Abstract
Backgroud:
To simulate the ischemia-reperfusion injury in vivo, hypoxia/reoxygenation injury model was established in vitro and primary cultured neonatal rat cardiomyocytes were underwent hypoxia with hydrosulfite (Na2S2O4) for 1 h followed by 1 h reoxygenation.
Materials and Methods:
Determination the cell viability by MTT colorimetric assay. We use kit to detect the activity of lactate dehydrogenase (LDH), Na+-K+-ATPase and Ca2+-ATPase. Do research on the effect which ferulic acid and its drug-containing plasma have to self-discipline, conductivity, action potential duration and other electrophysiological phenomena of myocardial cells by direct observation using a microscope and recording method of intracellular action potential.
Results:
The experimental datum showed that both can reduce the damage hydrosulfite to myocardial cell damage and improve myocardial viability, reduce the amount of LDH leak, increase activity of Na+-K+-ATPase, Ca2+-ATPase, and increase APA (Action potential amplitude), Vmax (Maximum rate of depolarization) and MPD (Maximum potential diastolic).
Conclusion:
Taken together, therefore, we can get the conclusion that ferulic acid drug-containing plasma has better protective effect injured myocardial cell than ferulic acid.
Keywords: ATPase, drug-containing plasma, electrophysiological phenomena, ferulic acid, hypoxia/reoxygenation, LDH, primary cultured neonatal rat cardiomyocytes, sodium hydrosulfite
INTRODUCTION
Ferulic acid, chemical name 4-hydroxy-3-metoxybenzene acrylic acid, is a kind of phenolic acid derived from a variety of plants, and in cell wall, it combines with polysaccharides and proteins to from cell wall skeleton. In some respects, ferulic acid has exact pharmacological activity and low toxicity, and it can also act as the active compound of some drugs[1,2,3] It has cis form and trans form,[3] it is slightly soluble in cold water and soluble in hot water, it has poor stability in aqueous solution, it is decomposable in light[3] and it has good pH stability.[4] We use DMEM high-glucose culture solution to prepare the ferulic acid solution. And we keep the ferulic acid solution in dark and cold place because of its poor stability. Its chemical structure as follows: [Figure 1]
Figure 1.
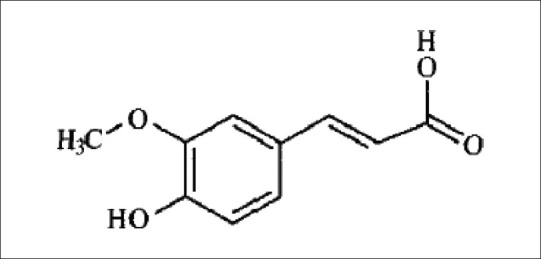
The chemical structure of ferulic acid[33]
Myocardial ischemia-reperfusion injury (MIRI) means cardiomyocyte injury induced during reperfusion after myocardial ischemia, and its injury degree is more severe than myocardial ischemia itself.[5] There are many Western medicines used clinically to treat coronary heart disease, but monomer composition is very rarely reported. Ferulic acid can resist platelet aggregation, inhibit platelet 5-hydroxytryptamine release, and inhibit platelet thromboxane A2 (TXA2) generation; it can reduce myocardial ischemia and oxygen consumption,[6] and is used in clinical to treat coronary heart disease and angina pectoris; mainly through inhibiting lipid oxidation, lowering cholesterol content in serum and antithrombotic effects, it can prevent atherosclerosis, so as to treat coronary heart disease.[2,7,8,9,10]
This paper adopts in vitro primary cultured neonatal rat cardiomyocytes and forms the hypoxia/reoxygenation injury model[11,12,13,14,15,16,17,18] to simulate in vivo ischemia-reperfusion injury, so as to study the effects of ferulic acid and its drug-containing plasma on cardiomyocyte injury and to explore its mechanism, and its advantages are that it can exclude internal neurohumor and the interaction between local different types of cells to a very great extent, and it can provide the basis for developing new drugs for coronary heart disease with clinical application value.
MATERIALS AND METHODS
Drugs and reagents
Ferulic acid (Batch No.: 0773-9910, purity higher than 98%, Chinese Biological Product Inspection Institute), MTT (GIBCO Company, USA), dimethyl sulfoxide (DMSO, Beijing Solarbio Science and Technology Co., Ltd.), trypsin (Difco Company, USA), collagenase I (GIBCO Company, USA), DMEM high-glucose and low-glucose culture solution (GIBCO Company, USA), sodium hydrosulfite (Tianjin Third Chemical Reagent Factory), verapamil hydrochloride tablets (Tianjin Central Pharmaceutical Co., Ltd.), Yangxinshi tablets (Qingdao Guofeng Pharmaceutical Co., Ltd.), lactate dehydrogenase (LDH) and ATP assay kit (Nanjing Jiancheng Bio Co., Ltd), other reagents are commercial analytical pure.
