Abstract
Although Mg2+ has a well-recognized role as an essential cofactor for all ATP-binding enzymes, its role as a signaling ion like Ca2+ has been controversial. A requirement for Mg2+ for optimal T lymphocyte stimulation was demonstrated more than 30 years ago, but the mechanism of its synergistic effect with Ca2+ in T cell activation remains elusive. Here, we summarize our recent discovery of a signaling role for Mg2+ in the T cell antigen receptor (TCR) signaling pathway from the study of a novel primary immunodeficiency now named X-linked immunodeficiency with Mg2+ defect, EBV infection and neoplasia (XMEN). XMEN patients were found to have a deficiency in Magnesium Transporter 1 (MAGT1), a Mg2+-specific transporter, which leads to the absence of a TCR-stimulated Mg2+ flux and attenuation of T cell activation. We further showed that this Mg2+ flux is required proximally for the temporal orchestration of phospholipase C-γ1 (PLCγ1) activation. Thus, our study not only provides a second messenger role for Mg2+ to explain its synergism with calcium in T cell signaling, it also identifies a potential extracellular therapeutic target for T cell-specific immunomodulation.
Introduction
Mg2+ is the most abundant divalent cation in mammalian cells. Although, cells exhibit high concentrations of intracellular Mg2+ (10-30 mM), most of it is bound to proteins, phospholipids, nucleic acids, and particularly ATP [1-3]. However, the free cytosolic Mg2+ (0.2-1 mM) represents only 1-5 % of the total Mg2+, and is actively maintained at 50 to 100-fold lower than its electrochemical equilibrium potential [4]. Unlike Ca2+, the role of Mg2+ as a second messenger is still controversial [5]. Nevertheless, within the last thirty years, an increasing number of observations have been reported that implicated Mg2+ in various cell functions in response to extracellular stimuli [6, 7]. For example, the role of intracellular free Mg2+ in growth factor-induced cell proliferation, originally thought to be strictly a co-factor effect, is now reevaluated as a possible second messenger role [8]. In the immune system, several studies have shown that extracellular Mg2+ acts synergistically with Ca2+ in T cell activation but not in B cells stimulated with lipopolysaccharide (LPS) [9-11]. Moreover, in suboptimal extracellular concentrations of Ca2+, extracellular Mg2+ is able to restore interleukin-2 (IL-2) receptor expression and cell proliferation in T cells stimulated by phytohemagglutinin (PHA) but not by ionomycin [12]. As ionomycin is a Ca2+ ionophore that bypasses proximal T cell receptor (TCR) signaling events to induce Ca2+ signaling, this result suggests that optimal T cell activation requires a Mg2+-dependent process upstream of the Ca2+ flux induction. In all these cases, the mechanism of action of the Mg2+ as a signaling ion remained elusive.
Recently, we reported a novel primary immunodeficiency named X-linked immunodeficiency with magnesium defect, EBV infection and neoplasia (XMEN) [13]. Our work unraveled a signaling role for Mg2+ in TCR signaling by identifying the loss of the Mg2+ transporter MAGT1 as the cause of this disease. This study demonstrated that TCR stimulation induced a Mg2+ flux through MAGT1 that was important for the activation of PLCγ1 and its downstream effects, including the production of inositol triphosphate (IP3) and the consequent release of Ca2+.
Description of the patients and TCR defect
Our study began with two young boys (patient A.1 and A.2) with a history of recurrent viral infections including chronic active Epstein-Barr virus (EBV) infections. These patients exhibited low CD4+ T cells counts, leading to an inverted CD4:CD8 ratio. Other lymphocytes populations did not show major disturbances. We first ruled out well-established causes of low CD4+ T cells counts, like infections with human immunodeficiency virus (HIV) or cytomegalovirus (CMV), Bare-Lymphocyte syndrome, Wiskott-Aldrich syndrome, and Di-George syndrome. Then we assessed the CD31+ cell population amongst the CD4+ CD27+CD45RO- naïve T cells and found this population reduced in the patients compared to normal controls, indicating a diminished thymic output of CD4+ T cells.
We assessed the function of the T cells by testing the ability of the patients’ T cells to normally activate upon TCR engagement using an agonist anti-CD3 antibody (OKT3). We found that patients’ T cells exhibited defective induction of several activation markers, such as CD69, CD25, Fas (CD95) and CTLA-4, in response to TCR stimulation (figure 1A). Looking at earlier signaling events, we observed that TCR-induced translocation of essential transcription factors, such as NF-κB and NFAT, were impaired as well in the patients’ cells (figure 1B). However, these defects were rescued by using the second messenger inducers phorbol 12-myristate 13-acetate (PMA) and ionomycin, showing that the patients had a proximal TCR signaling defect prior to the induction of the Ca2+ flux. Unlike T cells, the patients’ B cells showed normal activation upon B cell receptor (BCR) stimulation.
