Abstract
Prostaglandin E2 (PGE2), a prostanoid synthesized from arachidonic acid via the cyclooxygenase pathway, is a modulator of physiological responses including inflammation, fever, and muscle regeneration. Several patents have been filed that are related to PGE2, one of them being directly related to skeletal muscles. In this report, we first summarize the key patents describing inventions for the utilization of PGE2 for either diagnostic or therapeutic purposes, including skeletal muscle. In the second part of our work we present new and exciting data that demonstrates that PGE2 accelerates skeletal muscle myogenic differentiation. Our discovery resulted from our recent and novel concept of bone-muscle crosstalk. Bone and muscle are anatomically intimate endocrine organs and we aimed to determine whether this anatomical intimacy also translates into a biochemical communication from bone cells to muscle cells at the in vitro level. The effects of MLO-Y4 osteocyte-like cell conditioned medium (CM) and three osteocyte-secreted factors, PGE2, sclerostin and monocyte chemotactic protein (MCP-3), on C2C12 myogenic differentiation were evaluated using morphological analyses, a customized 96-PCR gene array, and measurements of intracellular calcium levels. MLO-Y4 CM and PGE2, but not sclerostin and MCP-3, induced acceleration of myogenesis of C2C12 myoblasts that was linked with significant modifications in intracellular calcium homeostasis. This finding should further stimulate the pursuit of new patents to explore the use of PGE2 and the new concept of bone-muscle crosstalk for the development and application of inventions designed to treat muscle diseases characterized by enhanced muscle wasting, such as sarcopenia.
Keywords: Bone-muscle crosstalk, PGE2, inventions, prostaglandins, prostanoid, EP Receptors, gene expression, MyoD, muscle regulatory factors, myogenic acceleration, calcium release, excitation-contraction coupling, bone cells, osteocytes, MLO-Y4 osteocytes cells like, MLO-Y4 conditioned media, bone secreted factors, muscle secreted factors, myokines, calcium-induced calcium release (CICR), patents
Introduction
PGE2 is one of the major products of arachidondic acid through cyclooxygenase-1/2 pathways. In the past decades, it has been related to inflammation, pain and cancer development. On the other hand, PGE2 is important in regulation of physiological functions, such as sodium and chloride transport in the kidney and smooth muscle tones, suggesting itself or its derivatives would be used in the treatment of diseases. Here we will summarize some patents associated with the application of PGE2 in diagnosis and therapeutics (Table 1)[1-9].
Table 1. Selected Patents related with usage of PGE2 in diagnosis and therapeutics.
| Patent # | Title | Target | Inventor(s) |
|---|---|---|---|
| US 6291528 | Prostaglandin E1/F2 in combination with prostaglandin F2.alpha. for enhancing female sexual arousal | Female sexual arousal dysfunction | Nathan Earl Scott |
| US 6476074 | Method and composition for treatment of erectile dysfunction | Impotence | Johan Stjernschantz, Bahram Resul |
| US 6545045 | Prostaglandin E agonists for treatment of glaucoma | Glaucoma and/or ocular hypertension | Peter G. Klimko, Najam A. Sharif, Brenda W. Griffin |
| US6841573 | Use of arachidonic acid as a method of increasing skeletal muscle mass | Skeletal muscle | William Charles Llewellyn |
| US 6610719 | Use of prostaglandin (PGE2) receptor a (EP4) selective agonists for the treatment of acute and chronic renal failure | Renal failure | Vishwas M. Paralkar, David D. Thompson |
| US 8168428 | Method to modulate hematopoietic stem cell growth | Stem cell | Leonard I. Zon, Trista E. North, Wolfram Goessling |
| US 2010/0267826 A1 | Treatment of ischemic episodes and cerebroprotection through Prostaglandin E2 (PGE2) EP2 and/or EP4 agonists | Ischemia | Katrin Andreasson |
| US 2010/0278875 A1 | Prostaglandin E2 (PGE2) as an adjuvant in monoclonal antibody generation | B cell | Jill Giles-Komar, Michael A. Rycyzyn |
| US 2011/0201684 A1 | EP2 Agonist from Non-Prostanoid Structures Designed as PGE2 Antagonists | Ocular hypertension | David F. Woodward, Jenny W. Wang |
To date, only one patent has been filed concerning with the effect of PGs on skeletal muscle[1]. Given the extensive usage of PGE2 in disorders in other tissues, there is a great potential for patenting the employment of PGE2 for the functional regulation of skeletal muscle and treatment of muscle diseases, such as muscle dystrophy. It is interesting to observe that patent US 8168428 describes an invention aimed at using PGE2 for the acceleration of growth of hematopoietic stem cells. Since skeletal muscle can use both resident satellite cells and hematopoietic stem cells for regeneration and repair, it would be interesting to test such approach in skeletal muscle cells. To a great extent, we shall present new and exciting data below that will present PGE2 as a potent signaling molecule in skeletal muscle cells.
