Abstract
Background
In contrast to the prominent function of the blood vasculature in promoting tissue inflammation, the role of lymphatic vessels in inflammation has been scarcely studied in vivo. To investigate whether modulating lymphatic vessel function might affect the course of chronic inflammation the major lymphangiogenic receptor vascular growth factor receptor 3 (VEGFR-3, FLT4) was blocked in an established model of inflammatory bowel disease.
Methods
Interleukin 10 (IL10)-deficient mice that spontaneously develop inflammatory bowel disease, were treated with a blocking antibody to VEGFR-3 for 2 weeks, and the inflammatory changes in colon tissue, as well as the blood and lymphatic vascularization were quantitatively analyzed.
Results
We found a significant increase in the severity of colon inflammation in anti-VEGFR-3 treated mice. This was accompanied by an increased number of enlarged and tortuous lymphatic vessels, and edema in colon submucosa, indicating impaired lymphatic function. In contrast, no major effects of the treatment on the blood vasculature were observed.
Conclusions
These results indicate that therapies aimed at promoting lymphatic function, e.g., with pro-lymphangiogenic factors such as VEGF-C, might provide a novel strategy for the treatment of inflammatory conditions such as inflammatory bowel disease.
Keywords: Lymphangiogenesis, VEGFR-3, colitis
Introduction
The major functions of the lymphatic vasculature are the drainage of interstitial tissue fluid and its return to the blood circulation as well as the mediation of the transport of immune cells and antigens to the lymph nodes (1). Lymphatic vessels facilitate the spread of cancer metastases to lymph nodes (2) and are involved in chronic inflammation (3) and transplant rejection (4).
Vascular endothelial growth factor receptor 3 (VEGFR-3, FLT4) is a tyrosine kinase receptor expressed on developing embryonic blood vessels (5), some angiogenic blood vessels (6) and adult lymphatic vessels. Binding of its ligand, vascular growth factor C (VEGF-C), initiates a signaling cascade crucial for lymphangiogenesis (7). The importance of VEGFR-3 for normal lymphatic vessel function was shown in some human lymphedema syndromes in which patients have VEGFR-3 mutations (8, 9) as wells as in several genetically engineered mouse models (10). Cutaneous-specific overexpression of a soluble VEGFR-3 – which captures VEGF-C and vascular growth factor D (VEGF-D, FIGF) and prevents their binding to the cell-bound VEGFR-3 – results in lymphedema (11). Blockade of VEGFR-3 signaling resulted in prolonged UVB-induced edema and skin inflammation (12), and stimulation of lymphatic vessel growth and function by VEGFR-3 ligands VEGF-C and VEGF-D inhibited development of chronic skin inflammation (3). Similarly, in a mouse model of chronic pulmonary infection, lymphangiogenesis was blocked by an anti-VEGFR-3 antibody, leading to prolonged mucosal edema (13). These results suggest that VEGFR-3 signals are required for lymphatic vessel function and lymphangiogenesis which serve to remove the excess fluid from tissues and to clear the immune cells and antigens from the site of inflammation (14, 15). In contrast, in several models of organ transplantation, inhibition of VEGR-3 inhibited inflammation and organ rejection (4, 16).
In the present study, we investigated the functional importance of VEGFR-3 signaling for the development and maintenance of inflammatory bowel disease. IL10-deficient mice with the C3.Bir genetic background (C3Bir.129P2(B6)-Il10tm1Cgn/J; hereafter referred to as C3Bir-Il10−/− mice) are a useful model for human inflammatory bowel disease (IBD) due to the strongly dysregulated colonic immune response that leads to severe colitis (17). IBD is known to be accompanied by blood vessel changes (18) and, as we have recently found (19), also by extensive inflammation-associated lymphatic vessel enlargement (lymphangiectasia). It is possible that blockade of VEGFR-3 might influence vascular function in IBD and therefore affect the course of the disease. Thus, we treated C3Bir-Il10−/− mice with an antibody blocking VEGFR-3 signaling, and we analyzed the degree of inflammation, lymphatic and blood vascularization after 18 days of treatment.