Animals
SD neonatal rats (1~3 d) and Kunming mice, provided by the Animal Center of Liaoning University of Traditional Chinese Medicine, reaching the national cleaning standard.
Main instruments
NUAIRETM US AUTOFLOW CO2 incubator (NUAIRE Company in Germany), AE31-type inverted fluorescence phase contrast microscope (Motic Company), UNRISE microplate reader (TECAN Company, in Switzerland). VDF-based microelectrode amplifiers (Shanghai Cathay Pacific Telecommunications Equipment Factory)
Primary cultured neonatal rat cardiomyocyte cultivation
We take several SD neonatal rats, take out the hearts under sterile conditions, wash off residual blood with PBS, cut the ventricles into 1m3 pieces, place them in a culture dish, add in appropriate amount of cold PBS, use trypsin and collagenase I mixture digestive fluid, repeat digestion in 37°C water bath, collect cell suspension, separate and purify cardiomyocytes with differential wall adhesion method, place cardiomyocyte suspension in culture flask, culture in 37°C and 5% CO2 incubator for 60 min, cardiac fibroblasts adhere to the wall quickly, extract cell suspension, regulate cell concentration to 5-10 ´ 105 cells/ml with high-glucose DMEM culture solution containing 10% fetal calf serum, transfer to 96-pore plate to culture, obtain purer cardiomyocytes, culture in 37°C and 5% CO2 condition, and select the cardiomyocytes with good growth for the experiment after 96 h.
Preparation of cardiomyocyte hypoxia/reoxygenation model[11,12,13,14,15,16,17,18]
The effects of different concentrations of sodium hydrosulfite on cardiomyocytes
Take primary cultured neonatal rat cardiomyocytes cultivated for 4 days and randomly divide into 7 groups, 10 pores each group: ① normal control group: normal high-glucose culture solution; ② low-glucose culture solution group; ③ 800 μM, 400 μM, 200 μM, 100 μM and 50 μM concentrations of sodium hydrosulfite solution + low-glucose culture solution group; place in 37°C and 5% CO2 incubator, and after culture for 1 h, replace with normal high-glucose culture solution to continue.[13,15,16]
The effects of different action time of sodium hydrosulfite on cardiomyocytes
Take primary cultured neonatal rat cardiomyocytes cultivated for 4 days and randomly divide into 7 groups, 10 pores each group: ① normal control group: normal high-glucose culture solution; ② low-glucose culture solution group; ③ 400 μM sodium hydrosulfite solution + low-glucose culture solution group; after cultivating for 30 min, 1 h, 2 h, 4 h and 24 h, respectively, replace with normal high-glucose culture solution, place in 37°C and 5% CO2 incubator, and continue cultivating for 30 min, 1 h, 2 h, 4 h and 24 h, respectively[13,15,16]
Preparation of drug-containing plasma
Experimental grouping
Experimental mice, conventional breeding, are given water, but not food for half day before blood sampling. Experimental grouping: they are randomly divided into 6 groups, 3 in each group: ① high-dose ferulic acid group: 7.8 mg/mL; ② medium-dose ferulic acid group: 2.6 mg/mL; ③ low-dose ferulic acid group: 0.87 mg/mL; ④ Chinese positive (Yangxinshi tablet) group: 0.0776 mg/mL; ⑤ Western positive group (verapamil hydrochloride tablet): 0.0776 mg/mL; ⑥ control group: natural drinking water; filling into stomach for 3 days, 0.5 mL/time, twice a day, take blood from eyeball, set Ep tube, prepare drug-containing plasma and blank plasma, and store for use.
Preparation of drug-containing plasma
Put blood sample in Ep tube, which is added with heparin (concentration of 0.25 mg/mL) in advance, shake slowly, centrifuge at 1000 r/min for 15 min, extract the supernatant, which is the drug-containing plasma, sub-package, and place in -20°C refrigerator for storage.
Protective effects of ferulic acid on cardiomyocytes with hypoxia/reoxygenation injury
Take primary cultured neonatal rat cardiomyocytes cultivated for 4 days and randomly divide into 6 groups, 10 pores each group: ① blank control group: 400 uM sodium hydrosulfite for hypoxia/reoxygenation 2 h, and add into DMEM culture solution; ② Chinese positive control group: 0.0776 mg/mL Yangxinshi tablet; ③ Western positive control group: 0.0776 mg/mL verapamil hydrochloride tablet; ④ different ferulic acid concentration (800 μM, 400 μM and 200 μM) groups; place into 37°C and 5% CO2 incubator for cultivating for 24 h.
Protective effects of drug-containing plasma on cardiomyocytes with hypoxia/reoxygenation injury
Take primary cultured neonatal rat cardiomyocytes cultivated for 4 days and randomly divide into 6 groups, 10 pores each group: ① blank control group: add 15% blank plasma; ② Chinese positive control group: add 15% Yangxinshi tablet plasma; ③ Western positive control group: add 15% verapamil hydrochloride tablet plasma; ④ drug-containing plasma containing different concentrations of ferulic acid (i.e., high, medium and low concentrations are, respectively, 7.8 mg/mL; 2.6 mg/mL; 0.87 mg/mL), and place into 37°C and 5% CO2 incubator for cultivating for 24 h.