In recent years, muscle work as endocrine organs has come to light. Skeletal muscles are the source of “myokines” with known effects on fat and body metabolism[10]. On the other hand, bone cells, and in particularly osteocytes are known to produce ATP, nitric oxide, prostaglandins (major one being PGE2), sclerostin, fibroblast growth factor 23, MCP-3, and many other factors [11-15]. Therefore, osteocytes can be viewed as a source and potential reservoir of factors in bone with the intrinsic potential of influencing other tissues. Given the intimate relationship between bone and muscle during development and life span[16], it is reasonable to assume that bone secreted factors might influence muscle behavior and function, but this hypothesis has not yet been investigated in a detailed and systematic manner.
To begin to address the potential biochemical relationship between bone and muscle, well established and recognized in vitro models of bone and muscle research were employed. C2C12 myoblasts, a well-accepted cell model for myogenic differentiation[17,18], and the osteocyte-like MLO-Y4 cells, a widely used cell model for osteocyte research, were used to investigate the potential effects of osteocyte signaling to muscle cells. As such, we focused our studies on the effects of MLO-Y4 CM and three potential factors derived from MLO-Y4 (PGE2, sclerostin, and MCP-3) on C2C12 myoblast differentiation into myotubes, gene PCR profile of 96 genes, and intracellular calcium homeostasis.
We found that MLO-Y4 CM (down to 10% CM) was a potent stimulant of myogenic differentiation, a phenomenon that was evidenced by morphological, functional, and genetic modifications that clearly indicate a myogenic effect of bone cells on muscle cells. Furthermore, our studies demonstrated that PGE2, but not sclerostin or MCP-3, induces significant myogenic differentiation that closely mirrors the effects of MLO-Y4 CM, suggesting that this major secreted prostaglandin from osteocytes could influence or modulate muscle function.
Methods
Cell culture
C2C12 myoblasts were maintained in high-glucose Dulbecco's modified Eagle's medium (Mediatech, Manassas, VA, USA) with 10% fetal bovine serum (FBS, Fisher, Pittsburgh, PA, USA), and 100U/ml penicillin and 100μg/ml streptomycin (Fisher) at 37°C in a humidified atmosphere with 5% CO2 for 24h. This media was removed and then, differentiation medium (2.5% horse serum, no FBS), was added to induce differentiation of myoblasts into contracting myotubes. MLO-Y4 cells were cultured in alpha modified essential medium with 2.5% fetal calf serum and 2.5% calf serum until 70% confluent before the collection of CM. Media without cells and osteoblasts CM were tested and found to have no effect on C2C12 cells (data not shown). The cells were treated with 25% MLO-Y4 osteocyte like cell conditioned media, 50nM PGE2 (Cayman Chemicals, Ann Arbor, Michigan, USA), 150μg/ml MCP-3 (R&D systems, Minneapolis, MN, USA), or 2μg/ml sclerostin (R&D systems) at the onset of differentiation. Images were taken after differentiation for 3 days using a Leica DMI 4000B microscope (Leica microsystems, Buffalo Grove, IL, USA), and myotube area (μm2) was automatically calculated and measured by the Leica Application Suite Advance Imaging fluorescence software package (Leica microsystems). At least 30 myotubes per well in each experiment were used for determination of cell area comparisons and experiments were repeated 6 times.
Intracellular Ca2+ measurements
A Photon Technology International (PTI, Birmingham, NJ, USA) imaging system was used to measure intracellular Ca2+ homeostasis. Each myotube imaged was loaded with 2μM Fura-2 AM for 30 min at 37°C, followed by application of 20mM caffeine (Fisher) with a perfusion system (Bioscience Tools, San Diego, CA, USA). Ratiometric analysis (350/375nm excitation ratio; 510nm emission) was performed using PTI's Felix 32 Photometry system. Both intracellular resting Ca2+ and the peak responses to caffeine treatment were measured in these studies. These experiments were repeated 3 times and at least 6 cells were tested on each experiment.
