Abstract
PCOS is a prevalent hyperandrogenic infertility and cardiometabolic disorder that increases a woman’s lifetime risk of type 2 DM. It is heritable and intensely familial. Progress towards a cure has been delayed by absence of an etiology. Evidence is mounting, however, for in utero T excess, together with gestational hyperglycemia, contributing to either early differentiation of PCOS or phenotypic amplification of its genotypes. Abnormal endocrine, ovarian and hyperinsulinemia traits are detectable as early as 2-months of age in daughters of women with PCOS, with adiposity enhancement of hyperinsulinemia during childhood potentially contributing to hyperandrogenism and LH excess by adolescence. These findings encourage increasing clinical focus on early childhood markers for adiposity and hyperinsulinemia accompanying ovarian and adrenal endocrine abnormalities that precede a diagnosable PCOS phenotype. They raise the possibility for lifestyle or therapeutic intervention prior to and during pregnancy or during childhood and adolescence alleviating the manifestations of a familial genetic predisposition to PCOS.
Keywords: fetal androgen excess, gestational hyperglycemia, developmental programming, childhood obesity, insulin resistance
Polycystic ovary syndrome (PCOS) phenotypes
Women with PCOS are diagnosed from at least two out of the following: [1] testosterone (T) excess, [2] intermittent or absent menstrual cycles (~90% accompaniment by LH excess), and [3] polycystic ovaries (>90% accompaniment by anti-mullerian hormone (AMH) excess) (1). This “Rotterdam consensus” diagnosis (2) was recently affirmed as the “gold standard” for PCOS at an evidence-based conference at NIH (3) (http://prevention.nih.gov/workshops/2012/pcos/docs/PCOS_Final_Statement.pdf). It permits four distinct PCOS phenotypes: (a) all three criteria; (b) testosterone excess and intermittent/absent menstrual cycles; (c) testosterone excess and polycystic ovaries; and (d) intermittent/absent menstrual cycles and polycystic ovaries. The first two phenotypes effectively comprise the NIH criteria for PCOS (4).
While a common origin for all PCOS phenotypes is debated (5–9), etiological clarity is confounded by phenotypic variability. For example, weight loss (9–11), insulin sensitizer treatment (12) or increasing age (13) can each ameliorate more severe PCOS phenotypes with all three criteria or testosterone excess and intermittent/absent menstrual cycles, into milder PCOS phenotypes with only testosterone excess and polycystic ovaries or intermittent/absent menstrual cycles and polycystic ovaries. PCOS symptomology is particularly alleviated by bariatric surgery-enabled weight loss (14). In contrast, obesity exaggerates phenotypic presentation in PCOS (14–16). Epigenetic and environmental factors can thus diminish or exacerbate PCOS phenotype independently of genetic predisposition. In this context, it is relevant to note that reproductive and hyperandrogenic dysfunction predominate in young women with PCOS, while PCOS women in their later years have more pronounced cardio-metabolic disorders (2, 13). Progress towards a cure for PCOS, and its increased lifetime risk of type 2 diabetes mellitus (type 2 DM) and cardiovascular disease (17, 18), is thus impeded by an obscured pathogenic origin. This mini-review will explore in utero origins for PCOS and accompanying insulin resistance (19–21), the ontogeny of adolescent PCOS, the role of insulin resistance in its pathogenesis, and early childhood factors related to the effect of obesity that may influence the impact of any hereditary component (Figure 1).
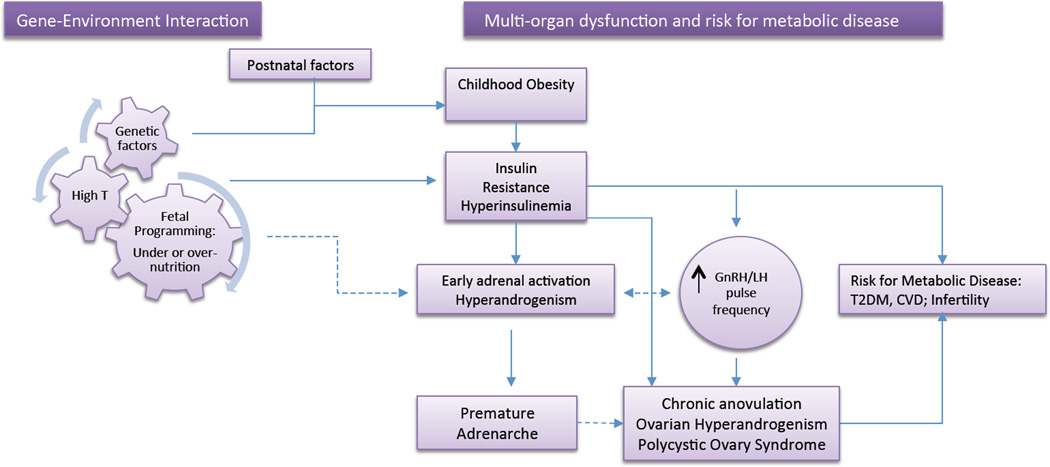
Figure 1.
Proposed ontogeny of PCOS with manifestations starting in childhood: Interaction of genetic and environmental factors shape the intrauterine environment leading to epigenetic changes and alteration of organ function at several levels. Childhood obesity and its associated insulin resistance further enhance the manifestations of genetic/epigenetic traits predisposing to hyperandrogenism, including effects on steroidogenesis and hypothalamic/pituitary function. The dashed lines represent relationships that have not been clearly established.
Genetic origins for PCOS
Heritability of PCOS, particularly hyperandrogenism (22), is readily apparent in twin (23) and genetic (24–26) studies, demonstrating considerable familial clustering of the syndrome (27). Currently, however, only a few PCOS susceptibility genes have been repeatedly identified in studies of women with Chinese or European ancestry: allelic variants of fibrillin-3 (FBN3) or DENN/MADD domain containing 1A (DENND1A) (28–31), and variants of luteinizing hormone receptor (LHR) (31–33).
FBN3 encodes for an extra-cellular matrix protein that regulates TGF-beta signaling. Its PCOS-associated allelic variant, A8, manifests a metabolically distinct phenotype, including insulin resistance (34). FBN3 expression, however, is confined to early-to-mid gestation in many organs and tissues, including the ovary (35, 36). Such a gestational stage includes a period of fetal developmental at which T-exposure induces altered DNA methylation of TGF-beta regulating genes and subsequent PCOS-like traits (37). Since the degree and type of fibrillin expression contributes to differences in elasticity of cell extracellular matrix interactions and storage of TGF-beta, fibrillins may provide gestationally relevant (35), tissue specific bases for cell-mediated engagement of extracellular matrix-stored TGF-beta in proliferation, differentiation, and apoptosis (38, 39). DENND1A regulates Rab GTPases (40) and is involved in intra-cellular vesicle trafficking, including calcium regulated exocytosis in pituitary cells that may include exocytosis of gonadotropins (41). In the ovary, variants of LHR may diminish or enhance pituitary LH stimulation of ovarian theca and stroma cell testosterone production, ovarian follicle development, LH surge-induced ovulation and corpus luteum function (42), while in adipocytes, LHR variants may alter LH stimulation of adipogenesis (43). Variants in these multi-organ system genes could contribute genetic determination of PCOS phenotypes for reproductive and metabolic pathophysiology. Neither these, nor other less robust gene candidates, however, are associated with the majority of PCOS subjects in any population study (44) potentially reflecting suspected multi-genic origins of PCOS with or without accompanying developmental environment contributions (27).
Adolescent PCOS: Insulin Resistance
Insulin resistance and/or hyperinsulinemia are major components of PCOS in obese, but also in lean, adult women affected by this condition (45). A similar profile is present in adolescent girls with PCOS with ~50% lower insulin sensitivity compared with obese controls of similar age, body composition and abdominal adiposity (46). This increased insulin resistance is associated with increased risk for type 2DM and cardiovascular disease in these young adolescents. In a clinic setting, adolescent girls with PCOS have a high prevalence of impaired glucose tolerance with 30% diagnosed with prediabetes and ~4% with type 2DM (47). In the National Health and Nutrition Examination Survey (NHANES III), girls with PCOS were 4.5 times more likely to fulfill the criteria for the metabolic syndrome than age matched girls after adjusting for BMI and indices of insulin resistance (48). The role of insulin resistance in the pathogenesis of adolescent PCOS is supported by the improvement of the hyperandrogenic profile with the use of insulin sensitizers such as metformin (49) or lifestyle changes with an aggressive weight loss program (50).
