, ribosomal RNA; lanes 3 and 4, corresponding autoradiograph; dots from top to bottom, 4.4, 2.9, 1.9, and 1.5 kb. Northern blots for K1, 10, and 14 were derived from the same gel. (B) In situ hybridization showed that in K10−/− epidermis, both K5 and 14 mRNA remained restricted to basal keratinocytes.
Figure 5.
Western blot analysis of epidermal keratins. (A) The loss of K10 expression in K10−/− epidermis was confirmed by Western blotting, and its reduced expression in heterozygote skin (+/–) was shown. Note a remarkable decrease in K1 in K10−/− pups and a slight decrease in the skin of heterozygotes. (B) The amount of K5 remained unaltered, whereas its partner K14 was slightly increased. The amount of the third basal keratin, K15, was unaltered. Equal loading was verified by quantitative comparison of Coomassie blue staining. Lanes 1–3, Coomassie blue–stained polyvinylidene difluoride membrane; lanes 4–6, corresponding Western blot; M, marker; dots from top to bottom, 66.4, 55.6, and 42.7 kDa.
The induction of K6, 16, and 17 in interfollicular epidermis is taken as one of the most sensitive indicators of an altered epidermal differentiation program as in hyperproliferation or wound healing (Coulombe, 1997; McGowan and Coulombe, 1998a). Remarkably, antibody staining revealed no differences between wild-type and K10−/− littermates as shown for K6 and 17 (Figure 2). These findings imply that the presence of keratins 5 and 14 in suprabasal cells as well as K15 and 17 are able to compensate for the absence of K1/10 IF in neonatal K10−/− mice. We cannot exclude that other, yet unknown mechanisms contribute to the integrity of the epidermis in K10−/− mice.
Ultrastructural and Biochemical Analysis Reveal Unexpected Properties of Epidermal Keratins
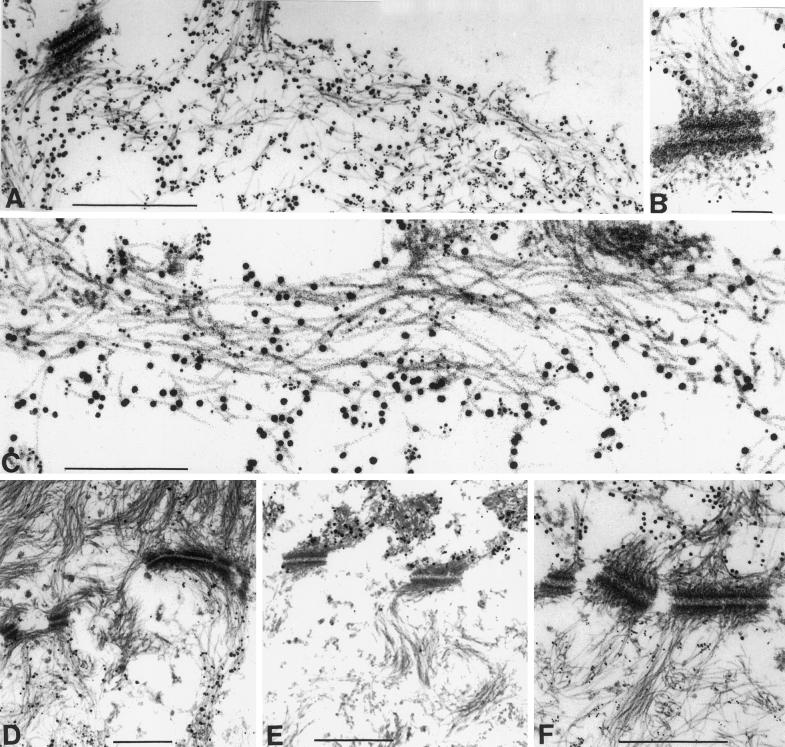
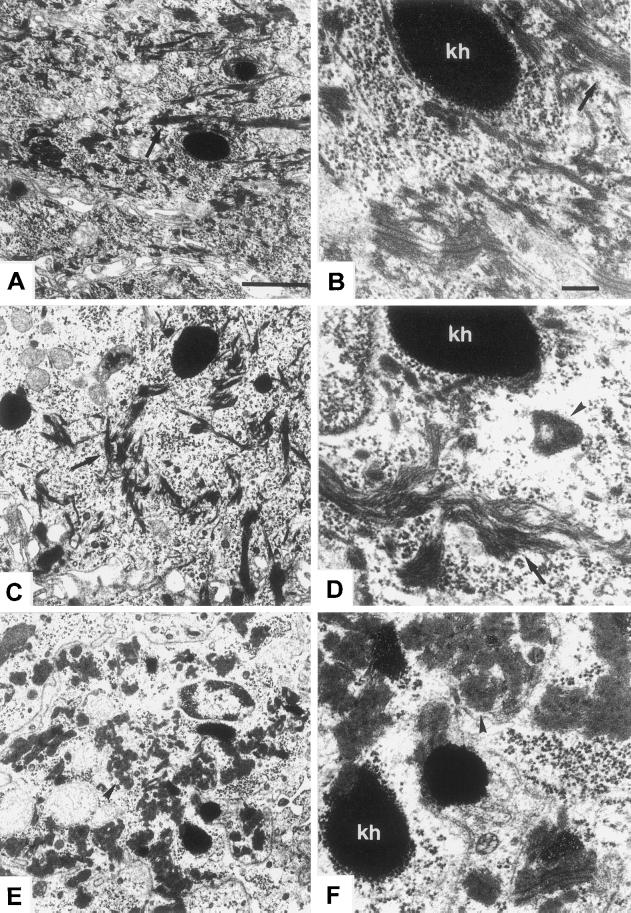
Normally, the change in keratin expression from basal keratins 5 and 14 to those indicating terminal differentiation, i.e., K1 and 10, is accompanied by a reorganization from a loosely bundled array of individual filaments in basal cells to a bundling of IF resulting in the “keratin pattern” typical of epidermis (see Figure 7D). EM revealed the presence of IF in suprabasal epidermis of K10−/− mice (Figure 6C) and normal desmosomes (our unpublished data). The former were very similar to wild-type IF (Figure 6A) with regard to their distribution but also exhibited the bundling otherwise typical of K1/10, although they predominantly consisted of K5/14 IF (Figure 6, B and D). Apart from normal filaments, we detected small, isolated keratin aggregates in rare granular cells (Figure 6D, arrow), which resembled those large abundant aggregates typical of those