Real-time PCR Array
Total RNA was extracted from C2C12 myoblasts after 24h treatment using an RNAqueous®-4PCR kit (Ambion, Austin, TX, USA) according to the manufacturer's instruction, and was quantified in a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA) by determining absorbance at 260nm in triplicates. 1μg of each RNA sample with the A260/280 nm absorbance ratio of 1.9-2.1 was reverse transcribed in a 20μl reaction volume using a commercial Quantitec Reverse Transcription Reagents kit (QiagenInc, Valencia, CA). The expression of 91 genes of interest and 5 reference genes was investigated using a customized Mouse RT2Profiler™ PCR Array system (SA Biosciences). Genes were grouped into 5 main groups: heat shock/stress, survival and metabolic, Ca2+ signaling, Ca2+ release, and hypertrophy genes. qPCR was performed using the Step-One Plus ™ Real-Time PCR System (ABI, Foster City, CA, USA) via standard fluorescent methodology and thermal cycling conditions following the manufacture's recommendations, including a threshold of 0.2 and validation of each gene tested by the identification of single peaks in melting curves. The real-time PCR reaction mixture contained 1μl of 50ng of cDNA, 12.5 μl of the RT2 Real-Time TM SYBR Green/Rox PCR master mix, 1μl of primer pairs and 10.5 μl of RNase free water to a complete reaction of 25 μl. Data was analyzed using RT² Profiler™ PCR Array software (SA Biosciences), and relative amounts were calculated by the 2−ΔΔCT method as previous described[19]. CT values were normalized to ActB as a reference gene. Gene expression was determined as up/down regulation of the gene of interest compared to the control.
Expression levels of the candidate reference genes: We also investigated the expression levels and variability for five candidate reference genes (B2m, GAPDH, Hprt1, Rplp1 and Actb), both for control and PGE2 treatment. CT values from ActB showed an average of 0.3 cycle difference and an efficiency of 94% therefore, this gene was selected for normalization purposes.
Statistical analysis
One-way ANOVA with post hoc Dunnett's test was performed to compare myotube area between control and treatments. Results were expressed as mean ± SD. Differences were considered significant at P < 0.05.
Results
Histological association between bone and muscle
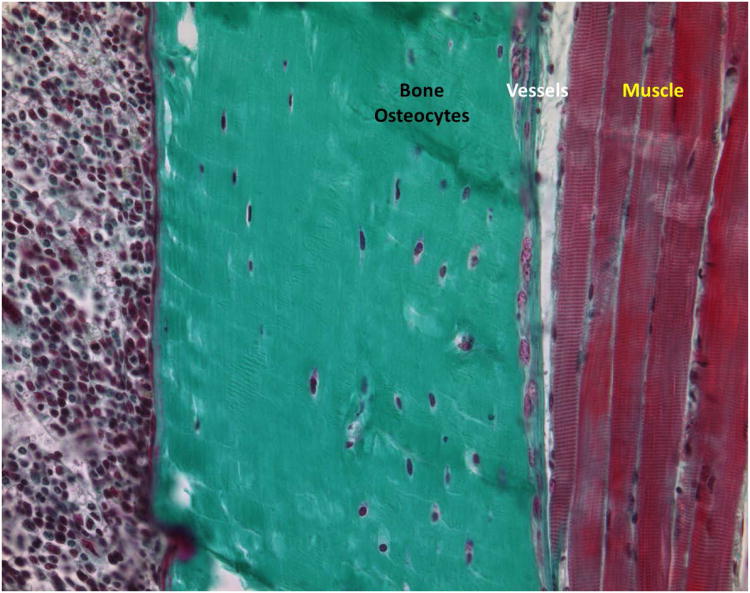
The intimate relationship between bone and muscle is clearly illustrated in Fig. 1. This figure also shows how closely associated these tissues are with the vascular tissue. The close association and functioning of these tissues suggests that they could reciprocally influence each other through secretion and uptake of specific molecules.
Figure 1. Histological evidence for the intimate association of bone-circulation-muscle.
Mineralized bone is shown in light green. The osteocytes stained in dark blue are the major cell component of mature bone. Vessels can be seen in between the bone and muscle interface. Given the overwhelming proof of tissues' crosstalk it seems feasible to infer that bone and muscle communicate biochemically either through paracrine or endocrine loops.
