Abstract
Background:
Buckwheat (Fagopyrum spp., Polygonaceae) is a widely planted food crop. Flavonoids, including quercetin, rutin, and kaempferol, are the main bioactive components in tartary buckwheat (Fagopyrum tataricum (L.) Gaertn). From the nutriological and pharmacological perspectives, flavonoids have great value in controlling blood glucose and blood pressure levels, and they also have antioxidant properties.
Objective:
To optimize the conditions for extraction of quercetin, rutin, and kaempferol from F. tataricum.
Materials and Methods:
A combination of ultrasound-assisted extraction (UAE) and response surface methodology (RSM) was used for flavonoid extraction and yield assessment. The RSM was based on a three-level, three-variable Box-Behnken design.
Results:
Flavonoids were optimally extracted from F. tataricum by using 72% methanol, at 60°C, for 21 minutes. Under these conditions, the obtained extraction yield of the total flavonoids was 3.94%.
Conclusion:
The results indicated that the UAE method was effective for extraction of flavonoids from tartary buckwheat.
Keywords: Buckwheat, flavonoids, Fagopyrum tataricum, response surface methodology, ultrasound-assisted extraction
INTRODUCTION
Buckwheat (Fagopyrum spp., Polygonaceae) is widely planted as a food crop. Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn), an edible and medicinal crop, is becoming increasingly popular because of its benefits to the human body.[1,2,3] Tartary buckwheat is receiving widespread attention as a functional food,[4] and a number of commercial buckwheat products are now being produced and distributed. Buckwheat contains many beneficial components, such as, flavonoids, fagopyrins, and D-chiro-inositol.[5,6] Such components have been reported to help control blood glucose[7] and blood pressure levels.[8] Moreover, buckwheat (Fagopyrum esculentum Moench) may be useful in the treatment of cancer.[9] Considerable research done currently on buckwheat is focused on its health efficacy and on component extraction processing.[10,11,12,13] The amount of total flavonoids (quercetin, rutin, and kaempferol) contained in F. tataricum is reported to be far higher than that in common buckwheat (F. esculentum Moench).[14] Rutin and quercetin are the most intensely studied flavonoids in tartary buckwheat, due to their functions and high concentrations.[15,16,17,18]
At present, various extraction techniques have been developed for the extraction of flavonoids from tartary buckwheat, including, ultrasound-assisted extraction (UAE), microwave-assisted extraction, and oscillation extraction.[19,20,21] The UAE method is a simple, rapid extraction technique, with high extraction efficiency, which is attributed to the effect of acoustic cavitation produced in the solvent by the passage of an ultrasound wave.
Response surface methodology (RSM) is a powerful statistical technique that is useful when optimizing processes in the fields of medicine and nutrition. It has been reported that RSM can be used to optimize complex processes used to extract compounds from plants.[22,23,24,25,26,27] To our knowledge, there are no reports on the use of RSM to optimize extraction conditions for quercetin, rutin, and kaempferol in buckwheat. In this study, we have focused on establishing a rapid and convenient method for extracting and quantifying three of the flavonoids (quercetin, rutin, and kaempferol) present in F. tataricum. The method uses UAE and RSM, which is based on a three-level, three-variable (extraction time, extraction temperature, and methanol concentration) Box-Behnken design (BBD). The results should be helpful in the further utilization of flavonoids from tartary buckwheat.
MATERIALS AND METHODS
Chemicals and reagents
Quercetin, rutin, and kaempferol used as reference standards were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Acetonitrile and methanol (high performance liquid chromatography (HPLC) grade) were purchased from Fisher Scientific Co. (USA). All others chemicals and solvents used in the study were of analytical grade.
Plant material
The samples of F. tataricum were harvested from the experimental farm of Chengdu University, Chengdu, Sichuan, China, in November 2011. The species identification was authenticated by Professor Zhao Gang (Chengdu University). The obtained tartary buckwheat seeds were dried, ground, and then passed through the sieve screen. The powder obtained from the 20 and 40 mesh sieve screens was subjected to UAE extraction.
Ultrasound-assisted extraction
Ultrasound-Assisted Extraction was performed by mixing 0.1 g of the sieved, dried powder with 25 ml of a predetermined concentration of ethanol in a single conical flask, followed by weighing and ultrasonic extraction for a predetermined time, at a predetermined temperature. The ultrasonic device parameters were 200 W of power at a frequency of 50 kHz. Following extraction, the mixture was cooled to room temperature. Subsequently, the extract was filtered and the filtrate collected for HPLC assessment.
