Abstract
Background
The current standard of practice for an athlete to return to sport after anterior cruciate ligament (ACL) reconstruction is varied. Attempt to return to activity is typically advised 6 months after surgery, but functional performance deficits and gait abnormalities are often still evident and may have important implications on future function.
Hypothesis
When comparing the involved and uninvolved limbs, patients who failed return-to-sport (RTS) criteria would demonstrate (1) smaller peak knee angles, extensor moments, and peak power absorption at the knee of the involved limb and (2) larger peak hip angles, extensor moments, and peak power generation of the involved limb.
Study Design
Controlled laboratory study.
Methods
A total of 42 patients completed functional and biomechanical gait assessment 6 months after ACL reconstruction. Functional testing involved an isometric quadriceps strength test, 4 single-legged hop tests, and 2 self-report questionnaires. Three-dimensional motion analysis was used to measure sagittal plane kinematics and kinetics of the hip and knee. A mixed-model analysis of variance and post hoc t tests were used to compare the limb symmetry of those who passed and those who did not pass RTS criteria. Minimal clinically important differences were calculated from healthy gait data and used to further define meaningful limb asymmetries.
Results
Twenty of the 42 (48%) patients passed RTS criteria 6 months after ACL reconstruction. Patients who did not pass the criteria demonstrated statistically significant differences between limbs on all kinematic and kinetic variables at the knee (P ≤ .027). Clinically meaningful asymmetries at the hip were also identified in this group. Only kinetic asymmetries at the knee were identified in the patients who passed RTS criteria.
Conclusion
Athletes who demonstrate superior functional performance 6 months after ACL reconstruction may have fewer abnormal and asymmetrical gait behaviors than their poorer performing counterparts. Patients who did not pass RTS criteria not only demonstrated larger kinematic and kinetic asymmetries between limbs but also appeared to use a gait strategy more closely aligned with athletes early after ACL rupture.
Clinical Relevance
Poor performance on a battery of functional performance measures may be related to the presence of movement asymmetries in athletes after ACL reconstruction. Objective RTS criteria have the potential to provide information to clinicians who determine when these athletes return to activity, and may aid in the prescription of targeted rehabilitation to address underlying movement asymmetry.
Keywords: ACL reconstruction, return to sport, function, gait mechanics, noncopers
Anterior cruciate ligament (ACL) ruptures often result in significant disability, both in activities of daily living and in sports. Because of recurrent episodes of knee instability, the majority of athletes who tear their ACL have difficulty resuming high-level activity without surgery.8 These patients are classified as noncopers14 and demonstrate aberrant and ineffective gait behaviors acutely after injury.39,40 In an attempt to protect the injured knee against repeated instability during daily activities, noncopers often employ an active knee-stiffening strategy, evidenced by truncated knee motion, lower knee extensor moments, and lower knee power absorption.2,39–41 Consequently, noncopers alter the neuromuscular behavior at the hip by way of increased joint extensor moments and increased hip power absorption.39 These maladaptations are also known to persist in spite of ACL reconstruction.17,38
With estimates of reinjury nearing 1 in 5 ACL-reconstructed athletes25,37,43 and the highest risk of reinjury within the first 7 months after surgery,25 there is legitimate concern over the criteria that define return-to-sport (RTS) “readiness.’” Often, medical clearance for return to activity occurs within 6 months after ACL reconstruction.24,29,49 Recent literature has challenged the emphasis for return to activity based on time alone,31 and many groups have advocated the use of objective criteria to inform clinical decision making.18,46 Adequate strength symmetry and performance on functional tests have been suggested as markers by which clearance for activity should be measured.12,13,24,32,33,42,46 Specifically, a battery of tests and measures, including quadriceps strength testing, 4 single-legged hop tests, and 2 self-report questionnaires, have been utilized to aid the objective determination of RTS readiness after ACL injury14 and reconstruction.18 By these standards, fewer than half of athletes who are classified as noncopers before surgery pass these criteria 6 months after ACL reconstruction.18
While the implications of poor clinical performance on RTS success after ACL reconstruction are clear, far less is known about the influence of abnormal movement patterns. Movement asymmetries in this population are nearly ubiquitous after ACL injury and reconstruction and have been reported to exist up to 2 years after ACL reconstruction.15–17,36–38 There is currently no evidence that establishes a link between movement asymmetries and poorer functional ability in ACL-reconstructed athletes; however, movement asymmetries are implicated in both the development of osteoarthritis3,47 and the predisposition for a second catastrophic injury.37 Specifically, abnormal joint motion has been suggested to be an instigating factor in the development of osteoarthritis in the ACL-injured knee.3,6
The first several months after ACL reconstruction appear to be a time of great vulnerability for athletes attempting to return to their previous level of activity. Not only are functional performance deficits4,18 and movement asymmetries9,17,37,38 commonplace, but the risk of reinjury is also the highest during this time frame.25 Athletes with multiplanar biomechanical asymmetries at the hip and knee 1 year after ACL reconstruction are at least 3 times more likely to suffer a second ACL injury than those without these asymmetries.37 Establishing a link between performance on clinical tests and movement asymmetries identified in a motion analysis laboratory could reduce the need for expensive biomechanical analyses and provide clinicians a surrogate, multipurpose assessment tool to inform critical RTS decision making.