No diagnostic clarity exists for pre-pubertal or adolescent girls with PCOS, however, as ovarian function is either in immature quiescence or too closely resembles PCOS in normal adolescence (51–53). Carmina and colleagues (54) propose delaying a PCOS diagnosis in adolescents until they are at least two years post-menarche and have at least two years of intermittent or absent menstrual cycles. Thus discerning care has to be given to reports involving PCOS adolescents. The origins of childhood insulin resistance and adolescent predisposition to PCOS have been linked to in utero adverse events (55, 56), a presentation with premature adrenarche in early childhood (57–60) together with major roles of family history and obesity (61) (Figure 1).
In utero exposure: effects of the hyperandrogenic PCOS environment
Animal studies, from rodents (62, 63) and sheep (64) to monkeys (8, 19), repeatedly demonstrate how fetal T excess, and possibly accompanying gestational hyperglycemia and hyperinsulinemia (65), determine a variety of PCOS-like phenotypes in adulthood, including the diversity encompassed by the “Rotterdam consensus” criteria (66). Current technology, however, prevents safe quantification of human fetal hormonal exposure during early-to-mid gestation (67), so investigations of fetal origins of PCOS in women rely on indirect assessments or postnatal outcomes of fetal T excess.
PCOS mothers contribute elevated maternal circulating levels of T to the gestational environment (68), and subtle perturbations in placental function (69), in addition to gestational hyperglycemia (70), which may compromise protection of their fetal daughters. Interestingly, elevated mid-gestation maternal T levels predict high AMH levels in adolescent daughters (71). Since elevated AMH is a characteristic of adolescents and women with PCOS (72) and newborn daughters of PCOS women (70, 73), such associations suggest a cross-generational relationship between the degree of maternal hyperandrogenism and development of PCOS in daughters. Mid-gestational daughters can contribute androgen excess when the fetal ovary can produce (74) and respond (75) to androgens. Elevated, mid-gestational amniotic fluid levels of T in fetal daughters of women with PCOS (76) are consistent with a fetal source for T excess in female offspring with an increased risk of PCOS.
Perinatal studies are mixed in their support of gestational T exposure as a fetal programming origin for PCOS, possibly because onset of labor variably reduces T levels in umbilical cord blood (77). In newborn daughters born to women with PCOS, one study shows elevated T levels in umbilical venous blood (78), whereas another two studies show reduced umbilical cord blood androstenedione levels (69, 79). In a third study involving adolescent girls diagnosed with PCOS including, as discussed below, an inherently high prevalence (~28%) of diagnosis at this young age, umbilical cord blood shows no elevation in T levels (80). With the ovary as a key fetal site for gestational T excess at a critical mid-gestational age for target organ differentiation (20), studies at the time of birth are likely too late to detect any remaining hormonal differences (67, 74). Perhaps consistent with this latter conclusion, two studies of non-pregnant PCOS women (81, 82) demonstrate positive correlations between adult T levels or hirsutism scores and the finger length ratio between the 2nd and 4th fingers, an anthropometric trait established in utero (83–85). PCOS-like monkeys show analogous positive correlations between the same finger length ratio and duration of T exposure during early-to-mid, but not late, gestation (41), implying that finger length associations in PCOS may indicate fetal T exposure during early-to-mid gestation.
Elusive In Utero Origins of PCOS in Humans
Notwithstanding a recent study showing mid-gestational elevations in amniotic fluid T levels in daughters of PCOS women (76), the basic problem with confirming developmental origins of PCOS in humans is an absence of evidence for fetal T excess (Table 1). Even though girls born to women with PCOS are at increased risk of PCOS in adulthood, studies have yet to link excess fetal T levels in mid-gestational girls with the onset of PCOS in adulthood (86, 87). The absence of evidence arises, in part, because of the technical and ethical challenges posed by blood sampling from mid-gestation human fetuses (67, 87). However, this “absence of evidence is not evidence of absence” (Carl Sagan, 1934–1996), but is at times considered as such (88). Furthermore, while the expression of aromatase in the PCOS placenta is indeed diminished (69), and potentially failing to prevent fetal T excess in PCOS pregnancies (68), the degree of diminished aromatase required to result in T excess in female fetuses is extremely rare (89). In recent studies of hypertensive preeclamptic pregnancies, on the other hand, findings suggest comprehensive reduction in placental ability to synthesize estrogens (90, 91), indicating a more common gestational impairment of T aromatization than is currently considered. It is therefore not too surprising that no association is found between high maternal, mid-gestational T levels and subsequent development of PCOS in daughters (80). It is fetal exposure to T that is key (92).
Table 1.
Human data most relevant to in utero origins of PCOS.
a. Human findings consistent with developmental origins for PCOS
|
b. Human findings inconsistent with developmental origins for PCOS
|
Other investigators have examined putative postnatal biomarkers of fetal T exposure in females. The latter, however, have not been validated against fetal T levels and can be inappropriately used to discount fetal T excess in PCOS women, including the sexually dimorphic ratio of 2nd to 4th finger length that can be diminutive in men (66, 85), but is inconsistently so in PCOS women (81, 82, 93). In monkeys, the same fetal T exposure that induces PCOS-like traits does not diminish this finger length ratio (66). Furthermore, women who gestated with a male co-twin show inconsistent expression of masculinized traits (94), thus an absence of PCOS prevalence among women with male co-twins (95) is not strong evidence for lack of fetal T exposure prior to PCOS onset. Variable phenotypes in women with PCOS also do not pose difficulties for a common fetal T origin (86), since variability in PCOS-like phenotypes ensues from fetal T exposure in animal models (37, 66).
There remains, however, an absence of evidence for mid-gestational T excess in human female fetuses, perhaps accompanied by gestational hyperglycemia, fetal hyperinsulinemia and their sequelae, that precedes PCOS phenotype development during adolescence. Until this information gap is closed, perhaps by determining T content in newborn baby hair and gaining a retrospective assessment of prior gestational T exposure, an in utero origin for PCOS will remain in dispute despite compelling animal evidence (8, 64) (Table 1).
Intrauterine Programming: Insulin Resistance
Epidemiologic and clinical studies conducted largely in adult populations suggest a link between in utero events, particularly fetal growth restriction, and subsequent risk of type 2DM and cardiovascular disease in humans (96, 97). This “Intrauterine Programming” or fetal origins hypothesis indicates that in utero factors lead to permanent changes in organ function and predisposes individuals exposed to fetal growth restriction to a variety of metabolic diseases. The increased risk for these metabolic diseases has been linked to increased insulin resistance in young individuals exposed to adverse in utero environment and born small for gestational age (SGA) (98, 99). A study of infants at 1 year of age demonstrates that SGA infants with catch-up growth are less insulin sensitive (with higher fasting insulin and higher triglycerides) than appropriate for gestational age (AGA) infants, despite their continuing lower weight and BMI (100). The picture is compounded by the influence of “catch-up” growth (101), with the highest levels of insulin resistance reported in children of low birth weight, but with subsequent high BMI and fat mass in childhood (102, 103). For example, in 9-year-old prepubertal children, hyperinsulinemic clamp studies show reduced insulin sensitivity in SGA children, compared with AGA, especially in SGA children with catch-up growth and a high BMI (104). These studies support an overall relationship between fetal growth restriction and increased adiposity and insulin resistance starting early in the childhood period.
In addition to in utero environmental factors, genetic polymorphisms modulate insulin resistance parameters in SGA individuals that may partly explain the variable degree of insulin resistance in subjects exposed to an adverse in utero environment (105). At the other extreme, over nutrition of the fetus appears to have long-term effects on obesity, insulin resistance, and predisposition to disorders of glycemic regulation. Offspring of mothers with diabetes during pregnancy have a higher frequency of childhood obesity and earlier onset of impaired glucose tolerance (106, 107) and type 2 DM (108). Rates of impaired glucose regulation are around 20% in adolescent offspring of mothers who have diabetes during pregnancy (107). The increased risk, however, is not restricted to mothers with diabetes during their gestation. In the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, a continuous relationship was observed between maternal hyperglycemia in the non-diabetic range and infant birth weight and cord blood C-peptide levels (109, 110). Given the effect of insulin on modulating ovarian (111) and adrenal steroidogenesis (112), a role of intrauterine adverse events which lead to insulin resistance and/or hyperinsulinemia may predispose adolescents to PCOS (Figure 1).
Intrauterine Programming: Under- and Over-Nutrition and PCOS
In addition to the relationship between fetal undernutrition and insulin resistance/hyperinsulinemia, such in utero environments can affect ovarian development and function, as well as adrenal function (55, 113) (Figure 1). Such potential fetal programming of PCOS, however, may be restricted to some populations such as those of Spanish or Iranian descent (55, 114, 115). Postmenarcheal Catalan girls with a history of low birth weight, catch-up growth, hyperinsulinemia and precocious pubarche have an exaggerated (50%) prevalence of PCOS that manifests as oligomenorrhea, hirsutism and hyperandrogenemia (55).
