Abstract
Background:
Many more fungal polysaccharides have been reported to exhibit a variety of biological activities, including anti-tumor, immunostimulation, anti-oxidation, and so on. The non-starch polysaccharides have emerged as an important class of bioactive natural products.
Objective:
To investigate the anti-microorganism, anti-tumor, and immune activities of a novel polysaccharide (TMP-A) isolated from Tricholoma matsutake.
Materials and Methods:
The anti-microorganism activity of purified polysaccharides (TMP-A) was evaluated by the inhibition zone diameter, the anti-tumor activity was evaluated by the S180 tumor cells that were implanted subcutaneously into the Kunming strain male mice in vivo, and the immune activity was evaluated by lymphocyte proliferation and macrophage stimulation, respectively.
Results:
In this study, the most susceptible bacteria of TMP-A at a concentration of 20 mg/ml was Micrococcus lysodeikticus (inhibition zone diameter 24.38 ± 1.19 mm) and the TMP-A did not show any antifungal activity for the tested stains of the fungi. In addition, the inhibitory rate in mice treated with 80 mg/kg TMP-A could reach 68.422%, being the highest in the three doses, which might be comparable to mannatide. The anti-tumor activity of the TMP-A was usually believed to be a consequence of the stimulation of the cell-mediated immune response, because it could significantly promote the lymphocyte and macrophage cells in the dose range of 50–200 μg/mL and in the dose range of 100 – 400 μg/mL in vitro, respectively.
Discussion and Conclusion:
The results obtained in the present study indicate that the purification polysaccharide of Tricholoma matsutake is a potential source of natural broad-spectrum, anti-microorganism, anti-tumor, and immunomodulation.
Keywords: Anti-microorganism activities, anti-tumor activities, immune activities, polysaccharide, Tricholoma matsutake
INTRODUCTION
A fungus is a member of a large group of eukaryotic organisms that include microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as a kingdom of Fungi, which are separate from plants, animals, and bacteria. One major difference is that fungal cells have cell walls that contain chitin, unlike the cell walls of plants, which contain cellulose. These and other differences show that the fungi form a single group of related organisms, named the Eumycota (true fungi or Eumycetes), that share a common ancestor (a monophyletic group). Fungi perform an essential role in the decomposition of organic matter and have fundamental roles in nutrient cycling and exchange. They also have long been used as a direct source of food, such as mushrooms and truffles.[1]
Fungal polysaccharide is a kind of active organic compound that is found in the fruiting bodies, mycelium, and fermentation broth of large edible and medicinal fungi.[2] Fungal polysaccharides are long carbohydrate molecules, of repeating units, joined together by glycosidic bonds. They are often linear, but may also be even highly branched.[2] In recent years, as many more fungal polysaccharides have been reported to exhibit a variety of biological activities, including anti-tumor, immunostimulation, anti-oxidation, and so on, the non-starch polysaccharides have emerged as an important class of bioactive natural products.[3,4,5,6,7,8] Certain mushrooms enjoy usage as therapeutics in folk medicines, such as Traditional Chinese Medicine. Notable medicinal mushrooms with a well-documented history of use include Agaricus subrufescens,[9,10] Ganoderma lucidum,[11] and Ophiocordyceps sinensis.[12] The shiitake mushroom is a source of lentinan, a clinical drug, approved for use in cancer treatments in several countries, including Japan.[13,14] In Europe and Japan, polysaccharide-K (brand name Krestin), a chemical derived from Trametes versicolor, is an approved adjuvant for cancer therapy.[15]
Tricholoma matsutake is a kind of fungi belonging to Subgenus Tricholoma, and is widely distributed in Asian countries, such as China, Japan, and Korea.[16,17] As a traditional edible fungus in oriental countries, it has been consumed as a vegetable and used in Traditional Chinese Medicine in single and compounding prescriptions for the prevention and treatment of diseases, for several thousand years.[18,19]
In our previous study, one novel water-soluble polysaccharide had been extracted and purified from the fruiting bodies of T. matsutake, using Diethylaminoethyl (DEAE)-cellulose column chromatography and Sephadex G-100 column chromatography. Its chemical structures had been characterized.[20] The present objective is to investigate the anti-microorganism, anti-tumor, and immune activities of the novel polysaccharide isolated from T. matsutake.
