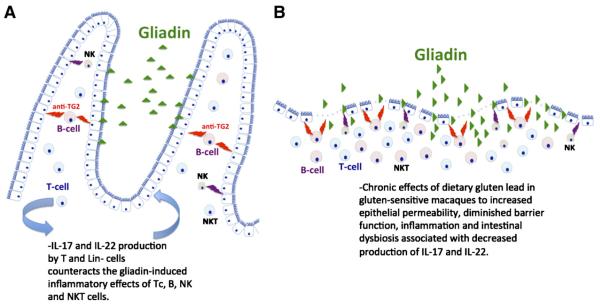
Abstract
Celiac disease (CD) is an autoimmune disorder caused by intolerance to dietary gluten. The interleukin (IL)-17 and IL-22 function as innate regulators of mucosal integrity. Impaired but not well-understood kinetics of the IL-17/22 secretion was described in celiac patients. Here, the IL-17 and IL-22-producing intestinal cells were studied upon their in vitro stimulation with mitogens in class II major histocompatibility complex-defined, gluten-sensitive rhesus macaques. Pediatric biopsies were collected from distal duodenum during the stages of disease remission and relapse. Regardless of dietary gluten content, IL-17 and IL-22-producing cells consisted of CD4+ and CD8+ T lymphocytes as well as of lineage-negative (Lin−) cells. Upon introduction of dietary gluten, capability of intestinal T cells to secrete IL-17/22 started to decline (p < 0.05), which was paralleled with gradual disruption of epithelial integrity. These data indicate that IL-17/22-producing cells play an important role in maintenance of intestinal mucosa in gluten-sensitive primates.
Keywords: Celiac disease, Th17, Rhesus, Tissue transglutaminase, Autoimmunity
1. Introduction
CD is an autoimmune disease characterized by production of antibodies against tissue transglutaminase 2 (TG2) — an intestinal enzyme that plays a multitude of roles including dietary gluten deamidation [1,2]. In individuals with major histocompatibility complex class II (MHC II) alleles DQ2 or DQ8, deamidated gluten residues trigger the CD4+ T-cell-mediated humoral and cellular immunity that can lead to chronic inflammation of not only small intestine but also other organs [3,4]. Histopathologically, CD appears as gluten-sensitive enteropathy (GSE) of small intestine that leads to damaged epithelium and partial or complete villous atrophy. We recently established the non-human primate (NHP) model of CD [5–10]. The presence of TG2 autoantibodies and gluten-sensitive enteropathy (GSE) was described. Remission and relapse of GSE can be accomplished in this model by the feeding of gluten-free (GFD) and gluten-containing (GD) diets, respectively. Consistent with human CD, GSE in macaques is characterized, by wide range of severity, from the subclinical to severe form that includes decreased resorption of nutrients, decreased xenobiotic metabolism and cancer predisposition [5,9]. Despite demonstrated involvement of TG2 in rhesus GSE, it is unknown whether the particular rhesus major histocompatibility complex class II (Mamu II) alleles trigger the T cell and cytokine responses analogous to human CD [11–15]. While interferon-gamma (IFN-γ) is secreted by celiac T cells [16] and it is capable of activating TG2 via the phosphatidylinosinol-3-kinase pathway [17], IL-17 and IL-22 cytokines are thought to function as innate regulators of mucosal integrity [18]. Impaired but not well-understood kinetics of IL-17/22 cytokine secretion was described in celiac patients [19–22]. In accord with epithelial integrity maintaining function of IL-17 and IL-22, we hypothesized that introduction of dietary gluten to TG2-antibody positive macaques will disrupt capability of intestinal lymphocytes to secrete these cytokines and lead to changes in intestinal tissue architecture.
2. Material and methods
2.1. Ethics approval
This study was performed with non-human primates. Ethics approval for veterinary procedures had been obtained from the Tulane University Animal Care and Use Committee, Animal Welfare Assurance A-4499-01. All veterinary procedures were performed only with sedated animals. Animal welfare and steps were taken to ameliorate suffering in accordance with the recommendations of the Guide to the Care and Use of Laboratory Animals (NIH) 78-23 (Revised, 1996).
2.2. AGA, TG2 antibody and MHC assays
Based on the prevalence of gluten sensitivity within the captive rhesus macaque population at the Tulane National Primate Research Center, ~100 randomly selected animals need to be tested for the presence of anti-gliadin antibodies (AGA) as well as TG2 antibodies [8] in order to identify one gluten-sensitive animal with CD-like symptoms. In this study, AGA and TG2 antibody-specific Enzyme-Linked Immuno Assays (ELISAs) were performed as described [5,6] to measure both antibodies in plasma from 1500 candidate rhesus macaques. All animals were Specific Pathogen-Free Indian Rhesus macaques, seronegative and virus negative for simian retrovirus type D, seronegative for simian T lymphotropic virus type 1, Simian Immunodeficiency Virus and herpes B viruses, and free of selected enteric pathogens [23]. All tuberculin skin tests, which were performed semi-annually, were negative for each animal involved. One ml of EDTA blood was collected from each animal during the semi-annual colony health maintenance inspections to extract the plasma and DNA [5,6]. Upon completion of AGA and TG2 assays and analysis, DNA samples from 50 TG2-AGA−, 31 TG2-AGA+ and 18 TG2+AGA+ antibody-defined macaques were selected for genetic evaluation of their rhesus MHC class II (Mamu II) composition as described previously [9,24].