Experimental design
The preliminary ranges of the extraction variables, namely, methanol concentration (X1), extraction time (X2), and extraction temperature (X3), were established by using a single-factor test. Subsequently, a Box-Behnken factorial design (BBD; Design-Expert software, version 7.1.6, Stat-Ease, Minneapolis, MN, USA) with three levels and three variables was applied, to determine the best UAE conditions for optimizing flavonoid yield. The adjusted R-squared (R2) values along with the F-test results and probability (p) values were used to evaluate the results of the model equations.
High performance liquid chromatography analysis
The UAE-obtained F. tataricum extracts were passed through 0.45 μm filters and then placed in an HPLC autosampler vial for immediate HPLC analysis. The rutin, quercetin, and kaempferol reference standard solution was prepared by dissolving rutin, quercetin, and kaempferol in 70% methanol. The HPLC system was comprised of two Shimadzu LC-20A pumps and a Shimadzu LC-20A autosampler (Kyoto, Japan). A Diamonsil-ODS C18 (250 mm × 4.6 mm × 5 μm) column was used. The temperature of the column was 30°C. Separation was performed by using a mixture of acetonitrile and distilled water containing 0.3% H3PO4, with a gradient elution: 0–8 minutes (20% acetonitrile), 8–13 minutes (20–40% acetonitrile), 13–29 minutes (40% acetonitrile), 29–29.1 minutes (40–20% acetonitrile), and 29.1–30 minutes (20% acetonitrile). The flow rate was set at 1 ml/minute. The eluent was obtained after the column was sent to a UV/VIS detector (Shimadzu, Kyoto, Japan). The detector wavelength was set at 365 nm.
RESULTS AND DISCUSSION
Chromatographic results
Chromatography images from the reference standard flavonoids and from the UAE-extracted tartary buckwheat sample are shown in Figure 1. Chromatographic results from the F. tataricum sample [Figure 1b] show that quercetin, rutin, and kaempferol were separated well, with a retention time of 9.337 minutes, 20.337 minutes, and 24.195 minutes, respectively. The total flavonoid yield was the total of the individual yields of the three assessed flavonoids: quercetin, rutin, and kaempferol.
Figure 1.
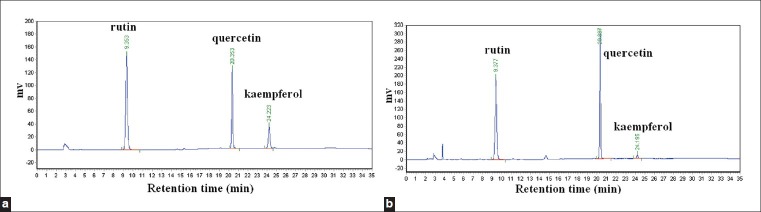
The HPLC profiles of quercetin, rutin, kaempferol standard substance (a) and tartary buckwheat extraction (b)
Selection of solvent
Flavonoids are normally extracted with methanol and ethanol. In this study, we attempted to determine the extraction solvent that produced the highest flavonoid yield. Our results indicated that the extraction yield of total flavonoids was higher when methanol was used as the extraction solvent (3.15%) than when ethanol (3.04%) was used. Moreover, methanol concentration was important to investigate the effect of methanol concentration on flavonoid yield; five different methanol concentrations were compared [Figure 2a]. When the methanol concentration was increased from 10 to 50%, the yields of quercetin and kaempferol reached their peak, but the yield of rutin was low. This could be because rutin could degrade quickly in low methanol concentrations due to the rutin-degrading enzyme contained in tartary buckwheat.[28] When the methanol concentration increased from 50 to 70%, the rutin yield reached a maximum. The yield slightly decreased at methanol concentrations over 70%. Hence, 70% methanol was chosen for testing in the subsequent optimization experiments.
Figure 2.