The Spanish investigators also report a relationship between low birth weight, premature adrenarche and subsequent early puberty (116, 117). Also, in a relatively small longitudinal study (from 2–8 years of age), SGA girls from Spain (birth weight < −2 SD) who had spontaneous catch-up growth compared with girls born AGA, are more likely to have increased body mass index (BMI) at age 8, increased fat mass and abdominal adiposity. This is associated with higher leptin levels, higher dehydroepiandrosterone sulphate (DHEAS) and lower sex hormone binding globulin (SHBG) and adiponectin in the SGA girls (118). Other investigators, however, report no relationship between low birth weight and serum DHEAS levels in short children 3–9 years of age (119). Studies from France do not show a relationship between low birth weight and premature adrenarche (120). Also, in studies of older French women, those with low birth weight were more hyperinsulinemic than controls, but did not show evidence of hyperandrogenism (121). On the other hand, in a large United Kingdom birth cohort evaluated at age 8 years, adrenal androgen levels were inversely related to birth weight SD score in each sex. After adjusting for childhood weight, children who showed rapid postnatal weight gain between 0 and 3 years of age had higher DHEAS and androstenedione levels at 8 years (122). In a retrospective Australian study of 89 children (79 girls) with precocious pubarche, 65% were overweight at diagnosis, 35% had a history of SGA and 24% had a history of prematurity. In this latter study, both prematurity and SGA were associated with precocious pubarche, as was obesity, irrespective of size at birth (123).
Consistent with an early origin of PCOS, studies by Ibanez and colleagues suggest that commencing metformin therapy before menarche in girls with premature pubarche and low birth weight, may prevent or delay the development of hirsutism, androgen excess, oligomenorrhea, and the diagnosis of PCOS more effectively than shorter-duration metformin therapy commencing after menarche (124). In the absence of other longitudinal studies clearly establishing a link between SGA, premature adrenarche and PCOS, however, these findings need to be interpreted with caution. Studies of SGA girls in northern Spain, evaluated at 14 to 18 years of age, report an ~20% smaller uterine size and an ~40% reduction in their ovarian volume compared with AGA controls (125). SGA girls also have elevated LH and fasting insulin, as well as an excess of abdominal fat compared with AGA peers. These SGA associations, however, are not confirmed by other researchers (126). In other studies of ovarian reserve, AMH levels in short 3–10 year old (average age of 6.24 years), prepubertal SGA girls are similar to those in AGA girls of similar age and gestational age, possibly indicating that the follicle pool is not affected in relationship to SGA status (127). This is unlike the findings of Sir-Petermann and colleagues who show higher AMH levels in SGA infants with catchup growth by 2–3 month of age (128). The discrepancy may be related to different ages and effect of early catch-up growth. It is unclear from these studies if there is altered follicular reserve or function in SGA girls, but a recent longitudinal study suggests that AMH levels vary little within individual healthy girls from childhood through adolescence, so that single AMH measurements may be representative of prepubertal ovarian populations of pre-antral and antral follicles (129).
In contradistinction to the effects of fetal growth restriction, over-nutrition in utero has also been related to higher risk of PCOS. Higher birth weight in girls born to overweight mothers is a risk factor for developing PCOS by 40–42 years of age (130). In a retrospective birth cohort study of singleton females, there is a 5% increase in the risk of developing adult hyperandrogenism when birth weight is high (56). On the other hand, thinness at birth, reflected in a low ponderal index (low birth weight for length), is associated with higher risk for developing all three PCOS diagnostic criteria in adulthood (56). As Davies and colleagues point out, higher birth weight may translate into higher risk for adiposity, and thus functional hyperandrogenism, as adipose tissue is androgenic (131, 132). In contrast, the risk of PCOS in relation to a low ponderal index may indicate a relationship between adverse in utero events and subsequent insulin resistance (133). Overall, these studies indicate that at least some metabolic components of the PCOS phenotype are programmed in utero, in particular the tendency for higher fat mass, visceral adiposity and insulin resistance (Figure 1).
Effect of Childhood Obesity
Obesity has been associated with early adrenarche and subsequent PCOS. Higher BMI Z-score has been associated with pubarche and early puberty in the National Health and Nutrition Examination survey (NHANES) (134). Childhood obesity, in particular visceral adiposity is a major determinant of insulin resistance in youth (135, 136). In the presence of normal beta cell function, insulin resistance is compensated by increased insulin secretion and hyperinsulinemia. The effect of obesity on insulin sensitivity is compounded by the physiologic insulin resistance of puberty (137). This hyperinsulinemia may drive premature adrenarche and the latter may be a precursor for subsequent PCOS in genetically predisposed girls, in particular in those in whom the phenotype is amplified by catch-up growth (Figure 1).
Daughters of women with PCOS evaluated during early childhood (age 4–8) and early puberty (age 9–13) have exaggerated adrenarche compared with girls of non-PCOS women of similar pubertal stage and BMI (57). This is in accordance with the role of hyperinsulinemia in ovarian (138) and adrenal hyperandrogenism (139). In high-risk obese girls with premature adrenarche of ethnic minority descent, androgen levels are inversely related to insulin sensitivity measured by intravenous glucose tolerance test (140). Moreover, girls with premature adrenarche and low insulin sensitivity compared with the group with relatively normal insulin sensitivity manifest higher ACTH stimulated androgen levels, higher free T and lower sex hormone binding globulin (140).
In other studies, peripubertal obesity in girls is associated with hyperandrogenemia (higher T, unbound (free) T, DHEAS and lower SHBG) and elevated insulin levels during each stage of pubertal development (141). Unbound T is five times as great in obese early-pubertal girls compared with normal weight peers of the same pubertal stage. Insulin levels correlated inversely with unbound T after adjusting for age, pubertal stage, insulin, LH, and DHEAS (141). The etiology of the hyperandrogenemia in obese girls is unclear. Knudsen and colleagues report wide variability in T levels across BMI Z-scores in obese girls (142) suggesting that obesity, alone, is not sufficient to produce hyperandrogenemia. In their study (142), the predictors of unbound T, after adjusting for BMI z-score, age and pubertal stage, were morning LH levels and fasting insulin. This is consistent with a role of obesity-related insulin resistance in driving hyperandrogenemia in these girls through an effect of insulin on adrenal and ovarian steroidogenesis (143), manifesting as early adrenarche (144) and subsequent PCOS (145). Such hyperandrogenemia appears to modulate gonadotropin levels. Obese peripubertal girls were found to have increased LH frequency, but low LH amplitude, and Tanner 3–5 girls have decreased overnight LH pulse amplitude compared with normal weight girls (146). These changes may reflect initial effect of obesity on LH pulses (147). Subsequently, hyperandrogenaemia reduces the inhibition of gonadotropin-releasing hormone pulse frequency by progesterone, causing rapid LH pulse secretion and further increasing ovarian androgen production (147–149). A similarly altered developmental trajectory in early-to-mid gestation T exposed PCOS-like monkeys (8) and comparable phenotype in peri-pubertally T-exposed female monkeys (150) are consistent with insulin-mediated weight gain amplifying postnatal expression of PCOS.
Effect of Family History: Endocrine and metabolic dysfunction starting in adolescence
PCOS prevalence rises to 20%–40% in families of women with PCOS (17, 151). Daughters and sisters of women with PCOS have higher levels of adrenal and ovarian androgens from adolescence into adulthood (57, 152–156). In addition, daughters, sisters, brothers, mothers, and fathers of women with PCOS exhibit insulin resistance, hyperinsulinemia, and/or impaired glucose tolerance (153–158). In adolescence, daughters of women with PCOS compared with controls of similar BMI z-score, waist to hip ratio and birth weight, exhibit a higher prevalence of hirsutism and greater ovarian volumes at different stages of pubertal development (155). Metabolic evaluation reveals increased 2-hour insulin levels during the oral glucose tolerance test with similar levels of glucose, an indication of insulin resistance, compared to their Tanner and BMI matched controls (155). Three additional studies confirm this pre-adolescent or adolescent onset of hyperinsulinemic responses in daughters or sisters of women with PCOS that accompany insulin resistance (153, 159, 160).