To date, no studies have evaluated whether functional deficits and movement asymmetry coincide in ACL-reconstructed athletes. Therefore, the purpose of this study was to compare the gait characteristics of athletes who pass and athletes who do not pass RTS criteria 6 months after ACL reconstruction. We hypothesized that only the noncopers who failed RTS criteria would demonstrate asymmetrical hip and knee mechanics. Specifically, we hypothesized that only the noncopers who failed RTS criteria would demonstrate (1) smaller peak knee angles, extensor moments, and peak absorption at the knee of the involved limb and (2) larger peak hip angles, extensor moments, and peak power generation of the involved limb.
MATERIALS AND METHODS
Forty-two athletes (30 male, 12 female; mean age, 29.3 ± 10.8 years) classified as noncopers, using a validated ACL-deficient screening examination (Table 1),13 completed a functional and biomechanical gait assessment 6 months after ACL reconstruction. All patients were between the ages of 14 and 50 years; had participated regularly (≥50 h/y) in cutting, jumping, and pivoting activities before their index injury; and had sustained a unilateral ACL rupture within 7 months of their initial clinical evaluation. An ACL rupture was confirmed by magnetic resonance imaging and clinical examination findings. Patients with symptomatic meniscal tears, large osteochondral defects (>1 cm2), or associated grade III ligament strains were excluded. Ligament reconstruction was performed using a soft tissue allograft or a semitendinosus/gracilis autograft by a single orthopaedic surgeon. Each patient underwent a supervised, progressive postoperative rehabilitation protocol that focused on the resolution of joint effusion, range of motion deficits, quadriceps strength impairments, and functional limitations.1
TABLE 1.
Noncoper Classification and Return-to-Sport Criteria as Measured by the ACL-Deficient Functional Examination13,18
| Noncoper Classification | Return-to-Sport Criteria | |
|---|---|---|
| Episodes of knee instability | >1 | ≤1 |
| 4 single-legged hop tests35 | <80% (6-m timed hop only) | ≥90% (All hop tests) |
| Knee Outcome Survey–Activities of Daily Living Scale | <80% | ≥90% |
| Global Rating Scale for Perceived Function | <60% | ≥90% |
Patients returned for functional testing and biomechanical gait analysis 6 months after surgery. Quadriceps strength index, performance on 4 single-legged hop tests, and scores from 2 self-report questionnaires, the Knee Outcome Survey–Activities of Daily Living Scale22 and the Global Rating Scale for Perceived Function,20 were used to determine whether athletes passed or failed RTS testing.18 Limb symmetry indexes were primarily calculated by dividing the performance of the involved limb by the performance of the uninvolved limb; in the case of the timed 6-m hop test, the performance of the uninvolved limb was divided by the performance of the involved limb. To pass RTS criteria, patients had to achieve at least 90% on each of the functional tests and measures (Table 1). Patients who demonstrated performance scores below the cutoff value on 1 or more of these tests did not pass RTS criteria.
Motion analysis was performed using a passive 8-camera system (120 Hz) (VICON, Oxford Metrics Ltd, London, United Kingdom) and an embedded 6-component force plate (1080 Hz) (Bertec Corp, Worthington, Ohio). Retroreflective markers were secured to the anatomic landmarks of the foot, ankle, shank, thighs, and pelvis of each patient to determine joint centers and segment pose; this marker set has been previously shown to have excellent intersession reliability.10 Rigid shell clusters were secured to the pelvis and distal-lateral aspect of the shanks and thighs to track segment motion during gait. Patients were instructed to walk at their self-selected speed through the capture volume over the embedded force place. Speed was monitored for consistency (±5%) using 2 infrared photocells placed 2.865 m apart. Gait trials were accepted if the patient maintained a consistent speed, avoided visual targeting of the force plate, and made isolated foot contact.
Data reduction was completed on 5 successful walking trials for each limb using custom LabVIEW (National Instruments, Austin, Texas) and Visual3D coding (C-Motion Inc, Germantown, Maryland). Kinematic and kinetic data were low pass filtered at 6 Hz and 40 Hz, respectively. Initial contact and end of stance were identified using a 50-N force plate threshold, and all trials were normalized to 100% of the stance phase and then ensemble-averaged before kinematic and kinetic data calculations. Joint angles were calculated using rigid body analysis with Euler angles, and internal joint moments and joint powers were calculated using inverse dynamics. Hip and knee kinematics and kinetics in the sagittal plane were examined from initial contact to peak knee flexion during the stance phase.
A mixed-model analysis of variance (ANOVA) was used to compare the limb symmetry of those who passed RTS criteria (“PASS”) and those who did not pass (“FAIL”). Where significant interactions were found, post hoc paired and independent t tests were used to evaluate the limb symmetry within each group and group differences, respectively. A priori significance level was set at .05. To identify whether clinically meaningful asymmetries or group differences coincided with statistically important differences, effect sizes (ES) were calculated when the limb asymmetries or limb differences between groups met or exceeded the minimal clinically important difference (MCID) values. Data collected from 10 uninjured athletes were used to determine the MCID between limbs for joint angles (knee and hip: 3°) and moments (knee: 0.04 Nm/kgm; hip: 0.06 Nm/ kg.m).11 Effect sizes were interpreted as small (0.2), medium (0.5), or large (0.8).7 This study was approved by the university’s review board. All patients provided written informed consent before their participation.