Determination of cell viability[19]
Adopt MTT method, after cell counting, inoculate 105 cells/pore in 96-pore plate, use for the experiment after cultivating for 72 h, add 20 μL (5 mg/mL) MTT reagent into each pore after hypoxia/reoxygenation, continue incubating in 37°C and 5% CO2 incubator for 4 h, remove the supernatant, add 150 μL dimethyl sulfoxide (DMSO), shake at room temperature for 10 min, and measure OD values with microplate reader of 492 nm wavelength. Survival rate = OD test/OD control, injury inhibition rate = (OD test-OD control)/OD control.
The detection of LDH activity in the myocardial cell culture supernatant
Catalytic lactic acid generate pyruvate, pyruvate and 2, 4-2 nitrobenzene hydrazine react and produce pyruvic acid nitrobenzene hydrazone, appear to be palm red in the alkaline solution. The enzyme activity can be obtained by colorimetric method. Take the cell culture supernatant 50 μL and test the tube absorbency value in 440 nm after reaction.
The determination of Na+-K+-ATPase and Ca2+-ATPase activity in the myocardial cell
ATPase can decompose ATP production ADP and inorganic phosphorus, by determination the amount of inorganic phosphorus we can know ATPase activity. After the experiment, abandon the medium and digestive with mixed digested liquid 2 mL for 1-2 min, after a few cells fall off, abandon the digest fluid, add 3 mL medium, wind down the cell, centrifugal and wash one time, add 1 mL PBS into the cell, use ultrasound machine to crush for 20 s. We all operate according to kit instructions, and test protein content with Coomassie brilliant blue method.
Electrophysiological Phenomena Experiments
Pulse experiments
After 24-48 hours, pseudopodia contact with each other and gradually form single cells and cell clusters. Beating rhythm gradually to be rules, after 48 hours, synchronization of beating rhythm has already formed gradually, during the experiments we chose myocardial cell clusters of 4-6 days which beat gradually with synchronization, after stabltion of 20 min, we make record of the change of the pulse frequency before and after administration. And the temperature of flast should be set at 36.5 ± 0.5°C.
The record and leading of action potential[20]
We chose myocardial cell clusters of 3-6 days which gradually beat synchronization and then we made use of the glass microelectrode of 10-25 mΩ resistance whose diameter is less than 0.5 μum, and fills with 3 mol/L of KCL. we use the microelectrode thrusters to penetrate into the cells in the right angle, lead to action potential, amplify through the microelectrode amplifier, one way entered the displayer crossing the line, the anther one entered the oscilloscope referral to show Vmax through differentiator. Keep the stable conditions for 20 min after leading to the action potential and make record of action potential before and after administration, t-test was used during the results processing.
Statistical analysis
Experimental data are expressed with x ± s, single-factor analysis of variance is adopted to make significance comparison between the groups, and t test is adopted to make mean comparison between the two groups.
RESULTS
Result of the effect of different concentrations of sodium hydrosulfite on cardiomyocytes
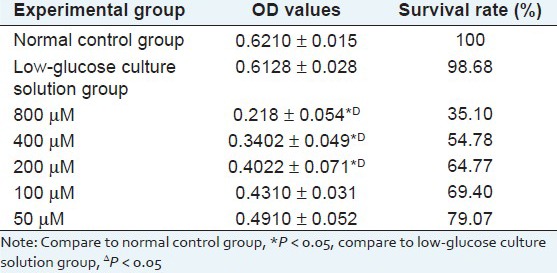
Experimental results show that as sodium hydrosulfite concentration increases, cell survival rate significantly decreases, and when the concentration is higher than 200 μM, the cell survival rate, measured with MTT method, has significant difference (*P < 0.05), compared with the normal control group, but considering the formation of myocardial injury model, 400 μM is selected as the best. As shown in Table 1.
Table 1.
Result of the effect of different concentrations of sodium hydrosulfite on cardiomyocytes (X̄ ± s)
Result of the effect of different action time of sodium hydrosulfite on cardiomyocytes
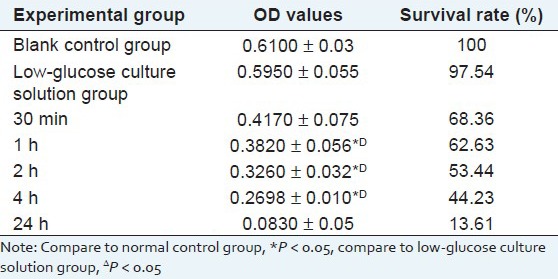
Experimental results show that in the same concentration, as sodium hydrosulfite injury time increases, the injury to cardiomyocytes significantly increases and cardiomyocyte survival rate significantly decreases, and the cell survival rate, measured with MTT method, has significant difference (*P < 0.05), compared with the normal control group. As shown in Table 2.