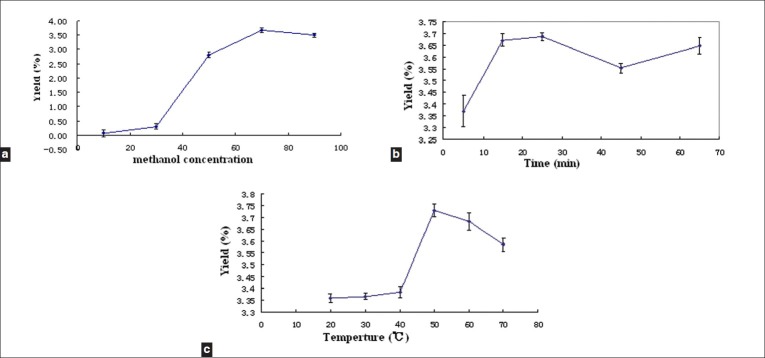
The effects of the extraction parameters on flavonoids yield: (a) Effect of methanol concentration on flavonoids yield. Other conditions were 50°C extraction temperature and 25 minutes extraction time. (b) Effect of extraction time on flavonoids yield. Other conditions were 70% methanol and 50°C extraction temperature. (c) Effect of extraction temperature on flavonoid yield. Other conditions were 70% methanol and 25 minute extraction time
Extraction time
Extraction time is another important factor that influences extraction yield, as usually, extraction yield increases with extraction time. In this study, UAE extraction was carried out for different durations (5 – 65 minutes). The extraction yield increased markedly when the duration increased from five minutes to 15 minutes [Figure 2b]. However, the yield remained approximately constant at durations from 15 minutes to 65 minutes. On the basis of these results, the extraction time was set at 15 minutes in the optimization experiments.
Extraction temperature
Temperature could also influence the extraction yield, as an increase in temperature could accelerate the extraction speed.[24] In this study, five different extraction temperatures were tested [Figure 2c]. The results indicated that the extraction yield increased markedly at extraction temperatures of 40°C and 50°C, but slightly decreased at temperatures above 50°C and higher. We speculated that, due to the actions of the rutin-degrading enzyme, the speed of rutin degradation may be accelerated when the temperature was lower than 50°C. Hence, 50°C was chosen for testing in the subsequent optimization experiments Figure 2.
Optimization of flavonoid yield
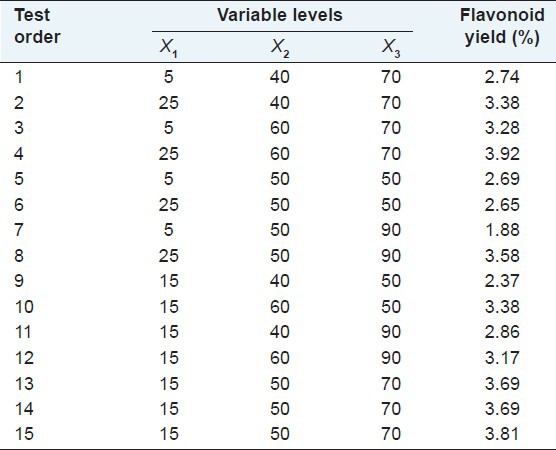
In order to determine the best extraction conditions, we performed parameter optimization through RSM. The RSM approach was based on the three levels and three variables described in Table 1. During our assessment, 12 factorial experiments were performed, along with three zero-point tests, to allow error estimation. Table 2 shows the results of those 15 experiments with the total flavonoid yield ranging from 1.88 to 3.92%. The results indicated that the best yield was obtained by performing UAE for 21 minutes at 60°C with a methanol concentration of 72%. The regression equation between the flavonoid yield (Y) and variables X1, X2, and X3 was:
Table 1.
The three levels of the three variables in the RSM assessment
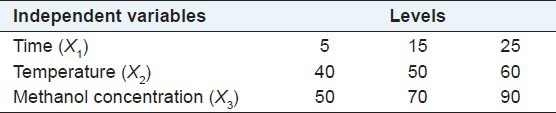
Table 2.
Response surface design and experimentally obtained data
Y = 3.73 + 0.37 X1 + 0.30 X2 + 0.050 X3 + 0.43 X1X3 - 0.17 X2X3 - 0.32 X12 - 0.077 X22 - 0.71 X32
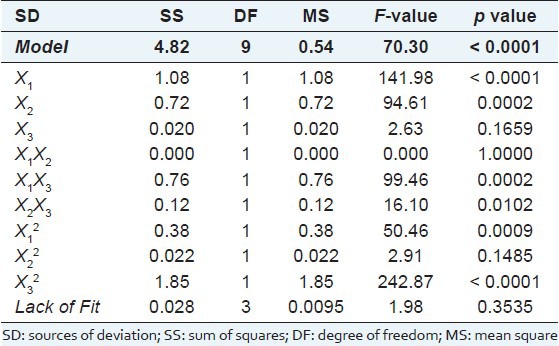
The analysis of variance (ANOVA) results for the regression model are shown in Table 3. The Model F-value of 70.30 implied that the model's relationships were significantly relative to the noise in the data, as there was only a 0.01% chance that a Model F-value this large could occur due to noise. The model's Lack of Fit F-value of 1.98 implied the model's lack of fit was not significant relative to pure error, as there was a 35.35% chance that a Lack of Fit F-value this large could occur due to noise. The non-significance of the Lack of Fit F-value indicated the validity of the regression model. The adjusted R-square for the equation was close to unity (R2 = 0.9922), indicating a high correlation between the observed and predicted values. The Design-Expert statistic ‘Adeq Precision’ was a measure of the model's signal-to-noise ratio, and a ratio greater than 4 indicated adequate model discrinination. The ratio obtained from our model was 28.781, which indicated model adequacy. Moreover, a low coefficient of variance (2.78) indicated a high degree of precision in the experimental values. In conclusion, the model (equation 1) was suitable for extracting flavonoid from tartary buckwheat.