In pubertal stages Tanner IV and V, the daughters of PCOS women have higher basal and leuprolide stimulated LH and 17-hydroxyprogesterone (17-OHP) levels, lower SHBG and higher and free androgen index (155). Elevated 17-OHP levels were replicated in separate study of adolescents (159). These studies indicate that metabolic abnormalities commonly associated with PCOS in women occur at least by the early pubertal stages and become more manifest with the progression of puberty in genetically predisposed females. Of note, the testosterone levels in stages IV and V correlate positively with 2-h insulin levels in adolescent daughters of women with PCOS (155). In addition, AMH levels, a marker of follicular development, are higher in daughters of women with PCOS at all Tanner stages and those with the highest AMH values have lower FSH concentrations and higher stimulated levels of insulin during Tanner stages I, II, and III (72). The PCOS adolescent daughter group with higher AMH levels may reflect a group at higher risk of metabolic abnormalities in adulthood. Overall, these studies are consistent with a genetic predisposition to PCOS manifesting in early childhood, with possible amplification of phenotype depending on the degree of accompanying metabolic dysfunction (Figure 1).
Conclusion
PCOS is a highly prevalent reproductive and cardiometabolic disorder that greatly increases a woman’s lifetime risk of infertility, type 2 DM and cardiovascular disease. It is heritable and intensely familial. Progress towards a cure has been hindered by absence of pathogenic mechanism. Circumstantial evidence is mounting, however, for in utero T excess, together with gestational hyperglycemia, contributing to either early differentiation of PCOS or amplification of its phenotypes. Abnormal endocrine, ovarian and hyperinsulinemic traits are detectable as early as 2-months of age in daughters of women with PCOS, with adiposity enhancement of hyperinsulinemia during childhood potentially contributing to hyperandrogenemia and LH excess expression by adrenarche and adolescence (Figure 1), abnormal developmental trajectories emulated by PCOS-like monkeys (8). These findings encourage increasing clinical focus on establishing childhood markers for increased adiposity and hyperinsulinemia accompanying ovarian and adrenal endocrine abnormalities that precedes PCOS in adolescence and young adulthood. They raise the possibility for lifestyle or therapeutic intervention prior to and during pregnancy (70), and/or during childhood and adolescence (124), preventing familial genetic predisposition to PCOS from manifesting a diagnostic phenotype in adulthood. The circumstantial nature of the evidence, however, that links fetal T exposure to postnatal development of PCOS in humans (Table 1), requires additional population studies to clarify androgenic beginnings.
Acknowledgments
Financial support: D.H.A. is supported, in part, by National Institutes of Health P50 HD044405 (to Andrea Dunaif, MD) and P51 RR000167 (Wisconsin National Primate Research Center). F.B. is supported, in part, by National Institutes of Health R03 HD059798 and USDA ARS 6250-5100-054.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: D.H.A. is a consultant for Viamet Pharmaceuticals, Inc., Morrisville, NC. F.B. has nothing to disclose.
References
- 1.Eilertsen TB, Vanky E, Carlsen SM. Anti-Mullerian hormone in the diagnosis of polycystic ovary syndrome: can morphologic description be replaced? Hum Reprod. 2012;27:2494–2502. doi: 10.1093/humrep/des213. [DOI] [PubMed] [Google Scholar]
- 2.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Hampton T. NIH Panel: Name Change, New Priorities Advised for Polycystic Ovary Syndrome. JAMA. 2013;309:863. doi: 10.1001/jama.2013.1236. [DOI] [PubMed] [Google Scholar]
- 4.Zawadski JK, Dunaif A. Diagnostic criteria for poly- cystic ovary syndrome; towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic Ovary Syndrome. Boston, MA: Blackwell Scientific Publications; 1992. pp. 377–384. [Google Scholar]
- 5.Xita N, Tsatsoulis A. Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies. The Journal of Clinical Endocrinology and Metabolism. 2006;91:1660–1666. doi: 10.1210/jc.2005-2757. [DOI] [PubMed] [Google Scholar]
- 6.Franks S. Animal models and the developmental origins of polycystic ovary syndrome: increasing evidence for the role of androgens in programming reproductive and metabolic dysfunction. Endocrinology. 2012;153:2536–2538. doi: 10.1210/en.2012-1366. [DOI] [PubMed] [Google Scholar]
- 7.Franks S, Berga SL. Does PCOS have developmental origins? Fertil Steril. 2012;97:2–6. doi: 10.1016/j.fertnstert.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbott DH, Nicol LE, Levine JE, Xu N, Goodarzi MO, Dumesic DA. Nonhuman primate models of polycystic ovary syndrome. Mol Cell Endocrinol. 2013 Jan 29; doi: 10.1016/j.mce.2013.01.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nybacka Å, Carlström K, Ståhle A, Nyrén S, Hellström PM, Hirschberg AL. Randomized comparison of the influence of dietary management and/or physical exercise on ovarian function and metabolic parameters in overweight women with polycystic ovary syndrome. Fertil Steril. 2011;96:1508–1513. doi: 10.1016/j.fertnstert.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Legro RS. Obesity and PCOS: implications for diagnosis and treatment. Semin Reprod Med. 2012;30:496–506. doi: 10.1055/s-0032-1328878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moran C, Arriaga M, Rodriguez G, Moran S. Obesity differentially affects phenotypes of polycystic ovary syndrome. Int J Endocrinol. 2012;2012:317241. doi: 10.1155/2012/317241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palomba S, Falbo A, Zullo F, Orio F., Jr. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev. 2009;30:1–50. doi: 10.1210/er.2008-0030. [DOI] [PubMed] [Google Scholar]
- 13.Carmina E, Campagna AM, Lobo RA. A 20-year follow-up of young women with polycystic ovary syndrome. Obstet Gynecol. 2012;119:263–269. doi: 10.1097/AOG.0b013e31823f7135. [DOI] [PubMed] [Google Scholar]
- 14.Escobar-Morreale HF. Surgical management of metabolic dysfunction in PCOS. Steroids. 2012;77:312–316. doi: 10.1016/j.steroids.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011 Jul 6;(7):CD007506. doi: 10.1002/14651858.CD007506.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95–109. doi: 10.1111/j.1467-789X.2012.01053.x. [DOI] [PubMed] [Google Scholar]
- 17.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95:2038–2049. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 18.Mani H, Levy MJ, Davies MJ, Morris DH, Gray LJ, Bankart J, Blackledge H, Khunti K, Howlett TA. Diabetes and cardiovascular events in women with polycystic ovary syndrome: a 20-year retrospective cohort study. Clin Endocrinol (Oxf) 2013;78:926–934. doi: 10.1111/cen.12068. [DOI] [PubMed] [Google Scholar]
- 19.Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW. Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab. 1998;9:62–67. doi: 10.1016/s1043-2760(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 20.Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome - a hypothesis. J Endocrinol. 2002;174:1–5. doi: 10.1677/joe.0.1740001. [DOI] [PubMed] [Google Scholar]
- 21.Franks S, McCarthy MI, Hardy K. Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl. 2006;29:278–285. doi: 10.1111/j.1365-2605.2005.00623.x. [DOI] [PubMed] [Google Scholar]
- 22.Legro RS, Spielman R, Urbanek M, Driscoll D, Strauss JF, 3rd, Dunaif A. Phenotype and genotype in polycystic ovary syndrome. Recent progress in hormone research. 1998;53:217–256. [PubMed] [Google Scholar]
- 23.Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91:2100–2104. doi: 10.1210/jc.2005-1494. [DOI] [PubMed] [Google Scholar]
- 24.Goodarzi MO. Looking for polycystic ovary syndrome genes: rational and best strategy. Semin Reprod Med. 2008;26:5–13. doi: 10.1055/s-2007-992919. [DOI] [PubMed] [Google Scholar]
- 25.Stewart DR, Dombroski BA, Urbanek M, Ankener W, Ewens KG, Wood JR, Legro RS, Strauss JF, 3rd, Dunaif A, Spielman RS. Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab. 2006;91:4112–4117. doi: 10.1210/jc.2006-0951. [DOI] [PubMed] [Google Scholar]