Table 2.
Result of the effect of different action time of sodium hydrosulfite on Cardiomyocytes (X̄ ± s)
Result of protective effects[21] of ferulic acid on cardiomyocytes with hypoxia/reoxygenation injury[11,12,13,14,15,16,17,18]
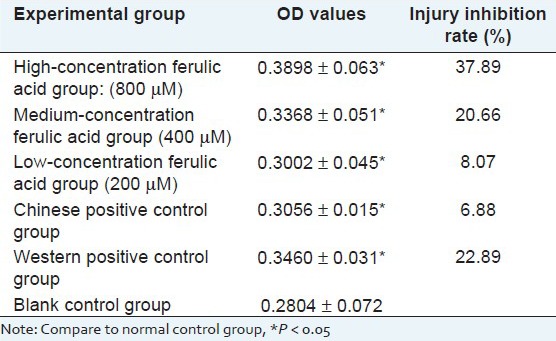
Experimental results show that as ferulic acid dose increases, the cell survival rate, measured with MTT method, significantly increases, indicating that ferulic acid has protective effects on the hypoxia/reoxygenation injury caused by sodium hydrosulfite, meanwhile, Chinese positive control group and Western positive control group have strong protective effects on injured cardiomyocytes, and high-dose ferulic acid group has significantly higher protective effects than the positive control groups. As shown in Table 3.
Table 3.
Result of protective effects of ferulic acid on cardiomyocytes with hypoxia/reoxygenation (X̄ ± s)
Result of protective effects of drug-containing plasma[22,23] on cardiomyocytes with hypoxia/reoxygenation injury
Experimental results show that high, medium and low-dose ferulic acid-containing plasma groups, Chinese positive plasma group and Western positive plasma group have protective effects on cardiomyocytes with hypoxia-reoxygenation injury, and the protective effects of ferulic acid-containing plasma are superior to Chinese and Western positive control groups; as ferulic acid-containing plasma concentration increases, the survival rate of cardiomyocytes has no dependence of concentration, and the survival rate in the low-dose ferulic acid-containing plasma group is the highest. As shown in Table 4.
Table 4.
Result of protective effects of drugcontaining plasma on cardiomyocytes with hypoxia/reoxygenation injury (X̄ ± s)
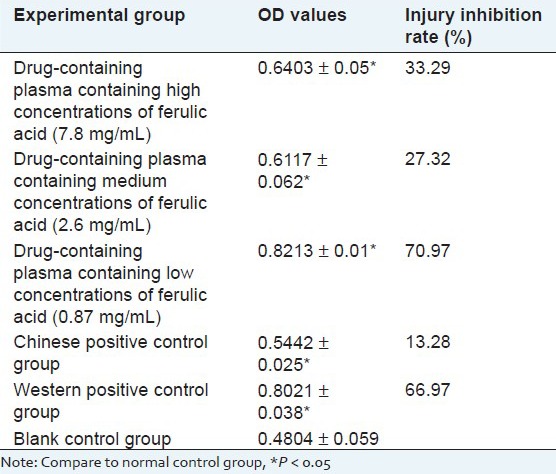
Result of effects of ferulic acid and its drug-containing plasma on the ldh activity in the myocardial cell culture supernatant and na+-k+-atpase and ca2+-atpase activity in the myocardial cell
Experiments show that Compared with control group, the LDH leakage of damage group increase, the activity of Na+-K+-ATPase and Ca2+-ATPase decline, ferulic acid and its drug-containing plasma both can reduce the leakage of LDH, increase the activity of Na+-K+-ATPase and Ca2+-ATPase and the drug-containing plasma has much better protective effect than ferulic acid. As shown in Table 5.
Table 5.
Result of effects of ferulic acid and its drug-containing plasma on the LDH activity in the myocardial cell culture supernatant and Na+−K+− ATPase and Ca2+− ATPase activity in the myocardial cell(X̄ ± s)
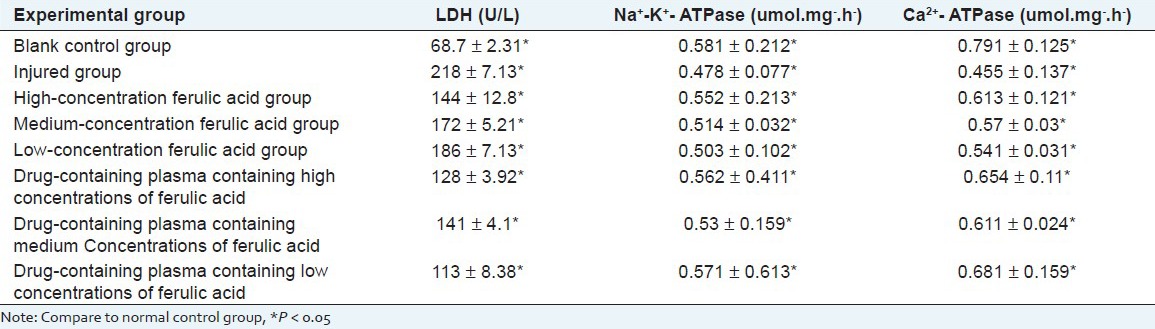
Result of effects of ferulic acid and its drug-containing plasma on spontaneous frequency of myocardial cell
Under inverted microscope, cardiomyocytes are round and suspended. Trypan blue stains for counting, and the unstained is viable cells, indicating that cardiomyocyte survival rate reaches 96%. After 4 h, they gradually adhere to wall, first in round shape and then in spindle shape, and the cells gradually extend on the wall, stretch out pseudopodia, and turn into triangle, polygon and other irregular shapes. After 24 h, it can be seen that the cells gradually stretch out pseudopodia. Cell mass firstly starts beating, and then single cell starts beating. Beating frequency varies, spontaneous frequency of myocardial cell before and administration is shown as Table 6. And ferulic acid-containing plasma group have better effect on strengthening the spontaneous frequency of cardiomyocytes cells.
