Abstract
Vascular remodeling, characterized by extracellular matrix deposition and increased media-to-lumen (M/L) ratio, contributes to the development of microvascular complications in diabetes. We have previously shown that selective ETA receptor blockade prevents whereas selective ETB receptor blockade augments medial thickening of mesenteric arteries via regulation of matrix metalloproteases (MMP) in type 2 diabetic Goto-Kakizaki (GK) rats. The goal of this study was to determine the effect of combined ETA and ETB receptor blockade on resistance vessel remodeling. Vessel structure, MMP activity and extracellular matrix proteins were assessed in control Wistar and diabetic GK rats treated with vehicle or bosentan (100 mg/kg/day) for 4 weeks (n=7–9 per group). Bosentan completely prevented the increase in M/L ratio and MMP-2 activity in diabetes but paradoxically increased M/L ratio and MMP activation in control animals. Collagenase (MMP-13) activity and protein levels were significantly decreased in diabetes. Accordingly, collagen deposition was augmented in GK rats. Dual ET receptor antagonism improved enzyme activity and normalized MMP-13 levels in diabetic group but blunted MMP-13 activity in control animals. In summary, current findings suggest that diabetes-mediated remodeling of resistance arteries is prevented by dual blockade of ETA and ETB receptors and the relative role of ET receptors in the regulation of vascular structure differs in control and disease states.
Introduction
Pathological changes in vascular function and structure underlie complications associated with diabetes. Endothelin-1 (ET-1) being a potent vasoconstrictor with profibrotic and proliferative properties that change vessel architecture has long been suggested as an important mediator of these vascular pathologies. A significant correlation has been observed between plasma ET-1 levels and diabetic complications (Haak, Jungmann, Felber, Hillmann, & Usadel, 1992; Takahashi, Ghatei, Lam, O'Halloran, & Bloom, 1990). ET-1 mediates its diverse effects via distinct G protein-coupled receptor subtypes, ETA and ETB. Previous studies showed that antagonism of the ETA receptor which is localized mainly on vascular smooth muscle cells (VSMC) prevents mesenteric vascular hypertrophy (Gilbert et al., 2000) and aortic extracellular matrix (ECM) deposition (Fukuda et al., 2005) in Type 1 diabetes. We extended these studies to Type 2 diabetes and reported that ETA antagonist partially prevents ECM deposition and matrix metalloprotease (MMP) activation in middle cerebral arteries but not in the kidney of Goto-Kakizaki (GK) rats, a non-obese Type 2 diabetes model (Harris et al., 2005; Portik-Dobos et al., 2005). Given that ETB receptors on endothelial vs VSM cells have opposing actions on vascular reactivity (Masaki, 1995), and that inhibition of the ETB receptor system in a knock-out mouse model or pharmacological blockade by an ETB antagonist leads to enhanced intimal hyperplasia observed in carotid arteries after injury induced by ligation suggesting a vasculoprotective effect (Murakoshi et al., 2002), we next asked the question whether ETB receptors contribute to or balance detrimental effects of ETA receptor activation in diabetic vascular remodeling. We recently reported that selective ETA antagonism prevents but selective ETB receptor blockade augments medial thickening of mesenteric arteries (Sachidanandam et al., 2007). Whether this effect was due to activation of ETA receptors in the presence of ETB receptor blockade or due to the loss of vasculoprotective effects of ETB receptors remained unknown.
Vascular ECM dynamics are tightly regulated by MMP family of proteases (Visse & Nagase, 2003). Accumulating evidence suggests that MMPs not only degrade matrix but also stimulate matrix protein synthesis via proteolytic activation of numerous growth factors (Ammarguellat, Gannon, Amiri, & Schiffrin, 2002; Eguchi, Dempsey, Frank, Motley, & Inagami, 2001; Harris et al., 2005; Portik-Dobos et al., 2005; Saito & Berk, 2001; Song & Ergul, 2006). However, regulation of the MMP activity of these resistance arteries under mild-to-moderate diabetic conditions as seen in Type 2 diabetes and the relative role of ET receptors in this process remained unknown. Accordingly, this study investigated vessel structure, MMP activity and ECM proteins in control Wistar and diabetic GK rats chronically treated with vehicle or dual ETA/ETB receptor antagonist bosentan. The central hypothesis was that due to the antagonism of the vasculoprotective ETB receptors, bosentan treatment will not be as effective as selective ETA receptor antagonism in reducing resistance artery remodeling in Type 2 diabetes.