Table 3.
Analysis of variance (ANOVA) results for regression equation 1
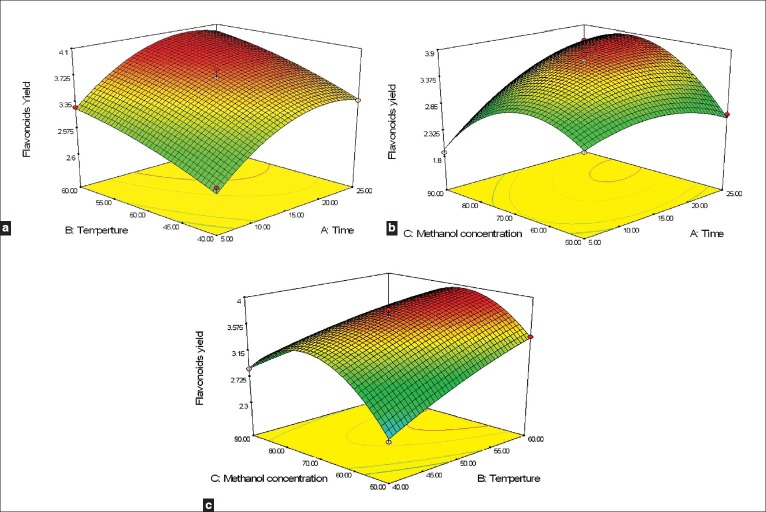
Three-dimensional response surface plots are presented in Figure 3. These results differed from those of the single-factor-test. The RSM results indicated that an increase in extraction temperature improved the extraction yield, which was not shown by the single-factor-test results. A possible explanation for the difference was that at a high methanol concentration, the extracted rutin was relatively stable, and as a result, with an increase in temperature, the extraction speed increased. On the basis of the RSM results, an increase in methanol concentration from 50 to 72% improved the extraction yield. However, when the methanol concentration was more than 72%, a slight decline in the response was observed. In the RSM plots in Figure 3, extraction times of over 21.4 minutes did not have an obvious effect on the extraction yield. A possible explanation for the result was that an increase in extraction time could accelerate rutin degradation during extraction, resulting in a lower yield.
Figure 3.
Response surface graphs for the effects of extraction time, extraction temperature, and methanol concentration on the flavonoid yield of tartary buckwheat: (a) Temperature and Time. (b) Time and methanol concentration. (c) Temperature and methanol concentration
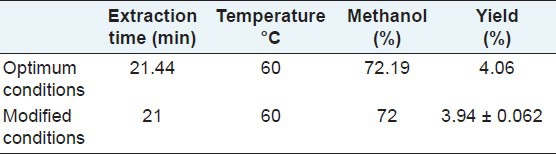
The maximum extraction yield of the three flavonoids combined was calculated by the Design-Expert software. The conditions that provided the highest percentage of extraction of total flavonoids were, a methanol concentration of 72%, extraction time of 21 minutes, and a temperature of 60°C. For these conditions, the corresponding theoretical maximum yield was 4.06%. To confirm the theoretical results, three parallel experiments were carried out under those optimized conditions. The average actual extraction yield obtained from the experiments was 3.94%, very close to the predicted results [Table 4]. By using UAE with these optimized conditions (extraction time, 21 minutes; temperature, 60°C; and solvent, 72% methanol), the yield of total flavonoids (%) in three varieties of tartary buckwheat were as follows: 3.98 ± 0.057 in Chuanqiao 1, 3.87 ± 0.065 in Xiqiao 1, and 4.04 ± 0.063 in Miqiao 1.
Table 4.