- 26.Ewens KG, Stewart DR, Ankener W, Urbanek M, McAllister JM, Chen C, Baig KM, Parker SC, Margulies EH, Legro RS, Dunaif A, Strauss JF, 3rd, Spielman RS. Family-based analysis of candidate genes for polycystic ovary syndrome. J Clin Endocrinol Metab. 2010;95:2306–2315. doi: 10.1210/jc.2009-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosova G, Urbanek M. Genetics of the polycystic ovary syndrome. Mol Cell Endocrinol. 2012 Oct 16; doi: 10.1016/j.mce.2012.10.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie GB, Xu P, Che YN, Xia YJ, Cao YX, Wang WJ, Qiao D, Wu XK, Yi L, Gao Q, Wang Y. Microsatellite polymorphism in the fibrillin 3 gene and susceptibility to PCOS: a case-control study and meta-analysis. Reprod Biomed Online. 2013;26:168–174. doi: 10.1016/j.rbmo.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Welt CK, Styrkarsdottir U, Ehrmann DA, Thorleifsson G, Arason G, Gudmundsson JA, et al. Variants in DENND1A are associated with polycystic ovary syndrome in women of European ancestry. J Clin Endocrinol Metab. 2012;97:E1342–E1347. doi: 10.1210/jc.2011-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodarzi MO, Jones MR, Li X, Chua AK, Garcia OA, Chen YD, et al. Replication of association of DENND1A and THADA variants with polycystic ovary syndrome in European cohorts. J Med Genet. 2012;49:90–95. doi: 10.1136/jmedgenet-2011-100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi Y, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43:55–59. doi: 10.1038/ng.732. [DOI] [PubMed] [Google Scholar]
- 32.Capalbo A, Sagnella F, Apa R, Fulghesu AM, Lanzone A, Morciano A, Farcomeni A, Gangale MF, Moro F, Martinez D, Ciardulli A, Palla C, Uras ML, Spettu F, Cappai A, Carcassi C, Neri G, Tiziano FD. The 312N variant of the luteinizing hormone/choriogonadotropin receptor gene (LHCGR) confers up to 2·7-fold increased risk of polycystic ovary syndrome in a Sardinian population. Clin Endocrinol (Oxf) 2012;77:113–119. doi: 10.1111/j.1365-2265.2012.04372.x. [DOI] [PubMed] [Google Scholar]
- 33.Mutharasan P, Galdones E, Peñalver Bernabé B, Garcia OA, Jafari N, Shea LD, Woodruff TK, Legro RS, Dunaif A, Urbanek M. Evidence for chromosome 2p16.3 polycystic ovary syndrome susceptibility locus in affected women of European ancestry. J Clin Endocrinol Metab. 2013;98:E185–E190. doi: 10.1210/jc.2012-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urbanek M, Sam S, Legro RS, Dunaif A. Identification of a polycystic ovary syndrome susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J Clin Endocrinol Metab. 2007;92:4191–4198. doi: 10.1210/jc.2007-0761. [DOI] [PubMed] [Google Scholar]
- 35.Hatzirodos N, Bayne RA, Irving-Rodgers HF, Hummitzsch K, Sabatier L, Lee S, Bonner W, Gibson MA, Rainey WE, Carr BR, Mason HD, Reinhardt DP, Anderson RA, Rodgers RJ. Linkage of regulators of TGF-β activity in the fetal ovary to polycystic ovary syndrome. FASEB J. 2011;25:2256–2265. doi: 10.1096/fj.11-181099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabatier L, Miosge N, Hubmacher D, Lin G, Davis EC, Reinhardt DP. Fibrillin-3 expression in human development. Matrix Biol. 2011;30:43–52. doi: 10.1016/j.matbio.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu N, Kwon S, Abbott DH, Geller DH, Dumesic DA, Azziz R, Guo X, Goodarzi MO. Epigenetic mechanism underlying the development of polycystic ovary syndrome (PCOS)-like phenotypes in prenatally androgenized rhesus monkeys. PLoS ONE. 2011;6:e27286. doi: 10.1371/journal.pone.0027286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massam-Wu T, Chiu M, Choudhury R, Chaudhry SS, Baldwin AK, McGovern A, Baldock C, Shuttleworth CA, Klelty CM. Assembly of fibrillin microfibrils governs extracellular deposition of latent TGF beta. J. Cell. Sci. 2010;123:3006–3018. doi: 10.1242/jcs.073437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sengle G, Ono RN, Sasaki T, Sakai LY. Prodomains of transforming growth factor beta (TGFbeta) superfamily members specify different functions: extracellular matrix interactions and growth factor bioavailability. J. Biol. Chem. 2011;286:5087–5099. doi: 10.1074/jbc.M110.188615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marat AL, Dokainish H, McPherson PS. DENN domain proteins: regulators of Rab GTPases. J Biol Chem. 2011;286:13791–13800. doi: 10.1074/jbc.R110.217067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tasaka K, Masumoto N, Mizuki J, Ikebuchi Y, Ohmichi M, Kurachi H, Miyake A, Murata Y. Rab3B is essential for GnRH-induced gonadotrophin release from anterior pituitary cells. J Endocrinol. 1998;157:267–274. doi: 10.1677/joe.0.1570267. [DOI] [PubMed] [Google Scholar]
- 42.Dickinson RE, Stewart AJ, Myers M, Millar RP, Duncan WC. Differential expression and functional characterization of luteinizing hormone receptor splice variants in human luteal cells: implications for luteolysis. Endocrinology. 2009;150:2873–2881. doi: 10.1210/en.2008-1382. [DOI] [PubMed] [Google Scholar]
- 43.Dos Santos E, Dieudonné MN, Leneveu MC, Pecquery R, Serazin V, Giudicelli Y. In vitro effects of chorionic gonadotropin hormone on human adipose development. J Endocrinol. 2007;194:313–325. doi: 10.1677/JOE-06-0101. [DOI] [PubMed] [Google Scholar]
- 44.Pau C, Saxena R, Welt CK. Evaluating reported candidate gene associations with polycystic ovary syndrome. Fertil Steril. 2013 Jan 29; doi: 10.1016/j.fertnstert.2012.12.033. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunaif A. Insulin action in the polycystic ovary syndrome. Endocrinology and metabolism clinics of North America. 1999;28:341–359. doi: 10.1016/s0889-8529(05)70073-6. [DOI] [PubMed] [Google Scholar]
- 46.Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. The Journal of Pediatrics. 2001;138:38–44. doi: 10.1067/mpd.2001.109603. [DOI] [PubMed] [Google Scholar]
- 47.Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. The Journal of Clinical Endocrinology and Metabolism. 2002;87:1017–1023. doi: 10.1210/jcem.87.3.8305. [DOI] [PubMed] [Google Scholar]
- 48.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. The Journal of Clinical Endocrinology and Metabolism. 2006;91:492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 49.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. The Journal of Clinical Endocrinology and Metabolism. 2002;87:1555–1559. doi: 10.1210/jcem.87.4.8398. [DOI] [PubMed] [Google Scholar]
- 50.Lass N, Kleber M, Winkel K, Wunsch R, Reinehr T. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima-media thickness in obese adolescent girls. The Journal of clinical endocrinology and metabolism. 2011;96:3533–3540. doi: 10.1210/jc.2011-1609. [DOI] [PubMed] [Google Scholar]
- 51.Nicandri KF, Hoeger K. Diagnosis and treatment of polycystic ovarian syndrome in adolescents. Curr Opin Endocrinol Diabetes Obes. 2012;19:497–504. doi: 10.1097/MED.0b013e32835a1a03. [DOI] [PubMed] [Google Scholar]
- 52.Roe AH, Prochaska E, Smith M, Sammel M, Dokras A. Using the Androgen Excess-PCOS Society Criteria to Diagnose Polycystic Ovary Syndrome and the Risk of Metabolic Syndrome in Adolescents. J Pediatr. 2012 Dec 19; doi: 10.1016/j.jpeds.2012.11.019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Williams RM, Ong KK, Dunger DB. Polycystic ovarian syndrome during puberty and adolescence. Mol Cell Endocrinol. 2013 Feb 4; doi: 10.1016/j.mce.2013.01.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol. 2010;203(201):e1–e5. doi: 10.1016/j.ajog.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Ibanez L, Potau N, Francois I, de Zegher F. Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. The Journal of clinical endocrinology and metabolism. 1998;83:3558–3562. doi: 10.1210/jcem.83.10.5205. [DOI] [PubMed] [Google Scholar]
- 56.Davies MJ, March WA, Willson KJ, Giles LC, Moore VM. Birthweight and thinness at birth independently predict symptoms of polycystic ovary syndrome in adulthood. Hum Reprod. 2012;27:1475–1480. doi: 10.1093/humrep/des027. [DOI] [PubMed] [Google Scholar]
- 57.Maliqueo M, Sir-Petermann T, Perez V, Echiburu B, de Guevara AL, Galvez C, et al. Adrenal function during childhood and puberty in daughters of women with polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 2009;94:3282–3288. doi: 10.1210/jc.2009-0427. [DOI] [PubMed] [Google Scholar]