previously described in K10T mice (Figure 6, E and F). We suspect that in K10−/− mice, these consisted of residual K1, which might either assemble into novel IF with the type I keratin 14 or form aggregates on its own. Immunogold EM with the use of antibodies directed against K1 and 14 confirmed the rare occurrence of suprabasal filament bundles, which contained both keratins (Figure 7, A–C). Additionally, we found that these filaments were able to attach to desmosomes (Figure 7B). K1/14 filaments were never detected in wild-type epidermis. They constituted only a small amount of the suprabasal IF network in K10−/− mice, indicating that the major part consisted of K5/14 filaments.
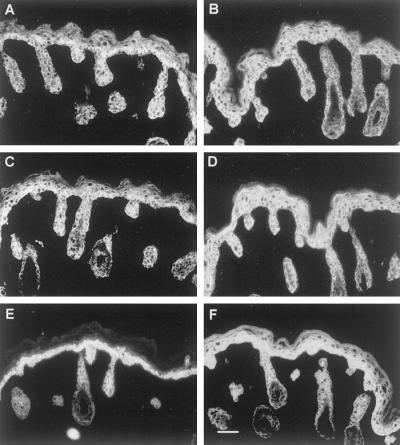
Figure 7.
Immunogold EM revealed the formation of K1/K14 filaments in K10−/− mice. (A–C) IFs in the granular layer of K10−/− epidermis. (D–F) Basal–suprabasal transition zone of wild-type epidermis, with the basal cell at the bottom. Desmosomes mark the level of the cell membranes, which have been lost upon fixation. (A) Survey of keratin bundles, which were labeled with both K1 and 14 antibodies. Filaments that were composed of K1 and 14 were also found attached to desmosomes (B). (C) Higher magnification of a filament bundle showing that K1 and 14 were in close proximity in the filaments. In single-antibody–labeling experiments in wild-type skin, K14 was detected in the basal epidermal layer (D), whereas K1 was exclusively found in subrabasal cells (E). This was confirmed in a double-labeling experiment with both antibodies (F). K1, 10-nm gold particles; K14, 5-nm gold particles. Bars: A and D–F, 0.5 μm; B, 0.1 μm; C, 0.24 μm.
Figure 6.
Ultrastructure of K10−/− epidermis. The granular layer of K10−/− epidermis maintained the typical content and distribution of IF bundles (C, arrow, survey EM). Higher magnification shows the regular shape of these filament bundles (D, arrow). For comparison, see survey micrograph of the wild type in A and a higher magnification in B (arrows on filament bundles) and the completely different setting in K10T mice, where the cytoplasm of granular layer cells was filled with a large amount of keratin aggregates (E, arrows, survey; F, details). The bundling otherwise typical of K1/10 was also noted for K5/14 in granular cells. Interestingly, in a few cells, we noted small keratin aggregates in the knockout epidermis (D, arrowhead), which were absent in wild-type littermates. These small aggregates closely resembled the aggregates observed in K10T mice (E and F, arrowheads). There were no signs of epidermal cytolysis in K10−/− neonates. Bars: A (also valid for C and E), 1 μm; B (also valid for D and F), 0.25 μm; kh, keratohyalin.