Materials and methods
C3Bir-Il10−/− (C3Bir.129P2(B6)-Il10tm1Cgn/J) mice were housed in a conventional SPF facility containing colitis-requisite microflora at The Jackson Laboratory-West (Sacramento, CA). At 6 weeks of age, mice were given injections of the blocking rat antimouse VEGFR-3 antibody mF4-31C1 (20) (a kind gift of Dr. B. Pytowski, ImClone Systems Inc, New York, NY). Control mice received phosphate buffered saline (PBS) injections. Each group consisted of 4 males and 4 females; mice were grouped according to similar body weights. Mice received intraperitoneal injections of 800 µg of mF4-31C1 or PBS injections every third day (in total 6 injections). Three days after the last injection, mice were euthanized and the entire colon was removed and fixed in Fekete’s acid-alcohol-formalin fixative. Tissues were then embedded in paraffin and cut into 6 µm sections (longitudinal sections of the rolled colon). Routine hematoxylin and eosin staining was performed on one section per case. Immunohistochemistry labeling on tissue sections was performed with rabbit anti-mouse LYVE1 antibody (kindly provided by Dr. N. Gale, Regeneron Pharmaceuticals, Tarrytown, NY) as previously described (19) and counterstained with hematoxylin. Tissue sections were also immunostained with a Meca32 antibody (BD Pharmingen, San Jose CA) and with an F40/80 antibody (Abcam, Cambridge UK), using the same procedure with an additional antigen retrieval step (proteinase K incubation; Dako, Glostrup, Denmark). To investigate the tissue distribution of the injected rat IgG antibody, immunohistochemical labeling on tissue sections was performed with a biotinylated anti-rat IgG (Vector Labs, Burlingame CA), followed by a streptavidin-bound horse radish peroxidase and the AEC substrate (Vector Labs). The mouse experiments were approved by the Institutional Animal Care and Use Committee.
LYVE1- immunostained sections of the entire colon were inspected by light microscopy at low magnification. The percentage of colon tissue with large and tortuous lymphatic vessels (LYVE1-positive structures with lumina) was estimated. The areas of tissue with the highest density of lymphatic vessels were selected (‘hot spots’). Four hot-spot images of each mouse colon were taken at 10× magnification and morphometric analyses were performed using the Photoshop Extended CS3 software. The area covered by lymphatic vessels (lymphatic vessel density), lymphatic vessel size and the width of the colon submucosa (inflammatory edema) were measured. The submucosal colon area was defined as the area that encompasses the lamina propria at the basis of the epithelial crypts, the muscularis mucosae, submucosa, and the muscle layers.
Three images of the Meca32 immunostained sections at 10× magnification were taken per sample (the areas with the highest density of blood vessels were selected (‘hot spots’)). Smaller blood vessels were located in the colon mucosa while larger blood vessels were located in the colon submucosa. The colon tissue area covered by blood vessels was measured including mucosa and submucosa using the Photoshop Extended CS3 software. The scoring of colon inflammation and of leukocyte infiltration was performed on hematoxylin-eosin stained paraffin sections in a blinded fashion. The histopathological colitis score was obtained according to a system previously shown to be very sensitive and robust (17, 21, 22). In brief, four general criteria were evaluated in sections, separately for the proximal, mid, and distal colon: severity of inflammation, degree of epithelial hyperplasia, degree of ulceration (if present), and percentage of area involved. Each of the criteria was graded on a 0–3 scale (0, absent; 1, mild; 2, moderate; 3, severe). The cumulative colitis score was obtained by summing up the scores for proximal, mid, and distal colon (0–36). The extent of leukocyte infiltration was also scored separately for the proximal, mid, and distal segment of colon. Each of the colon segments was graded on a 0–3 scale (0, absent; 1, mild; 2, moderate; 3, severe) and the cumulative score was obtained by summing the individual scores (0–9).