RESULTS
Twenty of the 42 (48%) patients passed RTS criteria 6 months after ACL reconstruction. There was no difference between the percentage of female patients (30%) and male patients (53%) who passed RTS criteria (P = .315), and mean gait speed did not differ between groups (PASS: 1.52 ± 0.11 m/s; FAIL: 1.57 ± 0.12 m/s; P = .143). Patients who failed RTS criteria were older, had a longer time period from injury to surgery, demonstrated lower strength and hop symmetry indexes, and reported poorer daily function when compared with patients who passed RTS criteria (Table 2).
TABLE 2.
Functional Testing Performance Scores of Patients Who Passed and Failed Return-to-Sport Criteriaa
| Pass | Fail | P Value | |
|---|---|---|---|
| Age, y | 25.4 ± 9.9 | 31.4 ± 12.2 | .09 |
| Time from injury to surgery, wk | 12.9 ± 7.1 | 21.5 ± 17.6 | .04 |
| Quadriceps strength index | 101.2 ± 8.1 | 91.5 ± 15.3 | .023 |
| Single hop for distance | 97.1 ± 4.6 | 86.6 ± 9.3 | <.001 |
| Crossover hop for distance | 96.1 ± 3.6 | 91.9 ± 6.4 | .018 |
| Triple hop for distance | 97.7 ± 7.6 | 91.3 ± 7.9 | .014 |
| 6-m timed hop | 99.8 ± 4.8 | 95.4 ± 8.0 | .043 |
| Knee Outcome Survey–Activities of Daily Living Scale | 97.8 ± 1.7 | 95.3 ± 4.1 | .016 |
| Global Rating Scale for Perceived Function | 95.5 ± 3.8 | 88.6 ± 7.5 | <.001 |
Values are expressed as mean ± standard deviation. Bolded values indicate statistical significance.
Joint Angles at Initial Contact and Peak Knee Flexion
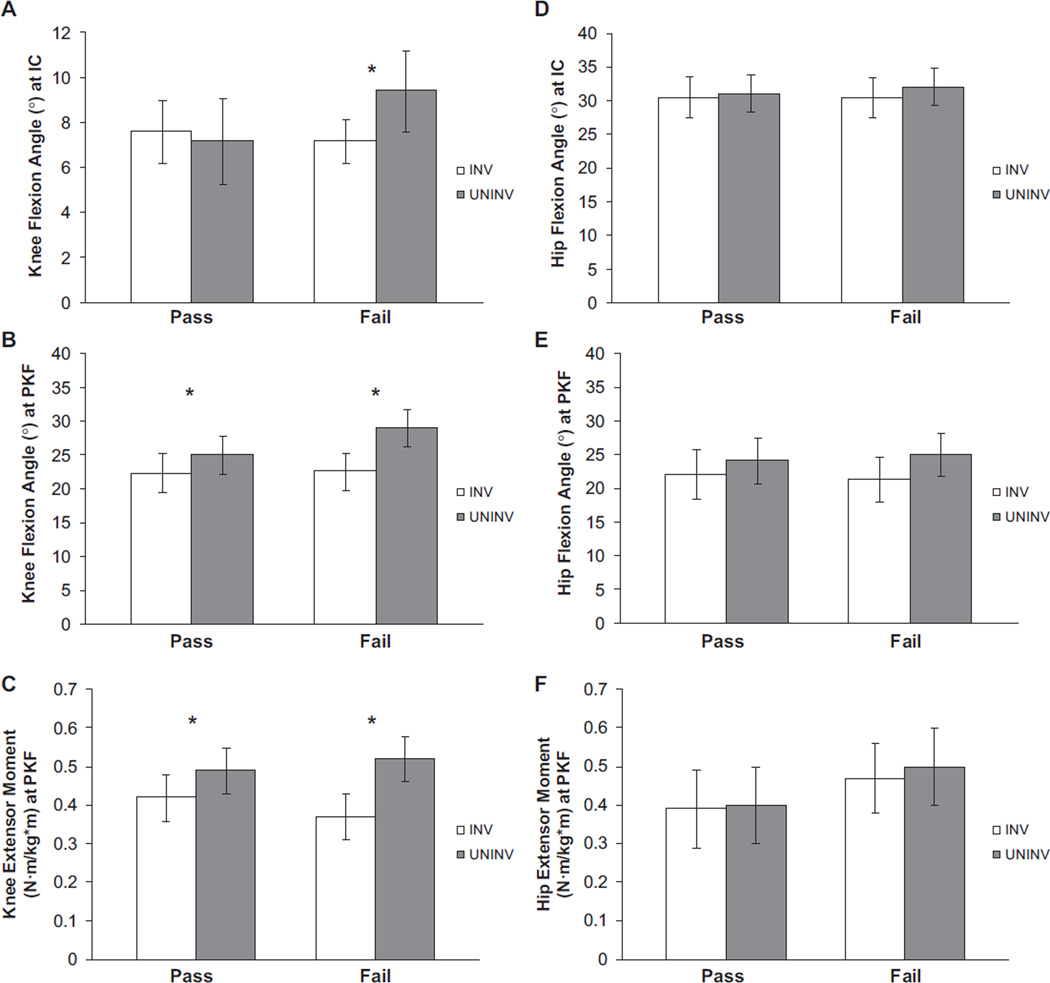
There was no significant limb × group interaction for the knee flexion angle at initial contact (P = .057). Only the FAIL group demonstrated a larger flexion angle on the uninvolved limb compared with the involved limb (P = .027), although this difference was not clinically meaningful (Figure 1 and Table 3). No limb × group interaction was found for hip flexion angle at initial contact (P = .368).