MATERIALS AND METHODS
Isolation for polysaccharide
The fruiting bodies of T. matsutake were collected in Xiaojing country of Sichuan province, China, and were authenticated by Prof. Zhirong Yang (College of Life Sciences, Sichuan University, Chengdu, China). At the same time, a voucher specimen had been preserved in the Key Laboratory of Southwest China Wildlife Resources Conservation, College of Life Sciences, China West Normal University. After the fruiting bodies of T. matsutake were soaked in 95% ethyl alcohol (EtOH), the residue was dried and then extracted with boiling water, thrice (five hours each). After the filtrate was concentrated, dialyzed, and centrifuged, the supernatant was added with three volumes of 95% EtOH, to precipitate the crude polysaccharides. Following this, the Sevag method[21] was used for the deproteinization, TMP (2 g) was subjected to a DEAE-cellulose column and eluted stepwise with 0, 0.1, 0.2, 0.3, 0.4, 0.5, and 1.0 M sodium chloride (NaCl). The eluate was monitored by the phenol–sulfuric acid method.[22] The 0 M NaCl elution was concentrated, lyophilized, and purified on a Sephadex G-100 column (2.6 cm × 60 cm). The resulting T. matsutake polysaccharide, named TMP-A, was obtained by the above processes and the yield rate of TMP-A was 0.215% (0.430 g) for the starting material. The structural features of the T. matsutake polysaccharide (TMP-A) were investigated by a combination of total hydrolysis, methylation analysis, gas chromatography–mass spectrometry (GC–MS), scanning electron microscopy (SEM), infrared (IR) spectra, nuclear magnetic resonance (NMR) spectroscopy, and the dynamic analysis of the atomic force microscope (AFM) studies. The results indicated that the T. matsutake polysaccharide (TMP-A) had a backbone of 1, 4-disubstituted-b-glucopyranos, which branched at O-6, and the branches were mainly composed of a (1→3)-a-galactopyranose residue, and it terminated with an a-xylopyranose residue [Figure 1].[20]
Figure 1.
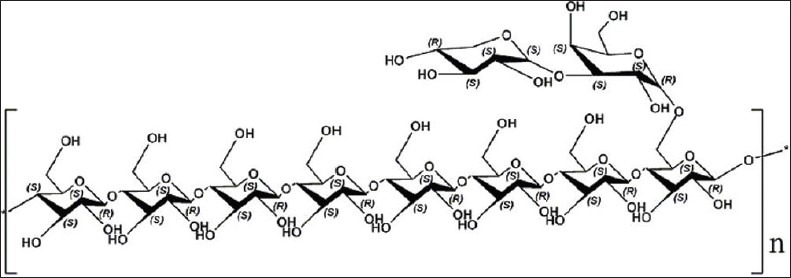
Predicted chemical structure of polysaccharide TMP-A
Preparation of test microorganisms
For the purpose of antimicrobial evaluation, eleven microorganisms were used. Escherichia coli (ATCC 8739, Gram-negative), Staphylococcus albus (ATCC 8799, Gram-positive), Brevibacillus laterosporus (ACCC 10274, Gram-negative), Bacillus subtilis (CGMCC 1.1507, Gram-positive), Micrococcus lysodeikticus (CGMCC 1.634, Gram-positive), Salmonella sp. (CGMCC 1.1552, G-), Gibberella fujikuroi (ACCC 30941), Fusarium graminearum (CGMCC 3.3631), Aspergillus niger (CMCC 98003), Penicillium sp. (ACCC 30106), and Aspergillus flavus (CGMCC 3.6434) microorganism strains were employed for determination of the antimicrobial activity. Bacteria and Fungi were obtained from the stock cultures of the Microbiology Laboratory, College of Life Sciences, Sichuan University. The bacterial and fungal stock cultures were maintained on Muller Hinton Agar (Oxoid CM 337, Basingstoke, Hampshire, UK) slants, respectively, which were stored at 4°C. For the purpose of antimicrobial evaluation, these bacteria were maintained on Blood agar base (Oxoid CM55, Basingstoke, Hampshire, UK). The Fungi was maintained on Sabouraud-dextrose agar (Oxoid CM41, Basingstoke, Hampshire, UK).