Results
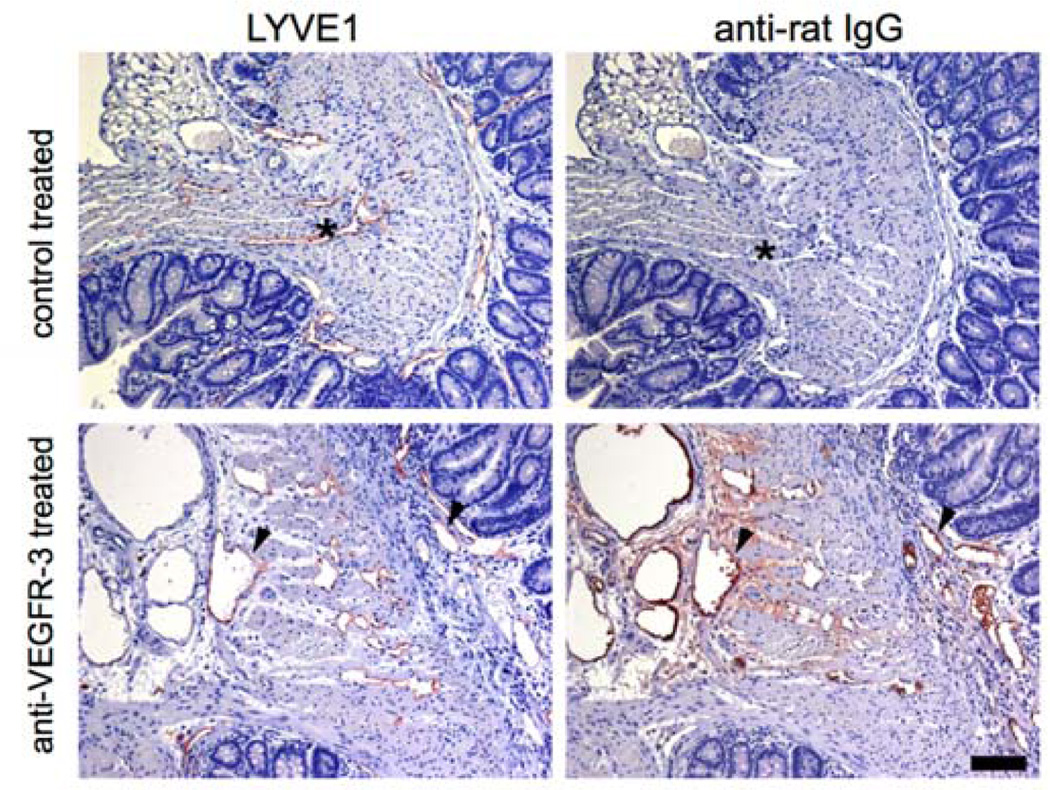
By 6 weeks of age, C3Bir-Il10−/− mice developed the first signs of colitis (17), accompanied by enlarged and tortuous lymphatic vessels and tissue edema (19). At this early stage of inflammation, treatment with the blocking anti-VEGFR-3 antibody mF4-31C1 was initiated. To determine if the injected antibody reached the tissue and to confirm the tissue localization of the injected antibody, colon sections of mF4-31C1-treated and control-treated mice were evaluated by immunohistochemistry. There was a strong immunoreactivity against the mF4-31C antibody in colon submucosa and lamina propria in the basis of the epithelial crypts, and also bound to the lymphatic vessel walls, as shown by co-localization with the LYVE1 signal on serial sections (Figure 1, arrowheads). Anti-rat IgG staining gave no signal in the colon tissue of the control mice (Figure 1, asterisks).
Figure 1. Detection of the injected anti-VEGFR-3 blocking antibody bound to lymphatic vessels.
Colon sections of mice that received either anti-VEGFR-3 antibody injections or control injections were immunostained with an anti-rat IgG antibody. The rat IgG signal was abundant in the colon lamina propria and submucosa and was co-localized with the LYVE1 signal on lymphatic vessels in serial sections (arrowheads). The rat IgG signal was absent in the colon tissue and lymphatic vessels of control-treated mice (asterisks). Scale bars: 100 µm.