Figure 1.
Knee (A–C) and hip (D–F) joint angles and moments during weight acceptance. Bars represent group means. Error bars represent 95% confidence interval. IC, initial contact; INV, involved limb; PKF, peak knee flexion; UNINV, uninvolved limb. *Denotes statistical significance (P ≤ .05).
TABLE 3.
Interlimb Symmetry of Knee Kinematics and Kinetics of Patients Who Passed and Failed Return-to-Sport Criteriaa
| Involved | Uninvolved | P Value | Effect Sizeb | |
|---|---|---|---|---|
| KFAIC, deg | ||||
| Pass | 7.6 (6.2–9.0) | 7.2 (5.3–9.0) | .659 | — |
| Fail | 7.2 (5.8–8.5) | 9.4 (7.6–11.2) | .027 | — |
| KFAPKF, deg | ||||
| Pass | 22.4 (19.5–25.3) | 25.1 (22.2–28.0) | .012 | — |
| Fail | 22.6 (19.8–25.3) | 29.1 (26.4–31.9) | <.001 | 1.06 |
| KEMPKF, N·m/kg·m | ||||
| Pass | 0.42 (0.35–0.48) | 0.49 (0.43–0.55) | <.001 | 0.58 |
| Fail | 0.37 (0.30–0.43) | 0.52 (0.46–0.57) | <.001 | 0.97 |
Values are expressed as mean (95% confidence interval). Bolded values indicate statistical significance. KEMPKF, internal knee extensor moment at peak knee flexion; KFAIC, knee flexion angle at initial contact; KFAPKF, knee flexion angle at peak knee flexion.
Effect sizes calculated when differences between limb means exceeded the minimal clinically important difference value.
A significant limb × group interaction was found for knee flexion angle at peak knee flexion (P = .023). Both the PASS (P = .012) and FAIL groups (P < .001) had a smaller peak knee flexion angle on the involved limb when compared with the uninvolved limb, but only the FAIL group demonstrated limb asymmetry that was clinically meaningful (ES = 1.06) (Table 3). There was no significant limb × group interaction for hip flexion angle at peak knee flexion (P = .243). A main effect of limb was identified (P < .001); however, only the FAIL group demonstrated clinically meaningful asymmetry between limbs (ES = 0.48).
Joint Moments at Peak Knee Flexion
A significant limb × group interaction was found for internal knee extensor moment (P = .030). Both the PASS and FAIL groups demonstrated smaller knee extensor moments on the involved limb compared with the uninvolved limb (P < .001), but the magnitude of the limb asymmetry was greater in the patients who failed RTS criteria than in those who passed (Table 3). There were no statistically significant interactions or main effects found for internal hip extensor moment (P ≥ .587) (Table 4). Similarly, clinically meaningful limb differences in hip extensor moment were not found in either group.
TABLE 4.
Interlimb Symmetry of Hip Kinematics and Kinetics of atients Who Passed and Failed Return-to-Sport Criteriaa
| Involved | Uninvolved | Effect Sizeb | |
|---|---|---|---|
| HFAIC, deg | |||
| Pass | 30.6 (27.6–33.8) | 31.1 (28.3–33.9) | — |
| Fail | 30.5 (27.5–33.5) | 32.1 (29.4–34.7) | — |
| HFAPKF, deg | |||
| Pass | 22.1 (18.5–25.7) | 24.1 (20.8–27.4) | — |
| Fail | 21.4 (18.0–24.8) | 25.0 (21.8–28.1) | 0.46 |
| HEMPKF, N·m/kg·m | |||
| Pass | 0.39 (0.30–0.49) | 0.40 (0.30–0.49) | — |
| Fail | 0.47 (0.38–0.56) | 0.50 (0.40–0.60) | — |
Values are expressed as mean (95% confidence interval). HEMPKF, internal hip extensor moment at peak knee flexion; HFAIC, hip flexion angle at initial contact; HFAPKF, hip flexion angle at peak knee flexion.
Effect sizes were calculated when differences between means exceeded the minimal clinically important difference value.