2.3. Rhesus macaques
Six TG2-antibody positive macaques and six healthy controls (Table A.1) were studied for the impact of dietary gluten on rhesus macaque intestinal IL-17 and IL-22 productions. Gluten-free (GFD) and gluten-containing (GD) diets were used [8]: GD is equivalent to “Purina Monkey Chow” that is routinely used to feed the captive NHPs. GFD was formulated as before [5]. Consistent with past studies where GFD was used to accomplish the immunological and clinical remission [5,8,9], GFD was fed to six gluten-sensitive animals. Remission was characterized by return of AGA and TG2 antibodies to baseline levels, no diarrhea, bloating or dehydration, and no (or only minimal) inflammation of small intestine. As these (CD-like) symptoms were not anticipated nor observed in control animals, only GD was fed to control group. Samples of peripheral blood and small intestine were obtained at a single time point from control macaques and at four time points from gluten-sensitive macaques: at the time of immunological remission, and at 5, 21 and 55 days following the initiation of GD.
2.4. Intestinal biopsy and peripheral blood samples
Biopsy samples of distal duodenum/proximal jejunum and peripheral blood were obtained as described [8]. Intestinal samples were divided and processed for the histopathological and confocal microscopy evaluation, for lamina propria lymphocyte isolation, in vitro stimulation and flow cytometry, while peripheral blood was used to obtain plasma.
2.5. Cell isolation and processing
Mononuclear cells from small intestinal tissues were isolated and processed as previously described [25,26]. Briefly, biopsy samples were collected from the distal duodenum and processed immediately for intestinal lamina propria lymphocyte (LPL) cell suspensions using the enzymatic digestion method.
2.6. Phenotyping
Flow cytometry for surface and intracellular staining of intestinal LPLs was performed using the standard protocols [26]. Cells were stained with antibodies from BD Biosciences Pharmingen (San Diego, CA) unless otherwise noted: CD3 (SP34), CD4 (L-200), CD8α (3B5, Caltag Laboratories, Burlingame, CA), CD14 (M5E2), CD20 (L27), IL-17 (eBio64CAP17, eBioscience, San Diego, CA), and IL-22 (IL22JOP, eBioscience, San Diego, CA), and LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen, Grand Island, NY). In order to detect IL-17 and IL-22 cytokines, lymphocytes were stimulated in vitro with 0.1 μM PMA and 0.5 μg/ml ionomycin (Sigma-Aldrich, St. Louis, MO) as described [26]. Samples were resuspended in BD Stabilizing Fixative (BD Biosciences), and data was acquired on a FACSAria flow cytometer (Becton Dickinson, San Jose, CA). Data was analyzed with Flowjo software (Tree star, Ashland, OR).
2.7. Confocal microscopy
Fresh jejunum biopsy tissues were obtained from rhesus macaques and cultured in complete RPMI medium (10% heat inactivated fetal calf serum, 1-glutamine, penicillin and streptomycin; Invitrogen) either alone or with 100 ng/ml PMA plus 0.5 mg/ml calcium ionophore (stimulation medium) for 4 h in the presence of 2 μM monensin (Sigma) to block protein transport and release, in order to detect IL-17+ and IL-22+ cells as described previously [26]. In brief, tissues were embedded and snap frozen in optimum cold temperature compound (OCT) and 7 μm frozen sections were stained using unconjugated primary antibodies (IL-17, eBio64CAP17; IL-22, IL22JOP, eBioscience, San Diego, CA) followed by appropriate secondary antibodies conjugated to Alexa 488 (green) or Alexa 568 (red) (Molecular Probes, Eugene, OR). Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with three lasers (Leica Microsystems, Exton, PA). Individual optical slices representing 0.2 μm and 32 to 62 optical slices were collected at 512 × 512 pixel resolution. NIH Image (version 1.62, Bethesda, MD) and Adobe Photoshop (version 7.0, San Jose, CA) were used to assign colors to the channels collected: HNPP/Fast Red (Roche, Indianapolis, IN), which fluoresces when exposed to a 568-nm wavelength laser, appears red; Alexa 488 (Molecular Probes) appears green.
2.8. Histopathological evaluation
Tissue architecture of small intestinal biopsy samples was microscopically evaluated following the hematoxylin and eosin (H&E) staining, as described [5,8,9].
2.9. Statistical analysis
Graphical presentation and statistical analysis of the cytokine-producing cell data were performed using the GraphPad Prism 4.0 (GraphPad Software, San Diego, CA). Comparisons between the groups were done by a one-way ANOVA and a non-parametric Mann–Whitney T-test. The values of p < 0.05 were considered statistically significant. The Fisher’s Exact Test analysis was used to determine if any of the Mamu II alleles were associated with TG2 antibodies in gluten-sensitive and control macaques.