Materials and methods
Animals
All experiments were performed on male Wistar (Harlan, Indianapolis, IN) and GK (in-house bred, derived from the Tampa colony) rats (Farese et al., 1994; Standaert et al., 2004). The animals were housed at the Medical College of Georgia animal care facility that is approved by the American Association for Accreditation of Laboratory Animal Care. All protocols were approved by the Institutional Animal Care and Use Committee. Animals were fed standard rat chow and tap water ad-libitum until sacrifice at 18 weeks of age. Weight and blood glucose measurements were monitored twice a week till sacrifice. Blood glucose was measured from the tail vein using a commercially available glucometer (Freesytle, Alameda, CA). Blood pressure was monitored either by telemetric method (as previously reported) (Harris et al., 2005) or via the tail cuff method (Kent Scientific, Torrington, CT) which we have previously validated on telemetry implanted animals. After the spontaneous onset of diabetes, starting at 14 weeks of age, animals received either vehicle or dual ETA/ETB receptor antagonist bosentan (Actelion) 100 mg/kg/day for 4 weeks. This treatment paradigm was based on our previous studies (Harris, Elgebaly, Li, Sachidanandam, & Ergul, 2008; Harris et al., 2005; Sachidanandam et al., 2008; Sachidanandam et al., 2007). Bosentan was mixed in a small amount of peach flavored low sugar baby food and given separately than regular chow. Vehicle groups received the same treat. Animals were anesthesized with sodium pentobarbital and exsanguinated via cardiac puncture. The mesenteric bed was then harvested and third order mesenteric arteries were isolated for morphometry and biochemical studies, snap frozen in liquid nitrogen, and stored at -80°C.
Plasma measurements
Plasma ET-1 was measured by specific ELISA kit from ALPCO Diagnostics (Windham, NH).
Tissue homogenization and MMP activity
Snap frozen mesenteric arteries were homogenized in modified RIPA buffer (50 mM Tris-HCl, 1 % Nonidet P-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1mM PMSF, 1 µg/ml aprotinin, leupeptin, pepstatin, 1 mM sodium orthovanadate, and 1 mM sodium fluoride) as we previously described (Harris et al., 2005). Gelatinolytic activity was assessed by densitometric analysis (Gel-Pro version 3.1, Media Cybernetics, Carlsbad, CA) (Harris et al., 2005). Recombinant MMP-2 and MMP-9 proteins (Calbiochem, San Diego, CA) were run in parallel with all samples and the band intensity on zymogram gels was normalized to that of standard to prevent gel-to-gel variability. Collagenase activity of vascular MMP-13 was determined using a fluorescein-conjugated collagen assay kit as recommended by the manufacturer (Molecular Probes, Eugene, Oregon). Briefly, homogenates (20 µg total protein) were incubated with the substrate and increased fluorescence that is directly proportional to the proteolytic activity of MMP-13 was measured at time 0, 2, 4, 8 and 24 h using a microplate fluorometer. Other serine proteases in the tissue extracts were blocked by using 50 mM phenylmethylsulfonylfluoride (PMSF).
Vessel morphometry
Fixed vessel segments were embedded in paraffin, sectioned at 4 microns, and mounted on treated slides. Sections were stained with Masson trichrome stain. Slides were viewed using a Zeiss Axiovert microscope (Carl Zeiss, Inc., Thornwood, NY) and media:lumen ratios (M/L) were analyzed using Spot software (Diagnostic Instruments, MI). 4 measurements were made per section and each animal had at least 3 sections.
Immunoblotting
Protein levels of MMP-2 was determined by immunoblotting as previously described (Ergul, Portik-Dobos, Giulumian, Molero, & Fuchs, 2003; Harris et al., 2005) and antibodies were from Calbiochem (Cambridge, MA). Collagen type 1 levels were evaluated by slot-blot analysis and antibodies were from BD Transduction Laboratories (San Jose, CA). All blots were restained with anti-actin antibody (Sigma, St. Louis, MO) for equal protein loading. Using a subset of samples, blots were hybridized with secondary antibody alone to evaluate nonspecific binding and there was no detectable signal.
Statistical analysis
A 2×2 analysis of variance was used to investigate the main effects of Disease (control vs. diabetic) and Drug (vehicle vs. bosentan) and the interaction between Disease and Drug. Effects were considered statistically significant at p<0.05. GraphPad Prism was used for all analyses.