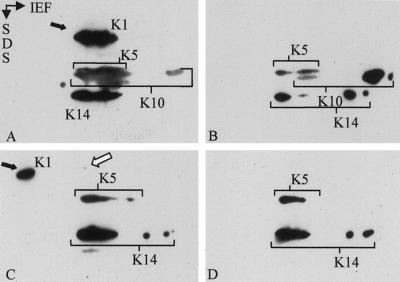
Previous data have shown that the stability of individual keratins depended on the expression of a partner keratin (Kulesh et al., 1989; Lersch et al., 1989). Given the persistence of K1 as demonstrated by IF and Western and Northern blotting, we examined whether K1 might become stabilized in the suprabasal epidermis by complex formation with the suprabasally increased K14. To that end, cytoskeletal keratin preparations were isolated from wild-type and K10−/− mice and dissolved in 4 M urea, which has been demonstrated to maintain oligomeric building blocks of keratins (Franke et al., 1983; Hatzfeld and Franke, 1985). Dialysis of in vivo keratin complexes against increasing concentrations of urea leads to the dissociation of individual keratin complexes. At appropriate urea concentrations, keratin complexes can be visualized by isoelectric focusing followed by SDS-gel electrophoresis (Franke et al., 1983; Hatzfeld and Franke, 1985). In accordance with previous data (Hatzfeld and Franke, 1985; Coulombe and Fuchs, 1990), K1, 5, 10, and 14 migrated in their authentic complex at 5.5 M urea in wild-type samples (Figure 8A). At higher urea concentrations, complexes dissolved, and keratins migrated to their individual isoelectric points (Figure 8B). In extracts from K10−/− mouse skin, most of K1 had already moved to its isoelectric point at 5.5 M urea, indicating the absence of its type II keratin partner K10 (Figure 8C). A minor portion, however, resided in a complex position together with K14 (white arrow). This indicates that in the absence of its natural partner, K1 can in part form novel heteromeric keratin complexes. These biochemical data are in agreement with immunogold EM findings and support the classical “promiscuity” concept of keratins (Hatzfeld and Franke, 1985).
Figure 8.
Two-dimensional gel electrophoresis of keratin complexes and subsequent Western blotting revealed that only a minor portion of the residual K1 formed filaments with K14. Keratin complexes were extracted from neonatal epidermis and resuspended in 5.5 M urea (A and C). To visualize keratins, the blots shown were incubated with a mixture of corresponding antibodies. At 5.5 M urea, the keratins of wild-type extracts still formed complexes with their partners and migrated at the complex-specific isoelectric point in the first dimension (A; IEF). Note that all keratins migrated at the same isoelectric position. Although K5 and 14 behaved in K10−/− null extracts (C), as in the control (A), and were exclusively migrating as a complex, most of K1 focused at its own basic IP (C, black arrow). Only a small amount of K1 (white arrow) was observed at the complex-specific IP. Dialysis to 6.5 M urea (B and D) resulted in almost complete dissociation of K1/K10 in the wild-type epidermis (exemplified by K10) and partial dissociation of K5/K14 complexes in both wild-type (B) and K10−/− (D) epidermis.
Altered Processing of Profilaggrin in K10T but Not in K10−/− Mice
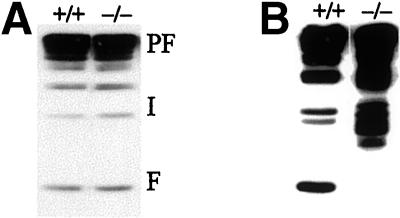
In contrast to the strong increase in keratohyalin, which we previously described in our K10T mice (Porter et al., 1996), K10−/− mice showed only a mild increase in the size of granules in the stratum granulosum. Although Western blot analysis revealed a normal amount of profilaggrin and filaggrin, similar to that of wild-type mice (Figure 9B), K10T mice showed an impairment of profilaggrin processing and an accumulation of the major filaggrin precursors (Figure 9B). As judged by its size, profilaggrin is processed down to the penultimate stage, i.e., a dimer, but is not cleaved to the monomer stage. This is compatible with a model suggesting that the final proteolytic cleavage requires the presence of intact keratin filaments.
Figure 9.
Normal profilaggrin processing in neonatal K10−/− epidermis. In contrast to the impairment of profilaggrin processing in K10T neonates (B), neither the amount of profilaggrin (PF) nor that of its processing intermediates (I) or mature filaggrin (F) was altered in neonatal K10−/− mice (A).
Barrier Formation in K10−/− Mice
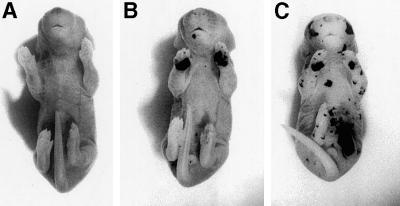
Finally, we examined whether K10−/− mice formed a normal epidermal barrier with the use of an assay described before (Hardman et al., 1998). When homozygous K10T mice (Porter et al., 1996) were analyzed, the previously reported barrier defect (Reichelt et al., 1999) was confirmed here in a dye penetration assay (Figure 10C). The epidermis showed multiple lesions at different sites and was highly susceptible to mechanical trauma. In contrast, K10−/− mice displayed a well-developed epidermal barrier all over the body, with the exception of the forepaw sole (Figure 10B). Here, a transient barrier disruption became apparent. After 2–3 d, however, the barrier recovered and did not seem to retard the normal development of the pups.
Figure 10.
Transient barrier defect of forepaw sole epidermis in K10−/− neonates. During the dye perfusion assay, the skin of control neonates did not take up the color (A), whereas the forepaw sole skin of K10 null neonates was stained (B). K10T neonates were much more affected than K10 null mice, showing dye penetration at multiple body sites (C).