Table 6.
Result of effects of ferulic acid and its drug-containing plasma on spontaneous frequency of myocardial cell (X̄ ± s)
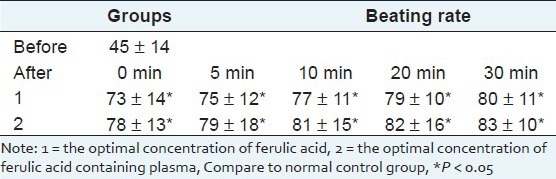
Result of effects of ferulic acid and its drug-containing plasma on potential across plasma membrane of myocardial cells
We chose myocardial cell clusters of 3-6 days which beat gradually synchronization, adding the optimal concentration of ferulic acid and its drug-containing plasma can make APA, MPD and Vmax decreased obviously. (P < 0.05) 5 min has little effect, 20 min has the most obvious effect, 30 min resumes. As shown in Table 7.
Table 7.
Result of effects of ferulic acid and its drug-containing plasma on potential across plasma membrane of myocardial cells(X̄ ± s)
Disscusion
One common problem in primary cultured cardiomyocyte cultivation is contamination of fibroblasts. After cultivated for 1~1.5 h, fibroblasts mostly adhere to the wall, while cardiomyocyte adhesion is slower. For this characteristic, differential adhesion method can be adopted to separate the two cells. Differential adhesion separation method can purify cardiomyocytes to 90%~95% purity, but fast proliferated fibroblasts can quickly overwhelm the cardiomyocytes in quantity, so how to make cardiomyocyte culture method simpler and make the separated cardiomyocytes reach the desired survival rate and purity is the key issues for cardiomyocyte culture in vitro. In operation, this paper makes the following improvements: ① Select the mixed digestion solution with moderate function and applicable to cardiomyocyte digestion and separation, and it can quickly digest the tissue and will not injure cardiomyocytes. ② During digestion, water bath temperature should be constant at 37°C appropriately, the first cardiomyocyte digestion time should be 10 min, and later the digestion time can be selected at 7~8 min, till the tissue is completely digested and turned into translucent state. Too long time and too high temperature for digestion will make cardiomyocytes lose activity. pH is adjusted within the range of 7.2~7.4, and higher or lower will affect the normal growth of cardiomyocytes and weaken or lose the ability of spontaneous beating. ③ Too long time or too large force of centrifugation will cause mechanical injury of cardiomyocytes, so 1000 r/min centrifugation for 10 min is appropriate. ④ After purification with the differential adherent separation method, 5-bromo-a-deoxyuridine is added to inhibit the synthesis of fibroblast DNA and protein, and by inhibiting the proliferation of the fibroblasts, it can ensure that cardiomyocytes still maintain high purity after cultured for 3~5 d.
Because in vivo model operation is relatively complicated, and modeled animal survival rate is relatively low, so cells are often selected as the primary means for efficacy screening, and neonatal rat cardiomyocyte hypoxia/reoxygenation is often used to simulate in vivo myocardial ischemia/reperfusion injury. Xu Daqiang etc[24] and Wang Feng,[25] Chen Yupei etc[26] Firstly use 99.99% nitrogen to presaturate medium for 10 min (nitrogen 1 L/min), and then fill with 99.99% nitrogen again for 30 s, drive away air of training bottle and continue which called hypoxia 1.5 h for oxygen, then to oxygen for 1 h, with normal medium nitrogen to replace the saturated medium, and continue to culture for 1 h, prepare the experiment model of hypoxia/reoxygenation. But during this method, nitrogen is easy to the loss and is not easy to control the content of nitrogen, and repeatability of operation is poorer. Zeng Jing etc[27] change for no sugar serum-free media, drop 2 mL sterilization liquid paraffin wax into each well of training board, and closed for 2, 4, 8, 12 h, respectively, suck out paraffin wax, and wash with sterilizated of PBS for three times. And then change for the serum containing 15% DMEM medium, and continue to culture for 2 h in CO2 incubator, that's reoxygenation. But the liquid paraffin wax may have certain effect to the growth of cells. And have bad effect to the accuracy of experimental results, Therefore, in this experiment we chose Na2S2O4 to cause the damage of myocardial cells, Na2S2O4, as an oxygen remover, does not injure cell membrane, and usually appropriate dose can remove oxygen in culture medium can cause cardiomyocyte hypoxia; replacing medium after hypoxia results in reoxygenation. This method is simple and easy to operate, and has better repeatability, is considered as a good method for preparation of cardiomyocyte hypoxia/reoxygenation.