Figure 1. XMEN patients have a proximal TCR activation defect with loss of Mg2+and Ca2+fluxes.

A) Graph representing the expression of the activation markers CD25, CD95 and CTLA4 in T cells after 3 days of stimulation with 1 ug/ml anti-CD3 (OKT3). The red box represents the normal range. B) Graph representing the nuclear translocation of NF-κB and NFAT after 30 min stimulation with OKT3. C) Mg2+ (upper panel) and Ca2+ (lower panel) fluxes in T cells from normal control or Patient A.1 after 5 ug/ml OKT3 stimulation.
Characterization of the genetic defect
As the two patients were boys, we hypothesized that the defect could be X-linked. To assess this possibility we tested for random lionization or inactivation of each of the mother’s X chromosomes [14]. When one of the X chromosomes carries a genetic mutation conferring a selective disadvantage to the cell (e.g. impacting proliferation or survival), then skewed lyonization reflects the loss of cells with an active X chromosome carrying the mutation. Based on that hypothesis, we performed a lyonization assay and showed that the mother’s T cells exhibited inactivation of only one of her X chromosomes instead of the expected random inactivation of both X chromosomes. This skewed lyonization led us to search for a genetic defect on the X chromosome. We performed X-chromosome exon-capture and single-end Solexa sequencing on genomic DNA from the mother and the two boys. According to our X-linked genetic model with skewed lyonization in the mother, we hypothesized that the defect should be present in both brothers, heterozygous in the mother and not present in the mother’s cDNA. Using these criteria, we initiated the analysis by looking for single nucleotide polymorphisms (SNPs) or 1-2 bp deletion-insertion polymorphisms (DIPs). We did not found any nonsense mutations or 1-2 bp DIPS. DIPs larger than 2 bp are not revealed by single-end Illumina sequencing, so we ruled out large DIPs (>1000 bp) using a comparative genomic hybridization array. For the intermediate DIPs (3-1000 bp), we searched for missing coverage in the patients but not in the mother by developing a new computational tool. This approach allowed us to identify a 10 bp deletion present in both patients in MAGT1, a gene coding for a Mg2+ transporter. Consistent with our genetic model, the deletion was not found in the mother’s cDNA nor in 100 normal controls. Finally, we confirmed the presence of the deletion by Sanger sequencing in both patients and showed it was heterozygous in the mother’s genomic DNA. Moreover, this mutation was also carried by the grandmother and the great-grandmother of the patients. The deletion was located in the 3’ exon-intron junction of the exon 7 of MAGT1 and induced the splicing of exon 6 with exon 9, creating a premature stop codon. This early termination led to the decrease of MAGT1 mRNA of ≈80 % by nonsense-mediated decay leading to the loss of MAGT1 protein expression in the patients.
MAGT1 deficiency impairs TCR-induced Mg2+ and Ca2+ influxes
Little was known about the function of MAGT1 in lymphocytes before our study. However, previous studies in transfected Xenopus laevis oocytes and the human embryonic kidney 293T cell line (HEK 293T) showed that MAGT1 induces a highly selective Mg2+ uptake with almost no permeability to other cations including Ca2+ [15, 16]. Using fluorescent probes for Mg2+ (Mag Fluo 4-AM) and Ca2+ (Fluo 4-AM), we assessed Mg2+ and Ca2+ uptake in T cells from the patients and normal controls after addition of 1 mM Ca2+ or Mg2+. We observed that the basal level of free Mg2+ was decreased in patients’ T cells and there was impaired Mg2+ uptake, which was consistent with MAGT1 as a Mg2+ transporter in lymphocytes. Nevertheless, although the patients’ T cells had defective Mg2+ uptake, the total amount of Ca2+ and Mg2+ in purified CD4+ and CD8+ T cells, measured by inductively coupled plasma mass spectrometry, was not different from normal control cells. As the free Mg2+ represents only a small fraction of the total cellular Mg2+ and the fluorescent probes only detect the free cytosolic Mg2+, the defect we observed was limited to the free Mg2+.