3. Results
3.1. Immunogenetic and histopathological evaluation
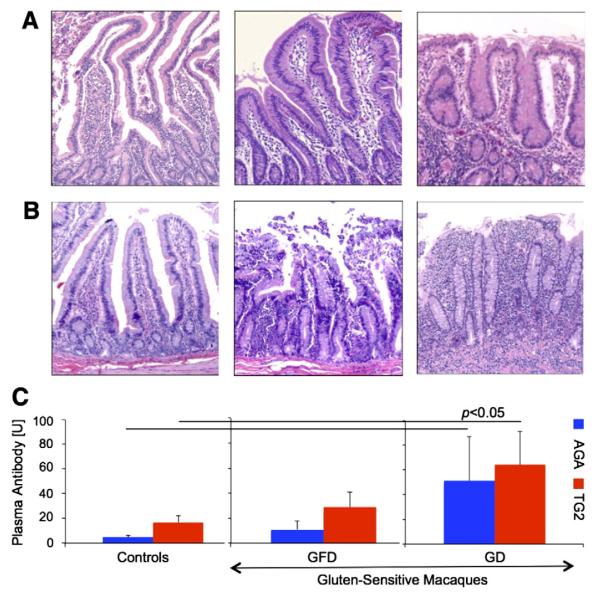
The six gluten-sensitive rhesus macaques and six healthy controls were used in this study (Table A.1). All six gluten-sensitive macaques responded to dietary gluten with production of AGA and TG2 plasma antibodies. Based on DNA analysis of their Mamu II (rhesus MHC II) three out of six gluten-sensitive macaques carried DQA1*01:05:01/DQB1*06:02 allelic pair. This particular allelic pair was associated (p = 0.008) with the presence of TG2 antibodies (Fig. 1). Consistent with clinical manifestations of CD in human patients [27], clinical and histopathological symptoms of rhesus GSE did not exhibit the same intensity in every animal (Table A.1). Administration of GFD to gluten-sensitive macaques for three consecutive months was sufficient to improve the small intestinal histopathology (Figs. 2A–B) as well as to decrease the AGA and TG2 antibody levels (Fig. 2C). Above-mentioned variability in responses of individual gluten-sensitive macaques was reflected by relatively high standard-error-bars representing these antibody responses. Despite such variability, group differences between gluten-sensitive and control animals were significant (Fig. 2C). Administration of GD to control animals had no impact on intestinal histopathology or AGA and TG2 antibody levels (Figs. 2A–C).
Figure 1.
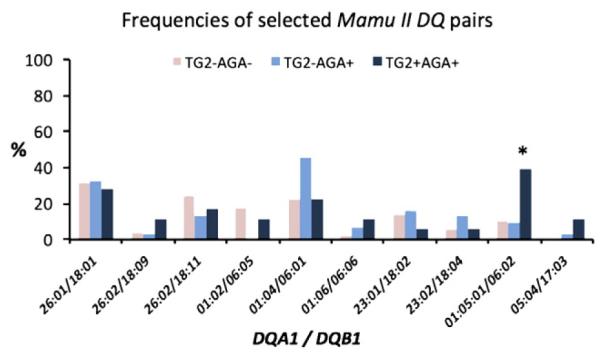
The plasma samples from 1500 captive rhesus macaques of Indian subspecies were tested for the presence of TG2 and AGA antibodies. The DNA isolated from peripheral blood of 99 antibody-defined macaques (50 TG2−AGA−, 31 TG2−AGA+, and 18 TG2+AGA+) was used to determine the frequencies of 21 Mamu II DQA1*/DQB1* allelic pairs within each of the three groups. The 10 allelic pairs with frequencies >5% are shown. Based on Fisher’s Exact Test analysis, DQA1*010501/DQB1*0602 pair was represented in TG2+ animals with significantly higher frequency (p = 0.008) than it was in rest of the macaques. From the presented data, it appears that allelic pair is present in 40% of TG2+ macaques vs. <10% in general population suggesting that there still might be additional, undiscovered predisposition alleles in macaques.
Figure 2.
Histopathological evaluation of H&E-stained duodenum (A) and jejunum (B) biopsy samples from control and gluten-sensitive rhesus macaques (50×). (A) Duodenum from a representative healthy control animal did not show any histopathology while on GD. Duodenum from a representative gluten-sensitive macaque on GFD showed, despite being in clinical and immunological remission, some lymphocytic and plasmacytic infiltration of lamina propria. Moderate enteropathy with shortened, fused and blunted villi appeared within three weeks after introduction of GD in the same animal. (B) Jejunum from the control animal did not show any histopathological responses to GD. Jejunum from this gluten-sensitive animal shows mild VA despite of being on GFD for almost a year. Upon introduction of dietary gluten in the same animal, rapid progression towards severe GSE characterized by disrupted epithelial layer, marked VA and lymphoplasmacytic enteritis took place. (C) AGA and TG2 plasma antibody levels in different cohorts are shown. In contrast to the control group, introduction of dietary gluten to gluten-sensitive group was associated with rise (p < 0.05) of AGA and TG2 plasma antibodies.