MLO-Y4 CM and PGE2 enhance myogenic differentiation of C2C12 myoblast
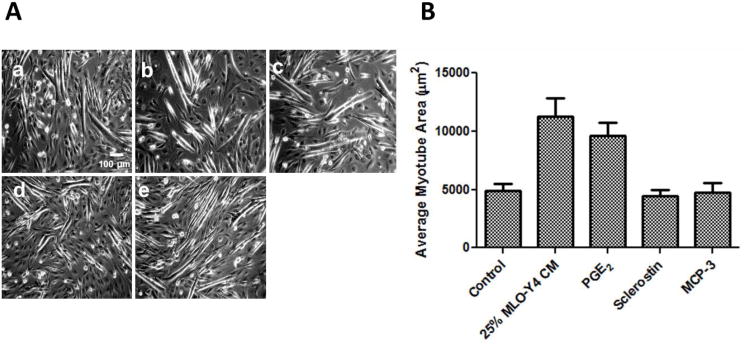
To examine the effect of bone-to-muscle signaling on myogenic differentiation, C2C12 cells were treated with MLO-Y4 osteocyte like cell CM, PGE2, MCP-3, or sclerostin when myoblast media was switched from complete growth medium to differentiation medium. Therefore, under all experimental conditions the effects of treatments were compared to the control effects of myogenic differentiation that are normally produced by removing FBS and replacing it with horse serum (2.5% in our study); therefore the effects of CM and bone specific factors are either seen as enhancing the already potent effects of differentiation media, or reducing, or having no effects. In this study, 25% CM and 50nM PGE2 were used in all experiments. We also performed dose response curves and found that 100, 50, 25, and 10% MLO-Y4 CM all promoted C2C12 myogenic differentiation, and 50nM PGE2, produced similar results to those produced by PGE2 at 0.25, 0.5, 1, and 5μM (Data not shown). Both 25% CM and 50nM PGE2 were selected because of the consistency and small intra-variability between experiments. After 72h of differentiation, C2C12 myotubes were visibly present in all conditions, but the number of developed myotubes was increased by both MLO-Y4 CM and PGE2 (number of visible myotubes per view: control: 7±2; 25% CM: 14±4, and PGE2: 13±3), but not by MCP-3 and sclerostin (9±3 and 10±4, respectively) (Fig. 2A). In addition, significant differences suggestive of cellular hypertrophy were observed in the newly formed myotubes (control cell area 4914±571μm2; CM cell area 10544±1466μm2; and PGE2 cell area 9833±1033 μm2) (Fig. 2B); however, MCP-3 and sclerostin did not have any effect on cell area (4741±831 and 4423±573 μm2, respectively).
Figure 2. MLO-Y4 CM and PGE2 significantly enhance myogenic differentiation of C2C12 myoblasts.
(A) Representative pictures of treatments taken 3 days after onset of differentiation. a) control; b) 25% MLO-Y4 CM; c) 50nM PGE2; d) 2μg/ml sclerostin; e) 150μg/ml MCP-3. (B) myotube area measurement shows that CM and PGE2, but not sclerostin and MCP-3 treatments significantly increase myotube area. *, significant different from control (P<0.05).
Change of gene expression profile after MLO-Y4 and PGE2 treatment
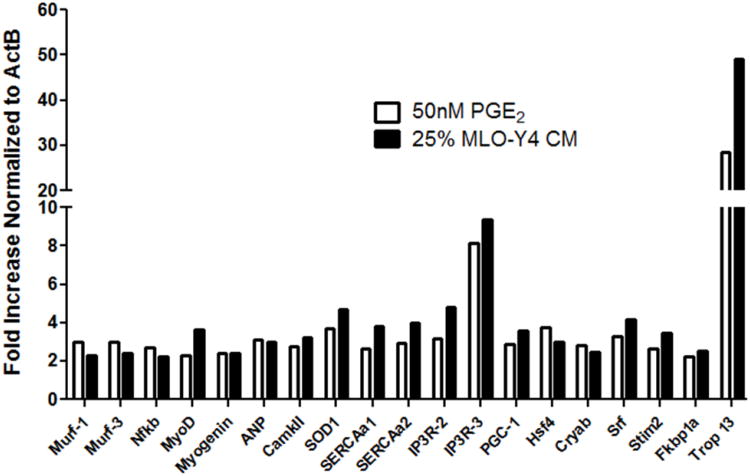
Next, to explore the mechanism(s) behind the effects of CM and PGE2, the expression of 91 genes related to cell myogenic differentiation, cell survival, Ca2+ signaling and homeostasis, cell metabolism, oxidative stress, and cell growth was measured. MyoD and myogenin, key transcriptional myogenic regulatory factors, were upregulated by 2-3 fold by CM and PGE2 24h after the onset of treatment (Fig. 3). Another important change in gene expression profile was the upregulation of Superoxide dismutase (SOD1), and Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), the master regulator of mitochondria biogenesis, by more than 3-fold by CM and PGE2, clearly demonstrating that cells treated with CM and PGE2 are adapting for higher metabolic demand. Last, the very high upregulation of troponin (49-fold and 28-fold by CM and PGE2, respectively) by 24h provided evidence that myoblasts were committed to differentiation into myotubes since they were developing their contractile machinery (Fig. 3).