Antimicrobial activity assays by inhibition zone diameter
The microorganism strains were cultured using ordinary broth for six to eight hours and then diluted using sterile broth, to contain bacteria of 106 cfu/ml (colony forming units). One hundred microliters of diluted bacteria were coated on ordinary agar plates and each bacteria had three repeats. The plates were dried naturally for 30 minutes, and then three sterilized Oxford Cups on were placed on each agar plates, equidistantly; to each was added 200 ml TMP-A of a concentration of 20 mg/ml[23] and in another plate water was added as a positive control. The temperature of the plates was raised to 37°C for 18 – 24 hours and then the inhibition zone diameter was measured using a Vernier calipers. The inhibition zone diameter was > 20 mm for high sensitivity, 10 – 19 mm for sensitivity, and < 10 mm for blunt sensitivity.[24]
Animals
S180 tumor cells were maintained in the peritoneal cavities of Kunming strain male mice, obtained from the Institute of Biochemistry and Molecular Immunology of North Sichuan Medical College (NSMC) (Nanchong, China). Male Kunming strain mice, weighing 25.0 ± 1.0 g, were purchased from NSMC, and housed six per plastic cage with wood chip bedding in an animal room, with a 12-hour light and 12-hour dark cycle, at room temperature (25 ± 2°C) and allowed free access to a standard laboratory diet (purchased from the Institute of Biochemistry and Molecular Immunology of the North Sichuan Medical College). The animal experiments were conducted according to the ‘Guidelines for Animal Experimentation’ of the North Sichuan Medical College.
Assay of anti-tumor activity in vivo
S180 tumor cells (3 ´ 106) were implanted subcutaneously into the right hind groin of the Kunming strain male mice. The mice were randomly divided into five groups (n = 6). A day after inoculation, TMP-A was dissolved in distilled water and administered intraperitoneally (i.p.) to the mice, at doses of 20, 40, and 80 mg/kg.[25] Positive and negative controls were set for comparison. The positive control was given with 0.2 ml mannatide (20 mg/kg) and the negative one with physiological saline instead of the test solution. The animals were sacrificed after two weeks. The body weights were measured. Tumors, spleens, and livers were excised and the tumor inhibitory ratios were calculated by the following formula:
Inhibition ratio (%) = [(A-B)/A] × 100
where A and B were the average tumor weights of the negative control and treated groups, respectively.
Preparation of Lymphocyte Cells
The extirpated spleens were treated in a germ-free condition. Single spleen cell suspension was prepared in 0.15 mol−1 NaCl/0.02 mol−1 sodium phosphate and pH 7.0 phosphate buffered saline containing 0.1% (w/v) bovine serum albumin, by forcing the spleen fragments through a fine wire mesh. The samples were washed twice in phosphate buffered saline/0.1% (w/v) bovine serum albumin, followed by low speed centrifugation, 200 rpm, for five minutes. The cells were resuspended to a concentration of 5 × 106 cells ml−1 in a RPMI1640 completely cultivated liquid (HyClone, USA).