Results
Animal data
Metabolic parameters for all study groups are summarized in Table 1. Diabetic animals were significantly smaller than control and ET receptor antagonism did not affect animal weight. GK animals displayed elevated blood glucose in all treatment groups.
Table 1.
Animal metabolic data
| Control (n=9) | Control + Bosentan (100 mg/kg) (n=8) |
Diabetes (n=9) | Diabetes + Bosentan (100 mg/kg) (n=8) |
|
|---|---|---|---|---|
| Weight (g) | 558 ± 14 | 528 ± 28 | 389 ± 15 * | 382 ± 9 * |
| Glucose (mg/dl) | 5.9 ± 0.2 | 5.4 ± 0.2 | 11.6± 1.7 * | 9.3 ± 0.7 |
| ET-1 (fmol/ml) | 0.4 ± 0.1 | 1.1 ± 0.3 | 1.2 ± 0.2 | 2.2± 0.4** |
| MAP (mm-Hg) | 102 ± 1 | 108 ± 3 | 110± 5* | 112± 2 |
p<0.05 vs control vehicle
p<0.05 vs diabetes vehicle
Vascular structure
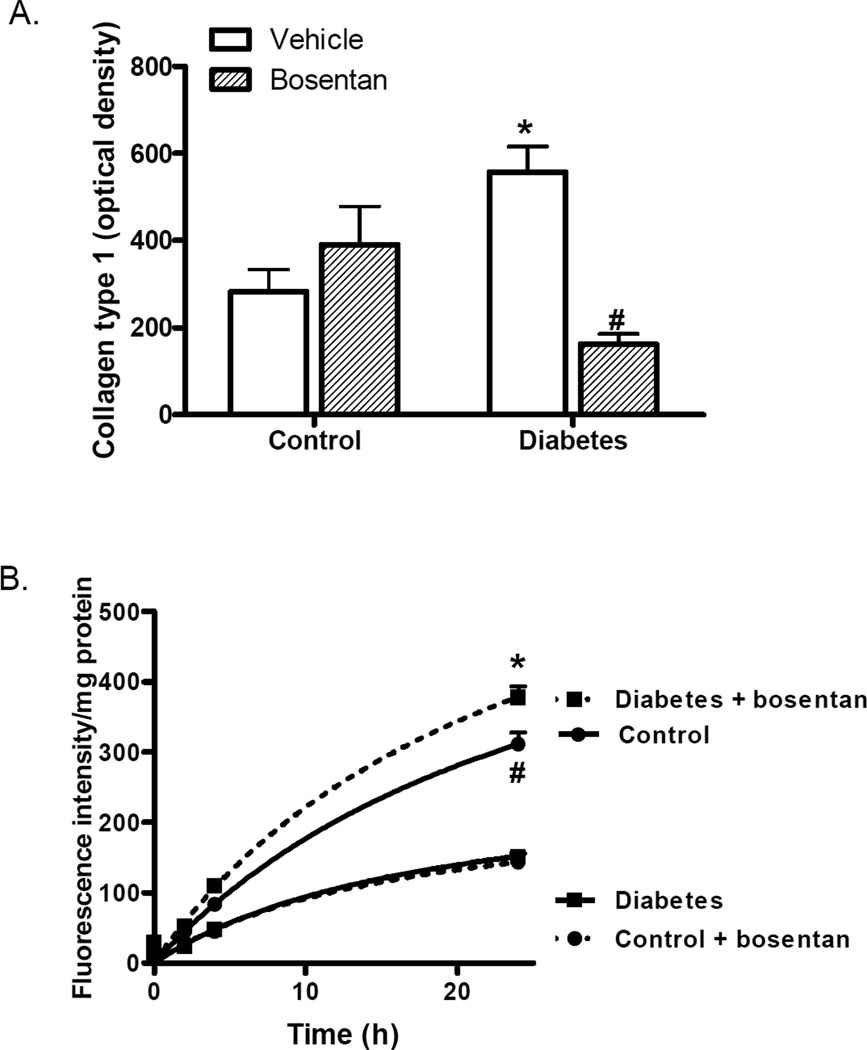
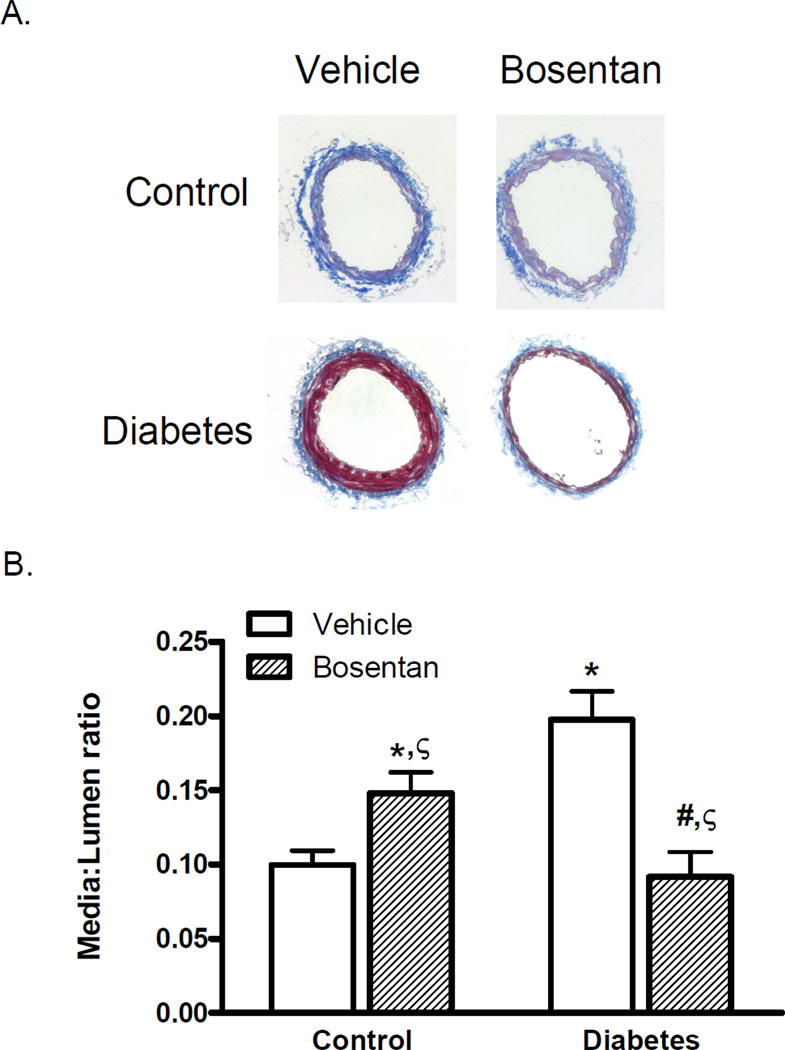
GK animals exhibited increased media thickness of mesenteric arteries with a significantly increased media:lumen (M/L) ratio as we previously reported. There was a disease and drug interaction such that dual ET receptor antagonism prevented the increase in M/L in diabetic animals but caused an increase in control animals. Total collagen type 1 levels were quantified by slot-blot analysis in addition to the qualitative assessment of collagen staining in Masson-trichrome stained sections. Densitometric analysis (Fig. 