Figure 3. Real time-PCR results of 91-gene array.
CM and PGE2 uprgulate the expression of myogenic regulatory factors, and genes related with Ca2+ homeostasis and mitochondria biogenesis in C2C12 myoblasts. Two arrays yielded similar results.
Monitoring modification of intracellular Ca2+ homeostasis caused by MLO-Y4 CM and PGE2
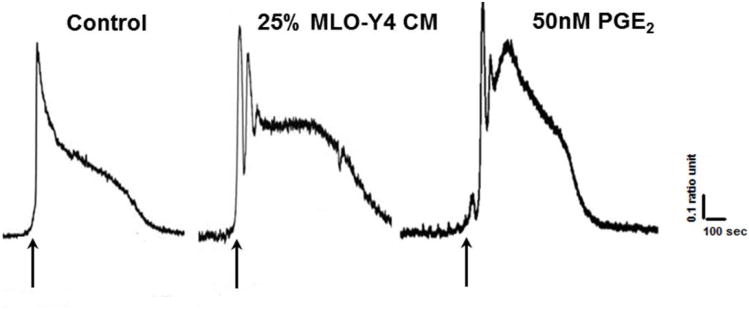
To physiologically link the morphometric and genetic observations of acceleration of myogenic differentiation produced by both CM and PGE2, intracellular calcium levels in the newly formed myotubes was measured. Our rationale was based on the fact that intracellular Ca2+ homeostasis is critical for muscle myogenesis and contractile function[20-22]. Therefore, intracellular calcium homeostasis could be used as an important functional surrogate between morphological and molecular modifications. The newly developed myotubes were loaded with the fluorescent dye Fura-2 AM, and exposed to 20mM caffeine to quickly and effectively release calcium from the sarcoplasmic reticulum of C2C12 myotubes[23-25]. This manipulation allows for monitoring of resting levels of calcium as well as the typical calcium transient that is produced in muscle cells in response to caffeine stimulation. As shown in Fig. 4, while control myotubes displayed the typical Ca2+ transient with a single peak in response to caffeine, myotubes in CM and PGE2 treated groups exhibited multiple and paced Ca2+ transients, suggesting first that more calcium is being release from the sarcoplasmic reticulum (SR), and second that the observed enhancement of calcium release might be related to stimulation of CICR, since caffeine acts in skeletal muscles by sensitizing the SR calcium release channels (Ryanodine receptor 1) to calcium, which is the operational molecular mechanism of CICR[26-28].
Figure 4. Modulation of Ca2+ homeostasis in myotubes by osteocyte signaling.
18-30 myotubes were tested for each condition. Arrows indicate addition of 20mM caffeine. While a single peak calcium transient is observed in response to caffeine under control conditions, multiple peaks are observed in cells treated with CM and PGE2.
Discussion
There are several examples of tissue crosstalk including fat-muscle, bone-kidney, and nerve-muscle[29-32], but a major gap remains in our knowledge regarding the likely important crosstalk between bone and muscle, two of the most intimately related tissues in animals and humans.
Despite the growing evidence of biochemical crosstalk between different body tissues, the traditional view of the muscle-bone relationship is that muscle applies load to the skeleton and that bone provides an attachment site for skeletal muscle – a relatively simple, mechanical relationship[16]. However, it is clear from pathological conditions, that muscle disease cannot fully explain concomitant bone disease. Some classical examples are sarcopenia where muscle atrophy cannot explain the totality of osteoporosis (e.g., muscle atrophy is much severer than bone loss during extended bed rest) and the well documented condition of loss of bone mass in astronauts in space that surpasses the loss in muscle mass and is difficult to recover upon return of astronauts to normal gravitational forces on earth[33-35].