Assay of Lymphocyte Proliferation
One hundred microliters (5 × 106 cells/ml) of a single spleen cell sample was placed on a 96-well microplate and cultured for 24 hours. Twenty microliters of TMP-A of different concentrations (25, 50, 100, 200 μg/ml)[26] were added to each test well as the experimental group, 20 μl ConA (50 μg/ml) was added as a positive control group, and 20 μl RPMI1640 cultivated liquid was added, as a blank control group. Subsequently, 80 μL of RPMI1640 cultivated liquid was added to each hole, for a total volume of 200 μl for each test. Eight repeated wells were used for each concentration. The cell samples were incubated in a 5% CO2–air mixture at 37°C for 68 hours. MTT cellular viability assay was used for lymphocyte proliferation analysis. Calculation of lymphocyte proliferation was carried out:
Rate of lymphocyte proliferation (%) = [(T − C)/C] × 100
where T is the optical density value of the test well and C that of the control well.
Preparation of peritoneal macrophage cells
BALB/c albino mice, six to eight weeks old, were injected intraperitoneally (i.p.) with 1 mL of 3% thioglycollate (Sigma Chemical Co., St. Louis, MO, USA). Four days after the injection, the mice were euthanized and peritoneal exudate cells were collected by lavage with 5 mL of sterile cold D-Hank's. The exudate cells were collected and cultured in 60 mm dishes with RPMI-1640 (Gibco, USA) containing 10% heat-inactivated FBS, penicillin (100 IU/mL), and streptomycin (100 lg/mL) (RPMI-FBS). After one hour of incubation at 37°C, the cultures were washed twice with RPMI-1640, to remove non-adherent cells, and the adherent cells were collected by gentle scraping. The viability of macrophages was assessed by trypan blue exclusion.
Assay of macrophage stimulation
Macrophages (1 × 106 cells/mL) were plated in a 96-well plate (Corning, NY). The cells were cultured in phenol red-free RPMIFBS medium, containing increasing concentrations of TMP-A from 50 to 200 μg/mL[27] (20 μL per well) as the experimental group, and 20 μl LPS (40 μg/mL) and 20 μl ConA (40 μg/ml) were added as the positive control group, respectively. Next 80 μL of phenol red-free RPMIFBS cultivated liquid was added to each hole for a total volume of 200 μl for each test. Eight repeated wells were used for each concentration. The cells samples were incubated in a 5% CO2–air mixture at 37°C for four hours. Then a neutral red solution of 50 μl was added to a final concentration of 0.72 mg/L, and it was continued to be cultured for 30 minutes. The neutral red solution was aspirated, washed thrice, and 200 μl of lysis buffer (glacial acetic acid: ethanol = 1:1) was added, and left standing overnight until cell lysis complete. It was evaluated for the absorbance value (OD).
Statistical analysis
All data were presented as means ± standard deviation (SD) of three replications. Statistical analyses were performed using student's t-test and one way analysis of variance (ANOVA). Values of P < 0.05 were considered to be a statistically significant finding and Values of P < 0.01 were considered to be a statistically very significant finding.
RESULTS AND DISCUSSION
Antimicrobial activity
It is well known that most of the new drugs discovered in the last few decades have originated from nature. Chemical constituents obtained from medicinal plants and other natural products have been increasingly used to treat many infectious diseases. The present study shows the antimicrobial activity of TMP-A, by inhibition zone diameter, on eleven microorganisms, shown in Table 1. Overall, TMP-A displays a broad antimicrobial spectrum and exerts a little stronger antimicrobial effect against Gram-positive bacteria than Gram-negative bacteria, at a concentration of 20 mg/ml. The most susceptible bacteria of TMP-A was Micrococcus lysodeikticus (inhibition zone diameter 24.38 ± 1.19 mm) and the inhibition zone diameters for S. albus, B. laterosporus, E. coli, B. Subtilis, and Salmonella sp. were also > 20 mm for high sensitivity [Table 1 and Figure 2]. The polysaccharide was inactive for the other two bacterial strains studied, which were G. fujikuroi and F. graminearum. The antimicrobial activity of the polysaccharide can be attributed to its interaction with the proteins in the outer cell wall of the bacteria, which largely consists of porins that coexist with lipopolysaccharide. However, a detailed study is required to fully understand the mechanistic steps of the interaction. The polysaccharide also did not show any antifungal activity for the tested stains of the fungi.