2A) demonstrated increased collagen type 1 in diabetes which was completely reduced by ET receptor antagonism. Bosentan treatment caused an increase in collagen levels in control animals but this effect did not reach significance.
Fig. 2.
Effects of ET receptor antagonism on collagen deposition in diabetes. (A) Mesenteric collagen type I levels, assessed by slot-blot analysis demonstrated increased deposition in diabetes which was attenuated by bosentan. n=7–9/group, *p<0.01 vs control vehicle, #p<0.001 vs diabetes vehicle, (B) MMP-13 collagenase activity was measured by incubating tissue homogenates with a fluorogenic MMP-13 substrate, which was decreased in diabetes. Similar to M:L ratio, bosentan treatment restored collagenase activity in diabetes but reduced enzyme activity even in control animals. n=5/group, *p9003C;0.001 vs diabetes vehicle, # p<0.001 vs control Bosentan. Treatment groups are indicated by broken lines.
Mesenteric MMP expression and activity
Since there is increased collagen accumulation in diabetes, MMP-13 activity, a collagenase that degrades fibrillar collagen, was measured using a fluorogenic assay. In diabetic animals, this activity was significantly decreased (Fig. 2B). Similar to morphometry results, ET receptor blockade with bosentan restored collagenase activity in diabetic animals but significantly reduced it in control animals.