To link the TCR activation defect and the loss of MAGT1 in the patients, we examined TCR stimulation with an OKT3 antibody affects free Mg2+ level in T cells. Interestingly, we showed that TCR stimulation induced a Mg2+ flux in normal T cells, which was impaired in the patients’ cells even with high doses of agonist (figure 1C). Moreover, the TCR-induced Ca2+ flux was drastically decreased in the patients’ T cells, raising the possibility that the Ca2+ flux was dependent on Mg2+ (figure 1C). We explored this possibility by modulating extracellular [Mg2+]e and [Ca2+]e. The TCR-induced Mg2+ and Ca2+ flux were both abrogated in absence of Mg2+, whereas only the Ca2+ flux was impaired in absence of Ca2+, showing that the Mg2+ flux was at least partially needed for the induction of the Ca2+ flux but not the converse. Moreover, we found that the BCR stimulation did not induce a Mg2+ flux, which is consistent with the fact that B cell activation was normal in the patients.
We were able to impair the TCR-induced Mg2+ flux as well as the NF-κB translocation by knocking down MGAT1 in normal T cells, recapitulating the patients’ phenotype. We also rescued the patients’ T cells with ectopic expression of MAGT1, restoring the TCR-induced Mg2+ flux and the upregulation of activation markers upon TCR stimulation. These results established that MAGT1 mediated the Mg2+ influx required for full T cell activation. Thus, MAGT1 deficiency was the proximate cause of the cellular defect in XMEN disease.
Impairment of PLCγ1 activation in MAGT1 deficient patients
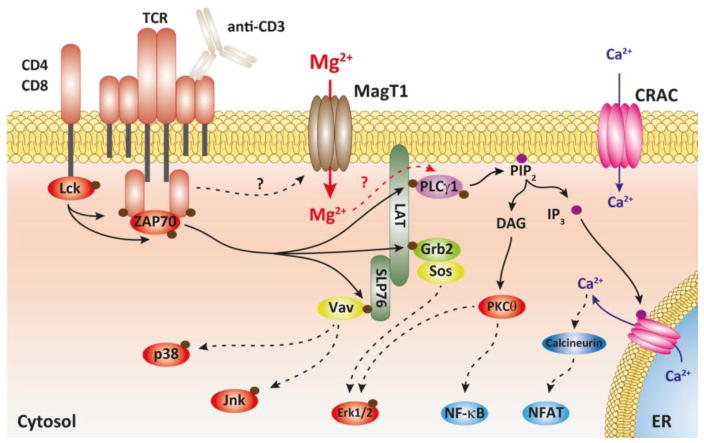
Since we showed that the Mg2+ flux was necessary to induce a full Ca2+ response, we next wanted to dissect the TCR signaling event leading to the generation of the Ca2+ flux [17, 18]. Stimulation of the TCR induces the activation of the Lck kinase, which initiates a cascade of phosphorylation events leading to the activation of the PLCγ1 which generates diacylglycerol (DAG) and IP3 (figure 2). IP3 then triggers the release of the Ca2+ from the endoplasmic reticulum initiating the store operated calcium entry (SOCE), which is the Ca2+ flux induced by TCR. By assessing the phosphorylation status of the different components of the TCR signaling apparatus, we showed that the phosphorylation of PLCγ1 was delayed in the patient cells after TCR stimulation. Indeed, the peak of phosphorylation of PLCγ1 induced by OKT3 stimulation was shifted from 5 minutes in normal control T cells to 60 minutes in the patients’ cells. As IP3 production was also delayed, we determined that PLCγ1 activation was the proximal step regulated by Mg2+. Thus, our findings suggested than Mg2+ acts as a second messenger in TCR signaling, leading to the activation of PLCγ1.
Figure 2. Hypothetical scheme depicting how the MagT1 mediated Mg2+ influx participates in TCR signaling.
Solid arrows indicate direct effects; dotted arrows indicate indirect effects.
Conclusion
Mg2+ plays a central role in many cellular functions by acting as a cofactor for all ATP consuming enzymes as well as a stabilizer for structural membrane phospholipids [19, 20]. However, our data showed that a transient, Mg2+ influx mediated by MAGT1 after TCR stimulation was able to modulate the activation of PLCγ1, and the subsequent Ca2+ influx. These observations revealed an essential role for Mg2+ in the TCR signaling.
The study of the XMEN patients also revealed that their lymphocytes exhibited a defective Mg2+ uptake leading to lower basal level of free Mg2+. However, this could not account for defective T cell activation, as the patient’s B cells activated normally compared to control. Recently, the role of TRPM7 in Mg2+ homeostasis in B cell has been described, and a lack of TRPM7 can be compensated by overexpressing MAGT1 in B cell line [21]. These observations taken together with ours makes us think that TRPM7 may play a greater role in Mg2+ homeostasis in B cells than MAGT1. This selective function of MAGT1 in T cells could be used to develop new immune modulator drugs to target T cell-dependent auto-immunity or other immunological diseases.