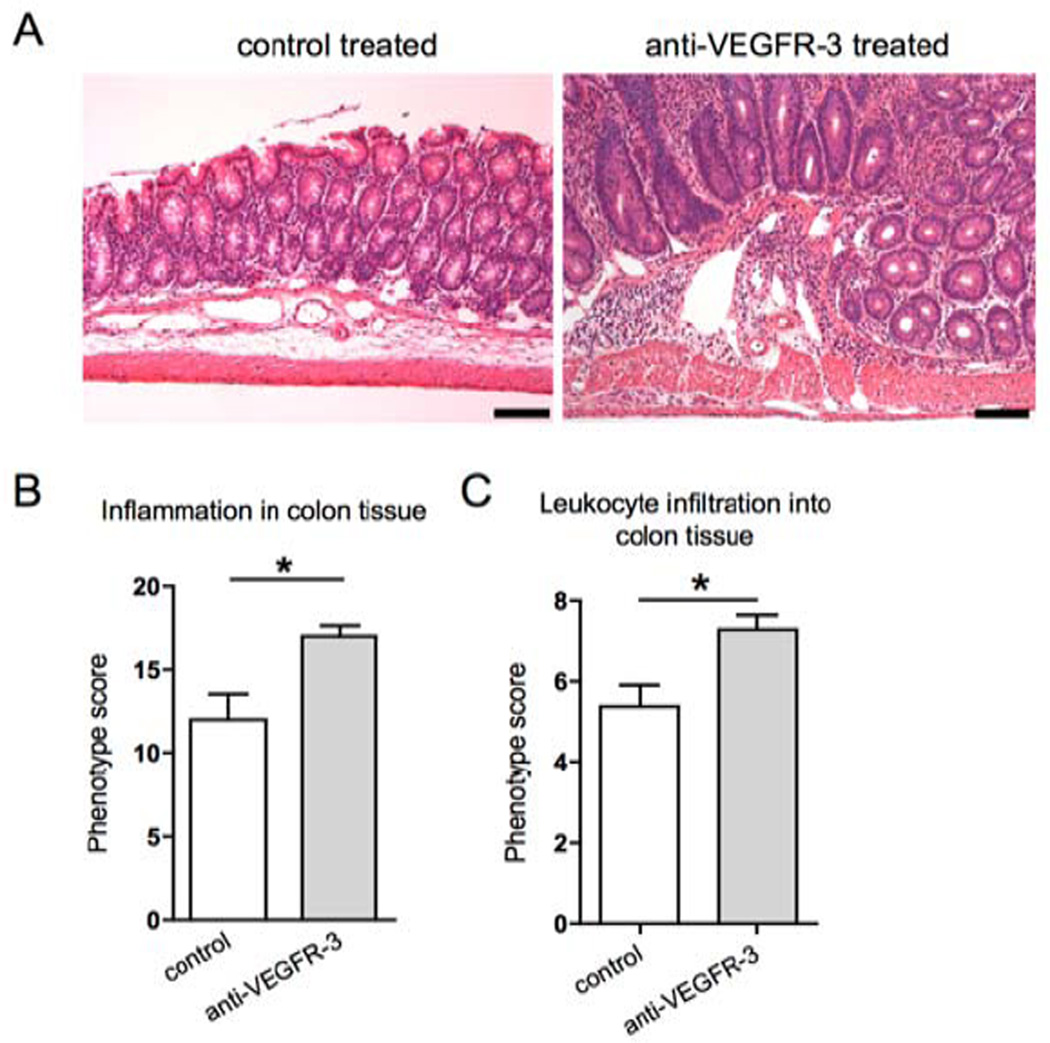
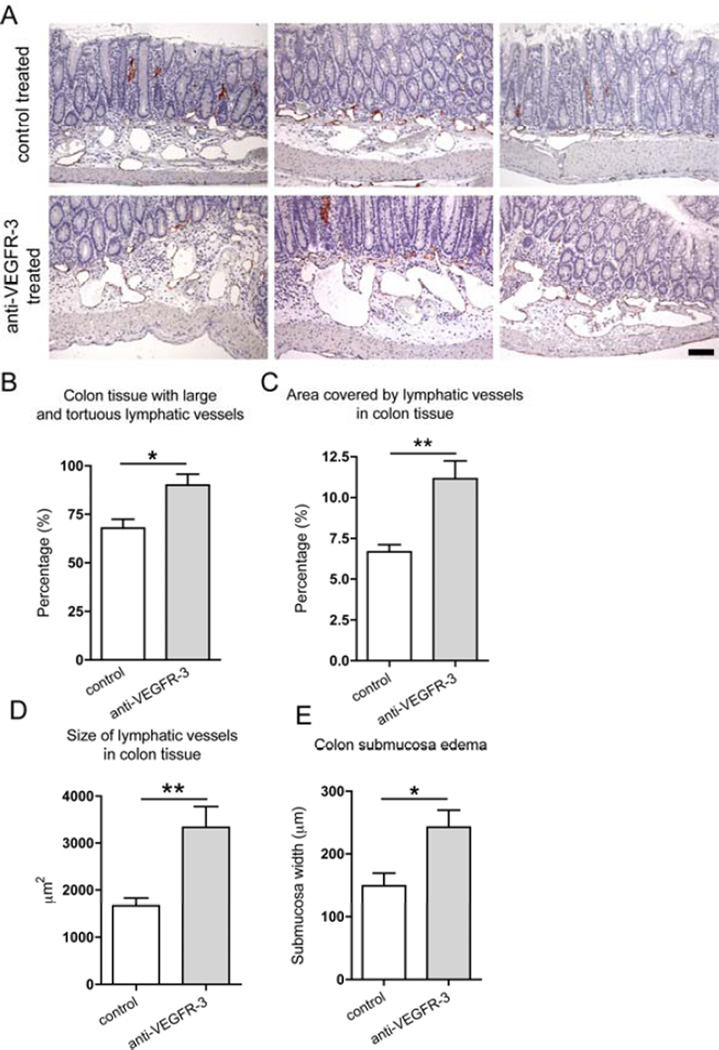
After 18 days of treatment, inflammatory changes were quantified in colons of anti-VEGFR-3 antibody treated mice and control mice. Surprisingly, we found that VEGFR-3 antibody treated mice had a higher colitis score (Figure 2B; control mice: 12.0±1.5, treated mice: 17.0±0.6, p=0.011). Leukocyte infiltration in the mucosa, lamina propria and submucosa of all of the colon segments was also more prominent in the anti-VEGFR-3 antibody treated mice (Figure 2C; control mice: 5.4±0.5, treated mice: 7.3±0.4, p=0.013). Importantly, anti-VEGFR-3 treated mice had a significant increase in the percentage of colon area with large and tortuous lymphatic vessels (Figure 3A and B, from 67.9±4.6% to 90.0±5.7%, p=0.01), the lymphatic vessel density (Figure 3C, from 6.7±0.4% to 11.2±1.1%, p=0.007), and lymphatic vessel size (Figure 2D, control mice: 1,666±157.4 µm2, treated mice: 3,334±442.4 µm2, p=0.009). Moreover, treated mice also had a marked increase in the colon submucosa edema (Figure 2E), indicating a decreased lymphatic vessel function.
Figure 2. Specific blockade of VEGFR-3 increases colon inflammation and leukocyte infiltration in the Il10−/− mouse model of IBD.