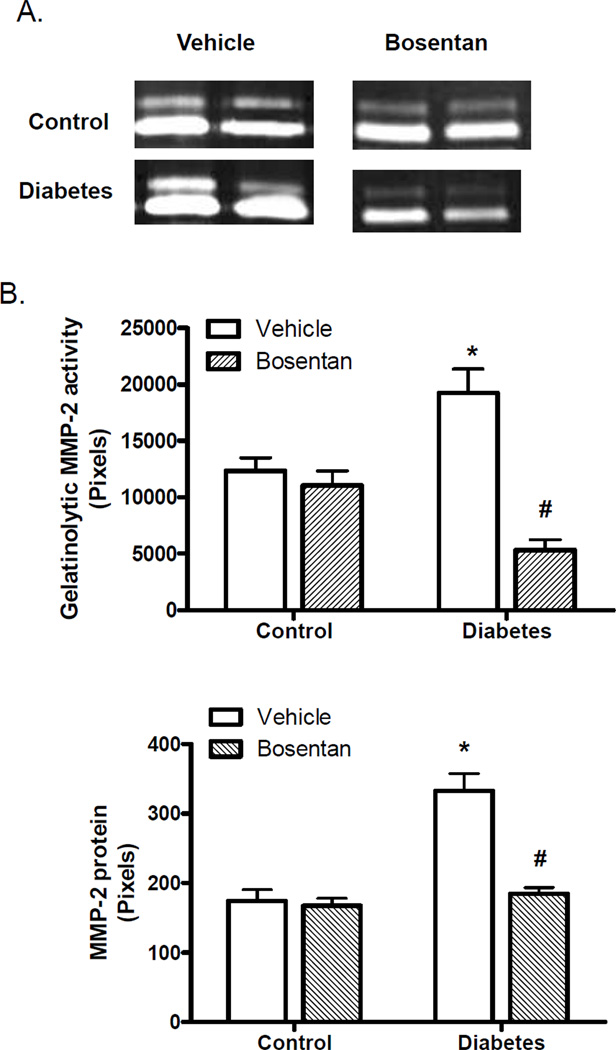
Gelatinolytic activity was evaluated using gelatin zymography, which detects MMP-2 and MMP-9-based lytic activity. Enzyme activity was detected mainly at 62 kDa and to a much lesser extent at 72 kDa corresponding to the active and latent forms of MMP-2, respectively. Densitometric analysis of the bands corresponding to active form demonstrated that MMP-2 activity was increased in diabetes and that ET receptor antagonism prevented this increase in activation (Fig. 3B). Unlike MMP-13 results, bosentan treatment did not have an effect on MMP-2 activity in control animals.
Fig. 3.
MP-2 activity is increased in diabetes. (A) Representative zymogram showing changes in vascular MMP-2 activity and (B) Densitometric analysis of lytic bands indicates an increase in MMP-2 activity that is ameliorated by ET receptor blockade. (C) MMP-2 protein is increased n=5/group, *p<0.001 vs control vehicle, #p<0.001 vs diabetes vehicle.
In order to determine whether increased MMP-2 activity results from an increase in protein levels, total MMP-2 protein was assessed by immunoblotting (Fig. 3C). MMP-2 levels were higher in diabetes contributing to increased enzyme activity and bosentan treatment prevented this increase.
Discussion
We previously showed that selective ETA receptor antagonism blunts whereas selective ETB receptor antagonisms exaggerates diabetes-mediated remodeling of resistance arteries suggesting a vasculoprotective effect (Sachidanandam et al., 2007). However the question remained whether this effect was due to the stimulation of unoccupied ETA receptors when ETB receptors are blocked or loss of vasculoprotection conferred by this receptor subtype. To address this question, control and diabetic animals were treated with vehicle or dual ET receptor antagonist bosentan using the same treatment paradigm employed in our previous study (Sachidanandam et al., 2007). The rationale was that 1) If ETA activation drives the remodeling response, we should see a similar effect to that of selective ETA blockade, or 2) If it is the loss of vasculoprotective effects of endothelial ETB receptors, we should not see a change in remodeling as ETB antagonism negates ETA antagonism effects. Given that pharmacological or genetic manipulation of ETB receptors results in augmented intimal remodeling in a carotid injury model (Murakoshi et al., 2002), the working hypothesis was that due to the antagonism of the vasculoprotective ETB receptors, treatment with nonselective ET receptor antagonist bosentan will not be as effective as selective ETA receptor antagonism in reducing resistance artery remodeling in Type 2 diabetes. Our intriguing findings demonstrate that the effect of ET receptor antagonism on matrix dynamics and vascular remodeling depends on the disease state. MMP activation and increased M/L ratio can be prevented by the administration of a dual ETA/ETB receptor antagonist in diabetes. On the other hand, same treatment worsens the remodeling process in control animals suggesting a vasculoprotective effect of this receptor subtype under physiological conditions.