Table 1.
Inhibition zone diameter of TMP-A on the growth of the eleven microorganisms (mean ± SD, n = 3)
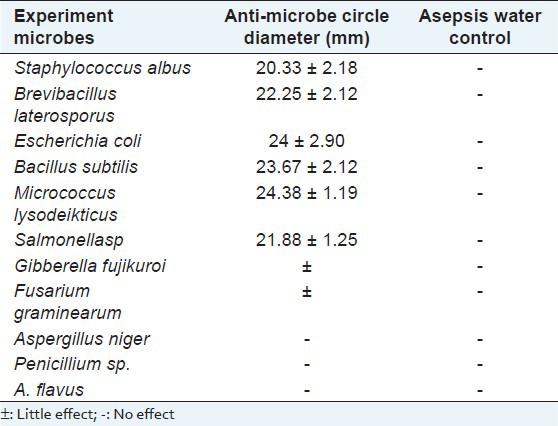
Figure 2.
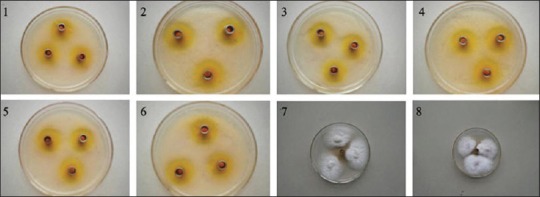
Inhibition zone diameter of TMP-A on the growth of the eleven microorganisms 1: Staphylococcus albus, 2: Brevibacillus laterosporus, 3: Escherichia coli, 4: Bacillus subtilis, 5: Micrococcus lysodeikticus, 6: Salmonellasp, 7: Gibberella fujikuroi, 8: Fusarium graminearum
Anti-tumor activity of TMP-A in vivo
A tumor is commonly used as a synonym for a neoplasm, a solid or fluid-filled (cystic) lesion that may or may not be formed by an abnormal growth of neoplastic cells, that appears enlarged in size. A tumor can be caused by an abnormal proliferation of tissues, which can be caused by genetic mutations and it can be benign, pre-malignant, or malignant, or can represent a lesion without any cancerous potential whatsoever. To detect the anti-tumor activity of TMP-A in vivo, we used the mice transplanted with S180, to evaluate the effects, and the results are summarized in Table 2. TMP-A could inhibit the growth of the tumors (P < 0.01) in a dose-dependent manner [Figure 3]. The inhibitory rate in mice treated with 80 mg/kg TMP-A was 70.089%, being the highest in the three doses. Furthermore, during the experiments, the appetite, activity, and coat luster of each animal in the TMP-A group were better than the mice treated with mannatide. The results showed little change in the average liver weight in the test groups, which indicated that TMP-A did not cause serious liver damage. On the fourteenth day, the average tumor weight of the negative control mice was 3.838 g, whereas, the average tumor weight of mice in the TMP-A group, at a dose of 80 mg/kg, was 1.149 g, and it was also significantly reduced in doses of 20 mg/kg and 40 mg/kg, which were 2.056 g and 1.542 g, respectively. It was noteworthy that the average weights of the spleens and thymus in the test groups were significantly greater in doses of 40 mg/kg than that in the mannatide mice, and even in that of the negative control mice, indicating that TMP-A could increase the weight of immune organs in moderate doses. These results suggest that activating immune responses in the host might be one of the mechanisms of the anti-tumor activity of TMP-A, as many anti-tumor polysaccharides are found in the world.
Table 2.