(A) Representative images of hematoxylin and eosin stained sections of 8-weeks old C3Bir-Il10−/− mice. Scale bars: 100 µm. (B) Histopathological colitis scoring system: evaluation of inflammation severity, epithelial hyperplasia, mucosal ulceration and extent of area involved along the entire colon. (C) The extent of leukocyte infiltration was evaluated along the entire colon length, inspecting the colon mucosa, lamina propria and submucosa areas (t-test *p ≤ 0.05, n=7–8).
Figure 3. Specific blockade of VEGFR-3 increases lymphatic vessel abnormalities in the Il10−/− mouse model of IBD.
(A) Colon sections of 8-weeks old C3Bir-Il10−/− mice were immunostained for LYVE1 (red) and counterstained with hematoxylin. While control mouse colons contained moderately enlarged lymphatic vessels, anti-VEGFR-3 treated mice contained larger areas with dense, enlarged and irregularly shaped lymphatic vessels, surrounded by the edematous tissue. Three representative images are shown, scale bar: 100 µm. Inflammatory changes of lymphatic vessels were evaluated by inspecting the colon tissue for the presence of large and tortuous vessels (B), by determining the area covered by lymphatic vessels in inflamed tissue (C), and by measuring the size of the lymphatic vessels in the inflamed tissue (D). (E) Edema was evaluated by measuring the width of the colon submucosa. (t-test *p ≤ 0.05, **p ≤ 0.01; n=7–8).