Driven by the finding that hyperglycemia stimulates the production of ET-1 (Hattori, Kasai, Nakamura, Emodo, & Shimoda, 1991), several studies employed ET receptor antagonists to identify the involvement of the ET system in diabetic vascular complications (Gilbert et al., 2000; Katakam, Pollock, Pollock, Ujhelyi, & Miller, 2001; Wu, Hopfner, McNeill, Wilson, & Gopalakrishnan, 2000). However, a big majority of these studies were conducted using experimental models of Type 1 diabetes or obese animals. For example, Gilbert and colleagues reported that ETA receptor antagonism prevents mesenteric vascular hypertrophy in Type 1 diabetes by inhibiting macrophage infiltration and epidermal growth factor signaling (Gilbert et al., 2000). Another study provided evidence that blockade of ET-1 action inhibits ECM deposition in the aorta as well (Fukuda et al., 2005). We extended these observations to a lean model of Type 2 diabetes and showed that treatment with atrasentan, an ETA antagonist, prevented diabetes-induced changes in expression of MMPs and procollagen type 1 in mesenteric arteries but not in aorta (Song & Ergul, 2006). Our previous studies also provided evidence that the ETA receptor antagonism prevents ECM deposition and MMP activation in middle cerebral arteries and mesenteric resistance arteries (Harris et al., 2005; Sachidanandam et al., 2007). However, selective ETB receptor blockade augmented the medial thickening and increased M/L ratio. This finding was consistent with a vasculoprotective role for this receptor subtype as reported by Murakoshi et al. (Murakoshi et al., 2002). In that particular study, inhibition of the ETB receptor genetically or pharmacologically resulted in enhanced intimal hyperplasia of carotid arteries after injury induced by ligation suggesting that blockade of the receptor subtype may be detrimental. In the current study, we used a pharmacological approach to block both ETA and ETB receptors with the idea that ETB antagonism will diminish the protective effects mediated by this receptor and thus inhibition of diabetic mesenteric artery remodeling will be less than that observed with selective ETA blockade in our previous study (Sachidanandam et al., 2007). To our surprise, bosentan treatment totally prevented medial thickening and restored MMP activity in diabetic rats. One explanation is that this effect is not due to the loss of vasculoprotection conferred by the ETB receptor but rather full activation of unopposed ETA receptors in our diabetes model. However, it must be recognized that the current study did not employ a direct comparison of selective ETA versus dual ETA/ETB antagonism and this explanation is based upon our previous finding that selective ETB blockade worsens medial thickening.
The relative role of the ETB receptor subtype in regulation of vascular structure, however, appears to be more complex than this explanation. Results obtained in the bosentan-treated control group showed that dual ET receptor antagonism caused an increase in M/L ratio and ECM deposition in otherwise healthy animals. In our previous study with selective ETB receptor blockade, we detected a similar response (Sachidanandam et al., 2007). Collectively, these results do support a protective role for this receptor in the regulation of vascular structure under physiological conditions. In disease states, receptor activation may be different. There is accumulating evidence that ET receptor subtypes can form homo and heterodimers (Gregan, Jurgensen et al., 2004; Gregan, Schaefer, Rosenthal, & Oksche, 2004; Sauvageau, Thorin, Caron, & Dupuis, 2006). It is suggested that when heterodimerized, the ETA receptor overrides ETB receptor activation and thus ETB receptor antagonism provides an ETA blockade-like effect (Gregan, Jurgensen et al., 2004; Gregan, Schaefer et al., 2004; Sauvageau et al., 2006). We have shown that ETA, but not ETB, receptor protein is increased in the mesenteric arteries in diabetes (Sachidanandam et al., 2007). It is possible that when there is an alteration in the balance of ETA and ETB receptors as occurs in disease states, dimerization patterns can change ultimately affecting the responses mediated by each receptor subtype.