- 58.Ibáñez L, Potau N, Carrascosa A. Insulin resistance, premature adrenarche, and a risk of the Polycystic Ovary Syndrome (PCOS) Trends Endocrinol Metab. 1998;9:72–77. doi: 10.1016/s1043-2760(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 59.McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S, Marshall JC. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab. 2007;92:430–436. doi: 10.1210/jc.2006-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenfield RL. Clinical review: Identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:787–796. doi: 10.1210/jc.2006-2012. [DOI] [PubMed] [Google Scholar]
- 61.Littlejohn EE, Weiss RE, Deplewski D, Edidin DV, Rosenfield R. Intractable early childhood obesity as the initial sign of insulin resistant hyperinsulinism and precursor of polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2007;20:41–51. doi: 10.1515/jpem.2007.20.1.41. [DOI] [PubMed] [Google Scholar]
- 62.Shi D, Vine DF. Animal models of polycystic ovary syndrome: a focused review of rodent models in relationship to clinical phenotypes and cardiometabolic risk. Fertil Steril. 2012;98:185–193. doi: 10.1016/j.fertnstert.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 63.Walters KA, Allan CM, Handelsman DJ. Rodent models for human polycystic ovary syndrome. Biol Reprod. 2012;86(149):1–12. doi: 10.1095/biolreprod.111.097808. [DOI] [PubMed] [Google Scholar]
- 64.Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2012 Oct 16; doi: 10.1016/j.mce.2012.10.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abbott DH, Bruns CR, Barnett DK, Dunaif A, Goodfriend TL, Dumesic DA, Tarantal AF. Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring. Am J Physiol Endocrinol Metab. 2010;299:E741–E751. doi: 10.1152/ajpendo.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abbott AD, Colman RJ, Tiefenthaler R, Dumesic DA, Abbott DH. Early-to-mid gestation fetal testosterone increases right hand 2D:4D finger length ratio in polycystic ovary syndrome-like monkeys. PLoS ONE. 2012;7:e42372. doi: 10.1371/journal.pone.0042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beck-Peccoz P, Padmanabhan V, Baggiani AM, Cortelazzi D, Buscaglia M, Medri G, Marconi AM, Pardi G, Beitins IZ. Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: circulating levels of gonadotropins, their common alpha-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J Clin Endocrinol Metab. 1991;73:525–532. doi: 10.1210/jcem-73-3-525. [DOI] [PubMed] [Google Scholar]
- 68.Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17:2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- 69.Maliqueo M, Lara HE, Sánchez F, Echiburú B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;166:151–155. doi: 10.1016/j.ejogrb.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 70.Crisosto N, Echiburú B, Maliqueo M, Pérez V, Ladrón de Guevara A, Preisler J, Sánchez F, Sir-Petermann T. Improvement of hyperandrogenism and hyperinsulinemia during pregnancy in women with polycystic ovary syndrome: possible effect in the ovarian follicular mass of their daughters. Fertil Steril. 2012;97:218–224. doi: 10.1016/j.fertnstert.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Hart R, Doherty DA, Norman RJ, Franks S, Dickinson JE, Hickey M, Sloboda DM. Serum antimullerian hormone (AMH) levels are elevated in adolescent girls with polycystic ovaries and the polycystic ovarian syndrome (PCOS) Fertil Steril. 2010;94:1118–1121. doi: 10.1016/j.fertnstert.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Sir-Petermann T, Codner E, Perez V, Echiburu B, Maliqueo M, Ladron de Guevara A, et al. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. The Journal of Clinical Endocrinology and Metabolism. 2009;94:1923–1930. doi: 10.1210/jc.2008-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sir-Petermann T, Ladron de Guevara A, Codner E, Preisler J, Crisosto N, Echiburu B, et al. Relationship between anti-Mullerian hormone (AMH) and insulin levels during different Tanner stages in daughters of women with polycystic ovary syndrome. Reproductive Sciences. 2012;19:383–390. doi: 10.1177/1933719111424444. [DOI] [PubMed] [Google Scholar]
- 74.Cole B, Hensinger K, Maciel GA, Chang RJ, Erickson GF. Human fetal ovary development involves the spatiotemporal expression of p450c17 protein. J Clin Endocrinol Metab. 2006;91:3654–3661. doi: 10.1210/jc.2006-0641. [DOI] [PubMed] [Google Scholar]
- 75.Fowler PA, Anderson RA, Saunders PT, Kinnell H, Mason JI, Evans DB, Bhattacharya S, Flannigan S, Franks S, Monteiro A, O'Shaughnessy PJ. Development of steroid signaling pathways during primordial follicle formation in the human fetal ovary. J Clin Endocrinol Metab. 2011;96:1754–1762. doi: 10.1210/jc.2010-2618. [DOI] [PubMed] [Google Scholar]
- 76.Palomba S, Marotta R, Cello AD, Russo T, Falbo A, Orio F, Tolino A, Zullo F, Esposito R, Sala GB. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clin Endocrinol (Oxf) 2012;77:898–904. doi: 10.1111/j.1365-2265.2012.04443.x. [DOI] [PubMed] [Google Scholar]
- 77.Keelan JA, Mattes E, Tan H, Dinan A, Newnham JP, Whitehouse AJ, Jacoby P, Hickey M. Androgen concentrations in umbilical cord blood and their association with maternal, fetal and obstetric factors. PLoS One. 2012;7(8):e42827. doi: 10.1371/journal.pone.0042827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barry JA, Kay AR, Navaratnarajah R, Iqbal S, Bamfo JE, David AL, Hines M, Hardiman PJ. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol. 2010;30:444–446. doi: 10.3109/01443615.2010.485254. [DOI] [PubMed] [Google Scholar]
- 79.Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL, Dunaif A. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J Clin Endocrinol Metab. 2010;95:2180–2186. doi: 10.1210/jc.2009-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hickey M, Sloboda DM, Atkinson HC, Doherty DA, Franks S, Norman RJ, Newnham JP, Hart R. The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. J Clin Endocrinol Metab. 2009;94:3714–3720. doi: 10.1210/jc.2009-0544. [DOI] [PubMed] [Google Scholar]
- 81.Lujan ME, Bloski TG, Chizen DR, Lehotay DC, Pierson RA. Digit ratios do not serve as anatomical evidence of prenatal androgen exposure in clinical phenotypes of polycystic ovary syndrome. Hum Reprod. 2010;25:204–211. doi: 10.1093/humrep/dep363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lujan ME, Podolski AJ, Chizen DR, Lehotay DC, Pierson RA. Digit ratios by computer-assisted analysis confirm lack of anatomical evidence of prenatal androgen exposure in clinical phenotypes of polycystic ovary syndrome. Reprod Biol Endocrinol. 2010;l8:156. doi: 10.1186/1477-7827-8-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manning JT, Scutt D, Wilson D, Lewis-Jones DI. The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum Reprod. 1998;13:3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]
- 84.McIntrye M. The use of digit ratios as markers for perinatal androgen action. Reprod Biol Endocrinol. 2006;4:10. doi: 10.1186/1477-7827-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dean A, Sharpe RM. Anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. J Clin Endocrinol Metab. 2013 Apr 8; doi: 10.1210/jc.2012-4057. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 86.Franks S, Berga SL. Does PCOS have developmental origins? Fertil Steril. 2012;97:2–6. doi: 10.1016/j.fertnstert.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 88.de Zegher F, Ibáñez L. Early Origins of polycystic ovary syndrome: hypotheses may change without notice. J Clin Endocrinol Metab. 2009;94:3682–3685. doi: 10.1210/jc.2009-1608. [DOI] [PubMed] [Google Scholar]
- 89.Jones ME, Boon WC, McInnes K, Maffei L, Carani C, Simpson ER. Recognizing rare disorders: aromatase deficiency. Nat Clin Pract Endocrinol Metab. 2007;3:414–421. doi: 10.1038/ncpendmet0477. [DOI] [PubMed] [Google Scholar]