DISCUSSION
How Little Keratin Is Enough to Maintain an Intact Epidermis?
In conjunction with previously established gene-targeted mice expressing a dominant-negative K10 mutation (K10T; Porter et al., 1996), to our knowledge, the present study provides the first comparison with the use of gene targeting of partial and complete IF protein deletions in the same tissue. The observation that K10−/− mice survived after birth and displayed a normal epidermis with all the hallmarks of terminal differentiation challenges some established beliefs and may be of significance for the treatment of dominant skin disorders, including epidermolytic hyperkeratosis.
Most importantly, K10−/− mice exhibited an epidermis stable enough to withstand the mechanical demands of birth and the stress exerted by the mother's care. This was obviously due to the suprabasally extended expression of K5/14/15, although being less abundant than K1/K10 filaments normally would be. Of note, K6, 16 and 17, three of the most sensitive indicators of a disturbed epidermal homeostasis (Weiss et al., 1984; McGowan and Coulombe, 1998a), were not induced in our mice. Because these keratins are otherwise detected in the interfollicular epidermis after wounding or in hyperproliferative conditions, their regular expression pattern in K10−/− neonates suggests a normal state of the epidermis. Although McGowan and Coulombe (1998a) described K17 expression as absent from the interfollicular epidermis, our mice showed patchy expression all over the epidermis. Because their mice had a genetic backgound different from those used in this study, we suggest that strain-specific differences account for the distinct K17 expression patterns.
Taking into consideration that no other keratins were induced and that epidermal thickness remained unaltered in K10−/− mice, we estimate that the amount of keratin per cell was reduced by more than half. Preliminary data indicate that this amount is also sufficient to sustain an intact, although slightly hyperkeratotic, epidermis in adult K10−/− animals. Our finding that the loss of K10 is not accompanied by extensive tissue damage is supported by the recent description of a K14 patient who represents the closest to a human knockout. This patient had a mild recessive form of epidermolysis bullosa simplex, probably caused by compensation of K14 by K15 (Batta et al., 2000). Collectively, results from K10 (this study), K18 (Magin et al., 1998), and K19 (Tamai et al., 2000) knockout mice and from the above-described patient demonstrate that the loss of a given keratin is far less detrimental than the expression of a mutant one, provided that compensation by another keratin of the same subfamily can take place. If this fails, loss of the keratin cytoskeleton prevails, leading to extensive tissue damage (Hesse et al., 2000; Peters et al., 2001).
How Specific Are Keratins in their Constituent Compartments?
Although our replacement experiment is one of nature, arguing that the K5/14 pair can complement K1/10 in suprabasal epidermis, experimental substitutions of a single keratin have been performed in the basal epidermis. In K14−/− mice, the highly related K16, a K16/14 hybrid, or the simple epithelial K18 did not fully rescue the phenotype of the knockout mice (Hutton et al., 1998; Paladini and Coulombe, 1999). Although the ectopically expressed proteins formed IF with the endogenous K5, the resulting filaments were possibly too weak to withstand mechanical stress, leading to a skin phenotype in those mice. To resolve the question of whether keratins have cell-type–specific functions or can complement each other, as suggested in certain internal epithelia (Magin et al., 1998), more refined experiments need to be performed. Our data suggest that even epithelia exposed to considerable stress, such as epidermis, do function with another set of keratins. Despite the fact that overall sequence identities between the type II keratins 1 and 5 and the type I keratins 10 and 14 were only 66 and 58%, respectively, both keratin sets contain sequence motifs typical of epidermal keratins in their head and tail domains. Therefore, we propose that, in functional terms, both the keratin type and the amount of keratin per cell do matter. This is supported by the phenotype of patients carrying functional K14 null alleles (Chan et al., 1994; Rugg et al., 1994; Jonkman et al., 1996; Batta et al., 2000), which is far less severe than that of patients with point mutations (Corden and McLean, 1996). Increasing the level of endogenous keratins by pharmaceutical intervention in the appropriate compartment of the epidermis might be a useful therapeutic approach for keratin disorders.
Fundamental Differences between Knockout Mice of Type I and II IF Proteins
In contrast to a former hypothesis established from cell transfection studies, which stated that individual keratins were unstable (Kulesh et al., 1989), a considerable amount of K1 persisted without its partner in K10−/− mice. Our analysis of keratin complexes has supported the view that most of keratin 1 remained stable without any other keratin and that a small part was able to form filaments with K14. This corresponded well with the presence of type I keratins in K5−/− mice (Peters et al., 2001) and K8 in K18−/− mice without a partner keratin (Magin et al., 1998), although the converse was not reported in K8−/− animals (Baribault et al., 1994). On the other hand, K13 was still detectable in esophagus extracts from K4−/− mice (Ness et al., 1998). The relative instability of some type I IFs against proteases is in agreement with the caspase-mediated cleavage of K18 at the linker L1/2 sequence VEVD/A (Caulin et al., 1997; Ku et al., 1997). It will be interesting to see whether individual keratins are stable per se, or whether other proteins (for candidates, see Nicholl and Quinlan, 1994) are involved. Moreover, the fact that neonatal K10−/− mice did not have cytolysis and were basically free of IF aggregates, whereas K10T knockout mice (Porter et al., 1996) and keratin transgenic mice (Fuchs et al., 1992) had extensive tissue damage raises again the issue of whether IF aggregates in general are involved in tissue disease or represent a byproduct (Eyer et al., 1998).