Inhibition of series of TMP-A on the growth of S180 tumor (mean ± SD, n = 8)
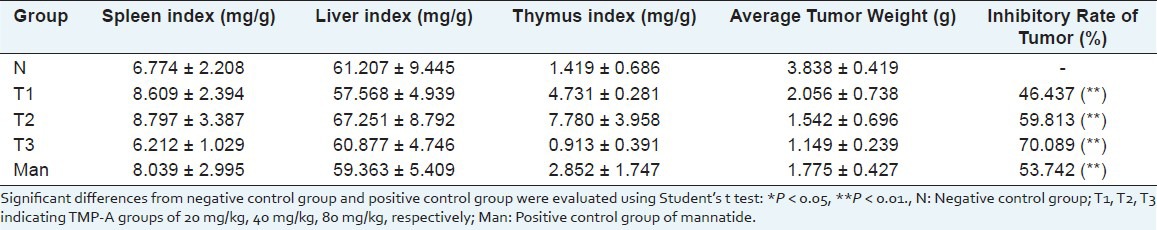
Figure 3.

Inhibition of the series of TMP-A on the growth of S180 tumor (mean ± SD, n = 8), N: negative control group; T1, T2, T3 indicating TMP-A groups of 20 mg/kg, 40 mg/kg, 80 mg/kg, respectively; Man: Positive control group of mannatide
Immune activity of TMP-A in vitro
The anti-tumor activity of the polysaccharide was usually believed to be a consequence of the stimulation of a cell-mediated immune response. The present study showed the immune activity of the TMP-A by assay of lymphocyte proliferation and macrophage stimulation. Proliferation of splenocytes is an indicator of immunoactivation. The purified polysaccharide TMP-A was able to proliferate splenocytes as shown in Figure 4. TMP-A could significantly promote the proliferation of spleen cells at a concentration of 5 μg/mL and 25 μg/mL dose (P < 0.05), while it could significantly promote the proliferation of spleen cells in a concentration of 50–200 μg/mL dose range, compared to the control group (P < 0.01).
Figure 4.
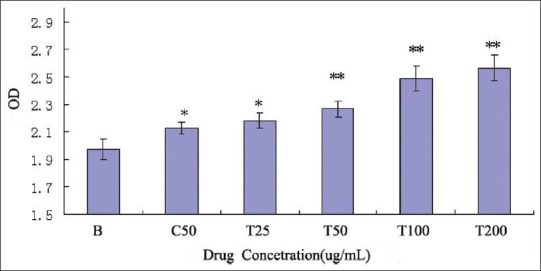
Effect of TMP-A on the proliferation of mouse spleen cell in vitro. (mean ± SD, n = 8), B: Blank control group; C: ConA group; T: TMP-A group, Significant differences from the negative control group and positive control group were evaluated using Student's t test: *P < 0.05, **P < 0.01
Polysaccharides are good stimulators of macrophages, owing to the presence of various receptors on the macrophage membrane. In this study, TMP-A has also significantly promoted the phagocytosis of mouse peritoneal macrophages, at a concentration 50 μg/mL dose (P < 0.05),and has very significantly promoted the phagocytosis of mouse peritoneal macrophages within the dose range of 100 – 400 μg/mL, compared to the control group (P < 0.01) [Figure 5].
Figure 5.
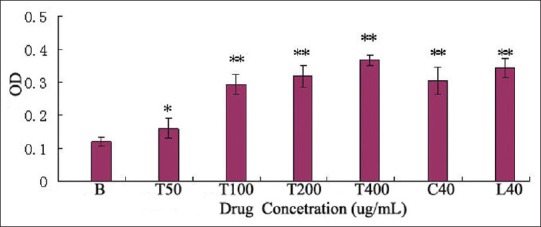
Effect of TMP-A, ConA, and LPS on mouse macrophage cell, B: Blank control group; C: ConA group; L: LPS group; T: TMP-A group, Significant differences from negative control group and positive control group were evaluated using Student's t test: *P < 0.05, **P < 0.01
The promoting capacity of both lymphocyte and macrophage cells and the concentration of TMP-A were positively correlated.