3.2. Characterization of small intestinal IL-17+ and IL-22+ cells
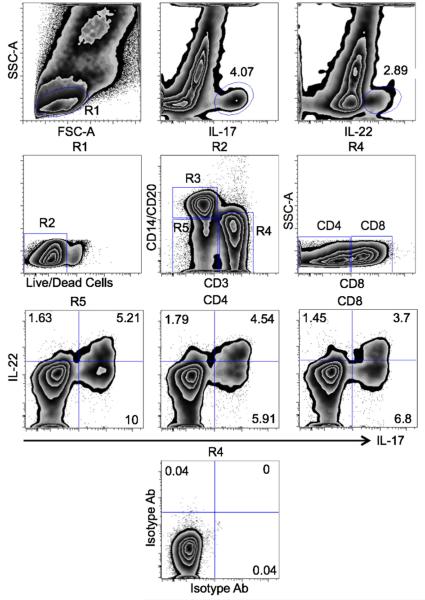
To define the intestinal IL-17+ and IL-22+ cells, duodenal LPLs of healthy control rhesus macaques were isolated, stimulated with PMA and ionomycin, and stained by use of IL-17 and IL-22 antibodies (Fig. 3). Both IL-17+ and IL-22+ cells were present within the gated lymphocyte-size cell population. The IL-17+ and IL-22+ cells were further defined by live/dead, CD3, CD4, CD8, CD14 and CD20 cell antibodies (Fig. 3). The population of interest (R2) contained not only IL-17+ but also IL-17+IL-22+ double positive cells (3.46%). The single positive IL-17+ and IL-22+ lymphocytes accounted for 4.81% and 3.89%, respectively. Besides typical T cells (R4), additional phenotypes such as monocytes/B lymphocytes (R3) and heterogeneous lineage negative or Lin− (R5) cells also secreted IL-17 and IL-22 (Fig. 3). Notably, the Lin− (R5) and T (R4) cell populations contained comparable proportions of IL-17+ and IL-22+ cells. Consistent with the above capability of mononuclear cells to produce the IL-17 and/or IL-22, the Lin−, CD3+ (CD4+ and CD8+) populations, all contained single positive IL-17+/IL-22+, as well as double positive IL-17+IL-22+ cells. These proportions of IL-17+/IL-22+ cells within the lymphocyte and Lin− subsets suggest that IL-17 and IL-22 cytokines play an important role in intestinal immunity while evoking individual and combined effects. Isotype controls for both IL-17 and IL-22 did not yield any significant proportions of positive cells (Fig. 3).
Figure 3.
Gating strategy of duodenal IL-17+ and IL-22+ cells. Lymphocyte population from control/healthy rhesus macaque (R1) contained both IL-17+ and IL-22+ cells. Live/dead cell staining was used to exclude the dead and to include the live cells (R2) into analysis. The monocyte/B cells (CD14+CD20+, R3), T cells (CD3+, R4) and lineage-negative (R5) populations were studied. The CD3+ T cells were further subdivided into CD4+ and CD8+ major subsets. While only few of the IL-17+/IL-22+ cells were detected in monocyte/B cell population (not shown), lineage-negative (R5) and T cell (R4) populations contained distinct subsets of IL-17+ and IL-22+ cells. In addition to single positive (IL-17+/IL-22+) T cells, both major subsets of duodenal T cells (CD4+ and CD8+) also contained double-positive (IL-17+IL-22+) cells. Isotype control antibodies for IL-17 and IL-22 corroborated the specificity of both cytokine markers.
3.3. Introduction of dietary gluten leads to decreased production of intestinal IL-17+ and IL-22+
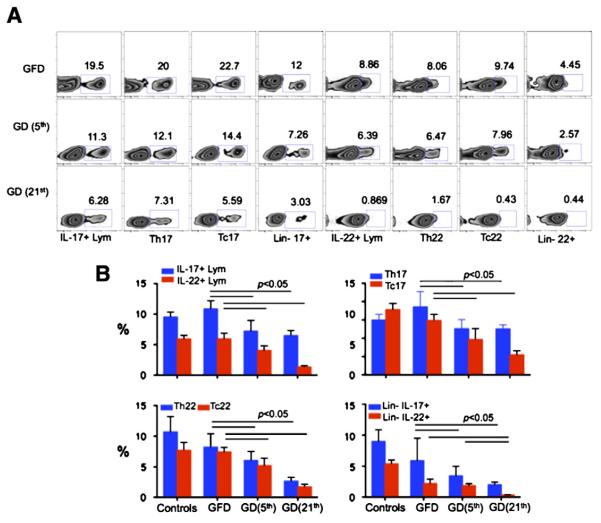
Once the remission, characterized by lowered levels of AGA and TG2 antibodies and absence of clinical symptoms was accomplished in gluten-sensitive macaques, duodenum biopsy samples were obtained, lamina propria lymphocytes (LPLs) were isolated and in vitro stimulated with PMA and ionomycin. Without such stimulation, IL-17 and IL-22 were undetectable at the spontaneous (in vivo) state. Additional biopsies were obtained after animals were placed on GD for 5 and 21 days (Fig. 4A). All cell populations involved in IL-17 and IL-22 secretion including the lymphocyte, Th (CD4+), Tc (CD8+), and Lin− populations were fewer in numbers with progression of time after initiation of GD (Fig. 4A). The Th17, Tc17, Th22 and Tc22 were significantly reduced by days 5 and 21 (p < 0.05), compared with GFD time point or healthy control animals (Fig. 4B). Consistent with our past reports [5,8,9], the differences between healthy controls and gluten-sensitive macaques while on GFD were measurable but not prominent. In contrast, the IL-17/IL-22-producing cells including the whole lymphocytes, Th17, Tc17, Th22, Tc22 and Lin− cells were significantly lowered by day 5 of GD (p < 0.05) and further decreased by day 21.
Figure 4.