In view of other keratin knockout mice (for review, see Magin et al., 2000) and patients (Corden and McLean, 1996), the normal appearance of K10−/− mice reported here supports the hypothesis that type I and II IF proteins are functionally different and that the deletion of type I is less damaging than that of type II keratins. In the epidermis, this could be due to the interaction of desmoplakin and cornified envelope proteins, which preferentially includes type II keratin interactions (Kouklis et al., 1994; Steinert and Marekov, 1995; Meng et al., 1997). Formal proof of this hypothesis has to await additional knockout mouse studies.
Altered Profilaggrin Processing in K10T but Not in K10−/− Mice
Filaggrin is a keratin-associated protein typical of mammalian epidermis (Harding and Scott, 1983). After its initial synthesis in granular keratinocytes, where its high molecular weight precursor profilaggrin is aggregated in insoluble keratohyalin granules, it becomes subsequently processed to functional filaggrin (Resing et al., 1984). Monomeric filaggrin is supposed to bundle keratin filaments via interaction between keratin rods and β-turn repeat motifs present in all mammalian filaggrins (Mack et al., 1993). Although previous studies have characterized the proteases and phosphatases involved in profilaggrin processing (Resing et al., 1984, 1989; Haugen-Scofield et al., 1988; Kam et al., 1993), the comparison of K10−/− and K10T mice has drawn attention to the subcellular topology of this process. We suggest that the penultimate cleavage leading to the release of the filaggrin monomer depends on the presence of intact keratin filaments. In keeping with in vitro–binding studies (Dale et al., 1978, 1989; Steinert et al., 1981), these can be formed not only by K1/10 but also by K5/14. The presence of keratin aggregates per se does not prevent profilaggrin processing, at least in humans (Ishida-Yamamoto et al., 1994). In patients with epidermolytic hyperkeratosis, an increase in profilaggrin, the processing of intermediates, and accumulation of filaggrin have been described (Ishida-Yamamoto et al., 1994). K10T mice, although they display keratin aggregates similar to patients, differ significantly with respect to keratin mutations in humans. Although the latter have keratin point mutations, K10T mice only express a head–coil 1A fragment (Porter et al., 1996), which is unable to form higher-order structures. Therefore, our results suggest that the last stage of profilaggrin processing requires interaction with keratin hetero-oligomers. This would be in agreement with recent data from transfection studies with filaggrin deletion constructs. These revealed that significant binding of filaggrin to keratin only occurred after disappearance of the granular profilaggrin morphology (Kuechle et al., 1999). It cannot be excluded, however, that the extent of cytolysis in K10T mice has an impact on the final stages of profilaggrin processing.
In conclusion, we have demonstrated that the deletion of K10, which is the most abundant epidermal protein, does not lead to epidermal fragility or to the up-regulation of the hyperproliferative keratins 6 and 17. The increased size of granular cells might be a sign that K5/14 do not fully replace K1/10 in suprabasal keratinocytes. Given that a lower amount of keratin seems sufficient to maintain an epidermis, these findings suggest that K1/10 might have additional functions. In the future, it will be possible to address additional issues, including wound healing, in those mice.
ACKNOWLEDGMENTS
This work is dedicated to Werner W. Franke on the occasion of his 60th birthday. We are grateful to T. Schwaluk for blastocyst injections and mouse care, R. Meier-Bornheim for help with 2-dimensional gels, and M. Lindemann for help with EM analysis. We thank M. Blessing, Johannes-Gutenberg-University, Mainz, Germany (K6 antiserum); P. Coulombe, Johns Hopkins University, Baltimore, MD (K17 antiserum); E. Fuchs, Howard Hughes Medical Institute, Chicago, IL (K15 antiserum); L. Langbein, German Cancer Research Centre, Heidelberg, Germany (K14 antiserum); and I. Leigh, Medical College of the Royal London Hospital, London, United Kingdom (LH2) for gifts of antibodies. This work was supported by Deutsche Forschungsgemeinschaft grant SFB 284; C7, a grant from the Fonds der Chemischen Industrie (to T.M.M.), and the Bonner Forum Biomedizin.