CONCLUSIONS
Purification polysaccharides prepared in our study were confirmed to be of high purity and indentificated the exact structure. Anti-microorganism test in vitro showed that it possessed a broad spectrum of antibacterial properties. The most susceptible bacteria of TMP-A of the concentration of 20 mg/ml was Micrococcus lysodeikticus (inhibition zone diameter 24.38 ± 1.19 mm) and the TMP-A did not show any antifungal activity for the tested stains of the fungi. In addition, the purification polysaccharides were also confirmed to have strong anti-tumor activities in vivo. The inhibitory rate in mice treated with 80 mg/kg TMP-A could reach 70.089%, being the highest in the three doses, which could be comparable to mannatide. The anti-tumor activity of the TMP-A was usually believed to be a consequence of the stimulation of a cell-mediated immune response, because it could significantly promote the lymphocyte and macrophage cells in the dose ranges of 50 – 200 μg/mL and 100–400 μg/mL in vitro, respectively. We could rationally assume that T. matsutake played its curative effect in traditional medicine partly by the mechanism of anti-microorganism, anti-tumor, and immune activities of polysaccharides in it.
Footnotes
Source of Support: This project was supported by the Application Foundation Project of Sichuan Province (2011JY0135), Foundation Project of the Educational Committee of Sichuan Province (10ZC120), National Natural Science Foundation of China (31200012) and Doctor Startup Foundation Project of China West Normal University (11B019) and (11B020)
Conflict of Interest: None declared.
REFERENCES
- 1.Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, et al. A higher level phylogenetic classification of the Fungi. Mycol Res. 2007;111:509–47. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Bertozzi CR, Kiessling LL. Chemical glycobioloy. Science. 2001;291:2357–64. doi: 10.1126/science.1059820. [DOI] [PubMed] [Google Scholar]
- 3.Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60:258–74. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 4.Borchers AT, Stern JS, Hackman RM, Keen CL, Gershwin ME. Mushrooms, tumors, immunity. Proc Soc Exp Biol Med. 1999;221:281–93. doi: 10.1046/j.1525-1373.1999.d01-86.x. [DOI] [PubMed] [Google Scholar]
- 5.Pauline MR, Time E, Peter C, Ian AW, Raymond AD. Glycosylation and the immune system. Science. 2001;291:2370–6. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Xie MY, Nie SP, Li C, Wang YX. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008;107:231–41. [Google Scholar]
- 7.Angeli JP, Ribeiro LR, Gonzaga ML, Soares Sde A, Ricardo MP, Tsuboy MS, et al. Protective effects of b-glucan extracted from Agaricus brasiliensis against chemically indueed DNA damage inhuman lymPhoeytes. Cell Boil Toxicol. 2006;22:285–91. doi: 10.1007/s10565-006-0087-z. [DOI] [PubMed] [Google Scholar]
- 8.Li SP, Zhao KJ, Ji ZN, Song ZH, Dong TT, Lo CK, et al. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003;73:2503–13. doi: 10.1016/s0024-3205(03)00652-0. [DOI] [PubMed] [Google Scholar]
- 9.Hetland G, Johnson E, Lyberg T, Bernardshaw S, Tryggestad AM, Grinde B. Effects of the medicinal mushroom Agaricus blazei Murill on immunity, infection and cancer. Scand J Immunol. 2008;68:363–70. doi: 10.1111/j.1365-3083.2008.02156.x. [DOI] [PubMed] [Google Scholar]