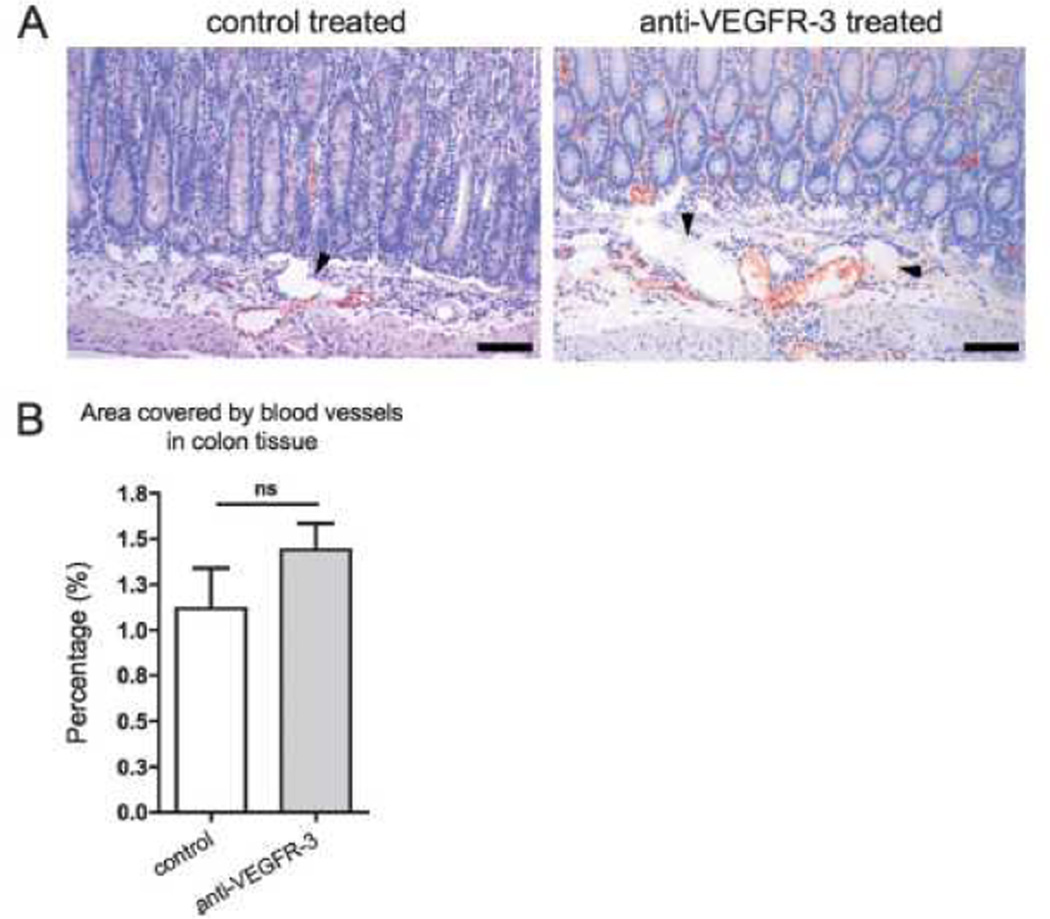
Blood vascular involvement was determined by immunostaining for the Meca32 endothelial antigen that is specifically expressed by blood vessels, but not by lymphatic vessels. A high density of blood vessels was present in the inflamed colons of both mouse groups (Figure 4A). However, there was no statistically significant difference between control and VEGFR-3 antibody treated mice in the extent of the blood vasculature-covered area (Figure 4B, p=0.34).
Figure 4. Blocking VEGFR-3 does not affect blood vessel density in colon inflammation.
(A) Colon sections of 8-weeks old C3Bir-Il10−/− mice were immunostained for the blood vascular marker Meca32 (red) and counterstained with hematoxylin. Arrowheads point to unstained lymphatic vessels. Representative images are shown. Scale bars: 100 µm. (B) Quantification of the tissue area covered by blood vessels revealed no significant difference between control-treated and anti-VEGFR-3-treated mice (t-test p-value: ns=not significant; n=7).
Since VEGFR-3 expression was previously reported on some tissue macrophages (23, 24), we investigated the colon sections of the control and the anti-VEGFR-3 treated mice for the presence of the F4/80 expressing cells and found no differences between the groups, indicating that macrophage numbers where not modulated by the anti-VEGFR-3 treatment (Fig., Supplemental Digital Content 1, http://links.lww.com/IBD/A177).
Taken together, the results of this study show that blockade of VEGFR-3 on lymphatic vessels enhances colon inflammation, most likely via impaired lymphatic vessel drainage and clearance of antigens, cytokines, and immune cells.
Discussion
Inflammatory bowel disease is a disease with rising incidence in the developed world and current therapeutic strategies have not been satisfactory (25). The prominent role of the vasculature in inflammation might provide a potential novel therapeutic target in this complex disease.
In the present study, we found that the blockade of VEGFR-3 signaling has a negative effect on colitis in a genetically engineered mouse model. Anti-VEGFR3 antibody treated mice had an increased inflammation and leukocyte infiltration that were accompanied by enlarged, tortuous and dense lymphatic vessels. The morphology of these vessels and the marked submucosal edema indicate poor lymphatic functionality. The measured increased density of the lymphatic vessels thus probably represents enlargement of the existing vessels as opposed to active lymphangiogenesis. Expansion of the lymphatic vessel network was previously also shown in the dextran sulfate sodium (DSS) and CD45RBhi/RAG-1−/− colitis models, indicating that lymphatic vessels play an important role both in acute and chronic models of IBD (26–28). Furthermore, it was previously shown that mice deficient for angiopoietin-2 - that have intestinal lymphatic dysplasia - have exacerbated colitis symptoms in the DSS colitis model, pointing to the importance of lymphatic vessel function in intestinal inflammation resolution (27).