Peak Joint Powers During Weight Acceptance
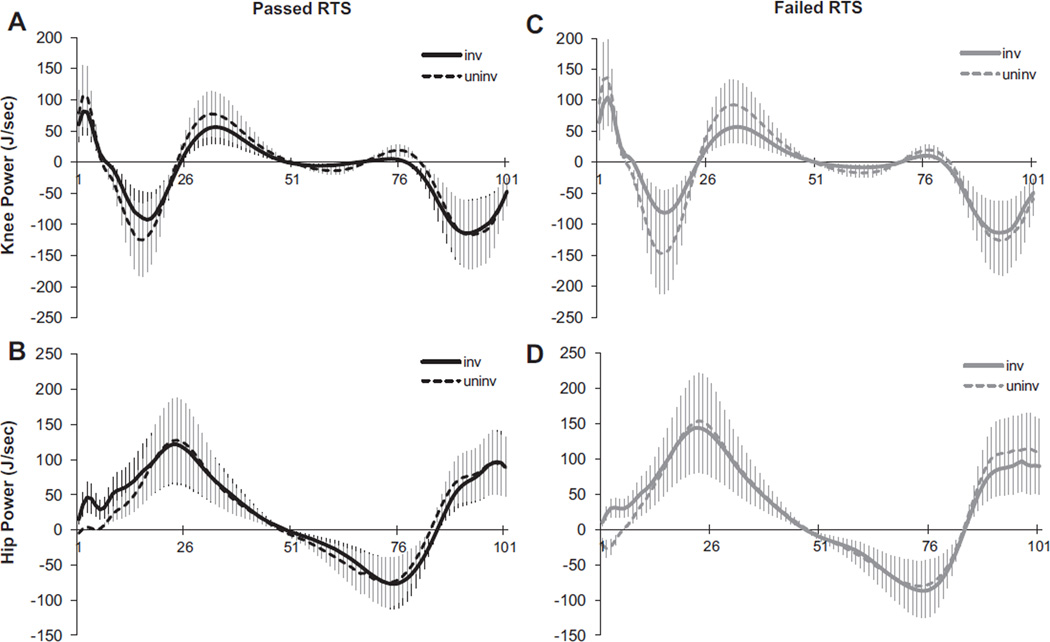
There was a main effect of limb for peak power absorption at the knee (P < .001), showing that the uninvolved knee of both groups absorbed more power during weight acceptance than did the involved limb (Figure 2). Peak power generation at the hip during weight acceptance was not different between the limbs of either group (P = .969). During the first 5% of the stance phase, however, only patients who failed RTS criteria had opposing hip strategies, generating power with their involved hip and absorbing power with their uninvolved hip (Figure 2).
Figure 2.
Knee (A) and hip (B) power curves during the stance phase of noncopers who passed return-to-sport (RTS) criteria and noncopers who failed RTS criteria (C, D). Positive power = power generation; negative power = power absorption. The x-axis represents the time-normalized stance (101 points); the y-axis represents joint powers.
DISCUSSION
The purpose of this study was to compare the gait characteristics of athletes who passed RTS criteria and athletes who did not pass RTS criteria 6 months after ACL reconstruction. Our data supported our initial hypotheses that patients who did not pass RTS criteria would demonstrate smaller peak knee angles and lower extensor moments on the involved knee. Interestingly, the expected compensatory strategy of increased ipsilateral hip extensor moments and power absorption was not found; rather, the poorly functioning patients appeared to rely on an altered contralateral hip strategy while simultaneously limiting motion and attenuating forces about the injured knee. The functional examination used to determine RTS readiness in this study appears most useful in identifying athletes with significant gait abnormalities after ACL reconstruction.
Patients who did not pass RTS criteria demonstrated statistically significant differences between limbs on all kinematic and kinetic variables at the knee. Specifically, lower peak knee flexion, knee extensor moment, and peak power absorption were characteristic of their reconstructed knee. Kinetic asymmetries at the knee were found in both groups of patients; however, the magnitude of knee extensor moment asymmetry in the patients who did not pass RTS criteria was more than twice that of the group who passed RTS criteria. Furthermore, knee joint motion asymmetries appeared to be characteristic of the group who did not pass RTS criteria. Early after injury, noncopers adopt a stiffened knee strategy by reducing the motion at the knee and co-contracting the supporting musculature in a likely attempt to avoid repeated episodes of joint instability.39 It is apparent from our data and from previous findings17,38 that this stiffened knee strategy can persist in athletes in spite of successful ligament reconstruction and may more accurately reflect the functional deficits of those who do not meet RTS criteria.
The compensatory movement behaviors of ACL-injured athletes are not confined to the reconstructed knee. The contrasting hip strategy found between the limbs of the patients who did not pass RTS criteria reinforces the concept that a unilateral ACL injury can elicit a “bilateral kinetic response”16 by which the injured patient also adopts an altered neuromuscular strategy for the uninvolved limb. This contralateral hip compensation appears to persist even after surgery and supervised rehabilitation for some athletes. In addition to absorbing power in the uninvolved hip early during the stance phase, the patients who did not pass our RTS criteria appeared to “overflex” their uninvolved knee during weight acceptance. This specific adaptation has not previously been described in this group of ACL-reconstructed athletes but may be another manifestation of the aberrant contralateral limb–loading patterns adopted by some patients after surgery.36,37
The hip strategy of the patients who failed RTS criteria partially supported our second hypothesis concerning asymmetries at the hip. While clinically meaningful limb differences in peak hip flexion angle and hip power were found, these asymmetries appeared to stem more from a compensatory strategy of the uninvolved hip rather than from an adaptation of the involved hip. Although not originally hypothesized, this adaptation was not totally unexpected. Roewer and colleagues38 also identified this strategy in noncopers 6 months after ACL reconstruction in which the uninvolved hip absorbed power and the involved hip generated power early in weight acceptance. Our findings indicate that this asymmetry may be specific to noncopers with poorer functional performance after ACL reconstruction and does not wholly define this cohort of athletes.