- 90.Hertig A, Liere P, Chabbert-Buffet N, Fort J, Pianos A, Eychenne B, Cambourg A, Schumacher M, Berkane N, Lefevre G, Uzan S, Rondeau E, Rozenberg P, Rafestin-Oblin ME. Steroid profiling in preeclamptic women: evidence for aromatase deficiency. Am J Obstet Gynecol. 2010;203(477):e1–e9. doi: 10.1016/j.ajog.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 91.Jobe SO, Tyler CT, Magness RR. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension. 2013;61:480–487. doi: 10.1161/HYPERTENSIONAHA.111.201624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holt HB, Medbak S, Kirk D, Guirgis R, Hughes I, Cummings MH, Meeking DR. Recurrent severe hyperandrogenism during pregnancy: a case report. J Clin Pathol. 2005;58:439–442. doi: 10.1136/jcp.2004.018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cattrall FR, Vollenhoven BJ, Weston GC. Anatomical evidence for in utero androgen exposure in women with polycystic ovary syndrome. Fertil Steril. 2005;84:1689–1692. doi: 10.1016/j.fertnstert.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 94.Tapp AL, Maybery MT, Whitehouse AJ. Evaluating the twin testosterone transfer hypothesis: a review of the empirical evidence. Horm Behav. 2011;60:713–722. doi: 10.1016/j.yhbeh.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 95.Kuijper EA, Vink JM, Lambalk CB, Boomsma DI. Prevalence of polycystic ovary syndrome in women from opposite-sex twin pairs. J Clin Endocrinol Metab. 2009;94:1987–1990. doi: 10.1210/jc.2009-0191. [DOI] [PubMed] [Google Scholar]
- 96.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 97.Hales CN, Barker DJ. The thrifty phenotype hypothesis. British medical bulletin. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 98.Jaquet D, Leger J, Czernichow P, Levy-Marchal C. The effect of in-utero undernutrition on the insulin resistance syndrome. Current diabetes reports. 2002;2:77–82. doi: 10.1007/s11892-002-0062-x. [DOI] [PubMed] [Google Scholar]
- 99.Leger J, Levy-Marchal C, Bloch J, Pinet A, Chevenne D, Porquet D, et al. Reduced final height and indications for insulin resistance in 20 year olds born small for gestational age: regional cohort study. Bmj. 1997;315:341–347. doi: 10.1136/bmj.315.7104.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soto N, Bazaes RA, Pena V, Salazar T, Avila A, Iniguez G, et al. Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: results from a prospective cohort. The Journal of clinical endocrinology and metabolism. 2003;88:3645–3650. doi: 10.1210/jc.2002-030031. [DOI] [PubMed] [Google Scholar]
- 101.Cianfarani S, Germani D, Branca F. Low birthweight and adult insulin resistance: the “catch-up growth” hypothesis. Archives of disease in childhood Fetal and neonatal edition. 1999;81:F71–F73. doi: 10.1136/fn.81.1.f71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ong KK, Petry CJ, Emmett PM, Sandhu MS, Kiess W, Hales CN, et al. Insulin sensitivity and secretion in normal children related to size at birth, postnatal growth, and plasma insulin-like growth factor-I levels. Diabetologia. 2004;47:1064–1070. doi: 10.1007/s00125-004-1405-8. [DOI] [PubMed] [Google Scholar]
- 103.Bavdekar A, Yajnik CS, Fall CH, Bapat S, Pandit AN, Deshpande V, et al. Insulin resistance syndrome in 8-year-old Indian children: small at birth, big at 8 years, or both? Diabetes. 1999;48:2422–2429. doi: 10.2337/diabetes.48.12.2422. [DOI] [PubMed] [Google Scholar]
- 104.Veening MA, Van Weissenbruch MM, Delemarre-Van De Waal HA. Glucose tolerance, insulin sensitivity, and insulin secretion in children born small for gestational age. The Journal of clinical endocrinology and metabolism. 2002;87:4657–4661. doi: 10.1210/jc.2001-011940. [DOI] [PubMed] [Google Scholar]
- 105.Jaquet D, Tregouet DA, Godefroy T, Nicaud V, Chevenne D, Tiret L, et al. Combined effects of genetic and environmental factors on insulin resistance associated with reduced fetal growth. Diabetes. 2002;51:3473–3478. doi: 10.2337/diabetes.51.12.3473. [DOI] [PubMed] [Google Scholar]
- 106.Silverman BL, Metzger BE, Cho NH, Loeb CA. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes care. 1995;18:611–617. doi: 10.2337/diacare.18.5.611. [DOI] [PubMed] [Google Scholar]
- 107.Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes care. 1998;21(Suppl 2):B142–B149. [PubMed] [Google Scholar]
- 108.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 109.Group HSCR. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. The New England journal of medicine. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 110.Group HSCR. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes. 2009;58(2):453–459. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocrine reviews. 1999;20(4):535–582. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- 112.Moghetti P, Castello R, Negri C, Tosi F, Spiazzi GG, Brun E, et al. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: apparent relative impairment of 17,20-lyase activity. The Journal of clinical endocrinology and metabolism. 1996;81:881–886. doi: 10.1210/jcem.81.3.8772544. [DOI] [PubMed] [Google Scholar]
- 113.Ibanez L, Potau N, Zampolli M, Prat N, Virdis R, Vicens-Calvet E, Carrascosa A. Hyperinsulinemia in postpubertal girls with a history of premature pubarche and functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1996;81:1237–1243. doi: 10.1210/jcem.81.3.8772605. [DOI] [PubMed] [Google Scholar]
- 114.Mehrabian F, Kelishadi R. Comparison of the metabolic parameters and androgen level of umbilical cord blood in newborns of mothers with polycystic ovary syndrome and controls. J Res Med Sci. 2012;17:207–211. [PMC free article] [PubMed] [Google Scholar]
- 115.Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburú B, Gazitúa R, Recabarren S, Cassorla F. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod. 2005;20:2122–2126. doi: 10.1093/humrep/dei009. [DOI] [PubMed] [Google Scholar]
- 116.Ibanez L, Ferrer A, Marcos MV, Hierro FR, de Zegher F. Early puberty: rapid progression and reduced final height in girls with low birth weight. Pediatrics. 2000;106:E72. doi: 10.1542/peds.106.5.e72. [DOI] [PubMed] [Google Scholar]
- 117.Ibanez L, Jimenez R, de Zegher F. Early puberty-menarche after precocious pubarche: relation to prenatal growth. Pediatrics. 2006;117:117–121. doi: 10.1542/peds.2005-0664. [DOI] [PubMed] [Google Scholar]
- 118.Ibanez L, Lopez-Bermejo A, Diaz M, de Zegher F. Catch-up growth in girls born small for gestational age precedes childhood progression to high adiposity. Fertility and sterility. 2011;96:220–223. doi: 10.1016/j.fertnstert.2011.03.107. [DOI] [PubMed] [Google Scholar]
- 119.Boonstra VH, Mulder PG, de Jong FH, Hokken-Koelega AC. Serum dehydroepiandrosterone sulfate levels and pubarche in short children born small for gestational age before and during growth hormone treatment. The Journal of clinical endocrinology and metabolism. 2004;89:712–717. doi: 10.1210/jc.2003-031160. [DOI] [PubMed] [Google Scholar]
- 120.Meas T, Chevenne D, Thibaud E, Leger J, Cabrol S, Czernichow P, et al. Endocrine consequences of premature pubarche in post-pubertal Caucasian girls. Clinical endocrinology. 2002;57:101–106. doi: 10.1046/j.1365-2265.2002.01579.x. [DOI] [PubMed] [Google Scholar]
- 121.Jaquet D, Leger J, Chevenne D, Czernichow P, Levy-Marchal C. Intrauterine growth retardation predisposes to insulin resistance but not to hyperandrogenism in young women. The Journal of clinical endocrinology and metabolism. 1999;84:3945–3949. doi: 10.1210/jcem.84.11.6106. [DOI] [PubMed] [Google Scholar]
- 122.Ong KK, Potau N, Petry CJ, Jones R, Ness AR, Honour JW, et al. Opposing influences of prenatal and postnatal weight gain on adrenarche in normal boys and girls. The Journal of Clinical Endocrinology and Metabolism. 2004;89:2647–2651. doi: 10.1210/jc.2003-031848. [DOI] [PubMed] [Google Scholar]
- 123.Neville KA, Walker JL. Precocious pubarche is associated with SGA, prematurity, weight gain, and obesity. Archives of disease in childhood. 2005;90:258–261. doi: 10.1136/adc.2004.053959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ibanez L, Lopez-Bermejo A, Diaz M, Marcos MV, de Zegher F. Early metformin therapy (age 8-12 years) in girls with precocious pubarche to reduce hirsutism, androgen excess, and oligomenorrhea in adolescence. The Journal of Clinical Endocrinology and Metabolism. 2011;96:E1262–E1267. doi: 10.1210/jc.2011-0555. [DOI] [PubMed] [Google Scholar]
- 125.Ibáñez L, Potau N, Enriquez G, Marcos MV, de Zegher F. Hypergonadotrophinaemia with reduced uterine and ovarian size in women born small-for-gestational-age. Hum Reprod. 2003;18:1565–1569. doi: 10.1093/humrep/deg351. [DOI] [PubMed] [Google Scholar]
- 126.Hernandez MI, Martinez A, Capurro T, Pena V, Trejo L, Avila A, et al. Comparison of clinical, ultrasonographic, and biochemical differences at the beginning of puberty in healthy girls born either small for gestational age or appropriate for gestational age: preliminary results. The Journal of Clinical Endocrinology and Metabolism. 2006;91:3377–3381. doi: 10.1210/jc.2005-2368. [DOI] [PubMed] [Google Scholar]