In conclusion, dual blockade of ET receptors completely prevents remodeling of resistance arteries in diabetic animals but paradoxically promotes vascular remodeling in control animals. These findings suggest that the relative roles of ET receptors in the regulation of vascular structure may differ in states, which has important clinical implications for the use of ET receptor antagonists.
Fig. 1.
Vessel segments were analyzed for morphological changes and collagen deposition by Masson staining. Diabetes induced a two-fold increase in media:lumen (M/L) ratio and ET receptor antagonism by bosentan prevented this increase. Representative sections are shown in Panel A and combined analysis is given in Panel B. n=7–9/group, *p<0.05 vs control vehicle,#p<0.001 vs diabetes vehicle or A-192621, ζp<0.001 drug-disease interaction with bosentan increasing M/L ratio in control but decreasing it in diabetic animals.
Acknowledgment
This work was supported by grants from NIH (RO1 DK074385) and American Heart Association (EIA 0740002N). The authors wish to thank Actelion for providing bosentan.
References
- Ammarguellat FZ, Gannon PO, Amiri F, Schiffrin EL. Fibrosis, matrix metalloproteinases, and inflammation in the heart of DOCA-salt hypertensive rats: role of ET(A) receptors. Hypertension. 2002;39(2 Pt 2):679–684. doi: 10.1161/hy0202.103481. [DOI] [PubMed] [Google Scholar]
- Eguchi S, Dempsey PJ, Frank GD, Motley ED, Inagami T. Activation of MAPKs by angiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p38 MAPK but not for JNK. J Biol Chem. 2001;276(11):7957–7962. doi: 10.1074/jbc.M008570200. [DOI] [PubMed] [Google Scholar]
- Ergul A, Portik-Dobos V, Giulumian AD, Molero MM, Fuchs LC. Stress upregulates arterial matrix metalloproteinase expression and activity via endothelin A receptor activation. Am J Physiol Heart Circ Physiol. 2003;285(5):H2225–H2232. doi: 10.1152/ajpheart.00133.2003. [DOI] [PubMed] [Google Scholar]
- Farese RV, Standaert ML, Yamada K, Huang LC, Zhang C, Cooper DR, et al. Insulin-induced activation of glycerol-3-phosphate acyltransferase by a chiro-inositol-containing insulin mediator is defective in adipocytes of insulin-resistant, type II diabetic, Goto-Kakizaki rats. Proc Natl Acad Sci U S A. 1994;91(23):11040–11044. doi: 10.1073/pnas.91.23.11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda G, Khan ZA, Barbin YP, Farhangkhoee H, Tilton RG, Chakrabarti S. Endothelin-mediated remodeling in aortas of diabetic rats. Diabetes Metab Res Rev. 2005;21(4):367–375. doi: 10.1002/dmrr.527. [DOI] [PubMed] [Google Scholar]
- Gilbert RE, Rumble JR, Cao Z, Cox AJ, van Eeden P, Allen TJ, et al. Endothelin receptor antagonism ameliorates mast cell infiltration, vascular hypertrophy, and epidermal growth factor expression in experimental diabetes. Circ. Res. 2000;86:158–165. doi: 10.1161/01.res.86.2.158. [DOI] [PubMed] [Google Scholar]
- Gregan B, Jurgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, et al. Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem. 2004;279(26):27679–27687. doi: 10.1074/jbc.M403601200. [DOI] [PubMed] [Google Scholar]
- Gregan B, Schaefer M, Rosenthal W, Oksche A. Fluorescence resonance energy transfer analysis reveals the existence of endothelin-A and endothelin-B receptor homodimers. J Cardiovasc Pharmacol. 2004;44:S30–S33. doi: 10.1097/01.fjc.0000166218.35168.79. [DOI] [PubMed] [Google Scholar]
- Haak T, Jungmann E, Felber A, Hillmann U, Usadel KH. Increased plasma levels of endothelin in diabetic patients with hypertension. Am. J. Hypertens. 1992;5:161–166. doi: 10.1093/ajh/5.3.161. [DOI] [PubMed] [Google Scholar]