Characterization of duodenal IL-17+ and IL-22+ cells. (A) Dietary gluten induced significant decrease of IL-17+/IL-22+ cells in duodenum of gluten-sensitive rhesus macaques. Differences in populations of IL-17+/22+ lymphocytes, IL-17+/22+CD4+ T helper (Th17+/22+) cells, IL-17+/22+CD8+ (Tc17+/22+) cells and IL-17+/22+ lineage-negative (Lin−) cells are shown concerning the GFD and GD periods. GD data are shown for days 5 and 21 following the introduction of GD. (B) Significant decreases of overall (group x ±STD) IL-17+/IL-22+ lymphocytes as well as Th, Tc and Lin− IL-17+/22+ cells are shown.
3.4. Distribution of intestinal IL-17+ and IL-22+ cells
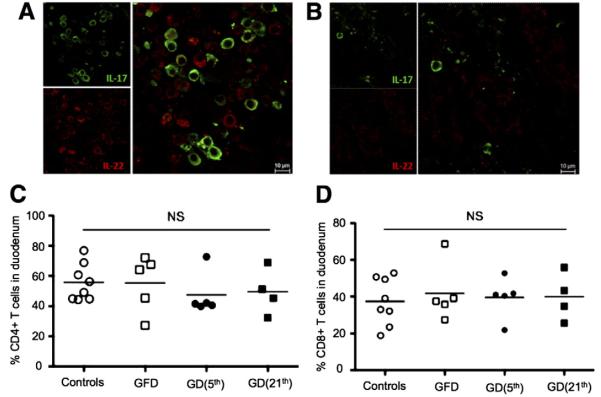
To support the flow cytometry findings and to visualize the changes in proportions of IL-17+/IL-22+ cells in duodenum lamina propria of gluten-sensitive macaques, the biopsy tissues were immunohistochemically processed and evaluated by confocal microscopy. Both of the IL-17+ (green) and IL-22+ (red) cells were found (Fig. 5). In case of tissues that were obtained from GFD-fed animals, the IL-17+, IL-22+ and IL-17+IL-22+ cells could be identified readily while extensive loss of these cells was observed in the same tissues during the GD-induced relapse period (Figs. 5A–B).
Figure 5.
IL-17+/IL-22+ cells vs. CD4+/CD8+ cells in duodenum. (A) Numerous IL-17+ (green) and IL-22+ (red) cells are seen in the intestinal biopsy tissue from gluten-sensitive macaque while in the stage of clinical and immunological remission. (B) In contrast, very few of the IL-17+/IL-22+ cells could be found in the same tissue and animal during the stage of clinical and immunological relapse induced by GD. (C, D) Despite changes in IL-17+/IL-22+ T cells, introduction of GD did not affect the total % of CD4+ and CD8+ T cells in duodenum of gluten-sensitive rhesus macaques. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. No change in the total counts of intestinal CD4+ and CD8+ cells
In order to determine if the decreased counts of IL-17+ and IL-22+ T cells in duodenum of gluten-sensitive macaques affected the total counts of CD4+ and/or CD8+ T cells, flow cytometry phenotyping of intestinal LPLs was performed with cells obtained from gluten-sensitive and control macaques (Figs. 5C–D). The total % of CD4+ and CD8+ T cells was not affected by dietary changes confirming that decrease in IL-17/IL-22-producing cells was not overall but it was linked to specific cell subsets (Figs. 5C–D).
4. Discussion
An extensive body of evidence has emerged during recent years concerning the role of IL-17 and IL-22 cytokines in the maintenance of mucosal integrity [18,19,21,28,29]. Although both IL-17 and IL-22 play roles in adaptive immunity, their functions in mucosal immunity are proinflammatory and tissue protective, respectively [30,31]. While Th cells were identified as a major source of IL-17/22, the Tc, NK, neutrophil and γδT also were described as additional sources [32,33]. Both IL-17 and IL-22 are thought to be involved in initiation of autoimmune diseases such as rheumatoid arthritis [34], multiple sclerosis [35], systemic lupus erythematosus [36] and inflammatory bowel disease [37]. As perturbed expression of autoimmunity-related genes in gluten-sensitive rhesus macaques was recently demonstrated by our group [9], the main objective of this study was to elucidate what role, if any, IL-17 and IL-22 played in GSE. The NHP model of GSE was used due to its availability and capacity to recapitulate remission and relapse stages by feeding the gluten-free and gluten-containing diets, respectively. In accord with epithelial integrity maintaining function of IL-17 and IL-22, it was expected that development of gluten-sensitive enteropathy will be associated with perturbed production of these cytokines. Recently, studies with HIV-infected patients and SIV-infected NHPs have shown that reduced Th17 compartment due to infection is predictive of dissemination of microbial products from the intestine, increased systemic immune activation, and disease progression [38].