Abbreviations used:
- EM
electron microscopy
- IF
intermediate filament
REFERENCES
- Baribault H, Penner J, Iozzo RV, Wilson HM. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 1994;8:2964–2973. doi: 10.1101/gad.8.24.2964. [DOI] [PubMed] [Google Scholar]
- Batta K, Rugg EL, Wilson NJ, West N, Goodyear H, Lane EB, Gratian M, Dopping-Hepenstal P, Moss C, Eady RA. A. keratin 14 “knockout” mutation in recessive epidermolysis bullosa simplex resulting in less severe disease. Br J Dermatol. 2000;143:621–627. doi: 10.1111/j.1365-2133.2000.03722.x. [DOI] [PubMed] [Google Scholar]
- Bickenbach JR, Longley MA, Bundman DS, Dominey AM, Bowden PE, Rothnagel JA, Roop DR. A transgenic mouse model that recapitulates the clinical features of both neonatal and adult forms of the skin disease epidermolytic hyperkeratosis. Differentiation. 1996;61:129–139. doi: 10.1046/j.1432-0436.1996.6120129.x. [DOI] [PubMed] [Google Scholar]
- Bussow H. Schwann cell myelin ensheathing CNS axons in the nerve fiber layer of the cat retina. J Neurocytol. 1978;7:207–214. doi: 10.1007/BF01217919. [DOI] [PubMed] [Google Scholar]
- Candi E, Tarcsa E, DiGiovanna JJ, Compton JG, Elias PM, Marekov LN, Steinert PM. A highly conserved lysine residue on the head domain of type II keratins is essential for the attachment of keratin intermediate filaments to the cornified cell envelope through isopeptide crosslinking by transglutaminases. Proc Natl Acad Sci USA. 1998;95:2067–2072. doi: 10.1073/pnas.95.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulin C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138:1379–1394. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y, Anton LI, Yu QC, Jackel A, Zabel B, Ernst JP, Fuchs E. A human keratin 14 “knockout”: the absence of K14 leads to severe epidermolysis bullosa simplex and a function for an intermediate filament protein. Genes Dev. 1994;8:2574–2587. doi: 10.1101/gad.8.21.2574. [DOI] [PubMed] [Google Scholar]
- Cheng J, Syder AJ, Yu QC, Letai A, Paller AS, Fuchs E. The genetic basis of epidermolytic hyperkeratosis: a disorder of differentiation-specific epidermal keratin genes. Cell. 1992;70:811–819. doi: 10.1016/0092-8674(92)90314-3. [DOI] [PubMed] [Google Scholar]
- Corden LD, McLean WH. Human keratin diseases: hereditary fragility of specific epithelial tissues. Exp Dermatol. 1996;5:297–307. doi: 10.1111/j.1600-0625.1996.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Coulombe PA. Towards a molecular definition of keratinocyte activation after acute injury to stratified epithelia. Biochem Biophys Res Commun. 1997;236:231–238. doi: 10.1006/bbrc.1997.6945. [DOI] [PubMed] [Google Scholar]
- Coulombe PA, Fuchs E. Elucidating the early stages of keratin filament assembly. J Cell Biol. 1990;111:153–169. doi: 10.1083/jcb.111.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale BA, Holbrook KA, Steinert PM. Assembly of stratum corneum basic protein and keratin filaments in macrofibrils. Nature. 1978;276:729–731. doi: 10.1038/276729a0. [DOI] [PubMed] [Google Scholar]
- Dale BA, Resing KA, Haydock PV, Fleckman P, Fisher C, Holbrook KA. Intermediate filament associated protein of epidermis. In: Rogers GE, Reis PJ, Ward KA, Marshall RC, editors. The Biology of Wool and Hair. London, United Kindgom: Chapman and Hall; 1989. pp. 97–116. [Google Scholar]
- Eyer J, Cleveland DW, Wong PC, Peterson AC. Pathogenesis of two axonopathies does not require axonal neurofilaments. Nature. 1998;391:584–587. doi: 10.1038/35378. [DOI] [PubMed] [Google Scholar]
- Franke WW, Kapprell HP, Mueller H. Isolation and symmetrical splitting of desmosomal structures in 9 M urea. Eur J Cell Biol. 1983;32:117–130. [PubMed] [Google Scholar]
- Fuchs E, Esteves RA, Coulombe PA. Transgenic mice expressing a mutant keratin 10 gene reveal the likely genetic basis for epidermolytic hyperkeratosis. Proc Natl Acad Sci USA. 1992;89:6906–6910. doi: 10.1073/pnas.89.15.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Geerts D, Fontao L, Nievers MG, Schaapveld RQ, Purkis PE, Wheeler GN, Lane EB, Leigh IM, Sonnenberg A. Binding of integrin alpha6beta4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J Cell Biol. 1999;147:417–434. doi: 10.1083/jcb.147.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Degenstein L, Dowling J, Yu QC, Wollmann R, Perman B, Fuchs E. Gene targeting of BPAG1: abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Harding CR, Scott IR. Histidine-rich proteins (filaggrins): structural and functional heterogeneity during epidermal differentiation. J Mol Biol. 1983;170:651–673. doi: 10.1016/s0022-2836(83)80126-0. [DOI] [PubMed] [Google Scholar]