- 10.Firenzuoli F, Gori L, Lombardo G. The medicinal mushroom Agaricus blazei Murrill: Review of literature and pharmaco-toxicological problems. Evid Based Complement Alternat Med. 2008;5:3–15. doi: 10.1093/ecam/nem007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paterson RR. Ganoderma–a therapeutic fungal biofactory. Phytochemistry. 2006;67:1985–2001. doi: 10.1016/j.phytochem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Paterson RR. Cordyceps: A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry. 2008;69:1469–95. doi: 10.1016/j.phytochem.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan R, Smith JE, Rowan NJ. Medicinal mushrooms and cancer therapy: Translating a traditional practice into Western medicine. Perspect Biol Med. 2006;49:159–70. doi: 10.1353/pbm.2006.0034. [DOI] [PubMed] [Google Scholar]
- 14.Halpern GM, Miller A. New York, New York: M. Evans and Co; 2002. Medicinal mushrooms: Ancient remedies for modern ailments; p. 116. [Google Scholar]
- 15.Fisher M, Yang LX. Anticancer effects and mechanisms of polysaccharide-K (PSK): implications of cancer immunotherapy. Anticancer Res. 2002;22:1737–54. [PubMed] [Google Scholar]
- 16.Gurein L, Vaario LM, Matsushita N, Shindo K, Suzuki K, Lapeyrie F. Growth stimulation of a Shiro-like, mycorrhiza forming, mycelium of Tricholoma matsutake on solid sub-strates by non- ionic surfactants or vegetable oil. Mycol Prog. 2003;2:37–44. [Google Scholar]
- 17.Mikio K, Kentaro K, Yuka N, Kazumttsu N, Koujirou M, Hideo N. Submerged Culture of Tricholoma matsutake Mycelium in Bubble Column Fermentors. J biosci bioeng. 1999;1:116–118. doi: 10.1016/s1389-1723(99)80020-6. [DOI] [PubMed] [Google Scholar]
- 18.Shuang Y, Xiaodong R, Jingxue S, Jiahui L, Tingting L, Fangrong T, et al. Preparation and the antitumor activity in vitro of polysaccharides from Tricholoma matsutake. World J Microbiol Biotechnol. 2010;26:497–503. [Google Scholar]
- 19.Ishihara Y, Iijima H, Yagi Y, Matsunaga K. Enhanced recovery of NK cell activity in mice under restraint stress by the administration of a biological response modifier derived from the mycelia of the basidiomycete Tricholoma matsutake. Stress. 2003;6:141–8. doi: 10.1080/1025389031000116479. [DOI] [PubMed] [Google Scholar]
- 20.Xiang D, Su F, Mei C, Maotao L, Jie T, Chunxiao G, et al. Structure cha99racterization of polysaccharide isolated from the fruiting bodies of Tricholoma Matsutake. Carbohydr Polym. 2010;81:942–7. [Google Scholar]
- 21.Chakraborty I, Mondal S, Pramanik M, Rout D, Islam SS. Structural investigation of a water-soluble glucan from an edible mushroom, Astraeus hygrometricus. Carbohydr Res. 2004;339:2249–54. doi: 10.1016/j.carres.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Dubois M, Gillis KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–6. [Google Scholar]
- 23.Riadh K, Hanen F, Wided M, Najla T, Baya M, Kamel C, et al. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem Toxicol. 2009;47:2083–91. doi: 10.1016/j.fct.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Kima YM, Farrah S, Baney RH. Structure-antimicrobial activity relationship for silanols, a new class of disinfectants, compared with alcohols and phenols. Int J Antimicrob Ag. 2007;29:217–22. doi: 10.1016/j.ijantimicag.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 25.Ye H, Wang KQ, Zhou CH, Liu J, Zeng XX. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008;111:428–32. doi: 10.1016/j.foodchem.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Shao BM, Dai H, Xu X, Lin ZB, Gao XM. Immune receptors for polysaccharides from Ganoderma lucidum. Biochem Biophys Res Commun. 2004;323:133–41. doi: 10.1016/j.bbrc.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 27.Xia L, Xiaoyan X, Mengyao Y, Zhirong Y, Linyong Z. Characterisation and immunostimulatory activity of an a-(1-6)-D-glucan from the cultured Armillariella tabescens mycelia. Food Chem. 2008;111:357–63. doi: 10.1016/j.foodchem.2008.03.076. [DOI] [PubMed] [Google Scholar]