Recently, it has been discovered that muscles, especially contracting muscles, are able to release peptides or proteins termed “myokines”, including interleukin(IL)-6[36], IL-8[37], IL-15[38] that appear to provide a reservoir of muscle derived factors for communication with other organs in the human body via autocrine, paracrine or endocrine mechanisms. The importance of these myokines for human fitness and their roles in diabetes and obesity is beginning to emerge and be recognized by the scientific community[39-41].
On the other hand, bone is also an endocrine organ. Osteocytes are the cells in skeleton that sense and respond to mechanical loads to regulate bone remodeling[42]. Upon sensing load, factors (a major one being PGE2) are produced and secreted by osteocytes which travel through their lacuna-canalicular system to the circulation, thereby reaching other tissues and organs[43]. These factors are also thought to be important for regulation of bone mass, resorption as well as bone formation. Intriguingly, the potential role of bone secreted factors on skeletal muscle function has not been considered an important research question. Given the proximity and similarities in developmental processes and their closely related functional aspects, i.e., bone and muscle work as a functional unit, it is important to investigate the possibility that bone and muscle crosstalk through secreted factors.
Obviously, a first level for these new studies is to investigate these potential effects of bone signaling to muscle using in vitro models. Our data strongly suggest that at least at the in vitro level, bone to muscle crosstalk occurs. Both MLO-Y4 CM and a major factor known to be secreted by osteocytes, PGE2, exert potent myogenic differentiation effects on C2C12 myoblasts as evidenced by morphological changes, robust modifications in gene expression profile, and very intriguing changes in intracellular calcium homeostasis. Thus, not only more and larger myotubes are formed in the presence of CM and PGE2, but also relevant functional and biochemical modifications occur within these newly formed myotubes that fully support the concept that osteocytes may induce or maintain myogenesis in muscles. Real-time PCR results provided genetic explanation for these effects. The upregulation of PGC-1α suggested mitochondria biogenesis was enhanced to match increased energy demand for myogenic differentiation. This finding was further supported by the upregulation of SOD1, likely reflecting adaptation to increased oxidative stress and the demands of larger myotubes. Moreover, the significant higher expression of troponin, a key maker for the organization and maturation of the contractile machinery in skeletal muscle, induced by CM and PGE2 treatments indicated that the differentiation from myoblasts into myotubes was promoted. Furthermore, our entire conclusion that myogenic differentiation was enhanced by CM and PGE2 was fully supported by upregulaiton of the two most important transcriptional regulatory factors, known as muscle regulatory factors, MyoD and myogenin.
Therefore, we believe that our results have added a new level of complexity to the bone-muscle loading relationship - the molecular coupling view. This view posits that the intimate association of muscle with bone allows for 1) soluble factors produced by osteocytes within bone to be targeted to muscle, and 2) muscle-derived soluble factors to be targeted to bone. In our study, the myogenic effect of MLO-Y4 CM was mimicked by PGE2 (but not by MCP-3 and sclerostin), suggesting that PGE2 could be an important and specific modulator of signaling from bone to muscle.
Conclusions
Using in vitro cell models allows insights into the bone-muscle relationship in a broader context. Sarcopenia and osteoporosis are normally related or simultaneously occurring conditions[35,36]. The current interpretation is that sarcopenia contributes to osteoporosis because muscles, through the loading forces of contraction and stretch, are anabolic stimulators of bones[37]. This view essentially negates the potential for a retrograde or backward signaling from bone to muscle. Our findings, from the basic in vitro level, demonstrate the potential for biochemical signaling from bone to muscle cells, suggesting that the bone-muscle relationship could be biologically more complex than previously thought and that bones might have much more influence on muscles than previously considered.
Obviously, additional studies in a diverse number of models, including primary cells, ex vivo and in vivo bone and muscle models of research will be required to provide an indepth view of bone to muscle biochemical crosstalk.
Acknowledgments
We thank Drs. Lara Nuria and Katharina Jaehn for maintaining MLO-Y4 cells and providing CM, and Dr.Sarah Dallas for the gift of sclerostin. This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institute of Health (NIAMS/NIH) grant RC2 AR058962 (LB, MB, MJ) and grants from Missouri Life Sciences Research Board(MB).