When studying drug efficacy, in vitro experiments are usually adopted to make high-throughput screening. Due to complex chemical composition and metabolic process of drug, simple use of monomer composition or extract to directly add into in vitro experiment system, it may affect the authenticity and reliability of experiment results. The pharmacological method of using animal drug-containing plasma to make in vitro experiments is to give drug to animal for certain time and take its plasma to make in vitro experiments, which is closer to actual in vivo situation, is a practical in vitro experiment system to study pharmacological efficacy, and can not only reflect the direct effect of the absorbable part in drug, but also can reflect the effects of the metabolites formed by drug composition in body and drug induced endogenous active substances, closer to the real process produced by drug in body environment, and providing a new method for studying Chinese medicine pharmacology from cellular and molecular levels.
LDH is the sign enzyme of myocardial cells, When membrane structure of cardiac muscle change, integrity destruction or permeability increase, a large amount of LDH of cells will leak, so the LDH content in the medium increase. A good method which can react the damage rate of the cell membrane indirectly
ATPase is protease that exists in cell membrane and organelles membrane, its dynamic react the cell energy metabolism and the loss of function. The activity of Na+-K+-ATPase in the hypoxia/reoxygenation injury myocardial cells is lower. Thus that causes abnormalities of ion transport of inside and outside of cell, and makes the Na+ in cells unable to discharge, and the gradient of Na+ across the plasma membrane descent, and activates Na+/Ca2+ exchange system, and activates the Ca2+ into the cell, forms calcium overload[28,29]. At the same time as Ca2+-ATPase activity reduce, due to dysfunction of the calcium pump in cell membrane and muscle plasma nets, which make Ca2+ extracellular loss in control and Ca2+ inside the cell and discharge, storage obstacles, finally cause the calcium overload in cells. Free calcium is one of the members which participates in the cell signal transmission pathways and is related to the cell apoptosis in the early signal. Therefore it shows that Ca2+ plays an important role in cell apoptosis and hypoxia/reoxygenation injury.
Studies show that ferulic acid causes positive inotropic role of myocardial cells through the cAMP as a second messenger mediated, this kind of action is the same with β agonists isopropyl adrenal, which is because cAMP increased, which causes the increase in the number of slow Ca2+ channels available. Make Ca2+ inward flow increase, and increase Ca2+ inside cells[30] the experimental observation shows that the ferulic acid and its drug- containing plasma can accelerate the beating frequency of myocardial cells, the increase of APA, Vmax, and MPD also support the idea above. Trautwein W. etc found that cAMP makes APA and Vmax increase.[31,32] The results are the same with observed one during the experiment that ferulic acid and its drug- containing plasma have the influence to action potential.
Yangxinshi tablet, followed by the Chinese Traditional Medicine therapeutic principle of “supportting healthy energy and promoting blood circulation to arrest pain”, is a kind of Chinese patent medicine adopted by Modern process technology, whcih is composed of 13 tastes of Chinese traditional medicine including Astragalus, dangshen, Salvia, Puerarin, Epimediumherb, hawthorn, Rehmannia, Angelica, Coptis, Corydalis, Ganoderma, Ginseng and Glycyrrhiza. And the complex prescription has the function of promoting blood circulation to arrest pain and is used to treat coronary heart disease of qi deficiency and blood stasis; angina, myocardial infarction with high blood fat, high blood sugar and so on with the same symptom as the above. In this prescription, Ginseng tonify vigour, benefit spirit and strengthen heart function; Astragalus tonify gas, impulse the stagnation; Salvia promote blood flow and remove blood stasis, promote and alleviate pain. With Dangshen tonify middle energizer and benefit gas; Angelica nourish and promote blood flow to alleviate the pain; Hawthorneliminate stasis to activate blood circulation; Corydalis promote blood flow, promote gas and alleviate pain; Total flavonoids contained in Puerarin can lower blood pressure and cerebral vascular resistance, increasing brain and coronary artery blood flow, reduce vascular resistance, reduce myocardial oxygen consumption, against the coronary artery vasospasm caused by hypophysin, improve myocardial metabolism, improve brain coronary circulation, etc. Complete with Epimedium herb tonify kidney Yang; Rehmannia nourish Yin liquid; Ganoderma tonify gas and benefit essence, calm the nerves; Coptis can clear away heart-fire and clear the vexation; Glycyrrhiza tonify middle energizer and benefit gas, alleviate the pain, to reconcile the medicine, for the role of adjuvant drug in the formula. All The medicine used together, can play the role of the supporting healthy energy, benefiting qi and activating blood circulation, and promoting pulse and alleviating the pain. And modern pharmacology study shows that, Yangxinshi tablet is based on the above pharmacological effects, so it can obtain the purpose to remove vascular smooth muscle spasms, expand the blood vessels, speed up the blood flow velocity, improve blood circulation, enhance the supply of blood and oxygen of the organization, thus it can improve heart head blood-vessel diseases. Therefore, we chose Yangxinshi tablet as the Chinese postive drug, in this case, which can reflect the better the protective effects of ferulic acid and its drug-containing plasma on primary cultured neonatal rat cardiomyocytes with hypoxia/reoxygenation injury.