The first several months after reconstruction represent a time of marked movement abnormalities5,9,17,37,38 often in spite of high-level sport performance.36,37 Ideally, a cluster of clinical tests and measures assessing the functional abilities of athletes would directly and robustly relate to the comparable measures of biomechanical impairments. Our data indicate that while performance on clinical tests and measures demonstrates some relationship with meaningful movement deficits and asymmetry, they may be only part of the ideal formula for informing RTS decision making. Neuromuscular asymmetries during dynamic movement may not only affect sport performance but also be highly predictive of the risk of a second knee injury. Paterno and colleagues37 prospectively followed 56 athletes for 1 year after their medical discharge to determine the biomechanical risk factors related to reinjury. Nearly one quarter of their patients experienced a second ACL injury. Increased knee abduction motion, asymmetry in internal hip rotation moment, asymmetry in knee extensor moments during a drop vertical jump, and deficits on single-legged postural assessment predicted the second injury with excellent sensitivity (92%) and specificity (88%). In a cohort of nearly 300 ACL-reconstructed athletes, Laboute and colleagues25 reported a nearly 3-fold increase in an athlete’s risk of reinjury if the patient returned to activity within the first 7 months after surgery. Regardless of functional ability, returning to sport within 6 months of surgery may place athletes at an increased risk for reinjury, particularly if movement asymmetries are also present. Concurrent assessments of functional ability and movement symmetry may be the ideal method of assessment for RTS readiness in a group of athletes at great risk for second knee injuries. Three-dimensional biomechanical analysis of human movement, however, is time intensive, highly technical, and expensive. Ultimately, identification of highly sensitive and specific clinical correlates to the biomechanical measures predictive of poor function and risk of a second injury will enhance RTS decision making for these athletes.
Perhaps of equal importance is the purported relationship between abnormal movement patterns and the development of osteoarthritis after ACL injury. Anterior cruciate ligament reconstruction alone cannot prevent degenerative joint changes,23,27,44,45,48 as it is possible that the intra-articular cellular response that occurs early after injury already initiates this process.26,28,34 The initiation and progression of osteoarthritis may be a result of a cyclic pattern of pathomechanics in the wake of ACL injury.3,47 By this proposed framework, the continued disturbance in the biomechanics of the joint by way of altered limb motion provides an intracapsular environment conducive to the degenerative process.3 The abnormal movement strategies adopted in response to ACL injury are also not ubiquitously resolved with surgery.15–17,36–38 The persistence of these maladaptations during tasks as simple as walking may have serious consequences to the long-term health of the injured joint.
Our study population was limited to noncopers, who represent the poorest performing group of ACL-deficient athletes. Although the majority of ACL-injured athletes are noncopers,21 these findings may not translate to all ACL-deficient patients who undergo reconstruction. Similarly, these data do not indicate that all noncopers who go on to fail RTS testing after ACL reconstruction demonstrate gross movement asymmetries, nor are all noncopers who go on to pass our RTS criteria after ACL reconstruction completely free of movement dysfunction. While concomitant assessment of functional performance and gait symmetry may enhance RTS decision making, widespread application of motion capture techniques is not feasible. The development and validation of RTS criteria that accurately identify athletes with movement asymmetries are warranted.
Noncopers are a notoriously variable group of ACL-injured athletes.18,30 Previous work has highlighted the difficulty with which these athletes resume normal function and adopt symmetrical movement strategies in the months after ACL reconstruction.10,17,19,38 In an attempt to more accurately characterize the movement patterns of these athletes after surgery, we stratified our cohort by functional performance on a validated clinical testing battery.13 By this method, the lower functioning patients (ie, those who did not pass RTS criteria) demonstrated more meaningful limb asymmetries during gait than did their higher functioning counterparts. Furthermore, patients who failed RTS criteria were older and had a longer time from injury to surgery. While these factors may also influence RTS success in this cohort, examination of this relationship was not the purpose of this study.
To our knowledge, this work is the first to distinguish the movement patterns between higher and lower functioning athletes after ACL reconstruction. Importantly, athletes who have been classified as noncopers before surgery and then demonstrate poorer function after ACL reconstruction may be more likely to have clinically significant neuromuscular asymmetries compared with those who pass RTS testing. Movement asymmetries, specifically, are identifiable and modifiable risk factors for a second ACL rupture in athletes who have already returned to activity.37 Additional targeted therapy may be warranted to address flawed movement mechanics and maximize function in some noncopers after ACL reconstruction. Neuromuscular interventions targeting abnormal and asymmetrical movement behaviors in athletes preparing to return to sport may not only enhance functional performance but also mitigate the risk for secondary injury and reduce the likelihood of developing debilitating osteoar-thritis of the knee joint.
CONCLUSION
Our findings indicate that athletes who have been classified as noncopers before ACL reconstruction and who demonstrate poorer functional performance 6 months after ACL reconstruction present with more abnormal and asymmetrical gait behaviors than do their higher functioning counterparts. Validating functional RTS criteria with biomechanical measures of symmetry may provide clinicians with a testing battery that would accurately identify athletes with meaningful movement asymmetries after ACL reconstruction. Furthermore, this validation would allow widespread application of sensitive and specific clinical testing tools for assessing RTS readiness and may enhance the ability of sports medicine specialists to identify those athletes at increased risk for a secondary injury. Future work should evaluate whether the incidence of reinjury and the development of osteoarthritis are related to high levels of functional performance in the absence of movement symmetry.
ACKNOWLEDGMENTS
Special thanks to Dr Erin Hartigan for her assistance with patient recruitment and clinical and biomechanical data collection as well as Greg Seymour for his help with biomechanical data collection and processing.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was funded in part by the National Institutes of Health (R01AR048212 and S10RR022396) and the Foundation for Physical Therapy Promotion of Doctoral Studies Scholarship awarded to Drs Di Stasi and Logerstedt during their doctoral work at the University of Delaware.