References
- 1.Cakmak I, Kirkby EA. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol Plant. 2008;133(4):692–704. doi: 10.1111/j.1399-3054.2007.01042.x. [DOI] [PubMed] [Google Scholar]
- 2.Cowan JA. Structural and catalytic chemistry of magnesium-dependent enzymes. Biometals. 2002;15(3):225–35. doi: 10.1023/a:1016022730880. [DOI] [PubMed] [Google Scholar]
- 3.Yang W, Lee JY, Nowotny M. Making and breaking nucleic acids: two-Mg2+-ion catalysis and substrate specificity. Mol Cell. 2006;22(1):5–13. doi: 10.1016/j.molcel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Romani AM. Cellular magnesium homeostasis. Arch Biochem Biophys. 2011;512(1):1–23. doi: 10.1016/j.abb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy E. Mysteries of magnesium homeostasis. Circ Res. 2000;86(3):245–8. doi: 10.1161/01.res.86.3.245. [DOI] [PubMed] [Google Scholar]
- 6.Vidair C, Rubin H. Mg2+ as activator of uridine phosphorylation in coordination with other cellular responses to growth factors. Proc Natl Acad Sci U S A. 2005;102(3):662–6. doi: 10.1073/pnas.0409082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takaya J, Higashino H, Kobayashi Y. Can magnesium act as a second messenger? Current data on translocation induced by various biologically active substances. Magnes Res. 2000;13(2):139–46. [PubMed] [Google Scholar]
- 8.Rubin H. The logic of the Membrane, Magnesium, Mitosis (MMM) model for the regulation of animal cell proliferation. Arch Biochem Biophys. 2007;458(1):16–23. doi: 10.1016/j.abb.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Abboud CN, Scully SP, Lichtman AH, Brennan JK, Segel GB. The requirements for ionized calcium and magnesium in lymphocyte proliferation. J Cell Physiol. 1985;122(1):64–72. doi: 10.1002/jcp.1041220111. [DOI] [PubMed] [Google Scholar]
- 10.Flynn A. Control of in vitro lymphocyte proliferation by copper, magnesium and zinc deficiency. J Nutr. 1984;114(11):2034–42. doi: 10.1093/jn/114.11.2034. [DOI] [PubMed] [Google Scholar]
- 11.Whitney RB, Sutherland RM. The influence of calcium, magnesium and cyclic adenosine 3’,5’-monophosphate on the mixed lymphocyte reaction. J Immunol. 1972;108(5):1179–83. [PubMed] [Google Scholar]
- 12.Modiano JF, Kelepouris E, Kern JA, Nowell PC. Requirement for extracellular calcium or magnesium in mitogen-induced activation of human peripheral blood lymphocytes. J Cell Physiol. 1988;135(3):451–8. doi: 10.1002/jcp.1041350312. [DOI] [PubMed] [Google Scholar]
- 13.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475(7357):471–6. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wengler GS, Parolini O, Fiorini M, Mella P, Smith H, Ugazio AG, Notarangelo LD. A PCR-based non-radioactive X-chromosome inactivation assay for genetic counseling in X-linked primary immunodeficiencies. Life Sci. 1997;61(14):1405–11. doi: 10.1016/s0024-3205(97)00686-3. [DOI] [PubMed] [Google Scholar]
- 15.Goytain A, Quamme GA. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genomics. 2005;6(1):48. doi: 10.1186/1471-2164-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou H, Clapham DE. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc Natl Acad Sci U S A. 2009;106(37):15750–5. doi: 10.1073/pnas.0908332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson EJ, Koretzky GA. Signal transduction in T lymphocytes. Clin Exp Rheumatol. 1999;17(1):107–14. [PubMed] [Google Scholar]
- 18.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76(2):263–74. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 19.Grubbs RD, Maguire ME. Magnesium as a regulatory cation: criteria and evaluation. Magnesium. 1987;6(3):113–27. [PubMed] [Google Scholar]
- 20.Permyakov EA, Kretsinger RH. Cell signaling, beyond cytosolic calcium in eukaryotes. J Inorg Biochem. 2009;103(1):77–86. doi: 10.1016/j.jinorgbio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Deason-Towne F, Perraud AL, Schmitz C. The Mg(2+) transporter MagT1 partially rescues cell growth and Mg(2+) uptake in cells lacking the channel-kinase TRPM7. FEBS Lett. 2011;585(14):2275–8. doi: 10.1016/j.febslet.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]