CONCLUSIONS
Ferulic acid and its drug-containing plasma can reduce the damage hydrosulfite to myocardial cell damage and improve myocardial viability, reduce the amount of LDH leak, increase the activity of Na+-K+-ATPase and Ca2+-ATPase, and increase APA, Vmax and MPD. And ferulic acid and its drug-containing plasma both have protective effect on injured myocardial cell. However, the drug-containing plasma has much better protective effect than ferulic acid.
Footnotes
Source of Support: This work was “Chinese Eleventh five-year plan” and “The special issues of major national science and technology”, “Component of Chinese herbal medicines sub-platform” (item number: 2010ZX09401-304-105C), “Combination components the new drug Salvia Xinmaitong capsule developement” (item number: 2010ZX09401-304-515) and it was supported by a grant from National Key New Drug Creation Special Management Office and and Liaoning Pharmaceutical Co., Ltd. P.R. China. The authors would like to thank the authorities in the Department of Pharmacy, Liaoning University of Traditional Chinese Medicine and Dianlian Medical University, P.R. China, for their support
Conflict of Interest: None declared.
REFERENCES
- 1.Hu YY, Xu XY. The chemical and pharmacological research of ferulic acid. Medicine. 2006;28:253–5. [Google Scholar]
- 2.Wang HW, Shi EX. The advance in the research on phamacology action of ferulic and its derivatives. Acta Academiae Medicinae Neimongol. 2007;29:601–3. [Google Scholar]
- 3.Liang N, Sun SP, Luo YE, Di LZ, Liu B. Research and process of ferulic acid. Heilongjiang Journal of Traditional Chinese Medicine. 2009;3:39–40. [Google Scholar]
- 4.Zhao DP, Yang WY. Research and process of ferulic acid. LiShizhen Medicine And Materia Medica Research. 2008;19:1839–41. [Google Scholar]
- 5.Li B, Du LJ, Wang YL, Sun ZR, Xu CQ, Zhang WH. The protective effect of ischemic preconditioning on neonatal rat myocardial ischemia/reperfusion injury. Journal of Qiqihar Medical College. 2008;29:416–8. [Google Scholar]
- 6.Zhang MF, Shen YQ. Anti-hypoxia effect of caffeic acid and ferulic acid. Northwest Pharmaceutical Journal. 1994;9:118. [Google Scholar]
- 7.Zhang MF. Research and process of ferulic acid's anti-atherosclerosis. Chinese Traditional and Herbal Drugs. 1990;21:41. [Google Scholar]
- 8.Huang WY, Chen SY, Qiu XS. Chinese medicine research of anti-atherosclerosis. Research of Traditional Chinese Medicine. 1998;14:58. [Google Scholar]
- 9.Kamal-Eldin A, Frank J, Razdan A, Tengblad S, Basu S, Vessby B. Effects of dietary phenolic compounds on tocopherol, cholesterol, and fatty acids in rats. Lipids. 2000;35:427–35. doi: 10.1007/s11745-000-541-y. [DOI] [PubMed] [Google Scholar]
- 10.Sakai S. Inhibitory effect of ferulic acid and isofenulic acid on the production of macrophage inflammatory protein-2 in response to respiratory syncytial virus infection in RAW264.7 ceils. Mediators Inflamm. 1999;8:173–5. doi: 10.1080/09629359990513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma QB, Gao W, Guo YH, Zhao CY, Xue L, Tang CS. Hypoxia/reoxygenation induced endoplasmic reticulum stress in cultured neonatal rat cardiomyocytea. Beijing Da Xue Xue Bao. 2005;37:386–8. [PubMed] [Google Scholar]
- 12.Matoba S, Tatsumi T, Keira N, Kawahara A, Akashi K, Kobara M. Cardioprotective effect of angiotensin-converting enzyme inhibition against hypoxia/reoxygenation injury in cultured rat cardiac myocytes. Circulation. 1999;99:817–22. doi: 10.1161/01.cir.99.6.817. [DOI] [PubMed] [Google Scholar]
- 13.Yu W, Xu B, Peng Y, Wu JL, Li L. Establisnment of hypoxia/reoxygenation model of rat myocardial induced by oxygen consumption of agent. LiShizhen Medicine And Materia Medica Research. 2010;21:1660. [Google Scholar]
- 14.Braunwald E, Kloner RA. Myocardial reperfusion: A double edged sward? J Clin Invest. 1985;76:1713–9. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J, Tu WF, Wang J, Fu YH, Lin QX. Lidocaine pretreatment on H 9 C 2 myocardial hypoxia-reoxygenation injury in rats. Guangdong Medical Journal. 2010;31:1077–80. [Google Scholar]