- Hardman MJ, Sisi P, Banbury DN, Byrne C. Patterned acquisition of skin barrier function during development. Development. 1998;125:1541–1552. doi: 10.1242/dev.125.8.1541. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M, Franke WW. Pair formation and promiscuity of cytokeratins: formation in vitro of heterotypic complexes and intermediate-sized filaments by homologous and heterologous recombinations of purified polypeptides. J Cell Biol. 1985;101:1826–1841. doi: 10.1083/jcb.101.5.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen-Scofield J, Resing KA, Dale BA. Characterization of an epidermal phosphatase specific for filaggrin phosphorylated by casein kinase II. J Invest Dermatol. 1988;91:553–559. doi: 10.1111/1523-1747.ep12476930. [DOI] [PubMed] [Google Scholar]
- Hesse M, Franz T, Tamai Y, Taketo MM, Magin TM. Targeted deletion of keratins 18 and 19 leads to trophoblast fragility and early embryonic lethality. EMBO J. 2000;19:5060–5070. doi: 10.1093/emboj/19.19.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton E, Paladini RD, Yu QC, Yen M, Coulombe PA, Fuchs E. Functional differences between keratins of stratified and simple epithelia. J Cell Biol. 1998;143:487–499. doi: 10.1083/jcb.143.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Eady RA, Underwood RA, Dale BA, Holbrook KA. Filaggrin expression in epidermolytic ichthyosis (epidermolytic hyperkeratosis) Br J Dermatol. 1994;131:767–779. doi: 10.1111/j.1365-2133.1994.tb08578.x. [DOI] [PubMed] [Google Scholar]
- Jonkman MF, et al. Effects of keratin 14 ablation on the clinical and cellular phenotype in a kindred with recessive epidermolysis bullosa simplex. J Invest Dermatol. 1996;107:764–769. doi: 10.1111/1523-1747.ep12365805. [DOI] [PubMed] [Google Scholar]
- Kam E, Resing KA, Lim SK, Dale BA. Identification of rat epidermal profilaggrin phosphatase as a member of the protein phosphatase 2A family. J Cell Sci. 1993;106:219–226. doi: 10.1242/jcs.106.1.219. [DOI] [PubMed] [Google Scholar]
- Kouklis PD, Hutton E, Fuchs E. Making a connection: direct binding between keratin intermediate filaments and desmosomal proteins. J Cell Biol. 1994;127:1049–1060. doi: 10.1083/jcb.127.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Liao J, Omary MB. Apoptosis generates stable fragments of human type I keratins. J Biol Chem. 1997;272:33197–33203. doi: 10.1074/jbc.272.52.33197. [DOI] [PubMed] [Google Scholar]
- Kuechle MK, Thulin CD, Presland RB, Dale BA. Profilaggrin requires both linker and filaggrin peptide sequences to form granules: implications for profilaggrin processing in vivo. J Invest Dermatol. 1999;112:843–852. doi: 10.1046/j.1523-1747.1999.00599.x. [DOI] [PubMed] [Google Scholar]
- Kulesh DA, Cecena G, Darmon YM, Vasseur M, Oshima RG. Posttranslational regulation of keratins: degradation of mouse and human keratins 18 and 8. Mol Cell Biol. 1989;9:1553–1565. doi: 10.1128/mcb.9.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lersch R, Stellmach V, Stocks C, Giudice G, Fuchs E. Isolation, sequence, and expression of a human keratin K5 gene: transcriptional regulation of keratins and insights into pairwise control. Mol Cell Biol. 1989;9:3685–3697. doi: 10.1128/mcb.9.9.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd C, Yu QC, Cheng J, Turksen K, Degenstein L, Hutton E, Fuchs E. The basal keratin network of stratified squamous epithelia: defining K15 function in the absence of K14. J Cell Biol. 1995;129:1329–1344. doi: 10.1083/jcb.129.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack JW, Steven AC, Steinert PM. The mechanism of interaction of filaggrin with intermediate filaments. The ionic zipper hypothesis. J Mol Biol. 1993;232:50–66. doi: 10.1006/jmbi.1993.1369. [DOI] [PubMed] [Google Scholar]
- Magin TM, Hesse M, Schroder R. Novel insights into intermediate-filament function from studies of transgenic and knockout mice. Protoplasma. 2000;211:140–150. [Google Scholar]
- Magin TM, Schroder R, Leitgeb S, Wanninger F, Zatloukal K, Grund C, Melton DW. Lessons from keratin 18 knockout mice: formation of novel keratin filaments, secondary loss of keratin 7 and accumulation of liver-specific keratin 8-positive aggregates. J Cell Biol. 1998;140:1441–1451. doi: 10.1083/jcb.140.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan K, Coulombe P. The wound repair-associated keratins 6, 16, and 17. Insights into the role of intermediate filaments in specifying keratinocyte cytoarchitecture. In: Herrmann H, Harris JR, editors. Subcellular Biochemistry. New York: Plenum Press; 1998a. pp. 173–204. [PubMed] [Google Scholar]
- McGowan K, Coulombe P. Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. J Cell Biol. 1998b;143:469–486. doi: 10.1083/jcb.143.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng JJ, Bornslaeger EA, Green KJ, Steinert PM, Ip W. Two-hybrid analysis reveals fundamental differences in direct interactions between desmoplakin and cell type-specific intermediate filaments. J Biol Chem. 1997;272:21495–21503. doi: 10.1074/jbc.272.34.21495. [DOI] [PubMed] [Google Scholar]