Analogous studies on the roles of IL-17 and IL-22 in celiacs have yielded mixed results [19–22]. In one study, gluten reactive duodenal T cells were obtained from celiac patients after an oral gluten challenge [19]. It was concluded that gluten-reactive T cells produced IFN-γ but no or very little IL-17 [19]. In another study that focused on direct detection of gliadin specific Th17 cells in untreated celiac patients, elevated numbers of both IL-17 and IL-22 producing cells were found in small intestinal mucosa [21]. In contrary, another study that focused on intraepithelial lymphocytes found reduced levels of IL-17A secretion in celiac patients on GD in comparison with patients on GFD [22]. No association was observed between the IL-17 production and histopathology. It is important to note that latest study measured IL-17A production by T cells that were in vitro stimulated with PMA/ionomycin mitogens — consistent with widely used methodology for intracellular cytokine detection [22]. It appears from these studies that depending on specificity of antigen used for in vitro stimulation, IL-17 production by intestinal T cells from celiac patients can vary [19–22]. Considering that intestinal T cells are encountering plethora of dietary and microbial antigens, it is likely that immune function of these cells is often immunosuppressive (oral tolerance) rather than immunostimulatory. Taken together, these studies underscore the value of a NHP model to study the gluten-dependent mucosal changes. NHP model as a research tool allows synchronized administration of GFD as well as multiple time point collections and thorough evaluation of intestinal tissues. Important similarities between the NHP/GSE system and human CD involve biomarkers such as AGA and TG2 antibodies, interferon-gamma (IFN-γ) secretion by gliadin-specific T cells (Fig. A1), villous atrophy, symptoms of diarrhea and dehydration, and reverse nature of these symptoms by adherence to GFD. Individual variability of GSE in NHPs is consistent with CD manifestations where majority of cases are subclinical while it is not well understood what environmental or intrinsic factors trigger the transition of subclinical to clinical form [27].
It was determined that the severity of GSE is in gluten-sensitive but not in healthy control macaques directly linked with number of intestinal IL-22+ cells. These findings are consistent with results from IL-22 knockout mice experiments, which demonstrated that diminished function of IL-22 causes increased intestinal epithelial damage, systemic bacterial infection and mortality [39]. An important unresolved question remains why production of both IL-22+ and IL-17+ cells decreases progressively in gluten-sensitive individuals after introduction of dietary gluten. A possible clue might come from studies that evaluated the benefits and detriments of IL-17 and IL-22 production in hosts with chronic GI bacterial infections. These studies imply that long-term IL-17/22-mediated intestinal inflammation is unfavorable because it promotes accumulation of metal ions like zinc and manganese, which function as essential micronutrients for bacteria [18]. It follows that accumulation of intestinal bacteria due to IL-17/22 production is not beneficial — due to potential displacement of normal GI microflora. Such a scenario may be particularly problematic in individuals with leaky gut. Furthermore, others have suggested that intestinal dysbiosis might be one of the predisposing factors for development of CD [40]. The fact that decreased production of IL-17 and IL-22 cell paralleled the progression of GSE and TG2 antibody responses is in agreement with reports that indicate that IL-17 synergizes with IL-22 to provide protective role of mucosa-associated tissues against extracellular pathogens by inducing the production of anti-bacterial peptides and/or anti-apoptotic signals [41–44]. Taken together, we propose that decreased production of IL-17 and IL-22 is in gluten-sensitive macaques associated with appearance of damaged epithelium and decreased function of small intestine (Fig. 6).
Figure 6.
Development of GSE in small intestine of gluten-sensitive rhesus macaque. (A, B) An interpretation of initial or pre-infiltrative stage and final or atrophic stage is illustrated.
Due to the large number of macaques prescreened serologically for AGA and TG2 antibodies as candidates for this study, it was possible to identify 50 TG2-AGA−, 31 TG2-AGA+ and 18 TG2+AGA+ antibody-defined macaques for DNA analysis and genetic evaluation of their Mamu II composition. Based on the assumption that elevated TG2 antibodies are accurate indicators of CD, the two Mamu II alleles (an allelic pair) that were identified to be statistically associated with TG2+ antibodies were DQA1*01:05:01 and DQB1*06:02 (Fig. 1). Three out of 6 gluten-sensitive animals were carriers of these alleles while all 6 had TG2 antibodies and were also responsive to dietary gluten by AGA (Table A.1). The fact that not all of the TG2+ macaques were carriers of above two alleles likely reflected the differences in polymorphism and high diversity of Mamu II in comparison to human MHC II (HLA II) [45,46]. This is reflected by the fact that ~40% of TG2+ macaques were of DQA1*01:05:01/DQB1*06:02 haplotype which corresponds to ~90% of human HLA-DQ2. Therefore, it is the higher diversity of rhesus MHC II diversity and not the mechanism of immune response, pathogenesis or clinical symptoms that represents the main difference between rhesus and human form of celiac disease. The capability of DQA1*01:05:01/DQB1*06:02 alleles to bind with deamidated gliadin residues was to some extent similar with human DQ2 but entirely different with DQ8 (Fig. A.2). Additional analyses of DNA samples from TG2+ macaques of Indian origin and T cell epitope mapping studies will provide additional clues. Although emphasis of this study was on innate immunity, our results also indicate that intestinal enzyme-digested gliadin can stimulate in vitro IFN-γ production by intestinal CD4+ and CD8+ T cells from gluten-sensitive macaques but not from controls (Fig. A1). Taken together, our findings suggest that NHP model of GSE represents a translation-able tool for evaluation of CD associated innate and adaptive immunity.
Supplementary Material
Acknowledgments
The authors thank to A. Tardo, X. Jin, C. Midkiff, C. Coyne, D. Kuebler, C. Lanclos and J. Bruhn for their technical support, and Drs. X. Alvarez, P. Kissinger and B. Pahar for their help and advice. This work was supported by the National Institutes of Health grants R01DK076653 and 3R01DK076653 to K.S. as well as the base operating grant of the Tulane National Primate Research Center RR000164.