- 16.Wang J, Yu J, Tu WF, Fu YH, Lin QX. Sufentanil pretreatment of H 9 C 2 cells after hypoxia-reoxygenation injury in cell viability and lactate dehydrogenase activity. Guangdong Medical Journal. 2010;31:948–50. [Google Scholar]
- 17.Wang RH, Lan XL, Cao GX, An R, Zhang YX. Methods to simplify and improve of SD rat myocardial cell culture. Chinese Heart Journal. 2008;20:154–7. [Google Scholar]
- 18.Guo J, Massaeli H, Li WT, Xu JM, Luo T, Shaw J. Identification of IKr and its trafficking disruption induced by probucol in cultured neonatal rat cardiomyocytes. J Pharmacol Exp Ther. 2007;321:911–20. doi: 10.1124/jpet.107.120931. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Li JD, Huang QF. PNS on AngII-induced myocardial apoptosis. China Journal of Chinese Materia Medica. 2005;30:778–81. [PubMed] [Google Scholar]
- 20.Yang YH, Wang WX. The effect of ranitidine on spontaneous beating and action potential of primary cultured neonatal rat cardiomyocytes. Chinese Pharmacological Bulletin. 1996;12:164. [Google Scholar]
- 21.Bian YF, Guo XX, Xiao CS. Protective effects of adiponectin against hypoxia/reoxygenation injury in neonatal rat cardiomyocytes. Sheng Li Xue Bao. 2010;62:149–55. [PubMed] [Google Scholar]
- 22.He SL. Questioned serum pharmacology, to strengthen multi-level semi-vivo studies. Chinese Pharmacological Bulletin. 2005;21:277–9. [Google Scholar]
- 23.Wang DS, Chen FP, He SL, Xie QZ, Fu B, Li X. Comparative study of the effect of rhubarb hut worm pill pharmacology plasma and serum pharmacological. Chinese Journal of Thrombosis and Hemostasis. 2005;11:5–8. [Google Scholar]
- 24.Xu DQ, Lei J, Peng XD, Lin R. Protective effect of Soybean nucleoside yuan on neonatal rat cardiomyocytes cells damaged by hypoxia-reoxygenation. Ningxia Medical Journal. 2007;29:755. [Google Scholar]
- 25.Wang F, Liu JC. Reasearch on the protective effect of Gallic catechin gallic acid ester pretreatment to neonatal rat cardiomyocytes cells damaged by hypoxia-reoxygenation and related mechanism. Jiangxi Medical Journal. 2009;49:14. [Google Scholar]
- 26.Chen YP, Min S, Yang MC, Zhang WS, Liu J, Xu Q. The protective effect of L calcium channel participate in emulsification isoflurane on neonatal rat cardiomyocytes cells damaged by hypoxia-reoxygenation. Chinese Pharmacological Bulletin. 2009;25:585. [Google Scholar]
- 27.Zeng WJ, Liu JY, Ke CB. The Establishment of the model of cardiomyocytes cells damaged by hypoxia-reoxygenation through Liquid paraffin wax closed method. Yunyang Medical Journal. 2010;29:108. [Google Scholar]
- 28.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial Stutming. Physiol Rev. 1999;79:609–34. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 29.Van Eyk JE, Powers F, Law W, Larue C, Hodges RS, Solaro RJ. Breakdown and release of myofilament proteins during ischemia and ischemia/reperfusion in rat hearts: Identification of degradation products and effects on the pCa-force relation. Circ Res. 1998;82:261–71. doi: 10.1161/01.res.82.2.261. [DOI] [PubMed] [Google Scholar]
- 30.Sperelakis N. Cyclic AMP and phosphory|ation in regulation of Ca2+ influx into myocardial cells and blockated by calcium antagonistic drugs. Am Heart J. 1984;107:347–57. doi: 10.1016/0002-8703(84)90385-5. [DOI] [PubMed] [Google Scholar]
- 31.Trautwein W, Taniguchi J, Noma A. The effect of intracellular cyclic nucleotides and calcium on the action potential and acetylcholine response of isolated cardiac cells. Pflugers Arch. 1982;392:307–14. doi: 10.1007/BF00581624. [DOI] [PubMed] [Google Scholar]
- 32.Liu B, Clanachan AS, Schulz R, Lopaschuk GD. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ Res. 1996;79:940–8. doi: 10.1161/01.res.79.5.940. [DOI] [PubMed] [Google Scholar]
- 33.Baidu Encyclopedia of introduction of ferulic acid [on line] [Last accessed on 2012 May 22]. Available: http://baike.baidu.com/view/337829.htm .