Footnotes
Conflict of Interest: Authors have no conflict of interest to declare
References
- 1.Scott NE. Prostaglandin E1/F2 in combination with prostaglandin F2.alpha for enhancing female sexual arousal. US6291528. 2001
- 2.Stjernschantz J. Method and composition for treatment of erectile dysfunction. US6476074. 2002
- 3.Klimko PG, Sharif NA, Griffin BW. Prostaglandin E agonists for treatment of glaucoma. US6545045. 2003
- 4.Llewellyn WC. Use of arachidonic acid as a method of increasing skeletal muscle mass. US6841573. 2005
- 5.Paralkar VM, Thompson DD. Use of prostaglandin (PGE2) receptor a (EP4) selective agonists for the treatment of acute and chronic renal failure. US6610719. 2003
- 6.Zon LI, North TE, Goessling W. Method to modulate hematopoietic stem cell growth. US8168428. 2012
- 7.Andreasson K. Treatment of ischemic episodes and cerebroprotection through Prostaglandin E2 (PGE2) EP2 and/or EP4 agonists. US2010/0267826 A1. 2010
- 8.Giles-Komar J, Rycyzyn MA. Prostaglandin E2 (PGE2) as an adjuvant in monoclonal antibody generation. US2010/0278875 A1. 2010
- 9.Woodward DF. EP2 Agonist from Non-Prostanoid Structures Designed as PGE2 Antagonists. US 2011/0201684 A1. 2011
- 10.Pedersen BK. Muscles and their myokines. J Exp Biol. 2011;214:337–46. doi: 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- 11.Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–14. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshiko Y, Wang H, Minamizaki T, Ijuin C, Yamamoto R, Suemune S, Kozai K, Tanne K, Aubin JE, Maeda N. Mineralized tissue cells are a principal source of FGF23. Bone. 2007;40:1565–73. doi: 10.1016/j.bone.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Tan SD, Bakker AD, Semeins CM, Kuijpers-Jagtman AM, Klein-Nulend J. Inhibition of osteocyte apoptosis by fluid flow is mediated by nitric oxide. Biochem Biophys Res Commun. 2008;369:1150–4. doi: 10.1016/j.bbrc.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Kitase Y, Barragan L, Qing H, Kondoh S, Jiang JX, Johnson ML, Bonewald LF. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the β-catenin and PKA pathways. J Bone Miner Res. 2010;25:2657–68. doi: 10.1002/jbmr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Löwik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–44. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 16.Schoenau E. From mechanostat theory to development of the “Functional Muscle-Bone-Unit”. J Musculoskelet Neuronal Interact. 2005;5:232–8. [PubMed] [Google Scholar]
- 17.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–7. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 18.Burattini S, Ferri P, Battistelli M, Curci R, Luchetti F, Falcieri E. C2C12 murine myoblasts as a model of skeletal muscle development: morpho-functional characterization. Eur J Histochem. 2004;48:223–33. [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Shen J, Yu WM, Brotto M, Scherman JA, Guo C, Stoddard C, Nosek TM, Valdivia HH, Qu CK. Deficiency of MIP/MTMR14 phosphatase induces a muscle disorder by disrupting Ca2+ homeostasis. Nat Cell Biol. 2009;11:769–76. doi: 10.1038/ncb1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X, Weisleder N, Thornton A, Oppong Y, Campbell R, Ma J, Brotto M. Compromised store-operated Ca2+ entry in aged skeletal muscle. Aging Cell. 2008;7:561–8. doi: 10.1111/j.1474-9726.2008.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hauser J, Saarikettu J, Grundström T. Calcium regulation of myogenesis by differential calmodulin inhibition of basic helix-loop-helix transcription factors. Mol Biol Cell. 2008;19:2509–19. doi: 10.1091/mbc.E07-09-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malgaroli A, Milani D, Meldolesi J, Pozzan T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987;105:2145–55. doi: 10.1083/jcb.105.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyrc K, Handran SD, Rothman SM, Goldberg MP. Ionized intracellular calcium concentration predicts excitotoxic neuronal death: observations with low-affinity fluorescent calcium indicators. J Neurosci. 1997;17:6669–77. doi: 10.1523/JNEUROSCI.17-17-06669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Struk A, Szücs G, Kemmer H, Melzer W. Fura-2 calcium signals in skeletal muscle fibresloaded with high concentrations of EGTA. Cell Calcium. 1998;23:23–32. doi: 10.1016/s0143-4160(98)90071-9. [DOI] [PubMed] [Google Scholar]