- Ming ME, Daryanani HA, Roberts LP, Baden HP, Kvedar JC. Binding of keratin intermediate filaments (K10) to the cornified envelope in mouse epidermis: implications for barrier function. J Invest Dermatol. 1994;103:780–784. doi: 10.1111/1523-1747.ep12413024. [DOI] [PubMed] [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Ness SL, Edelmann W, Jenkins TD, Liedtke W, Rustgi AK, Kucherlapati R. Mouse keratin 4 is necessary for internal epithelial integrity. J Biol Chem. 1998;273:23904–23911. doi: 10.1074/jbc.273.37.23904. [DOI] [PubMed] [Google Scholar]
- Nicholl ID, Quinlan RA. Chaperone activity of alpha-crystallins modulates intermediate filament assembly. EMBO J. 1994;13:945–953. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini RD, Coulombe PA. The functional diversity of epidermal keratins revealed by the partial rescue of the keratin 14 null phenotype by keratin 16. J Cell Biol. 1999;146:1185–1201. doi: 10.1083/jcb.146.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, B., Kirfel, J., Büssow, H., Vidal, M., and Magin, T.M. (2001). Complete cytolysis and neonatal lethality in keratin 5 knockout mice reveal its fundamental role in skin integrity and in EBS. Mol. Biol. Cell 12. [DOI] [PMC free article] [PubMed]
- Porter RM, Leitgeb S, Melton DW, Swensson O, Eady RAJ, Magin TM. Gene targeting at the mouse cytokeratin 10 locus: severe skin fragility and changes of cytokeratin expression in the epidermis. J Cell Biol. 1996;132:925–936. doi: 10.1083/jcb.132.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt J, Bauer C, Porter RM, Lane EB, Herzog V, Magin TM. Out of balance: consequences of a partial keratin 10 knockout. J Cell Sci. 1997;110:2175–2186. doi: 10.1242/jcs.110.18.2175. [DOI] [PubMed] [Google Scholar]
- Reichelt J, Doering T, Schnetz E, Fartasch M, Sandhoff K, Magin TM. Normal ultrastructure, but altered stratum corneum lipid and protein composition in a mouse model for epidermolytic hyperkeratosis. J Invest Dermatol. 1999;113:329–334. doi: 10.1046/j.1523-1747.1999.00702.x. [DOI] [PubMed] [Google Scholar]
- Resing KA, Walsh KA, Dale BA. Identification of two intermediates during processing of profilaggrin to filaggrin in neonatal mouse epidermis. J Cell Biol. 1984;99:1372–1378. doi: 10.1083/jcb.99.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resing KA, Walsh KA, Haugen-Scofield J, Dale BA. Identification of proteolytic cleavage sites in the conversion of profilaggrin to filaggrin in mammalian epidermis. J Biol Chem. 1989;264:1837–1845. [PubMed] [Google Scholar]
- Rose O, Grund C, Reinhardt S, Starzinski-Powitz A, Franke WW. Contactus adherens, a special type of plaque-bearing adhering junction containing M-cadherin, in the granule cell layer of the cerebellar glomerulus. Proc Natl Acad Sci USA. 1995;92:6022–6026. doi: 10.1073/pnas.92.13.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothnagel JA, Dominey AM, Dempsey LD, Longley MA, Greenhalgh DA, Gagne TA, Huber M, Frenk E, Hohl D, Roop DR. Mutations in the rod domains of keratins 1 and 10 in epidermolytic hyperkeratosis. Science. 1992;257:1128–1130. doi: 10.1126/science.257.5073.1128. [DOI] [PubMed] [Google Scholar]
- Rugg EL, McLean WH, Lane EB, Pitera R, McMillan JR, Dopping HP, Navsaria HA, Leigh IM, Eady RA. A functional “knockout”of human keratin 14. Genes Dev. 1994;8:2563–2573. doi: 10.1101/gad.8.21.2563. [DOI] [PubMed] [Google Scholar]
- Steinert PM, Cantieri JS, Teller DC, Lonsdale EJ, Dale BA. Characterization of a class of cationic proteins that specifically interact with intermediate filaments. Proc Natl Acad Sci USA. 1981;78:4097–4101. doi: 10.1073/pnas.78.7.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995;270:17702–17711. doi: 10.1074/jbc.270.30.17702. [DOI] [PubMed] [Google Scholar]
- Steinert PM, Marekov LN. Direct evidence that involucrin is a major early isopeptide cross-linked component of the keratinocyte cornified cell envelope. J Biol Chem. 1997;272:2021–2030. doi: 10.1074/jbc.272.3.2021. [DOI] [PubMed] [Google Scholar]
- Tamai Y, Ishikawa T, Bösl MR, Mori M, Nozaki M, Baribault H, Oshima RG, Taketo MM. Cytokeratins 8 and 19 in the mouse placental development. J Cell Biol. 2000;151:563–572. doi: 10.1083/jcb.151.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RA, Eichner R, Sun TT. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984;98:1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JM, Chipev CC, DiGiovanna JJ, Bale SJ, Marekov LN, Steinert PM, Compton JG. Mutations in the H1 and 1A domains in the keratin 1 gene in epidermolytic hyperkeratosis. J Invest Dermatol. 1994;102:17–23. doi: 10.1111/1523-1747.ep12371725. [DOI] [PubMed] [Google Scholar]