Theoretical yield of total flavonoids based on RSM-derived optimum extraction conditions and actual yield from modified experimental conditions
In conclusion, a new optimization method based on a combination of UAE and RSM was investigated for the extraction of total flavonoids from tartary buckwheat. The RSM method was based on a three-level, three-variable (extraction time, extraction temperature, and methanol concentration) BBD. The maximum extraction yield of total flavonoids was obtained by performing UAE with 72% methanol at 60°C, for 21 minutes. Under these conditions, the experimental yield of total flavonoids was 3.94%, close to the theoretical yield of 4.06%. The results indicated that the UAE-RSM approach was effective for maximizing the extraction of total flavonoids from tartary buckwheat.
ACKNOWLEDGMENTS
This research was financially supported by the National Spark Program of China (2010GA812002), a fund earmarked for the China Agriculture Research System (CARS-08-B-3), and by the Administration Foundation of Traditional Chinese Medicine of Sichuan (2010-78).
Footnotes
Source of Support: This research was financially supported by the National Spark Program of China (2010GA812002), a fund earmarked for the China Agriculture Research System (CARS-08-B-3, CARS-08-D-3), and by the Administration Foundation of Traditional Chinese Medicine of Sichuan
Conflict of Interest: None declared.
REFERENCES
- 1.He J, Klag MJ, Whelton PK, Mo JP, Chen JY, Qian MC, et al. Oats and buckwheat intakes and cardiovascular disease risk factors in an ethnic minority of China. Am J Clin Nutr. 1995;61:366–72. doi: 10.1093/ajcn/61.2.366. [DOI] [PubMed] [Google Scholar]
- 2.Guo X, Zhu K, Zhang H, Yao H. Anti-tumor activity of a novel protein obtained from tartary buckwheat. Int J Mol Sci. 2010;11:5201–11. doi: 10.3390/ijms11125201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Liu JR, Gao JM, Parry JW, Wei YM. Antioxidant activity of Tartary buckwheat bran extract and its effect on the lipid profile of hyperlipidemic rats. J Agric Food Chem. 2009;57:5106–12. doi: 10.1021/jf900194s. [DOI] [PubMed] [Google Scholar]
- 4.Min B, Lee SM, Yoo SH, Inglett GE, Lee S. Functional characterization of steam jet-cooked buckwheat flour as a fat replacer in cake-baking. J Sci Food Agric. 2010;90:2208–13. doi: 10.1002/jsfa.4072. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda K. Buckwheat: Composition, chemistry, and processing. Adv Food Nutr Res. 2002;44:395–434. doi: 10.1016/s1043-4526(02)44008-9. [DOI] [PubMed] [Google Scholar]
- 6.Yang N, Ren G. Determination of D-chiro-Inositol in tartary buckwheat using high-performance liquid chromatography with an evaporative light-scattering detector. J Agric Food Chem. 2008;56:757–60. doi: 10.1021/jf0717541. [DOI] [PubMed] [Google Scholar]
- 7.Yao Y, Shan F, Bian J, Chen F, Wang M, Ren G. D-chiro-inositol-enriched tartary buckwheat bran extract lowers the blood glucose level in KK-Ay mice. J Agric Food Chem. 2008;56:10027–31. doi: 10.1021/jf801879m. [DOI] [PubMed] [Google Scholar]
- 8.Kim DW, Hwang IK, Lim SS, Yoo KY, Li H, Kim YS, et al. Germinated Buckwheat extract decreases blood pressure and nitrotyrosine immunoreactivity in aortic endothelial cells in spontaneously hypertensive rats. Phytother Res. 2009;23:993–8. doi: 10.1002/ptr.2739. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Cui CB, Kang IJ, Kim SY, Ham SS. Cytotoxic effect of buckwheat (Fagopyrum esculentum Moench) hull against cancer cells. J Med Food. 2007;10:232–8. doi: 10.1089/jmf.2006.1089. [DOI] [PubMed] [Google Scholar]
- 10.Zhou XL, Zhou YM. Study on antioxidative activities of crude and pure flavoniods from buckwheat bran in vitro. Sci Technol Food Ind. 2008;10:78–80. [Google Scholar]
- 11.Huang YM, Li X, Zhang L. Protective Effects of Flavones of Buckwheat on Cerebral Ischemia-reperfusion Injury in Rats. J Sichuan Normal Univ. 2006;29:499–501. [Google Scholar]