- 26.Reber BF, Stucki JW, Reuter H. Unidirectional interaction between two intracellular calcium stores in rat phaeochromocytoma (PC12) cells. J Physiol. 1993;468:711–27. doi: 10.1113/jphysiol.1993.sp019796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoesch RE, Weinreich D, Kao JP. A novel Ca(2+) influx pathway in mammalian primary sensory neurons is activated by caffeine. J Neurophysiol. 2001;86:190–6. doi: 10.1152/jn.2001.86.1.190. [DOI] [PubMed] [Google Scholar]
- 28.Endo M. Calcium-induced calcium release in skeletal muscle. Physiol Rev. 2009;89:1153–76. doi: 10.1152/physrev.00040.2008. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Schulz TJ, Espinoza DO, Huang TL, Emanuelli B, Kristiansen K, Tseng YH. Cross talk between insulin and bone morphogenetic protein signaling systems in brown adipogenesis. Mol Cell Biol. 2010;30:4224–33. doi: 10.1128/MCB.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab. 2010;95:4795–801. doi: 10.1210/jc.2010-1030. [DOI] [PubMed] [Google Scholar]
- 31.Yadav VK, Oury F, Tanaka KF, Thomas T, Wang Y, Cremers S, Hen R, Krust A, Chambon P, Karsenty G. Leptin-dependent serotonin control of appetite: temporal specificity, transcriptional regulation, and therapeutic implications. J Exp Med. 2011;208:41–52. doi: 10.1084/jem.20101940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol. 2009;310:21–9. doi: 10.1016/j.mce.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith SM, Wastney ME, O'Brien KO, Morukov BV, Larina IM, Abrams SA, Davis-Street JE, Oganov V, Shackelford LC. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the mir space station. J Bone Miner Res. 2005;20:208–18. doi: 10.1359/JBMR.041105. [DOI] [PubMed] [Google Scholar]
- 34.Sibonga JD, Evans HJ, Sung HG, Spector ER, Lang TF, Oganov VS, Bakulin AV, Shackelford LC, LeBlanc AD. Recovery of spaceflight-induced bone loss: bone mineral density after long-duration missions as fitted with an exponential function. Bone. 2007;41:973–8. doi: 10.1016/j.bone.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Zwart SR, Pierson D, Mehta S, Gonda S, Smith SM. Capacity of omega-3 fatty acids or eicosapentaenoic acid to counteract weightlessness-induced bone loss by inhibiting NF-kappaB activation: from cells to bed rest to astronauts. J Bone Miner Res. 2010;25:1049–57. doi: 10.1359/jbmr.091041. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen BK, Fischer CP. Beneficial health effects of exercise--the role of IL-6 as a myokine. Trends Pharmacol Sci. 2007;28:152–6. doi: 10.1016/j.tips.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Gray SR, Kamolrat T. The effect of exercise induced cytokines on insulin stimulated glucose transport in C2C12 cells. Cytokine. 2011;55:221–8. doi: 10.1016/j.cyto.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Argilés JM, López-Soriano FJ, Busquets S. Therapeutic potential of interleukin-15: a myokine involved in muscle wasting and adiposity. Drug Discov Today. 2009;14:208–13. doi: 10.1016/j.drudis.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen BK. The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem. 2006;42:105–17. doi: 10.1042/bse0420105. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen BK. The diseasome of physical inactivity--and the role of myokines in muscle--fat cross talk. J Physiol. 2009;587:5559–68. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoene M, Weigert C. The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obes Rev. 2008;9:20–9. doi: 10.1111/j.1467-789X.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- 42.Bonewald L. Osteocytes as multifunctional cells. J Musculoskelet Neuronal Interact. 2006;6:331–3. [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Wang Y, Han Y, Henderson SC, Majeska RJ, Weinbaum S, Schaffler MB. In situ measurement of solute transport in the bone lacunar-canalicular system. Proc Natl Acad Sci U S A. 2005;102:11911–6. doi: 10.1073/pnas.0505193102. [DOI] [PMC free article] [PubMed] [Google Scholar]