It has been suggested that blockade of lymphangiogenesis might be beneficial in some inflammatory diseases and in renal transplant rejection because of the inhibition of proinflammatory immune cell transport to the draining lymph node (4, 29). However, these findings focused on the early stages of inflammation where lymphatic vessels participate in the initiation of the immune response, whereas inflammatory bowel disease is a chronic and long lasting inflammatory disease where lymphatic vessel function might be reduced.
VEGFR-3 expression has been thought to be largely restricted to the lymphatic endothelium. However, tumor macrophages were also found to express VEGFR-3 (24) and VEGF-C was shown to stimulate migration of VEGFR-3 positive macrophages (23). Thus, some of the observed anti-VEGFR-3 effects might have been influenced by the inhibition of macrophage functions since macrophages have been implicated in inflammation resolution (15) and also in the regulation of lymphatic vessel diameter (30). To investigate this possibility, we analyzed the tissue for the presence of F4/80-positive cells. However, we observed no differences in their abundance in the colons of treated mice. These findings are in agreement with a recent study in a skin psoriasis-like inflammation model (3) where VEGFR-3 expression was shown to be restricted to lymphatic vessels in the inflamed skin, indicating that only tumor-associated macrophages, but not inflammation-associated macrophages might express VEGFR-3.
Recently, expression of VEGFR-3 was also reported on some tumor blood vessels and VEGFR-3 was shown to contribute to tumor angiogenesis in some experimental models of cancer (6, 31), Thus, it would be conceivable that blood vessels in IBD-affected colons might also express VEGFR-3, in which case the anti-VEGFR-3 antibody might also have inhibited angiogenesis. However, we did not find any significant effect of VEGFR-3 blockade on the blood vessel density in our IBD model, indicating that VEGFR-3 signaling might not play a major role in the mediation of angiogenesis in experimental colitis.
Our findings are the first to reveal that pharmacological inhibition of lymphangiogenesis exacerbates inflammation in experimental IBD. We found increased leukocyte numbers in the colons of anti-VEGFR-3 treated mice. The underlying mechanism of this finding is most likely the reduced lymphatic vessel tissue draining and cell transport function. However, reduced lymphatic vessel function may also indirectly cause a higher rate of leukocyte entry into the tissue via the blood vessels, due to the persistent presence of proinflammatory mediators that attract leukocytes and maintain the blood vascular endothelium in an activated and hyperpermeable state, thereby facilitating leukocyte extravasation. Consequently, the episodic nature of human IBD may be partly due the active lymphatic function during the remission periods.
Recently, inhibition of lymphangiogenesis and lymphatic drainage via VEGFR-3 blocking was found to aggravate inflammation in a mouse rheumatoid arthritis model (32) and mouse chronic skin inflammation (3). These unanticipated results, together with the results of our current study indicate that an active lymphatic vessel function is needed for the resolution of inflammation.
Taken together, we conclude that therapies that aim to promote lymphatic function, e.g., with pro-lymphangiogenic factors such as VEGF-C, might provide a novel strategy for the treatment of inflammatory conditions. In particular, in inflammatory bowel disease patients, submucosal edema, enlarged lymphatic vessels and lymphatic vessel obstruction were found (28, 33, 34), suggesting that stimulating lymphatic function might be a promising clinical approach.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health grant CA69184, Swiss National Science Foundation grant 31003A_130627, Advanced European Research Council Grant LYVICAM (to M.D.), and by National Institutes of Health grant DK44240 and Broad Biomedical Research Program (to J.P.S).