- Harris AK, Elgebaly MM, Li W, Sachidanandam K, Ergul A. Effect of chronic endothelin receptor antagonism on cerebrovascular function in type 2 diabetes. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1213–R1219. doi: 10.1152/ajpregu.00885.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AK, Hutchinson JR, Sachidanandam K, Johnson MH, Dorrance AM, Stepp DW, et al. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes. 2005;54(9):2638–2644. doi: 10.2337/diabetes.54.9.2638. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Kasai K, Nakamura T, Emodo T, Shimoda S-I. Effects of glucose and insulin on immunoreactive endothelin-1 release from cultured bovine endothelial cells. Metabolism. 1991;40:165–169. doi: 10.1016/0026-0495(91)90168-v. [DOI] [PubMed] [Google Scholar]
- Katakam PV, Pollock JS, Pollock DM, Ujhelyi MR, Miller AW. Enhanced endothelin-1 response and receptor expression in small mesenteric arteries of insulin-resistant rats. Am J Physiol Heart Circ Physiol. 2001;280(2):H522–H527. doi: 10.1152/ajpheart.2001.280.2.H522. [DOI] [PubMed] [Google Scholar]
- Masaki T. Possible role of endothelin in endothelial regulation of vascular tone. Annu. Rev. Pharmacol. Toxicol. 1995;35:235–255. doi: 10.1146/annurev.pa.35.040195.001315. [DOI] [PubMed] [Google Scholar]
- Murakoshi N, Miyauchi T, Kakinuma Y, Ohuchi T, Goto K, Yanagisawa M, et al. Vascular endothelin-B receptor system in vivo plays a favorable inhibitory role in vascular remodeling after injury revealed by endothelin-B receptor-knockout mice. Circulation. 2002;106(15):1991–1998. doi: 10.1161/01.cir.0000032004.56585.2a. [DOI] [PubMed] [Google Scholar]
- Portik-Dobos V, Harris AK, Song W, Hutchinson J, Johnson MH, Imig JD, et al. Endothelin antagonism prevents early EGFR transactivation but not increased matrix metalloproteinase activity in diabetes. Am J Physiol Regul Integr Comp Physiol. 2005;290(2):R435–R441. doi: 10.1152/ajpregu.00300.2005. [DOI] [PubMed] [Google Scholar]
- Sachidanandam K, Elgebaly MM, Harris AK, Hutchinson JR, Mezzetti EM, Portik-Dobos V, et al. Effect of chronic and selective endothelin receptor antagonism on microvascular function in Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2008;294(6):H2743–H2749. doi: 10.1152/ajpheart.91487.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidanandam K, Portik-Dobos V, Harris AK, Hutchinson JR, Muller E, Johnson MH, et al. Evidence for vasculoprotective effects of ETB receptors in resistance artery remodeling in diabetes. Diabetes. 2007;56(11):2753–2758. doi: 10.2337/db07-0426. [DOI] [PubMed] [Google Scholar]
- Saito Y, Berk BC. Transactivation: a novel signaling pathway from angiotensin II to tyrosine kinase receptors. J Mol Cell Cardiol. 2001;33(1):3–7. doi: 10.1006/jmcc.2000.1272. [DOI] [PubMed] [Google Scholar]
- Sauvageau S, Thorin E, Caron A, Dupuis J. Evaluation of endothelin-1-induced pulmonary vasoconstriction following myocardial infarction. Exp Biol Med (Maywood) 2006;231(6):840–846. [PubMed] [Google Scholar]
- Song W, Ergul A. Type-2 diabetes-induced changes in vascular extracellular matrix gene expression: Relation to vessel size. Cardiovasc Diabetol. 2006;5(1):3. doi: 10.1186/1475-2840-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert ML, Sajan MP, Miura A, Kanoh Y, Chen HC, Farese RV, Jr, et al. Insulin-induced activation of atypical protein kinase C, but not protein kinase B, is maintained in diabetic (ob/ob and Goto-Kakazaki) liver. Contrasting insulin signaling patterns in liver versus muscle define phenotypes of type 2 diabetic and high fat-induced insulin-resistant states. J Biol Chem. 2004;279(24):24929–24934. doi: 10.1074/jbc.M402440200. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Ghatei MA, Lam HC, O'Halloran DJ, Bloom SR. Elevated plasma endothelin in patients with diabetes mellitus. Diabetologia. 1990;33:306–310. doi: 10.1007/BF00403325. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Wu S, Hopfner RL, McNeill JR, Wilson TW, Gopalakrishnan V. Altered paracrine effect of endothelin in blood vessels of the hyperinsulinemic, insulin resistant obese Zucker rat. Cardiovasc. Res. 2000;45:994–1000. doi: 10.1016/s0008-6363(99)00417-4. [DOI] [PubMed] [Google Scholar]