Abbreviations
- CD
celiac disease
- IL
interleukin
- TG2
tissue transglutaminase
- MHC
major histocompatibility complex
- GSE
gluten-sensitive enteropathy
- NHP
non-human primate
- IFN-γ
interferon-gamma
- AGA
anti-gliadin antibodies
- GFD
gluten-free diet
- GD
gluten-containing diet
- LPL
intestinal lamina propria lymphocytes
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.clim.2013.02.012.
Conflict of interest statement The authors declare that no competing interests exist.
References
- [1].Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature’s biological glues. Biochem. J. 2002;368:377–396. doi: 10.1042/BJ20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- [3].Stamnaes J, Dorum S, Fleckenstein B, Aeschlimann D, Sollid LM. Gluten T cell epitope targeting by TG3 and TG6; implications for dermatitis herpetiformis and gluten ataxia. Amino Acids. 2010;39:1183–1191. doi: 10.1007/s00726-010-0554-y. [DOI] [PubMed] [Google Scholar]
- [4].Abadie V, Sollid LM, Barriero LB, Jabri B. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu. Rev. Immunol. 2011;29:493–525. doi: 10.1146/annurev-immunol-040210-092915. [DOI] [PubMed] [Google Scholar]
- [5].Bethune MT, Borda JT, Ribka E, Liu MX, Phillippi-Falkenstein K, Jandacek RJ, Doxiadis GGM, Gray GM, Khosla C, Sestak K. A non-human primate model for gluten sensitivity. PLoS One. 2008;3:e1614. doi: 10.1371/journal.pone.0001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bethune MT, Ribka E, Khosla C, Sestak K. Transepithelial transport and enzymatic detoxification of gluten in gluten-sensitive rhesus macaques. PLoS One. 2008;3:e1857. doi: 10.1371/journal.pone.0001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bethune MT, Crespo-Bosque M, Bergseng E, Mazumdar K, Doyle L, Sestak K, Sollid LM, Khosla C. Noninflammatory gluten peptide analogs as biomarkers for celiac sprue. Chem. Biol. 2009;16:868–881. doi: 10.1016/j.chembiol.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mazumdar K, Alvarez X, Borda JT, Dufour J, Martin E, Bethune MT, Khosla C, Sestak K. Visualization of transepithelial passage of the immunogenic 33-residue peptide from α-2 gliadin in gluten-sensitive macaques. PLoS One. 2010;5:e10228. doi: 10.1371/journal.pone.0010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sestak K, Conroy L, Aye PP, Mehra S, Doxiadis GG, Kaushal D. Improved xenobiotic metabolism and reduced susceptibility to cancer in gluten-sensitive macaques upon introduction of a gluten-free diet. PLoS One. 2011;6:e18648. doi: 10.1371/journal.pone.0018648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sestak K, Mazumdar K, Midkiff CC, Dufour J, Borda JT, Alvarez X. Recognition of epidermal transglutaminase by IgA and tissue transglutaminase 2 antibodies in a rare case of rhesus dermatitis. JOVE. 2011;58:e3154. doi: 10.3791/3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lundin KEA, Scott H, Hansen T, Paulsen G, Halstensen TS, Fausa O, Thorsby E, Sollid LM. Gliadin-specific HLA-DQ(α1*0501, β1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J. Exp. Med. 1993;178:187–196. doi: 10.1084/jem.178.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jabri B, Kasarda DD, Green PH. Innate and adaptive immunity: the yin and yang of celiac disease. Immunol. Rev. 2005;206:219–231. doi: 10.1111/j.0105-2896.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- [13].Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–1933. doi: 10.1053/j.gastro.2009.09.008. [DOI] [PubMed] [Google Scholar]
- [14].Sollid LM, Vaage J. Cross-dressing T cells go wild. Nat. Med. 2006;12:611–612. doi: 10.1038/nm0606-611. [DOI] [PubMed] [Google Scholar]
- [15].Brandtzaeg P. The changing immunological paradigm in coeliac disease. Immunol. Lett. 2006;105:127–139. doi: 10.1016/j.imlet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- [16].Nilsen EM, Lundin KE, Krajci P, Scott H, Sollid LM, Brandtzaeg P. Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Tho profile dominated by interferon-γ. Gut. 1995;37:766–776. doi: 10.1136/gut.37.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].DiRaimondo TR, Klock C, Khosla C. Interferon-γ activates transglutaminase 2 via a phosphatidylinositol-3-kinase-dependent pathway: implications for celiac sprue therapy. JPET. 2012;341:104–114. doi: 10.1124/jpet.111.187385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Blaschitz C, Raffatellu M. Th17 cytokines and the gut mucosal barrier. J. Clin. Immunol. 2010;30:196–203. doi: 10.1007/s10875-010-9368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bodd M, Raki M, Tollefsen S, Fallang LE, Bergseng E, Lundin KE, Sollid LM. HLA-DQ2-restricted gluten-reactive T cells produce IL-21 but not IL-17 or IL-22. Mucosal Immunol. 2010;3:594–601. doi: 10.1038/mi.2010.36. [DOI] [PubMed] [Google Scholar]