- 12.Xu BC, Ding XL. Study on the extraction processing of flavon from duck wheat husk. Sci Technol Food Ind. 2002;23:40–3. [Google Scholar]
- 13.Bai BL, Cao BY, Zheng HY, Chang YQ. Extraction and Purification of Flavonoids from Tartary Buckwheat Leaves. Food Sci. 2008;29:181–5. [Google Scholar]
- 14.Naghski J, krewson CF, porter WL, couch JF. Factors affecting the rutin contents of dried buckwheat meals. J Am Pharm Assoc Am Pharm Assoc. 1950;39:696–8. doi: 10.1002/jps.3030391215. [DOI] [PubMed] [Google Scholar]
- 15.Vogrincic M, Timoracka M, Melichacova S, Vollmannova A, Kreft I. Degradation of rutin and polyphenols during the preparation of tartary buckwheat bread. J Agric Food Chem. 2010;58:4883–7. doi: 10.1021/jf9045733. [DOI] [PubMed] [Google Scholar]
- 16.Kalinova J, Vrchotova N. Level of catechin, myricetin, quercetin and isoquercitrin in buckwheat (Fagopyrum esculentum Moench), changes of their levels during vegetation and their effect on the growth of selected weeds. J Agric Food Chem. 2009;57:2719–25. doi: 10.1021/jf803633f. [DOI] [PubMed] [Google Scholar]
- 17.Zhao G, Zou L, Wang Z, Hu H, Hu Y, Peng L. Pharmacokinetic profile of total quercetin after single oral dose of tartary buckwheat extracts in rats. J Agric Food Chem. 2011;59:4435–41. doi: 10.1021/jf1049529. [DOI] [PubMed] [Google Scholar]
- 18.Dadáková E, Kalinová J. Determination of quercetin glycosides and free quercetin in buckwheat by capillary micellar electrokinetic chromatography. J Sep Sci. 2010;33:1633–8. doi: 10.1002/jssc.200900809. [DOI] [PubMed] [Google Scholar]
- 19.Liu CH, Gao JF, Wang PK, Feng BL, Cai Y. Research on Ultrasonic Wave Extraction of Flavone from Tartary Buckwheat. Acta Agric Boreali-occidentalis Sinica. 2009;18:281–4. [Google Scholar]
- 20.Du MH, Wang QL. Studies on the Chemical Change in the Process of Microwave-Assisted Extraction of Flavonoids from Tartary Buckwheat Bran. J Huazhong Agric Univ. 2008;27:536–9. [Google Scholar]
- 21.Liu BG, Zhu YY. Study on the Extraction Technology of Tartary-buckwheat Flavonoid. Cereal Feed Ind. 2004;4:23–5. [Google Scholar]
- 22.Mehrnoush A, Mustafa S, Sarker MZ, Yazid AM. Optimization of the conditions for extraction of serine protease from kesinai plant (Streblus asper) leaves using response surface methodology. Molecules. 2011;16:9245–60. doi: 10.3390/molecules16119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryad A, Lakhdar K, Majda KS, Samia A, Mark A, Corinne AD, et al. Optimization of the Culture Medium Composition to Improve the Production of Hyoscyamine in Elicited Datura stramonium L. Hairy Roots Using the Response Surface Methodology (RSM) Int J Mol Sci. 2010;11:4726–40. doi: 10.3390/ijms11114726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Yu Y, Yang R, Wan C, Xu B, Cao S. Optimization of Total Flavonoid Compound Extraction from Gynura medica Leaf Using Response Surface Methodology and Chemical Composition Analysis. Int J Mol Sci. 2010;11:4750–63. doi: 10.3390/ijms11114750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou TB, Wang M, Gan RY, Ling WH. Optimization of ultrasound-assisted extraction of anthocyanins from mulberry, using response surface methodology. Int J Mol Sci. 2011;12:3006–17. doi: 10.3390/ijms12053006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao LC, He Y, Deng X, Yang GL, Li W, Liang J, et al. Response surface modeling and optimization of accelerated solvent extraction of four lignans from fructus schisandrae. Molecules. 2012;17:3618–29. doi: 10.3390/molecules17043618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao LC, Liang J, Li W, Cheng KM, Xia X, Deng X, et al. The use of response surface methodology to optimize the ultrasound-assisted extraction of five anthraquinones from Rheum palmatum L. Molecules. 2011;16:5928–37. doi: 10.3390/molecules16075928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang GL, Zhou L, Liang R, Geng HL. Effect of Different Extraction Conditions on Rutin Hydrolysis of Fagopyrum tataricum Seeds. Acta Bot Boreali-Occidentalia Sin. 2005;25:1035–8. [Google Scholar]