We thank Jeanette Scholl for excellent technical assistance, Sinem Karaman for helping with immunohistochemistry and Dr. Bronek Pytowski, Imclone Systems Inc. for providing the mF4-31C1 antibody.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 2.Skobe M, Hawighorst T, Jackson DG, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 3.Huggenberger R, Ullmann S, Proulx ST, et al. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J Exp Med. 2010;207:2255–2269. doi: 10.1084/jem.20100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerjaschki D, Regele HM, Moosberger I, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 5.Kaipainen A, Korhonen J, Mustonen T, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tammela T, Zarkada G, Wallgard E, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 7.Makinen T, Veikkola T, Mustjoki S, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. Embo J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karkkainen MJ, Ferrell RE, Lawrence EC, et al. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat Genet. 2000;25:153–159. doi: 10.1038/75997. [DOI] [PubMed] [Google Scholar]
- 9.Ghalamkarpour A, Morlot S, Raas-Rothschild A, et al. Hereditary lymphedema type I associated with VEGFR3 mutation: the first de novo case and atypical presentations. Clin Genet. 2006;70:330–335. doi: 10.1111/j.1399-0004.2006.00687.x. [DOI] [PubMed] [Google Scholar]
- 10.Karkkainen MJ, Saaristo A, Jussila L, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci U S A. 2001;98:12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makinen T, Jussila L, Veikkola T, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 12.Kajiya K, Detmar M. An important role of lymphatic vessels in the control of UVB-induced edema formation and inflammation. J Invest Dermatol. 2006;126:919–921. doi: 10.1038/sj.jid.5700126. [DOI] [PubMed] [Google Scholar]
- 13.Baluk P, Tammela T, Ator E, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angeli V, Randolph GJ. Inflammation, lymphatic function, and dendritic cell migration. Lymphat Res Biol. 2006;4:217–228. doi: 10.1089/lrb.2006.4406. [DOI] [PubMed] [Google Scholar]
- 15.Kataru RP, Jung K, Jang C, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 16.Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 17.Bristol IJ, Farmer MA, Cong Y, et al. Heritable susceptibility for colitis in mice induced by IL-10 deficiency. Inflamm Bowel Dis. 2000;6:290–302. doi: 10.1002/ibd.3780060407. [DOI] [PubMed] [Google Scholar]
- 18.Deban L, Correale C, Vetrano S, et al. Multiple pathogenic roles of microvasculature in inflammatory bowel disease: a Jack of all trades. Am J Pathol. 2008;172:1457–1466. doi: 10.2353/ajpath.2008.070593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jurisic G, Sundberg JP, Bleich A, et al. Quantitative lymphatic vessel trait analysis suggests Vcam1 as candidate modifier gene of inflammatory bowel disease. Genes Immun. 2010;11:219–231. doi: 10.1038/gene.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pytowski B, Goldman J, Persaud K, et al. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- 21.Bleich A, Mahler M, Most C, et al. Refined histopathologic scoring system improves power to detect colitis QTL in mice. Mamm Genome. 2004;15:865–871. doi: 10.1007/s00335-004-2392-2. [DOI] [PubMed] [Google Scholar]
- 22.de Buhr MF, Hedrich HJ, Westendorf AM, et al. Analysis of Cd14 as a genetic modifier of experimental inflammatory bowel disease (IBD) in mice. Inflamm Bowel Dis. 2009;15:1824–1836. doi: 10.1002/ibd.21030. [DOI] [PubMed] [Google Scholar]
- 23.Skobe M, Hamberg LM, Hawighorst T, et al. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoppmann SF, Birner P, Stockl J, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 26.Halin C, Detmar M. Chapter 1. Inflammation, angiogenesis, and lymphangiogenesis. Methods Enzymol. 2008;445:1–25. doi: 10.1016/S0076-6879(08)03001-2. [DOI] [PubMed] [Google Scholar]
- 27.Ganta VC, Cromer W, Mills GL, et al. Angiopoietin-2 in experimental colitis. Inflamm Bowel Dis. 2010;16:1029–1039. doi: 10.1002/ibd.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander JS, Chaitanya GV, Grisham MB, et al. Emerging roles of lymphatics in inflammatory bowel disease. Ann N Y Acad Sci. 2010;1207(Suppl 1):E75–E85. doi: 10.1111/j.1749-6632.2010.05757.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 30.Gordon EJ, Rao S, Pollard JW, et al. Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation. Development. 2010;137:3899–3910. doi: 10.1242/dev.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laakkonen P, Waltari M, Holopainen T, et al. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593–599. doi: 10.1158/0008-5472.CAN-06-3567. [DOI] [PubMed] [Google Scholar]
- 32.Guo R, Zhou Q, Proulx ST, et al. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum. 2009;60:2666–2676. doi: 10.1002/art.24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heatley RV, Bolton PM, Hughes LE, et al. Mesenteric lymphatic obstruction in Crohn's disease. Digestion. 1980;20:307–313. doi: 10.1159/000198452. [DOI] [PubMed] [Google Scholar]
- 34.Alexander JS, Ganta VC, Jordan PA, et al. Gastrointestinal lymphatics in health and disease. Pathophysiology. 2010;17:315–335. doi: 10.1016/j.pathophys.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.