- 127.Lem AJ, Boonstra VH, Renes JS, Breukhoven PE, de Jong FH, Laven JS, et al. Anti-Mullerian hormone in short girls born small for gestational age and the effect of growth hormone treatment. Human Reproduction. 2011;26:898–903. doi: 10.1093/humrep/deq391. [DOI] [PubMed] [Google Scholar]
- 128.Sir-Petermann T, Marquez L, Carcamo M, Hitschfeld C, Codner E, Maliqueo M, et al. Effects of birth weight on anti-mullerian hormone serum concentrations in infant girls. The Journal of Clinical Endocrinology and Metabolism. 2010;95:903–910. doi: 10.1210/jc.2009-1771. [DOI] [PubMed] [Google Scholar]
- 129.Hagen CP, Aksglaede L, Sørensen K, Mouritsen A, Andersson AM, Petersen JH, Main KM, Juul A. Individual serum levels of anti-Müllerian hormone in healthy girls persist through childhood and adolescence: a longitudinal cohort study. Hum Reprod. 2012;27:861–866. doi: 10.1093/humrep/der435. [DOI] [PubMed] [Google Scholar]
- 130.Cresswell JL, Barker DJ, Osmond C, Egger P, Phillips DI, Fraser RB. Fetal growth, length of gestation, and polycystic ovaries in adult life. Lancet. 1997;350:1131–1135. doi: 10.1016/s0140-6736(97)06062-5. [DOI] [PubMed] [Google Scholar]
- 131.Puche C, José M, Cabero A, Meseguer A. Expression and enzymatic activity of the P450c17 gene in human adipose tissue. Eur J Endocrinol. 2002;146:223–229. doi: 10.1530/eje.0.1460223. [DOI] [PubMed] [Google Scholar]
- 132.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 133.Larnkjær A, Ingstrup HK, Schack-Nielsen L, Mølgaard C, Michaelsen KF. Thin newborns are more insulin resistant at 10 years of age. Acta Paediatr. 2011;100:511–514. doi: 10.1111/j.1651-2227.2010.02087.x. [DOI] [PubMed] [Google Scholar]
- 134.Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics. 2001;108:347–353. doi: 10.1542/peds.108.2.347. [DOI] [PubMed] [Google Scholar]
- 135.Arslanian S, Suprasongsin C. Insulin sensitivity, lipids, and body composition in childhood: is "syndrome X" present? The Journal of clinical endocrinology and metabolism. 1996;81:1058–1062. doi: 10.1210/jcem.81.3.8772576. [DOI] [PubMed] [Google Scholar]
- 136.Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. The Journal of clinical endocrinology and metabolism. 2003;88:2534–2540. doi: 10.1210/jc.2002-021267. [DOI] [PubMed] [Google Scholar]
- 137.Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatric research. 2006;60:759–763. doi: 10.1203/01.pdr.0000246097.73031.27. [DOI] [PubMed] [Google Scholar]
- 138.Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab. 1998;83:2001–2005. doi: 10.1210/jcem.83.6.4886. [DOI] [PubMed] [Google Scholar]
- 139.Rosenfield RL. Plasma 17-ketosteroids and 17-beta hydroxysteroids in girls with premature development of sexual hair. The Journal of pediatrics. 1971;79:260–266. doi: 10.1016/s0022-3476(71)80111-7. [DOI] [PubMed] [Google Scholar]
- 140.Vuguin P, Linder B, Rosenfeld RG, Saenger P, DiMartino-Nardi J. The roles of insulin sensitivity, insulin-like growth factor I (IGF-I), and IGF-binding protein-1 and −3 in the hyperandrogenism of African-American and Caribbean Hispanic girls with premature adrenarche. The Journal of Clinical Endocrinology and Metabolism. 1999;84:2037–2042. doi: 10.1210/jcem.84.6.5722. [DOI] [PubMed] [Google Scholar]
- 141.McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, et al. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. The Journal of Clinical Endocrinology and Metabolism. 2006;91:1714–1722. doi: 10.1210/jc.2005-1852. [DOI] [PubMed] [Google Scholar]
- 142.Knudsen KL, Blank SK, Burt Solorzano C, Patrie JT, Chang RJ, Caprio S, et al. Hyperandrogenemia in obese peripubertal girls: correlates and potential etiological determinants. Obesity. 2010;18:2118–2124. doi: 10.1038/oby.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rosenfield RL. Polycystic ovary syndrome and insulin-resistant hyperinsulinemia. Journal of the American Academy of Dermatology. 2001;45(3 Suppl):S95–S104. doi: 10.1067/mjd.2001.117430. [DOI] [PubMed] [Google Scholar]
- 144.Jabbar M, Pugliese M, Fort P, Recker B, Lifshitz F. Excess weight and precocious pubarche in children: alterations of the adrenocortical hormones. Journal of the American College of Nutrition. 1991;10:289–296. doi: 10.1080/07315724.1991.10718155. [DOI] [PubMed] [Google Scholar]
- 145.Apter D, Butzow T, Laughlin GA, Yen SS. Metabolic features of polycystic ovary syndrome are found in adolescent girls with hyperandrogenism. The Journal of Clinical Endocrinology and Metabolism. 1995;80:2966–2973. doi: 10.1210/jcem.80.10.7559882. [DOI] [PubMed] [Google Scholar]
- 146.McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. The Journal of Clinical Endocrinology and Metabolism. 2009;94:56–66. doi: 10.1210/jc.2008-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rosenfield RL, Bordini B. Evidence that obesity and androgens have independent and opposing effects on gonadotropin production from puberty to maturity. Brain Research. 2010;1364:186–197. doi: 10.1016/j.brainres.2010.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Burt Solorzano CM, McCartney CR, Blank SK, Knudsen KL, Marshall JC. Hyperandrogenaemia in adolescent girls: origins of abnormal gonadotropin-releasing hormone secretion. BJOG. 2010;117:143–149. doi: 10.1111/j.1471-0528.2009.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Blank SK, McCartney CR, Chhabra S, Helm KD, Eagleson CA, Chang RJ, et al. Modulation of gonadotropin-releasing hormone pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls--implications for regulation of pubertal maturation. The Journal of Clinical Endocrinology and Metabolism. 2009;94:2360–2366. doi: 10.1210/jc.2008-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McGee WK, Bishop CV, Bahar A, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Hum Reprod. 2012;27:531–540. doi: 10.1093/humrep/der393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril. 2001;75:53–58. doi: 10.1016/s0015-0282(00)01662-9. [DOI] [PubMed] [Google Scholar]
- 152.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 153.Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS. Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2008;93:1662–1669. doi: 10.1210/jc.2007-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sir-Petermann T, Maliqueo M, Codner E, Echiburu B, Crisosto N, Perez V, et al. Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:4637–4642. doi: 10.1210/jc.2007-1036. [DOI] [PubMed] [Google Scholar]
- 155.Sir-Petermann T, Codner E, Perez V, Echiburu B, Maliqueo M, Ladron dG, et al. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:1923–1930. doi: 10.1210/jc.2008-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yildiz BO, Yarali H, Oguz H, Bayraktar M. Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2031–2036. doi: 10.1210/jc.2002-021499. [DOI] [PubMed] [Google Scholar]
- 157.Baillargeon JP, Carpentier AC. Brothers of women with polycystic ovary syndrome are characterised by impaired glucose tolerance, reduced insulin sensitivity and related metabolic defects. Diabetologia. 2007;50:2424–2432. doi: 10.1007/s00125-007-0831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sir-Petermann T, Angel B, Maliqueo M, Carvajal F, Santos JL, Perez-Bravo F. Prevalence of type II diabetes mellitus and insulin resistance in parents of women with polycystic ovary syndrome. Diabetologia. 2002;45:959–964. doi: 10.1007/s00125-002-0836-3. [DOI] [PubMed] [Google Scholar]
- 159.Trottier A, Battista MC, Geller DH, Moreau B, Carpentier AC, Simoneau-Roy J, Baillargeon JP. Adipose tissue insulin resistance in peripubertal girls with first-degree family history of polycystic ovary syndrome. Fertil Steril. 2012;98:1627–1634. doi: 10.1016/j.fertnstert.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Raissouni N, Kolesnikov A, Purushothaman R, Sinha S, Bhandari S, Bhangoo A, Malik S, Mathew R, Baillargeon JP, Hernandez MI, Rosenbaum M, Ten S, Geller D. Altered glucose disposition and insulin sensitivity in peri-pubertal first-degree relatives of women with polycystic ovary syndrome. Int J Pediatr Endocrinol. 2012;2012:14. doi: 10.1186/1687-9856-2012-14. [DOI] [PMC free article] [PubMed] [Google Scholar]