REFERENCES
- 1.Adams D, Logerstedt DS, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J Orthop Sports Phys Ther. 2012;42(7):601–614. doi: 10.2519/jospt.2012.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkjaer T, Simonsen EB, Jorgensen U, Dyhre-Poulsen P. Evaluation of the walking pattern in two types of patients with anterior cruciate ligament deficiency: copers and non-copers. Eur J Appl Physiol. 2003;89(3–4):301–308. doi: 10.1007/s00421-002-0787-x. [DOI] [PubMed] [Google Scholar]
- 3.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 4.Ardern CL, Webster KE, Taylor NF, Feller JA. Return to the preinjury level of competitive sport after anterior cruciate ligament reconstruction surgery: two-thirds of patients have not returned by 12 months after surgery. Am J Sports Med. 2011;39(3):538–543. doi: 10.1177/0363546510384798. [DOI] [PubMed] [Google Scholar]
- 5.Button K, van Deursen R, Price P. Recovery in functional non-copers following anterior cruciate ligament rupture as detected by gait kinematics. Phys Ther Sport. 2008;9(2):97–104. doi: 10.1016/j.ptsp.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008;40(2):215–222. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 8.Daniel DM, Fithian DC. Indications for ACL surgery. Arthroscopy. 1994;10(4):434–441. doi: 10.1016/s0749-8063(05)80196-3. [DOI] [PubMed] [Google Scholar]
- 9.DeVita P, Hortobagyi T, Barrier J. Gait biomechanics are not normal after anterior cruciate ligament reconstruction and accelerated rehabilitation. Med Sci Sports Exerc. 1998;30(10):1481–1488. doi: 10.1097/00005768-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Di Stasi SL, Hartigan EH, Snyder-Mackler L. Unilateral stance strategies of athletes with ACL deficiency. J Appl Biomech. 2012;28(4):374–386. doi: 10.1123/jab.28.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Stasi SL, Snyder-Mackler L. The effects of neuromuscular training on the gait patterns of ACL-deficient men and women. Clin Biomech. 2012;27(4):360–365. doi: 10.1016/j.clinbiomech.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eastlack ME, Axe MJ, Snyder-Mackler L. Laxity, instability, and functional outcome after ACL injury: copers versus noncopers. Med Sci Sports Exerc. 1999;31(2):210–215. doi: 10.1097/00005768-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald GK, Axe MJ, Snyder-Mackler L. A decision-making scheme for returning patients to high-level activity with nonoperative treatment after anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):76–82. doi: 10.1007/s001670050190. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald GK, Axe MJ, Snyder-Mackler L. Proposed practice guidelines for nonoperative anterior cruciate ligament rehabilitation of physically active individuals. J Orthop Sports Phys Ther. 2000;30(4):194–203. doi: 10.2519/jospt.2000.30.4.194. [DOI] [PubMed] [Google Scholar]
- 15.Gao B, Zheng NN. Alterations in three-dimensional joint kinematics of anterior cruciate ligament-deficient and -reconstructed knees during walking. Clin Biomech. 2010;25(3):222–229. doi: 10.1016/j.clinbiomech.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Hart JM, Ko JW, Konold T, Pietrosimone B. Sagittal plane knee joint moments following anterior cruciate ligament injury and reconstruction: a systematic review. Clin Biomech. 2010;25(4):277–283. doi: 10.1016/j.clinbiomech.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Hartigan E, Axe MJ, Snyder-Mackler L. Perturbation training prior to ACL reconstruction improves gait asymmetries in non-copers. J Orthop Res. 2009;27(6):724–729. doi: 10.1002/jor.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartigan EH, Axe MJ, Snyder-Mackler L. Time line for noncopers to pass return-to-sports criteria after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2010;40(3):141–154. doi: 10.2519/jospt.2010.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartigan EH, Zeni J, Jr, Di Stasi S, Axe MJ, Snyder-Mackler L. Pre-operative predictors for non-copers to pass return to sports criteria after ACL reconstruction. J Appl Biomech. 2012;28:366–373. doi: 10.1123/jab.28.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopper DM, Goh SC, Wentworth LA, et al. Test-retest reliability of knee rating scales and functional hop tests one year following anterior cruciate ligament reconstruction. Phys Ther Sport. 2002;3:10–18. [Google Scholar]
- 21.Hurd WJ, Axe MJ, Snyder-Mackler L. Influence of age, gender, and injury mechanism on the development of dynamic knee stability after acute ACL rupture. J Orthop Sports Phys Ther. 2008;38(2):36–41. doi: 10.2519/jospt.2008.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132–1145. doi: 10.2106/00004623-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Kessler MA, Behrend H, Henz S, Stutz G, Rukavina A, Kuster MS. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):442–448. doi: 10.1007/s00167-008-0498-x. [DOI] [PubMed] [Google Scholar]
- 24.Kvist J. Rehabilitation following anterior cruciate ligament injury: current recommendations for sports participation. Sports Med. 2004;34(4):269–280. doi: 10.2165/00007256-200434040-00006. [DOI] [PubMed] [Google Scholar]