- [20].Monteleone I, Sarra M, Blanco GDV, Paoluzi OA, Franze E, Fina D, Fabrizi A, MacDonald TT, Pallone F, Monteleone G. Characterization of IL-17A producing cells in celiac disease mucosa. J. Immunol. 2010;184:2211–2218. doi: 10.4049/jimmunol.0901919. [DOI] [PubMed] [Google Scholar]
- [21].Fernandez S, Molina IJ, Romero P, Gonzalez R, Pena J, Sanchez F, Reynoso FR, Perez-Navero JL, Estevez O, Ortega C, Santamaria M. Characterization of gliadin-specific Th17 cells from the mucosa of celiac disease patients. Am. J. Gastroenterol. 2011;106:528–538. doi: 10.1038/ajg.2010.465. [DOI] [PubMed] [Google Scholar]
- [22].La Scaleia R, Barba M, Nardo G. Di, Bonamico M, Oliva S, Nenna R, Valitutti F, Mennini M, Barbato M, Montuori M, Porzia A, Petrarca L, Battella S, Cucchiara S, Piccoli M, Santoni A, Mainiero F, Palmieri G. Size and dynamics of mucosal and peripheral IL-17A+ T-cell pools in pediatric age, and their disturbance in celiac disease. Mucosal Immunol. 2012;5:513–523. doi: 10.1038/mi.2012.26. [DOI] [PubMed] [Google Scholar]
- [23].Sestak K, Merritt CK, Borda J, Saylor E, Schwamberger SR, Cogswell F, Didier ES, Didier PJ, Plauche G, Bohm RP, Aye PP, Alexa P, Ward RL, Lackner AA. Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus macaques. Infect. Immun. 2003;71:4079–4086. doi: 10.1128/IAI.71.7.4079-4086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Doxiadis GG, Otting N, de Groot NG, de Groot N, Rouweler AJ, Noort R, Verschoor EJ, Bontjer I, Bontrop RE. Evolutionary stability of MHC class II haplotypes in diverse rhesus macaque populations. Immunogenetics. 2003;55:540–551. doi: 10.1007/s00251-003-0590-9. [DOI] [PubMed] [Google Scholar]
- [25].Xu H, Wang X, Pahar B, Moroney-Rasmussen T, Alvarez X, Lackner AA, Veazey RS. Increased B7-H1 expression on dendritic cells correlates with PD-1 expression on T cells in SIV-infected macaques and may contribute to T cell dysfunction and disease progression. J. Immunol. 2010;185:7340–7348. doi: 10.4049/jimmunol.1001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xu H, Wang X, Liu DX, Rasmussen T, Lackner AA, Veazey RS. IL- 17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucosal Immunol. 2012;5:658–669. doi: 10.1038/mi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Green PH, Cellier C. Celiac disease. N. Engl. J. Med. 2007;357:1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- [28].Akdis M, Palomares O, van de Veen W, van Splunter M, Akdis CA. Th17 and Th22 cells: a confusion of antimicrobial response with tissue inflammation versus protection. J. Allergy Clin. Immunol. 2012;129:1438–1449. doi: 10.1016/j.jaci.2012.05.003. [DOI] [PubMed] [Google Scholar]
- [29].Rubino SJ, Geddes K, Girardin SE. Innate IL-17 and IL-22 responses to enteric bacterial pathogens. Trends Immunol. 2012;33:112–118. doi: 10.1016/j.it.2012.01.003. [DOI] [PubMed] [Google Scholar]
- [30].Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, Ramon M, Bergman R, Krueger JG, Guttman-Yassky E. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J. Allergy Clin. Immunol. 2009;123:1244–1252. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- [33].Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J. Allergy Clin. Immunol. 2007;120:247–254. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- [34].Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Klotz L, Burgdorf S, Dani I, Saijo K, Flossdorf J, Hucke S, Alferink J, Nowak N, Beyer M, Mayer G, Langhans B, Klockgether T, Waisman A, Eberl G, Schultze J, Famulok M, Kolanus W, Glass C, Kurts C, Knolle PA. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J. Exp. Med. 2009;106:2079–2089. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–593. doi: 10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- [37].Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hartigan-O’Connor DJ, Hirao LA, McCune JM, Dandekar S. Th17 cells and regulatory T cells in elite control over HIV and SIV. Curr. Opin. HIV AIDS. 2011;6:221–227. doi: 10.1097/COH.0b013e32834577b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- [40].Sanz Y, De Palma G, Laparra M. Unraveling the ties between celiac disease and intestinal microbiota. Int. Rev. Immunol. 2011;30:207–218. doi: 10.3109/08830185.2011.599084. [DOI] [PubMed] [Google Scholar]
- [41].Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- [44].Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Doxiadis GG, Otting N, de Groot NG, Bontrop RE. Differential evolutionary MHC class II strategies in humans and rhesus macaques: relevance for biomedical studies. Immunol. Rev. 2001;183:76–85. doi: 10.1034/j.1600-065x.2001.1830106.x. [DOI] [PubMed] [Google Scholar]
- [46].de Groot N, Doxiadis GG, de Groot NG, Otting N, Heijmans C, Rouweler AJM, Bontrop RE. Genetic makeup of the DR region in rhesus macaques: gene content, transcripts, and pseudogenes. J. Immunol. 2004;172:6152–6157. doi: 10.4049/jimmunol.172.10.6152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.