- 25.Laboute E, Savalli L, Puig P, et al. Analysis of return to competition and repeat rupture for 298 anterior cruciate ligament reconstructions with patellar or hamstring tendon autograft in sportspeople. Ann Phys Rehabil Med. 2010;53(10):598–614. doi: 10.1016/j.rehab.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Lohmander LS, Ionescu M, Jugessur H, Poole AR. Changes in joint cartilage aggrecan after knee injury and in osteoarthritis. Arthritis Rheum. 1999;42(3):534–544. doi: 10.1002/1529-0131(199904)42:3<534::AID-ANR19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 27.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 28.Lohmander LS, Saxne T, Heinegard D. Increased concentrations of bone sialoprotein in joint fluid after knee injury. Ann Rheum Dis. 1996;55(9):622–626. doi: 10.1136/ard.55.9.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McRae SM, Chahal J, Leiter JR, Marx RG, Macdonald PB. Survey study of members of the Canadian Orthopaedic Association on the natural history and treatment of anterior cruciate ligament injury. Clin J Sport Med. 2011;21(3):249–258. doi: 10.1097/JSM.0b013e318219a649. [DOI] [PubMed] [Google Scholar]
- 30.Moksnes H, Snyder-Mackler L, Risberg MA. Individuals with an anterior cruciate ligament-deficient knee classified as noncopers may be candidates for nonsurgical rehabilitation. J Orthop Sports Phys Ther. 2008;38(10):586–595. doi: 10.2519/jospt.2008.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myer GD, Martin L, Jr, Ford KR, et al. No association of time from surgery with functional deficits in athletes after anterior cruciate ligament reconstruction: evidence for objective return-to-sport criteria. Am J Sports Med. 2012;40:2256–2263. doi: 10.1177/0363546512454656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myer GD, Paterno MV, Ford KR, Quatman CE, Hewett TE. Rehabilitation after anterior cruciate ligament reconstruction: criteria-based progression through the return-to-sport phase. J Orthop Sports Phys Ther. 2006;36(6):385–402. doi: 10.2519/jospt.2006.2222. [DOI] [PubMed] [Google Scholar]
- 33.Myer GD, Schmitt LC, Brent JL, et al. Utilization of modified NFL combine testing to identify functional deficits in athletes following ACL reconstruction. J Orthop Sports Phys Ther. 2011;41(6):377–387. doi: 10.2519/jospt.2011.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson F, Billinghurst RC, Pidoux I, et al. Early post-traumatic osteo-arthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthritis Cartilage. 2006;14(2):114–119. doi: 10.1016/j.joca.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513–518. doi: 10.1177/036354659101900518. [DOI] [PubMed] [Google Scholar]
- 36.Paterno MV, Ford KR, Myer GD, Heyl R, Hewett TE. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin J Sport Med. 2007;17(4):258–262. doi: 10.1097/JSM.0b013e31804c77ea. [DOI] [PubMed] [Google Scholar]
- 37.Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–1978. doi: 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roewer BD, Di Stasi SL, Snyder-Mackler L. Quadriceps strength and weight acceptance strategies continue to improve two years after anterior cruciate ligament reconstruction. J Biomech. 2011;44(10):1948–1953. doi: 10.1016/j.jbiomech.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudolph KS, Axe MJ, Buchanan TS, Scholz JP, Snyder-Mackler L. Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surg Sports Traumatol Arthrosc. 2001;9(2):62–71. doi: 10.1007/s001670000166. [DOI] [PubMed] [Google Scholar]
- 40.Rudolph KS, Eastlack ME, Axe MJ, Snyder-Mackler L. 1998 Basmajian Student Award Paper. Movement patterns after anterior cruciate ligament injury: a comparison of patients who compensate well for the injury and those who require operative stabilization. J Electro-myogr Kinesiol. 1998;8(6):349–362. doi: 10.1016/s1050-6411(97)00042-4. [DOI] [PubMed] [Google Scholar]
- 41.Rudolph KS, Snyder-Mackler L. Effect of dynamic stability on a step task in ACL deficient individuals. J Electromyogr Kinesiol. 2004;14(5):565–575. doi: 10.1016/j.jelekin.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt LC, Paterno MV, Hewett TE. The impact of quadriceps femoris strength asymmetry on functional performance at return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2012;42(9):750–759. doi: 10.2519/jospt.2012.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37(2):246–251. doi: 10.1177/0363546508325665. [DOI] [PubMed] [Google Scholar]
- 44.Streich NA, Zimmermann D, Bode G, Schmitt H. Reconstructive versus non-reconstructive treatment of anterior cruciate ligament insufficiency: a retrospective matched-pair long-term follow-up. Int Orthop. 2011;35(4):607–613. doi: 10.1007/s00264-010-1174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Struewer J, Frangen TM, Ishaque B, et al. Knee function and prevalence of osteoarthritis after isolated anterior cruciate ligament reconstruction using bone-patellar tendon-bone graft: long-term follow-up. Int Orthop. 2012;36(1):171–177. doi: 10.1007/s00264-011-1345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomee R, Kaplan Y, Kvist J, et al. Muscle strength and hop performance criteria prior to return to sports after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1798–1805. doi: 10.1007/s00167-011-1669-8. [DOI] [PubMed] [Google Scholar]
- 47.Van de Velde SK, Bingham JT, Hosseini A, et al. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis Rheum. 2009;60(12):3693–3702. doi: 10.1002/art.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wells L, Dyke JA, Albaugh J, Ganley T. Adolescent anterior cruciate ligament reconstruction: a retrospective analysis of quadriceps strength recovery and return to full activity after surgery. J Pediatr Orthop. 2009;29(5):486–489. doi: 10.1097/BPO.0b013e3181aa2197. [DOI] [PubMed] [Google Scholar]